Summary
Little is known regarding the long-term adverse effects of COVID-19 on female-specific cancers, nor the shared genetic influences underlying these conditions. We performed a comprehensive genome-wide cross-trait analysis to investigate the shared genetic architecture between COVID-19 (infection, hospitalization, and critical illness) with three female-specific cancers (breast cancer (BC), epithelial ovarian cancer (EOC), and endometrial cancer (EC)). We identified significant genome-wide genetic correlations with EC for both hospitalization ( = 0.19, p = 0.01) and critical illness ( = 0.29, p = 3.00 × 10−4). Mendelian randomization demonstrated no valid association of COVID-19 with any cancer of interest, except for suggestive associations of genetically predicted hospitalization (ORIVW = 1.09, p = 0.04) and critical illness (ORIVW = 1.06, p = 0.04) with EC risk, none withstanding multiple correction. Cross-trait meta-analysis identified 20 SNPs shared between COVID-19 with BC, 15 with EOC, and 5 with EC; and transcriptome-wide association studies revealed multiple shared genes. Findings support intrinsic links underlying these complex traits, highlighting shared mechanisms rather than causal associations.
Subject areas: Virology, Public health, Cancer
Graphical abstract

Highlights
-
•
There is no valid causal association between COVID-19 and female-specific cancers
-
•
Multiple pleiotropic SNPs and genes link COVID-19 with female-specific cancers
-
•
Shared mechanisms rather than causality underlies COVID-19 and female-specific cancers
Virology; Public health; Cancer
Introduction
The global spread of coronavirus disease 2019 (COVID-19) has caused more than 760 million registered infections and 6.9 million deaths since December 2019 (World Health Organization (WHO): https://covid19.who.int/). Despite lungs being the organ predominately affected, a multi-system involvement of COVID-19 is well-characterized, with extra-pneumatic manifestations documented in hematologic, cardiovascular, neurological tissues, and others, possibly caused by direct viral virulence or as a result of immunopathological reactions.1,2 Moreover, while most COVID-19 patients recover within a couple of weeks after infection, a non-negligible proportion of individuals experience chronic symptoms lasting for months, especially in women.3,4 Given the enormous scale of the initial pandemic and its extensive impact on multiple bodily systems, there is increasing attention and concern regarding the potential long-term consequences faced by the millions of individuals who have recovered from COVID-19.3,5 This concern persists despite the WHO declaring on May 5, 2023 that COVID-19 is no longer a global public health emergency.6
Strong evidence has been raised reflecting the disparities in COVID-19 pandemic, potentially mediated through unique social determinants of health.7,8 Women, especially those with high health burdens are affected disproportionally by COVID-19.7,9 For example, individuals with breast cancer (BC) were nearly three times more likely to die from COVID-19 than their non-cancer referents (odds ratio, OR = 3.30; 95% confidence interval, 95%CI = 1.96–5.57).10 Among women with gynecological cancers, mainly epithelial ovarian cancer (EOC) and endometrial cancer (EC), a significantly increased mortality due to COVID-19 (14.0%)11 was found compared to general population (5.6%).12 Indeed, several shared signaling pathways, including cytokine, immunosuppression, coagulation disorders, inflammatory reactions, and hormone secretion,13,14,15,16 have been reported. Nevertheless, whether COVID-19 increases the susceptibility to cancer in those without prior malignancies remains unclear due to the hitherto restricted length of observational time. It is concerned that COVID-19 may predispose recovered patients to cancer development based on the growing evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in modulating oncogenic pathways, promoting chronic low-grade inflammation, and causing tissue damage.13,17
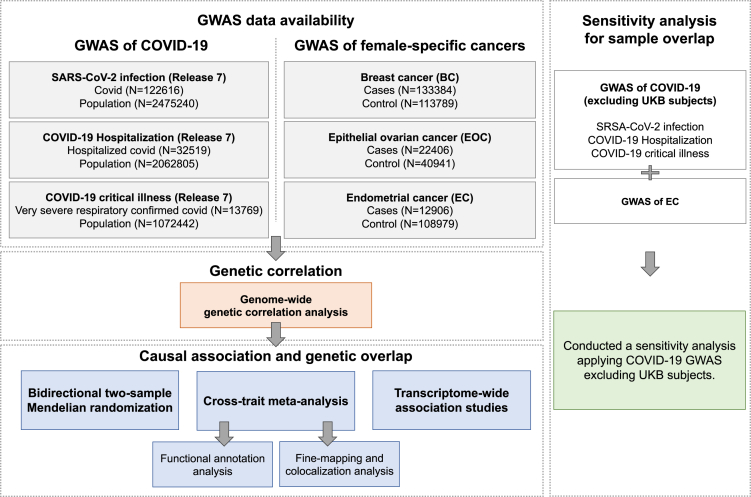
One way of evaluating the putative causal association underlying two phenotypes is to apply Mendelian randomization (MR),18 a framework leveraging genetic variants as instruments to overcome the limitation of conventional epidemiological designs, such as restricted observational duration, environmental confounder, and reverse association.19 Using other genetic methods including genetic correlation analysis,20 cross-trait meta-analysis,21 and transcriptome-wide association study (TWAS),22 shared genetic influences across traits can also be quantified, driving forward epidemiologic associations with novel insights into the underlying biological mechanisms. Here, we apply these methods to perform a comprehensive genome-wide cross-trait analysis,19 with an overarching goal of characterizing the shared genetic architecture and the putative associations underpinning COVID-19 and female-specific cancers (BC, EOC, and EC). Three COVID-19 phenotypes were included, namely SARS-CoV-2 infection, COVID-19 hospitalization, and COVID-19 critical illness. The overview of study design is shown in Figure 1. Details on the characteristics of each included dataset are presented in Table S1.
Figure 1.
Overall study design of genome-wide cross-trait analysis
GWAS summary statistics for each trait of interest were retrieved from publicly available GWAS(s). GWAS: genome-wide association study; UKB: UK Biobank.
Results
Genome-wide genetic correlation
For COVID-19 susceptibility, we found no evidence on a shared genetic basis with any of the female-specific cancers (BC: = 0.01, p = 0.90; EOC: = 0.01, p = 0.91; EC: = 0.09, p = 0.23; Table 1) using pairwise linkage-disequilibrium score regression (LDSC) (STAR Methods).20 For COVID-19 severity, we identified a suggestive genetic correlation for hospitalization with EC ( = 0.19, p = 0.01), as well as a significant genetic correlation for critical illness with EC ( = 0.29, p = 3.00 × 10−4). No significant result was found for COVID-19 severity with either BC (hospitalization: = 0.06, p = 0.16; critical illness: = 0.05, p = 0.28) or EOC (hospitalization: = 0.04, p = 0.55; critical illness: = 0.02, p = 0.77). Interestingly, for EC and COVID-19, both the magnitude and the significance of increased as the disease developed, from infection (0.09) to hospitalization (0.19) to critical illness (0.29).
Table 1.
Genetic correlation between female-specific cancers and COVID-19 phenotypes
| Cancer | COVID-19 phenotype | 95%CI | p-value | |
|---|---|---|---|---|
| BC | Infection | −0.01 | (-0.09,0.08) | 0.90 |
| Hospitalization | 0.06 | (-0.02,0.14) | 0.16 | |
| Critical illness | 0.05 | (-0.04,0.13) | 0.28 | |
| EOC | Infection | 0.01 | (-0.16,0.18) | 0.91 |
| Hospitalization | −0.04 | (-0.19,0.10) | 0.55 | |
| Critical illness | −0.02 | (-0.17,0.13) | 0.77 | |
| EC | Infection | 0.09 | (-0.06,0.24) | 0.23 |
| Hospitalization | 0.19 | (0.04,0.34) | 0.01 | |
| Critical illness | 0.29 | (0.14,0.45) | 3.00 × 10−4∗ |
Bold-face: p < 0.05; ∗p < 5.56 × 10−3.
: genetic correlation; CI: confidence interval; Infection: reported SARS-CoV-2 infection vs. population; Hospitalization: COVID-19 hospitalization patients vs. population; Critical illness: very severe respiratory confirmed COVID-19 patients vs. population; BC: breast cancer; EOC: overall invasive epithelial ovarian cancer; EC: endometrial cancer.
Bidirectional Mendelian randomization
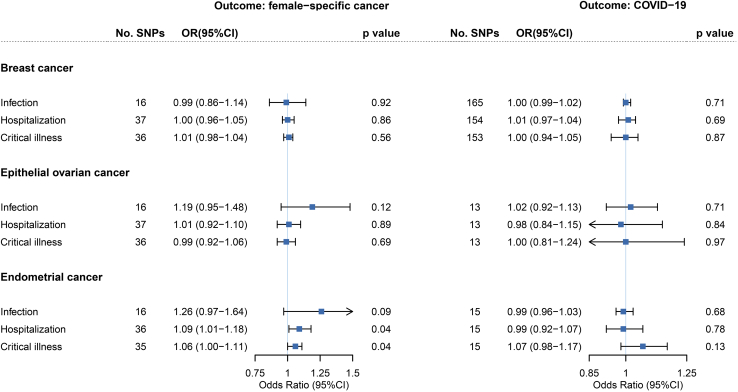
We continued to conduct an MR to evaluate potential associations of genetically predicted COVID-19 phenotypes on female-specific cancers risk, motivated by the significant shared genetic basis (STAR Methods). We identified and selected 16, 38, and 37 SNPs as instrumental variables (IVs) for infection, hospitalization, and critical illness of COVID-19. F-statistics suggested minimal weak instrument bias (Table S2). For COVID-19 susceptibility, we did not find any association with female-specific cancer (BC: ORIVW = 0.99, 95%CI = 0.86–1.14, p = 0.92; EOC: ORIVW = 1.19, 95%CI = 0.95–1.48, p = 0.12; EC: ORIVW = 1.26, 95%CI = 0.97–1.64, p = 0.09; Figure 2, Tables S3 and S4). For COVID-19 severity, genetically predicted hospitalization (ORIVW = 1.09, 95%CI = 1.01–1.18, p = 0.04) and critical illness (ORIVW = 1.06, 95%CI = 1.00–1.11, p = 0.04) were associated with the risk of EC under suggestive significance, none of which withstood multiple correction. The estimates remained directionally consistent using the MR-Egger regression (hospitalization: OR = 1.06, 95%CI = 0.91–1.24; critical illness: OR = 1.03, 95%CI = 0.93–1.13) or the weighted median approach (hospitalization: OR = 1.05, 95%CI = 0.95–1.16; critical illness: OR = 1.03, 95%CI = 0.97–1.10). No substantial alteration was found after excluding palindromic SNPs or pleiotropic SNPs, and the leave-one-out analysis demonstrated that the pooled estimate was not driven by any outlying variant. MR-PRESSO (MR-Pleiotropy Residual Sum and Outlier) yielded to similar findings. However, CAUSE (causal analysis using summary effect estimates) could not distinguish a model of causality from correlated pleiotropy for either hospitalization (p = 0.44) or critical illness (p = 0.13; Figures S1-S18). No association of genetically predicted COVID-19 severity was found for BC (hospitalization: ORIVW = 1.00, 95%CI = 0.96–1.05; critical illness: ORIVW = 1.01, 95%CI = 0.98–1.04) or EOC (hospitalization: ORIVW = 1.01, 95%CI = 0.92–1.10; critical illness: ORIVW = 0.99, 95%CI = 0.92–1.06).
Figure 2.
Bidirectional Mendelian randomization associations between COVID-19 phenotypes and female-specific cancers
On the left are the MR effect estimates of genetically predicted COVID-19 phenotypes on each female-specific cancer by the inverse-variance weighted approach. On the right are the MR effect estimates of genetically predicted female-specific cancer on COVID-19 phenotypes by the inverse-variance weighted approach. Boxes represent the point estimates of MR effects, and error bars represent 95% confidence intervals. p value is for MR estimates (IVW method).
In the reverse-direction MR where female-specific cancers were considered as exposures, we selected 168, 13, and 16 SNPs as IVs to proxy BC, EOC, and EC. F-statistics for these IVs suggested strong instruments (Table S5). None of the three genetically predicted female-specific cancers appeared to affect COVID-19 susceptibility or severity (Figure 2 and Table S6).
Cross-trait meta-analysis and pleiotropic loci
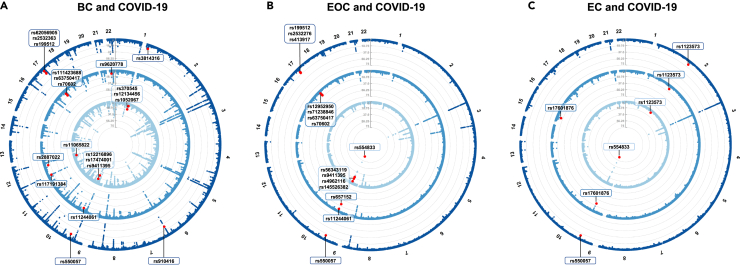
With little sign of vertical pleiotropy, we continued to perform cross-trait meta-analysis to reveal horizontal pleiotropic effect of individual variant using cross phenotype association (CPASSOC) (STAR Methods).21 A total of 20 independent pleiotropic SNPs were identified as shared by BC with at least one COVID-19 phenotype, including seven for infection, seven for hospitalization, and six for critical illness (Figure 3 and Tables S7-S10). These 20 SNPs were mainly located at genomic regions 17q21.31 (harboring WNT3, MAPT, CRHR1, and PLEKHM1), 9q34.2 (harboring ABO, LCN1P2, and REXO4), and 1q22 (harboring THBS3, GON4L, and PMF1). Notably, SNP rs910416 located at 6q25.1 showed the most significance (pCPASSOC = 1.90 × 10−29), followed by SNP rs17474001 at 9q34.2 (pCPASSOC = 2.48 × 10−26), and SNP rs9411395 at 9q34.2 (pCPASSOC = 4.71 × 10−26). We also identified a novel shared locus (index SNP rs1052067, pCPASSOC = 2.76 × 10−8) located at 1q22.
Figure 3.
Pleiotropic loci between female-specific cancers and COVID-19 phenotypes identified from cross-trait meta-analysis
(A) Pleiotropic loci identified for breast cancer and COVID-19 phenotypes.
(B) Pleiotropic loci identified for epithelial ovarian cancer and COVID-19 phenotypes.
(C) Pleiotropic loci identified for endometrial cancer and COVID-19 phenotypes. In each circular Manhattan plot, the circle from center to periphery shows the cross-trait meta-analysis results between each female-specific cancer and the three COVID-19 phenotypes (light blue: SRAS-CoV-2 infection, blue: COVID-19 hospitalization, dark blue: COVID-19 critical illness). The outermost numbers represent chromosomes 1–22. The red dots represent significant pleiotropic SNPs in cross-trait meta-analysis (pCPASSOC < 5 × 10−8 and psingle-trait<1 × 10−3 in both traits).
A total of 15 independent pleiotropic SNPs were identified as shared by EOC with at least one COVID-19 phenotype, including five for infection, six for hospitalization, and four for critical illness. These 15 SNPs were mainly distributed at two loci, 9q34.2 (harboring ABO, SURF4, and LCN1P2) and 17q21.31 (harboring WNT3, MAPT, CRHR1, and PLEKHM1). All top-three most significant SNPs were located at 9q34.2, including SNP rs554833 (pCPASSOC = 1.28 × 10−88), SNP rs657152 (pCPASSOC = 6.25 × 10−25), and SNP rs56343119 (pCPASSOC = 1.28 × 10−22).
A total of five independent pleiotropic SNPs were identified as shared by EC with at least one COVID-19 phenotype, including two for infection, three for hospitalization, and two for critical illness. Three out of the five shared SNPs were located at 9q34.2 (harboring ABO). Among the rest, SNP rs1123573 was located at 2p16.1 (harboring BCL11A), and SNP rs17601876 was located at 15q21.2 (harboring CYP19A1, RP11-108K3.1). Index SNP rs554833 at 9q34.2 showed the most significance (pCPASSOC = 3.29 × 10−85), followed by SNP rs657152 at 9q34.2 (pCPASSOC = 3.77 × 10−26), and SNP rs17601876 at 15q21.2 (pCPASSOC = 3.07 × 10−14).
Identification of causal variants and colocalization
For all identified pleiotropic SNPs, we determined a 99% credible set of causal SNPs using FM-summary (STAR Methods). A total of 4568 candidate SNPs were identified as the credible set of shared causal SNPs for BC and COVID-19 phenotypes. Corresponding figures in EOC and EC were 4893 and 106. Particularly, we identified only one candidate causal SNP in the 99% credible set for BC/infection (rs12216896), BC/hospitalization (rs2887022), and EC/hospitalization (rs1123573). Lists of candidate causal SNPs at each pleiotropic locus were shown in Table S11. List of SNPs in the 99% credible set identified from fine-mapping analysis for each CPASSOC-identified locus shared between COVID-19 and breast cancer, related to Figure 3, Table S12. List of SNPs in the 99% credible set identified from fine-mapping analysis for each CPASSOC-identified locus shared between COVID-19 and epithelial ovarian cancer, related to Figure 3, Table S13. List of SNPs in the 99% credible set identified from fine-mapping analysis for each CPASSOC-identified locus shared between COVID-19 and endometrial cancer, related to Figure 3.
We next performed colocalization analysis to determine whether genetic variants driving the association between different traits are the same (STAR Methods). We identified several loci to colocalize at the same candidate SNPs (PPH4 > 0.5, PPH4, the probability that both traits are associated through sharing a single causal variant), including four shared loci for BC and COVID-19 phenotypes, eight shared loci for EOC and COVID-19 phenotypes, and three shared loci for EC and COVID-19 phenotypes (Table S14).
Transcriptome-wide association studies and shared genes
We identified multiple independent gene-tissue pairs shared between female-specific cancers and COVID-19 phenotypes (Table 2) (STAR Methods).
Table 2.
TWAS-identified shared gene-tissue pairs between COVID-19 and female-specific cancers after conditional and joint analysis
| Gene | Tissue Type | CHR | No. SNPs | Female-specific cancer |
COVID-19 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | BEST. GWAS.ID | Z | pBonferroni | Subtype | BEST. GWAS.ID | Z | pBonferroni | ||||
| Breast cancer and COVID-19 | |||||||||||
| GBAP1 | Adrenal Gland | 1 | 328 | BC | rs4971059 | −6.73 | 3.89 × 10−6 | Infection | rs11264339 | 7.05 | 4.06 × 10−7 |
| GBAP1 | Artery Aorta | 1 | 328 | BC | rs4971059 | −6.49 | 1.96 × 10−5 | Infection | rs11264339 | 7.24 | 1.04 × 10−7 |
| GBAP1 | Artery Coronary | 1 | 328 | BC | rs4971059 | −5.68 | 3.10 × 10−3 | Infection | rs11264339 | 7.09 | 3.02 × 10−7 |
| GBAP1 | Artery Tibial | 1 | 328 | BC | rs4971059 | −6.30 | 7.05 × 10−5 | Infection | rs11264339 | 6.97 | 7.24 × 10−7 |
| GBAP1 | Cells Transformed fibroblasts | 1 | 328 | BC | rs4971059 | −6.44 | 2.72 × 10−5 | Infection | rs11264339 | 7.13 | 2.34 × 10−7 |
| GBAP1 | Esophagus Muscularis | 1 | 328 | BC | rs4971059 | −6.71 | 4.68 × 10−6 | Infection | rs11264339 | 6.80 | 2.39 × 10−6 |
| GBAP1 | Heart Atrial Appendage | 1 | 328 | BC | rs4971059 | −5.68 | 3.10 × 10−3 | Infection | rs11264339 | 7.09 | 3.02 × 10−7 |
| GBAP1 | Skin Not Sun Exposed Suprapubic | 1 | 328 | BC | rs4971059 | −6.84 | 1.79 × 10−6 | Infection | rs11264339 | 7.00 | 5.92 × 10−7 |
| GBAP1 | Skin Sun Exposed Lower leg | 1 | 328 | BC | rs4971059 | −6.62 | 8.19 × 10−6 | Infection | rs11264339 | 7.05 | 4.04 × 10−7 |
| ABO | Whole Blood | 9 | 595 | BC | rs495828 | −5.34 | 2.15 × 10−2 | Infection | rs612169 | −10.05 | 2.24 × 10−18 |
| KANSL1-AS1 | Artery Coronary | 17 | 29 | BC | rs17763086 | −6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| KANSL1-AS1 | Brain Anterior cingulate cortex BA24 | 17 | 29 | BC | rs17763086 | −6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| RP11-259G18.1 | Brain Cortex | 17 | 66 | BC | rs17763086 | −6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| CRHR1-IT1 | Stomach | 17 | 93 | BC | rs17763086 | −6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| RPS26P8 | Pituitary | 17 | 106 | BC | rs17763086 | −6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| RP11-707O23.5 | Artery Tibial | 17 | 111 | BC | rs17763086 | −6.15 | 1.77 × 10−4 | Hospitalization | rs8072451 | −7.87 | 7.98 × 10−10 |
| RP11-707O23.5 | Brain Hypothalamus | 17 | 111 | BC | rs17763086 | −6.03 | 3.72 × 10−4 | Hospitalization | rs8072451 | −7.58 | 7.84 × 10−9 |
| LRRC37A4P | Adrenal Gland | 17 | 138 | BC | rs17763086 | 6.03 | 3.93 × 10−4 | Hospitalization | rs8072451 | 7.57 | 8.91 × 10−9 |
| LRRC37A4P | Heart Left Ventricle | 17 | 138 | BC | rs17763086 | 6.07 | 3.05 × 10−4 | Hospitalization | rs8072451 | 7.65 | 4.79 × 10−9 |
| ABO | Whole Blood | 9 | 595 | BC | rs495828 | −5.34 | 2.15 × 10−2 | Hospitalization | rs657152 | −5.70 | 2.80 × 10−3 |
| RP11-707O23.5 | Artery Tibial | 17 | 111 | BC | rs17763086 | −6.15 | 1.77 × 10−4 | Critical illness | rs16940665 | −7.59 | 7.13 × 10−9 |
| RP11-707O23.5 | Brain Hypothalamus | 17 | 111 | BC | rs17763086 | −6.03 | 3.72 × 10−4 | Critical illness | rs16940665 | −7.40 | 3.10 × 10−8 |
| LRRC37A4P | Brain Nucleus accumbens basal ganglia | 17 | 138 | BC | rs17763086 | 6.03 | 3.82 × 10−4 | Critical illness | rs16940665 | 7.45 | 2.13 × 10−8 |
| MSTO2P | Muscle Skeletal | 1 | 236 | BC | rs11264372 | −6.45 | 2.65 × 10−5 | Critical illness | rs11803917 | −5.70 | 2.73 × 10−3 |
| MSTO2P | Pancreas | 1 | 236 | BC | rs11264372 | −6.28 | 7.94 × 10−5 | Critical illness | rs11803917 | −5.43 | 1.32 × 10−2 |
| HCN3 | Brain Nucleus accumbens basal ganglia | 1 | 298 | BC | rs4971059 | −7.75 | 2.13 × 10−9 | Critical illness | rs35154152 | −5.38 | 1.73 × 10−2 |
| GBAP1 | Brain Cerebellum | 1 | 328 | BC | rs4971059 | −7.01 | 5.75 × 10−7 | Critical illness | rs35154152 | −5.44 | 1.23 × 10−2 |
| MUC1 | Pancreas | 1 | 339 | BC | rs4971059 | 6.47 | 2.24 × 10−5 | Critical illness | rs35154152 | 5.25 | 3.59 × 10−2 |
| ABO | Whole Blood | 9 | 595 | BC | rs495828 | −5.34 | 2.15 × 10−2 | Critical illness | rs657152 | −5.57 | 5.98 × 10−3 |
| Epithelial ovarian cancer and COVID-19 | |||||||||||
| ABO | Artery Aorta | 9 | 595 | EOC | rs495828 | 5.60 | 4.92 × 10−3 | Infection | rs612169 | 17.33 | 6.75 × 10−62 |
| KANSL1-AS1 | Brain Anterior cingulate cortex BA24 | 17 | 29 | EOC | rs4566211 | 7.08 | 3.35 × 10−7 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| KANSL1-AS1 | Vagina | 17 | 29 | EOC | rs4566211 | 7.10 | 2.87 × 10−7 | Hospitalization | rs8072451 | −7.54 | 1.05 × 10−8 |
| RP11-259G18.2 | Small Intestine Terminal Ileum | 17 | 59 | EOC | rs4566211 | 7.16 | 1.82 × 10−7 | Hospitalization | rs8072451 | −7.58 | 7.80 × 10−9 |
| CRHR1-IT1 | Artery Aorta | 17 | 93 | EOC | rs17631676 | 7.11 | 2.66 × 10−7 | Hospitalization | rs8072451 | −7.55 | 9.99 × 10−9 |
| CRHR1-IT1 | Prostate | 17 | 93 | EOC | rs17631676 | 7.10 | 2.87 × 10−7 | Hospitalization | rs8072451 | −7.54 | 1.05 × 10−8 |
| RPS26P8 | Breast Mammary Tissue | 17 | 106 | EOC | rs17631676 | 7.08 | 3.35 × 10−7 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| RPS26P8 | Pituitary | 17 | 106 | EOC | rs17631676 | 7.08 | 3.35 × 10−7 | Hospitalization | rs8072451 | −7.57 | 8.91 × 10−9 |
| LRRC37A4P | Adrenal Gland | 17 | 138 | EOC | rs17631676 | −7.08 | 3.35 × 10−7 | Hospitalization | rs8072451 | 7.57 | 8.91 × 10−9 |
| PLEKHM1 | Brain Cortex | 17 | 162 | EOC | rs17631676 | 7.25 | 9.99 × 10−8 | Hospitalization | rs8072451 | −5.19 | 5.00 × 10−2 |
| ABO | Artery Aorta | 9 | 595 | EOC | rs495828 | 5.60 | 4.92 × 10−3 | Hospitalization | rs657152 | 8.79 | 3.49 × 10−13 |
| KANSL1-AS1 | Pancreas | 17 | 29 | EOC | rs4566211 | 7.12 | 2.61 × 10−7 | Critical illness | rs16940665 | −7.42 | 2.83 × 10−8 |
| KANSL1-AS1 | Vagina | 17 | 29 | EOC | rs4566211 | 7.10 | 2.87 × 10−7 | Critical illness | rs16940665 | −7.45 | 2.18 × 10−8 |
| RP11-259G18.2 | Small Intestine Terminal Ileum | 17 | 59 | EOC | rs4566211 | 7.16 | 1.82 × 10−7 | Critical illness | rs16940665 | −7.52 | 1.29 × 10−8 |
| RP11-259G18.1 | Brain Hippocampus | 17 | 65 | EOC | rs4566211 | 7.11 | 2.66 × 10−7 | Critical illness | rs16940665 | −7.46 | 1.94 × 10−8 |
| CRHR1-IT1 | Artery Aorta | 17 | 93 | EOC | rs17631676 | 7.11 | 2.66 × 10−7 | Critical illness | rs16940665 | −7.46 | 1.94 × 10−8 |
| LRRC37A4P | Brain Amygdala | 17 | 137 | EOC | rs17631676 | −7.11 | 2.66 × 10−7 | Critical illness | rs16940665 | 7.46 | 1.94 × 10−8 |
| ABO | Artery Aorta | 9 | 595 | EOC | rs495828 | 5.60 | 4.92 × 10−3 | Critical illness | rs657152 | 6.68 | 5.70 × 10−6 |
Infection: reported SARS-CoV-2 infection vs. population; Hospitalization: hospitalized COVID-19 patients vs. population; Critical illness: very severe respiratory confirmed COVID-19 patients vs. population; BC: breast cancer; EOC: overall invasive epithelial ovarian cancer; TWAS: transcriptome-wide association study; GWAS: genome-wide association study; SNP: single nucleotide polymorphism; CHR: Chromosome; ID: identifier; No. SNPs: number of SNPs in the locus; Z: Z value for TWAS.
A total of 11 genes were TWAS-significant for BC with at least one COVID-19 phenotype, including two with infection, seven with hospitalization, and seven with critical illness, enriched in tissues of adrenal gland, artery, brain, heart, pancreas, skin, stomach, and whole blood. Two genes were located at pleiotropic loci identified in cross-trait meta-analysis, including ABO (enriched in whole blood and shared by BC with all three COVID-19 phenotypes) and MSTO2P (enriched in muscle skeletal and pancreas).
A total of eight genes were TWAS-significant for EOC with at least one COVID-19 phenotype, including one with infection, seven with hospitalization, and six with critical illness, enriched in tissues of artery, adrenal gland, brain, breast mammary, pancreas, and vagina. Among these TWAS significant genes, ABO (enriched in artery aorta and shared by EOC with all three COVID-19 phenotypes), CRHR1-IT1 (enriched in artery aorta and prostate), and PLEKHM1 (enriched in brain cortex) were located at pleiotropic loci identified in cross-trait meta-analysis.
Sensitivity analysis for sample overlap
A significant genetic correlation between COVID-19 severity and EC as well as a suggestive effect of genetically predicted COVID-19 severity on EC risk were identified in the main analysis (STAR Methods). Given the sample overlap (both genome-wide association studies (GWASs) contained UK Biobank (UKB) individuals), we additionally conducted a sensitivity analysis applying COVID-19 GWAS excluding UKB subjects. Results of the sensitivity analysis remained consistent with the main analysis, including directionally consistent genome-wide genetic correlation (infection: = 0.04, p = 0.59; hospitalization: = 0.14, p = 0.03; critical illness: = 0.22, p = 1.70 × 10−3), marginal associations between genetically predicted COVID-19 phenotypes with EC risk (infection: ORIVW = 1.33, 95%CI = 1.06–1.66; hospitalization: ORIVW = 1.11, 95%CI = 1.03–1.1; critical illness: ORIVW = 1.06, 95%CI = 1.00–1.12), as well as two replicated pleiotropic SNPs (SNP rs1123573 and SNP rs550057, Table S16. Details of instrumental variables selected for COVID-19 phenotypes in sensitivity analysis using COVID-19 GWASs excluding individuals from UK Biobank, related to Figure 2, Table S17. Bidirectional Mendelian randomization estimates between COVID-19 phenotypes with female-specific cancers in sensitivity analysis using COVID-19 GWASs excluding individuals from UK Biobank, related to Figure 2, Table S18. Pleiotropic loci between COVID-19 phenotypes and endometrial cancer in sensitivity analysis using COVID-19 GWASs excluding individuals from UK Biobank, related to Figure 3).
Discussion
Leveraging the hitherto largest genetic data and novel statistical approaches, the current study performed a comprehensive genome-wide cross-trait analysis to systematically investigate the shared genetic influences underpinning COVID-19 and female-specific malignancies. Our study covered both susceptibility and severity of COVID-19, as well as the three most common female cancers, BC, EOC, and EC. From a genetic perspective, our work demonstrated biological links underlying these complex traits, highlighting shared mechanisms rather than potential causal associations.
COVID-19 presents a broad spectrum of clinical manifestations ranging from asymptomatic infection to death, with the host genetic determinants one of the main influential factors.2,23 For COVID-19 susceptibility, our study suggested no apparent genetic association with any of the examined female-specific cancers. While for COVID-19 severity, we found a significant genome-wide genetic correlation for EC with both COVID-19 hospitalization and critical illness, highlighting a non-trivial genetic component that is shared by cancer and a worse symptom of COVID-19. Notably, as the severity of infection develops, the overall COVID-19-EC genetic correlation increases, even with a decreasing sample size of corresponding COVID-19 GWAS. One conjecture is that the level of plasma cytokines and immune responses are higher in severe COVID-19 patients, both of which are well-established hallmarks for cancer initiation.24,25
A shared genetic basis can be the result of vertical pleiotropy and/or horizontal pleiotropy. In our downstream analysis performed to explore these alternatives, we identified no association of genetically predicted COVID-19 susceptibility with any cancer of interest, largely in line with the overall null genetic correlation. Two previous studies reported suggestive associations between genetically predicted SARS-CoV-2 infection with EC risk (OR = 1.37, 95%CI = 1.11–1.69; OR = 1.17, 95%CI = 1.01–1.34).26,27 However, these studies applied a limited number of IVs generated from an older version of COVID-19 GWAS (Release 4), which might reduce the precision of MR estimates due to insufficient power. With an eight-times augmented sample size of COVID-19 cases (122,616 vs. 14,134) and a larger number of IVs (16 vs. 3 or 13), our MR did not support for such potential associations. For COVID-19 severity, despite suggestive associations identified for genetically predicted hospitalization or critical illness on EC risk, none withstood multiple testing. While these findings were consistent with a previous MR reporting marginal associations (hospitalization: OR = 1.15, 95%CI = 1.00–1.31; critical illness: OR = 1.08, 95%CI = 1.01–1.15),27 both the significance and the magnitude of estimates in our study attenuated with a nearly four-times augmented sample size of severe cases (hospitalization: 32,519 vs. 6,404; critical illness: 13,769 vs. 4,336) and a five-times enlarged number of IVs (hospitalization: 38 vs. 7; critical illness: 37 vs. 7). Based on our current findings, a worse symptom of COVID-19 does not seem to represent a risk factor for EC development, while future investigations are warranted to further establish or rule out our findings. By applying a reverse directional MR design, we further confirmed that genetically predicted female-specific cancers appear not to affect either the susceptibility or the severity of COVID-19, concordant with a previous MR study.28
Taken together, our MR analysis delivers timely messages that may have important clinical and public health implications. We provide evidence suggesting that COVID-19 is not likely to pose a direct effect on the immediate risk of the examined female-specific cancers, suggesting that extra cancer screening in those recovered from COVID-19 may not confer a substantial public health benefit. In fact, the potential impact of COVID-19 pandemic on routine cancer screening should be given attention, which may lead to an increased burden of cancer mortality.29 Regarding the inconclusive effect of genetically predicted COVID-19 severity on EC risk, the marginal effect size reflects limited clinical significance. Our study, however, has not ruled out the possibility of subsequent increased risks of other chronic diseases, which, like cancer, is crucial for reducing disease burden and promoting health equity in post-COVID era.4,5 To identify other potential long-term sequelae of COVID-19, cancers in other tissues or of other sites, cardiovascular, hematological, neurological diseases, as well as possible long-term chronic inflammation, also require attention.
Contrary to the limited genetic evidence observed for vertical pleiotropy, our cross-trait meta-analysis revealed multiple horizontal pleiotropic loci shared between cancers and COVID-19, suggesting that the previously reported phenotypic links could be largely explained by common biological mechanisms. The shared signals identified for both the susceptibility and the severity of COVID-19 further validate the notion that overall genetic correlation may fail to detect pleiotropic effects at individual variant level. Notably, many of our identified cross-trait effects were previously implicated in hematologic systems (ABO, THBS3),23,30 immune response (WNT3, PLEKHM1, BCL11A, GON4L),13 cell proliferation (PMF1, TTC28, KANSL1), and hormone secretion (CRHR1, ESR1, CYP19A1),14,15 reflecting potential mechanistic pathways linking COVID-19 to tumorigenesis. Via colocalization analysis, multiple genes (ABO, WNT3, CUX2, SURF4, LCN1P2, CTD-2612A24.1, RP11-430N14.4) showed strong evidence of a shared causal mechanism (PPH4 > 0.5). Here, we highlight two interesting examples, ABO and WNT3, both are shared by COVID-19 with more than one investigated cancer.
ABO, a protein-coding gene involved in blood group systems biosynthesis and coagulation, is a well-known COVID-19 risk gene.23,31 In COVID-19 patients, ABO contributes to hypercoagulation states and thromboses by affecting plasma glycoproteins,31,32,33,34 meanwhile, such hypercoagulation states also frequently occurs in many cancer patients.35,36 By regulating the circulating levels of several pro-inflammatory and immune adhesion molecules, ABO might contribute to both tumorigenesis and COVID-19 development.37,38 WNT3 represents a typical immune-related gene and was identified as a shared gene for COVID-19 severity (rather than susceptibility) with cancer. By activating the WNT/β-catenin pathway, WNT3 plays a shaping role in tumor proliferation, migration and invasion, and functions in a variety of pathological processes including inflammation, metabolism, neurological development, and fibrosis processes.39,40 Demonstrated by previous studies, upregulation of the canonical WNT/β-catenin pathway in COVID-19 patients is associated with inflammation and cytokine storm,41 and such inflammatory immune responses are more likely to occur in patients with severe COVID-19.24,25 This may explain why immune-related genes such as WNT3 were mainly identified to be shared with COVID-19 severity. Our findings suggest critical roles of coagulation and immune responses in both COVID-19 and female-specific cancers regulations, which help pinpoint therapeutic targets for both diseases.
Integrating GWAS and GTEx tissue-specific expression data, our TWAS analysis further revealed biological pleiotropy at a gene-tissue pair level. Similar with findings from CPASSOC, we found shared genes between COVID-19 and cancers that are related to hematologic systems (ABO), immune function (MUC1, PLEKHM1), and cell proliferation (KANSL1-AS1). The multiple genes identified in blood vessel or heart tissues indicate a biological mechanism through the cardiovascular system, which corroborates well with the established knowledge as both COVID-19 and cancer are associated with a number of cardiovascular complications.34,42 In addition, we identified shared regulatory features in the nervous system, especially for COVID-19 severity. In fact, the neuro-invasiveness and neuro-invasion of SARS-CoV-2 have been well-characterized by previous studies, with more than 80% of severe COVID-19 patients showing neurological manifestations during the acute stage of their disease.43 Through peripheral nerves and/or the hematogenous route, viruses can access the cranial nerves and influence disease manifestation.43 Moreover, the importance of nervous system in cancer development has also been increasingly recognized.44 Cancer cells transduce neurotransmitter-mediated intracellular signaling pathways which may lead to their activation, growth, and metastasis.44 To sum up, these shared biologic pathways for COVID-19 and female-specific cancers implicate therapeutic strategies in clinical practice of the coexisting groups. More studies are needed to fully disclose the complex mechanisms.
Overall, our genetic analysis extends previous findings by highlighting an intrinsic link underlying female-specific cancers and COVID-19. COVID-19 is not likely to elevate the immediate risk of female-specific cancers (BC, EOC, EC), but rather appears to share mechanistic pathways with these conditions. Such common biological mechanisms are specifically substantiated by the pleiotropic loci and shared genes identified in our study, implicating therapeutic strategies for future clinical practice.
Limitations of study
Several limitations need to be acknowledged. First, due to unavailability of data, we conduct our analysis using sex-combined GWAS summary data of COVID-19 which may introduce sex heterogeneity. Future investigations leveraging large-scale sex-specific data may reduce this bias. Second, to avoid bias from population stratification, we chose GWAS data restricted to the European ancestry, limiting the generalizability to other ethnic groups. Third, the power of our MR analyses could still be limited by sample size, case proportion, and heritability of IVs, leading to the overall negative findings. However, by using data from the hitherto largest GWAS for COVID-19, our overall statistical power was considerably raised compared with previous genetic studies. We had 80% power at an alpha level of 0.05 to detect a 33% increased cancer risk with infection, a 43% increased risk with hospitalization, and a 47% increased risk with critical illness.45,46 Larger GWAS data are needed to validate our results in the future. Finally, we only used summary-level data rather than individual-level data due to data limitation. Compared with individual-level data, summary-level data, especially those from large consortia, often offer a larger body of sample size and scope, which enhance statistical power and precision of the causal estimates.18 However, the drawbacks of summary-level data are also obvious, as it is unable to consider important potential confounders for each individual, such as medication used in treatments, local social and medical conditions, etc. Ideally, future studies should validate our results using independent data.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| GWAS summary statistics for COVID-19 | COVID-19 Host Genetics Initiative | https://www.covid19hg.org/ |
| GWAS summary statistics for breast cancer | Zhang et al.47 | http://bcac.ccge.medschl.cam.ac.uk/bcacdata/ |
| GWAS summary statistics for epithelial ovarian cancer | Phelan et al.48 | http://ocac.ccge.medschl.cam.ac.uk/ |
| GWAS summary statistics for endometrial cancer | O'Mara et al.49 | https://www.ebi.ac.uk/gwas/publications/30093612 |
| Software and algorithms | ||
| LDSC | Bulik-Sullivan et al.20 | https://github.com/bulik/ldsc |
| TwoSampleMR | Hemani et al.50 | https://mrcieu.github.io/TwoSampleMR/ |
| CAUSE | Morrison et al.51 | https://github.com/jean997/cause |
| MR-PRESSO | Verbanck et al.52 | https://github.com/rondolab/MR-PRESSO |
| CPASSOC | Zhu et al.21 | http://hal.case.edu/∼xxz10/zhu-web/ |
| FM-summary | Farh et al.53 | https://github.com/hailianghuang/FM-summary |
| Coloc | Giambartolomei et al.54 | https://chr1swallace.github.io/coloc/ |
| PLINK | Purcell et al.55 | https://www.cog-genomics.org/plink/1.9/ |
| VEP | Howe et al.56 | https://grch37.ensembl.org/info/docs/tools/vep/index.html |
| FUSION | Gusev et al.22 | http://gusevlab.org/projects/fusion/ |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Xia Jiang (xiajiang@scu.edu.cn, xia.jiang@ki.se).
Materials availability
The study did not generate any new materials.
Experimental model and study participant details
Datasets of cancer
Three common female malignant tumors, the BC, EOC, and EC,57 were included in our study. GWAS summary data of BC was obtained from a meta-analysis of the Breast Cancer Association Consortium (BCAC) and 11 other BC genetic studies,47 involving 133,384 cases and 113,789 controls. GWAS summary data of overall invasive EOC was obtained from the Ovarian Cancer Association Consortium (OCAC) meta-analysis,48 involving 22,406 cases and 40,941 controls. GWAS summary data of EC was obtained from a meta-analysis of the Endometrial Cancer Association Consortium (ECAC), the Epidemiology of Endometrial Cancer Consortium (E2C2), and the UK Biobank,49 involving 12,906 cases and 108,979 controls. All individuals were of European ancestry.
Datasets of COVID-19
For COVID-19 phenotypes, we used the hitherto largest GWAS summary data of European ancestry conducted by the COVID-19 Host Genetics Initiative, release 7 (https://www.covid19hg.org/), from which subjects of 23andMe were excluded due to data restrictions.58 Three phenotypes were selected and further divided into two categories, representing COVID-19 susceptibility and severity. SARS-CoV-2 infection, defined as “cases with reported SARS-CoV-2 infection regardless of symptoms (N = 122,616) vs. population (N = 2,475,240)”, was used to index COVID-19 susceptibility. COVID-19 hospitalization, defined as “moderate or severe COVID-19 patients who were hospitalized due to COVID-19 symptoms (N = 32,519) vs. population (N = 2,062,805)”, and COVID-19 critical illness, defined as “severe COVID-19 patients who needed respiratory support or who died due to the disease (N = 13,769) vs. population (N = 1,072,442)” were used to index COVID-19 severity. Considering a potential sample overlap between GWAS of EC and GWAS of COVID-19 phenotypes (both involving UKB subjects), we further performed a sensitivity analysis using trans-ancestry COVID-19 GWAS excluding individuals of UKB.
Ethics statement
The ethical approval for each summary-level data can be found from the corresponding studies.
Method details
Genome-wide genetic correlation analysis
To describe the average shared genetic effect between female-specific cancers and COVID-19 phenotypes, we quantified their genome-wide genetic correlation using pairwise LDSC.20 The genetic correlation estimates range from −1 to +1, with +1 indicating a complete positive correlation and −1 indicating a complete negative correlation. We used pre-computed LD-scores obtained from ∼1.2 million common single nucleotide polymorphisms (SNPs) of European ancestry represented in the Hapmap3 reference panel. Bonferroni correction was applied to account for multiple testing.
Bidirectional Mendelian randomization analysis
The average shared genetic effects can be decomposed into vertical pleiotropy and/or horizontal pleiotropy, where vertical pleiotropy (or a putative causal association) refers to genetic variants affecting one trait (outcome) via its effect on an intermediate trait (exposure), and horizontal pleiotropy, often simplified as pleiotropy, refers to genetic variants affecting both traits independently.19 To further explore these alternatives, we first conducted a bidirectional two-sample MR between COVID-19 phenotypes and female-specific cancers. As no significant SNP was reported in the original COVID-19 GWAS, we selected independent IVs by clumping all variants that reached genome-wide significance (p < 5×10-8) according to a strict criterion (r2 ≤ 0.001 within a 1.0Mb window). For cancers, we collected all previously reported independent index SNPs reaching genome-wide significance (p < 5×10-8) from corresponding GWAS. We calculated the F-statistic to evaluate instrument strength, with an F-statistic < 10 indicating a weak instrument.59
We applied inverse-variance weighted (IVW) method as our primary approach,18,50 complemented with MR-Egger60 and weighted median61 to evaluate its robustness. To validate MR model assumptions, we conducted several important sensitivity analyses. First, we excluded palindromic IVs that have the same alleles on forward and reverse strands, and pleiotropic IVs that are associated with potential confounders according to GWAS catalog (https://www.ebi.ac.uk/gwas/, accessed on 05/08/2021). Next, we performed a leave-one-out analysis in which we excluded one IV at a time and conducted IVW using the remaining SNPs to identify outlying instruments. Finally, we used MR-PRESSO and CAUSE approaches to detect and correct for the potential uncorrelated and correlated horizontal pleiotropy.51,52
Cross-trait meta-analysis
To identify pleiotropic loci affecting both traits, we further performed a cross-trait meta-analysis using CPASSOC.21 We chose SHet, a statistic that is more powerful for heterogonous effects (common when meta-analysing different traits), to combine summary statistics across traits. We used PLINK 1.9 “clumping” function to obtain independent loci with parameters: --clump-p1 5e-8 --clump-p2 1e-5 --clump-r2 0.2 --clump-kb 500.55 Index SNPs, satisfying pCAPSSOC < 5×10-8 and psingle-trait < 1×10-3 (both traits), were considered as significant pleiotropic SNPs. An index SNP satisfying the following conditions was considered as a novel shared SNP: (1) did not reach genome-wide significance (5×10-8 < psingle-trait < 1×10-3) in single-trait GWAS; and (2) was not in LD (r2 < 0.05) with any of the previously reported genome-wide significant SNPs in single-trait GWAS. To further investigate biological insights for the shared variants, we use Ensemble Variant Effect Predictor (VEP) to annotate the linear closest genes of the identified pleiotropic SNPs.56
Fine-mapping credible set and colocalization analysis
Due to the complex LD patterns among SNPs, index SNPs are not necessarily causal variations.62 We conducted a fine-mapping analysis using FM-summary to identify a credible set of variants that were 99% likely to contain causal variants at each of the shared loci. FM-summary is a fine-mapping algorithm in Bayesian framework which maps the primary signal and uses a flat prior with steepest descent approximation.53
To assess whether the same variants are responsible for two GWAS signals or are distinct variants close to each other, we conducted a colocalization analysis using Coloc.54 Coloc is a tool in Bayesian framework that provides intuitive posterior probabilities of five hypotheses (H0-H4). We extracted summary statistics for variants within 500 kb of each shared index SNP and calculated the posterior probability for H4 (PPH4, the probability that both traits are associated through sharing a single causal variant). A locus was considered colocalized if PPH4 was greater than 0.5.
Transcriptome-wide association studies
Many genetic variants implement their function by modulating gene expression in different tissues, thus, considering gene expression and tissue specificity help clarify common biological mechanisms. We performed TWAS to identify genes whose expression is significantly associated with traits using FUSION.22 We integrated GWAS summary data with expression weights across 48 tissues from GTEx (Genotype-Tissue Expression, version 7), with one tissue-trait pair at a time. To identify an independent set of gene-tissue pairs, we conducted joint/conditional tests (an extension of TWAS) among regions with multiple identified signals.63 Shared gene-tissue pairs were determined through intersection across traits.
Quantification and statistical analysis
We used LDSC software (v1.0.1) for genome-wide genetic correlation analysis,20 R software (v4.1.0) with packages including “TwoSampleMR” (v0.5.4), “MRPRESSO” (v1.0), “MendelianRandomization” (v0.7.0), and “CAUSE” (v1.2.0) for MR analysis,51,52,60,61 CPASSOC software (v1.01) for cross-trait meta-analysis,21 and FUSION software for TWAS analysis.22
For genome-wide genetic correlation analysis and MR analysis, we set a statistical significance threshold of p < 5.56×10−3 (α = 0.05/9, number of phenotype pairs) after applying Bonferroni correction, while results with 5.56×10−3 ≤ p < 0.05 were defined as suggestive significance.64 We considered an MR effect estimate as robust if it was statistically significant in IVW and remained directional consistence across both the MR-Egger and the weighted median approaches. Regarding TWAS analysis, we applied Bonferroni-correction for all gene-tissue pairs tested (∼230,000 in total) within each trait, and defined pBonferroni < 0.05 as the significance threshold.64 For more information on each analysis (e.g., definition of significant pleiotropic SNPs), please refer to the “method details” section.
Acknowledgments
We thank all the patients, staff and investigators who contributed to the COVID-19 Host Genetics Initiative, BCAC consortium, OCAC consortium and ECAC consortium. This study was supported by funds from the National Natural Science Foundation of China (81874283, 81673255, 81874282), the National Key R&D Program of China (2020YFC2006505), the Health Commission of Sichuan Province (20PJ093), the Key R&D Program of Sichuan, China (2022YFS0055), the Recruitment Program for Young Professionals of China, the Promotion Plan for Basic Medical Sciences, the Development Plan for Cutting-Edge Disciplines, Sichuan University, and other Projects from West China School of Public Health and West China Fourth Hospital, Sichuan University. The Funders had no role in study design, data collection, data analyses, interpretation, or writing of the study.
Author contributions
Conceptualization: X.Z., X.W. Data curation: X.Z., X.W. Formal analysis: X.Z., X.W., J.X. Funding acquisition: J.L., X.J., B.Z. Investigation: X.Z., X.W., J.X., L.Z., Y.H. Methodology: J.X., L.Z., C.X. Project administration: X.Z., X.W., X.J. Supervision: B.Z., J.L., X.J. Writing-original draft: X.Z., X.W. Writing-review & editing: J.X., Y.H., X.J.
Declaration of interests
The authors have declared that no competing interests exist.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 29, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107497.
Supplemental information
Data and code availability
-
•
This paper analyzes existing, publicly available data. GWAS summary statistics of the COVID-19 Host Genetics Initiative are accessible at https://www.covid19hg.org/. GWAS summary statistics for breast cancer, epithelial ovarian cancer, and endometrial cancer can be downloaded from the GWAS catalog (https://www.ebi.ac.uk/gwas/) or from the websites of the consortium (http://bcac.ccge.medschl.cam.ac.uk/bcacdata/, http://ocac.ccge.medschl.cam.ac.uk/).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 4.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenharo M. WHO declares end to COVID-19's emergency phase. Nature. 2023 doi: 10.1038/d41586-023-01559-z. [DOI] [PubMed] [Google Scholar]
- 7.Hawkes S., Tanaka S., Pantazis A., Gautam A., Kiwuwa-Muyingo S., Buse K., Purdie A. Recorded but not revealed: exploring the relationship between sex and gender, country income level, and COVID-19. The Lancet. Global health. 2021;9:e751–e752. doi: 10.1016/s2214-109x(21)00170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans N.G., Berger Z.D., Phelan A.L., Silverman R.D. Covid-19, equity, and inclusiveness. BMJ (Clinical research ed.) 2021;373:n1631. doi: 10.1136/bmj.n1631. [DOI] [PubMed] [Google Scholar]
- 9.Ball E., Willmott F., Rivas C., Talati C. COVID-19 in Women's health: pre-operative gynaecological assessment and shared decision making. Best practice & research. Best Pract. Res. Clin. Obstet. Gynaecol. 2021;73:12–21. doi: 10.1016/j.bpobgyn.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugge M., Zorzi M., Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nat. Cancer. 2020;1:784–788. doi: 10.1038/s43018-020-0104-9. [DOI] [PubMed] [Google Scholar]
- 11.Lara O.D., Smith M., Wang Y., O'Cearbhaill R.E., Blank S.V., Kolev V., Carr C., Knisely A., McEachron J., Gabor L., et al. COVID-19 outcomes of patients with gynecologic cancer in New York City: an updated analysis from the initial surge of the pandemic. Gynecol. Oncol. 2022;164:304–310. doi: 10.1016/j.ygyno.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., Yee N.T.S., Liu C., Nerurkar S.N., Kai J.C.Y., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021;93:1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zong Z., Wei Y., Ren J., Zhang L., Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol. Cancer. 2021;20:76. doi: 10.1186/s12943-021-01363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmar H.S., Nayak A., Gavel P.K., Jha H.C., Bhagwat S., Sharma R. Cross Talk between COVID-19 and breast cancer. Curr. Cancer Drug Targets. 2021;21:575–600. doi: 10.2174/1568009621666210216102236. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari S., Dey Pereira S., Asare-Warehene M., Naha R., Kabekkodu S.P., Tsang B.K., Satyamoorthy K. Comorbidities and inflammation associated with ovarian cancer and its influence on SARS-CoV-2 infection. J. Ovarian Res. 2021;14:39. doi: 10.1186/s13048-021-00787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai C., Ahmed O.A., Shen H., Zeng S. Which cancer type has the highest risk of COVID-19 infection? J. Infect. 2020;81:647–679. doi: 10.1016/j.jinf.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini G., Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. 2021;43 doi: 10.1002/bies.202000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess S., Scott R.A., Timpson N.J., Davey Smith G., Thompson S.G., EPIC- InterAct Consortium Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015;30:543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z., Hasegawa K., Camargo C.A., Jr., Liang L. Investigating asthma heterogeneity through shared and distinct genetics: insights from genome-wide cross-trait analysis. J. Allergy Clin. Immunol. 2021;147:796–807. doi: 10.1016/j.jaci.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulik-Sullivan B., Finucane H.K., Anttila V., Gusev A., Day F.R., Loh P.R. ReproGen Consortium, Psychiatric Genomics Consortium, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3, Duncan L., et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X., Feng T., Tayo B.O., Liang J., Young J.H., Franceschini N., Smith J.A., Yanek L.R., Sun Y.V., Edwards T.L., et al. Meta-analysis of correlated traits via summary statistics from GWASs with an application in hypertension. Am. J. Hum. Genet. 2015;96:21–36. doi: 10.1016/j.ajhg.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gusev A., Ko A., Shi H., Bhatia G., Chung W., Penninx B.W.J.H., Jansen R., de Geus E.J.C., Boomsma D.I., Wright F.A., et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016;48:245–252. doi: 10.1038/ng.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao R., Xu Y., Zhu G., Zhou S., Li H., Han G., Su W., Wang R. Genetic variation associated with COVID-19 is also associated with endometrial cancer. J. Infect. 2022;84:e85–e86. doi: 10.1016/j.jinf.2022.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X., Peng H., Xiong S., Li C., Zhong R., He J., Liang W. Novel evidence revealed genetic association between COVID-19 infection, severity and endometrial cancer. J. Infect. 2022;85:e1–e3. doi: 10.1016/j.jinf.2022.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z., Wei Y., Zhu G., Wang M., Zhang L. Cancers and COVID-19 risk: a Mendelian randomization study. Cancers. 2022;14:2086. doi: 10.3390/cancers14092086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janda M., Paul C., Horsham C., PoCoG Cancer Prevention Special Interest Group Changes in cancer preventive behaviours, screening and diagnosis during COVID-19. Psycho Oncol. 2021;30:271–273. doi: 10.1002/pon.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C., Hu C., Su K., Wang K., Du X., Xing B., Liu X. The integrative analysis of Thrombospondin Family genes in Pan-cancer reveals that THBS2 Facilitates Gastrointestinal cancer metastasis. J. Oncol. 2021;2021 doi: 10.1155/2021/4405491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernández Cordero A.I., Li X., Milne S., Yang C.X., Bossé Y., Joubert P., Timens W., van den Berge M., Nickle D., Hao K., Sin D.D. Multi-omics highlights ABO plasma protein as a causal risk factor for COVID-19. Hum. Genet. 2021;140:969–979. doi: 10.1007/s00439-021-02264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui T., Titani K., Mizuochi T. Structures of the asparagine-linked oligosaccharide chains of human von Willebrand factor. Occurrence of blood group A, B, and H(O) structures. J. Biol. Chem. 1992;267:8723–8731. [PubMed] [Google Scholar]
- 33.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchini M., Favaloro E.J., Targher G., Lippi G. ABO blood group, hypercoagulability, and cardiovascular and cancer risk. Crit. Rev. Clin. Lab Sci. 2012;49:137–149. doi: 10.3109/10408363.2012.708647. [DOI] [PubMed] [Google Scholar]
- 35.Falanga A., Marchetti M., Vignoli A. Coagulation and cancer: biological and clinical aspects. J. Thromb. Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues C.A., Ferrarotto R., Kalil Filho R., Novis Y.A.S., Hoff P.M.G. Venous thromboembolism and cancer: a systematic review. J. Thromb. Thrombolysis. 2010;30:67–78. doi: 10.1007/s11239-010-0441-0. [DOI] [PubMed] [Google Scholar]
- 37.Barbalic M., Dupuis J., Dehghan A., Bis J.C., Hoogeveen R.C., Schnabel R.B., Nambi V., Bretler M., Smith N.L., Peters A., et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010;19:1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paterson A.D., Lopes-Virella M.F., Waggott D., Boright A.P., Hosseini S.M., Carter R.E., Shen E., Mirea L., Bharaj B., Sun L., et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler. Thromb. Vasc. Biol. 2009;29:1958–1967. doi: 10.1161/atvbaha.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grainger S., Willert K. Mechanisms of Wnt Signaling and Control. Wiley interdisciplinary reviews. Systems biology and medicine. 2018 doi: 10.1002/wsbm.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J., Xiao Q., Xiao J., Niu C., Li Y., Zhang X., Zhou Z., Shu G., Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022;7:3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallée A., Lecarpentier Y., Vallée J.N. Interplay of Opposing effects of the WNT/β-Catenin pathway and PPARγ and implications for SARS-CoV2 treatment. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.666693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roh J.D., Kitchen R.R., Guseh J.S., McNeill J.N., Aid M., Martinot A.J., Yu A., Platt C., Rhee J., Weber B., et al. Plasma Proteomics of COVID-19-associated cardiovascular Complications: implications for Pathophysiology and therapeutics. JACC. Basic Transl. Sci. 2022;7:425–441. doi: 10.1016/j.jacbts.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer L., Laksono B.M., de Vrij F.M.S., Kushner S.A., Harschnitz O., van Riel D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. 2022;45:358–368. doi: 10.1016/j.tins.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuol N., Stojanovska L., Apostolopoulos V., Nurgali K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018;37:5. doi: 10.1186/s13046-018-0674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim H., Chasman D.I., Smith J.D., Mora S., Ridker P.M., Nickerson D.A., Krauss R.M., Stephens M. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brion M.J.A., Shakhbazov K., Visscher P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013;42:1497–1501. doi: 10.1093/ije/dyt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Ahearn T.U., Lecarpentier J., Barnes D., Beesley J., Qi G., Jiang X., O'Mara T.A., Zhao N., Bolla M.K., et al. Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 2020;52:572–581. doi: 10.1038/s41588-020-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phelan C.M., Kuchenbaecker K.B., Tyrer J.P., Kar S.P., Lawrenson K., Winham S.J., Dennis J., Pirie A., Riggan M.J., Chornokur G., et al. Identification of 12 new susceptibility loci for different histotypes of epithelial ovarian cancer. Nat. Genet. 2017;49:680–691. doi: 10.1038/ng.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Mara T.A., Glubb D.M., Amant F., Annibali D., Ashton K., Attia J., Auer P.L., Beckmann M.W., Black A., Bolla M.K., et al. Identification of nine new susceptibility loci for endometrial cancer. Nat. Commun. 2018;9:3166. doi: 10.1038/s41467-018-05427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hemani G., Zheng J., Elsworth B., Wade K.H., Haberland V., Baird D., Laurin C., Burgess S., Bowden J., Langdon R., et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7 doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison J., Knoblauch N., Marcus J.H., Stephens M., He X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 2020;52:740–747. doi: 10.1038/s41588-020-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verbanck M., Chen C.Y., Neale B., Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farh K.K.H., Marson A., Zhu J., Kleinewietfeld M., Housley W.J., Beik S., Shoresh N., Whitton H., Ryan R.J.H., Shishkin A.A., et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giambartolomei C., Vukcevic D., Schadt E.E., Franke L., Hingorani A.D., Wallace C., Plagnol V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howe K.L., Achuthan P., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Azov A.G., Bennett R., Bhai J., et al. Ensembl 2021. Nucleic Acids Res. 2021;49:D884–D891. doi: 10.1093/nar/gkaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of Incidence and mortality Worldwide for 36 cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 58.COVID-19 Host Genetics Initiative The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur. J. Hum. Genet. 2020;28:715–718. doi: 10.1038/s41431-020-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burgess S., Thompson S.G., CRP CHD Genetics Collaboration Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 60.Bowden J., Davey Smith G., Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowden J., Davey Smith G., Haycock P.C., Burgess S. Consistent estimation in Mendelian randomization with Some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016;40:304–314. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaid D.J., Chen W., Larson N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018;19:491–504. doi: 10.1038/s41576-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gusev A., Mancuso N., Won H., Kousi M., Finucane H.K., Reshef Y., Song L., Safi A., Schizophrenia Working Group of the Psychiatric Genomics Consortium. McCarroll S., et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat. Genet. 2018;50:538–548. doi: 10.1038/s41588-018-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bland J.M., Altman D.G. Multiple significance tests: the Bonferroni method. BMJ (Clinical research ed.) 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
This paper analyzes existing, publicly available data. GWAS summary statistics of the COVID-19 Host Genetics Initiative are accessible at https://www.covid19hg.org/. GWAS summary statistics for breast cancer, epithelial ovarian cancer, and endometrial cancer can be downloaded from the GWAS catalog (https://www.ebi.ac.uk/gwas/) or from the websites of the consortium (http://bcac.ccge.medschl.cam.ac.uk/bcacdata/, http://ocac.ccge.medschl.cam.ac.uk/).
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.