Abstract
Vision is an important source of information about other minds for sighted children, especially prior to the onset of language. Visually observed actions, eye gaze, and facial expressions of others provide information about mental states, such as beliefs, desires, and emotions. Does such experience contribute causally to the development of cortical networks supporting social cognition? To address this question we compared functional development of brain regions supporting theory of mind (ToM), as well as behavioral ToM reasoning, across congenitally blind (n=17) and sighted (n=114) children and adolescents (4–17 years old). We find that blind children in this age range show slightly lower ToM behavioral performance relative to sighted children. Likewise, the functional profile of ToM brain regions is qualitatively similar, but quantitatively weaker in blind relative to sighted children. Alongside prior research, these data suggest that vision facilitates, but is not necessary for, ToM development.
Keywords: Blindness, Theory of Mind, Development, fMRI
1. Introduction
When we try to understand others’ actions and motivations, we use our “theory of mind” (ToM): an intuitive theory, or set of structured and systematically interconnected representations, that specifies the causal connections between mental states (beliefs, desires, emotions), actions, and events (Gopnik and Wellman, 1992). ToM enables us to predict that someone will search for something they want and to understand that failed searches are followed by disappointment. As children get older, their ToM reasoning improves: children reason about increasingly sophisticated mental state concepts (Peterson et al., 2012, Wellman and Liu, 2004) and develop an understanding of their causal connections (Harris et al., 2014). In parallel, brain regions associated with ToM reasoning show functional development. Adults and children reliably recruit a network of brain regions when they engage in ToM reasoning, including bilateral temporoparietal junction (R/LTPJ), precuneus (PC), and dorsal, middle, and ventromedial prefrontal cortex (D/M/VMPFC) (Adolphs, 2009, Carrington and Bailey, 2009, Fehlbaum et al., 2021). By three years of age, “ToM brain regions” are engaged during mental state reasoning, functionally correlated with one another, and functionally distinct from other brain regions (e.g., brain regions that process bodily sensations; Richardson et al., 2018). Throughout early to middle childhood, “ToM brain regions” become increasingly functionally correlated with one another (Richardson, 2019, Richardson et al., 2018, Xiao et al., 2019) and the right TPJ becomes increasingly selective for reasoning about minds, relative to other information about people (e.g., their physical appearances, relationships, and other non-mentalistic internal states; Gweon et al., 2012; Saxe et al., 2009; Saxe and Kanwisher, 2003; Saxe and Powell, 2006; Spunt et al., 2015).
An outstanding question is what drives neurocognitive ToM development (Carey and Spelke, 1996, Gopnik et al., 1994, Meltzoff, 1999). On the one hand, spontaneous looking and helping tasks have revealed a rich sensitivity to others’ goals, perceptions, and knowledge in toddlers (Knudsen and Liszkowski, 2011, Knudsen and Liszkowski, 2012, Onishi and Baillargeon, 2005; though see Poulin-Dubois et al., 2018; Powell et al., 2017). Moreover, functional near-infrared spectroscopy evidence suggests that right TPJ preferentially responds to scenarios depicting agents with false beliefs even in 7-month-old infants (Hyde et al., 2018). The adult theory of mind may be, in some respects, qualitatively similar to that of infants (Fodor, 1992, Scholl and Leslie, 1999).
At the same time, children’s representations of the mind are influenced by their experiences. ToM develops differently in children who have siblings (Perner et al., 1994, Jenkins and Astington, 1996, McAlister and Peterson, 2006) and depending on local cultural traditions (Selcuk et al., 2018, Shahaeian et al., 2011, Wellman et al., 2006). The clearest evidence of a causal influence concerns the role of linguistic experience in ToM development. Children who experience delayed access to language (i.e., d/Deaf children born to parents who do not know sign language at the time of their child’s birth) show delayed ToM development relative to hearing children and to d/Deaf children exposed to sign language from birth (Gale et al., 1996; Peterson et al., 2005; Schick et al., 2007), even when measured using non-linguistic ToM tasks (Figueras-Costa and Harris, 2001, Meristo et al., 2012). These children show corresponding delayed development of mental-state selective responses in RTPJ (Richardson, Koster-Hale et al., 2020) – suggesting that early linguistic experience facilitates neurocognitive ToM development. Remarkably, d/Deaf adults without access to a signed language continue to show differences in performance on ToM tasks with minimal linguistic demands; their performance improves with increased language access (Pyers and Senghas, 2009).
Can non-linguistic aspects of experience similarly drive neurocognitive ToM development? Complementary insights come from studies with children and adults who grow up without vision, i.e., people who are congenitally blind (Bedny and Saxe, 2012). There are many ways that visual experience could in principle contribute to ToM development: through first-person seeing/knowing experiences, through visually observing the expressions and actions of others, or through visually mediated social interactions. It is possible that people first directly experience gaining visual knowledge and subsequently use that experience to inform their metacognitive understanding of how seeing leads to knowing. For example, the experience of seeing yourself in the mirror and realizing that you are wearing a stained shirt may facilitate your understanding of someone else’s visual and emotional experience in an analogous situation (Bedny et al., 2009, Meltzoff and Brooks, 2008, Sommerville et al., 2005). Vision may also contribute to ToM development by providing access to the observable manifestations of others’ mental states and their causes and consequences (e.g., someone who frowns upon opening the cabinet likely had a false belief about its contents; Wu et al., 2018; Wu and Schulz, 2018). Finally, vision may facilitate early social interactions, especially when children are too young to engage in linguistic conversation (i.e., through eye contact and joint attention). These early social interactions may scaffold subsequent social development throughout childhood, including development of ToM (Wellman et al., 2008).
For any or all of these reasons, ToM development might be different in blind children. Yet by adulthood, blind and sighted individuals show similar behavior on ToM tasks and similar neural responses in ToM brain regions when reasoning about other minds – even on ToM tasks that specifically target visual experience (Bedny et al., 2009, Koster-Hale et al., 2014; for broader meta-analysis of social cognitive neuroscience studies with blind individuals, see Arioli et al., 2021). Unlike language, vision is thus not required to achieve typical adult behavioral and neural signatures of ToM. These studies leave open the possibility that vision plays a role in ToM development that is eventually compensated by language and other sources of information. By this account, the timecourse of development of ToM might differ among children who are blind.
Consistent with this hypothesis, multiple behavioral studies suggest that blind children pass explicit (linguistic) false-belief tasks later than sighted children (Begeer et al., 2014, Brambring, 2011, Brambring and Asbrock, 2010, Green et al., 2004, McAlpine and Moore, 1995, Minter et al., 1998, Pérez-Pereira and Conti-Ramsden, 2013, Peterson et al., 2000). These tasks typically test children’s ability to predict and/or explain the actions of a character who has a belief that is false. To “pass” these tasks, children must consider the beliefs of the character, rather than their own perspective/reality (Wellman et al., 2001). One methodological challenge inherent to conducting this research with blind children is that most traditional ToM tasks rely on visual aids and/or illustrations (Pérez-Pereira and Conti-Ramsden, 2005) and even non-visual ToM tasks can tap experiences and knowledge that are less available to blind children (Minter et al., 1998; see Brambring and Asbrock, 2010, for further discussion). Many prior studies on this topic also suffer from other methodological flaws, including failure to screen blind children for other disabilities and lack of a matched control group. Nevertheless, one study tested a relatively large sample of children (n=45 4–10 year old blind children and n=37 3–6 year old sighted children) using a battery of carefully controlled false-belief tasks based on tactile or auditory experience and found evidence for delayed ToM development among blind children (Brambring and Asbrock, 2010). For example, in one task, children listened to a familiar song and, upon pausing mid-song, were asked what they expected to hear next. Children then heard an unexpected ending to the song. Children were asked what their friend (who was not present) would expect to hear when she listens to the beginning of the song. Whereas sighted children reliably passed such false belief tasks at 5.1 years, blind children passed at 6.7 years. These results suggest that visual experience facilitates false belief task performance, and thus plausibly, ToM development more generally.
Complementary neuroimaging evidence can help characterize the contribution of visual experience to the development of cognitive and neural mechanisms involved in ToM. Specifically, if visual experience contributes to ToM development, then blind children might show transient delays in the development of brain regions that support ToM reasoning – e.g., delayed development of the expected functional response profile in ToM brain regions and mental-state selective responses in RTPJ. To our knowledge, there have not been fMRI studies of ToM development with blind children.
Here, we used complementary behavioral and neural measures to study ToM development in blind children. To characterize children’s ToM development behaviorally, we developed and validated a task that does not rely on the use of visual aids or illustrations, for use with four- to twelve-year-old children. During this task, an experimenter read short stories and asked questions that require children to reason about the characters’ mental states (beliefs, desires, emotions), moral blameworthiness, and lying behavior https://osf.io/pavdg/). We varied the modality of the evidence for a character’s beliefs (e.g., visual, aural, amodal) across items in order to test if blind children show differential comprehension of visual experiences in particular. To complement this new task, we also captured parents’ impressions of their child’s social development using the Children’s Social Understanding Scale (Tahiroglu et al., 2014).
To characterize children’s ToM development neurally, we used a fMRI experiment with aurally presented stories that does not rely on visual aids or illustrations and has been used in prior pediatric fMRI studies of ToM (Gweon et al., 2012, Richardson et al., 2020, Richardson et al., 2020). During this experiment, children listened to short stories that describe characters’ mental states (Mental condition), characters’ appearance and enduring relationships (Social condition), or non-social control stories that do not include agents (Physical condition).
We compared the functional response profile of the ToM network across blind and sighted children using previously identified functional signatures. First, we examined the overall response profile in ToM brain regions to the different story conditions. Previous studies have found that RTPJ, in particular, shows a highly selective response to mentalizing content, i.e., a larger response when reasoning about mental states as opposed to other information about people and reasoning about physical objects (Saxe et al., 2009, Saxe and Kanwisher, 2003, Saxe and Powell, 2006, Spunt et al., 2015). Based on prior research with sighted and blind adults and sighted children, we expected the Mental state stories to evoke the highest response, followed by the Social and then Physical control stories (Bedny et al., 2009, Gweon et al., 2012, Richardson et al., 2020). Second, we measured the extent to which responses were selective for mentalizing content, relative to non-mentalistic social content. Children with more selective RTPJ responses perform better on ToM behavioral tasks, controlling for age (Gweon et al., 2012). This selectivity measure is also reduced in d/Deaf children who experience delayed access to language (Richardson, Koster-Hale et al., 2020). Third, we examined inter-region correlations between ToM brain regions, which is reduced in young children and in children who fail explicit false-belief tasks, relative to those who pass, controlling for age (Richardson et al., 2018). Weaker inter-region correlations between ToM brain regions may reflect less communication between these regions and/or less functionally mature responses within any/all of these regions. Finally, we measured the extent to which the social content of stories predicts neural response pattern similarity in ToM brain regions. Prior research suggests that this measure is reduced in RTPJ in autistic children, relative to neurotypical children (Richardson, Gweon et al., 2020).
We compared these functional signatures of ToM brain regions across blind children and a large sample of sighted children who completed the same fMRI experiment, which included a subset of sighted children who were blindfolded during the scan and matched to the blind children on age, handedness, and MRI head coil.
2. Methods
2.1. Participants
Participants in the fMRI experiment were 17 congenitally blind children (4–17 years old, M(SD)=8.16(3.5); 8 girls) and 114 sighted children (4.5–16 years old, M(SD)= 8.77(2.2); 39 girls). Blind participants were blind due to pathology of the eye or optic nerve. All but one blind child had at most minimal light perception since birth; one child lost vision gradually between ages two and five years, ending with minimal light perception (see Supplementary Table 1 for details). All sighted participants had normal or corrected-to-normal vision. One blind child experienced a seizure early in life. None of the participants had known cognitive or neurological disabilities or history of head trauma.
A subset of the sighted children wore a total light-exclusion blindfold during the fMRI scan to reduce visual cortex responses to visual input during the scan, for a different study (n=18, 4.5–16 years old, M(SD)= 8.99(2.9); Bedny et al., 2015). Blindfolded and non-blindfolded sighted children are primarily treated as a single group for the current study, which focuses on responses in higher-cognitive networks. However, because blindfolded sighted children were closely matched on MRI head coil, age, and handedness, we also report statistics comparing blind children to blindfolded children in the Supplementary Materials and visualize their data separately.
Due to the low prevalence of congenital blindness, blind participants were recruited from across the United States of America over the course of four years (2010–2014). Information about the study was sent out to listservs and blindness organizations including the National Federation of the Blind, Perkins School for the Blind, the National Association for Parents of the Visually Impaired, Wonderbaby.org, and to local pediatric ophthalmologists. Sighted children were recruited locally.
An additional five blind and thirteen sighted (including six blindfolded) children were recruited but excluded from neuroimaging data analyses due to excessive motion and/or sleeping in the scanner (n=3 blind, n=7 sighted, see Methods for analysis of participant motion), completing only a single run of the fMRI experiment (n=5 sighted), incidental finding of abnormal brain anatomy (n=1 sighted), and suspected atypical early social experience in a group home (n=1 blind; did not complete behavioral testing) or diagnosis of an autism spectrum disorder and inability to complete the fMRI task (n=1 blind; did not complete behavioral testing).
All children were fluent English speakers at the time of the study. Two blind children were adopted from countries outside of the United States and learned English in monolingual environments upon their arrival in the United States at ages 28.5 and 27 months. All sighted children were native English speakers and over 70% were monolingual (Supplementary Table 1).
This study was approved by the institutional review board at MIT. All participants gave assent and their parents gave written informed consent.
Data from all participants have been reported in prior publications on visual cortex plasticity in blindness and theory of mind in sighted children (Bedny et al., 2015, Gweon et al., 2012, Lane et al., 2017, Richardson et al., 2020). None of these prior studies examined ToM responses in blind (or blindfolded) children.
2.2. Behavioral ToM task administered outside of the scanner
We developed a non-visual story-based task to measure ToM reasoning abilities in blind children aged four to twelve years (publicly available for download: https://osf.io/pavdg/), based on an existing task developed for sighted children (Gweon et al., 2012; https://osf.io/g5zpv/). During the non-visual ToM task, children listened to an experimenter read twenty short stories and answered 51 questions. Of these, 22 questions were two-alternative forced choice (2AFC) questions. The remaining 29 questions were open-ended and asked children to explain why a character acted as they did (explanation questions). Answering these questions correctly required children to consider the desires, expectations, beliefs, intentions, perceptual access, and knowledge of the characters. To investigate whether blind children’s lack of personal experience with vision leads to differences in reasoning about vision, the task included questions that targeted reasoning about the source modality of mental states (i.e., visual or aural sources; 9 questions each); the remaining questions involved amodal sources or both visual and aural sources. Nineteen control questions were included to verify children attended to and understood the story. Overall performance was calculated as the number of questions answered correctly out of all 2AFC and explanation questions; we additionally calculated performance on false-belief items and on items targeting particular source modalities of mental states, separately.
The behavioral ToM task was collected in a majority of blind children (n=12, 4–11 years, M(SD)=7.4(2.1), 4 girls) and sighted children who were blindfolded during the scan (n=21, 4–11 years, M(SD)=7.9(2.0), 14 girls). For one blind child, behavioral ToM data were collected opportunistically a year and a half after fMRI data collection. Additional sighted children over age twelve years (n=2, ages 13.5 and 16.2 years) and under age four years (n=1, 3.98 years) completed the behavioral task but were excluded from main analyses to tightly match groups in age; differences in results based on these exclusions are noted.
We validated the behavioral ToM task by testing if performance correlated with performance on a previously used ToM task (Gweon et al., 2012) in sighted children who completed both tasks (n=21; blindfolded). Performance on both tasks correlated with age (non-visual ToM task: rτ(19)=0.52, CI=[0.26,0.71], p=.001; Gweon et al., 2012’s ToM task: rτ(19)=0.37, CI=[0.08,0.61], p=.02). Performance on Gweon et al. (2012)’s ToM task correlated with performance on the non-visual ToM task even when controlling for age and verbal and non-verbal IQ (b=0.46, t=2.6, CI=[0.08,0.84], p=.02), as measured by verbal and non-verbal subtests of the Kaufman Brief Intelligence Test (KBIT-2; Kaufman, 1997).
2.3. Parent report measure of social reasoning
We additionally collected parent impressions of their child’s social understanding. Using complementary measures that vary in informant (child and parent) and format (lab-based task and questionnaire) provides a fuller picture of children’s social development (Campbell and Fiske, 1959, Whitcomb, 2013). Parents completed the Children’s Social Understanding Scale (CSUS), which has previously been validated as a measure of ToM (Tahiroglu et al., 2014). The CSUS involves reading statements about various social behaviors and rating how true those statements are of their child. Statements are designed to fall into one of six concept categories that are often studied in research on ToM development: perception, intention, knowledge, desire, emotion, and belief. CSUS data were collected for 16 blind children (4–18 years old, M(SD)=8.6(3.9) years) and 24 sighted (blindfolded) children (n=24, 4–16 years old, M(SD)=8.3(2.9) years). For five blind children, CSUS data were collected a year and a half after fMRI data collection via mailed-in questionnaires. CSUS summary scores correlated with performance on the non-visual ToM task, controlling for age (b=0.49, t=2.1, CI=[0.02,0.96], p=.04) in (sighted and blind) children with both measures (n=34).
2.4. Mock scan session
Prior to the MRI session, all children practiced lying still in an MRA Incorporated mock scanner (http://mra1.com/) while listening to a story, listening to music, or watching a cartoon (non-blindfolded sighted children) for 10–15 minutes. The sighted children who wore a blindfold during the scan also wore the blindfold during the mock scan to prepare them for this experience. Recordings of scanner noises played in the background to acclimate children to the scanner sounds. Head motion was monitored using an MRA Inc. Digital Video Motion Detector. When head motion was detected, the child’s story/music/cartoon paused for three seconds such that children learned to stay very still. Mock scans tend to reduce participant motion and increase MRI data retention in pediatric samples (de Bie et al., 2010).
2.5. FMRI experiment
Children listened to stories read in child-directed speech while undergoing fMRI. Stories either described a protagonist’s mental states (Mental condition), a protagonist’s social relationships and appearance (Social condition), or physical events with objects, not involving people (Physical condition). Only the Mental stories referenced mental states (beliefs, desires, and emotions). Each story was recorded by one of three native English-speaking women. Stories were matched across conditions for number of words (M=52.5 words), number of sentences (M=4.7), and Flesch Reading Ease Level (M=85.7), as well as duration and volume. Story stimuli have been described in previous publications (Bedny et al., 2015, Gweon et al., 2012, Richardson et al., 2020) and are publicly available for download (https://osf.io/cbw6f/).
In order to ensure attention to the stories, children performed a “Does this come next?” task. On each trial, children listened to a 20-second story followed by the question “Does this come next?” (1.5 s). They then heard a probe sentence (3 s) that was either a continuation of the same story or a sentence from a different story in the experiment. Children judged whether the probe sentence was the correct continuation of the initial story. The correct answer was “yes” 50% of the time. Each probe sentence was followed by a 6.5-second pause during which the child’s response was collected via a button press. Following each trial children heard a 5-second motivational auditory clip which said “Great Job! Get ready for the next one” for correct responses or “Alright. Here comes another one!” for incorrect responses. Children completed seven practice trials of this task prior to the fMRI scan.
In addition to the English stories, children listened to 20-second clips of foreign speech and instrumental music. The foreign speech and music conditions were included in the experiment for analyses of cortical responses to language (Bedny et al., 2015, Lane et al., 2017); responses to these conditions were not analyzed for the present study and so are not further described. Non-blindfolded sighted children saw colorful swirl images during the stories, foreign speech, music clips, and rest periods. During the prompt, story ending, and response portion of the experiment, these children saw an image of a check (left) and an “X” (right), which corresponded to the “yes” and “no” response options. Behavioral responses were not collected from 4 blindfolded children and 2 sighted (non-blindfolded) children due to technical error, nor from 1 blind child who was not provided with the button box due to her young age. All children were monitored for attention during the scan by an experimenter who stood near their feet. Both groups performed at above-chance levels in all conditions on the “Does this come next?” task (M(SE) accuracy, Mental: blind:.75(0.07), sighted:.88(0.02); Social: blind:.76(0.07), sighted:.88(0.02); Physical: blind:.68(0.09), sighted:.91(0.01); one-tailed t-tests: all ts> 2.1, ps< 0.05), indicating engagement with the experiment. Performance improved with age (full sample: b=0.32, t=6.6, CI=[0.22,0.41], p=1.8−10; blind and blindfolded: b=0.21, t=2.1, CI=[0.01,0.42], p=.04) and blind children performed worse than sighted children (full sample: b=−0.72, t=−5.1, CI=[−1,−0.44], p=5.7−7; blind and blindfolded: b=−0.55, t=−2.7, CI=[−0.95,−0.15], p=.008). There were no significant interactions among group, condition, or age variables on task accuracy (see Supplementary Figure 1).
Stimuli were presented through Matlab 7.6, 7.10, or 2010a running on an Apple MacBook Pro. The order of all five conditions was counterbalanced across runs and participants and palindromic within a run; rest blocks occurred at the start, halfway point, and end of each run (i.e., Rest A B C D E Rest E D C B A Rest). Each run consisted of ten experimental trials (36 s each) and three rest blocks (12 s each), for a total run-time of 6.6 minutes. The full experiment consisted of four runs of the functional task, with 2 trials per run per condition, for a total of 8 trials per condition in the full experiment.
2.6. FMRI data acquisition
Participants were scanned on a 3T Siemens scanner at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT using the standard 12-channel head coil (blind and blindfolded participants, n=20 non-blindfolded sighted children) or one of two custom 32-channel phased-array head coils made for younger (n=18 non-blindfolded sighted) or older children (n=37 non-blindfolded sighted; Keil et al., 2011), or the standard Siemens 32-channel head coil (n=19 non-blindfolded sighted; coil unknown for n=2 non-blindfolded sighted children). Given differences in head coil across samples, we control for head coil in analyses that compare the full sample of sighted children to blind children. We also report results comparing blind and blindfolded sighted children, who were scanned using the same head coil, in the Supplementary Materials.
T1-weighted structural images were collected in 128 (12ch coil) or 176 (all others) interleaved sagittal slices with 1.33 mm (12ch coil) or 1 mm (all others) isotropic voxels (GRAPPA parallel imaging, acceleration factor of 3; adult coil: FOV: 256 mm; pediatric coils: FOV: 192 mm). Four runs of functional data were collected with a gradient-echo EPI sequence sensitive to Blood Oxygen Level Dependent (BOLD) contrast in 3×3×4 mm (12ch coil) or 3 mm isotropic voxels (all others) in 30 (12ch coil) or 32 (all others) interleaved near-axial slices aligned with the anterior/posterior commissure, and covering the whole brain (with the exception of the cerebellum; TR=2 s, TE = 30 ms, flip angle=90°, 198 volumes). Prospective acquisition correction (PACE) was used to adjust the position of the gradients based on the participant’s head motion one TR back (Thesen et al., 2000). Four dummy scans were collected to allow for steady state magnetization.
A researcher remained inside the scanner room during the scan. If the participant moved noticeably, this researcher placed her hand on the child’s leg as a reminder to stay still.
2.7. FMRI data analysis
FMRI data were analyzed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and custom software written in Matlab. All preprocessing decisions match those used in recent publications of similar pediatric fMRI data (https://osf.io/wzd8a/; Richardson, Gweon et al., 2020). Each individual’s functional data were realigned to the first functional image of the first run, which was registered to each individual’s anatomical image. Anatomical images were normalized to the standard MNI template. All data were smoothed using a 5 mm kernel (Gaussian filter, full width half max).
We used the artifact rejection toolbox to identify motion artifacts as timepoints in which a participant moved 2 mm or more relative to the previous timepoint, and/or timepoints that showed a global signal change greater than three standard deviations from the mean (https://www.nitrc.org/projects/artifact_detect/; Whitfield-Gabrieli et al., 2011). For each participant, we dropped runs in which 1/3 or more of the timepoints were identified as motion spikes. Participants were excluded from further neuroimaging analyses if they had fewer than two runs of usable data. The number of excluded timepoints and the mean translation between functional images (“motion”, calculated prior to the exclusion of motion spikes) did not differ between blind and sighted children (excluded timepoints: Cohen’s d=0.17, negligible effect; motion: Cohen’s d=0.04, negligible effect; Supplementary Figure 2). There were medium effects of group on both of these measures in the blind vs. blindfolded children subset (excluded timepoints: Cohen’s d=−0.53, medium effect; motion: Cohen’s d=−0.56, medium effect; Supplementary Figure 2). The effect sizes for correlations between these measures and age (excluded timepoints: r(129)=−0.16, small effect; motion: r(129)=−0.18, small effect) and ToM behavior, controlling for age (excluded timepoints: r(24)=0.06, negligible effect; motion: r(24)=−0.10, negligible effect) were small or negligible. Motion was included as a covariate in all linear regression analyses that tested for between-subject and between-group differences in neural responses.
A general linear model was used to analyze BOLD data from each participant as a function of condition. Data were modeled using a boxcar regressor convolved with a canonical hemodynamic response function (HRF). Nuisance regressors for run effects, motion spikes, and five aCompCor regressors defined from individual white matter masks (Behzadi et al., 2007), eroded in two voxels in each direction, were included in the model. Data were high-pass filtered (128 seconds/cycle, or 0.0078 Hz, applied after interpolation over artifact timepoints) and underwent SPM’s image scaling.
2.7.1. Whole-brain analysis
Second-level random effects analyses were conducted to examine contrast activation (Mental > Physical) within each group and to test for significant differences between the groups. To correct for multiple comparisons, non-parametric whole-brain analyses were performed using the SnPM toolbox for SPM5, which estimates the false-positive rate directly from the data via 5000 Monte Carlo permutations (3 mm variance smoothing, no global normalization, grand mean scaling, or threshold masking). The corrected p-value for filtering was 0.05, with an uncorrected T-value minimum threshold of 3 and a voxel-cluster combining theta value of 0.5. Voxel-cluster combining was performed jointly using Fisher, Tippet, and Mass voxel-cluster combining functions. Results of these analyses were additionally viewed at a more lenient threshold (voxel-wise p<.05 and cluster-wise k=10, uncorrected).
2.7.2. Response magnitude and selectivity in theory of mind regions of interest
Following previous fMRI studies of neural correlates of theory of mind development, we measured the neural response to each condition (Mental, Social, Physical) and the selectivity of responses for mental state content, relative to non-mentalistic social content (Gweon et al., 2012, Richardson et al., 2020), in brain regions previously identified as important for ToM reasoning (“ToM brain regions”): bilateral TPJ, precuneus (PC) and dorso-, mid-, and ventromedial prefrontal cortex (D/M/VMPFC) (Adolphs, 2009, Carrington and Bailey, 2009, Fehlbaum et al., 2021). Since previous studies have observed brain-behavior correlations with RTPJ selectivity, we examined responses in this region separately (Gweon et al., 2012, Richardson et al., 2020).
Region of interest (ROI) analyses were performed in group-defined and individual-subject ROIs. Group ROIs were defined by drawing 10 mm spheres around ToM responsive peaks within search spaces created in a large adult dataset (https://saxelab.mit.edu/use-our-theory-mind-group-maps; Dufour et al., 2013), excluding voxels that overlapped with an independent set of language responsive regions (described below and visualized in Supplementary Figure 4; to download ROIs: https://osf.io/pavdg/). Beta values were extracted from group ROIs in order to examine the effect of each condition (Mental, Social, Physical) on the response magnitude in ToM ROIs. Within group ROIs, selectivity was defined as the average beta estimate to Mental > Social * 100. These exact group ROIs and selectivity measure have been used in prior studies (Richardson, Koster-Hale et al., 2020; https://osf.io/wzd8a).
To define threshold-independent individual-subject ROIs within each child, we identified the 80 voxels with the highest t-value to the Mental > Physical contrast within the same search spaces described above (Dufour et al., 2013). The choice of 80 voxels was based on prior work (Kliemann et al., 2018, Richardson et al., 2020, Skerry and Saxe, 2014). Beta values were extracted from individual-subject ROIs and visualized (Supplementary Figure 5); statistics were not run on these beta values as they were non-independent from individual-subject ROI definition. Within individual-subject ROIs, selectivity was defined as the average value of the Mental>Social contrast*100. See Supplementary Materials for similar results in threshold-dependent individual-subject ROIs, defined according to prior research (Richardson, Koster-Hale et al., 2020; https://osf.io/wzd8a) and for further details about individual-subject ROI analysis decisions.
2.7.3. Inter-region correlation analyses of functional responses within and across ToM and language brain regions
We explored two additional aspects of the ToM neural response. First, we used inter-region correlation analyses to measure correlations in functional responses within and across ToM and language brain regions (Paunov et al., 2019). Prior research with children (using a different task) has found that responses in ToM brain regions become more correlated with one another with age and are less correlated with one another in children who fail explicit false-belief tasks, controlling for age (Richardson et al., 2018). We extracted inter-region correlations within and across ToM and language brain regions using an approach previously used with non-blindfolded sighted children (https://osf.io/wzd8a) and in prior studies (Richardson et al., 2018, Richardson et al., 2020). ToM regions were the group ROIs described above; language brain regions were eleven 10 mm spheres drawn around peak coordinates from a prior study (Fedorenko et al., 2010), excluding voxels that overlapped with the ToM group ROIs. Language brain regions included left inferior and orbital inferior frontal gyrus, middle and superior frontal gyrus, anterior temporal lobe, posterior temporal lobe, and bilateral middle posterior and middle anterior temporal lobe, and angular gyrus (cerebellar ROIs were excluded due to lack of coverage). Preprocessed, scaled timecourses were extracted from each voxel per ROI. The five PCA-based noise regressors and motion artifact timepoint regressors (included as nuisance regressors in the story task) were regressed from these timecourses and the residual timecourses were high-pass filtered with a cut-off of 100 seconds/cycle (0.01 Hz). Timecourses from voxels within an ROI were averaged, creating one timecourse per ROI, and artifact timepoints were subsequently NaNed. Each ROI timecourse was correlated with every other ROI timecourse, per subject, and these correlation values were Fisher z-transformed. Within-ToM and within-language network correlations were calculated as the average correlation value between brain regions within each network. Across-ToM-language network correlations were calculated as the average correlation value between ToM and language brain regions. In order to test if these two brain networks were functionally distinct in blind and sighted children, we used t-tests to compare within- versus across-network correlations. We also directly compared within- and across-network correlations across blind and sighted children.
2.7.4. Multivariate representational similarity analysis
Second, we used representational similarity analyses to measure the extent to which a “condition model” (i.e., a model that represents the similarity [Euclidean distance] of story stimuli in terms of their condition label) predicted multivariate pattern similarity in ToM brain regions. Prior research using the same fMRI experiment suggests that the “condition model fit” in RTPJ is reduced in autistic children (Richardson et al., 2020). The analysis procedures for this metric followed those used in the prior research (ibid.). Blind and sighted children did not differ on this metric; for brevity the methods and results are described only in the Supplementary Materials.
2.8. Statistical analyses
All statistical analyses were conducted in RStudio (Version 1.4.1106; R version 4.0.4). For all regression analyses, we examined residuals to determine whether they were normally distributed and to detect the presence of outliers using Shapiro-Wilk Normality tests and Q-Q normal plots. We additionally assessed homogeneity of variance in dependent variables across blind and sighted children using Levene’s tests (‘leveneTest’ function in car package; Fox et al., 2007).
We conducted robust linear regressions to test for an effect of group on ToM performance and on neural measures of ToM brain region function, controlling for age. Analyses tested for significant group-by-age interactions; non-significant interactions were removed. For ROI analyses, we conducted statistical tests across all ToM regions, together (bilateral TPJ, precuneus [PC], and dorso-, mid-, and ventromedial prefrontal cortex [D/M/VMPFC]; ‘rlmer’ function in the robustlmm package (Koller, 2016), including ROI as a fixed effect with RTPJ as the reference ROI and subject identifier as a random effect) and in RTPJ, separately (‘lmrob’ function in the robustbase package (Maronna and Suggests, 2007)). Robust linear regressions are appropriate for use with small sample sizes and when assumptions of traditional regression analyses are not met (e.g., presence of outliers, heteroscedasticity). For neural measures, statistical analyses controlled for handedness, motion, and MRI head coil.
Statistical analyses in the main text compare blind children to all sighted children (n=114, including blindfolded children); see Supplementary Materials for analyses comparing blind children to blindfolded children (n=18) and analyses of lateralized neural measures in right-handed participants only (n=10 blind and n=92 sighted children). In both sets of analyses, the power of our statistical tests is limited by the small number of blind children; the main benefit of the large sample of sighted children is in visualizing the neuroimaging data from blind children against a large distribution of values obtained in sighted children.
Finally, we tested for neural correlates of behavioral ToM performance outside of the scanner in children who completed the ToM task and contributed usable fMRI data (n=11 blind and n=16 blindfolded sighted children). Based on prior research that has found such relationships in other samples of children, our primary neural outcome measure of interest was RTPJ selectivity (Gweon et al., 2012, Richardson et al., 2020), but we explored brain-behavior correlations with selectivity indices in other ToM brain regions and with the two exploratory neural ToM measures (inter-region correlations and “condition model fit”; Supplementary Figure 10). Given the small sample size of blind children (and therefore weak power for studying individual differences among blind children), we tested for brain-behavior correlations in the combined sample of blind and sighted children. This analysis was likely underpowered to detect group differences in brain-behavior correlations and does not test for an effect in the blind group specifically.
2.9. Data and resource availability
Summary data and code for reproducing statistical analyses are publicly available for download, alongside novel experimental materials (https://osf.io/pavdg/). Data were collected prior to the popularization of public data sharing and so the conditions of the informed consent given by participants and their families do not allow for public archiving of individual raw MRI or behavioral data. Individuals seeking access to raw data should contact Dr. Rebecca Saxe (saxe@mit.edu). Access will be granted to individuals who complete a data usage agreement through the Committee on the Use of Humans as Experimental Subjects (COUHES) at MIT.
3. Results
3.1. Behavioral performance on the ToM task administered outside of the scanner
Across all 4–12-year-old children (n=12 blind and n=21 sighted), performance on the behavioral ToM task increased with age (b=0.54, t=6.6, CI=[0.37,0.70], p<.0001). Blind children performed slightly less accurately than sighted children (blind M(SE)=0.70(0.05); sighted M(SE)=0.80(0.04); effect of group, controlling for age: b=−0.53, t=−2.2, CI=[−1.0,−0.03], p=.04; Fig. 1). When the oldest sighted children (n=2, ages 13.5 and 16.2 years) or youngest sighted child (3.98-year-old) were included, the group difference was not significant (bs>−0.44, ts>−1.8, CIs=[−1,0.15], ps>0.07; Fig. 1).
Fig. 1.
ToM behavior outside of the scanner and parent report. Scatterplots show proportion correct on the behavioral ToM task (left) and summary score on the Children’s Social Understanding Scale (CSUS; parent report measure; right) by age (x-axes). Blind children (n=12 ToM; n=16 CSUS) are shown in orange; sighted children (n=21 ToM; n=24 CSUS) are shown in blue. Violin plots to the right of each scatterplot illustrate the distribution of these two measures by group.
When looking at the false-belief items separately, blind and sighted children did not differ in performance (group effect, controlling for age: b=−0.10, t=−1.5, CI=[−0.23,0.04], p=.15; Supplementary Figure 3). Considering exclusively the items that required reasoning about characters’ visual experiences, blind children did not perform significantly differently from sighted children (group effect, controlling for age: b=−0.15, t = −1.7, CI=[−0.33,0.03], p=.09; see Supplementary Figure 3 for visualization of proportion correct by source modality). The pattern of results was the same when sighted children younger/older than 4–12 years of age were included. In sum, although performance of blind children on ToM task was lower, there was no evidence that this was related to whether the source modality of mental states was visual.
3.2. Parent report measure of social reasoning
Parent ratings on the Children’s Social Understanding Scale (CSUS) increased with age (b=0.47, t = 3.8, CI=[0.22,0.72], p=.0005) and were non-significantly lower for blind children (n=16, summary score: M(SE)= 3.42(0.09)), relative to sighted children (n = 24, M(SE)= 3.65(0.06); group effect, controlling for age: b= −0.56, t = −1.8, CI= [− 1.2,0.09], p =.09; see Supplementary Figure 3 for visualization of parent ratings by concept category). The same pattern of results was observed in the 4–12-year-old children who completed the NV ToM task (n=33).
3.3. FMRI results
3.3.1. Whole-brain random effects analysis
In a whole-brain random effects analysis of the Mental > Physical contrast, sighted children (n = 114 in total; n = 18 blindfolded) showed the expected profile of activation in bilateral temporoparietal junction, precuneus, and medial prefrontal cortex (p < .05, corrected for multiple comparisons, Supplementary Figure 4). Among blind children (n = 17), no clusters survived correcting for multiple comparisons via permutation tests. At a more lenient statistical threshold (p < .05 voxel-wise uncorrected, k = 10) blind children showed activity in precuneus, medial prefrontal cortex, bilateral middle superior temporal sulcus as well as a small cluster in left temporoparietal junction (Fig. 2).
Fig. 2.
Whole-brain random effects analysis of the Mental > Physical contrast. Blind children (n = 17) are shown in orange at p < .05, k = 10 uncorrected for multiple comparisons. Sighted children are shown in blue; overlap across groups is shown in pink. Top row shows results from all sighted children (n = 114, including n = 18 blindfolded), corrected for multiple comparisons at p<.05; bottom row shows results in a size-, handedness-, coil-, and activation threshold-matched subset of sighted children who were blindfolded during the scan (n= 18; p < .05, k=10 uncorrected).
When blind and sighted groups were directly compared to each other on the Mental > Physical contrast, there were no between-group differences in the typical ToM network (bilateral TPJ, precuneus, D/M/VMPFC). Sighted children had significantly more activation to the Mental > Physical contrast than blind children in right caudate nucleus (peak voxel: [14,10,20]) and right anterior cerebellum (peak voxel: [10,−54,−26]); blindfolded sighted children had significantly more activation than blind children in right anterior temporal pole (peak voxel: [46,20,−32]; Supplementary Figure 4). No clusters showed significantly more activity in blind children relative to sighted children (full sample or blindfolded subset).
3.3.2. Magnitude and selectivity of response in ToM regions of interest
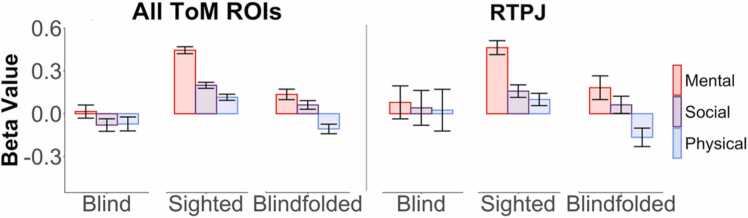
Sighted children showed the expected response profile in ToM regions of interest: responses were highest to the mental state stories, followed by stories including general social information, and lowest to the physical control stories (Fig. 3; effect of Social & Physical conditions (relative to Mental) in all group-defined ToM ROIs: bs< −0.3, ts< −9, ps< 0.0001; RTPJ: bs< 0.5, ts< −7, ps< 0.0001). This response profile was not apparent among blind children (Fig. 3; effect of Social & Physical conditions in all ToM ROIs: bs>−0.15, ts>−1.3, ps>0.1; RTPJ: bs=−0.08, ts=−0.5, ps>0.5). In a direct comparison of blind and sighted children, there were significant condition-by-group interactions such that condition differences were larger among sighted children (all ToM ROIs: bs<−0.2, ts<−2.2, ps<0.05; RTPJ: bs<−0.4, ts<−2.5, ps<0.05). There was also a main effect of group, such that sighted children had larger beta values overall (all ToM ROIs: b=0.57, t = 3.8, p < .0005; RTPJ: b=0.40, t = 1.7, p = .10) and of age, such that response magnitude increased with age (all ToM ROIs: b=0.18, t = 2.3, p < .05; RTPJ: b=0.40, t = 3.2, p < .005). We observed a group-by-age interaction such that sighted children showed less change with age than blind children (all ToM ROIs: b=−0.32, t = −3.4, p < .001; RTPJ: b=−0.55, t = −3.8, p < .0005; Supplementary Figure 6). A similar pattern of results was obtained when comparing blind children to the blindfolded subset of sighted children (see Supplementary Materials).
Fig. 3.
Responses to fMRI experiment in group-defined ToM regions of interest. Bar charts show the beta value per story condition (Mental, Social, Physical) extracted from group-defined ToM ROIs in blind (n=17), all sighted (n = 114), and blindfolded sighted (n = 18) children; error bars indicate standard error from the mean. “All ToM ROIs” beta values reflect the average beta value across all ToM ROIs (R/LTPJ, PC, D/M/VMPFC). See Supplementary Figure 5 for beta value plots in individually-defined ROIs and in each group-defined ToM ROI, separately.
Response selectivity for mental state content (Mental > Social contrast value) did not differ between blind and sighted children, across all ToM ROIs (individual-subject ROIs: b=−0.08, t = −0.41, CI=[−0.46,0.30], p = .68; group ROIs: b=−0.38, t = −1.6, CI=[−0.85,0.08], p = .11), nor in RTPJ specifically (individual-subject ROI: b=−0.11, t = −0.56, CI=[−0.50,0.28], p = .58; group ROI: b=−0.38, t = −1.7, CI=[−0.84,0.08], p=.10; Fig. 4). There were no significant effects of age or group-by-age interactions on response selectivity; however, there were significant group-by-ROI interactions. In sighted children, relative to blind children, RTPJ was more selective than PC (individual-subject ROIs: b=0.56, t = 2.7, CI=[0.14,0.97], p < .01; group ROIs: b=0.70, t = 2.7, CI=[0.20,1.2], p < .0005) and VMPFC (individual-subject ROIs: b=0.41, t = 2.0, CI=[0.003,0.82], p < .05; group ROIs: b=0.60, t = 2.3, CI=[0.09,1.1], p<.05; Supplementary Figures 5 and 6). A similar pattern of results was obtained when comparing blind children to the blindfolded subset of sighted children (see Supplementary Materials).
Fig. 4.
Selectivity of response to mental state content relative to general social information in individual-subject ToM regions of interest. Box and violin plots show the Mental > Social contrast value in individual-subject ToM regions of interest. “All ToM ROIs” contrast values reflect the average contrast value across all ToM ROIs (R/LTPJ, PC, D/M/VMPFC). Blind children (n=17) are shown in orange and sighted children (n = 114, including n = 18 blindfolded) are shown in blue. Center line indicates median, box reflects interquartile range (IQR), whiskers show first quartile/third quartile -/+ 1.5 *IQR, black diamonds indicate group average value. See Supplementary Figure 7 for plots in group-defined ROIs and in each ToM ROI, separately.
3.4. Inter-region correlation analysis
Among blind children, ToM and language networks were functionally distinct: brain regions within each network were more correlated with other brain regions in the same network than with brain regions in the other functional network (within-ToM [M(SE)= 0.23(0.03)] vs. across-ToM-Language [M(SE)= 0.18(0.02)]: t(16) = 3.72, CI= [0.03,Inf], p=.0009; within-Language [M(SE)= 0.27(0.02)] vs. across-ToM-language: t(16) = 6.45, CI= [0.07,Inf], p = 4.04 × 10−6, one-tailed paired t-tests). Sighted children showed the same pattern of data (within-ToM [M(SE)= 0.26(0.01)] vs. across-ToM-Language [M(SE)= 0.20(0.01)]: t(113)=10.7, CI= [0.06,Inf], p =2.8 × 10−19; within-Language [M(SE)= 0.25(0.01)] vs. across-ToM-language: t(113)=10.0, CI= [0.04,Inf], p=1.3 × 10−17; see Supplementary Materials for similar results in blindfolded sighted children). All age effects (and group-by-age interactions) were non-significant (all bs<0.35, ts<1.7, ps>0.1).
Nevertheless, inter-region correlations within the ToM network were significantly lower among blind children, relative to all sighted children (b=−0.72, t=−2.7, CI=[−1.2,−0.19], p=.008; Fig. 5). Inter-region correlations within the language network did not differ between blind and sighted children (b=−0.18, t=−0.67, CI=[−0.73,0.36], p = .51); inter-region correlations across the ToM and language networks were also similar across groups (b=−0.52, t=−1.6, CI=[−1.2,0.13], p=.12). None of the inter-region correlation measures differed significantly between blind and blindfolded children (see Supplementary Materials).
Fig. 5.
Inter-region correlations in ToM and language networks. Correlation matrices show z-scored correlations between each brain region within the ToM and language networks in blind children (n=17, left), all sighted children (n=114, middle) and blindfolded sighted children (n=18, right); within-ToM network correlations are outlined in red; within-language network correlations are outlined in orange. Brain regions are in the same order along the x- and y-axes: ToM regions: R/L temporoparietal junction (TPJ), precuneus (PC), dorso-, middle- and ventromedial prefrontal cortex (D/M/VMPFC); Language regions: R/L middle anterior temporal lobe (MAT), L anterior temporal lobe (LAT), R/L middle posterior temporal lobe (MPT), L posterior temporal lobe (LPT), L angular gyrus (LAngG), L superior frontal gyrus (LSFG), L middle frontal gyrus (LMFG), L orbital inferior frontal gyrus (LIFGorb), L inferior frontal gyrus (LIFG). Box plots show the inter-region z-scored correlation value within ToM (red) and language (orange) brain regions, and across the two networks (blue). Center line indicates median, box reflects interquartile range (IQR), whiskers show first quartile/third quartile –/+ 1.5 *IQR, black diamonds indicate group average value. Dots show individual subject correlation values.
3.5. Relationship between ToM performance outside of the scanner and functional maturation in ToM brain regions
Across all participants with both behavioral and fMRI data (n=27), RTPJ selectivity (Mental > Social) correlated with ToM task performance (correlation controlling for age and motion, individual-subject ROI: b=0.30, t = 2.8, CI=[0.08,0.52], p = .01; group ROI: b=0.27, t = 2.3, CI=[0.02,0.52], p=.03; Fig. 6). In a regression that additionally included group as a covariate, this brain-behavior correlation was not significant (individual-subject ROI: b=0.28, t = 1.8, CI=[−0.03,0.59], p = .08; group ROI: b=0.15, t = 0.98, CI=[−0.17,0.46], p=.34). There was no evidence for a difference in this correlation between blind and sighted children (i.e., the interaction term was non-significant and removed from the regression); though note that we were likely underpowered to detect this effect. There were similar correlations between selectivity and ToM task performance in other ToM regions (left TPJ and medial prefrontal cortex; see Supplementary Figure 10). Within-ToM inter-region correlation was not correlated with ToM task performance, controlling for age and motion (b=0.15, t = 0.76, CI=[−0.26,0.56], p = .45; with group included as main effect: b=0.19, t = 1.9, CI=[−0.02,0.41], p=.07; Supplementary Figure 10).
Fig. 6.
Selectivity for mental state content in individual-subject RTPJ correlates with ToM behavior. Scatterplot shows proportion correct on the ToM behavioral task (administered outside of the scanner; x-axis) by RTPJ selectivity (Mental > Social contrast value, y-axis) in blind children (orange, n=11) and sighted children (blue, n=16) who contributed both measures. Violin plots illustrate the distribution of proportion correct on the ToM behavioral task (top) and selectivity values (right) by group.
4. Discussion
This study investigated the impact of visual experience on children’s theory of mind development, behaviorally and neurally. We extend prior behavioral research on ToM development in children born blind by developing and validating a novel non-visual story-based behavioral ToM task and we use fMRI to provide the first description of functional responses in ToM brain regions in blind children.
Behaviorally, blind children’s performance on our ToM task was slightly lower from that of sighted children. When we examined performance on false belief items only, we found similar performance among blind and sighted children. Our results are in line with those of Brambring and Asbrock (2010), who found a relatively short (19–26 months) delay in passing classic false-belief tasks among 4–10-year-old congenitally blind children (n = 45). Brambring and Asbrock (2010) found that blind children perform similarly to sighted children on false belief tasks by seven years of age (Brambring and Asbrock, 2010). Unlike Brambring and Asbrock (2010), we did not find smaller differences between blind and sighted children at older ages (i.e., a group-by-age interaction) in overall performance or in performance on false belief items. However, our sample size was small (n = 12 blind children), so we were likely underpowered to detect such effects. Relatedly, the statistical significance of the group difference in overall performance varied depending on whether the youngest/oldest sighted children were included in analyses. Given this, we interpret our results cautiously and in light of prior evidence.
Blind children performed similarly to sighted children on items that specifically required reasoning about vision. This observation is consistent with one prior study with d/Deaf children, whose reasoning about auditory experiences is similar to hearing children’s (Schmidt and Pyers, 2014). These results suggest that by preschool years, children understand how sensory experiences lead to knowing, regardless of whether they themselves have had those sensory experiences.
Along with evidence from Brambring and Asbrock (2010), our data are in conflict with previous reports that blind children experience severe ToM delays that persist into early adolescence (Green et al., 2004, McAlpine and Moore, 1995, Minter et al., 1998, Peterson et al., 2000). Reports of drastic delays likely reflect lack of adequate screening of blind children for other disabilities, lack of appropriately adapted tasks (e.g., inclusion of objects with which blind children have less experience), or absence of appropriate control groups. Once these factors are taken into account, both the current evidence and the prior literature suggest that blindness per se does not severely impact ToM development.
This pattern of evidence contrasts with evidence for long-lasting effects of the absence of language on ToM development. In particular, d/Deaf individuals who do not have access to rich, conversational language perform worse on ToM tasks even in adulthood (Pyers and Senghas, 2009). While language may be necessary for the development of ToM, visual access to others’ actions, expressions, and nonverbal dyadic interactions appear to have a more subtle and transient role in ToM development.
Though our sample of blind children who completed the behavioral ToM task was small (n = 12), there were some strengths of our approach, including the breadth of ToM concepts tested (including concepts that develop after children pass false-belief tasks) and the relatively large number of test items. Performance on the ToM task correlated with age among blind children, captured individual differences in ToM in an independent sample of sighted children, and correlated with the selectivity of the RTPJ response for mental state content (relative to social content), controlling for age – a conceptual replication of prior research (Gweon et al., 2012). Performance on our ToM task also correlated with parent ratings of children’s social understanding, which were comparable across blind and sighted children. The parent report results suggest that the small difference in ToM task performance between blind and sighted children may not translate to obvious differences in everyday social behavior.
In addition to measuring blind children’s ToM development behaviorally, we sought to characterize their functional responses in brain regions that are typically recruited for ToM reasoning. Compared to behavioral evidence, fMRI evidence is significantly more challenging and expensive to collect. But from a scientific perspective, fMRI evidence offers a complementary approach for understanding the impact of vision on ToM development as well as novel insight into the role of vision on the development of ToM brain regions.
Consistent with evidence for subtle behavioral delays, we found evidence for different development of ToM brain regions in blind children on some but not all neural measures. Relative to sighted children, blind children showed weaker responses in expected cortical areas (e.g., RTPJ), while engaging in ToM reasoning. The whole-brain random effects analysis with blind children showed weak responses overall to the Mental > Physical contrast – which reliably evokes activity in ToM brain regions in adults and children (Gweon et al., 2012, Richardson et al., 2020). When we used prior group-level data to define regions of interest, blind children did not show a significant effect of condition on the magnitude of response in ToM brain regions. Moreover, the effect of condition among sighted children – in whom responses in ToM regions were significantly larger to the Mental state condition, relative to the Social and Physical conditions – was significantly larger than the effect of condition among blind children. A follow up analysis suggested that this pattern may be related to smaller portions of the expected ToM regions being selective for ToM content in blind children (Supplementary Materials and Supplementary Figure 8). Finally, the strength of the functional correlation between ToM brain regions was larger in sighted children, relative to blind children. Lower inter-region correlations among ToM brain regions have been observed in young children and in children who fail false belief tasks, relative to those who pass (Richardson et al., 2018). Though our sample of blind children was small (n = 17 in fMRI analyses), we observed the expected neural response profiles in ToM brain regions in a similar sample size of age- and coil-matched sighted children (n = 18 blindfolded sighted children).
However, when we identified individual-subject regions of interest, response selectivity values for mental state content (relative to non-mentalistic social information) in ToM brain regions in blind children were in the same range as those of sighted children and did not differ statistically across groups (Fig. 4, Supplementary Figures 5 and 7). Similar selectivity values among blind children, alongside preferential responses in some ToM brain regions to Mental state stories, as revealed by lenient whole-brain analyses (Fig. 2), provides positive evidence for qualitatively similar responses in ToM brain regions. The extent to which condition labels predicted multivariate response patterns in ToM brain regions also did not differ across blind and sighted children (Supplementary Materials and Supplementary Figure 9). Additionally, in analyses that directly compared whole-brain activation across blind and sighted children, there were no group differences in the recruitment of ToM brain regions. Such null results are difficult to interpret and in the present study are further complicated by our small sample size; additional research with larger samples is necessary. In the meantime, we suggest that neural ToM responses in blind children are qualitatively similar to, but quantitatively weaker than, ToM responses in sighted children.
Prior research suggests that, by adulthood, the neural ToM response is similar across blind and sighted people (Bedny et al., 2009, Koster-Hale et al., 2014; for broader meta-analysis of social cognitive neuroimaging studies in blind individuals, see Arioli et al., 2021). Taken together with the current results, we hypothesize that delayed development of the ToM neural response resolves sometime during childhood.
One incidental finding in our fMRI data is that wearing a blindfold reduces the magnitude of the univariate response across all conditions as well as the Mental > Social condition effect in ToM brain regions. That is, while blindfolded sighted children showed the predicted condition differences (Mental > Social > Physical) in ToM brain regions, the response to all conditions, and the difference in response between Mental and Social conditions, are reduced relative to non-blindfolded sighted children (Fig. 3). These differences are difficult to interpret, though consistent with evidence that the magnitude of activation (often estimated via the amplitude of activity in the low frequency range of the BOLD response) in several brain regions, including precuneus and parietal cortex, varies in adults depending on whether their eyes are open or closed (e.g., Rączy et al., 2022). One possibility is that these differences are secondary consequences of previously observed differences in response magnitude in lower-level visual brain regions, including the fusiform gyrus, in these same children (Bedny et al., 2015). While wearing a blindfold helps to match the visual input during the scan to that of blind children, it is a novel and potentially distracting experience for sighted children. It is therefore difficult to know the full set of consequences of blindfolding on brain activity, especially in children. We cannot exclude the possibility that the differences observed in ToM brain regions between blindfolded and non-blindfolded sighted children are due to absence of visual input per se; additional research is necessary to investigate this possibility. Regardless, as ToM responses in blind children were weaker than both groups of sighted children, the differences in response between blindfolded and non-blindfolded sighted children do not impact our conclusions.
The approach taken in this study represents just one way to investigate neural ToM development in blind children. Given that the functional response profile of ToM brain regions in blind adults is similar to that of sighted adults (Bedny et al., 2009, Koster-Hale et al., 2014), and that these same regions are recruited during ToM reasoning in sighted children (Fehlbaum et al., 2021, Gweon et al., 2012), we asked: what does the functional response profile of these specific regions look like in blind children? This approach is hypothesis-driven and provides insight into the impact of experience on the development of these particular functional brain regions. This approach is reasonable given the necessarily small sample of blind children, but it is also limited. In particular, it would not be sensitive to functional reorganization of brain regions recruited for ToM across development (e.g., whether the ToM network splits off from other networks during development; whether other regions are part of the ToM network earlier in development). Studies that implement data-driven analyses with large pediatric datasets are better suited to investigate these questions (Yates et al., 2021).
One strength of the approach taken in this study is that it uses the same fMRI experiment to build directly on prior research of neural ToM development. This research suggests that one neural correlate of ToM development is RTPJ selectivity: in younger children and in children who experience delayed ToM development due to delayed access to language, RTPJ responses are high to both Mental and Social stories, whereas in adults and older children, RTPJ responses to Social stories are suppressed, reflecting a more mental-state selective response (Gweon et al., 2012, Richardson et al., 2020). By contrast, in the blind children here, responses to all stories (including Mental stories) were relatively low in RTPJ and across all ToM regions of interest. Responses to all three story conditions increased more with age in blind children, relative to sighted children. Additionally, the inter-region correlation among ToM brain regions was reduced in blind children – an effect not present among children with delayed access to language (Richardson, Koster-Hale et al., 2020), but observed in young children and in children who fail explicit false-belief tasks, relative to those who pass (Richardson et al., 2018). While additional research is necessary to replicate these results, this could suggest that lack of vision affects neural ToM development differently than delayed access to language.
It is worth noting that different neural responses across blind and sighted populations should not be interpreted as evidence of a ‘worse’ outcome. Adults and children who are blind also show functional plasticity in ‘visual’ cortices, related to blindness (Bedny, 2017, Bedny et al., 2011, Bedny et al., 2015). These changes are deviations for ‘typical’ neural profiles but are associated with behavioral advantages, rather than impairments (Loiotile et al., 2020, Röder et al., 2003, Röder and Rösler, 2003). Blind adults and children also show changes in language lateralization, which are not associated with any group differences in behavior (Lane et al., 2017). It is possible that differences in ToM brain regions of blind children are partially related to plasticity in other neural systems (e.g., visual or language systems). More generally, more common neural profiles are not necessarily optimal. Additional research is necessary to understand the behavioral correlates (if any) of the observed neural differences between blind and sighted children.
In sum, this study provides initial evidence for transient influence of vision on the development of the neural ToM response by identifying differences between sighted and blind children. Additional research with larger samples and wider age ranges is necessary to fully understand the contribution of vision to ToM development. FMRI studies may be particularly useful, as they enable pinpointing the neural correlates of putative behavioral delays and have the potential to reveal different mechanisms of development across populations. In the meantime, our evidence, alongside prior evidence for typical ToM behavior in blind children by age seven years (Brambring and Asbrock, 2010) and typical ToM neural responses in blind adults (Bedny et al., 2009, Koster-Hale et al., 2014), suggests that vision facilitates, but is not necessary for, the development of ToM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank participants and families for engaging with the research, Swetha Dravida, Ben Deen, and Nicholas Dufour for assisting with data collection and the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT for support. We would also like to thank Lindsey Yazzolino, the National Federation of the Blind, Perkins School for the Blind, the National Association for Parents of the Visually Impaired, Wonderbaby.org, and local pediatric ophthalmologists, and especially Dr. Anne Fulton, for assisting with recruitment. We gratefully acknowledge support from the David and Lucile Packard Foundation (#2008–333024 to R.S.) and a grant from the Harvard/MIT Joint Research Grants Program in Basic Neuroscience (to R.S. and Dr. Anne Fulton). For the purposes of open access, the author has applied a ‘Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101285.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
Summary data for reproducing statistical analyses are publicly available for download (https://osf.io/pavdg/). Data were collected prior to the popularization of public data sharing and so the conditions of the informed consent given by participants and their families do not allow for public archiving of individual raw MRI or behavioral data. Individuals seeking access to raw data should contact Dr. Rebecca Saxe (saxe@mit.edu). Access will be granted to individuals who complete a data usage agreement through the Committee on the Use of Humans as Experimental Subjects (COUHES) at MIT.
References
- Adolphs R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 2009;60(1):693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arioli M., Ricciardi E., Cattaneo Z. Social cognition in the blind brain: a coordinate-based meta-analysis. Hum. Brain Mapp. 2021;42(5):1243–1256. doi: 10.1002/hbm.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M. Evidence from blindness for a cognitively pluripotent cortex. Trends Cogn. Sci. 2017;21(9):637–648. doi: 10.1016/j.tics.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Bedny M., Saxe R. Insights into the origins of knowledge from the cognitive neuroscience of blindness. Cogn. Neuropsychol. 2012;29(1–2):56–84. doi: 10.1080/02643294.2012.713342. [DOI] [PubMed] [Google Scholar]
- Bedny M., Pascual-Leone A., Saxe R.R. Growing up blind does not change the neural bases of theory of mind. Proc. Natl. Acad. Sci. 2009;106(27):11312–11317. doi: 10.1073/pnas.0900010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Pascual-Leone A., Dodell-Feder D., Fedorenko E., Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc. Natl. Acad. Sci. 2011;108(11):4429–4434. doi: 10.1073/pnas.1014818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M., Richardson H., Saxe R. ‘Visual’ cortex responds to spoken language in blind children. J. Neurosci. 2015;35(33):11674–11681. doi: 10.1523/JNEUROSCI.0634-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S., Dik M., Voor De Wind M.J., Asbrock D., Brambring M., Kef S. A new look at theory of mind in children with ocular and ocular-plus congenital blindness. J. Vis. Impair. Blind. 2014;108(1):17–27. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie H.M.A., Boersma M., Wattjes M.P., Adriaanse S., Vermeulen R.J., Oostrom K.J., Huisman J., Veltman D.J., Delemarre-van de Waal H.A. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur. J. Pediatr. 2010;169(9):1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambring M. Response to Hobson’s letter: congenital blindness and autism. J. Autism Dev. Disord. 2011;41(11):1595–1597. doi: 10.1007/s10803-011-1187-z. [DOI] [PubMed] [Google Scholar]
- Brambring M., Asbrock D. Validity of false belief tasks in blind children. J. Autism Dev. Disord. 2010;40(12):1471–1484. doi: 10.1007/s10803-010-1002-2. [DOI] [PubMed] [Google Scholar]
- Campbell D.T., Fiske D.W. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol. Bull. 1959;56:81–105. doi: 10.1037/h0046016. [DOI] [PubMed] [Google Scholar]
- Carey S., Spelke E. Science and core knowledge. Philos. Sci. 1996;63(4):515–533. [Google Scholar]
- Carrington S.J., Bailey A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour N., Redcay E., Young L., Mavros P.L., Moran J.M., Triantafyllou C., Gabrieli J.D., Saxe R. Similar brain activation during false belief tasks in a large sample of adults with and without autism. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Hsieh P.-J., Nieto-Castanon A., Whitfield-Gabrieli S., Kanwisher N. New method for fMRI investigations of language: defining ROIs functionally in individual subjects. J. Neurophysiol. 2010;104(2):1177–1194. doi: 10.1152/jn.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum L.V., Borbás R., Paul K., Eickhoff S. b, Raschle N. m. Early and late neural correlates of mentalizing: ALE meta-analyses in adults, children and adolescents. Soc. Cogn. Affect. Neurosci., nsab105. 2021 doi: 10.1093/scan/nsab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueras-Costa B., Harris P. Theory of mind development in deaf children: a nonverbal test of false-belief understanding. J. Deaf Stud. Deaf Educ. 2001;6(2):92–102. doi: 10.1093/deafed/6.2.92. [DOI] [PubMed] [Google Scholar]
- Fodor J.A. A theory of the child’s theory of mind. Cognition. 1992 doi: 10.1016/0010-0277(92)90004-2. [DOI] [PubMed] [Google Scholar]
- Fox, J., Friendly, G.G., Graves, S., Heiberger, R., Monette, G., Nilsson, H., Ripley, B., Weisberg, S., Fox, M.J., & Suggests, M. (2007). The car package. R Foundation for Statistical Computing.
- Gopnik A., Wellman H.M. Why the child’s theory of mind really is a theory. Mind Lang. 1992;7(1–2):145–171. [Google Scholar]
- Gopnik A., Slaughter V., Meltzoff A. Changing your views: how understanding visual perception can lead to a new theory of the mind. Children’s Early Underst. Mind: Orig. Dev. 1994:157–181. [Google Scholar]
- Green S., Pring L., Swettenham J. An investigation of first-order false belief understanding of children with congenital profound visual impairment. Br. J. Dev. Psychol. 2004;22(1):1–17. [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012;83(6):1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Harris P.L., Rosnay M. de, Ronfard S. The mysterious emotional life of little red riding hood. Child. Emot. 2014;26:106–118. doi: 10.1159/000354364. [DOI] [Google Scholar]
- Hyde D.C., Simon C.E., Ting F., Nikolaeva J. Functional organization of the temporal-parietal junction for theory of mind in preverbal infants: a near-infrared spectroscopy study. J. Neurosci. 2018 doi: 10.1523/JNEUROSCI.0264-17.2018. 0264–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J.M., Astington J.W. Cognitive factors and family structure associated with theory of mind development in young children. Dev. Psychol. 1996;32(1):70. [Google Scholar]
- Kaufman A.S. MN: NCS. Pearson; 1997. KBIT-2: Kaufman brief intelligence test. Minneapolis. [Google Scholar]
- Keil B., Alagappan V., Mareyam A., McNab J.A., Fujimoto K., Tountcheva V., Triantafyllou C., Dilks D.D., Kanwisher N., Lin W. Size-optimized 32-channel brain arrays for 3 T pediatric imaging. Magn. Reson. Med. 2011;66(6):1777–1787. doi: 10.1002/mrm.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliemann D., Richardson H., Anzellotti S., Ayyash D., Haskins A.J., Gabrieli J.D., Saxe R.R. Cortical responses to dynamic emotional facial expressions generalize across stimuli, and are sensitive to task-relevance, in adults with and without Autism. Cortex. 2018;103:24–43. doi: 10.1016/j.cortex.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen B., Liszkowski U. Eighteen- and 24-month-old infants correct others in anticipation of action mistakes. Dev. Sci. 2011;15(1):113–122. doi: 10.1111/j.1467-7687.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- Knudsen B., Liszkowski U. 18-month-olds predict specific action mistakes through attribution of false belief, not ignorance, and intervene accordingly. Infancy. 2012;17(6):672–691. doi: 10.1111/j.1532-7078.2011.00105.x. [DOI] [PubMed] [Google Scholar]
- Koller M. robustlmm: an R package for robust estimation of linear mixed-effects models. J. Stat. Softw. 2016;75:1–24. doi: 10.18637/jss.v075.i06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J., Bedny M., Saxe R. Thinking about seeing: perceptual sources of knowledge are encoded in the theory of mind brain regions of sighted and blind adults. COGNITION. 2014;133(1):65–78. doi: 10.1016/j.cognition.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C., Kanjlia S., Richardson H., Fulton A., Omaki A., Bedny M. Reduced left lateralization of language in congenitally blind individuals. J. Cogn. Neurosci. 2017;29(1):65–78. doi: 10.1162/jocn_a_01045. [DOI] [PubMed] [Google Scholar]
- Loiotile R., Lane C., Omaki A., Bedny M. Enhanced performance on a sentence comprehension task in congenitally blind adults. Lang., Cogn. Neurosci. 2020;35(8):1010–1023. doi: 10.1080/23273798.2019.1706753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronna, M., & Suggests, M. (2007). The robustbase Package.
- McAlister A., Peterson C.C. Mental playmates: Siblings, executive functioning and theory of mind. Br. J. Dev. Psychol. 2006;24(4):733–751. [Google Scholar]
- McAlpine L.M., Moore C.L. The development of social understanding in children with visual impairments. J. Vis. Impair. Blind. 1995;89 349–349. [Google Scholar]
- Meltzoff A.N. Origins of theory of mind, cognition, and communication. J. Commun. Disord. 1999;32(4):251–269. doi: 10.1016/s0021-9924(99)00009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A.N., Brooks R. Self-experience as a mechanism for learning about others: a training study in social cognition. Dev. Psychol. 2008;44(5):1257–1265. doi: 10.1037/a0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meristo M., Morgan G., Geraci A., Iozzi L., Hjelmquist E., Surian L., Siegal M. Belief attribution in deaf and hearing infants. Dev. Sci. 2012;15(5):633–640. doi: 10.1111/j.1467-7687.2012.01155.x. [DOI] [PubMed] [Google Scholar]
- Minter M., Hobson R.P., Bishop M. Congenital visual impairment and’theory of mind. Br. J. Dev. Psychol. 1998;16:183. [Google Scholar]
- Onishi K.H., Baillargeon R. Do 15-month-old infants understand false beliefs? Science. 2005;308(5719):255–258. doi: 10.1126/science.1107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunov A.M., Blank I.A., Fedorenko E. Functionally distinct language and theory of mind networks are synchronized at rest and during language comprehension. J. Neurophysiol. 2019;121(4):1244–1265. doi: 10.1152/jn.00619.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pereira M., Conti-Ramsden G. Do blind children show autistic features. Autism Blind.: Res. Reflect. 2005:99–127. [Google Scholar]
- Pérez-Pereira M., Conti-Ramsden G. Psychology Press; 2013. Language development and social interaction in blind children. [Google Scholar]
- Perner J., Ruffman T., Leekam S.R. Theory of mind is contagious: you catch it from your sibs. Child Dev. 1994;65(4):1228–1238. doi: 10.1111/j.1467-8624.1994.tb00814.x. [DOI] [Google Scholar]
- Peterson C.C., Peterson J.L., Webb J. Factors influencing the development of a theory of mind in blind children. Br. J. Dev. Psychol. 2000;18(3):431–447. [Google Scholar]
- Peterson C.C., Wellman H.M., Slaughter V. The mind behind the message: advancing theory-of-mind scales for typically developing children, and those with deafness, autism, or Asperger syndrome. Child Dev. 2012;83(2):469–485. doi: 10.1111/j.1467-8624.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin-Dubois D., Rakoczy H., Burnside K., Crivello C., Dörrenberg S., Edwards K., Krist H., Kulke L., Liszkowski U., Low J. Do infants understand false beliefs? We don’t know yet–A commentary on Baillargeon, Buttelmann and Southgate’s commentary. Cogn. Dev. 2018;48:302–315. [Google Scholar]
- Powell, L.J., Hobbs, K., Bardis, A., Carey, S., & Saxe, R. (2017). Replications of implicit theory of mind tasks with varying representational demands. Cognitive Development.
- Pyers J.E., Senghas A. Language promotes false-belief understanding: evidence from learners of a new sign language. Psychol. Sci. 2009;20(7):805–812. doi: 10.1111/j.1467-9280.2009.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rączy K., Hölig C., Guerreiro M.J., Lingareddy S., Kekunnaya R., Röder B. Typical resting-state activity of the brain requires visual input during an early sensitive period. Brain. Communications. 2022;4(4) doi: 10.1093/braincomms/fcac146. fcac146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H. Development of brain networks for social functions: confirmatory analyses in a large open source dataset. Dev. Cogn. Neurosci. 2019;37 doi: 10.1016/j.dcn.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., Lisandrelli G., Riobueno-Naylor A., Saxe R. Development of the social brain from age three to twelve years. Nat. Commun., 9(1), Artic. 2018:1. doi: 10.1038/s41467-018-03399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., Koster-Hale J., Caselli N., Magid R., Benedict R., Olson H., Pyers J., Saxe R. Reduced neural selectivity for mental states in deaf children with delayed exposure to sign language. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-17004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., Gweon H., Dodell-Feder D., Malloy C., Pelton H., Keil B., Kanwisher N., Saxe R. Response patterns in the developing social brain are organized by social and emotion features and disrupted in children diagnosed with autism spectrum disorder. Cortex. 2020;125:12–29. doi: 10.1016/j.cortex.2019.11.021. [DOI] [PubMed] [Google Scholar]
- Röder B., Rösler F. Memory for environmental sounds in sighted, congenitally blind and late blind adults: evidence for cross-modal compensation. Int. J. Psychophysiol. 2003;50(1–2):27–39. doi: 10.1016/s0167-8760(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Röder B., Demuth L., Streb J., Rösler F. Semantic and morpho-syntactic priming in auditory word recognition in congenitally blind adults. Lang. Cogn. Process. 2003;18(1):1–20. [Google Scholar]
- Saxe R., Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in theory of mind. NeuroImage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. It’s the thought that counts specific brain regions for one component of theory of mind. Psychol. Sci. 2006;17(8):692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R., Whitfield-Gabrieli S., Scholz J., Pelphrey K.A. Brain regions for perceiving and reasoning about other people in school-aged children. Child Dev. 2009;80(4):1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Schmidt E., Pyers J. First-hand sensory experience plays a limited role in children’s early understanding of seeing and hearing as sources of knowledge: evidence from typically hearing and deaf children. Br. J. Dev. Psychol. 2014;32(4):454–467. doi: 10.1111/bjdp.12057. [DOI] [PubMed] [Google Scholar]
- Scholl B.J., Leslie A.M. Modularity, development and ‘theory of mind’. Mind Lang. 1999;14(1):131–153. doi: 10.1111/1468-0017.00106. [DOI] [Google Scholar]
- Selcuk B., Brink K.A., Ekerim M., Wellman H.M. Sequence of theory-of-mind acquisition in Turkish children from diverse social backgrounds. Infant Child Dev. 2018 [Google Scholar]
- Shahaeian A., Peterson C.C., Slaughter V., Wellman H.M. Culture and the sequence of steps in theory of mind development. Dev. Psychol. 2011;47(5):1239. doi: 10.1037/a0023899. [DOI] [PubMed] [Google Scholar]
- Skerry A.E., Saxe R. A common neural code for perceived and inferred emotion. J. Neurosci.: Off. J. Soc. Neurosci. 2014;34(48):15997–16008. doi: 10.1523/JNEUROSCI.1676-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville J.A., Woodward A.L., Needham A. Action experience alters 3-month-old infants’ perception of others’ actions. Cognition. 2005;96(1):B1–B11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Kemmerer D., Adolphs R. The neural basis of conceptualizing the same action at different levels of abstraction. Soc. Cogn. Affect. Neurosci., nsv0. 2015:84. doi: 10.1093/scan/nsv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiroglu D., Moses L.J., Carlson S.M., Mahy C.E.V., Olofson E.L., Sabbagh M.A. The children’s social understanding scale: construction and validation of a parent-report measure for assessing individual differences in children’s theories of mind. Dev. Psychol. 2014;50(11):2485–2497. doi: 10.1037/a0037914. [DOI] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Liu D. Scaling of theory-of-mind tasks. Child Dev. 2004;75(2):523–541. doi: 10.1111/j.1467-8624.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Cross D., Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Fang F., Liu D., Zhu L., Liu G. Scaling of theory-of-mind understandings in Chinese children. Psychol. Sci. 2006;17(12):1075–1081. doi: 10.1111/j.1467-9280.2006.01830.x. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Lopez-Duran S., LaBounty J., Hamilton B. Infant attention to intentional action predicts preschool theory of mind. Dev. Psychol. 2008;44(2):618–623. doi: 10.1037/0012-1649.44.2.618. [DOI] [PubMed] [Google Scholar]
- Whitcomb S. Routledge,; 2013. Behavioral, social, and emotional assessment of children and adolescents. [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A., Ghosh S. Artifact detection tools (ART). Cambridge. Ma. Release Version. 2011;7(19):11. [Google Scholar]
- Wu Y., Schulz L.E. Inferring beliefs and desires from emotional reactions to anticipated and observed events. Child Dev. 2018;89(2):649–662. doi: 10.1111/cdev.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Baker C.L., Tenenbaum J.B., Schulz L.E. Rational inference of beliefs and desires from emotional expressions. Cogn. Sci. 2018;42(3):850–884. doi: 10.1111/cogs.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Geng F., Riggins T., Chen G., Redcay E. Neural correlates of developing theory of mind competence in early childhood. NeuroImage. 2019;184:707–716. doi: 10.1016/j.neuroimage.2018.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T.S., Ellis C.T., Turk-Browne N.B. Emergence and organization of adult brain function throughout child development. NeuroImage. 2021;226 doi: 10.1016/j.neuroimage.2020.117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Summary data for reproducing statistical analyses are publicly available for download (https://osf.io/pavdg/). Data were collected prior to the popularization of public data sharing and so the conditions of the informed consent given by participants and their families do not allow for public archiving of individual raw MRI or behavioral data. Individuals seeking access to raw data should contact Dr. Rebecca Saxe (saxe@mit.edu). Access will be granted to individuals who complete a data usage agreement through the Committee on the Use of Humans as Experimental Subjects (COUHES) at MIT.