Abstract
Attention following (AF) is a cornerstone of social cognitive development and a longstanding topic of infancy research. However, there is conflicting evidence regarding the development of AF. One reason for discrepant findings could be that infants’ AF responses do not generalize across settings, and are influenced by situational factors. Theories of AF development based on data collected in laboratory paradigms might skew our understanding of infants' everyday AF. To reveal more generalizable patterns of infant AF development, we compared healthy, North American infants' (N = 48) AF developmental trajectories between a controlled laboratory paradigm and a naturalistic, home-based, parent-directed paradigm. Longitudinal micro-behavioral coding was analyzed to compare individual infants' AF between the two settings every month from 6 to 9 months of age. We aimed to (1) examine longitudinal development of infant AF in two settings; (2) compare AF development between settings, and (3) explore differences in adult cueing behaviors that influence AF. We found that longitudinal trajectories of AF differed between home and lab, with more AF at home in earlier months. Additionally, AF at home was related to maternal cueing variables including bid duration and frequency. These results have implications for the assessment of infants' developing social attention behaviors.
Keywords: Gaze following, Infant social development, Joint attention, Longitudinal, Parenting, Social context
During the first year of life, infants start to engage more in triadic interactions, in which infants and caregivers attend to objects in coordinated transations. These attention-sharing episodes give infants opportunities for social referencing (Feinman, 1982), language learning (Tomasello & Todd, 1983), and learning about others’ action-patterns (Bakeman & Adamson, 1984). Despite great interest in this phenomenon, attempts to study the developmental trajectories of infant attention-following (AF) skills have yielded mixed results. One source of difficulty in pinpointing the emergence and trajectory of AF stems from the interactive effects of situational variables such as a cue-giver’s behaviors, physical properties of the testing environment, and the social context of the interaction. Weisz (1978) suggested that to identify robust features of human development, we should identify behaviors that are trans-contextually valid. Moreover, Baumrind (1968) and Robinson and Rackstraw (1967) warned that observations made in laboratory settings are not representative of everyday caregiver-infant interactions, and might seriously distort parental behavior. This argument emphasizes that in order to get a more precise picture of typical infant AF, we should obtain representative slices of everyday interactions (Bronfenbrenner, 1977).
Studies of infant AF have been conducted in both naturalistic (Deák et al., 2017; Yu & Smith, 2013) and controlled laboratory (Butterworth and Jarrett, 1991, Deák et al., 2000) settings. Each setting has its advantages and disadvantages. AF behaviors in a home setting are more likely to resemble everyday, natural triadic interactions. Bronfenbrenner (1977) suggested that observations in a home setting provide a more ecologically valid sample of behaviors. Conversely, laboratory studies tend to minimize distractions, control extraneous variables, and standardize protocols and observational conditions across participants, sessions, and trials (Lytton, 1977). Notably, indirect evidence suggests that AF differs between naturalistic and laboratory settings.
Previous research has investigated the effects of situational variables on maternal and infant behaviors around AF. Studies show that WEIRD (western, educated, industrialized, rich, and democratic; Henrich et al., 2010) parents use various cueing behaviors to attract and redirect infants’ attention, as opposed to the scripted gaze and/or pointing cues typically used in controlled lab studies (e.g., Butterworth & Jarrett, 1991). Deák et al. (2017) observed that during unscripted at-home dyadic play, North American mothers tended to employ a variety of behavioral cues to direct infants’ attention, including verbalizations, gaze, pointing, manual actions, and non-speech sounds. Further, caregivers' touching behaviors can modify infants' attention (Jean et al., 2014). Research paradigms that restrict the cues by which adults can direct infants' attention will have limited scope to support conclusions about the development of infant AF skills (Boyer et al., 2020). Micro-behavioral analyses also have revealed that caregivers’ object-handling actions are more reliable attention-recruiters than the explicit gestures (e.g. pointing) typically used in lab studies (Deák et al., 2014, Deák et al., 2017, Yu and Smith, 2013). Relatedly, Deák et al. (2014) and Morissette et al. (1995) suggested that laboratory studies overestimate the importance of gaze cues in directing infants’ attention, and that infant AF differs in laboratory versus naturalistic settings. For example, Deák et al. (2008) showed that 1-year-old infants, who can follow gaze in laboratory paradigms, do not spontaneously follow gaze in a cluttered lab environment.
Possible setting-based differences in infant AF are also important for diagnostic purposes. Atypical AF skills are often used in diagnosing autism spectrum disorder (ASD) (Dawson et al., 2004, Grzadzinski et al., 2021). Standardized tests such as the Early Social Communication Scales (ESCS, Mundy et al., 2003) have been used to assess infants' social-attention skills, including AF. Yet it is unclear whether such clinic-based assessments predict everyday AF in informal settings. More generally, if lab paradigms elicit atypical behaviors, they may skew our inferences about everyday AF and its development. A direct comparison of infants' AF between laboratory and home settings would inform our understanding of the development of AF more broadly, and potentially suggest improvements in the assessment of infants' early social skills.
Given these considerations, it is important to assess how infant AF differs in laboratory versus home settings, using paradigms that represent previous studies. A guiding assumption is that laboratory paradigms are less naturalistic, but also less variable, than home-based paradigm. In fact it is possible to simulate aspects of naturalistic environments in carefully-designed laboratory studies (see, e.g., Sun & Yoshida, 2022, for an innovative example), but most published and influential studies in the literature generally fit the above distinction.
To obtain a more precise characterization of the early development of AF, we tested a group of infants monthly from 6 to 9 months in a state-of-the-science laboratory paradigm (Tang et al., 2022), and tested the same infants monthly in a naturalistic home-based paradigm. Further we coded and analyzed caregivers' spontaneous attention-modulating cues in the home setting.
Prior studies indicate that AF develops from 6 to 9 months (e.g., Mundy & Gomes, 1998). To further our understanding of this transitional age, we recruited infants (N = 48) and their mothers in a Southern California city. Infants' AF behaviors were tested monthly in two settings: (1) a scripted laboratory task with an experimenter who produced controlled attention-directing cues (gaze, point, and gaze-and-point); and (2) an unscripted AF task at home with their mother. Our home paradigm offers a compromise between naturalism and familiarity of setting on the one hand, and control over parameters like targets and target-locations on the other. The controls implemented in the home permitted meaningful comparisons across participants and sessions. Differences in the two (lab and home) paradigms included the physical environment, the task structure, the adult interlocutor, and details of the behavioral interactions. We hypothesized that some of these differences would influence the emergence of infant AF between settings. In order to meaningfully compare infants' AF between the two settings, we defined an interval of scripted cue actions by an experimenter toward a pre-specified target, carried out in the lab, as a trial. Correspondingly, we defined bouts of unscripted, successive (i.e., temporally-bound) cue actions by a mother towards a spontaneously selected target, carried out at home, as a bid. We assessed infants' AF efficacy as the ratio of trials or bids within a session for which an infant efficiently reoriented attention to the specified target of that trial or bid.
To better understand any differences in infants' AF between settings, we analyzed infants' contingent responses to the experimenter's or mother's cues via micro-behavioral coding methods and sequential analyses. Three behavioral variables were considered in more detail for this report: bid density (bid duration and frequency; Tomasello, 1988), and two bid strategies by caregivers at home: touching infants (presumably to recruit their attention), and speech (i.e., verbalizations to influence infants' attention) (Suarez-Rivera et al., 2019; Tomasello & Farrar, 1986).
By examining infant AF development during an important developmental period in both laboratory and naturalistic settings, we hope to identify robust cross-situational trends in infant AF development, and to evaluate the validity of standardized assessments of infants’ social attention skills. The results might therefore inform behavioral assessment instruments for infants referred for possible ASD or other developmental disorders (e.g., Grzadzinski et al., 2021; see also Leekam et al., 1998). However, we consider this a preliminary comparison of setting effects on infant AF. It cannot determine the independent roles of all variables that differ between lab and home settings – rather, it is a first attempt to understand the effects of setting, and some relevant factors that differ between most laboratory versus most home studies, on infant-adult attention-sharing.
1. Method
1.1. Participants
Forty-eight infant-mother dyads were recruited in San Diego, California. Families were recruited as a convenience sample through a birth record database from the State of California, fliers posted in local businesses that serve young families, online postings to parents’ groups, and by word-of-mouth. Five infants withdrew from the study and were dropped from analyses, leaving 43 infants (20 female). Infants participated monthly from 6 to 9 months,4 at mean ages for home sessions of 186.5 (SD = 7.4), 217.1 (9.1), 247.6 (6.8), and 277.3 (7.6) days, respectively; and for lab sessions at mean ages of 188.8 (SD = 6.5), 219.4 (8.8), 248.8 (9.3), and 278.5 (8.2) days, respectively. All caregivers were biological mothers, averaging 32.4 years of age at the 6 month session (range = 21–42), with a mean of 16.1 years of formal education (range = 12–21). The average intervals in days between monthly home and lab sessions were, respectively from 6 to 9 months, 5.7 (SD = 4.4), 6.3 (9.7), 5 (6.5), and 4.5 (3.5).
Parents reported that thirty-two infants (74.4 %) were of primarily European descent, two (4.7%) of primarily Hispanic/Latinx ethnicity, and nine (20.9 %) of multiple or “other” racial or ethnic backgrounds. Twenty-six (60.5 %) infants were first-born; the rest had one or more siblings. Thirty-three infants (76.7 %) lived with two adults, three (7 %) lived with one adult, and seven (16.3 %) lived with 3 + adults.
2. Materials
2.1. Lab
2.1.1. Test environment
Note that the lab results reported here are a subset of the data reported in Tang et al. (2022).5 Infants were tested in a sound-attenuated room (4.0 m × 3.6 m) while sitting in a Bumbo soft seat on their mother’s lap, facing Experimenter 1 (E1; see Fig. 1). Experimenters were trained, English-fluent, female, advanced undergraduates or staff members aged 19–22 years. None were biological parents, but all had experience working with infants.
Fig. 1.
A pointing trial in the lab vs a bid at home. Note: a. Lab session: the infant sits on mother's lap, facing E1 (seen pointing cue to a side monitor). The infant has followed the cue to the target monitor, so the reward video has been initiated. b. Home session: the infant sits in a walker across from the caregiver (who is making a bid to the side puppet and is turning in the direction of the target).
Mothers wore opaque glasses and noise-canceling headphones playing music so that they could not perceive the cues or their infant's reaction. Six monitors (33 cm diagonal) were positioned approximately 2 m from the infant, at the average height of the infants’ eyes. Two monitors were in front of the infant ( ± 33° from midline), two were in the infant’s periphery ( ± 78° from midline), and two were behind the infant ( ± 126° from midline). A soundbar was mounted beneath each monitor to play the audio when the corresponding video played on that monitor. Five video cameras (4 JVC Everios; 1 Panasonic CCTV camera) were set up to capture different views of the session, as summarized in Table 1.
Table 1.
Camera Views At Home and Laboratory Settings.
| Camcorder | Scene captured | Position | |
|---|---|---|---|
| Home | Contextual | 3 target puppets; heads & upper bodies of Inf and Mom; nearby environment | 1.7 m from Inf; ± 270°; 80 cm high |
| Infant | Inf head & upper body, tray | 1.2 m from Inf; ± 45°; 60 cm high | |
| Caregiver | Mom head & upper body; left tray | 1.3 m from Inf; ± 155°; 60 cm high | |
| Lab | Front (2: left & right) | Inf head & upper body; One zoomed out to capture E1 | 2 m from Inf; ± 33° |
| Back (2) | Inf head & upper body; One zoomed out to capture E1 | 2 m from Inf; ± 126° | |
| Overhead | Inf, E1 & monitors | 2 m from Inf |
Note: Inf = infant; E1 = experimenter 1. Radial angle is relative to Inf's midline.
An adjacent control room displayed the output of the cameras via an AV system designed to push videos to selected monitors and imprint timestamps, a RAID computer customized to capture videos from the cameras, a video synthesizer to create a backup file, and a two-way radio to communicate with E1.
2.1.2. Rewards videos
Based on pilot testing with a variety of video clips from different sources we selected video clips from the commercial Baby Einstein series. The clips all show high-contrast toys or animals moving to synchronized, synthesized, simplified arrangements of European classical music (mostly mid-tempo, with diatonic harmonizations, simple meters, and common Western modalities). The videos use perspectives, focal distances, and motion patterns that infants find engaging. The videos capture infants’ attention (DeLoache & Chiong, 2009), including in joint-viewing contexts (Demers et al., 2013). Two experienced infant researchers selected and rendered a set of 8-sec video clips from the series, featuring a variety of dynamic visual stimuli and musical selections.
2.2. Home
2.2.1. Test environment
Infants were seated in a comfortable, commercially available walker in the usual play area of their home. Mothers sat on a pillow on the floor, facing the infant. See Fig. S1 for details of participant, target, and camera placements. The walker height was modified so that infants’ and mothers’ eye heights were nearly equal. Three Canon Optura mini-DV camcorders on tripods captured different views of the session, as summarized in Table 1 (see also Fig. 1).
Four tripods covered in beige cloth, for the camcorders and distractor toys, were placed at predetermined angles and distances, (see Table 1, top, and Fig. S1). The target puppets were placed in front of three of the tripods (Fig. S1).
2.2.2. Toys
Three animal puppets (controlled for familarity and size) were used during the play sessions. Puppets were replaced every month to control for novelty. They were placed upright against tripods covered with beige cloth for a neutral background, facing the dyad (Fig. S1). Puppets were moderately complex (Deák et al., 2000) and age-appropriate.
Two additional age-appropriate toys were placed on the contextual camera tripod and on the side tripod, respectively. These served as distractors, to test whether infants precisely followed mothers’ cues or were simply attracted by toys.
3. Procedure
3.1. Lab
Infants sat on their mother's lap facing E1, who the infant knew from 1 to 2 previous sessions (Fig. 1). After an orientation phase (described below), E1 conducted 18 test trials by directing infants’ attention to specific monitors, and 2 baseline trials. Each test trial featured one of three cue types: gaze (G), point (P), or gaze-and-point (GP). The order of trials and monitors was quasi-randomized and consistent across infants. A different trial order was used every month. Randomization was constrained so that each combination of cue type and target location (left and right front, periphery, and back) was used once in the 18 test trials. Additionally, locations and cues were constrained to be relatively balanced across the first and second half of the trials. E1 was signaled by another experimenter (E2) in the control room, via earphones and two-way radios, on the timing and order of trials.
3.1.1. Orientation phase
E1 socialized with infants until they seemed comfortable, then began showing infants each monitor using natural social cues (voice, gesture, gaze, touch). E2 triggered a video reward as the infant attended to each monitor. Video rewards were different for each monitor and in each session. This procedure was designed to teach infants to expect visual rewards from the monitors. There was a brief pause after the last orientation trial.
3.1.2. Test phase
In each of 18 test trials E1 silently produced one of three cues: (1) Gaze: turned head to look at the target monitor; (2) Point: extended arm and hand in a full index-point toward the target monitor; (3) Gaze+Point: produced both gaze and point cues simultaneously. In each trial E1 first called the infant’s name to draw her/his attention, and only produced the cue when this was achieved. E1 produced the cue for 4 s, then turned back to the infant for 1 s, then repeated the cue for another 4 s before terminating the cue(s) and turning back to midline with her hands in her lap.
E2 monitored infants’ behaviors and played the video reward if and when the infant turned to the cued monitor. Otherwise, the video played approximately 2 s after the trial ended, when E1 was no longer producing a cue. This ensured that all infants had the same number of opportunities to associate the monitors with the video rewards.
In two additional baseline trials (one in each half of the trials). E1 called the infant’s name, then looked down at her lap for 4 s. These trials were included to estimate infants’ tendency to look at the monitors without any directional cue.
3.1.3. Coding
Trained research assistants, blind to specific hypotheses, watched synchronized video recordings and coded (at 30 Hz precision) the onset and offset of each cue, and the time and location of infants' visual fixations to E1's face or hand (social cues), any video monitor, their mother (who infants occasionally tried to engage), or other locations in the room.
3.2. Home
After obtaining informed consent and instructing the mother, infants and mothers were seated in pre-specified locations and the researchers left the room. After a 6-minute free play session (described elsewhere; de Barbaro et al., 2016), the mother attempted to direct infants' attention to target puppets (described below) placed at controlled distances and angles across sessions and dyads.
For the AF task the toys used during free play were removed by researchers, and mothers were instructed to “try to get your child to look at all three of the puppets in front, to the side of, and behind you. Do this how you would normally direct your infant’s attention to an object.” Because coding showed that mothers typically pointed to start a bid, a mother’s first point to a target was coded as the start of the AF task (and of the first bid). The researchers stayed out of the room during the session, but re-entered if the baby became fussy.
3.2.1. Coding
Videotapes were captured at 30 fps using VirtualDub software, clipped at a common sync point, and downsampled to 10 fps for coding.6 Coders used Mangold INTERACT (Mangold, 2022) to code three minutes from the AF task, annotating all of the mother’s manual actions and gaze shifts, and infants' visual fixations to the tray, puppets, or mother. In another coding pass, all maternal utterances were transcribed (Alonzo et al., in preparation); these times of maternal utterances were compiled with the manual action and gaze data.
3.2.2. Statistical analyses
Analyses were run using R [v4.3.0; R Core Team 2023].
Current analyses focus on mothers' behavioral cues to draw infants’ attention, and the frequency and duration of these cues. Mothers typically first drew infants’ attention by touching their hands and/or face, calling their names, or tapping the plastic tray while making eye contact. After getting infants’ attention, mothers typically used three types of cues: (1) Point: arm extended with the index finger pointing to a target; (2) Vocalize: calling the infant's name and/or using attention-eliciting and/or -attracting speech or vocal sounds; (3) Gaze: turning head and eyes to fixate a target. Mothers used unscripted combinations of these cues during a bid.
4. Results
4.1. Dependent measures
All analyses are focused on infants’ AF responses, or "hits," defined as switching gaze from the adult (i.e., E1 [lab] or mother [home]) to the target specified by the adult's cue. Hits can be defined using more stringent or more lenient criteria (Tang et al., 2022). Here we apply a stringent criterion: infants' first fixation away from the adult must focus on the cued target. This criterion was applied to both lab and home data. Tang et al. (2022) showed that the results (for lab data) are only modestly affected by the criterion.
4.2. Missing data and data retention
Lab data were retained if infants completed at least 17 trials, with at least one trial for each of the nine cue/location combinations. Home data were retained if mothers produced at least two bids for each target location. Preliminary analyses showed that these criteria eliminated 16.8 % of home sessions (29 out of 172) and 15.7% of lab sessions (27 out of 172) – without affecting the results.
4.3. Attention following by age and setting
4.3.1. Overall cue following performance
Because the number of trials varied slightly across sessions, results are reported as standardized proportions of hits. Cues in the lab were G, P, or GP (trial-wise, within-subjects), whereas cues at home were unscripted, and typically included combinations of gaze, pointing gestures, vocalizations, and other actions (e.g., touch; see below). Boxplots in Fig. 2 show distributions of proportions of hits in home and lab settings from 6 to 9 months of age. Infants overall followed attention more at home than in the lab.
Fig. 2.
Box plots: total proportions of hits by month in home and lab settings. Note: Boxes show proportion of hits each month, for retained sessions (see text). Blue lines: best-fitting linear trends for mean hits at home (y = −0.029x + 0.199), and in the lab (y = 0.377x - 2.81). Shaded region: 95 % CI.
To capture these differences a three-way ANOVA was conducted on infants’ session-wise AF performance (i.e., hit ratios), with age, target location, and setting varied within-subjects (Table 2). Main effects were found for age, target location, and setting. Significant two-way interactions were found between age and setting, age and target location, and target location and setting. Each significant effect is described below.
Table 2.
Three-way ANOVA results based on age, target location, and setting.
| dfnum | F | p | |
|---|---|---|---|
| Age | 3 | 3.38 | .0179* |
| Target location | 2 | 145 | < .001* ** |
| Setting | 1 | 84.9 | < .001* ** |
| Age: target location | 6 | 2.21 | .040** |
| Age: setting | 3 | 4.04 | .007** |
| Target location: setting | 2 | 10.6 | < .001* ** |
| Age: target location: setting | 6 | 1.41 | .21 |
Note: * p < .05; * * p < .01; * ** p < .001.
The month-by-month mean hit rates in the lab, from 6 to 9 months, were 0.095 (SD=0.096), 0.148 (0.104), 0.172 (0.121), and 0.231 (0.119). Month-by-month mean hit rates in the home were 0.283 (SD=0.172), 0.270 (0.187), 0.262 (0.215), and 0.256 (0.174). Hit proportions were higher at home than in the lab. Home hit rates also had a wider interquartile range, suggesting greater variability at home across months. To elaborate the age-by-setting interaction, one-way ANOVAs were conducted for each setting. They revealed a significant increase in AF in the lab from six to nine months (F(3, 141) = 9.74, p < 0.001); but no significant change at home (F(3, 126) = 0.23, p = 0.879).
4.3.2. Attention-following by target location
Infant AF to each target-location pair (front, side, back) increased with age in the lab setting, although at every age and setting they followed most readily to front targets (Fig. 3). In both settings infants followed cues to front more than side targets (Home: Z = −1.95, p < .05; Lab: Z = −8.03, p < .0001) or back targets (Home: Z = −8.16, p < .0001; Lab: Z = −12.6, p < .0001). However, developmental trajectories in the lab were significantly steeper for front than side targets, t(64) = 4.79, p < .0001, or back targets, t(51) = 5.63, p < .0001 (the latter did not differ, p = .481). By contrast, trajectories at home did not differ for front vs. side targets, p = .704, front vs. back targets, p = .446, or side vs. back targets, p = .724 (all t values <1).
Fig. 3.
Hit Proportions by month by target location and setting. Note: Points indicate proportions of hits for each target location: Front (left panel), Side (middle), and Back (right). Top: Home data; bottom: Lab data. Blue lines: best-fitting linear regression trends, with 95 % CI cloud.
4.3.3. Individual differences in following
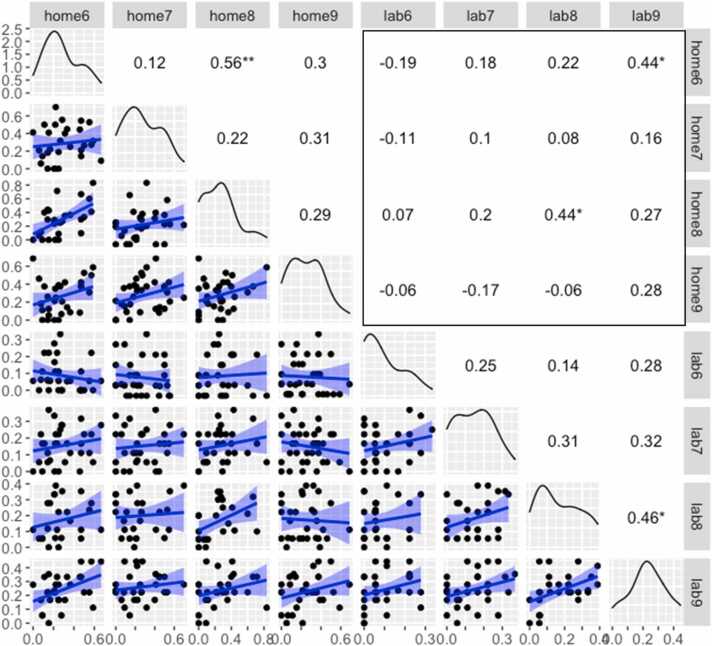
Between-month stability of AF at home was positive but low-moderate, with inter-month correlations ranging from .12 to .56 (Pearson rs), and only one reliable association. By contrast, between-month AF stability in the lab increased from 6 to 9 months (rs: .25 to .46). Pearson's r coefficients and p values, scatterplots, and histograms are shown in Fig. 4. AF between lab and home (boxed region of Fig. 4) did not show overall reliable stability, with coefficients averaging r = .116.
Fig. 4.
Correlations of hit proportions from 6 to 9 months in home and lab settings. Note: Above the diagonal: Pearson's correlations between stringent hit rates across months in home and lab settings. The boxed area shows correlations across settings. Below the diagonal: Scatterplots, with best-fitting linear trends for infants with valid data in both months (shaded region: 95% CI). Diagonal: smoothed histograms for that month and setting. Axis labels at left and bottom indicate scale. *p < .05; **p < .01; ***p < .001.
Additional Linear Mixed-Effects models were run on session-wise hit rates, to estimate variance related to individual differences. The results are reported in Supplemental Tables S1 and S2.
4.3.4. Maternal behavioral predictors of following at home
The above results suggest that infants follow adults' attention cues more reliably at home than in the lab, especially during the earliest months of AF. The following analyses describe mothers' bids and attention-directing cue combinations (Deák et al., 2017), and consider how these behavioral variables predict infants' AF. The first results concern bid duration and frequency, and address whether infants more reliably followed longer bids and/or mothers who initiated more bids.
4.4. Bid duration and frequency
In the lab setting E1 produced each cue (gaze, point, or gaze and point) twice per trial, each time for 4 s. By contrast, in the naturalistic setting mothers produced bids of various durations and frequencies. The month-by-month trial duration means in the lab were constant from 6 to 9 months (4 s per cue, 9–10 s per trial). However, bid duration at home significantly decreased from 6 to 9 months: respectively, means = 3.5 s (SD=2.8), 3.6 (3.0), 3.7 (3.1), and 3.2 (2.8) (y = −1.15x + 43.3, p = .018, see Fig. S3). In contrast, whereas the monthly trial frequency in the lab remained constant at 18 trials per session, the mean bid frequency at home increased from 6 to 9 months: respectively, 19.3 (SD=6.5), 19.9 (7.5), 19.7 (7.2), and 21.6 (7.3) (Fig. S4).
4.5. Bid orienting actions
Mothers' actions to orient the infant to an attention-directing bid were noted, during extensive preliminary observations, to include touches of the infant and verbalizations to the infant. Both of these were commonly used to attract and "center" the infant's attention before and during an attention-directing cue toward a target.
4.5.1. Touch
Mothers often touched infants to "center" their attention before redirecting them to a target. All mothers used touch at least once per session before a directing cue. The proportions of bids initiated with a touch within 500 msec before the first directing cue did not reliably differ with age: 11.2% (SD= 9.44%), 12.8 (15.0), 12.7 (14.4), and 7.58 (7.16) from 6 through 9 months, respectively (Fig. S5).
4.5.2. Verbal cue use and duration
Mothers spontaneously combine multiple cues to direct infants’ attention (Deák et al., 2017), often including verbal cues. The average proportions of time spent verbalizing during a bid were consistent from 6 to 9 months in home session: .486 (SD= .254), .513 (.263), .453 (.290), .504 (.274) (Fig. S7). The proportions of bids incorporating verbal cues were relatively constant from 6 to 9 months: .915 (SD= .279), .907 (.290), .833 (.373), .888 (.316) (Fig. S6). The only verbal cue in the lab setting was when E1 called infants’ names to center their attention before each trial.
4.6. Relations of settings to infant AF differences between home and lab
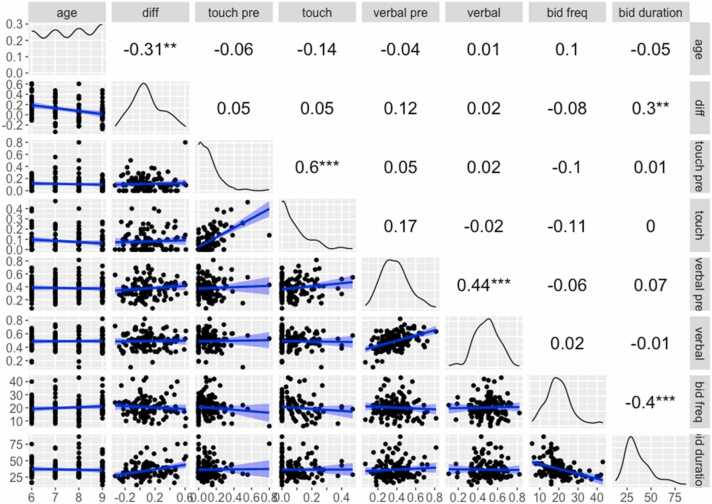
To understand the relation between bid behaviors and differences in an infant's hit rate between home and lab, we explored correlations between these variables (Fig. 5). Differences in infants’ AF hit proportions between settings were calculated for each month. Age was negatively associated with differences in AF between settings (r = −.31, p < .001), indicating that as infants got older, AF became more similar between home and lab settings. Also, bid duration (at home) was positively associated with between-setting differences (r = .30, p < .001), suggesting that longer bids predicted higher hit rates at home, and thus a larger difference between settings.
Fig. 5.
Correlations of home bid variables with differences between home and lab AF rates. Notes: Diff = [Home - Lab] hit rate difference; touch pre = % of bids preceded by maternal touches; touch = % of bid time with maternal touches; verbal pre = % of bids preceded by maternal verbalization; verbal = % of bid time with maternal verbalizations; bid freq = frequency of each mother's bids, averaged session-wise for individual subjects; bid duration = mean bid duration within a session. Above the diagonal: Pearson's correlations among the aforementioned variables and infant age. Below the diagonal: Scatter plots with best-fitting linear trends (shaded region: 95% CI). On the diagonal: smoothed histogram for that variable. Axis labels at left and bottom indicate scale. *p < .05; **p < .01; ***p < .001.
In addition, mothers' spontaneous attention-orienting actions were reflected in the attention-directing cues they used during bids. Mothers' tendency to initiate a bid with a touch was correlated with touching infants during the bid (r = .60, p < .001); similarly, mothers' tendency to initiate a bid with a verbalization predicted their further verbalization during the bid (r = .44, p < .001).
To further explore these relations, a forward stepwise linear regression quantified predictors of the difference in infants’ AF between home and lab. Age and bid variables (those shown in Fig. 5) were entered as predictors. At each step variables were chosen based on p values, and AIC was used to limit the number of variables in the final model. The final model reduced the predictors to three, two of which were significant: age (smaller between-setting AF differences with increasing age) and bid duration (larger differences with longer bids). Rate of prior verbalizations was a non-significant predictor (Table 3).
Table 3.
Forward stepwise model.
| Beta | SE | t | p | |
|---|---|---|---|---|
| Intercept | 0.257 | 0.157 | 1.639 | .10 |
| Age | -0.050 | 0.017 | -2.945 | .004** |
| Bid duration | 0.005 | 0.002 | 2.826 | .006** |
| Verbal info before cue | 0.154 | 0.139 | 1.107 | .27 |
Note: *p < .05; **p < .01; ***p < .001.
5. Discussion
Despite active research for over 40 years on the development of attention-following (AF) in infancy, disagreements persist about critical issues such as the age of emergence of AF, and the effect of contextual factors (many of which would differ across settings) on AF (see Astor & Gredebäck, 2022). Some researchers (Bard et al., 2021, Belsky, 1980, Brookhart and Hock, 1976, McGuire, 1983, Meehl, 1978; Parry, 1972) have pointed out that ignoring the effects of setting (and accompanying contextual factors) on infants' behaviors hinders our understanding of complex social processes. We believe that it also limits our understanding of developmental trajectories and the factors that affect these trajectories. For example, although there is evidence that cultural practices and settings influence infant AF development (e.g., Cohen et al., 2017; Keller, 2007), the literature on AF seldom compares AF behaviors across settings, and virtually never has done so with the same infants. This absence of data comparing the same infant's attention patterns in different settings or tasks, across developmental time, precludes any comprehensive theory or even a generalizable description of the development of AF (but see Teuscher & Triesch, 2007). Moreover, it precludes informed predictions of how an infant's social attention might vary across settings, thereby limiting clinical efforts (see Grzadzinski et al., 2021). Most studies have been conducted in controlled laboratory settings, with little discussion or understanding of the differences between these paradigms and naturalistic episodes of attention-sharing (Astor and Gredebäck, 2022, Suarez-Rivera et al., 2022). Other studies have been conducted in homes or other naturalistic settings, but most of these have small samples, "rough" coding schemes that do not precisely specify caregivers' behaviors, and/or vague methodological documentation.
The current study sought to address these limitations by observing a group of 43 infants monthly from 6 to 9 months using systematic and well-documented methods (https://osf.io/m64bk/), both in a modified laboratory paradigm that addresses some limitations of previous lab studies (Tang et al., 2022), and in an unscripted home paradigm that constrains some general aspects of the interaction (e.g., visual targets; seating arrangement), but leaves the caregivers' behaviors minimally unconstrained. This allowed us to document systematic age-related and individual differences across two historically influential but quite dissimilar paradigms, and test whether the lab paradigm predicts AF in a more naturalistic home-based paradigm. This design is pertinent to the interpretation of prior research.
Previous studies suggest that infants and caregivers behave differently in laboratories than at home or daycare centers. For example, play and exploration, as well as affect and responses to parents, are influenced by setting in infants as young as 1 year old (Fein, 1975, Parry, 1972). Tamis-LeMonda et al. (2017) found that mothers’ infant-directed speech differs between home and structured lab settings, and that controlled paradigms can elicit more language and shared attention in mother-infant dyads. Also, unfamiliar settings may elicit more negative reactions from infants to strangers (Ross et al., 1975, Skarin, 1977): i.e., less smiling, more crying, and more wary expressions have been reported in lab than home settings (Skarin, 1977, Sroufe et al., 1974).
Attention-sharing, and AF in particular might also be affected by situational factors that vary across settings. For example, infants' responsiveness to attention-directing social cues might be modulated by ecological factors. For example, the repetition of cues and the complexity or uniqueness of targets (Deák et al., 2000) predict infants' AF. Notably, also, 1-year-olds showed minimal gaze-following in a lab setting that was cluttered, like a home setting (albeit unfamiliar; Deák et al., 2008). Deák et al.'s (2008) results also imply that some caregiver cues, like pointing, might be more robust to setting differences than others, like gaze shifts. Such results suggest that infants' response to particular combinations of attention-directing cues will depend partly on little-understood contextual factors that vary across settings.
5.1. Age effects across settings
The results suggest that longitudinal changes in infants' AF were modulated by setting. 'Hit' rates (i.e., proportion of infants’ first look, after the adult's first cue, to the target) were reliably higher at home than in the lab. Additionally, the trajectory of change across months was flat in homes, but increased significantly in the lab, consistent with previous lab studies (e.g., Butterworth & Cochran, 1980; Butterworth & Jarrett, 1991). Previous findings suggested that unfamiliar settings may elicit more negative stranger responses from infants (Ross et al., 1975, Skarin, 1977), which might explain the lower hit rates at 6 months in the lab. In addition, the experimenters might have shown less sensitive or affectional behaviors with infants due to their age and experience with infants (Landy et al., 1983), and/or due to the less-flexible lab protocol. However, novel settings and adults can elicit more focused attention from infants: Striano & Bertin (2005) and Gredebäck et al. (2010) found that infants coordinated attention more with strangers than with mothers, and demonstrated an increasing stranger preference from 2 to 9 months of age. These findings suggest that age effects in stranger responses might facilitate infant AF in the lab, and contribute to increasing AF from 6 to 9 months.
Another possible reason for the lower lab AF rate is that the more novel environment might have induced more extensive visual scanning, consistent with norepinephrine-mediated heightened vigilance (deBarbaro et al., 2011). Higher scanning rates due to vigilance would accompany more broadly distributed attention, and thus a tendency to look at multiple targets, as opposed to focused attention to a single target. This might have modestly reduced AF in the lab.
It is also possible that the controlled lab paradigm was a more challenging environment than home for infants, resulting in lower overall hit rates. One type of challenge might have been emotional regulation in a strange setting (e.g., Walden & Baxter, 1989). As infants develop affect regulation processes with social partners, from 6 to 9 months (Striano & Bertin, 2005), their AF might tend to improve in novel settings (e.g., laboratories). This hypothesis also suggests that individual variability in affect regulation should affect infants' AF – a potential question for future research.
It also might be assumed that the lab setting was more challenging (thus reducing AF) because there were more targets: six, versus only three in home sessions. Hypothetically this would lower the base-rate of success in any given trial. This assumption, however, seems implausible. First, there were two additional distractor objects at home, which infants could (and occasionally did) look at, reducing the difference in number of possible targets (lab:home) from 6:3 to 6:5. Second, homes were far more cluttered than the stark lab testing room, with unspecified (but large) numbers of additional informal visual targets. Although it is difficult to estimate the total number of possible targets in a given home, it was certainly greater, not less, than the total in the lab. Moreover, the infant seat used for home sessions had a visually interesting and potentially distracting tray, unlike the seat in the lab. Thus it was probably overall harder in homes than the lab to search for and fixate a specific visual target.
Finally, we considered whether our hit rates in the lab were lower than in comparable lab studies - if so, perhaps some aspect of our paradigm suppressed AF. As a comparison, for example, Morissette et al. (1995) reported 30.4% and 8.3% hit rates for front and side targets, respectively, at 6 months, and 52.1% and 18.7% hits at 9 months, for GP trials. However, they used only 4 targets (with no "lure" distracting from the side target), and infants' mothers delivered cues, so their task had some advantages. Yet the task garnered AF levels comparable to ours, suggesting that our task was not particularly disadvantageous.
5.2. Individual differences: Stability within and across settings
Our results show low-moderate stability of individual AF in the lab between most months, with a reliable association only between 8 and 9 months. These associations are slightly lower but in the same range as most between-month correlations reported by Morales et al. (2000), though ours were between shorter intervals across a narrower age range. They also are slightly lower than the correlation from 6 to 8 months reported by Saxon et al. (1997). A comparable range of correlations across months was found at home, with a trend of more stability in later months. This is roughly consistent with previous reports (Mundy et al., 2007).
Correlations between home and lab settings (boxed area in Fig. 4) were low and non-significant. This suggests that an infant's AF in a lab task predicts only 1–2 % of variance in their AF at home, even in the same month. This raises questions about the generalizability and external validity of standardized/scripted tests of infant social attention administered by clinicians or experimenters in unfamiliar settings (e.g., clinics, labs).
5.3. Attention cues and bid structures as mediators of AF
Mothers at home spontaneously produced sequences of unscripted social cues to direct infants' attention. Notably, mothers tended (non-significantly) to initiate more and shorter bids with infants' increasing age, potentially making AF more challenging as infants got older. This is consistent with previous evidence that caregivers support infants' AF with social cues (Bornstein and Tamis-LeMonda, 1990, Sun and Yoshida, 2022, Yoshida et al., 2020). Infants also are expected to react faster to bids at home with increasing age. The relation between infants' developing AF skills and mothers' changing expectations and strategies, however, makes the setting-by-age effect difficult to interpret. It is also possible that maternal behaviors did not change in a linear relation to infants’ developing AF and other (e.g., motor) skills - this possibility requires further research. A hit latency analysis (Supplemental Fig. S2) showed that infants' hits became faster from 6 to 9 months, indicating growing refinement of AF skill with age.
The results also reveal how mothers' spontaneous bids to direct infants' attention differ systematically from scripted laboratory cues. Mothers typically made at least one attention recruiting action before a bid, usually verbally addressing and often also touching the infant. Although rates of these actions did not reliably predict infants' AF, the fact that mothers almost always verbalized during, and often before, bids suggests that verbalizations characteristically play a role in infant AF at home. Though the lab protocol also included scripted attention-recruiting verbalizations (i.e., calling the infant's name just before the cue), mothers at home used varied utterance content (e.g., infant's name, imperatives; references to the target; vocal sound effects relevant to the target; etc.), touch, and extraneous actions (e.g., snapping or fluttering fingers; waving; leaning in to the infant; changing affect; etc.) - both to recruit infants' attention before a bid, and to direct their attention during the bid. This array of cues might have facilitated AF at home, particularly at 6–7 months when infants are just starting to follow gaze and points (Deák, 2014; Tang et al., 2022). In future studies we will attempt to relate specific communicative content – verbal content and nonverbal actions, and their temporal and sequential distribution to infants' attention shifts, including AF.
5.4. Interactions between setting and situational variables
There was evidence that maternal cueing tendencies (or strategies), such as controlling bid duration, modulated infant AF performance, suggesting that caregivers can scaffold infants’ attention in ways that go beyond scripted laboratory protocols (see Suarez-Rivera et al., 2019). In addition, the regression model indicated that in earlier months infants were more heavily influenced by maternal cueing strategies.
Infant AF rates in home and lab settings converged in later months, suggesting that AF skills become less susceptible to setting-based differences from 6 to 9 months. Moreover, individual infants' stability increased in later months - but only within the lab setting (Tang et al., 2022). Infants' AF in one setting never predicted AF in the other, suggesting that increasing cross-situational similarity was only at the group level, not at the individual level.
Another situational variable – target location – interacted with setting. Infants followed more to targets within their visual field in both settings, consistent with previous reports that infants follow cues to targets near their midline earlier, to peripheral targets later, and finally to targets behind them (Butterworth and Jarrett, 1991, Deák et al., 2000). The current results offer the first evidence (to our knowledge) that this trend generalizes across settings, even in highly familiar environments. This is consistent with the argument (Deák et al., 2000) that infants rarely follow cues to targets behind them partly because the cues are usually subtler and the responses require more effort (and therefore greater motivation).
5.5. Future direction and limitations
The results show situational influences on infant AF development from 6 to 9 months of age. The findings do not allow us to pinpoint specific factors that differentiate home- vs. lab-based AF, but rather provide a holistic picture of how two commonly-studied settings and paradigms - home and lab - characterized by distinct sets of contextual factors - can influence infant AF. They also reveal a structured and complex "choreography" in mothers' unscripted attempts to recruit and direct infants' attention. The results also suggest that infants' lab AF tendencies do not predict their AF in naturalistic settings. Of course, AF in just two settings, home and lab, does not exhaust the manifestations of this complex social pattern. AF and other triadic interactions in other settings (e.g., larger family groups, day care settings, playgrounds, etc.) might robustly predict individual differences (e.g., Morales et al., 2000; see also Mateus et al., 2013).
Another limitation is that the home settings were standardized and manipulated in some regards, for purposes of control, comparability, and fidelity of measurement. Thus, home sessions were not fully naturalistic. Further research is needed to determine whether, and how, our manipulations in infants' homes contributed to any deviations from natural, everyday AF. Additionally, the focused face-to-face toy-play interactions that were distilled in the home sessions do not represent how caregivers around the world spend most of their time interacting with infants (e.g., Suarez-Rivera et al., 2022). Thus, our home sessions can be considered a modified, semi-controlled representation of a narrow 'slice' of at-home interactions that is simulated by dedicated AF paradigms in labs, clinics, etc.
Our findings should not be interpreted to show that home settings yield generally "better" assessments than lab or clinical paradigms. Rather, the differences between settings can reveal contextual variables that affect infants' social attention and action. Also, the differences alert us to the risk of overgeneralizing from infant behavior in a single setting. The present findings also have the potential to improve tools for evaluating early social development, such as diagnostic assessments of ASD or other developmental syndromes (e.g., Perkovich et al., 2022).
This study has other limitations. First, this sample, like most infant social cognition development studies, consisted of predominantly "WEIRD" families (Keller, 2018). To claim generalizability, the results must be compared across diverse cultural groups, and across settings with different perceptual, social, and affect-modulating factors. Also, even the home paradigm was artificial in some regards: for example, the use of cameras, experimenters nearby, and novel toys might limit the generalizability of results to everyday settings. Finally, perhaps other maternal behaviors not yet coded, or finer details of the behaviors that were coded, might explain more variance in infants' AF. We therefore advocate additional studies of the micro-behavioral structure of AF episodes by infants and caregivers from diverse populations, across a wider range of settings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Note: The data are available at https://osf.io/4nqbc/. This research was supported by a grant from the National Science Foundation (SES-0527756 to G. Deák and J. Triesch) and from the University of California - San Diego's Academic Senate (to G. Deák). J. Triesch was supported by the Johanna Quandt foundation. The authors state no conflict of interests. The project's human research participants treatment protocol was approved by the university's institutional review board. We thank the students of the Cognitive Development Lab for assistance in data collection and coding, and we thank the families who participated in this research.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2023.101283.
Families also participated in sessions at 4 and 5 months, and in the lab at 10–12 months; data from those sessions will be reported elsewhere (e.g., Tang et al., 2022).
Note that the current results report unweighted following rates by infants in the lab, whereas results reported in Tang et al. (2022) focus on weighted following rates - thus the results are not identical.
Preliminary analyses indicated that a 10 Hz sampling rate was adequate. The sampling rate difference between the lab and home videos does not affect the current analyses or conclusions.
Contributor Information
Yueyan Tang, Email: yyt005@ucsd.edu.
Gedeon O. Deák, Email: gdeak@ucsd.edu.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Astor K., Gredebäck G. Gaze following in infancy: five big questions that the field should answer. Adv. Child Dev. Behav. 2022;63:191–223. doi: 10.1016/bs.acdb.2022.04.003. [DOI] [PubMed] [Google Scholar]
- Bakeman R., Adamson L.B. Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Dev. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Bard K.A., Keller H., Ross K.M., Hewlett B., Butler L., Boysen S.T., Matsuzawa T. Joint attention in human and chimpanzee infants in varied socio-ecological contexts. Monogr. Soc. Res. Child Dev. 2021;86:7–217. doi: 10.1111/mono.12435. [DOI] [PubMed] [Google Scholar]
- Baumrind D. American Psychological Association; San Francisco: 1968. Naturalistic Observation in the Study of Parent-child Interaction. [Google Scholar]
- Belsky J. Mother-infant interaction at home and in the laboratory: a comparative study. J. Genet. Psychol. 1980;137(1):37–47. doi: 10.1080/00221325.1980.10532800. [DOI] [PubMed] [Google Scholar]
- Bornstein M.H., Tamis-LeMonda C.S. Activities and interactions of mothers and their firstborn infants in the first six months of life: covariation, stability, continuity, correspondence, and prediction. Child Dev. 1990;61(4):1206. doi: 10.1111/j.1467-8624.1990.tb02854.x. [DOI] [PubMed] [Google Scholar]
- Boyer T.W., Harding S.M., Bertenthal B.I. The temporal dynamics of infants' joint attention: effects of others' gaze cues and manual actions. Cognition. 2020;197 doi: 10.1016/j.cognition.2019.104151. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. Toward an experimental ecology of human development. Am. Psychol. 1977;32:513–531. [Google Scholar]
- Brookhart J., Hock E. The effects of experimental context and experiential background on infants' behavior toward their mothers and a stranger. Child Dev. 1976;47(2):333–340. [Google Scholar]
- Butterworth G.E., Cochran E. Towards a mechanism of joint visual attention in human infancy. Int. J. Behav. Dev. 1980;3:253–272. [Google Scholar]
- Butterworth G.E., Jarrett N. What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. Br. J. Dev. Psychol. 1991;9:55–72. [Google Scholar]
- Cohen A.S., Sasaki J.Y., German T.C., Kim H.S. Automatic mechanisms for social attention are culturally penetrable. Cogn. Sci. 2017;41(1):242–258. doi: 10.1111/cogs.12329. [DOI] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Law J. Early social attention impairments in autism: social orienting, joint attention and attention to distress. Dev. Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- de Barbaro K., Chiba A., Deák G.O. Micro‐analysis of infant looking in a naturalistic social setting: insights from biologically based models of attention. Dev. Sci. 2011;14(5):1150–1160. doi: 10.1111/j.1467-7687.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- de Barbaro K., Johnson C.M., Forster D., Deák G.O. Sensorimotor decoupling contributes to triadic attention: a longitudinal investigation of mother-infant-object interactions. Child Dev. 2016;87(2):494–512. doi: 10.1111/cdev.12464. [DOI] [PubMed] [Google Scholar]
- Deák G.O. In: Benson J., editor. Vol. 46. 2014. Development of adaptive tool-use in early childhood: sensory-motor, social, and conceptual factors; pp. 149–181. (Advances in Child Development and Behavior). [DOI] [PubMed] [Google Scholar]
- Deák G.O., Flom R.A., Pick A.D. Effects of gesture and target on 12- and 18-month-olds’ joint visual attention to objects in front of or behind them. Dev. Psychol. 2000;36(4):511–523. org/10.1037/0012-1649.36.4.511. [PubMed] [Google Scholar]
- Deák G.O., Walden T.A., Kaiser M.Y., Lewis A. Driven from distraction: how infants respond to parents’ attempts to elicit and re-direct their attention. Infant Behav. Dev. 2008;31:34–50. doi: 10.1016/j.infbeh.2007.06.004. org/10.1016/j.infbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Deák G.O., Krasno A., Jasso H., Triesch J. What leads to shared attention? Action and gaze sequences in naturalistic infant-caregiver interactions. Infancy. 2017 doi: 10.1111/infa.12204. [DOI] [Google Scholar]
- Deák G.O., Krasno A.M., Triesch J., Lewis J., Sepeta L. Watch the hands: infants can learn to follow gaze by seeing adults manipulate objects. Dev. Sci. 2014;17(2):270–281. doi: 10.1111/desc.12122. [DOI] [PubMed] [Google Scholar]
- DeLoache J.S., Chiong C. Babies and baby media. Am. Behav. Sci. 2009;52(8):1115–1135. [Google Scholar]
- Demers L.B., Hanson K.G., Kirkorian H.L., Pempek T.A., Anderson D.R. Infant gaze following during parent-infant coviewing of baby videos. Child Dev. 2013;84(2):591–603. doi: 10.1111/j.1467-8624.2012.01868.x. [DOI] [PubMed] [Google Scholar]
- Fein G.G. Children's sensitivity to social contexts at 18 months of age. Dev. Psychol. 1975;11(6):853–854. [Google Scholar]
- Feinman S. Social referencing in infancy. Merrill-Palmer Q. 1982;28(4):445–470. [Google Scholar]
- Gredebäck G., Fikke L., Melinder A. The development of joint visual attention: a longitudinal study of gaze following during interactions with mothers and strangers. Dev. Sci. 2010;13:839–848. doi: 10.1111/j.1467-7687.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- Grzadzinski R., Amso D., Landa R., et al. Pre-symptomatic intervention for autism spectrum disorder (ASD): defining a research agenda. J. Neurodev. Disord. 2021;13:49. doi: 10.1186/s11689-021-09393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J., Heine S.J., Norenzayan A. The weirdest people in the world? Behav. Brain Sci. 2010;33(2–3):61–135. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Jean A.D., Stack D.M., Arnold S. Investigating maternal touch and infants' self‐regulatory behaviours during a modified face‐to‐face Still‐Face with Touch procedure. Infant Child Dev. 2014;23(6):557–574. [Google Scholar]
- Keller H. Lawrence Erlbaum Associates Publishers; 2007. Cultures of Infancy. [Google Scholar]
- Keller H. Universality claim of attachment theory: Children’s socioemotional development across cultures. Proc. Nat. Acad. Sci. 2018;115(45):11414–11419. doi: 10.1073/pnas.1720325115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy S., Montgomery J.S., Schubert J., Cleland J.F., Clark C. Mother‐infant interaction of teenage mothers and the effect of experience in the observational sessions on the development of their infants. Early Child Dev. Care. 1983;10(2–3):165–186. [Google Scholar]
- Leekam S.R., Hunnisett E., Moore C. Targets and cues: gaze-following in children with autism. J. Child Psychol. Psychiatry. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Lytton H. Observation studies of parent-child interaction: a methodological review. Child Dev. 1977;42:651–684. [Google Scholar]
- Mangold, P., 2022. INTERACT User Guide. Mangold International GmbH (Ed.).
- Mateus V., Martins C., Osório A., Martins E.C., Soares I. Joint attention at 10 months of age in infant–mother dyads: contrasting free toy-play with semi-structured toy-play. Infant Behav. Dev. 2013;36(1):176–179. doi: 10.1016/j.infbeh.2012.09.001. [DOI] [PubMed] [Google Scholar]
- McGuire William J. A contextualist theory of knowledge: its implications for innovation and reform in psychological research. Adv. Exp. Soc. Psychol. 1983;16:1–47. [Google Scholar]
- Meehl P.E. Theoretical risks and tabular asterisks: Sir Karl, Sir Ronald, and the slow progress of soft psychology. J. Consult. Clin. Psychol. 1978;46(4):806–834. [Google Scholar]
- Morales M., Mundy P., Delgado C.E., Yale M., Messinger D., Neal R., Schwartz H.K. Responding to joint attention across the 6-through 24-month age period and early language acquisition. J. Appl. Dev. Psychol. 2000;21(3):283–298. [Google Scholar]
- Morissette P., Ricard M., Decarie M.G. Joint visual attention and pointing in infancy: a longitudinal study of comprehension. Br. J. Dev. Psychol. 1995;13:163–175. [Google Scholar]
- Mundy P., Gomes A. Individual differences in joint attention skill development in the second year. Infant Behav. Dev. 1998;21(3):469–482. [Google Scholar]
- Mundy, P.; Delgado, C.; Block, J.; Venezia, M.; Hogan, A.; Seibert, J., 2003. A manual for the abridged early social communication scales (ESCS).
- Mundy P., Block J., Delgado C., Pomares Y., Van Hecke A.V., Parlade M.V. Individual differences and the development of joint attention in infancy. Child Dev. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry M.H. Infants’ responses to novelty in familiar and unfamiliar settings. Child Dev. 1972;43(1):233–237. [Google Scholar]
- Perkovich E., Sun L., Mire S., Laakman A., Sakhuja U., Yoshida H. What children with and without ASD See: similar visual experiences with different pathways through parental attentional strategies. J. Autism Dev. Lang. Impair. 2022;7 doi: 10.1177/23969415221137293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W.P., Rackstraw M.S.J. Variations in mothers’ answers to children’s questions, as a function of social class, verbal intelligence test scores and sex. Sociology. 1967;1(3):259–276. [Google Scholar]
- Ross G., Kagan J., Zelazo P., Kotelchuck M. Separation protest in infants in home and laboratory. Dev. Psychol. 1975;11(2):256–257. [Google Scholar]
- Saxon T.F., Frick J.E., Colombo J. A longitudinal study of maternal interactional styles and infant visual attention. Merrill-Palmer Q. 1997;(1982-):48–66. [Google Scholar]
- Skarin K.S. Cognitive and contextual determinants of stranger fear in six- and eleven-month-old infants. Child Dev. 1977;48(2):537–544. [PubMed] [Google Scholar]
- Sroufe L.A., Waters E., Matas L. In: The Origins of Fear. Lewis M., Rosenblum L., editors. Wiley; New York: 1974. Contextual determinants of infant affective response; pp. 49–52. [Google Scholar]
- Striano T., Bertin E. Coordinated affect with mothers and strangers: a longitudinal analysis of joint engagement between 5 and 9 months of age. Cogn. Emot. 2005;19:781–790. doi: 10.1080/02699930541000002. [DOI] [Google Scholar]
- Suarez-Rivera C., Smith L.B., Yu C. Multimodal parent behaviors within joint attention support sustained attention in infants. Dev. Psychol. 2019;55(1):96–109. doi: 10.1037/dev0000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Rivera C., Smith L.B., Yu C. Multimodal parent behaviors within joint attention support sustained attention in infants. Dev. Psychol. 2019;55:96–109. doi: 10.1037/dev0000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Rivera C., Schatz J., Herzberg O., Tamis-LeMonda C. Joint engagement in the home environment is frequent, multimodal, timely, and structured. Infancy. 2022;27:232–254. doi: 10.1111/infa.12446. [DOI] [PubMed] [Google Scholar]
- Sun L., Yoshida H. Why the parent's gaze is so powerful in organizing the infant's gaze: the relationship between parental referential cues and infant object looking. Infancy. 2022;27(4):780–808. doi: 10.1111/infa.12475. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda C.S., Kuchirko Y., Luo R., Escobar K., Bornstein M.H. Power in methods: language to infants in structured and naturalistic contexts. Dev. Sci. 2017;20 doi: 10.1111/desc.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y., Gonzalez, M. R., Deák, G.O., 2022. The Slow Emergence of Gaze- and Point-Following: A Longitudinal Study of Infants From 4 to 12 Months. [Manuscript submitted for publication]. [DOI] [PubMed]
- Teuscher C., Triesch J. To each his own: the caregiver's role in a computational model of gaze following. Neurocomputing. 2007;70(13–15):2166–2180. [Google Scholar]
- Tomasello M. The role of joint attentional processes in early language development. Lang. Sci. 1988;10:69–88. [Google Scholar]
- Tomasello M., Todd J. Joint attention and lexical acquisition style. First Lang. 1983;4(12):197–211. [Google Scholar]
- Tomasello M., Farrar M.J. Joint attention and early language. Child Dev. 1986;57(6):1454–1463. doi: 10.2307/1130423. [DOI] [PubMed] [Google Scholar]
- Walden T.A., Baxter A. The effect of context and age on social referencing. Child Dev. 1989;60:1511–1518. [PubMed] [Google Scholar]
- Weisz J. Transcontextual validity in developmental research. Child Dev. 1978;49:1–12. [Google Scholar]
- Yoshida H., Cirino P., Burling J.M., Sunbok L., Lee S. Parents’ gesture adaptations to children with autism spectrum disorder. J. Child Lang. 2020;47(1):205–224. doi: 10.1017/S0305000919000497. [DOI] [PubMed] [Google Scholar]
- Yu C., Smith L.B. Joint attention without gaze following: human infants and their parents coordinate visual attention to objects through eye-hand coordination. PLOS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.