Abstract
Background and aim
The temporomandibular joint (TMJ) is a synovial joint that allows the complex movements essential for life. It connects the jawbone to the skull, working as a sliding hinge. Moreover, pluripotent stem cells are a source of precursors and tissue-specific cells in developing organisms, however, their biodistribution in developing fetal tissues is weakly studied. The aim of our study was analyse immunohistochemical expression of Nanog, Oct-4, Sox-2 and Stat-3 and Sox-5, in TMJ tissue samples from human fetuses aged between the 12th and 20th weeks of intrauterine life.
Materials and methods
We fixed and processed TMJ tissue samples from human fetuses, histological sections and immunohistochemical procedures were carried out.
Results
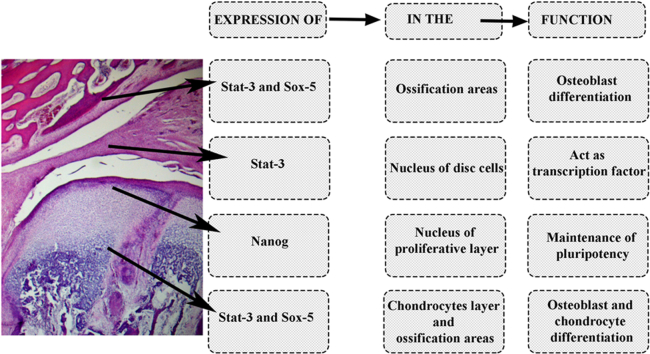
TMJ histological studies examination did not reveal any difference in the tissue organization between the samples in the studied periods. Immunohistochemical analysis demonstrated that Oct-4 and Sox-2 lack their expression in TMJ. In contrast, Nanog was expressed in nucleous of proliferative layer of mandibular condyle, Stat-3 was expressed in nuclear cells of articular disc, Stat-3 and Sox-5 showed positive nuclear and cytoplasmic immunostaining in codrocyte layers and in ossification areas.
Conclusions
Nanog acts in maintanence of pluripotency, Stat-3 in articular disc acts as a transcriptional factor. Stat-3 and Sox-2 act in chondrocyte and osteoblast diferentiation. Distribution of the cells, which express Nanog, Stat-3, and Sox-5 in TMJ tissue during fetal development, can help further understand its physiology, pathology, and repairing capacities.
Keywords: Temporomandibular joint, Transcription factor, Stem cells, Human fetus
Graphical abstract
1. Introduction
The temporomandibular joint (TMJ) is an interdependent bilateral synovial joint, allowing rotation and translation movements of the jaw around a fixed bone.1,2 In adulthood, TMJ comprises bone structures, mandibular condyle (MC), articular tubercle, and mandibular fossa (MF) of the temporal bone, covered by articular fibrocartilage. The articular disc (AD) regulates the discrepancy between the anatomic joint surfaces, absorbs shock, and promotes smooth movements of the TMJ.3 The fibrous capsule allows broad movements of the joint, and the synovial membrane produces a viscous lubricant liquid that nourishes fibrocartilage.4 The development of the human TMJ is a unique process and Mérida-Velasco et al. (1999)5 identified three phases in TMJ development. Our study focused on the twelfth to twentieth weeks of intrauterine life, which is the third phase identified as maturation, with the differentiation of cells present in the proliferation layer.
The TMJ has several developmental stimuli, including intrauterine and postnatal ones. Intrauterine stimuli include sucking, tongue expulsion, and facial movements,6 and postnatal ones also include dental occlusion, masticatory forces, facial morphology, and also pathological stimulus like vitamins, hormonal deficits, and infections or inflammatory diseases, all leading to TMJ cellular response and in some cases to temporomandibular disorders (TMD).7,8
The TMJ response in all developmental stages is associated with cell response. In early development, pluripotent stem cells transcriptional factors (TF) mainly rule this responsiveness. TMJ, like most craniofacial structures, is originated from the neural crest. Neural crest stem cells are known to migrate and subsequently participate in the morphogenesis of all craniofacial structures such as cartilage, bone, ligaments, cranial sutures, muscles, tendons, teeth, and periodontium.9
Pluripotent cells show a vast differentiation capacity in early development due to the expression of such TFs as, Sox-2, Nanog, Stat-3, and Oct-4.10, 11, 12 In addition, some TFs, like Sox-5, plays an important role in chondrogenesis.13,14 However, the expression of these TFs in TMJ during prenatal human development is unknown.1 Therefore, in this study, we investigate the expression of such TFs as Sox-2, Nanog, Stat-3, Oct-4 and Sox-5 in TMJ in human fetuses aged 12–20 weeks.
2. Methods
According to the Ethical Committee of São Paulo Federal University/São Paulo Hospital (#1906/09), fetuses were collected in the Pathology Department Archives of São Paulo Federal University. Samples of both genders, ranging from 12 to 20 weeks of development were used, and those presenting aberrations or genetic changes were excluded. Twenty fetal TMJ were dissected and fixed in neutral buffered formalin solution at 10%. The decalcification process was in formic acid at 10% for two days. After that, the tissue was processed and embedded in paraffin. Paraffin-embedded samples were cut by cross-section using the microtome Accu-Cut® SRMTM (Sakura Finetek, USA) into thin slices of 3 μm. StarFrost® adhesive microscope slides silane coating (Knittel Glass, Germany) were used to keep the section attached to the slide. The histological sections were stained with hematoxylin and eosin (H/E) and analyzed under a microscope before immunohistochemical staining.
2.1. Immunohistochemistry
Sections (3 μm thick) from specimens were deparaffinized and rehydrated. The slides were heated to 95 °C for 35 min in citrate buffer pH 6.0 for antigen retrieval and incubated in methanol and in 6% hydrogen peroxide solution (1:1) for 30 min to quench endogenous peroxidase activity. The sections were incubated with 1% BSA (Bovine Serum Albumin; Sigma Aldrich) for 45 min to block nonspecific reactions. The slides were incubated with primary antibodies: Oct-4 (C52G3, Cell Signaling Technology, Danvers, MA) 1:25, Sox-2 (D6D9 XP, Cell Signaling Technology, Danvers, MA) 1:50, Nanog (1E6C4, Cell Signaling Technology, Danvers, Massachusetts) 1:60, Stat-3 (79D7, Cell SignalingTechnology, Danvers, Massachusetts) 1:50 and Sox-5 (AV33323, Sigma Aldrich, St Louis, Missouri) 1:50, overnight at 4 °C. The Advanced HRP system (Dako, Glostrup, Denmark) detected antibodies. The specimens were counterstained with Harris's hematoxylin (Sigma Aldrich, St Louis, Missouri) dehydrated and mounted for observation. Nonimmune serum was used as a negative control, bell stage of dental formation was used as a positive control for Oct-4, Sox-5 and Nanog, lung carcinoma for Sox-2 and colon adenocarcinoma for Stat-3. Immunohistochemistry was not evaluated by software, staining was analyzed by three researchers specialized in morphology. The assessment was carried out independently.
3. Results
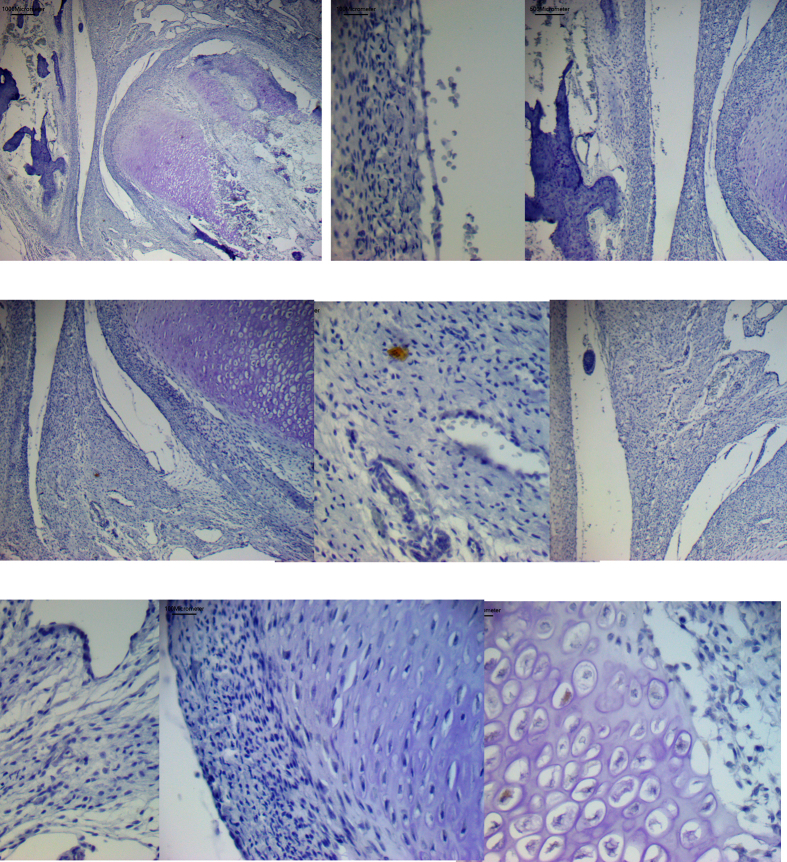
Histological analysis confirmed that between 12 and 20 weeks of human fetus gestational age, the TMJ (Fig. 1, Fig. 2, Fig. 3a) comprises the mandibular condyle (MC), mandibular fossa (MF) belonging to the squamous portion of the temporal bone (TB) and articular disc (AD). No changes were observed in TMJ structures between the fetuses of different gestation ages.5
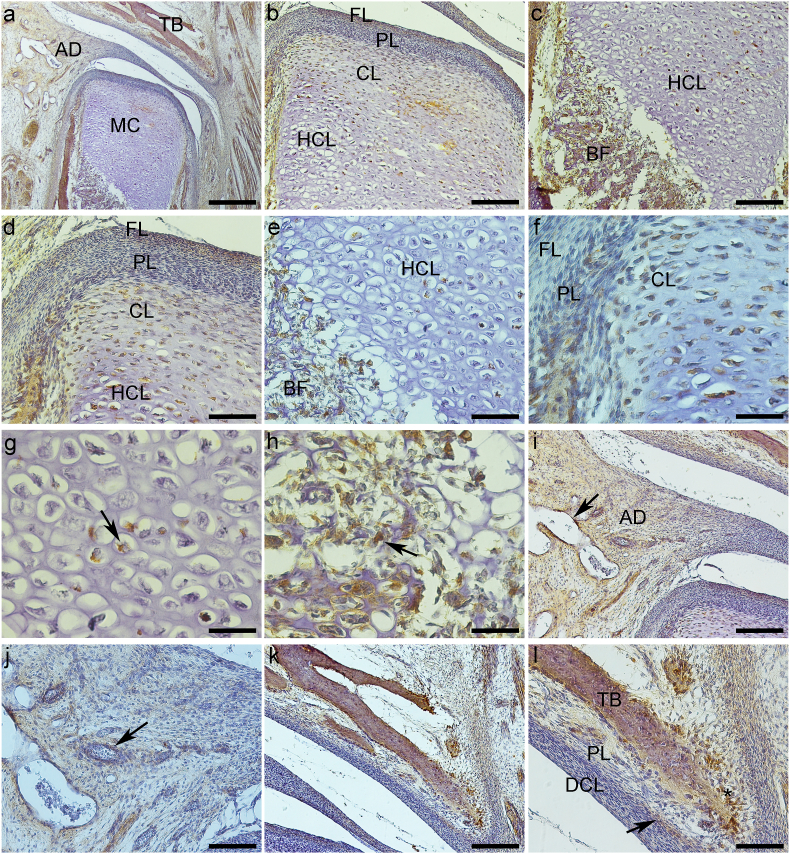
Fig. 1.
Expression of Nanog protein (a–l). a – General TMJ image with mandibular condyle (MC), articular disc (AD) and temporal bone (TB). b and d – Mandibular condyle with fibrous (FL), proliferative (PL) and chondrocytes layers (CL). d and f- Chondrocytes layer (CL) of mandibular condyle. e and g- Hypertrophic chondrocytes layer (HCL) of mandibular condyle with nuclear staining (arrow). c, e and h – Bone formation (BF) in the mandibular condyle (MC), with ostoblastic expression (arrow). i and j – Articular disc showing imunostaining surrounding vessels (arrow). k and l- Mandibular fossa of temporal bone (TB) with expression in osteoblasts (*), fibroblasts of dense connective layer (DCL) and in undifferentiated cells of proliferative layer (PL) (arrow). a-Bar = 500 μm b, c, i and k - Bar = 200 μm d, e, j and l-Bar = 100 μm. f, g and h- Bar = 50 μm.
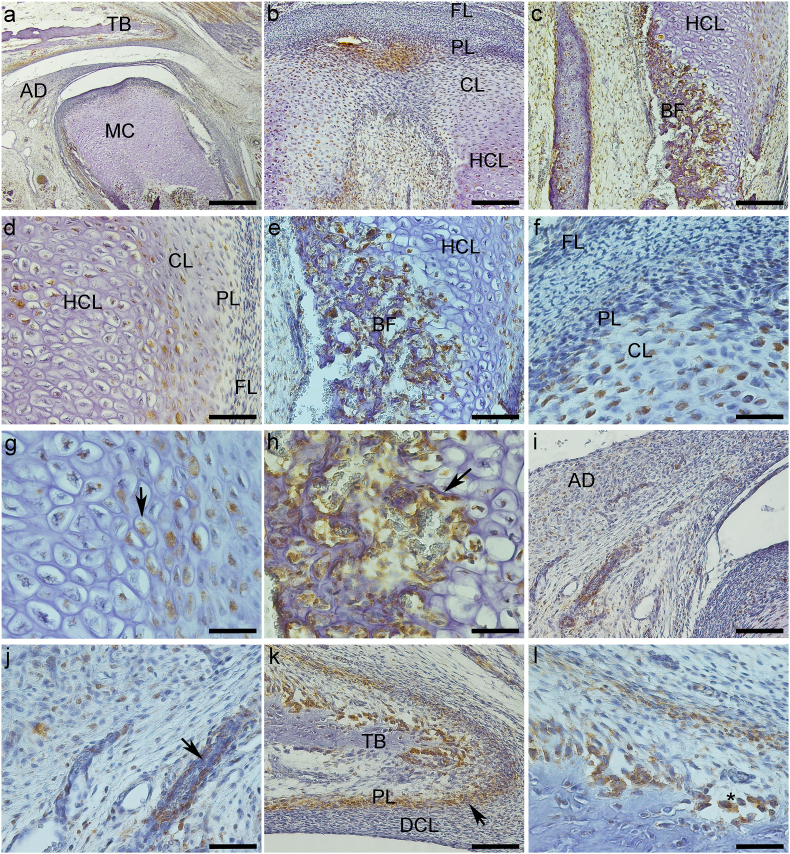
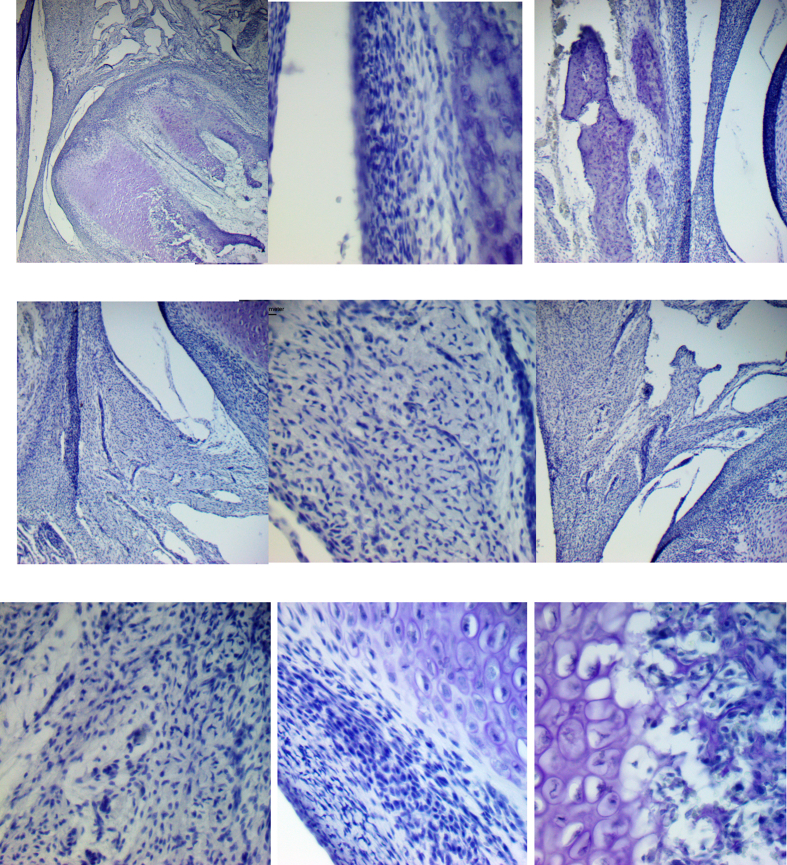
Fig. 2.
Expression of Stat-3 protein (a–l). a – General TMJ image with mandibular condyle (MC), articular disc (AD) and temporal bone (TB). b and d – Mandibular condyle with fibrous (FL), proliferative (PL) and chondrocytes layers (CL). d and f- Chondrocytes layer (CL) of mandibular condyle. e and g- Hypertrophic chondrocytes layer (HCL) of mandibular condyle with nuclear staining (arrow). c, e and h – Bone formation (BF) in the mandibular condyle (MC), with ostoblastic expression (arrow). i and j – Articular disc showing imunostaining surrounding vessels (arrow). k and l- Mandibular fossa of temporal bone (TB) with expression in osteoblasts (*), and in undifferentiated cells of proliferative layer (PL) (arrow). a-Bar = 500 μm. b and c - Bar = 200 μm d,e, i and k-Bar = 100 μm. f, g, h, j and l- Bar = 50 μm.
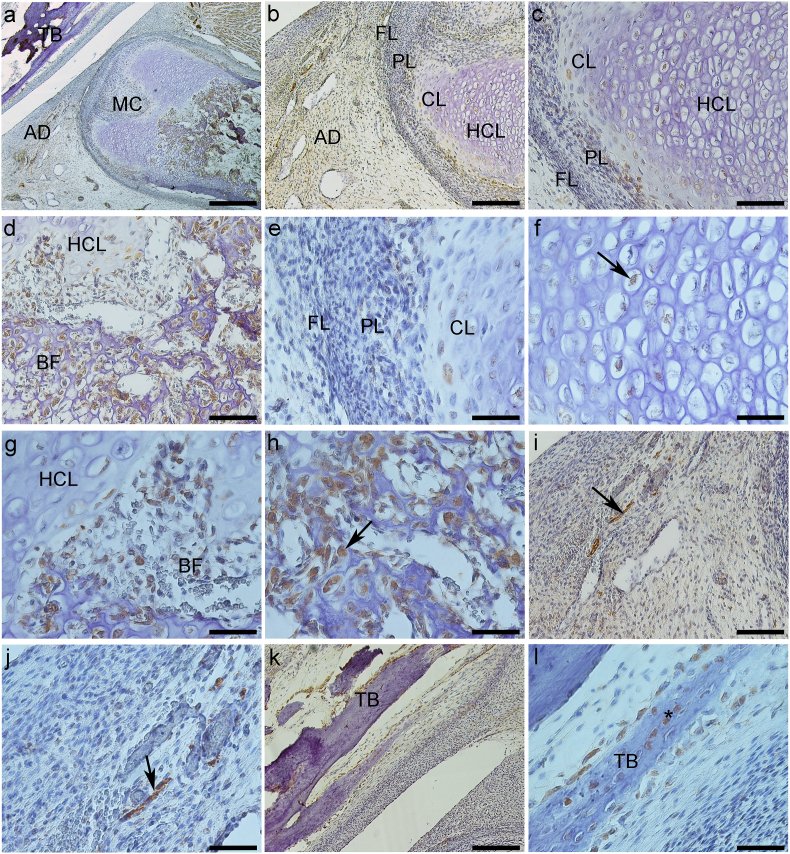
Fig. 3.
Expression of Sox-5 protein (a–l). a – General TMJ image with mandibular condyle (MC), articular disc (AD) and temporal bone (TB). b, c and e − Mandibular condyle with fibrous (FL), proliferative (PL) and chondrocytes layers (CL). c and e− Chondrocytes layer (CL) of mandibular condyle. f and g- Hypertrophic chondrocytes layer (HCL) of mandibular condyle with nuclear staining (arrow). d, g and h – Bone formation (BF) in the mandibular condyle (MC), with ostoblastic expression (arrow). i and j – Articular disc showing imunostaining surrounding vessels (arrow). k and l- Mandibular fossa of temporal bone (TB) with expression in osteocytes (*). a-Bar = 500 μm. b and k - Bar = 200 μm. c, d and i-Bar = 100 μm. e, f, g, h, j and l- Bar = 50 μm.
The articular disc was between the mandibular condyle and the mandibular fossa of the temporal bone and divided the articular cavity into supra-and infra-discal cavities. The disc proved to be thinner in the center and thicker at the periphery. Its upper portion was flat to slightly convex, and its bottom was concave. In TMJ sagittal sections, the articular disc was connected to the capsule, and two divisions were seen; its upper portion was directed to the temporal bone and the lower portion to the mandible condyle. The lateral pterygoid muscle was attached in the anterior region of these two portions. The articular disc is composed of dense fibrous connective tissue and fibrocartilage, similar to the tissue surrounding the condylar process of the mandible and the mandibular fossa. Its structure consists of bundles of collagen fibers, fibroblasts, fibrochondrocytes, and undifferentiated cells (Fig. 1, Fig. 2i). The mandibular condyle, in sagittal sections, is a convex structure, we can observe the fibrous layer (FL), consisting of dense connective tissue, proliferative layer (PL), which is composed of undifferentiated cells, chondrocytes layer (CL), hypertrophic chondrocytes layer (HCL) and formation of bone tissue (BT) (Fig. 1b–f; 2b-f; 3b-e, g). The mandibular fossa of the temporal bone (MF) presents its lower surface as slightly concave, a dense connective layer (DCL) which is very similar to the fibrous layer of the condyle, a fibrocartilage proliferative layer (PL) with undifferentiated cells, and temporal bone (TB) (Fig. 1, Fig. 2k).
3.1. Immunohistochemistry (Table 1)
Table 1.
Expression of Nanog, Stat 3 and Sox-5 in TMJ analyzed layers/areas.
| TMJ layers/areas |
Transcriptional Factors/Intracellular |
||
|---|---|---|---|
| Localization | |||
| NANOG | STAT-3 | SOX-5 | |
| Mandibular Condyle | |||
| Fibrous Layer | N+C (Strong) | C (Weak) | – |
| Proliferative Layer | N+C (Strong) | C (Weak) | C (Weak) |
| Chondrocyte Layer | N | N | N |
| Hypertrophic Chondrocyte Layer | N | N | N |
| Ossification Area (Osteoblast) | C | C | C |
| Articular Disc | |||
| Fibroblast/fibrocondrocytes | C | N | C (Weak) |
| Perivascular | C | N | C (Strong) |
| Mandibular Fossa | |||
| Dense Conective Layer | C | – | – |
| Proliferative Layer | C | C | – |
| Ossification Area (Osteoblast) | C | C | C |
(N-nuclear, C-cytoplasmic, N+C similar expression in nucleus and cytoplasm, - no immunostainning).
3.1.1. Oct-4 and Sox-2
We did not observe immunostaining for Oct-4 and Sox-2 in TMJ of human fetuses (See Suplementary Material-Fig. S1 e S2).
3.1.2. Nanog
More precisely, in the mandibular condyle in the FL (fibroblasts and fibrochondrocytes) and PL, there was intense cytoplasmic and nuclear staining (Fig. 1b, d, and f). CL (Fig. 1b, d, and f) and HCL presented predominantly nuclear staining (Fig. 1b, c, e, and g). The mandibular condyle ossification area showed intense immunostaining mainly in the cytoplasm of osteoblasts and in the extracellular matrix (Fig. 1c, e, and h).
In the articular disc, fibroblasts, fibrochondrocytes, undifferentiated cells, and cells surrounding blood vessels showed mainly cytoplasmic staining (Fig. 1i and j). Analyzing the mandibular fossa (temporal bone), Nanog presented essentially cytoplasmic staining in DCL fibroblasts and in undifferentiated cells of PL (Fig. 1k and l). Osteoblasts surrounding recently formed temporal bone expressed Nanog in the cytoplasm (Fig. 1l) (See Table I).
3.1.3. Stat-3
Weak immunostaining was observed in FL and PL fibroblasts and fibrochondrocytes (Fig. 2b, d, and f). On the other hand, CL (Fig. 2b, d, and f) and HCL (Fig. 2b, c, d, e, and g) expressed Stat-3 in the nucleus while the ossification area (Fig. 2c, e, and h) was positive in cytoplasm of osteoblasts and the extracellular matrix.
Cells of the articular disc presented Stat-3 essentially in the nucleus of fibroblasts, fibrochondrocytes, and cells surrounding blood vessels (Fig. 2i and j). Mandibular fossa showed Stat-3 immunoexpression mainly in undifferentiated cells cytoplasm of proliferative layer (Fig. 2k and l). Stat-3 was observed in osteoblasts’ cytoplasm surrounding the recently formed bone matrix (Fig. 2k and l) (See Table 1).
3.1.4. Sox-5
The mandibular condyle showed weak immunoreactivity in the cytoplasm of proliferative layer cells (Fig. 3b, c, and e). CL and HCL showed weak nuclear staining (Fig. 3c, e, and f). The ossification area presented intense immunostaining in the cytoplasm of osteoblasts, surrounding the recently formed bone matrix (Fig. 3d and h). It was possible to see positive Sox-5 immunostaining in the nucleus of cells near HCL (Fig. 3d and g).
Fibroblasts, fibrochondrocyte, and poorly differentiated cells of the articular disc showed weak staining in the cytoplasm (Fig. 3i and j). Cells in the perivascular region showed intense cytoplasmic immunoexpression (Fig. 3i and j). Sox-5 did not show immunostaining in DCL fibroblasts and PL of the mandibular fossa (Fig. 3k); on the other hand, osteoblasts surrounding recently formed temporal bone and osteocytes were positive for Sox-5 (Fig. 3l) (See Table 1).
4. Discussion
TMJ is a synovial joint that allows the complex movements necessary for life. Therefore, we focused our study on the morphological features of TMJ development in human fetuses aged between the 12th and 20th weeks of intrauterine life, which has not been studied previously. TMJ starts to develop at the 7th week, thus adopting its postnatal form around the 12th week of intrauterine life.5 We demonstrated that TMJ histological characteristics showed no differences in studied period of fetal development; for that reason, we analyzed the specimens as a single group. In addition to morphological analysis and taking into account the use of human fetuses, we also for the first time investigated the expression of the pattern of selective markers of pluripotency and those related to normal chondrogenesis, bone growth, and ossification. The limitation of our study was that we were not able to study the immunohistochemical expression of these transcription factors in the stages prior to the maturation stage that is, the blastemastic stage (weeks 7–8 of development) and the cavitation stage (week 9–11 of development), according to Mérida-Velasco et al.(1999).5 Another limitation refers to the fact that it was not possible to use other techniques, such as q-PCR, as the tissue studied was previously fixed in formalin and embedded in paraffin, so future studies using molecular techniques should be necessary.
Our immunohistochemical studies demonstrate that Sox-2 and Oct-4 were not expressed in this period of TMJ development. Sox-2 and Oct-4 express at high level in early embryo and in pluripotent stem cells and loss of expression of these proteins results in progressive loss of pluripotency and cell differentiation.15,16 The lack of staining for Oct-4 in areas of cell proliferation may be associated with the presence of osteochondrogenically-committed cells or their precursors and may confirm that it is not required for self renewal and differentiation ability of somatic cells.17 Sox-2 is expressed at the earliest nervous system developmental stages, and it functions as a marker protein for neural development and also regulates a variety of other tissue-specific stem/progenitor cells.18,19 Thus, the lack of expression of Sox-2 suggests that in TMJ this protein is possibly not involved in regulation of stem cells self-renewal potential.
We also showed that Nanog had a robust nuclear and cytoplasmic localization in the PL and FL of the mandibular condyle. In addition, its cytoplasmic localization was observed in articular disc cells and DCL and PL cells of MF. Our data suggest that Nanog showed differential expression in TMJ tissues, thus indicating its ambiguous role in pluripotency regulation events that occur during TMJ development.20,21 Active Nanog is typically localized in the cell nucleus of pluripotent stem cells.22 Recently, cytoplasmic, more precisely centrosome localization of Nanog, during the cell cycle was detected in various tumor and non-tumor cell types.23 Fan et al., 202124 recently described a fibrocartilage stem cell population in a fibrous superficial zone of the adult human mandibular condyle. The formation of these cells occurs during the late period of the embryonic stage,25 which corroborate with our data.
Stat-3 is an important TF mediating intracellular signaling in osteoblasts and osteoclasts.26 In humans, Stat-3 mutations reduce bone mass and increase bone resorption and trauma fractures, caused by rises in osteoclast number, thus suggesting it's important role in osteoclast recruitment and activity. The literature has shown that Stat-3 is required for osteoblast and chondrocyte differentiation.26 Stat-3 nuclear trafficking is pivotal to its function as a TF; to influence transcription Stat-3 should enter the nucleus.27 According to Stat-3 biological function, we observed its expression in ossification areas of MF and MC. In articular disc cells, Stat-3 presented nuclear localization in fibrochondrocytes suggesting its transcriptionally active state. While in PL and FL of the mandibular condyle, Stat-3 showed weak cytoplasmic staining.
Our results demonstrate Sox-5 nuclear positive immunostaining in CL and HCL and in the cells near HCL. As reported, Sox-5 belongs to the Sox family. Its normal function avoids defects in chondrogenesis, bone growth, and ossification.13,14,28, 29, 30
We may conclude that Sox-2 and Oct-4 aparently are not involved in signaling pathway that regulates self-renewal potential and undifferentiated state of cells in fetus TMJ. Nanog, Stat-3 and Sox-5 are transcriptionally active in the chondrocyte hypertrophic and chondrocyte layers of the mandibular condyle. Additionally, Nanog was active in the fibrous and proliferative layers of the mandibular condyle. Stat-3 was expressed in fibroblast/fibrocondrocytes and perivascular region of the articular disc (Table 1). All these markers are implicated with cells proliferation, thus Sox-5 regulates cell proliferation, apoptosis and migration in Kaposi's sarcoma cells.31 Nanog is a player in intranscriptional networks in embryonic stem cell pluripotency,32 and Stat-3 is a signal transducer that regulates multiple cell processes, including proliferation.33 Therefore, we may suppose that TMJ layers/areas positive for all three markers are areas of cell proliferation and growth. This conclusion follows the biological function of the condyle, which provides structural support to the overlying cartilage with a meager self-repair potential.
Ethical approval
Ethical Committee of Sao Paulo Federal University/Sao Paulo Hospital (#1906/09)
Declaration of competing interest
The authors declare they have no competing interests.
List of abreviations
- AD
Articular Disc
- BSA
Bovine Serum Albumine
- BT
Bone tissue
- CL
Condrocytes Layer
- DCL
Dense connective Layer
- FL
Fibrous Layer
- H/E
Hematoxilin and Eosin
- HCL
Hypertrofic Condrocytes Layer
- HRP
Horseradish Perixidase
- MC
Mandibular Condyle
- MF
Mandibular Fossa
- NCSC
eural Crest Stem Cells
- Oct
4 Octamer-binding - 4
- PL
Proliferative Layer
- Sox-2
SRY (sex determining region Y)-box 2
- Sox-9
SRY (sex determining region Y)-box 2
- Stat-3
signal transducers and activators of transcription)
- TB
Temporal BoneTF-Transcriptional Factors
- TMD
Temporomandibular Disorders
- TMJ
Temporomandibular Joint
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jobcr.2023.08.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Immunostaining for Oct-4 was not observed in TMJ tissue.
Fig. S2.
Immunostaining for Sox-2 was not observed in TMJ tissue.
References
- 1.Shibukawa Y., Young B., Wu C., et al. Temporomandibular joint formation and condyl growth require Indian hedgehog signaling. Dev Dynam. 2007;236:426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 2.Terhune C.E., Cooke S.B., Otárola-Castillo E. Form and function in the platyrrhine skull: a three-dimensional analysis of dental and TMJ morphology. Anat Rec. 2015;298:29–47. doi: 10.1002/ar.23062. [DOI] [PubMed] [Google Scholar]
- 3.de Moraes L.O.C., Lodi F.R., Gomes T.S., et al. Immunohistochemical expression of types I and III collagen antibodies in the temporomandibular joint disc of human foetuses. Eur J Histochem. 2011;55 doi: 10.4081/ejh.2011.e24. E-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Farias J.F.G., Melo S.L.S., Bento P.M., Oliveira L.S.A.F., Campos P.S.F., de Melo D.P. Correlation between temporomandibular joint morphology and disc displacement by MRI. Dentomaxillofacial Radiol. 2015;2015(44) doi: 10.1259/dmfr.20150023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mérida-Velasco J.R., Rodríguez-Vázquez J.F., Mérida-Velasco J.A., Sánchez-Montesinos I., Espín-Ferra J., Jiménez-Collado J. Development of the human temporomandibular joint. Anat Rec. 1999;255:20–33. doi: 10.1002/(SICI)1097-0185(19990501)255:1<20::AID-AR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Kurjak A., Stanojevic M., Andonotopo W., Scazzocchio-Duenas E., Azumendi G., Carrera J.M. Fetal behavior assessed in all three trimesters of normal pregnancy by four-dimensional ultrasonography. Croat Med J. 2005;46:772–780. [PubMed] [Google Scholar]
- 7.Shen M., Luo Y., Niu Y., et al. 1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokines. Bone. 2013;55:400–409. doi: 10.1016/j.bone.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Vilanova L.S., Gonçalves T.M., Meirelles L., Garcia R.C. Hormonal fluctuations intensify temporomandibular disorder pain without impairing masticatory function. Int J Prosthodont (IJP) 2015;28:72–74. doi: 10.11607/ijp.4040. [DOI] [PubMed] [Google Scholar]
- 9.Mao J.J., Giannobile W.V., Helms J.A., et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;2006(85):966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferro F., Spelat R., D'Aurizio F., et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PLoS One. 2012;2012(7) doi: 10.1371/journal.pone.0041774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh Y.H., Ng J.H., Ng H.H. Molecular framework underlying pluripotency. Cell Cycle. 2008;7:885–891. doi: 10.4161/cc.7.7.5636. [DOI] [PubMed] [Google Scholar]
- 12.Rui Y., Xu L., Chen R., et al. Epigenetic memory gained by priming with osteogenic induction medium improves osteogenesis and other properties of mesenchymal stem cells. Sci Rep. 2015;5 doi: 10.1038/srep11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefebvre V. The Sox D transcription factors--Sox5, Sox6, and Sox13--are key cell fatemodulators. Int J Biochem Cell Biol. 2010;42:429–432. doi: 10.1016/j.biocel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.M., Im G.I. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–2024. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Liu L., Wei X., Huang R., Ling J., Wu L., Xiao Y. Effect of bone morphogenetic protein-4 on the expression of Sox2, Oct-4, and c-Myc in human periodontal ligament cells during long-term culture. Stem Cell Dev. 2013;2013(22):1670–1677. doi: 10.1089/scd.2012.0548. [DOI] [PubMed] [Google Scholar]
- 16.Bhartiya D. Intricacies of pluripotency. J Stem Cells Regen Med. 2015;11:2–6. doi: 10.46582/jsrm.1101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierantozzi E., Gava B., Manini I., et al. Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cell Dev. 2011;20:915–923. doi: 10.1089/scd.2010.0353. [DOI] [PubMed] [Google Scholar]
- 18.Pevny L.H., Nicolis S.K. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Thiel G. How Sox2 maintains neural stem cell identity. Biochem J. 2013;450:e1–e2. doi: 10.1042/BJ20130176. [DOI] [PubMed] [Google Scholar]
- 20.Huang C.E., Hu F.W., Yu C.H., et al. Concurrent expression of Oct4 and Nanog maintains mesenchymal stem-like property of human dental pulp cells. Int J Mol Sci. 2014;15:18623–18639. doi: 10.3390/ijms151018623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T.M., Wu Y.N., Guo X.M., Hui J.H.P., Lee E.H., Lim B. Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cell Dev. 2009;18:1013–1022. doi: 10.1089/scd.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do H.-J., Lim H.-Y., Kim J.-H., Song H., Chung H.-M., Kim J.-H. An intact homeobox domain is required for complete nuclear localization of human Nanog. BBRC (Biochem Biophys Res Commun) 2007;353(3):770–775. doi: 10.1016/j.bbrc.2006.12.100. [DOI] [PubMed] [Google Scholar]
- 23.Mikulenkova E., Neradil J., Vymazal O., Skoda J., Veselska R. NANOG/NANOGP8 localizes at the centrosome and is spatiotemporally associated with centriole maturation. Cells. 2020;9:692. doi: 10.3390/cells9030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Y., Cui C., Li P.B.R., Lyu P., Li Y., Zhu S. Fibrocartilage stem cells in the temporomandibular joint: insights from animal and human studies. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.665995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruscitto A., Scarpa V., Morel M., Pylawka S., Shawber C.J., Embree M.C. Notch regulates fibrocartilage stem cell fate and is upregulated in inflammatory TMJ arthritis. J Dent Res. 2020;99:1174–1181. doi: 10.1177/0022034520924656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J. JAK-STAT and bone metabolism. JAK-STAT. 2013;2 doi: 10.4161/jkst.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cimica V., Chen H.-C., Iyer J.K., Reich N.C. Dynamics of the STAT3 transcription factor: nuclear import dependent on ran and importin-β1. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefebvre V., Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Research Part C. Embryo Today: Review. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre V. Roles and regulation of SOX transcription factors in skeletogenesis. Curr Top Dev Biol. 2019;133:171–193. doi: 10.1016/bs.ctdb.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuliani N., Lisignoli G., Magnani M., et al. New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cell Int. 2013;2013 doi: 10.1155/2013/312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan W.M., Fan Y.G., Cui M., et al. SOX5 regulates cell proliferation, apoptosis, migration and invasion in KSHV-infected cells. Virol Sin. 2021;36:449–457. doi: 10.1007/s12250-020-00313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan G., Thomson J. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y., Wang D., Xu J., et al. Stat3 activation is critical for pluripotency maintenance. J Cell Physiol. 2019;234:1044–1051. doi: 10.1002/jcp.27241. [DOI] [PubMed] [Google Scholar]