Abstract
BACKGROUND:
The incidence of rectal cancer in patients younger than 50 years is increasing. To test the hypothesis that the biology in this younger cohort may differ, this study compared survival patterns, stratifying patients according to National Comprehensive Cancer Network (NCCN) guideline–driven care and age.
METHODS:
The National Cancer Data Base was queried for patients treated with curative-intent transabdominal resections with negative surgical margins for stage I to III rectal cancer between 2004 and 2014. Outcomes and overall survival for patients younger than 50 years and patients 50 years old or older were compared by subgroups based on NCCN guideline–driven care.
RESULTS:
A total of 43,106 patients were analyzed. Younger patients were more likely to be female and minorities, to be diagnosed at a higher stage, and to have travelled further to be treated at academic/integrated centers. Short- and long-term outcomes were significantly better for patients younger than 50 years, with age-specific survival rates calculated. Younger patients were more likely to receive radiation treatment outside NCCN guidelines for stage I disease. In younger patients, the administration of neoadjuvant chemoradiation for stage II and III disease was not associated with an overall survival benefit.
CONCLUSIONS:
Age-specific survival data for patients with rectal cancer treated with curative intent do not support an overall survival benefit from NCCN guideline–driven therapy for stage II and III patients younger than 50 years. These data suggest that early-onset disease may differ biologically and in its response to multimodality therapy.
Keywords: National Cancer Data Base (NCDB), rectal cancer, survival, treatment appropriateness
INTRODUCTION
Colorectal cancer is the third most common cancer and second leading cause of cancer death in the United States. Although the overall incidence of rectal cancer is decreasing in patients older than 50 years,1 likely because of improved screening adherence,2 there has been a disproportionate increase in rectal cancer incidence in patients younger than 50 years.3,4 Furthermore, overall mortality for colorectal cancer is improving, but not for these younger patients; their mortality rate from rectal cancer over time has increased from 1970 to 2014.5,6 On the basis of single-institution studies, this is thought to primarily be a result of delays in diagnosis in the younger cohort.7 The notion that these early-onset cases represent differences in epidemiology and perhaps responses to therapy is emerging.
National Comprehensive Cancer Network (NCCN) guidelines define the current standard of care for the intended cure of rectal cancer as surgical resection alone for stage I disease8 and as neoadjuvant chemoradiation therapy with subsequent surgical resection and systemic chemotherapy for stage II and III disease.8,9 However, these recommendations and current survival estimates are predominantly influenced by trials or databases with patients older than 50 years, with younger patients not well studied. To address this gap in our knowledge and to test the hypothesis that the biology in patients younger than 50 years may differ, we aimed to analyze survival patterns in the younger cohort. This work queried a large national database for patients with rectal cancer who underwent curative-intent treatment to compare outcomes in cohorts defined by adherence to NCCN guidelines and age.
MATERIALS AND METHODS
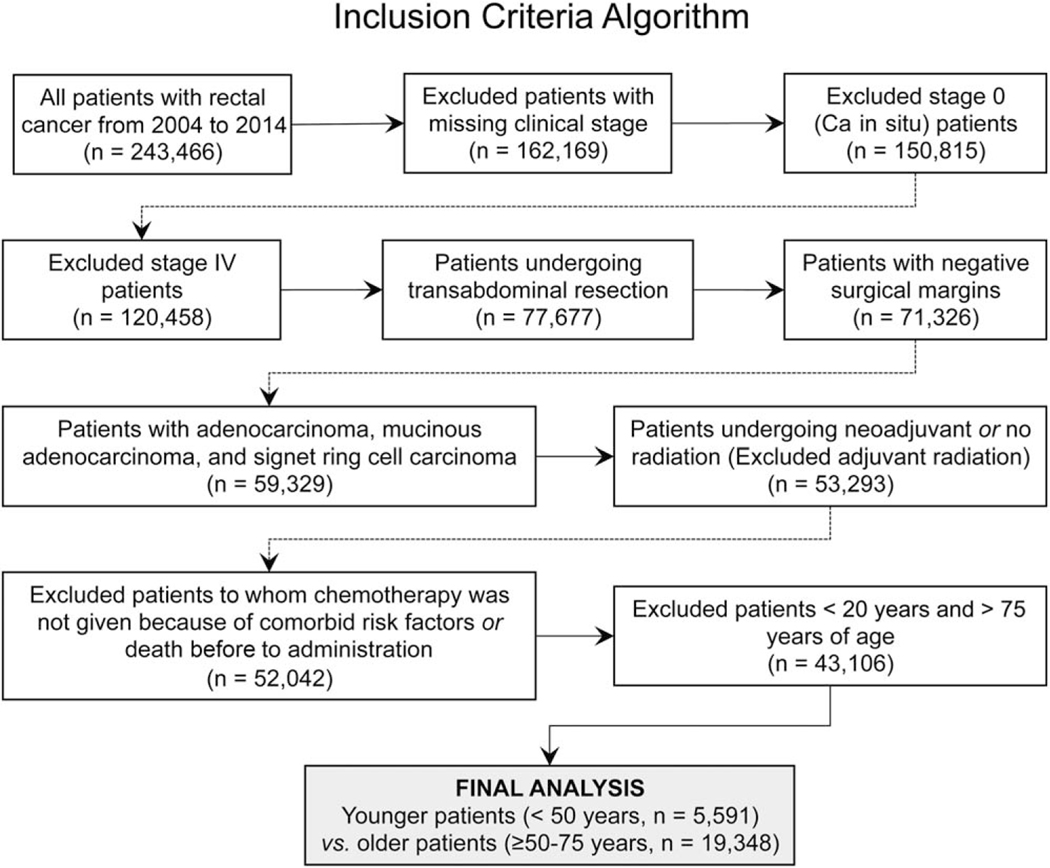
A retrospective review of the National Cancer Data Base (NCDB) was performed after approval by the institutional review committee. Briefly, the NCDB is a hospital-based, national registry of de-identified cancer patients and a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The participant user file analyzed in this study included all patients diagnosed with rectal cancer from 2004 through 2014. Inclusion criteria consisted of histologically proven rectal adenocarcinoma in patients undergoing curative-intent surgical resection for stage I to III disease. Patients with carcinoma in situ and metastatic disease were excluded. Patients undergoing local excision and those with positive surgical margins were also excluded to control for the type and quality of surgical resection because no variable for the quality of total mesorectal excision was available in the NCDB. Patients receiving adjuvant radiation were excluded because this was uncommon, patients were equally distributed across age groups, and reasons for adjuvant radiation were undocumented. Patients at the extremes of age (<20 and >75 years) and those who did not receive chemotherapy because of comorbidities or death were excluded to control for age-based survival differences. Finally, patients with pertinent, missing variables were excluded. A detailed inclusion diagram is shown in Figure 1. Patients were cohorted by adherence to NCCN-based guidelines (as defined previously) for treatment and by age (< or ≥50 years).
Figure 1.
Inclusion criteria algorithm. Ca indicates carcinoma.
The statistical analysis was conducted with IBM SPSS Statistics (version 23). Variables were analyzed with the exact chi-square test and the independent sample t test for categorical and continuous variables, respectively. Survival curves were estimated with Kaplan-Meier methodology, and differences between groups were assigned with logrank, Breslow, and Tarone-Ware tests. Time-specific mortality rates for 3-, 5-, 7-, and 10-year mortality were calculated with life tables. Relative survival was calculated with expected survival values obtained from a time- and age-matched cohort in the Surveillance, Epidemiology, and End Results database.10 Survival curves were re-estimated for the younger (<50 years) and older cohorts (≥50 years) and were substratified by patients who did or did not receive NCCN guideline–driven care. Variables that were significant in a univariate analysis for overall survival were included in a multivariate Cox proportional hazards regression model. The univariate and multivariate analyses were repeated for the age-based cohorts. A P value <.05 was considered statistically significant. All analysis involving neoadjuvant therapy groups was repeated for clinical and pathologic stages to confirm the absence of a statistical difference in the results. Clinical and pathologic TNM staging was performed according to American Joint Committee on Cancer guidelines, with 54% and 46% of the patients classified according to the sixth and seventh editions, respectively.8
RESULTS
Patient Population Overall
Of the 243,666 patients with rectal cancer over the 10-year period in the NCDB, 43,106 patients were included in the final analysis. Only 9126 patients (21%) were younger than 50 years. Baseline demographic and clinical data stratified by age are presented in Table 1. The younger cohort had a significantly higher proportion of females and minorities (African Americans, Hispanics, and Asians), was more likely to be uninsured, and had fewer comorbid conditions. The younger patients were more likely to reside in a metropolitan area, earn a higher income, travel further for medical care, and be treated at integrated and/or academic cancer centers. Oncologically, the younger patients were significantly more likely to be diagnosed at a higher pathologic stage (stage III, 40% vs 31%; P<.001) and were more likely to have poorly differentiated lesions and mucinous or signet ring cell lesions. No significant difference was noted in the clinicodemographic information within the younger population, even when deciles were being compared.
TABLE 1.
Age-Based Comparison of Clinicodemographic Data for Patients With Rectal Cancer
| Characteristic | <50 y Old | 50–75 y Old | |||
|---|---|---|---|---|---|
|
| |||||
| No. | % of Total | No. | % of Total | P | |
|
| |||||
| Sex | |||||
| Male | 5335 | 58.5 | 21,711 | 63.9 | <.001a |
| Female | 3791 | 41.5 | 12,269 | 36.1 | |
| Race | |||||
| White | 7626 | 85.5 | 29,429 | 87.9 | <.001b |
| African American | 838 | 9.4 | 2629 | 7.9 | |
| Native American | 43 | 0.5 | 164 | 0.5 | |
| Asian/Pacific Islander | 415 | 4.6 | 1245 | 3.7 | |
| Spanish origin | |||||
| Non-Hispanic | 7984 | 92.2 | 30,309 | 94.3 | <.001b |
| Hispanic | 677 | 7.8 | 1831 | 5.7 | |
| Treatment site | |||||
| Community center | 544 | 7.8 | 3200 | 9.4 | <.001c |
| Comprehensive community center | 2757 | 39.6 | 14,843 | 43.7 | |
| Academic center | 2900 | 41.7 | 12,394 | 36.5 | |
| Integrated network | 760 | 10.9 | 3543 | 10.4 | |
| Insurance status | |||||
| Uninsured | 590 | 6.6 | 1469 | 4.4 | <.001d |
| Private/managed care | 7022 | 78.2 | 17,555 | 52.4 | |
| Medicaid | 962 | 10.7 | 1991 | 5.9 | |
| Medicare | 283 | 3.2 | 12,080 | 36.0 | |
| Other government | 120 | 1.3 | 435 | 1.3 | |
| Income level | |||||
| <$38,000/y | 1385 | 15.3 | 5932 | 17.6 | <.001e |
| $38,000–47,999/y | 2037 | 22.5 | 8240 | 24.5 | |
| $48,000–62,999/y | 2426 | 26.8 | 9197 | 27.3 | |
| ≥$63,000/y | 3202 | 35.4 | 10,299 | 30.6 | |
| Population density | |||||
| Urban | 1294 | 14.6 | 5850 | 17.7 | <.001f |
| Metro | 7403 | 83.6 | 26,387 | 79.8 | |
| Rural | 160 | 1.8 | 838 | 25.8 | |
| Charlson comorbidity score | |||||
| 0 | 8202 | 89.9 | 26,330 | 77.5 | <.001g |
| 1 | 796 | 8.7 | 6147 | 18.1 | |
| ≥2 | 128 | 1.4 | 1503 | 4.4 | |
| Stage at diagnosis | |||||
| I | 2194 | 33.2 | 9586 | 39.1 | <.001h |
| II | 1756 | 26.6 | 7296 | 29.7 | |
| III | 2663 | 40.3 | 7656 | 31.2 | |
| Tumor differentiation | |||||
| Well differentiated | 657 | 8.3 | 2583 | 8.7 | <.001i |
| Moderately differentiated | 6178 | 77.7 | 23,391 | 79.0 | |
| Poorly differentiated | 1034 | 13.0 | 3362 | 11.3 | |
| Undifferentiated, anaplastic | 83 | 1.0 | 290 | 1.0 | |
| Histology | |||||
| Adenocarcinoma | 8521 | 93.4 | 32,130 | 94.6 | <.001j |
| Mucinous | 500 | 5.5 | 1677 | 4.9 | |
| Signet ring cell | 105 | 1.2 | 173 | 0.5 | |
| Interval between diagnosis and start of treatment, mean ± SD, d | 118.9 ± 0.6 | — | 117.3 ± 0.3 | — | <.001 |
| Interval between diagnosis and definitive surgery, mean ± SD, d | 120.6 ± 0.3 | — | 122.1 ± 0.6 | — | <.001 |
The P value indicates that the younger cohort had a significantly higher proportion of females.
The P value indicates that the younger cohort had a significantly higher proportion of minorities.
The P value indicates that the younger cohort had a significantly higher proportion of patients treated at academic/integrated centers.
The P value indicates that the younger cohort had a significantly higher proportion of uninsured patients.
The P value indicates that the younger cohort had a significantly higher proportion of higher income earners.
The P value indicates that the younger cohort had a significantly higher proportion of patients with a metropolitan residence.
The P value indicates that the younger cohort had a significantly lower comorbidity index.
The P value indicates that the younger cohort had a significantly higher proportion of patients with a greater stage at diagnosis.
The P value indicates that the younger cohort had a significantly higher proportion of patients with undifferentiated or poorly differentiated lesions.
The P value indicates that the younger cohort had a significantly higher proportion of patients with mucinous or signet ring cell lesions.
Patient Population by Age
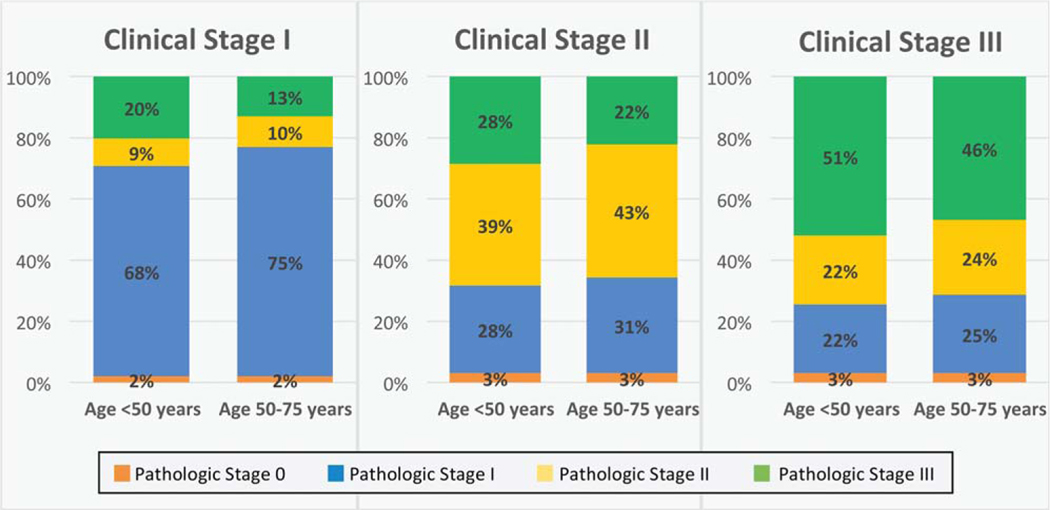
We sought to evaluate differences in the accuracy of clinical staging (stage I) and the degree of downstaging noted with neoadjuvant therapy (stages II and III) across age cohorts. The concordance of clinical and pathologic stages stratified by age revealed no difference across age groups, and this suggested equal accuracy of clinical staging (Fig. 2). For internal validity, a high concordance (95%) was noted in patients with clinical and pathologic stage I disease who did not receive radiation or chemotherapy. In terms of treatment, younger patients were more likely to receive radiation therapy for all stages. This was applicable to stage I disease (radiation therapy, 41.9% for young patients vs 31.7% for old patients; P<.001) and stage II and III disease (chemoradiation therapy, 93.6% vs 88.1%; P<.001). Thus, fewer patients in the younger cohort received NCCN guideline–directed therapy for stage I disease, but a greater percentage received it for stage II and III disease.
Figure 2.
Concordance of clinical and pathologic stages stratified by age and stage. No difference was noted across age cohorts; this signified equivalent accuracy of clinical staging across groups.
Postoperative Outcomes by Age
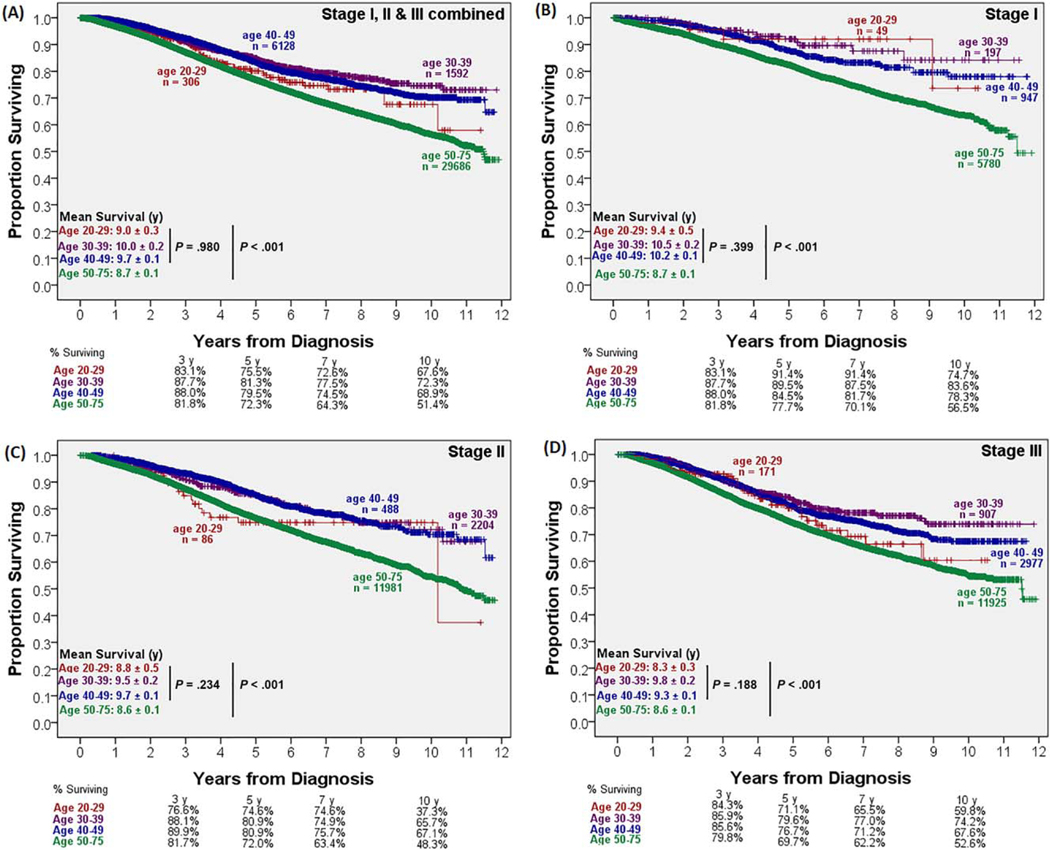
Younger patients experienced shorter hospital stays (6.7 vs 8.0 days; P<.001), but 30-day readmission rates were similar between groups. The younger cohort had better short-term mortality and long-term survival rates than their older counterparts. Both the 30-day mortality rate (2.0% vs 0.2%; P<.001) and the 90-day mortality rate (3.7% vs 0.5%; P<.001) were higher in the older cohort. This difference became more pronounced with 3-, 5-, and 10-year survival rates, as shown in Figure 3A, and it persisted independently for stage I, II, and III disease (Fig. 3B–D). The Kaplan-Meier analysis revealed a significant survival advantage for younger patients at 12 years (9.8 vs 8.7 years; P<.001). No significant survival difference was noted within the younger population by deciles (Fig. 3).
Figure 3.
Overall survival by age.
A univariate and multivariate analysis of overall survival for the entire group was performed to investigate the impact of individual factors on patients’ survival, and this demonstrated relations with known clinicopathologic factors. The analysis revealed a hazard ratio of 2.2 for patients who were 50 years of age or older (confidence interval, 2.0–2.2; P< 0.01). Factors associated with poorer overall survival were male sex, African American race, a higher comorbidity index, nonprivate insurance, a lower income level, treatment at community hospitals (vs academic centers or integrated networks), a higher pathologic stage, a poorly differentiated/undifferentiated tumor, and a mucinous or signet ring cell histology.
To determine the contributory role of age to overall survival in the context of other demographic and pathologic factors, a Cox regression multivariate analysis was performed for both age cohorts (Table 2). The aforementioned factors associated with survival for the study population maintained significance across age cohorts, except for the following differences: For patients younger than 50 years, the survival benefits of being Asian and Hispanic and being treated at academic/integrated networks and the survival detriments of African American race, being uninsured, and an increasing numeric age disappeared.
TABLE 2.
Age-Based Multivariate Analysis of Overall Mortality for Patients With Rectal Cancer
| Characteristic | <50 y Old | 50–75 y Old | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
|
| ||||||
| Age (y) | 1.01 | 0.98–1.04 | NS | 1.03 | 1.02–1.04 | <.001 |
| Sex: female (ref male) | 0.70 | 0.59–0.84 | <.001 | 0.80 | 0.75–0.87 | <.001 |
| Race (ref white) | ||||||
| African American | 1.11 | 0.83–1.48 | NS | 1.15 | 1.02–1.30 | .028 |
| Native American | 1.53 | 0.61–3.83 | NS | 0.83 | 0.47–1.48 | NS |
| Asian/Pacific Islander | 0.86 | 0.55–1.35 | NS | 0.87 | 0.71–1.06 | NS |
| Hispanic (ref non-Hispanic) | 1.05 | 0.74–1.48 | NS | 0.86 | 0.72–1.01 | NS |
| Treatment site (ref community center) | ||||||
| Comprehensive community center | 1.05 | 0.75–1.45 | NS | 0.93 | 0.83–1.04 | NS |
| Academic center | 0.89 | 0.64–1.23 | NS | 0.81 | 0.72–0.92 | .001 |
| Integrated network | 1.00 | 0.67–1.47 | NS | 0.81 | 0.70–0.95 | .007 |
| Insurance status (ref private insurance) | ||||||
| Uninsured | 0.89 | 0.69–1.37 | NS | 1.47 | 1.25–1.74 | <.001 |
| Medicaid | 1.60 | 1.24–2.07 | <.001 | 1.44 | 1.23–1.67 | <.001 |
| Medicare | 1.56 | 1.05–2.31 | .026 | 1.23 | 1.11–1.36 | <.001 |
| Other government | 1.06 | 0.43–2.56 | NS | 1.82 | 1.38–2.40 | <.001 |
| Income level (ref < $38,000/y) | ||||||
| $38,000–47,999/y | 0.97 | 0.74–1.27 | NS | 0.913 | 0.82–1.01 | NS |
| $48,000–62,999/y | 0.94 | 0.72–1.23 | NS | 0.835 | 0.75–0.93 | .001 |
| ≥$63,000 | 0.66 | 0.49–0.87 | .004 | 0.81 | 0.73–0.91 | <.001 |
| Population density (ref urban) | ||||||
| Metro | 0.99 | 0.78–1.26 | NS | 1.01 | 0.92–1.12 | NS |
| Rural | 0.67 | 0.35–1.27 | NS | 1.10 | 0.88–1.40 | NS |
| Charlson comorbidity score (ref 0) | ||||||
| 1 | 1.10 | 0.82–1.47 | NS | 1.34 | 1.23–1.46 | <.001 |
| ≥2 | 1.80 | 1.04–3.10 | .008 | 2.11 | 1.84–2.41 | <.001 |
| Clinical stage at diagnosis: III (ref II) | 1.02 | 0.84–1.23 | NS | 0.98 | 0.91–1.05 | NS |
| Pathologic stage at diagnosis (ref I) | ||||||
| II | 1.92 | 1.40–2.65 | <.001 | 1.5 | 1.35–1.66 | <.001 |
| III | 3.78 | 2.81–5.07 | <.001 | 2.39 | 2.16–2.64 | <.001 |
| Tumor differentiation (ref well differentiated) | ||||||
| Moderately differentiated | 1.05 | 0.74–1.49 | NS | 0.944 | 0.83–1.08 | NS |
| Poorly differentiated | 1.55 | 1.04–2.30 | <.001 | 1.37 | 1.17–1.59 | <.001 |
| Undifferentiated, anaplastic | 1.52 | 0.70–3.30 | NS | 1.54 | 1.15–2.06 | .004 |
| Histology (ref adenocarcinoma) | ||||||
| Mucinous | 1.20 | 0.86–1.67 | NS | 1.05 | 0.91–1.20 | NS |
| Signet ring cell | 3.44 | 2.03–5.83 | <.001 | 1.56 | 1.08–2.25 | <.001 |
| Neoadjuvant therapy for stage II/III (ref no neoadjuvant therapy) | 1.11 | 0.60–1.42 | NS | 0.77 | 0.70–0.85 | <.001 |
Abbreviations: CI, confidence interval; NS, not statistically significant (P≥.05); Ref, reference variable for the multivariate analysis.
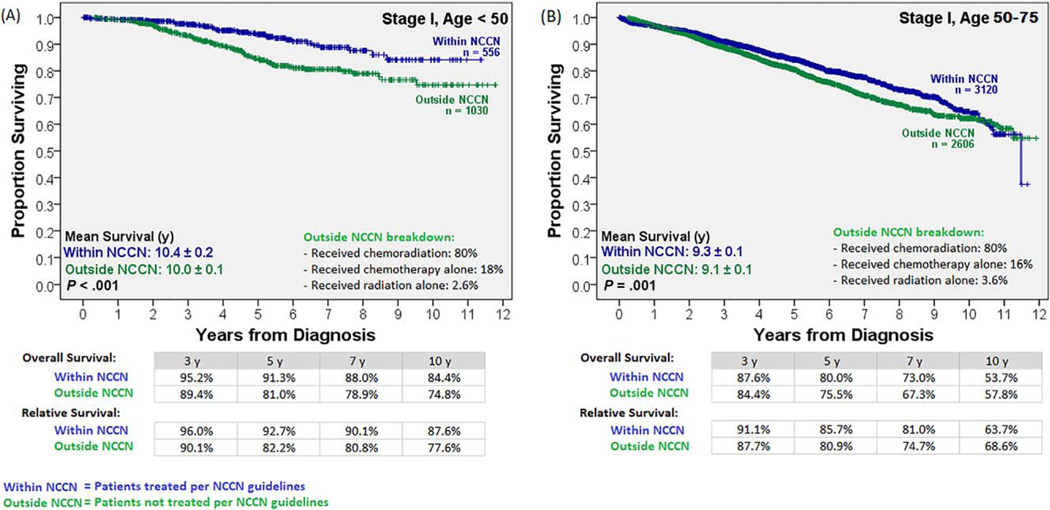
Survival by NCCN Guideline–Driven Care and Age
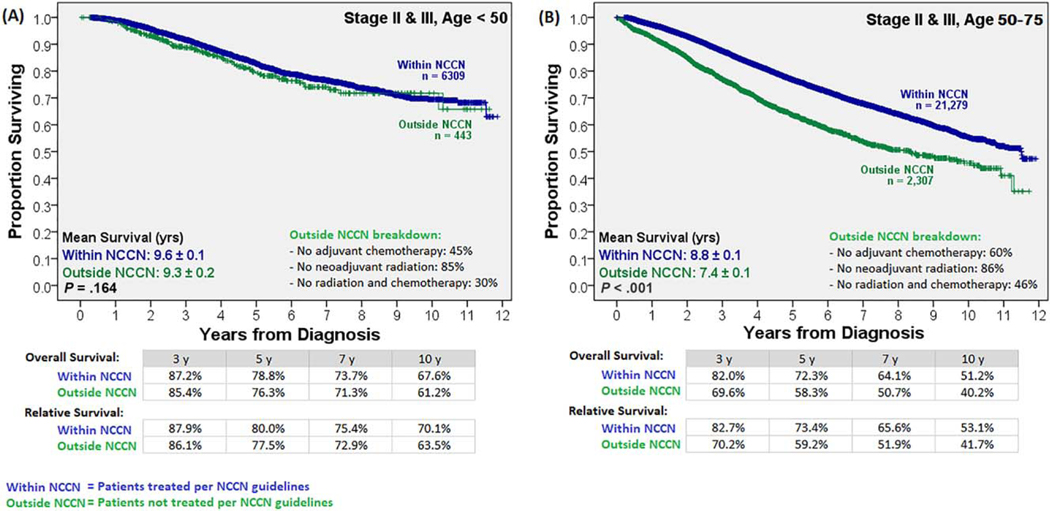
To determine the impact of age and stage-appropriate NCCN guideline–driven care on survival, the Kaplan-Meier analysis was stratified by these 2 variables (Supporting Figs. 1 and 2). In the older group, survival improved with the receipt of NCCN guideline–driven care for stage II and III disease (Figs. 4A and 5A). The 14% survival benefit for the older patients with stage II and III disease receiving stage-appropriate therapy at 5 years persisted over the long term (11% survival benefit in 10-year overall survival). In contrast, although the younger population showed a survival benefit from NCCN guideline–driven care for stage I disease (Fig. 4B), it failed to show a survival benefit when NCCN guidelines were followed for stage II and III disease (Fig. 5B). Treatment details for the patients in the outside-NCCN group are shown in Figures 4 and 5; the majority of clinical stage I patients in that group received neoadjuvant chemoradiation therapy (80%), whereas the majority of clinical stage II and III patients in that group failed to receive neoadjuvant radiation therapy (85%). A separate subgroup analysis for stage II and III disease patients was performed. Further age substratification revealed that following NCCN-guideline–driven care led to significantly reduced survival in patients younger than 45 years (P<.03), no actuarial survival benefit until an age ≥50 years, and a statistically significant survival benefit only at an age>54 years (Supporting Fig. 3). A Cox regression multivariate analysis stratified by both age cohorts (Table 2), when limited to patients with stage II and III disease, confirmed a lack of a survival benefit for the younger cohort when NCCN guideline–driven care was followed.
Figure 4.
Overall and relative survival with stage I disease stratified by age and treatment adherence to NCCN guidelines. A survival benefit was noted from following NCCN guideline–driven care in both age cohorts. NCCN indicates National Comprehensive Cancer Network.
Figure 5.
Overall and relative survival with clinical stage II and III disease stratified by age and chemoradiation therapy: Unlike their older counterparts, patients younger than 50 years showed no survival benefit from NCCN guideline–directed therapy. NCCN indicates National Comprehensive Cancer Network.
DISCUSSION
Survival data specific to younger patients with rectal cancer are lacking in the literature. Furthermore, current recommendations for therapy are based on results from trials that predominantly accrued patients of older ages. Using a large, national database to evaluate more than 43,000 patients with rectal cancer over a 10-year time period, our study provides data showing that early-onset rectal cancer may differ in its epidemiology, biology, and response to current treatment regimens. Specifically, our analysis suggests that survival advantages associated with stage-appropriate NCCN guideline–driven care for stage II and III disease do not appear to be realized in patients younger than 50 years. We also provide age-specific survival data for the younger group in the largest cohort studied to date.
Our data suggest that patients younger than 50 years with rectal cancer differ at presentation in comparison with the more aged cohort. They are more likely to be female, minorities, and uninsured. They are more likely to be diagnosed at a higher stage and have poor tumor differentiation and other histological features associated with worse outcomes (mucinous or signet ring cell histology). In contrast, they are more likely to travel further to receive medical treatment and are more likely to receive treatment at academic centers/integrated treatment centers. They have better overall survival in comparison with the cohort 50 years of age and older.
Younger patients appear to have better short-term (shorter hospital stays and better 30- and 90-day mortality) and long-term outcomes (higher 3-, 5-, 7-, and 10-year survival rates). Although survival was previously known to be better in young patients, our study shows no difference in survival by deciles up to the age of 50 years, after which the prognosis worsens by each decile. This held true in the multivariate analysis. This is an interesting finding because the younger group fares better over the long term despite a higher incidence of several factors associated with poor outcomes in the multivariate analysis for the entire group, such as African American race, an uninsured/Medicaid status, a higher pathologic stage, a poorly differentiated/undifferentiated tumor, and a mucinous/signet ring cell histology.
Because enrollment in prior randomized controlled trials has been limited to patients older than 50 years, this age group primarily drives outcome data. Our study is the largest to report current long-term survival data for the younger population for prognostic and research purposes. In addition, not only is there a difference in the prevalence of stage-appropriate NCCN guideline–driven care for the younger group, younger patients also appear to respond differently to such treatment. For stage II and III disease, younger patients are more likely to receive NCCN guideline–driven care (chemoradiation and surgical resection), but this does not seem to affect their survival. In contrast, older patients show a large and significant survival benefit from it. A higher incidence of microsatellite instability, which has been demonstrated in younger patients with colon cancer,11 could explain the reduced effectiveness of conventional adjuvant therapy in our study. However, genetic data are not available in the NCDB, and the exclusion of more proximal colonic tumors in our study, which are more likely microsatellite instability–high,12 makes conclusions difficult to achieve.
A treatment-selection bias in favor of aggressive treatment for the younger cohort, in turn affecting survival rates, cannot be excluded, but that would lead to the opposite results (ie, a bigger survival benefit for the younger cohort). Previous studies have shown patients older than 75 years to be less likely to receive optimal NCCN guideline–driven care.13,14 We attempted to control for this by requiring all patients to have undergone transabdominal resection with negative margins to emulate treatment with an intent to cure and by excluding patients older than 75 years from the analysis. Regardless, the study findings, including the fact that NCCN guideline–driven care did not affect survival in the younger population with stage II and III disease, were similar when the age group older than 75 years was not excluded, except that the survival differences were more pronounced. Moreover, the proportion of patients adhering to therapeutic guidelines in our study is consistent with previous studies (approximately 74%) by the Optimizing the Surgical Treatment of Rectal Cancer (OSTRiCh) consortium.15
This study has several limitations. First, the large, national, retrospective database affords little control over locoregional practices such as different surgical techniques and chemotherapy and radiation regimens. We were unable to stratify outcomes by specific surgical techniques or quality of resection, but our exclusion criteria should have mitigated this limitation: we considered only transabdominal resections that had a negative surgical margin because an independent variable evaluating this (quality of the total mesorectal excision dissection) does not exist in the NCDB. Controlling for chemotherapy regimens was also not possible; however, the majority of 5-flourouracil (5-FU)–based chemotherapy regimens used during the study period have resulted in similar long-term survival.16 To investigate a time-dependent effect on the study findings from a possible national transition from 5-FU to folinic acid, fluorouracil, and oxaliplatin (FOLFOX) over the study period, the survival curves were recalculated for before and after 2008 (Supporting Fig. 4). Because the 2 major trials showing significant improvements in disease-free survival with the addition of oxaliplatin to 5-FU were published in 2007 and 2011,17,18 we decided on 2008 as an appropriate time cutoff for the analysis. This showed no difference in the pre-2008 and post-2008 survival comparisons for the overall group (Supporting Fig. 4A) or for stage II and III patients (Supporting Fig. 4B,C). Regarding radiation regimens, studies with overlapping time periods have estimated long-course external-beam radiation to be used in more than 99% of the population,19 and this is unlikely to have significantly affected our results. In addition, the number of patients excluded because of missing data, which amounts to approximately half of the stage I to III patients, is a limitation. Nevertheless, we have captured the largest number of patients to be studied in this manner to date.
A major limitation of this study is the absence of disease-free survival data in the NCDB. As a result, it may be difficult to show a distinction between an actuarial benefit of following NCCN guidelines and an age-driven difference in overall survival because of the low rate of mortality in the younger cohort. Certain subgroups within the younger cohort may benefit from following NCCN guidelines, and this could be a topic of further investigation. The absence of local recurrence and disease-free survival data in the NCDB also limits our ability to recognize the reason for poor survival among patients. However, overall survival and disease-free survival should more closely mirror each other for the younger population, and this makes these results more oncologically reliable in this age group. We attempted to overcome these limitations by excluding patients at a higher risk of mortality from age-based nononcologic causes. This included the exclusion of patients at the extremes of age (<20 and >75 years) and those who did not receive chemotherapy because of comorbidities and/or death before administration. Moreover, relative survival was calculated in addition to overall survival.
In summary, this study uses a large national cancer database to demonstrate that patients younger than 50 years diagnosed with rectal cancer represent a unique demographic group in which the survival advantage of receiving NCCN guideline–driven therapy for stage II and III disease does not materialize. Moreover, progressive age within this younger cohort is not a significant determinant of survival or a response to adjuvant therapies. This analysis supports the notion that early-onset rectal cancer may differ in its biology and response to therapy, as has been previously shown in colon cancer.11 These data may help to stimulate future trial proposals to investigate the possibility of the exclusion or selective use of adjuvant therapies for stage II and III disease in the younger cohort to help to decrease treatment toxicity. We further provide age-specific survival data by decile for young patients. These data provide practicing physicians the ability to offer prognostic data tailored to the younger population, which do not exist at present and can greatly improve discussions with these patients regarding their long-term prognosis.
Supplementary Material
FUNDING SUPPORT
Supported in part by funds from the National Institutes of Health Division of Loan Repayment (to Daniel Delitto), the American Cancer Society, and the National Cancer Institute and by research support from Elekta AB to the Medical College of Wisconsin (to William A. Hall). It was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences (National Institutes of Health) through grant UL1TR001436. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672).
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
Thomas J. George reports acting as a consultant for Merck and Bayer for work performed outside of the current study. His institutional research has been supported by Incyte, Bristol-Myers Squibb, Bayer, Merck, NewLink, AstraZeneca/Med-Immune, and Tesaro.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Pignone M, Rich M, Teutsch SM, et al. Screening for colorectalcancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer JE, Narang T, Schnoll-Sussman FH, et al. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2010;116:4354–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates inadults aged 20 to 54 years in the United States, 1970–2014. JAMA. 2017;318:572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216–224. [DOI] [PubMed] [Google Scholar]
- 7.Scott RB, Rangel LE, Osler TM, et al. Rectal cancer in patientsunder the age of 50 years: the delayed diagnosis. Am J Surg. 2016; 211:1014–1018. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Rectal cancer (version 3.20170). https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed August 25, 2017.
- 10.Surveillance, Epidemiology, and End Results Program. SEER*Stat database: mortality—all COD, aggregated with state, total U.S. (1969-2014) <Katrina/Rita population adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2016. Underlying mortality data provided by NCHS; (www.cdc.gov/nchs). https://seer.cancer.gov/data-software/documentation/seerstat/. Accessed December 27, 2017. [Google Scholar]
- 11.Tricoli JV, Boardman LA, Patidar R, et al. A mutational comparison of adult and adolescent and young adult (AYA) colon cancer. Cancer. 2018;124:1070–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boland CR. Clinical uses of microsatellite instability testing in colorectal cancer: an ongoing challenge. J Clin Oncol. 2007;25:754–756. [DOI] [PubMed] [Google Scholar]
- 13.Cho MM, Morgan JW, Knutsen R, et al. Outcomes of multimodality therapies for patients with stage II or III rectal cancer in California, 1994–2009. Dis Colon Rectum. 2013;56:1357–1365. [DOI] [PubMed] [Google Scholar]
- 14.Schiphorst AH, Verweij NM, Pronk A, et al. Age-related guideline adherence and outcome in low rectal cancer. Dis Colon Rectum. 2014;57:967–975. [DOI] [PubMed] [Google Scholar]
- 15.Monson JR, Probst CP, Wexner SD, et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg. 2014;260:625–631. [DOI] [PubMed] [Google Scholar]
- 16.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. [DOI] [PubMed] [Google Scholar]
- 17.De Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. 2007 ASCO annual meeting proceedings (post-meeting edition). J Clin Oncol. 2007;25(suppl):4007. [Google Scholar]
- 18.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohiuddin M, Marks J, Marks G. Management of rectal cancer: short- vs. long-course preoperative radiation. Int J Radiat Oncol Biol Phys. 2008;72:636–643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.