Abstract
By using an animal model in which inflammatory cytokines are induced in lipopolysaccharide (LPS)-injected rat brain, we investigated the induction of tumor necrosis factor alpha (TNFα), interleukin-1beta (IL-1β), and IL-6. Immunoblotting and immunohistochemistry revealed that all three cytokines were transiently induced in the cerebral cortex at about 12 h after LPS injection. To clarify which glial cell type induced the cytokines, we examined the respective abilities of astrocytes and microglia in vitro. Primary microglia largely induced TNFα, IL-1β and IL-6 in response to LPS, but primary astrocytes induced only limited levels of TNFα. Thus, we used specific inhibitors to focus on microglia in surveying signaling molecules involved in the induction of TNFα, IL-1β, and IL-6. The experiments using mitogen-activated protein kinases (MAPK) inhibitors revealed that c-Jun N-terminal kinase (JNK)/p38, external signal regulated kinase (ERK)/JNK, and ERK/JNK/p38 are necessary for the induction of TNFα, IL-1β, and IL-6, respectively. The experiments using protein kinase C (PKC) inhibitor clarified that PKCα is required for the induction of all these cytokines in LPS-stimulated microglia. Furthermore, LPS-dependent IL-1β/IL-6 induction was suppressed by pretreatment with a nitric oxide (NO) scavenger, suggesting that NO is involved in the signaling cascade of IL-1β/IL-6 induction. Thus, an inducible NO synthase induced in the LPS-injected cerebral cortex might be related to the induction of IL-1β/IL-6 through the production of NO in vivo. Taken together, these results demonstrated that microglia induce different kinds of inflammatory cytokine through specific combinations of MAPKs and by the presence or absence of NO.
Keywords: Microglia, lipopolysaccharide, inflammatory cytokines, tumor necrosis factor alpha, interleukin 1beta, interleukin 6
Introduction
Inflammatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin 1beta (IL-1β), and IL-6 have been detected in various brain diseases such as Alzheimer's disease,1–3 Parkinson's disease,4,5 amyotrophic lateral sclerosis, 6 multiple sclerosis, 2 and acquired immunodeficiency syndrome dementia. 1 They have also been found in damaged regions including ischemia, 7 stroke, 8 and injury.9–11 These three cytokines have been generally thought to exacerbate inflammatory and immune reactions and, in the worst cases, to cause cell death. In fact, TNFα has been reported to kill oligodendrocytes and neurons in the nervous system.12,13 IL-1β caused neuronal cell death in the hippocampus 14 and indirectly caused neuronal cell death with other factors.15–17 IL-6 has been reported to induce cell death in cerebellar granule neurons. 18 Thus, these inflammatory cytokines were often regarded as the targets for the amelioration or improvement of symptoms in many brain degenerative diseases or injuries. However, the mechanism underlying the induction of inflammatory cytokines has not been systemically analyzed, and thus ambiguous issues remain. For example, the point at which each inflammatory cytokine is induced after stimulation is still uncertain. The relationship between the induction of inflammatory cytokines and glial proliferation/activation is unclear. Which glial cell type is responsible for the induction of inflammatory cytokines has not been sufficiently analyzed. Whether all inflammatory cytokines are induced by a common signaling cascade also remains to be investigated.
Therefore, we investigated the time course of the induction of inflammatory cytokines and glial proliferation/activation as well as the ability of astrocytes and microglia in vitro to induce inflammatory cytokines, and further analyzed signaling molecules that are relevant to the induction of inflammatory cytokines. These analyses revealed notable findings.
Material and methods
Reagents and antibodies
Lipopolysaccharide (LPS) was supplied by Difco Laboratories (Detroit, MI). Dulbecco's modified phosphate-buffered saline (PBS) was supplied from Kanto Chemical (Tokyo). Enzyme-linked immunosorbent assay (ELISA) kits for rat TNFα, IL-1β, and IL-6 were purchased from R&D Systems (Minneapolis, MN), Thermo Fisher Scientific (Rockford, IL), and Cloud-Clone (Katy, TX), respectively.
The cell-permeable and selective mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor PD98059, 19 cell-permeable p38MAPK inhibitor SB203580, 20 and cell-permeable protein kinase C (PKC) inhibitor myristoylated pseudosubstrate (20–28) (MP) 21 were purchased from Calbiochem-Novabiochem (LaJolla, CA). The c-Jun N-terminal kinase (JNK) inhibitor SP600125 22 was supplied by Funakoshi (Tokyo). The NO assay kit, 2-(4-carboxyphenyl)-4,4,5, 5-tetramethylimidazoline-1-oxyl 3-oxide, sodium salt (Carboxy-PTIO), 23 and S-nitroso-N-acetyl-DL-penicillamine (SNAP) 24 were obtained from Dojindo Laboratories (Kumamoto, Japan).
Antibodies against TNFα (sc-1351), IL-1β (sc-1251), IL-6 (sc-1265), NO synthase 2 (NOS2) (corresponds to iNOS, sc-7271), actin (sc-1615), cFms (sc-692), and L-selectin (sc-390756) were supplied by Santa Cruz Biotechnology (Dallas, TX). Another anti-IL-6 antibody (ARC0062) was purchased from Thermo Fisher Scientific. Anti-glial fibrillary acidic protein (GFAP) (MAB5628) antibody was purchased from Millipore (Temecula, CA). Anti-ionized Ca2+ binding adapter molecule 1 (Iba1) (016–20001) antibody was supplied by Fuji Film Wako Pure Chemical Industries (Osaka, Japan). Antibodies against PCNA (NA03) and CD68 (ED1) (MCA341R) were obtained from Calbiochem-Novabiochem and Bio-Rad, respectively.
Horseradish peroxidase (HRP)-conjugated anti-mouse IgG (sc-2055), HRP-conjugated anti-rabbit IgG (sc-2374), and HRP-conjugated anti-goat IgG (sc-2020) were purchased from Santa Cruz Biotechnology. Alexa Fluor 488-conjugated anti-mouse IgG (A11001) and Alexa Fluor 568-conjugated anti-rabbit IgG (A11011) were obtained from Life Technology Corp. Alexa Fluor 488-conjugated anti-rabbit IgG (711-545-152) was purchased from Jackson ImmunoResearch Lab Inc (West Grove, PA). Alexa Fluor 568-conjugated anti-goat IgG (ab175704) was supplied by Abcam (Cambridge, UK).
Animals and operation
Male Wistar rats (8 weeks old) were obtained from Clea Japan (Tokyo) and kept on a 12-h daylight cycle with food and water. The animal experiments were carried out in accordance with the guidelines laid down by the US National Institutes of Health regarding the care and use of animals, and were approved by the ethics committee of Soka University (approval code: 2012).
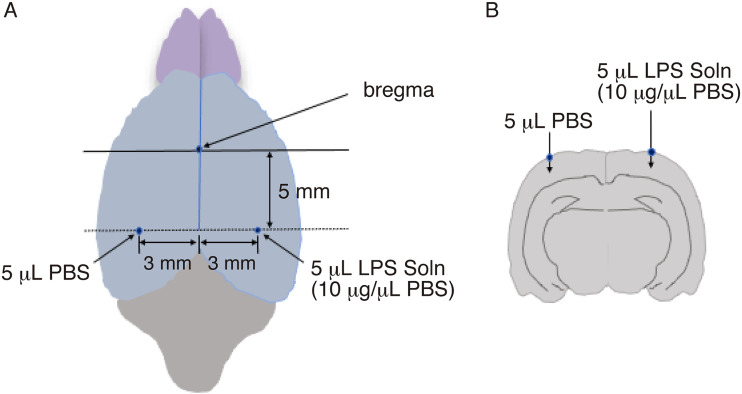
Rats were anesthetized with isoflurane and fixed to a corkboard. In each hemisphere, a hole was made in the skull at 5 mm posterior and 3 mm lateral to the bregma, which are the coordinates in the stereotaxic atlas of Paxinos and Watson (1982). 25 In the left hole, 5 μL of phosphate-buffered saline (PBS) (vehicle) was injected at 5 mm depth. In the right hole, 5 μL of LPS solution (10 μg/μL PBS) was injected at 5 mm depth by a Hamilton syringe (Figure 1A.1B). By using this method, we intended to obtain the effect of LPS alone. This LPS concentration (10 μg/μL PBS) did not exhibit cytotoxic effects and was suitable for obtaining stable and reproducible results in vivo. After the surgical skin was closed, the animals were left in normal conditions. The rats were reared for 3, 6, 12, 24 h, and 3 days, and decapitated under anesthesia. The whole brains were removed, frozen on dry ice, and stored at −80°C until use.
Figure 1.
Schema indicating LPS-injection site
A. Overhead view of rat brain. Five microliters of PBS (vehicle) was injected at 5 mm posterior to and 3 mm to the left of the bregma, and 5 μL of LPS solution (10 μg/μL PBS) was injected at 5 mm posterior to and 3 mm to the right of the bregma.
B. Coronal view of rat brain. The view around the dotted line in A is shown. .
For the preparation of a tissue extract of the LPS/PBS-injected region, the brains were kept at −15°C, and a 2-mm-thick tissue around the LPS injection hole was cut out. A tissue on the PBS-injected side was similarly taken from the opposite hemisphere.
Immunoblotting
The tissue samples recovered from the LPS/PBS-injected sites and a PBS-injected site were solubilized with nonreducing sodium dodecyl sulphate (SDS) sample solution [62.5 mM Tris HCl (pH 6.8), 2.3% SDS, 10% glycerol] and centrifuged (100,000 g, 30 min). The supernatant was recovered and the protein content was determined by the method of Lowry et al. 26 The supernatant (tissue extract) was adjusted to contain 5% 2-mercaptoethanol, and the resultant sample was subjected to immunoblotting for TNFα (1:100), IL-1β (1:100), IL-6 (1:100), iNOS (1:1000), actin (1:2000), GFAP (1:1000), Iba1 (1:1000), PCNA (1:500), cFms (1:1000), CD68 (1:500), and L-selectin (1:1000).
The freeze-dried MCM and ACM (see above) were solubilized with reducing sample buffer [62.5 mM Tris HCl (pH 6.8), 5% 2-mercaptoethanol, 2.3% SDS, 10% glycerol], and an aliquot of each medium was subjected to immunoblotting for TNFα (1:1000), IL-1β (1:1000), and IL-6 (1:1000).
Immunohistochemical staining
The coronal sections 20 μm thick were cut on a cryostat (Leica CM1860, Germany) from a brain region in which PBS (vehicle) and PBS/LPS were injected as shown in Figure 1. The sections were air-dried for 20 min and fixed in 4% paraformaldehyde in 10 mM PBS for 10 min. The fixed sections were treated sequentially with 50%, 100%, and 50% acetone for 2, 3, and 2 min, respectively. These sections were further soaked in 0.1% Triton X-100/10 mM PBS for 10 min and blocked with 0.2% bovine serum albumin or 0.2% skim milk for 60 min.
To examine whether microglia proliferate in an LPS/PBS-injected region, the sections were stained by fluorescent dual staining with anti-PCNA antibody and anti-Iba1 antibody. The sections were incubated first with anti-PCNA antibody (1:100) at 4°C for 16 h, and then with Alexa Fluor 488-conjugated anti-mouse IgG (1:500) at 4°C for 16 h. The sections were further incubated with anti-Iba1 antibody (1:500) at 4°C for 16 h, and then with Alexa Fluor 568-conjugated anti-rabbit IgG (1:500) at 4°C for 16 h.
To investigate whether the inflammatory cytokines are expressed in the LPS/PBS injected region, the sections were incubated first with anti-TNFα antibody (1:100), anti-IL-1β antibody (1:100), or anti-IL-6 antibody (1:100) at 4°C for 16 h, and then with anti-Iba1 antibody (1:1000) at 4°C for 16 h. The sections were further incubated with Alexa Fluor 568-conjugated anti-goat IgG (1:500) and Alexa Fluor 488-conjugated anti-rabbit IgG (1:500) at room temperature for 3 h. These sections were sufficiently washed and mounted with PermaFluor (Thermo Scientific). The specimens were observed under a fluorescence microscope (Nikon ECLIPSE TS100).
Preparation of primary microglia and astrocytes
To obtain microglia and astrocytes in vitro, we prepared primary mother cultures of the cerebral hemispheres of newborn rat. The primary cultures were made essentially according to a method described previously, 27 and both microglia and astrocytes were prepared from the cultures.
Briefly, microglia were floated by gentle shaking of primary mother cultures that had been maintained for 10–20 days, and seeded on 60-mm dishes (Nunclon, Sigma-Aldrich) at a density of 1.5 × 106 (for immunoblotting). The purity was over 99.9% based on the assessment of Iba1 staining.
Astrocytes were prepared from a primary mother culture maintained for 3 weeks, essentially as described. 28 The astrocytes were subcultured onto 60-mm dishes (Nunclon) at a density of 1.5 × 106 cells/dish (for immunoblotting). The cell purity was estimated as 98% based on staining with anti-GFAP antibody.
Stimulation of microglia and astrocytes and recovery of their conditioned media
The microglial and astrocytic cultures prepared as described above were rinsed with serum-free DMEM and maintained with the same medium for 16 h. These cells were stimulated with LPS (0.5 μg LPS/mL) as necessary.
To test the effects of an MEK inhibitor (PD98059), a JNK inhibitor (SP600125), a p38MAPK inhibitor (SB203580), a protein kinase Cα inhibitor (MP), and Carboxy-PTIO on LPS-stimulated microglia, microglia were pretreated with the respective reagents for 1 h prior to LPS stimulation.
The conditioned media of microglia and astrocytes were recovered at suitable time points (0, 6, 12, and 24 h) and referred to as microglial conditioned medium (MCM) and astrocytic conditioned medium (ACM), respectively. For the immunoblotting of TNFα, IL-1β, or IL-6, MCM and ACM were concentrated by Centricut V-10 (Kurabo, Osaka, Japan) and freeze-dried.
ELISA for rat TNFα, IL-1β, and IL-6
The amounts of TNFα, IL-1β, and IL-6 secreted from microglia into the MCM were also determined by an ELISA kit. Fifty microliters of each MCM sample and each standard rat TNFα (0, 12.5, 25, 50, 100, and 200 pg/mL), rat IL-1β (0, 25.6, 64, 160, and 400 pg/mL), or rat IL-6 (0, 7.8, 15.6, 31.2, 62.5, 125, and 250 pg/mL) were concomitantly poured into the wells of a 96-well plate precoated with anti-rat TNFα, IL-1β, or IL-6 antibody. After 2 h (in the cases of TNFα and IL-1β) or 1 h (in the case of IL-6), the bound cytokine was detected according to each protocol. Using these assay kits, it was possible to detect a minimum of 10 pg TNFα/mL, 5 pg IL-1β/mL, and 3pg IL-6 /mL.
Statistical analysis
The contents of TNFα, IL-1β, or IL-6 in MCM determined by ELISA were expressed as means ± SDs of three separate experiments. The densities of the TNFα, IL-1β, and IL-6 protein bands in immunoblotting were measured by densitometry using ImageJ software (NIH, Bethesda, MD) and expressed as means ± SDs of three separate experiments. The value relative to that in the control (defined as 100) was assessed via Student's t-test In all cases, P values less than 0.05 were considered significant (*P < 0.05, **P < 0.01).
Results
Induction of inflammatory cytokines in LPS-injected rat cerebral cortex
To examine the induction of inflammatory cytokines in the brain, we administered a bacterial endotoxin LPS in rat cerebral cortex (see Material and methods; Figure 1). After the LPS injection, we compared the levels of inflammatory cytokines between the LPS/PBS-injected region and the PBS (vehicle)-injected region. Immunoblotting indicated that TNFα, IL-1β, and IL-6 were clearly induced in the LPS/PBS-injected region at 6–12 h after injection, after which the levels declined (Figure 2A). In contrast, in the PBS-injected region these cytokines were not significantly induced at any time point (Figure 2A). We thus confirmed that the inflammatory cytokines TNFα, IL-1β, and IL-6 are inducible-type cytokines in the brain.
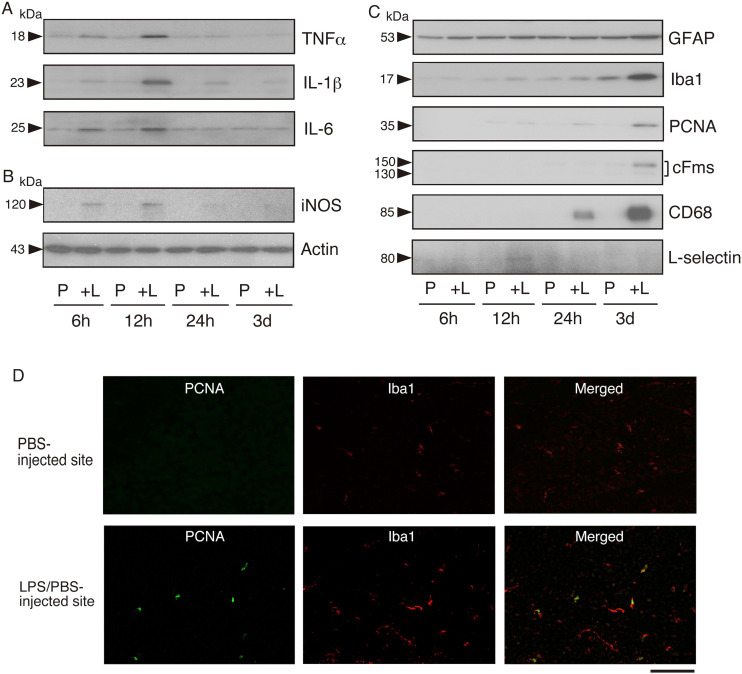
Figure 2.
Induction of inflammatory cytokines in LPS-injected rat brain
A. Induction of inflammatory cytokines. PBS was injected into the left cerebral cortex and LPS/PBS was administered in the right cerebral cortex (see Material and methods). Both areas were cut out at 6, 12, and 24 h, and at 3 days post-LPS injection, and the tissue extracts were prepared as described in Material and methods. The tissue extracts (20 μg protein) of the PBS (vehicle)-injected site (P) and the LPS/PBS-injected site ( + L) were immunoblotted for TNFα, IL-1β, and IL-6. A representative result is shown.
Molecular size (kDa) is indicated on the left side.
B. Induction of iNOS. The same samples as in A were immunoblotted for iNOS and actin. A typical result is shown.
C. Induction of glial markers, proliferation marker, monocyte/macrophage marker, and neutrophil marker. The same samples as in A were immunoblotted for GFAP, Iba1, PCNA, cFms, CD68, and L-selectin. A typical result is shown.
D. Immunohistochemical detection of proliferating cells in the LPS/PBS-injected region. Cryosections were cut from the brain into which LPS had been injected 3 days prior and stained dually with anti-PCNA antibody (PCNA; Green) and anti-Iba1 (Iba1; red) antibody. PBS-injected and LPS/PBS-injected sites are shown at the upper and lower positions, respectively. Merged images are shown on the right side. Scale bar = 50 μm.
In addition to inflammatory cytokines, we examined the expression of inducible NO synthase (iNOS), because the iNOS product NO is associated with the induction of inflammatory cytokines.29,30 Immunoblotting indicated that iNOS protein was transiently induced in the LPS/PBS-injected region at 6–12 h after LPS injection (Figure 2B).
To ascertain the relationship between the induction of inflammatory cytokines and glial proliferation/activation, we examined the expression of astrocytic marker GFAP and microglial marker Iba1 in LPS/PBS-injected cerebral cortex. At 3 days post-injection, the GFAP level was slightly promoted (Figure 2C), and the Iba1 level was largely increased (Figure 2C). An S-phase-specific protein, proliferating cell nuclear antigen (PCNA), 31 was detected at 24 h after LPS/PBS-injection, and the level was increased at 3 days post-injection (Figure 2C). A receptor for M-CSF (cFms) that is expressed in proliferating microglia 32 was induced in the LPS/PBS-injected region at 3 days (Figure 2C). CD68 is known to be expressed predominantly on the lysosomal membrane of monocytes/macrophages,33,34 and thus was used as a marker of these cells. This CD68 band was detected at 24 h in the LPS/PBS-injected site, and the level was elevated at 3 days (Figure 2C). L-selectin was used as a neutrophil marker because it is expressed on most neutrophils and monocytes. 35 The L-selectin was temporally detected at 12 h in the LPS/PBS-injected site (Figure 2C). These results indicated that inflammatory cytokines (TNFα, IL-1β, and IL-6) were induced at 6–12 h in advance of the upregulation of Iba1 and GFAP levels by microglia/astrocytes as well as infiltrated monocytes/macrophages, which occurred at 24 h-3 days post-LPS stimulation.
To confirm that microglia proliferate in response to LPS stimulation, we immunohistochemically compared the expression/localization of PCNA in microglia between PBS-injected and LPS/PBS-injected regions (Figure 2D). The cryosection cut from the brain into which LPS had been injected 3 days prior was dually stained by anti-PCNA antibody and anti-Iba1 antibody. In the PBS-injected site, PCNA was hardly present and Iba1 was poorly expressed (Figure 2D, upper panels). On the other hand, in the LPS/PBS-injected site, some pointlike PCNA stainings were seen and Iba1 was strongly detected. The merged image shows that all of the nuclear PCNA consisted of Iba1-stained microglia(Figure 2D, lower panel). Therefore, the results of PCNA (Figure 2C and 2D) strongly suggested that microglia did not proliferate at 6–12 h but did at 3 days post-LPS injection.
Immunohistochemical analysis of inflammatory cytokines in LPS-injected brain
To confirm the induction of inflammatory cytokines in vivo, we prepared coronal cryosections at the site where LPS had been injected 12 h prior, as shown in Figure 1B, and dually stained the sections with each anti-inflammatory cytokine antibody and anti-Iba1 antibody (Figure 3).
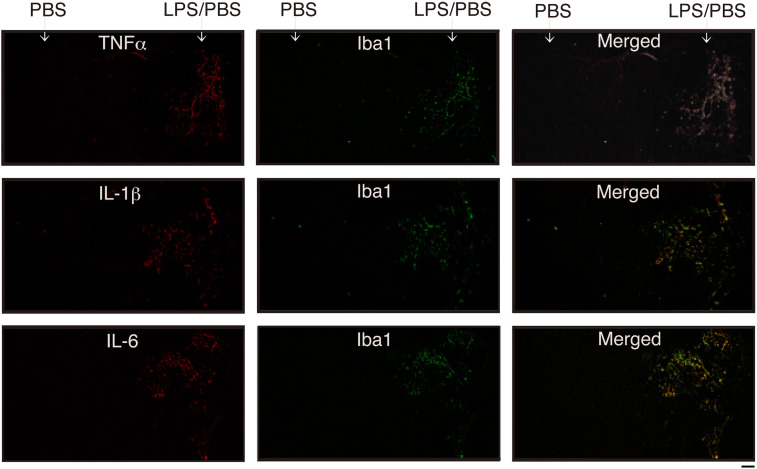
Figure 3.
Detection of inflammatory cytokines by immunohistochemical staining
coronal sections were prepared from the brain in which PBS (vehicle) and LPS/PBS had been injected 12 h prior, as shown in Figure 1B. The cryosections were dually stained with anti-TNFα antibody and anti-Iba1 antibody (top), anti-IL-1β antibody and anti-Iba1 antibody (medium), and anti-IL-6 antibody and anti-Iba1 antibody (bottom). Merged images are shown on the right side. Cytokine and Iba1 can be seen as red (Alexa Fluor-568) and Green (Alexa Fluor-488), respectively. Scale bar = 500 μm.
The three antibodies stained many cells in the LPS/PBS-injected region but few in the PBS-injected region (Figure 3). Iba1 was weakly detected in the LPS/PBS-injected region. Dual staining using anti-inflammatory cytokine antibody and anti-Iba1 antibody indicated that a lot of cells positive for each anti-inflammatory cytokine antibody were anti-Iba1 antibody-positive (Figure 3). These results suggested that TNFα, IL-1β, and IL-6 were induced mostly by microglia. It also remained a possibility that only a small number of astrocytes induced a low level of inflammatory cytokines in vivo.
Induction of inflammatory cytokines in microglia and astrocytes stimulated with LPS in vitro
As described above (Figures 2, 3), inflammatory cytokines were induced in the cerebral cortex in response to LPS. Since we anticipated that glial cells mainly induced those cytokines, we investigated the abilities of astrocytes and microglia to induce each cytokine in the LPS-stimulated in vitro system.
In advance of the determinations, we examined the purity of the astrocytes and microglia used in this study. Immunoblotting indicated that GFAP (an astrocytic marker) alone and Iba1 (a microglial marker) alone were detected in astrocyte extract and microglial extract, respectively (Figure 4A) under similar levels of protein content (Figure 4B). These results indicated that neither cell type is contaminated by the other.
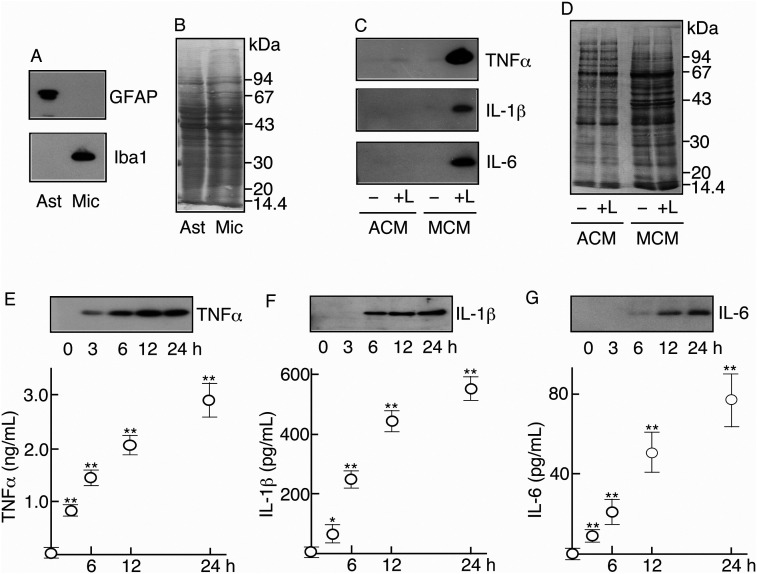
Figure 4.
Ability of glial cells to induce inflammatory cytokines
A. Purity of astrocytes and microglia. The cell extracts of astrocytes and microglia were immunoblotted for GFAP and Iba1.
B. Protein profile of glial cell extract. Cellular extracts of astrocytes and microglia were subjected to SDS-PAGE and transblotting. The transblotted PVDF membrane was stained with 0.1% Coomassie Brilliant Blue (CBB).
C. Detection of inflammatory cytokines. Two astrocytic cultures and two microglial cultures were prepared. Each culture was stimulated with LPS (0.5 μg/mL) ( + L) and the others remained as nonstimulated control (–). At 24 h, each medium of astrocytes and microglia was recovered as astrocytic conditioned medium (ACM) and microglial conditioned medium (MCM), respectively. Two ACMs (–, + L) and two MCMs (–, + L) were concentrated, freeze-dried, and immunoblotted for each of TNFα, IL-1β, and IL-6.
D. Protein profiles of ACM and MCM. Two ACMs (–, + L) and two MCMs (–, + L) were subjected to SDS-PAGE and transblotted to PVDF membrane. The membrane was stained with Coomassie Brilliant Blue (CBB).
E, F, G. Determination of inflammatory cytokines in LPS-stimulated microglia. Five microglial cultures (2 × 106 cells) were prepared and then stimulated with LPS (0.5 μg/mL) as described in the Material and methods. At 0, 3, 6, 12, and 24 h, the medium was recovered and the media were concentrated, freeze-dried, and immunoblotted for TNFα (E), IL-1β (F), and IL-6 (G). At the same time, the medium taken from each culture was used to determine TNFα (E), IL-1β (F), and IL-6 (G) levels by ELISA.
The abilities of these astrocytes and microglia to induce inflammatory cytokines were compared under both nonstimulated and LPS-stimulated conditions. In the nonstimulated condition, TNFα was not detected in astrocytic conditioned medium (ACM) or in microglial conditioned medium (MCM) (Figure 4C). When astrocytes were stimulated with LPS, small amounts of TNFα were detected in the ACM (Figure 4C). On the other hand, microglia were found to induce large amounts of TNFα when stimulated with LPS (Figure 4C).
IL-1β and IL-6 were not significantly detected in ACMs of nonstimulated and LPS-stimulated astrocytes or in MCM of nonstimulated microglia (Figure 4C). However, these cytokines were largely induced in MCMs of LPS-stimulated microglia (Figure 4C). The protein profiles of ACM and MCM suggested that astrocytes and microglia released similar levels of proteins (Figure 4D).
As shown in Figure 4C, microglia were found to be a major cell type inducing TNFα, IL-1β, and Il-6 in response to LPS. We thus focused on the abilities of microglia and quantitatively determined the amounts of the inflammatory cytokines in MCM of LPS-stimulated microglia. Immunoblotting revealed that the amounts of TNFα, IL-1β, and IL-6 increased in MCM of LPS-stimulated microglia depending on the culture time (Figure 4E-G). ELISA estimated that microglia (106 cells) released 23.1 ± 7.5 pg, 819.0 ± 96.3 pg, 1420.1 ± 147.9 pg, 2049.7 ± 204.3 pg, and 2880.2 ± 364.8 pg TNFα at 0, 3, 6, 12, and 24 h, respectively (Figure 4E). Similarly, the amounts of IL-1β and IL-6 were quantified. Microglia (106 cells) released 4.75 ± 4.0 pg, 63.3 ± 23.1 pg, 250.0 ± 26.5 pg, 445.0 ± 35.0 pg, and 553.1 ± 35.1 pg IL-1β at 0, 3, 6, 12, and 24 h, respectively (Figure 4F), and 0.75 ± 0.35 pg, 8.8 ± 1.6 pg, 21.3 ± 5.5 pg, 51.3 ± 11.8 pg, and 76.2 ± 13.7 pg IL-6 at 0, 3, 6, 12, and 24 h, respectively (Figure 4G). Microglia in vitro were verified to have potencies sufficient to induce all three kinds of inflammatory cytokines in response to LPS.
Signaling molecules involved in the induction of inflammatory cytokines in microglia
We have preliminarily explored signaling molecules related to the induction of inflammatory cytokines in LPS-stimulated microglia by using specific inhibitors. Such inhibition experiments highlighted MAPKs and PKC as important signaling molecules.
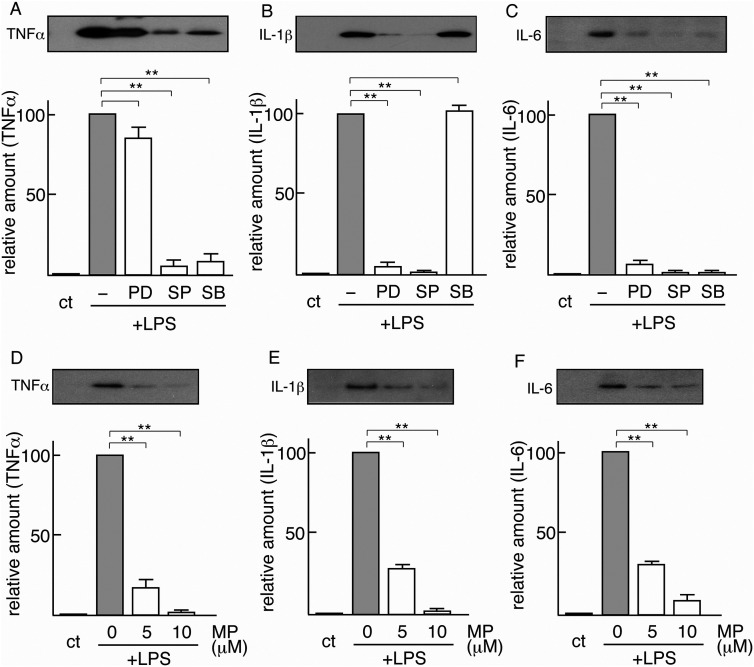
First, using specific inhibitors, we examined the relevance of MAPKs to the induction of inflammatory cytokines (Figures 5A-C). Immunoblotting indicated that LPS-dependent TNFα induction was suppressed by pretreatment with a JNK inhibitor (SP) or a p38MAPK inhibitor (SB), but not with an ERK inhibitor (PD) (Figure 5A). Quantification of the bands led us to estimate that the amounts of LPS-induced TNFα were suppressed to 83.8 ± 8.6%, 5.0 ± 4.7%, and 8.6 ± 6.5% by pretreatment with 20 μM PD, 20 μM SP, and 20 μM SB, respectively (Figure 5A). This suggested that JNK and p38 are strongly associated with the induction of TNFα in LPS-stimulated microglia. LPS-inducible IL-1β was significantly inhibited by pretreatment with an ERK inhibitor (PD) or a JNK inhibitor (SP) (Figure 5B). Quantification of the results indicated that LPS-induced IL-1β was reduced to 5.4 ± 2.9%, 1.4 ± 0.4%, and 102.9 ± 3.5% by pretreatment with 20 μM PD, 20 μM SP, and 20 μM SB, respectively, suggesting that ERK and JNK are related to the signaling cascade to induce IL-1β in LPS-stimulated microglia. On the other hand, LPS-inducible IL-6 was strongly depressed by pretreatment with an ERK inhibitor (PD), a JNK inhibitor (SP), and a p38MAPK inhibitor (SB) (Figure 5C). Quantification revealed that LPS-inducible IL-6 was decreased to 6.9 ± 1.9%, 1.3 ± 1.1%, and 1.3 ± 1.1% by pretreatment with 20 μM PD, 20 μM SP, and 20 μM SB, respectively. This suggested that ERK, JNK, and p38MAPK are all linked to the induction cascade of IL-6. These inhibition experiments revealed that each inflammatory cytokine is induced by a specific signaling cascade in which a different combination of MAPKs is working.
Figure 5.
Effects of MAPK inhibitors and protein kinase Cα inhibitor
A. B, C. Effects of MAPK inhibitors. Five microglial cultures were prepared, four of which were pretreated with vehicle (–), 20 μM PD98059 (PB), 20 μM SP600125 (SP), or 20 μM SB203580 (SB) for 1 h. These four microglia were then stimulated with LPS (0.5 μg/mL). The one remaining microglia was used as the control (ct). After 24 h, the medium of each culture was collected, concentrated, and freeze-dried as described in Material and methods. These samples were immunoblotted for TNFα (A), IL-1β (B), and IL-6 (C). The intensity of each inflammatory cytokine band in immunoblotting was quantified and is expressed as a value relative to that of microglia stimulated with LPS alone (defined as 100). The results are each the mean ± SD of three independent experiments. Differences between the inhibitor nonpretreated group (–) and the 20 μM inhibitor (PD, SP, SB)–pretreated groups were assessed by Student's t-test *P < 0.05, **P < 0.01.
D, E, F. Effects of PKCα. Four microglial cultures were prepared, three of which were pretreated with myristoylated pseudosubstrate (20–28) (MP) (0, 5, and 10 μM) for 1 h prior to LPS stimulation (0.5 μg/mL). The remaining microglial culture was used as the control (ct). These four cultures were maintained for 24 h, and each medium was recovered, concentrated, and freeze-dried. These samples were immunoblotted for TNFα (D), IL-1β (E), and IL-6 (F). The intensity of each inflammatory cytokine band in immunoblotting was quantified and is expressed as a value relative to that of microglia stimulated with LPS alone (defined as 100). The results are each the mean ± SD of three independent experiments. Differences between the inhibitor nonpretreated group (0 μM MP) and the inhibitor (5 μM, 10 μM)–pretreated groups were assessed by Student's t-test *P < 0.05, **P < 0.01.
We next investigated the involvement of PKCα in the induction of TNFα, IL-1β, and IL-6 in LPS-stimulated microglia (Figures 5D-F). LPS-dependent TNFα induction was significantly inhibited by pretreatment with a PKCα inhibitor, MP (Figure 5D). We estimated that the amounts of LPS-induced TNFα were suppressed to 17.3 ± 5.1% and 1.4 ± 0.1% by pretreatment with 5 μM and 10 μM MP, respectively (Figure 5D). LPS-dependent IL-1β was efficiently inhibited by MP. The LPS-inducible IL-1β levels were suppressed to 27.5 ± 1.5% and 2.1 ± 0.9% by pretreatment with 5 μM and 10 μM MP, respectively (Figure 5E). Similarly, LPS-dependent IL-6 inductions were downregulated to 29.3 ± 1.2% and 9.2 ± 4.0% by pretreatment with 5 μM and 10 μM MP (Figure 5F). These results indicated that PKCα activity is required in a cascade leading to the induction of TNFα, IL-1β, and IL-6 in microglia.
Role of iNOS induced in LPS-injected cerebral cortex
As shown in Figure 2A, iNOS was induced at 6–12 h together with inflammatory cytokines (TNFα, IL-1β, and IL-6) in the LPS/PBS-injected cerebral cortex. Considering the biological significance of NO derived from iNOS, we investigated the influence of NO on the induction of inflammatory cytokines (TNFα, IL-1β, and IL-6) in LPS-stimulated microglia in vitro (Figures 6A-C).
Figure 6.
Effects of NO scavenger and NO generator on the induction of inflammatory cytokines
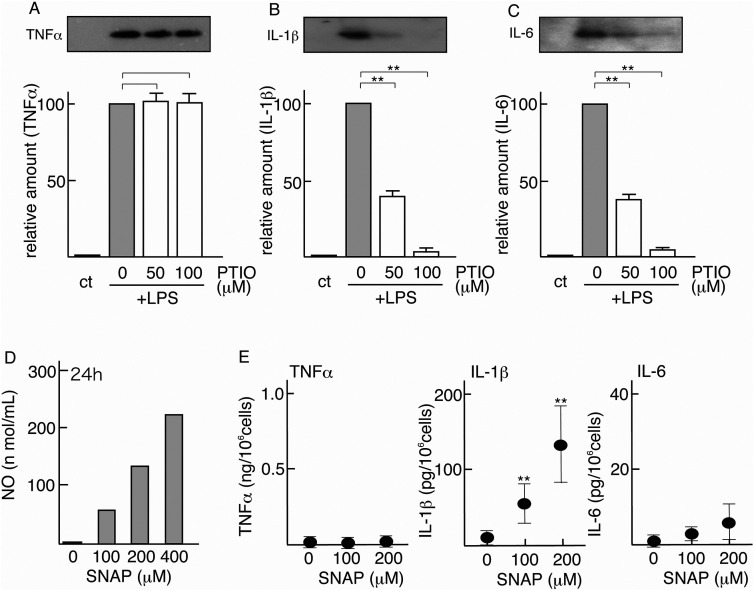
A, B, C. Effects of NO scavenger on LPS-inducible inflammatory cytokines. Of four microglial cultures, three were pretreated with Carboxy-PTIO (PTIO) (0, 50, 100 μM) for 1 h prior to LPS stimulation (0.5 μg/mL). One culture was left untreated as a control (ct). These four cultures were maintained for 24 h, and their MCM samples were concentrated and immunoblotted for TNFα (A), IL-1β (B), and IL-6 (C). A typical profile is shown in the upper panel. The intensity of each inflammatory cytokine band in immunoblotting was quantified and is expressed as a value relative to that of the microglia stimulated with LPS alone (defined as 100). The results are each the mean ± SD of three independent experiments. Differences between the PTIO nonpretreated group (0 μM) and the PTIO (50 μM and 100 μM)–pretreated groups were assessed by Student's t-test *P < 0.05, **P < 0.01.
D. Production of NO in SNAP-administered culture medium. SNAP was added to the culture dishes (0, 100, 200 and 400 μM) on which DMEM alone was present, and the amount of NO in each medium was assayed at 24 h after SNAP addition.
E. Effects of NO donor on the induction of inflammatory cytokines. Microglial cultures were treated with SNAP (0, 100, and 200 μM) for 24 h. At the end of the stimulation, the amounts of TNFα, IL-1β, and IL-6 in each MCM were determined by ELISA. The results are each the mean ± SD of three to four separate experiments. Differences between the control (0 μM SNAP) and stimulated groups (100 and 200 μM SNAP) were assessed by Student's t-test *P < 0.05, **P < 0.01.
Immunoblotting indicated that NO scavenger Carboxy-PTIO did not affect the induction of TNFα in LPS-stimulated microglia (Figure 6A), whereas it significantly suppressed the inductions of LPS-dependent IL-1β and IL-6 (Figure 6B, 6C). The quantified results indicated that LPS-inducible TNFα levels reached 102.5 ± 5.9% and 101.0 ± 6.6% by pretreatment with 50 μM and 100 μM Carboxy-PTIO, respectively, prior to LPS stimulation (Figure 6A). On the other hand, LPS-inducible IL-1β was downregulated to 40.1 ± 3.9% and 3.0 ± 1.6% by pretreatment with 50 μM and 100 μM Carboxy-PTIO, respectively (Figure 6B). Similarly, LPS-inducible IL-6 was suppressed to 38.0 ± 3.6 and 4.9 ± 0.1% by pretreatment with 50 μM and 100 μM Carboxy-PTIO, respectively (Figure 6C). These results suggested that NO is involved in the induction of IL-1β and IL-6 in LPS-stimulated microglia.
These results allowed us to predict that NO induced in the LPS/PBS-injected region serves to induce IL-1β and IL-6 production in vivo. To investigate this possibility, we planned to add NO generator SNAP in microglial culture in vitro. In advance, we examined whether SNAP actually produced NO. In the culture system, we confirmed that the level of enhancement of the NO level in culture medium depended on the SNAP concentration (Figure 6D).
The effects of SNAP on the induction of inflammatory cytokines in microglial culture were determined by ELISA. The results indicated that SNAP significantly enhanced IL-1β but not TNFα or IL-6 (Figure 6E). In the nonstimulated microglial conditioned medium, 14.8 ± 7.3 pg IL-1β/mL was detected, but when microglia were stimulated with 100 μM and 200 μM SNAP, the amounts of IL-1β in the conditioned medium were 64.4 ± 18.7 pg/mL and 133.0 ± 48.7 pg/mL, respectively (Figure 6E). These results suggested that NO is directly associated with a signaling cascade leading to IL-1β expression.
Discussion
Inflammatory cytokines including TNFα, IL-1β, and IL-6 have been regarded as targets for improving clinical condition, because these cytokines have been believed to cause deleterious actions including neuronal cell death and/or inflammation. TNFα exhibits cytotoxic effects on oligodendrocytes 12 and neurons. 13 This cytokine has been known to enhance neuronal cell death mediated by glutamate/glutamate receptor.36–38 IL-1β has various biological activities. It enhances NMDA-evoked cell death, 16 hippocampal neuronal cell death, 14 glia-triggered dopaminergic cell death, 15 and proNGF-mediated neuronal cell death. 17 IL-6 is considered an inflammatory cytokine, and has been reported to lead to cell death in cerebellar granule neurons. 18 However, in many experiments IL-6 has been shown to exhibit neurotrophic/survival actions.39–42 Although IL-6 appears to be a dichotomic cytokine or a doubleedged cytokine,43,44 in this study we treated IL-6 as an inflammatory cytokine.
These inflammatory cytokines have been thought to be induced together in the pathological state of the brain through a common mechanism. However, these points have not been systematically analyzed and have remained ambiguous. Thus, in this study we aimed to clarify these points.
Various LPS injection methods, including intraperitoneal, intraventricular, intracerebral, and subcutaneous injection, have been used as models for inducing inflammatory cytokines in vivo. In this study, we used an intracerebral injection method in which endotoxin LPS was injected into the adult rat cerebral cortex (Figure 1). LPS is a component of the cell membrane in Gram-negative bacteria.4546 and can mimic bacterial infection. In this model, TNFα, IL-1β, and IL-6 were actually detected in the LPS/PBS-injected region. Thus, this animal model was found to be effective for analyzing the induction of inflammatory cytokines.
As shown in this study, TNFα, IL-1β, and IL-6 were transiently induced at 6–12 h after LPS injection (Figure 2). Initially, we speculated that these inflammatory cytokines were produced by activated astrocytes and/or growing microglia, consistent with the upregulated GFAP and/or Iba1 levels. However, the inflammatory cytokines were induced at 6–12 h following LPS injection, when the levels of GFAP/Iba1 were not elevated (Figure 2A,C). PCNA, an indicator of the S phase, was not detected at 6–12 h post-LPS injection. This suggested that the glial proliferation did not occur at that time point (Figure 2C). It was at 3 days that microglia actually expressed PCNA and proliferated in response to LPS (Figure 2D). A leukocyte (neutrophil) marker, L-selectin, was slightly detected at 12 h after LPS-injection (Figure 2C), suggesting that some neutrophils infiltrated in the LPS-injected region. Although neutrophils have long been believed not to produce inflammatory cytokines, in recent year they were recognized to produce some kinds of cytokines. 47 Thus, we considered that some inflammatory cytokines (TNFα, IL-1β, and IL-6) detected at 6–12 h post-LPS injection may be derived from the leukocytes (neutrophils). On the other hand, the profile of CD68 suggested that monocytes/macrophages infiltrated at 24 h-3 days after LPS injection but not at 6–12 h (Figure 2C). On the basis of these results, we speculated that the inflammatory cytokines (TNFα, IL-1β, and IL-6) detected at an early point (6–12 h) were induced primarily by functionally activated microglia and/or astrocytes prior to their upregulation of Iba1/GFAP levels. In fact, our immunohistochemical method indicated that the three inflammatory cytokines were predominantly produced by microglia in vivo (Figure 3).
Regarding glial activation and its relevance to the production of inflammatory cytokines, many studies have been carried out and discussed. For instance, Norden et al. 48 reported that LPS challenge induced inflammatory cytokine mRNA in microglia at 2–4 h and in astrocytes at 12 h. On the other hand, this LPS stimulation caused an increase in Iba1 immunoreactivity at 24–48 h after LPS administration. These profiles roughly resembled our results in which the inflammatory cytokines were induced at 6–12 h but Iba1 increased at 24 h-3 days post-LPS injection (Figure 2). Bowyer et al. 49 observed that the size of microglia was changed at 6–12 h after LPS stimulation in rat into which LPS had been subcutaneously injected. This report indicated that microglia were morphologically activated at 6–12 h after LPS administration and also supported our results that microglia are functionally activated and induce inflammatory cytokines at 6–12 h post-LPS injection prior to the upregulation of Iba1 (Figure 2). In intraventricularly LPS-infused brain, Quan et al. 50 reported that IL-1 was detected in the brainstem and diencephalon at 2 h and in all the regions (except the cerebellum) at 6 h after LPS injection. This is generally consistent with our results indicating that inflammatory cytokines were induced 6–12 h post-LPS administration. Hang et al. 51 reported that TNFα and IL-6 were upregulated as early as 3 h after LPS administration in the rat cortex. The relatively early induction of inflammatory cytokines by LPS-stimulation is similar to our results. Herber et al. 52 analyzed microglial responses in LPS-injected mouse hippocampus and observed that microglia were activated for 28 days after LPS injection. We also observed a similar phenomenon in which the level of Iba1 was enhanced even 2 weeks post-LPS injection. However, at 1–2 weeks post-LPS injection, no significant amount of inflammatory cytokines was detected. Hong et al. 53 immunohistochemically analyzed the expression of TNFα and IL-1β in LPS-administered mouse cerebellum and demonstrated that these cytokines were co-localized mainly with Iba1-positive cells and were rarely co-localized with GFAP-positive cells. Their results showed that, unlike with our results, some astrocytes expressed IL-1β in the cerebellum. However, this issue can be explained as a difference between species. Regarding the ability of astrocytes/microglia to produce inflammatory cytokines, there is a difference between rat and mouse. For example, Tarassishin et al. 54 reported that astrocytes in mouse can produce an amount of IL-1β in response to LPS, but rat astrocytes do not have the ability to produce the cytokine, as shown in this study (Figure 4). Consequently, the LPS injection experiment in vivo clarified that inflammatory cytokines including TNFα, IL-1β, and IL-6 were induced at an early time (at 6–12 h post-LPS injection) mainly by functionally activated microglia/astrocytes and partially by infiltrated neutrophils, after which (at 24 h-3 days post-LPS injection) microglial proliferation, astrocytic activation, and infiltration of monocytes/macrophages would occur.
Since the results of the in vivo study above suggested that glial cells are a primary producer of inflammatory cytokines, we examined the ability of astrocytes/microglia to induce TNFα, IL-1β, and IL-6 in vitro (Figure 4), and demonstrated that microglia are the main cell type inducing these cytokines in the LPS-stimulated condition (Figure 4). In this condition, astrocytes did not induce IL-1β or IL-6 and did induce only a limited amount of TNFα (Figure 4). Thus, microglia are suggested to be a main producer of inflammatory cytokines in vivo.
We subsequently investigated signaling molecules involved in the induction of inflammatory cytokines in microglia. First, we demonstrated that a specific combination of MAPKs is related to the induction of each inflammatory cytokine in LPS-stimulated microglia (Figure 5). MAPKs are Ser/Thr kinases, and are involved in various biological responses including metabolism, growth, differentiation, apoptosis, and stress. MAPKs generally include ERK, JNK, and p38MAPK.55–57 In the present study, we found that JNK/p38MAPK, ERK/JNK, and ERK/JNK/p38MAPK were necessary for the induction of TNFα, IL-1β, and IL-6, respectively, in LPS-stimulated microglia (Figure 5A-C). Thus, it is notable that the data showed that a specific combination of MAPKs distinguishes the expression of each inflammatory cytokine.
We next showed the necessity of PKCα in the induction of inflammatory cytokines (Figure 5D-F). PKC is a member of the family of Ser/Thr kinases, many of which need diacyl glycerol as an activator; and its involvement in various cellular functions, such as cytokine release and proliferation, has been shown.58–60 PKC is generally classified into three groups: conventional (cPKC), novel (nPKC), and atypical (aPKC). Thus far, we detected PKCα (cPKC), PKC δ, ε, (nPKC), and PKCι, ζ, ρ (aPKC) in our primary microglia. Among cPKCs, PKCβ and PKCγ (neuron specific) were not detected (not shown). The inhibition experiments revealed that PKCα is involved in the induction of TNFα, IL-1β, and IL-6 in LPS-stimulated microglia (Figure 5A-C). Thus, PKCα is thought to be a main cPKC functioning in the signaling cascade and leading to the induction of inflammatory cytokines in primary microglia. LPS signaling starts by forming the LPS/LBP/CD14/TLR4 complex on the cell surface, 61 and the signaling is propagated downstream and activates transcription factors including nuclear factor kB (NFκB) and interferon regulatory factor (IRF).62,63 Somewhere in the signaling transduction processes, PKCα may serve to induce TNFα, IL-1β, and IL-6 in microglia.
We previously examined the role of NFκB in the production of inflammatory cytokines in LPS-stimulated microglia. 64 When microglia were stimulated with LPS, TNFα and IL-1β were induced, and simultaneously IκBα and IκBβ were degraded time-dependently, suggesting the occurrence of NFκB activation. These results allowed us to investigate the relation between the induction of TNFα/IL-1β and the NFκB activation. As a result, we found that the NFκB inhibitor ammonium pyrrolidine dithiocarbamate (APDC) 65 suppressed the induction of IL-1β, but not that of TNFα, in LPS-stimulated microglia. In a separate experiment, we clarified that LPS-dependent IL-6 induction was not downregulated by pretreatment with APDC. We thus concluded that NFκB is associated with the induction of IL-1β in LPS-stimulated microglia.
Furthermore, we showed that NO is associated with the induction of IL-1β/IL-6 in LPS-stimulated microglia (Figure 6). Administration of NO scavenger in LPS-stimulated microglia significantly reduced the induction of IL-1β/IL-6 (Figure 6B,6C), suggesting an association between NO and a signaling cascade inducing IL-1β/IL-6. On the other hand, in microglia the NO generator induced IL-1β but not IL-6 (Figure 6E), suggesting that NO can directly activate a signaling cascade inducing IL-1β. In a signaling cascade inducing IL-6, a certain requisite component serving downstream of NO may be lacking in nonstimulated microglia, and thus NO alone could not stimulate the cascade even if generated.
We indicated here that microglia are a major glial cell type responsible for the induction of inflammatory cytokines, and that their induction of each inflammatory cytokine advances via a different signaling pathway. We believe that the results will contribute to a study controlling inflammation in vivo and/or a study leading to the amelioration or improvement of inflammation in the pathological state of the brain.
Conclusions
We demonstrated that microglia induce inflammatory cytokines including TNFα, IL-1β, and IL-6 in response to LPS in vivo. Each inflammatory cytokine was found to be induced by a specific combination of MAPKs. PKCα was required in LPS-dependent TNFα, IL-1β, and IL-6 induction. NO was suggested to be involved in the induction of IL-1β and IL-6 in LPS-stimulated microglia.
Together, the previous and present results strongly suggested that microglia in vivo are the major cell type for inducing inflammatory cytokines including TNFα, IL-1β, and IL-6, each of which is separately induced by the function of a specific combination of MAPKs and the presence or absence of NO.
Acknowledgements
We thank Yoko Tohyama for her excellent technical assistance.
Author biographies
Takashi Ishijima is a graduate student in Graduate school of Science and Engineering, Soka University. His research interests include “Signaling mechanism in injured nervous system”.
Kazuyuki Nakajima received his PhD in the medical school in 1986. He is a Professor in Glycan & Life Systems Integration Center (GaLSIC), Soka University, Tokyo, Japan. His research interests include “Interaction between neurons and glial cells in the nervous system”.
Footnotes
Author contribution: T. Ishijima performed the operation on the animals and was primarily responsible for analyzing the TNFα, IL-1β, and IL-6 proteins by immunoblotting. K. Nakajima participated in designing the study and performed some supporting experiments.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Kazuyuki Nakajima https://orcid.org/0000-0003-4108-6018
References
- 1.Dickson DW, Lee SC, Mattiace LA, et al. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's Disease. Glia 1993; 7: 75–83. [DOI] [PubMed] [Google Scholar]
- 2.Patterson PH. Cytokines in Alzheimer's Disease and multiple sclerosis. Curr Opin Neurobiol 1995; 5: 642–646. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev 1995; 21: 195–218. [DOI] [PubMed] [Google Scholar]
- 4.Phani S, Loike JD, Przedborski S. Neurodegeneration and inflammation in Parkinson's Disease. Parkinsonism Relat Disord 2012; 18: S207–S209. [DOI] [PubMed] [Google Scholar]
- 5.Stojkovska I, Wagner BM, Morrison BE. Parkinson's disease and enhanced inflammatory response. Exp Biol Med (Maywood) 2015; 240: 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans MC, Couch Y, Sibson N, et al. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci 2013; 53: 34–41. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol 2001; 14: 89–94. [DOI] [PubMed] [Google Scholar]
- 8.Slowik A, Lammerding L, Hoffmann S, et al. Brain inflammasomes in stroke and depressive disorders: regulation by oestrogen. J Neuroendocrinol 2018; 30: 1–11. doi: 10.1111/jne.12482 [DOI] [PubMed] [Google Scholar]
- 9.Arvin B, Neville LF, Barone FC, et al. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev 1996; 20: 445–452. [DOI] [PubMed] [Google Scholar]
- 10.Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury 2003; 34: 397–404. [DOI] [PubMed] [Google Scholar]
- 11.Bergold PJ. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp Neurol 2016; 275 Pt 3: 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol 1988; 23: 339–346. [DOI] [PubMed] [Google Scholar]
- 13.Venters HD, Dantzer R, Kelley KW. A new concept in neurodegeneration: tNFalpha is a silencer of survival signals. Trends Neurosci 2000; 23: 175–180. [DOI] [PubMed] [Google Scholar]
- 14.Lu KT, Wang YW, Yang JT, et al. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma 2005; 22: 885–895. [DOI] [PubMed] [Google Scholar]
- 15.Long-Smith CM, Collins L, Toulouse A, et al. Interleukin-1β contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J Neuroimmunol 2010; 226: 20–26. [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Huang Y, Zhao L, et al. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem 2013; 125: 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S, Friedman WJ. Interleukin-1β enhances neuronal vulnerability to proNGF-mediated apoptosis by increasing surface expression of p75(NTR) and sortillin. Neuroscience 2014; 257: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conroy SM, Nguyen V, Quina LA, et al. Interleukin-6 produces neuronal loss in developing cerebellar granule neuron cultures. J Neuroimmunol 2004; 155: 43–54. [DOI] [PubMed] [Google Scholar]
- 19.Pang L, Sawada T, Decker SJ, et al. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem 1995; 270: 13585–13588. [DOI] [PubMed] [Google Scholar]
- 20.Kramer RM, Roberts EF, Um SL, et al. P38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J Biol Chem 1996; 271: 27723–27729. [DOI] [PubMed] [Google Scholar]
- 21.Eichholtz T, de Bont DB, de Widt J, et al. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem 1993; 268: 1982–1986. [PubMed] [Google Scholar]
- 22.Bennett BL, Sasaki DT, Murray BW, et al. SP600125, An anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 2001; 98: 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer S, Leopold E, Hemmens B, et al. Interference of carboxy-PTIO with nitric oxide- and peroxynitrite-mediated reactions. Free Radic Biol Med 1997; 22: 787–794. [DOI] [PubMed] [Google Scholar]
- 24.Barrachina D, Calatayud S, Esplugues J, et al. Nitric oxide donors preferentially inhibit neuronally mediated rat gastric acid secretion. Eur J Pharmacol 1994; 262: 181–183. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier, 1982. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–275. [PubMed] [Google Scholar]
- 27.Oshiro S, Kawamura K, Zhang C, et al. Microglia and astroglia prevent oxidative stress-induced neuronal cell death: implications for aceruloplasminemia. Biochim Biophys Acta 2008; 1782: 109–117. [DOI] [PubMed] [Google Scholar]
- 28.Maeda S, Nakajima K, Tohyama Y, et al. Characteristic response of astrocytes to plasminogen/plasmin to upregulate transforming growth factor beta 3 (TGF(3) production/secretion through proteinase-activated receptor-1 (PAR-1) and the downstream phosphatidylinositol 3-kinase (PI3K)-Akt/PKB signaling cascade. Brain Res 2009; 1305: 1–13. [DOI] [PubMed] [Google Scholar]
- 29.Ianaro A, O'Donnell CA, Di Rosa M, et al. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology 1994; 82: 370–375. [PMC free article] [PubMed] [Google Scholar]
- 30.Pahan K, Sheikh FG, Namboodiri AM, et al. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 1997; 100: 2671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 2003; 116: 3051–3060. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto S, Nakajima K, Kohsaka S. Macrophage-colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. J Neurochem 2010; 115: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 33.Dijkstra J, van Galen M, Scherphof G. Effects of (dihydro)cytochalasin B, colchicine, monensin and trifluoperazine on uptake and processing of liposomes by kupffer cells in culture. Biochim Biophys Acta 1985; 845: 34–42. [DOI] [PubMed] [Google Scholar]
- 34.Damoiseaux JG, Döpp EA, Calame W, et al. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology 1994; 83: 140–147. [PMC free article] [PubMed] [Google Scholar]
- 35.Ivetic A. A head-to-tail view of L-selectin and its impact on neutrophil behaviour. Cell Tissue Res 2018; 371: 437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelbard HA, Dzenko KA, DiLoreto D, et al. Neurotoxic effects of tumor necrosis factor alpha in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci 1993; 15: 417–422. [DOI] [PubMed] [Google Scholar]
- 37.Chao CC, Hu S. Tumor necrosis facror-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci 1994; 16: 172–179. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi H, Jin S, Wang J, et al. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 2006; 281: 21362–21368. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, Hatanaka H. Interleukin-6 protects cultured rat hippocampal neurons against glutamate-induced cell death. Brain Res 1994; 643: 173–180. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda S, Wen TC, Morita F, et al. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett 1996; 204: 109–112. [DOI] [PubMed] [Google Scholar]
- 41.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 1998; 18: 176–179. [DOI] [PubMed] [Google Scholar]
- 42.Their M, März P, Otten U, et al. Interleukin-6 (IL-6) and its soluble receptor support survival of sensory neurons. J Neurosci Res 1999; 55: 411–422. [DOI] [PubMed] [Google Scholar]
- 43.Gadient RA, Otten UH. Interleukin-6 (IL-6)–a molecule with both beneficial and destructive potentials. Prog Neurobiol 1997; 52: 379–390. [DOI] [PubMed] [Google Scholar]
- 44.Spooren A, Kolmus K, Laureys G, et al. Interleukin-6, a mental cytokine. Brain Res Rev 2011; 67: 157–183. [DOI] [PubMed] [Google Scholar]
- 45.Galanos C, Freudenberg MA. Bacterial endotoxins: biological properties and mechanisms of action. Mediators Inflamm 1993; 2: S11–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston A, Mandrell RE, Gibson BW, et al. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit Rev Microbiol 1996; 22: 139–180. [DOI] [PubMed] [Google Scholar]
- 47.Kasama T, Miwa Y, Isozaki T, et al. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy 2005; 4: 273–279. [DOI] [PubMed] [Google Scholar]
- 48.Norden DM, Trojanowski PJ, Villanueva E, et al. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2016; 64: 300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowyer JF, Sarkar S, Burks SM, et al. Microglial activation and responses to vasculature that result from an acute LPS exposure. Neurotoxicology 2020; 77: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol 1994; 49: 125–134. [DOI] [PubMed] [Google Scholar]
- 51.Hang C-h, Shi J-x, Tian J, et al. Effect of systemic LPS injection on cortical NF-kappaB activity and inflammatory response following traumatic brain injury in rats. Brain Res 2004; 1026: 23–32. [DOI] [PubMed] [Google Scholar]
- 52.Herber DL, Maloney JL, Roth LM, et al. Diverse microglial responses after intrahippocampal administration of lipopolysaccharide. Glia 2006; 53: 382–391. [DOI] [PubMed] [Google Scholar]
- 53.Hong J, Yoon D, Nam Y, et al. Lipopolysaccharide administration for a mouse model of cerebellar ataxia with neuroinflammation. Sci Rep 2020; 10: 13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarassishin L, Suh H-S, Lee SC. LPS And IL-1 differentially activate mouse and human astrocytes: role of CD14. Glia 2014; 62: 999–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Liu HT. MAPK Signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12: 9–18. [DOI] [PubMed] [Google Scholar]
- 56.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912. [DOI] [PubMed] [Google Scholar]
- 57.Strnisková M, Barancík M, Ravingerová T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen Physiol Biophys 2002; 21: 231–255. [PubMed] [Google Scholar]
- 58.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of proteinkinase C. Science 1992; 258: 607–614. [DOI] [PubMed] [Google Scholar]
- 59.Quest AF. Regulation of protein kinase C: a tale of lipids and proteins. Enzyme Protein 1996; 49: 231–261. [DOI] [PubMed] [Google Scholar]
- 60.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J 1999; 13: 1658–1676. [PubMed] [Google Scholar]
- 61.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013; 45: e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu YC1, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42: 145–151. [DOI] [PubMed] [Google Scholar]
- 63.O'Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors - redefining innate immunity. Nat Rev Immunol 2013; 13: 453–460. [DOI] [PubMed] [Google Scholar]
- 64.Nakajima K, Matsushita Y, Tohyama Y, et al. Differential suppression of endotoxin-inducible inflammatory cytokines by nuclear factor kappa B (NFkappaB) inhibitor in rat microglia. Neurosci Lett 2006; 401: 199–202. [DOI] [PubMed] [Google Scholar]
- 65.Schreck R, Meier B, Männel DN, et al. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med 1992; 175: 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]