Abstract

Efforts to lower the operating temperature of solid oxide fuel cells include producing electrolytes that are sufficiently conductive and stable below 600 °C. Doped ceria is one such electrolyte being considered. During this study, codoped ceria powders (Ce0.8Sm0.2–xMxO2−δ, M = Bi3+, Zn2+ and x = 0, 0.05, 0.1, 0.15, 0.2) were prepared via coprecipitation by the addition of sodium carbonate and annealed at 800 and 1200 °C, respectively. Poor solubility of the codopants in the ceria was observed for samples annealed at 800 °C, resulting in a mixed-phase product including stable phases of the oxides of these codopants. A second-stage partial incorporation of these codopants into the ceria lattice was observed when the annealing temperature was increased to 1200 °C, with both codopants forming cubic-type phases of their respective oxides. Materials were characterized using X-ray diffraction (XRD), Raman spectroscopy, and Fourier transform infrared spectroscopy (FTIR), as well as scanning electron microscopy (SEM) for structural and morphological investigations. The oxide ion conductivity was evaluated using electrochemical impedance spectroscopy between 550 and 750 °C. Fuel cell performance tests of selected samples (annealed at 1200 °C) showed remarkable improvement in peak power densities when the test temperature was increased from 500 to 600 °C (∼720 mW/cm2 for Ce0.8Sm0.15Bi0.05O2−δ and ∼1230 mW/cm2 for Ce0.8Sm0.15Zn0.05O2−δ), indicating possible contribution from the distinct cubic-type oxide phases of the codopants in performance enhancement.
Keywords: fuel cell, electrolyte, codoping, phase change, conductivity, power density
1. Introduction
Solid oxide fuel cells (SOFCs) can have a huge positive impact on the world’s changing environment due to global warming and play an important role in realizing the United Nations’ Sustainable Development Goal 7, calling to “ensure access to affordable, reliable, sustainable and modern energy for all”.1 The ability of SOFCs to operate directly on hydrocarbon fuels eliminates the need for reformers, making them appealing for commercial applications.2,3 This is possible if the bottlenecks toward their commercialization can be tackled, one of them being their high operating temperature (800–1000 °C), which itself brings associated disadvantages, such as rapid deterioration of cell components and their compatibility. A component of prime importance is the electrolyte that plays a significant role in determining the SOFC operating temperature. Traditionally used yttria-stabilized zirconia (YSZ) shows acceptable ionic conduction but only at high temperature (≥800 °C), with an unsatisfactory conductivity at low temperature (≤600 °C).4,5
Ceria is an important oxide ion conductor. However, it shows poor conductivity in pure form at low temperature (≤600 °C).6 Doped ceria has received much attention in recent decades for electrolytic applications in SOFCs due to its notable ionic conductivity in the low-temperature regime (600 °C or lower) and good compatibility with fuel electrode materials.7 Nanostructured ceria shows high thermal stability as well as catalytic activity and impedes carbon formation when hydrocarbon fuels are used.8,9 Although Ce0.8Sm0.2O2−δ (SDC) has demonstrated impressive oxide ion conductivity with appreciable fuel cell performance,10 efforts have been made and are still in progress to introduce codopants to replace costly rare earth constituent metals and to further enhance the ion migration activity at lower temperature while improving other features like density, electrode–electrolyte compatibility, durability, and performance.11−13 Various examples exist where ceria doped with aliovalent cations has shown appreciable results.11,12,14,15 For example, Wu et al.14 observed notable ionic conduction for aliovalent cations (Sm3+, Mg2+, Ca2+, Sr2+, Ba2+) codoped ceria electrolytes. Ceria carbonate composite electrolytes have attracted considerable attention in recent years for low-temperature applications due to the possible role of carbonate in conductivity enhancement. A novel core–shell SDC/Na2CO3 composite electrolyte prepared by Wang et al.16 obtained a conductivity of over 0.1 S/cm at a temperature slightly above 300 °C, ascribing it to the development of an interface between carbonate and ceria phases for fast ion transport. Various mechanisms of oxide ion transport through carbonate are discussed in (17). A unique advantage of carbonate is that it allows hybrid conduction of ions (H+/O2–),18 and the presence of carbonate around ceria transforms it into a multi-ion [O2–, H+, CO32–] conductive electrolyte.19 Apart from its beneficial role in conductivity enhancement, it acts as a sintering aid, a pore filler to increase the density of the electrolytic material, an electron insulator, an electrode/electrolyte glue, and an accelerator for the oxygen reduction reaction.20
Bi2O3 received a lot of attention for SOFC electrolytic applications due to its exceptionally high oxide ion conductivity, but due to certain serious drawbacks, interest has declined.21 Some studies attempted to dope Bi3+ into the ceria lattice or introduce Bi3+ into ceria electrolytes to make use of the high oxide conducting ability of Bi2O3.22,23 For example, Zhao et al.22 observed a notable increase in oxide transport by incorporating a small amount of Bi3+ into SDC (conductivity of Ce0.8Sm0.1Bi0.1O1.9 ≈ 3.98 × 10–2 S/cm at 750 °C) compared to that of SDC (conductivity of Ce0.8Sm0.2O1.9 ≈ 2.2 × 10–2 S/cm at 750 °C). However, Bi3+ solubility in the ceria lattice, and hence the formation of a single-phase Bi3+-doped ceria system, is dependent on temperature and the synthetic methodology. As noted by Accardo et al.,23 limited solubility of Bi3+ in the ceria lattice is expected because of the general violation of the Hume–Rothery rules for solid solutions, also applicable to ceramic systems. Moreover, the gradual evolution of Bi3+-related phases was observed for CeO2–Bi2O3 systems over time or upon high-temperature treatment.
Among the transition metals, Zn2+ has hardly been considered as a dopant or codopant for ceria electrolytes, which may also be due to its expected limited solubility in the ceria lattice according to the Hume–Rothery rules. There are reports of using ZnO as a sintering aid since it lowers the sintering temperature as well as enhances the grain boundary conductivity in doped ceria.24,25 Ge et al.24 showed that a 1 mol % addition of ZnO to Ce0.8Ln0.2O2−δ (Ln = Y, Sm, Gd) lowered the sintering temperature by ∼200 °C and enhanced the grain boundary conductivity.
This study attempts to investigate the effect of Bi3+ and Zn2+ as codopants for Ce0.8Sm0.2–xMxO2−δ (where M = Bi3+ or Zn2+ and x = 0.05–0.2). The structure–property relationship, where the ionic conductivity is the property of interest for electrolytic applications, was studied for the codoped samples and compared to that of Ce0.8Sm0.2O2−δ. The anticipated role of carbonate in enhancing ionic conductivity was also considered.
2. Experimental Section
The solid solutions of Ce0.8Sm0.2–xMxO2−δ/carbonate (M = Bi3+ or Zn2+ where x = 0, 0.05, 0.1, 0.15, and 0.2) were prepared using the coprecipitation route. For Ce0.8Sm0.2–xBixO2−δ/carbonate where x = 0, 0.05, 0.1, 0.15, and 0.2, the sample IDs used are SBC0, SBC5, SBC10, SBC15, and SBC20, respectively. Similarly, SZC0, SZC5, SZC10, SZC15, and SZC20 were used for Ce0.8Sm0.2–xZnxO2−δ/carbonate. The precursors Ce(NO3)3·6H2O, Sm(NO3)3·6H2O, and Bi(NO3)3·5H2O or Zn(NO3)2·6H2O (Sigma-Aldrich, 99.99% purity) were mixed according to their stoichiometric amounts in deionized water and stirred to dissolve all salts, followed by the dropwise addition of 2 mol L–1 of sodium carbonate solution (Sigma-Aldrich and 99.99% pure) to yield a total metal-ion-to-carbonate mole ratio of 1:2. The white precipitates obtained by vacuum filtration were dried in an oven at 120 °C and annealed at 400 °C for 4 h. The samples were then annealed at 800 °C, and part of the sample was further annealed at 1200 °C (for 4 h at each step). The obtained samples were ground into fine powders using a mortar and pestle for further characterization. A schematic of the synthetic method is shown in Figure S1.
3. Sample Characterization
The crystal structure was investigated using powder X-ray diffraction (XRD, Bruker D8, Germany) with Cu Kα radiation (λ = 1.54056 Å). Scanning electron microscopy (SEM, ThermoScientific Verios G4 UC or Regulus 8230, depending on availability) was used to record the micrographs of prepared samples. Raman spectrometry was also used to probe the structure of the powder samples (Renishaw inVia Reflex, λ = 532 nm). Fourier transform infrared spectroscopy (FTIR, ThermoFisher Scientific, Nicolet 6700) in the range from 4000 to 400 cm–1 was performed using the KBr disc method. The electrical properties of prepared pelletized samples were measured between 550 and 750 °C using electrochemical impedance spectroscopy (CorrTest Electrochemical using Corrosion Studio, Ver. 5.3, China) over a frequency range of 0.1 Hz–1 MHz and applying an amplitude of 10 mV. Circular pellets were prepared by pressing the powdered samples in an 11 mm diameter die at 250 MPa and then sintering in air for 4 h at 800 °C and 1200 °C depending on the annealing temperature. Pt paste was coated on both sides for current collection. Zview (Scribner) was used for equivalent circuit fitting.
3.1. Fuel Cell Measurement
Fuel cell performance (between 500 and 600 °C) was determined by a dc electronic load instrument (ITECH8511, ITECH Electrical Co., Ltd.). For this purpose, symmetrical button cells were prepared by sandwiching powders of selected samples (SBC5 and SZC5 annealed at 1200 °C) between commercially purchased Ni0.8Co0.15Al0.05LiO2−δ (NCAL) electrodes (for both the anode and the cathode) and pressed in a 13 mm diameter die at 250 MPa. The button cells (∼1 mm thick with an active area of 0.64 cm2) were sintered at 600 °C for 1 h to dry and densify the Ag paste before testing. The performance of fuel cell was recorded at an elevated temperature using hydrogen as a fuel and air as an oxidant under laboratory conditions. Hydrogen (fuel, 99% pure) was fed at the anode with a flow rate of 120 mL/min, while air (oxidant) was supplied at the cathode with a flow rate of 100 mL/min.
4. Results and Discussion
4.1. Powder X-ray Diffraction (PXRD)
The room-temperature XRD diffraction patterns of SBC and SZC samples annealed at 800 °C are shown in Figure 1a,b. The diffraction patterns characteristic of face-centered cubic (fcc) ceria (space group Fm3̅m) are shown by all samples (ICSD # 00–034–0394).26 However, additional phases appear when larger amounts of Bi3+ or Zn2+ were added (above ∼10 mol %), which corresponded to a monoclinic α-Bi2O3 (space group P21/c; ICSD # 00–041–1449)26 for SBC and a hexagonal wurtzite ZnO (space group P63mc; ICSD # 00–036–1451)26 for SZC. This indicates that for the prepared samples that were annealed at 800 °C, the doping limit of the codopants (Bi3+ or Zn2+) into the Sm3+-doped ceria is ∼5 mol % (certainly less than 10 mol %) and the rest separates out to form a stable room-temperature α-Bi2O3 or ZnO phases. To assess the effect of higher-temperature annealing on the incorporation of codopants (Bi3+, Zn2+) into Sm3+-doped ceria, all samples were further annealed at 1200 °C and XRD patterns were recorded as depicted in Figure 1c,d. All samples now indicate the presence of only the fcc phase with no extra peaks. None of the XRD results picked up a carbonate phase, probably because this would be more amorphous.
Figure 1.
Room-temperature XRD patterns of samples annealed at 800 °C, showing the major fcc phase (indexed peaks) and the minor phases of (a) α-Bi2O3 in the SBC samples and (b) ZnO in the SZC samples as indicated. Upon further annealing at 1200 °C, the XRD patterns indicate a pure fcc phase for both (c) SBC and (d) SZC samples across the dopant range.
The lattice parameters of the fcc phase were calculated
using 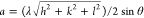 , and the results are presented in Table 1. As the ionic radius
of Bi3+ (1.17 Å27) in an
octahedral oxide arrangement is greater than that of Ce4+ (0.97 Å27) and Sm3+ (1.079
Å27), a slight systematic increase
in the lattice parameter would have been expected as the amount of
Bi3+ was increased with a corresponding decrease in Sm3+. Instead, a decrease in the lattice parameter was observed.
For SBC samples annealed at 800 °C, Bi3+ was not fully
incorporated in the ceria lattice due to the formation of the α-Bi2O3 phase and the decreasing Sm3+ content
would justify the decrease in lattice parameter. Even for SBC5, the
lattice parameter decreased, which may mean that not all of the added
Bi3+ was incorporated in the lattice and that any additional
phase was below the detection limit of XRD.
, and the results are presented in Table 1. As the ionic radius
of Bi3+ (1.17 Å27) in an
octahedral oxide arrangement is greater than that of Ce4+ (0.97 Å27) and Sm3+ (1.079
Å27), a slight systematic increase
in the lattice parameter would have been expected as the amount of
Bi3+ was increased with a corresponding decrease in Sm3+. Instead, a decrease in the lattice parameter was observed.
For SBC samples annealed at 800 °C, Bi3+ was not fully
incorporated in the ceria lattice due to the formation of the α-Bi2O3 phase and the decreasing Sm3+ content
would justify the decrease in lattice parameter. Even for SBC5, the
lattice parameter decreased, which may mean that not all of the added
Bi3+ was incorporated in the lattice and that any additional
phase was below the detection limit of XRD.
Table 1. Lattice Parameters (a in Å) for SBC and SZC Samples with Varied Dopant Concentrations That Were Annealed at 800 and 1200 °C, Respectively.
| sample | 800 °C | 1200 °C | sample | 800 °C | 1200 °C |
|---|---|---|---|---|---|
| SBC0 | 5.435 | 5.432 | SZC0 | 5.435 | 5.432 |
| SBC5 | 5.418 | 5.431 | SZC5 | 5.427 | 5.447 |
| SBC10 | 5.406 | 5.423 | SZC10 | 5.419 | 5.421 |
| SBC15 | 5.402 | 5.421 | SZC15 | 5.400 | 5.427 |
| SBC20 | 5.401 | 5.405 | SZC20 | 5.391 | 5.403 |
When annealed at 1200 °C, the lattice parameters for SBC0 and SBC5 showed a very small difference indicating the incorporation of more Bi3+ into the ceria lattice. In fact for SBC5–SBC20, the lattice parameter increased when annealed at 1200 °C with respect to that for the samples annealed at 800 °C. This supports that the higher annealing temperature results in more successful doping of Bi3+ into the ceria lattice. SBC20 exhibited the smallest difference in the lattice parameter at the two annealing temperatures, which could indicate that a dissolution limit was reached and could also point to the role of Sm3+ in the lattice assisting in the incorporation of Bi3+ into the ceria lattice.
Different phases of Bi2O3 (e.g., α (monoclinic), β (tetragonal), γ (body-centered cubic), and δ (fcc)) are stable in different temperature ranges. α-Bi2O3 changes to δ-Bi2O3 at 730 °C,28 and this δ-phase can be stabilized to room temperature by doping.29,30 For the SBC samples annealed at 1200 °C, it is thus also possible that the small amount of the α-Bi2O3 phase was transformed into a δ-Bi2O3 phase and stabilized by the incorporation of Sm3+ or Ce4+ into the Bi2O3 lattice producing XRD peaks that would overlap with that of the fcc ceria phase. However, further characterization is required to verify this.
The SZC samples showed similar behavior to that of SBC. The ionic radius of Zn2+ (0.90 Å27) is smaller than that of Ce4+ and Sm3+; thus, an increasing amount of Zn2+ added (with a simultaneous decrease in Sm3+) would result in a decrease in the lattice constant. For samples annealed at 800 °C, the expected decrease in lattice parameter was observed. However, this clearly does not imply the successful incorporation of Zn2+ in the ceria lattice as the formation of a hexagonal wurtzite ZnO phase was observed to a small extent for SZC10–SZC20 and the decreasing Sm3+ content alone would cause a decrease in the cell parameter. Upon further annealing at 1200 °C, the hexagonal phase disappeared but the lattice parameters unexpectedly increased for all Zn2+-containing samples. Lin et al.31 also observed an expansion of the lattice parameter for 20 mol % of Zn-doped ceria upon increasing the annealing temperature (to 1300 °C). They suggested that Zn2+ could be incorporated into the ceria lattice either by substituting Ce4+ to create oxygen vacancies or by occupying interstitial sites causing the lattice to expand.
Lin et al.31 also noted that some Zn2+ crystallized out as ZnO for Zn-doped ceria calcined at 800 °C. After annealing at 1300 °C, a second-stage incorporation into the host ceria was observed, but using synchrotron-based XRD, they proved that the second-stage incorporation was still incomplete and the hexagonal ZnO phase was still present, although at much lower concentrations. It has also been shown that a cubic zinc-blende ZnO structure (space group F4̅3m) can be stabilized by growth on cubic substrates or with high-temperature and -pressure treatments.32−34 It is thus possible that as the SZC samples were annealed at 1200 °C, the wurtzite ZnO could also have transformed into a cubic-type ZnO where the XRD peaks would overlap with that for the doped ceria.
4.2. Raman Spectroscopy
Figure 2a,b shows the room-temperature Raman spectra of SBC samples annealed at 800 and 1200 °C, respectively. For samples annealed at 800° C, the F2g band can be seen to shift from 460.6 to 462.5 cm–1 (see Table 2) as the amount of Bi3+ added was increased together with a decrease in the amount of Sm3+ added. For pure ceria, the F2g band was reported to occur at 462 cm–1 by Hebert and Stöwe35 and 465 cm–1 by Shirbhate et al.,36 noting that the morphology of the material can also affect the position of the band to a small extent.37 According to Sun et al.,38 ceria can compensate for high levels of oxygen vacancies due to the substitution of lower-valent elements in cation sublattice, leading to increased oxide ion conductivity. With the limited incorporation of Bi3+ in the ceria lattice, as evidenced by the presence of the α-Bi2O3 phase for SBC10–SBC20, it is expected that the F2g band positions would be based mostly on the systematically decreasing Sm3+ content; hence, they shift towards the peak position for ceria. A similar trend was noted for the samples annealed at 1200 °C, with the F2g bands occurring at a slightly higher wavenumber (see Table 2).
Figure 2.
Raman spectra of SBC (a, b) and SZC (c, d) samples annealed at 800 and 1200 °C, respectively. The insets show enlarged regions of the spectra.
Table 2. F2g Band Positions (in cm–1) with Varying Compositions for SBC and SZC Samples Annealed at 800 and 1200 °C, Respectively.
| sample | 800 °C | 1200 °C | sample | 800 °C | 1200 °C |
|---|---|---|---|---|---|
| SBC0 | 460.9 | 461.5 | SZC0 | 460.9 | 461.5 |
| SBC5 | 460.9 | 462.7 | SZC5 | 460.9 | 462.5 |
| SBC10 | 461.5 | 463.3 | SZC10 | 460.9 | 462.8 |
| SBC15 | 461.9 | 464.1 | SZC15 | 462.6 | 464.3 |
| SBC20 | 462.4 | 465.5 | SZC20 | 464.3 | 465.5 |
For SBC0 (i.e., 20% Sm3+-doped ceria) annealed at 800 °C, the two peaks at ∼550 and ∼600 cm–1 represent the oxygen vacancies.37 With the increased Bi3+ addition and a coupled decreased Sm3+ content, the band at ∼550 cm–1 decreases in intensity, while the band at ∼600 cm–1 becomes broad and increases in intensity, and for SBC20, it shifts to a slightly lower wavenumber (∼570 cm–1). Another band at ∼510 cm–1 is also present for SBC20. Hebert and Stöwe35 also observed bands at 510 and 570 cm–1 for Ce0.8Bi0.2Ox and Ce0.9Bi0.1Ox, respectively, and assigned them to oxygen vacancy formation in ceria based on results from density functional theory (DFT) calculations by Schilling et al.39 A weak Raman peak ranging between 513 and 535 cm–1 for α-Bi2O3 has been reported,28,40 but other peaks at lower wavenumbers (e.g., 314 and 411 cm–135) that are not overlapping with the ceria F2g band were not present. For the samples annealed at 1200 °C, the peaks at ∼550 and ∼600 cm–1 remained in those positions for all samples with decreasing intensity as the ratio of Bi3+ to Sm3+ added increased, except for the SBC20 sample. On zooming in to this region for SBC20 (see the inset in Figure 2b), very low intensity peaks at ∼510 and ∼600 cm–1 were noted. No α-Bi2O3 was detected by XRD for samples annealed at 1200 °C. It thus suggests that the peak at ∼570 cm–1 for the 800 °C-annealed samples signifies that it is related to the α-Bi2O3 phase, which grows to become more dominant as the amount of Bi3+ is increased.
A broad band at ∼660 cm–1 for samples annealed at 800 °C and at ∼665 cm–1 for samples annealed at 1200 °C also appeared with the introduction of Bi3+, although not evident for SBC20. This can be ascribed to δ-Bi2O3 and which could not be detected using XRD due to the overlapping peaks with that for doped ceria. The broad band is due to a large amount of disorder in the structure and was observed by Vila et al. at around 610–640 cm–1,28 by Díaz-Guerra et al.41 at 630 cm–1 with a shoulder band at 655 cm–1 for a δ-Bi2O3 film, and by Rubbens et al.42 at ∼640 cm–1 for doped-Bi2O3. This band is not evident for SBC20 (and is very small for samples annealed at 1200 °C), which indicates that Sm3+ plays a role in stabilizing this δ-phase.
Deconvolution of the spectra up to 700 cm–1 was performed using a Gaussian amplitude function to determine more quantitative data (Figure S2). This was unfortunately not possible with the spectra for SBC5–SBC15 annealed at 800 °C due to the growing peak at ∼570 cm–1 overlapping the bands at both ∼550 and ∼600 cm–1. The defect concentration, determined as the area ratio between the ∼550 cm–1 and F2g bands,43 the relative intensities of the oxygen vacancy band (shoulder band ∼550 cm–1), and the full width at half-maximum (FWHM) of the F2g bands are compared schematically for the 1200 °C-annealed samples (Figure S3). The decreasing defect concentration with the systematically decreasing Sm3+ content highlights that the corresponding increase in the added codopant (Bi3+) does not result in the effective incorporation in the ceria lattice. The F2g band becomes sharper and more intense from SBC0 to SBC20, supporting the hypothesis of the formation of more crystalline ceria phases due to minimal intrusion of impurities in the lattice.
The room-temperature Raman spectra of SZC samples annealed at 800 and 1200 °C are shown in Figure 2c,d, respectively. For SZC0–SZC10 annealed at 800 °C, the F2g band is essentially at the same position (460.7 cm–1) (see the inset in Figure 2c and Table 2). For SZC15 and SZC20, the F2g band position appears at higher wavenumbers (462.6 and 464.3 cm–1, respectively), possibly indicating a smaller extent of substitution into the ceria lattice, and this is supported by the XRD results showing the additional wurtzite ZnO. A similar trend was seen for the F2g band positions for SZC samples annealed at 1200 °C (see Table 2).
The intensities of the oxygen vacancy bands at ∼550 and ∼600 cm–1 decrease with an increase in the Zn2+-to-Sm3+ ratio added, which again denotes lesser incorporation of Zn2+ into the ceria lattice. If Zn2+ were in the ceria lattice the intensity of the oxygen vacancy bands would have increased since fewer oxygens would be required to maintain charge neutrality. For SZC20, a minor band was seen at ∼600 cm–1 reflecting partial intrusion of Zn2+ to generate oxygen vacancies. Again, a similar trend was noted for samples annealed at 1200 °C. For SZC20, a closer look (see the inset in Figure 2d) reveals the presence of a few of very low intensity peaks at 507, 517, 531, and 550 cm–1 and a broad band at 661 cm–1, which are in reasonable agreement with those observed by Lima et al. for ZnO.44 These bands were not detected for SZC10 and SZC15, which was most likely due to the superimposition of these bands with the more pronounced shoulder bands in that region. Although not observed for SZC0–SZC15, SZC20 shows a slightly higher intensity peak at ∼1072 cm–1, which was ascribed to the presence of ZnO as noted according to the RRUFF Project.45
Deconvolution of the spectra for the SZC samples was performed as before (Figures S4 and S5), and the quantitative data was extracted (Table S2). The shoulder band at ∼550 cm–1 gradually weakened from SZC0 to SZC15, and for SZC20, a sharp F2g band was present with no considerable shoulder band in the spectra of samples annealed at 800 and 1200 °C (Figure 2c,d). This clearly shows that a minimal amount of Zn2+ was incorporated in the ceria lattice (a separate ZnO phase was rather formed) and that only Sm3+ was substituted into the lattice resulting in the reduction of the defect concentration as the Sm3+ content was decreased. The defect concentration did not change significantly upon increasing the annealing temperature. The stepwise difference between the parameters (Table S2) upon changing the sample composition is not as large between SZC5 and SZC10 as compared to the others. This possibly signifies better intrusion of Zn2+ along with Sm3+ into ceria lattice for SZC10 compared to others.
The bands at ∼1240, ∼1310, and ∼1360 cm–1 in the spectra in Figure 2 are due to Sm3+ photoluminescence. These are clearly absent in the SBC20 and SZC20 samples where no Sm3+ is present. It is noted that the intensity of these bands increases with a decreasing Sm3+ content in all cases.
For samples annealed at 800 °C, there was evidence of some carbonate present. For the SBC samples, a characteristic band for carbonates in the ν1 region was located at ∼1065 cm–143 (the broad band at ∼1160 cm–1 was attributed to the 2LO mode of ceria46). For the SBC samples, the peaks at ∼850 and ∼950 cm–1 were assigned to the presence of carbonate in the ν2 region.47 As these were all very low intensity peaks, the presence of carbonate was further investigated by FTIR. In all samples annealed at 1200 °C, these peaks disappeared, an expected outcome of high-temperature annealing.
4.3. FTIR Spectroscopy
Figure 3 shows the FTIR spectra of the SBC (a) and SZC (b) samples annealed at 800 °C, respectively. The bands appearing at ∼3460, ∼2920, ∼2850, and 1625 cm–1 in (a) have been assigned to the O–H stretching, C–H stretching, and H–O–H bending, respectively.48,49 Another band only seen for SBC0 at 1731 cm–1 has been assigned to the presence of Na2CO3 on a ceria surface.50,51 Although not observed in Raman spectroscopy, FTIR also shows a band at ∼1465 cm–1, indicating the antisymmetric stretching of carbonate in the ν3 mode, similar to the 1444 cm–1 band for SDC/Na2CO3 reported by Yin et al.51 The presence of nitrates on the ceria surface was detected by the band located at ∼1380 cm–1.46 Raman spectroscopy indicated the presence of carbonate in the ν1 mode (symmetric stretching = 1000–1100 cm–1) at ∼1065 cm–1 and is observed at ∼1068 cm–1 in the FTIR spectra. Another band representing the out-of-plane bending of carbonate in the ν2 region (800–900 cm–1) was observed at ∼850 cm–1; however, it was not observed in Raman spectroscopy.51 The features below 700 cm–1 represent M–O vibrations.50 The FTIR spectra of SBC samples annealed at 1200 °C are represented in Figure S6a, where the bands associated with carbonates have completely vanished as previously noted due to the volatilization of the carbonates at this high temperature. The dominant bands observed are due to the physically adsorbed moisture (∼3460 and ∼1640 cm–1) and nitrates (∼1382 cm–1).50−52 As before, the M–O vibrations are present below 700 cm–1.50
Figure 3.
FTIR spectra of (a) SBC and (b) SZC samples annealed at 800 °C.
The FTIR spectra of SZC samples (b) are similar to that of the SBC samples except for the ∼1533 and ∼1480 cm–1 bands, which appear to evolve and increase in intensity with increasing Zn2+ content. The former resembles the 1513 cm–1 band observed by Hu et al.53 who assigned it to polydentate carbonate on ZnO, while the latter, seen when the Zn2+ content is increased to 10 mol %, is in good agreement with the 1445 cm–1 band, indicating the presence of carbonate on a ZnO surface reported elsewhere.54 The FTIR spectra of SZC 1200 °C-annealed samples are like SBC samples (Figure S6b).
4.4. SEM
Figure 4 depicts the microstructure of SZC samples annealed at 800 and 1200 °C, recorded using the ThermoScientific Verios G4 UC SEM. SZC0 comprised a range of shapes, with many being cubic along with some round and polygonal-shaped particles (see Figure S7a). For SZC5–SZC15 (800 °C annealed (a–d)), Zn2+ addition (with the corresponding Sm3+ removal) greatly affects the microstructure of ceria. The grain size distribution differs greatly with Zn2+ addition causing inhomogeneity. SZC20 shows particles of varying sizes. As XRD has detected the presence of ZnO for SZC10–SZC20, we may speculate that the unusual shaped particles may belong to ZnO, while the small round-shaped ones are those of ceria. Gao et al.25 studied the effect of ZnO on yttria-doped ceria and speculated that, after a certain solubility limit, excess ZnO tends to separate out in the form of larger zinc-rich particles or agglomerates.
Figure 4.
SEM micrographs of SZC5–SZC20 samples annealed at 800 °C (a–d) and 1200 °C (e–h), with (II) showing a possible ZnO-related phase.
The SEM micrographs of SZC samples annealed at 1200 °C (e–h) were taken using the Regulus SEM. The most obvious effect of high-temperature annealing is the particle growth. SZC0 comprises particles having polygonal and round morphologies (Figure S7b), while for SZC5, the introduction of Zn2+ affects the microstructure where particles tend to stick to each other leading to the formation of rod-shaped structures.
As the Zn2+ content is increased to 10 mol % (SZC10), small agglomerates are formed and do not display any particular morphology. With the further addition of the Zn2+ content up to 15 mol % (SZC15), the microstructure becomes relatively clear, with the majority being circular polygonal-shaped particles. For SZC20, particles of mixed morphologies can be seen, though the majority are polygonal.
Energy-dispersive system (EDS) analysis of samples SZC5–SZC20 annealed at 1200 °C was carried out and is represented in Figure S8. These indicate that even for the samples with the lowest Zn2+ content, i.e., SZC5, ZnO is observed. So, the solubility limit of Zn2+ in the ceria lattice is less than 5 mol %. For SZC10–SZC20, the separated ZnO-rich areas have clearly been shown by the EDS results in Figure S8.
Figure 5a–d shows the surface morphologies of SBC5–SBC20 samples annealed at 800 °C and were recorded using the ThermoScientific Verios SEM. SBC0 is equivalent to SZC0 (both annealed at 800 and 1200 °C). For SBC5, the introduction of the Bi3+ content caused the formation of wire-like agglomerated structures. For SBC10–SBC15, particles of different sizes are visible with no distinctive morphology. The SBC20 micrograph depicts two different sized particles, which may represent two different phases, as indicated by both XRD and Raman spectroscopy.
Figure 5.
SEM micrographs of SBC5–SBC20 samples annealed at 800 °C (a–d) and 1200 °C (e–h). The insets labeled (II) in SBC5–SBC15 of 1200 °C-annealed samples are supposed impurity phases.
The surface morphologies of the SBC samples annealed at 1200 °C were acquired using the Regulus 8230 SEM (Figure 5e–h). The high-temperature annealing again led to particle growth, evident from the SEM micrographs of all samples, and polygonal-shaped particles dominate the surface morphology. With the introduction of the Bi3+ content, some unusual particles begin to appear, which increase in quantity with increasing Bi3+ content. Although it is difficult to predict with certainty without EDS results, based on the results obtained by Raman spectroscopy and reasonably supported by XRD findings, we may speculate that these unusual particles may belong to the Bi2O3 impurity phase.
4.5. Electrochemical Impedance Spectroscopy (EIS)
The electrochemical properties of SBC and SZC samples were measured over a temperature range of 550–750 °C in open air. The Nyquist plots of SBC samples annealed at 800 and 1200 °C are reproduced in Figure S9. For 800 °C-annealed samples in the frequency region used for measurements, SBC0 displayed predominantly the response due to the bulk and grain boundary, showing a decrease in resistance with an increase in the test temperature. At lower temperatures, the bulk region often did not completely resolve due to the high-frequency limitation of the instrument at 1 MHz. With the introduction of the Bi3+ content, particularly for SBC5–SBC15, predominantly the response due to the bulk and grain boundary region is observed. SBC15 shows a comparatively high resistance at low temperature. SBC20 shows a sharp decrease in resistance when the temperature is increased from 550 to 600 °C. This is evident in Figure 6a, where the Arrhenius plot of SBC20 indicates a stepped function in conductivity, where the reported conductivities incorporate both the bulk and grain boundary resistances.
Figure 6.
Arrhenius plots of SBC samples annealed at (a) 800 °C and (b) 1200 °C.
As the presence of α-Bi2O3 was confirmed by XRD and Raman spectroscopy, the sudden increase in conductivity of SBC20 strongly suggests a phase change and the conductivity values recorded are most likely related to the more conductive δ-Bi2O3 phase. Interestingly, SBC10–SBC15 shows comparatively low conductivities among all samples (see Figure 6a). This may imply a combination of the ceria lattice having fewer oxide vacancies (due to the lower amount of Sm3+ added and Bi3+ not being significantly incorporated), as well as the secondary bismuth oxide phase not being present at a significant quantity to affect the conductivity. The conductivity was thus hampered by this α-Bi2O3 phase, which is not an ionic conductor.55 It is noted that the conductivity values at the lowest temperature are almost the same for SBC15 and SBC20. It appears that no phase change occurred in the case of SBC15 upon heating.
As XRD lattice parameters did not show the expected expansion and Raman spectra also indicated the presence of other Bi3+-related phases, the Nyquist plots of SBC samples annealed at 1200 °C will be affected by both the doped ceria phases and other Bi3+-related phases that are present (Figure S9). Compared to SBC0, there was a decrease in resistance for the 5 mol % doped Bi3+, i.e., SBC5. Comparable lattice parameter values for SBC0 and SBC5 mean the intrusion of Bi3+ into the ceria lattice to generate oxygen vacancies, which in turn means an increase in oxide ion conductivity. Interestingly, SBC10 and SBC15 indicate higher conductivity than SBC0, which might be due to the contribution from the Bi3+-related phase (δ-Bi2O3).
SBC20 showed a relatively different behavior with a significantly higher resistance at 550 °C being noted, so it has not been included. Even at 600 and 650 °C, it showed a comparatively higher resistance compared to SBC0. However, an abrupt change was noticed at 650 °C or above. A sudden drop in resistance is noticed, strongly suggesting the conduction due to the δ-Bi2O3-related phase, which is a high oxygen ion conductor. From Arrhenius plots in Figure 6b, the small step function of conductivity is particularly evident for SBC10 and SBC20, with SBC5–SBC15 showing higher conductivity values than SBC0 due to Bi3+addition, suggestive of ionic conduction due to the Bi3+-related phase.
In general, Figure 6 shows a decreasing trend in conductivities above 5 mol % Bi3+ content, particularly for 1200 °C-annealed samples, which might be due to nonincorporation of Bi3+ into ceria lattice and separation into a different phase, evident from the XRD lattice parameter trend and Raman spectroscopy results. The conductivities of 800 °C-annealed samples remain higher than those of 1200 °C-annealed samples pointing toward the possible contribution of carbonate in conductivity enhancement. Li et al.56 noted that the addition of Li2CO3 increases the grain boundary conductivity of SDC and lowers its sintering temperature. Different mechanisms for ion transport through the carbonate have been reviewed in detail in (17).
Figure 7 shows the Nyquist plots of the SZC samples annealed at 800 and 1200 °C. SZC0 and SBC0 are the same samples (both 800 and 1200 °C annealed and see Figure S9). For the 800 °C-annealed samples, SZC5–SZC15 (Figure 7a–c) showed a noticeable bulk and grain boundary response with considerably higher resistances with increasing Zn2+ (and simultaneous decreasing Sm3+) content, which decreases significantly with increasing test temperature. The bulk response at lower temperatures could not be fully measured due to the limited test frequency. As XRD has indicated the presence of wurtzite ZnO and energy-dispersive X-ray (EDX) analysis also confirmed the Zn-rich regions, even for SZC5, it is believed that ZnO hinders the oxide ion conduction, thus decreasing the conductivity. SZC20 showed very high resistance, not suitable for electrolyte application and thus was not included here.
Figure 7.
Nyquist plots of SZC5–SZC15 samples annealed at 800 °C (a–c) and 1200 °C (d–f), respectively.
However, high-temperature annealing (1200 °C) introduces significant changes in Nyquist plots of SZC samples, depicted in Figure 7d–f. The charge-transfer resistance is considerably reduced for Zn2+-doped samples, particularly for SZC5, and with SZC10 and SZC15 being very similar (Figure 8b). The conductivities were now all higher than that of SZC0. The noticeable decrease in resistance might be due to the second-stage incorporation of Zn2+ in ceria to generate oxygen vacancies, as supported by XRD findings. It is interesting to note that the conductivity values follow a similar trend to the XRD lattice parameters of 1200 °C-annealed samples, i.e., increase in conductivity with increasing lattice parameter.
Figure 8.
Arrhenius plots of SZC samples annealed at (a) 800 °C and (b) 1200 °C.
The Arrhenius plots of SZC samples annealed at 800 °C, shown in Figure 8a, indicate an overall decrease in conductivities with Zn2+ addition. As the presence of wurtzite ZnO was indicated for SZC5–SZC15, it appears that this phase, which is not an ionic conductor (rather it is a semiconductor32), together with the fact that the Sm3+ concentration is decreased in the ceria lattice, both lead to lower ionic conductivities compared to that for SZC0. SZC10 and SZC15 behaved similarly with activation energies of 1.56 and 1.77 eV, respectively, between 550 and 650 °C. The mechanism of conduction then changed as indicated by the decreasing slope and activation energies of 0.71 and 1.16 eV between 650 and 750 °C for SZC10 and SZC15, respectively. SZC5 showed an activation energy of 1.20 eV between 550 and 650 °C, with an abrupt change in conductivity when the temperature was increased from 650 to 750 °C, normally indicative of a phase change. However, variable temperature phase analysis would be required to fully confirm this.
The Arrhenius plots of SZC samples annealed at 1200 °C, depicted in Figure 8b, now show the Zn2+-doped samples having comparatively higher conductivity values than SZC0. SZC5–SZC15 gives steeper slopes when the temperature is increased from 600 to 650 °C, pointing toward the possible role of cubic-type ZnO in ionic conduction over 600 °C, particularly visible for Zn2+ content-containing samples.
The conductivities of Zn2+-doped samples annealed at 800 °C remain considerably lower compared to those annealed at 1200 °C (Table S5). SZC5 (1200 °C annealed) shows the best conductivity, with comparable values shown by SZC10 and SZC15, although reasonably higher than SZC0. The impedance data reveals that low-temperature annealing might be helpful to exploit the beneficial trait of carbonate in ionic conduction; however, it is not suitable for the effective incorporation of codopants into the host ceria lattice.
4.6. Fuel Cell Performance
The fuel cell performance of selected samples annealed at 1200 °C (SBC5 and SZC5) was recorded at a temperature range of 500–600 °C using hydrogen as a fuel and air as an oxidant under laboratory conditions. SBC5 showed slightly lower OCV at 500 °C, which increased with increasing test temperature, depicted in Figure 9, which might be due to the possible presence of α-Bi2O3 impeding the ionic conduction; however, it transforms into the δ phase at 600 °C, showing a sudden increase in OCV. The test temperature was confined to 600 °C. Above this temperature, Ni-foam shows stability issues, which may result in performance degradation. This is also sufficient as the aim is to look at the performance in the lower-temperature range (600 °C or lower). Figure 9 reflects a sharp increase in peak power densities for both samples when the test temperature was increased from 550 to 600 °C, strongly suggesting the performance improvement due to the presence of cubic-type phases of Bi2O3 and ZnO in SBC5 and SZC5, respectively. A remarkable increase in the peak power density (Pmax = over 1230 mW/cm2) was recorded for SZC5, higher than SDC or other codoped samples reported in the literature, compared in Table S7.
Figure 9.
I–V and I–P curves of (a) SBC5 and (b) SZC5 both annealed at 1200 °C and measured from 500 to 600 °C.
5. Conclusions
Attempts were made to prepare codoped ceria powders via coprecipitation (using sodium carbonate) by systematically replacing Sm3+ with non-rare earth metals Bi3+ and Zn2+. For both dopant series annealed at 800 °C, a minimal incorporation of the codopants into host ceria occurred. This was observed by the decrease in lattice parameters and the F2g band positions shifting to the pure ceria value with a decrease in defect concentration when Sm3+ was supposedly systematically replaced with Bi3+ or Zn2+. For 1200 °C-annealed samples, the incorporation of the codopants was slightly higher and additional δ-Bi2O3 and cubic-type ZnO phases appeared were present in the SBC and SZC powders, respectively.
EIS analysis of the SZ5–SZC15 codoped samples annealed at 800 °C showed lower conductivities compared to SZC0 (i.e., Ce0.8Sm0.2O2−δ). However, the same samples annealed at 1200 °C showed higher conductivity values than SZC0, suggesting that the cubic-type ZnO may assist in enhancing the ionic conductivity. SBC annealed at 800 and 1200 °C exhibited a similar trend. Exceptions were noted for the 800 °C-annealed SBC samples. SBC5 showed slightly higher conductivity than SBC0 (i.e., Ce0.8Sm0.2O2−δ) and SBC20 showed comparable conductivities to SBC0 when the test temperature was increased to 600 °C and above, which is believed to be due to the δ-Bi2O3 phase being fully formed as evident from the step function in the Arrhenius plot. Similar behavior was seen while measuring the fuel cell performance. For SBC5 and SZC5, the peak power densities recorded at 550 °C were close to 350 and 650 mW/cm2, respectively, which increased to ∼720 and ∼1230 mW/cm2 at 600 °C. This suggests that the performance improvement is due to the temperature-dependent phase change of the codopant oxide phases. This study suggests that the conductivity and performance of a solid electrolyte material can be tailored by the presence of multiple phases together with a ceria solid solution. However, the successful formation and effective control of these phases need further investigation in terms of thermal stability, mechanical stability (due to thermal expansion parameters), and the effect of aging on the conductivity properties.
Acknowledgments
The financial assistance of the National Research Foundation (NRF) toward this research is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the author and are not necessarily to be attributed to the NRF. This work was supported by NRF equipment grants 78555 and 99003 and CPRR Running expenses 05852 and 141966. The financial support from the National Natural Science Foundation of China (NSFC) under grant no. 22209191 is also acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.3c08146.
Schematic of synthetics method and theoretical densities; multiple peak-fitted Raman spectra of SBC and SZC samples; quantitative data obtained by deconvolution of Raman spectra; FTIR spectra of SBC and SZC samples annealed at 1200 °C; SEM micrographs of SZC0; EDS images of SZC5–SZC20; Nyquist plots of SBC samples; total ionic conductivity values of SBC and SZC samples; error range of fitted resistances of SBC and SZC samples for EIS measurements; and a comparison of fuel cell performance data in a tabular form (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- UN General Assembly . Transforming our world: the 2030 Agenda for Sustainable Development 2015, A/RES/70/1, available at: https://www.un.org/en/development/desa/population/migration/generalassembly/docs/globalcompact/A_RES_70_1_E.pdf (accessed July 07, 2023).
- Sun C.; Xie Z.; Xia C.; Li H.; Chen L. Investigations of Mesoporous CeO2–Ru as a Reforming Catalyst Layer for Solid Oxide Fuel Cells. Electrochem. Commun. 2006, 5, 833–838. 10.1016/j.elecom.2006.03.018. [DOI] [Google Scholar]
- Yang W.; Ma Z.; Sun C.; Chen L. Core-Shell Structured Sr0.88Y0.08TiO3-Ce0.8Sm0.2O1.9 Composite as an Anode for Solid Oxide Fuel Cells Operating with CH4. ECS Trans. 2013, 57, 1313–1319. 10.1149/05701.1313ecst. [DOI] [Google Scholar]
- Zakaria Z.; Hassan S. H. A.; Shaari N.; Yahaya A. Z.; Kar Y. B. A Review on Recent Status and Challenges of Yttria Stabilized Zirconia Modification to Lowering the Temperature of Solid Oxide Fuel Cells Operation. Int. J. Energy Res. 2020, 44, 631–650. 10.1002/er.4944. [DOI] [Google Scholar]
- Ma Z.; Sun C.; Ma C.; Wu H.; Zhan Z.; Chen L. Ni Doped La0.6Sr0.4FeO3-δ Symmetrical Electrode for Solid Oxide Fuel Cells. Chin. J. Catal. 2016, 8, 1347–1353. 10.1016/S1872-2067(15)61116-0. [DOI] [Google Scholar]
- Liu Y.; Tang Y.; Ma Z.; Singh M.; He Y.; Dong W.; Sun C.; Zhu B. Flowerlike CeO2 Microspheres Coated with Sr2Fe1.5Mo0.5Ox Nanoparticles for an Advanced Fuel Cell. Sci. Rep. 2015, 5, 11946 10.1038/srep11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Wei J.; Yin F.; Sun C. Recent Advances in Carbon-Resistant Anodes for Solid Oxide Fuel Cells. Mater. Chem. Front. 2023, 7, 1943–1991. 10.1039/D2QM01366E. [DOI] [Google Scholar]
- Xian C.; Wang S.; Sun C.; Li H.; Chan S.; Chen L. Effect of Ni Doping on the Catalytic Properties of Nanostructured Peony-like CeO2. Chin. J. Catal. 2013, 34, 305–312. 10.1016/S1872-2067(11)60466-X. [DOI] [Google Scholar]
- Zhou Y.; Luo T.; Du X.-L.; Wang J.; Yang W.; Sun C.; Xia C.; Wang S.; Zhan Z. High Activity of Nanoporous-Sm0.2Ce0.8O2-δ@430L Composites for Hydrogen Electro-Oxidation in Solid Oxide Fuel Cells. Adv. Energy Mater. 2014, 17, 140088 10.1002/aenm.201400883. [DOI] [Google Scholar]
- Zhan Z.; Wen T.-L.; Tu H.; Lu Z.-Y. AC Impedance Investigation of Samarium-Doped Ceria. J. Electrochem. Soc. 2001, 148, A427–A432. 10.1149/1.1359198. [DOI] [Google Scholar]
- Raza R.; Wang X.; Ma Y.; Zhu B. Study on Calcium and Samarium Co-Doped Ceria Based Nanocomposite Electrolytes. J. Power Sources 2010, 195, 6491–6495. 10.1016/j.jpowsour.2010.04.031. [DOI] [Google Scholar]
- Zheng Y.; Gu H.; Chen H.; Gao L.; Zhu X.; Guo L. Effect of Sm and Mg Co-Doping on the Properties of Ceria-Based Electrolyte Materials for IT-SOFCs. Mater. Res. Bull. 2009, 44, 775–779. 10.1016/j.materresbull.2008.09.021. [DOI] [Google Scholar]
- Tarancón A. Strategies for Lowering Solid Oxide Fuel Cells Operating Temperature. Energies 2009, 2, 1130–1150. 10.3390/en20401130. [DOI] [Google Scholar]
- Wu Y.-C.; Lin C. The Microstructures and Property Analysis of Aliovalent Cations (Sm3+, Mg2+, Ca2+, Sr2+, Ba2+) Co-Doped Ceria-Base Electrolytes after an Aging Treatment. Int. J. Hydrogen Energy 2014, 39, 7988–8001. 10.1016/j.ijhydene.2014.03.063. [DOI] [Google Scholar]
- Preethi S.; Babu K. S. Divalent Cations Modified Grain Boundary Scavenging in Samarium Doped Ceria Electrolyte for Solid Oxide Fuel Cells. J. Alloys Compd. 2019, 792, 1068–1078. 10.1016/j.jallcom.2019.04.062. [DOI] [Google Scholar]
- Wang X.; Ma Y.; Raza R.; Muhammed M.; Zhu B. Novel Core–Shell SDC/Amorphous Na2CO3 Nanocomposite Electrolyte for Low-Temperature SOFCs. Electrochem. Commun. 2008, 10, 1617–1620. 10.1016/j.elecom.2008.08.023. [DOI] [Google Scholar]
- Raza R.; Zhu B.; Rafique A.; Naqvi M. R.; Lund P. Functional Ceria-Based Nanocomposites for Advanced Low-Temperature (300–600 °C) Solid Oxide Fuel Cell: A Comprehensive Review. Mater. Today Energy 2020, 15, 100373 10.1016/j.mtener.2019.100373. [DOI] [Google Scholar]
- Raza R.; Qin H.; Fan L.; Takeda K.; Mizuhata M.; Zhu B. Electrochemical Study on Co-Doped Ceria–Carbonate Composite Electrolyte. J. Power Sources 2012, 201, 121–127. 10.1016/j.jpowsour.2011.10.124. [DOI] [Google Scholar]
- Wang X.; Ma Y.; Zhu B. State of the Art Ceria-Carbonate Composites (3C) Electrolyte for Advanced Low Temperature Ceramic Fuel Cells (LTCFCs). Int. J. Hydrogen Energy 2012, 37, 19417–19425. 10.1016/j.ijhydene.2011.09.096. [DOI] [Google Scholar]
- Fan L.; He C.; Zhu B. Role of Carbonate Phase in Ceria-Carbonate Composite for Low Temperature Solid Oxide Fuel Cells: A Review: Review of Carbonate Role in Ceria-Carbonate Composite for LT-SOFC. Int. J. Energy Res. 2017, 4, 465–481. 10.1002/er.3629. [DOI] [Google Scholar]
- Zhang Y.; Knibbe R.; Sunarso J.; Zhong Y.; Zhou W.; Shao Z.; Zhu Z. Recent Progress on Advanced Materials for Solid-Oxide Fuel Cells Operating below 500 °C. Adv. Mater. 2017, 29, 1700132 10.1002/adma.201700132. [DOI] [PubMed] [Google Scholar]
- Zhao W.; An S.; Ma L. Processing and Characterization of Bi2O3 and Sm2O3 Co-doped CeO2 Electrolyte for Intermediate-Temperature Solid Oxide Fuel Cell: Processing and Characterization of CeO2 Electrolyte. J. Am. Ceram. Soc. 2011, 94, 1496–1502. 10.1111/j.1551-2916.2010.04270.x. [DOI] [Google Scholar]
- Accardo G.; Spiridigliozzi L.; Dell’Agli G.; Yoon S. P.; Frattini D. Morphology and Structural Stability of Bismuth-Gadolinium Co-Doped Ceria Electrolyte Nanopowders. Inorganics 2019, 7, 118 10.3390/inorganics7100118. [DOI] [Google Scholar]
- Ge L.; Li S.; Zheng Y.; Zhou M.; Chen H.; Guo L. Effect of Zinc Oxide Doping on the Grain Boundary Conductivity of Ce0.8Ln0.2O1.9 Ceramics (Ln = Y, Sm, Gd). J. Power Sources 2011, 196, 6131–6137. 10.1016/j.jpowsour.2011.03.032. [DOI] [Google Scholar]
- Gao L.; Zhou M.; Zheng Y.; Gu H.; Chen H.; Guo L. Effect of Zinc Oxide on Yttria Doped Ceria. J. Power Sources 2010, 195, 3130–3134. 10.1016/j.jpowsour.2009.11.117. [DOI] [Google Scholar]
- Gates-Rector S.; Blanton T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. 10.1017/S0885715619000812. [DOI] [Google Scholar]
- Giacovazzo C.; Monaco H. L.; Viterbo D.; Scordari F.; Gilli G.; Zanotti G.; Catti M.; Giacovazzo C.; Paufler P. Fundamentals of Crystallography. International Union of Crystallography, Oxford Univ. Press 1992, 654 p. Pb. £ 27.50.ISBN0-19-855578-4. Cryst. Res. Technol. 1993, 28, 370. 10.1002/crat.2170280318. [DOI] [Google Scholar]
- Vila M.; Díaz-Guerra C.; Piqueras J. Laser Irradiation-Induced α to δ Phase Transformation in Bi2O3 Ceramics and Nanowires. Appl. Phys. Lett. 2012, 101, 071905 10.1063/1.4747198. [DOI] [Google Scholar]
- Takahashi T.; Esaka T.; Iwahara H. Oxide Ion Conduction in the Sintered Oxides of MoO3-Doped Bi2O3. J. Appl. Electrochem. 1977, 7, 31–35. 10.1007/BF00615527. [DOI] [Google Scholar]
- Jung D. W.; Lee K. T.; Wachsman E. D. Terbium and Tungsten Co-Doped Bismuth Oxide Electrolytes for Low Temperature Solid Oxide Fuel Cells. J. Korean Ceram. Soc. 2014, 51, 260–264. 10.4191/kcers.2014.51.4.260. [DOI] [Google Scholar]
- Lin F.; Alxneit I.; Wokaun A. Structural and Chemical Changes of Zn-Doped CeO2 Nanocrystals upon Annealing at Ultra-High Temperatures. CrystEngComm 2015, 17, 1646–1653. 10.1039/C4CE02202E. [DOI] [Google Scholar]
- Özgür Ü.; Alivov Y. I.; Liu C.; Teke A.; Reshchikov M. A.; Doğan S.; Avrutin V.; Cho S.-J.; Morkoç H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301 10.1063/1.1992666. [DOI] [Google Scholar]
- Morkoc H.; Ozgur U.. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH Verlag: Weinheim, Germany, 2009; pp 1–76. [Google Scholar]
- Wu Y.; Kang J.; Liu F. Pressure Induced Wurtzite-to-Zinc Blende Phase Transition in ZnO at Finite Temperature. J. Mater. Res. 2008, 23, 3347–3352. 10.1557/JMR.2008.0410. [DOI] [Google Scholar]
- Hebert S. C.; Stöwe K. Synthesis and Characterization of Bismuth-Cerium Oxides for the Catalytic Oxidation of Diesel Soot. Materials 2020, 13, 1369 10.3390/ma13061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirbhate S.; Yadav A. K.; Acharya S. Investigation of In-Situ Oxygen Vacancies Dissociation Mechanism and Associated Atomic Scale Reshuffling during Oxy-Ion Migration in Nanostructured Co-Doped Ceria. Solid State Ion. 2020, 345, 115157 10.1016/j.ssi.2019.115157. [DOI] [Google Scholar]
- Preethi S.; Abhiroop M.; Babu K. S. Low Temperature Densification by Lithium Co-Doping and Its Effect on Ionic Conductivity of Samarium Doped Ceria Electrolyte. Ceram. Int. 2019, 45, 5819–5828. 10.1016/j.ceramint.2018.11.251. [DOI] [Google Scholar]
- Sun C.; Li H.; Chen L. Nanostructured Ceria-Based Materials: Synthesis, Properties, and Applications. Energy Environ. Sci. 2012, 5, 8475–8505. 10.1039/c2ee22310d. [DOI] [Google Scholar]
- Schilling C.; Hofmann A.; Hess C.; Ganduglia-Pirovano M. V. Raman Spectra of Polycrystalline CeO2: A Density Functional Theory Study. J. Phys. Chem. C 2017, 121, 20834–20849. 10.1021/acs.jpcc.7b06643. [DOI] [Google Scholar]
- Salazar-Pérez A. J.; Camacho-López M. A.; Morales-Luckie R. A.; Sánchez-Mendieta V.; Ureña-Núñez F.; Arenas-Alatorre J. Structural Evolution of Bi2O3 Prepared by Thermal Oxidation of Bismuth Nano-Particles. Superficies Vacio 2005, 3, 4–8. [Google Scholar]
- Díaz-Guerra C.; Almodóvar P.; Camacho-López M.; Camacho-López S.; Piqueras J. Formation of β-Bi2O3 and δ-Bi2O3 during Laser Irradiation of Bi Films Studied in-Situ by Spatially Resolved Raman Spectroscopy. J. Alloys Compd. 2017, 723, 520–526. 10.1016/j.jallcom.2017.06.263. [DOI] [Google Scholar]
- Rubbens A.; Drache M.; Roussel P.; Wignacourt J. P. Raman Scattering Characterization of Bismuth Based Mixed Oxides with Bi2O3 Related Structures. Mater. Res. Bull. 2007, 42, 1683–1690. 10.1016/j.materresbull.2006.11.036. [DOI] [Google Scholar]
- Uthayakumar A.; Pandian A.; Mathiyalagan S.; Kumar A.; Keshri A. K.; Omar S.; Balani K.; Krishna Moorthy S. B. Interfacial Effect of the Oxygen-Ion Distribution on the Conduction Mechanism in Strontium-Added Ce0.8Sm0.2O2−δ/Na2CO3 Nanocomposite. J. Phys. Chem. C 2016, 120, 25068–25077. 10.1021/acs.jpcc.6b07915. [DOI] [Google Scholar]
- Lima L.; Caldas L. de S.; Alí A.; Barreto J.; Freitas R.; Mazzarella A.; Felix G.; Carozo V.; Stavale F. Growth and Raman Spectroscopy of Ultrathin ZnO(0001) Films on Ag(001). Surf. Sci. 2021, 704, 121748 10.1016/j.susc.2020.121748. [DOI] [Google Scholar]
- Lafuente B.; Downs R. T.; Yang H.; Stone N.. 1. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography; De Gruyter: Berlin, Germany, 2015; pp 1–30. [Google Scholar]
- Mihaylov M. Y.; Zdravkova V. R.; Ivanova E. Z.; Aleksandrov H. A.; Petkov P. S.; Vayssilov G. N.; Hadjiivanov K. I. Infrared Spectra of Surface Nitrates: Revision of the Current Opinions Based on the Case Study of Ceria. J. Catal. 2021, 394, 245–258. 10.1016/j.jcat.2020.06.015. [DOI] [Google Scholar]
- Brooker M. H.; Bates J. B. Raman and Infrared Spectral Studies of Anhydrous Li2CO3 and Na2CO3. J. Chem. Phys. 1971, 54, 4788–4796. 10.1063/1.1674754. [DOI] [Google Scholar]
- Khan I.; Tiwari P. K.; Basu S. Development of Melt Infiltrated Gadolinium Doped Ceria-Carbonate Composite Electrolytes for Intermediate Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2019, 294, 1–10. 10.1016/j.electacta.2018.10.030. [DOI] [Google Scholar]
- Agarwal S.; Zhu X.; Hensen E. J. M.; Lefferts L.; Mojet B. L. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. 10.1021/jp409989y. [DOI] [Google Scholar]
- Ristoiu T.; Petrisor T. Jr; Gabor M.; Rada S.; Popa F.; Ciontea L.; Petrisor T. Electrical Properties of Ceria/Carbonate Nanocomposites. J. Alloys Compd. 2012, 532, 109–113. 10.1016/j.jallcom.2012.03.098. [DOI] [Google Scholar]
- Yin S.; Zeng Y.; Li C.; Chen X.; Ye Z. Investigation of Sm0.2Ce0.8O1.9/Na2CO3 Nanocomposite Electrolytes: Preparation, Interfacial Microstructures, and Ionic Conductivities. ACS Appl. Mater. Interfaces 2013, 5, 12876–12886. 10.1021/am403198x. [DOI] [PubMed] [Google Scholar]
- Seo J.; Lee J. W.; Moon J.; Sigmund W.; Paik U. Role of the Surface Chemistry of Ceria Surfaces on Silicate Adsorption. ACS Appl. Mater. Interfaces 2014, 6, 7388–7394. 10.1021/am500816y. [DOI] [PubMed] [Google Scholar]
- Hu J.; Li Y.; Zhen Y.; Chen M.; Wan H. In Situ FTIR and Ex Situ XPS/HS-LEIS Study of Supported Cu/Al2O3 and Cu/ZnO Catalysts for CO2 Hydrogenation. Chin. J. Catal. 2021, 42, 367–375. 10.1016/S1872-2067(20)63672-5. [DOI] [Google Scholar]
- Edwards J. F.; Schrader G. L. Methanol, Formaldehyde, and Formic Acid Adsorption on Methanol Synthesis Catalysts. J. Phys. Chem. A 1985, 89, 782–788. 10.1021/j100251a015. [DOI] [Google Scholar]
- Azad A. M.; Larose S.; Akbar S. A. Bismuth Oxide-Based Solid Electrolytes for Fuel Cells. J. Mater. Sci. 1994, 29, 4135–4151. 10.1007/BF00414192. [DOI] [Google Scholar]
- Li S.; Xian C.; Yang K.; Sun C.; Wang Z.; Chen L. Feasibility and Mechanism of Lithium Oxide as Sintering Aid for Ce0.8Sm0.2Oδ Electrolyte. J. Power Sources 2012, 205, 57–62. 10.1016/j.jpowsour.2012.01.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











