Significance
Ectomycorrhizal fungi are important and ubiquitous symbionts of most temperate and boreal trees that form interaction networks with multiple hosts of the same or different species. Using DNA metabarcoding of root-associated ectomycorrhizal fungal communities, we show that soil moisture and tree host carbon status serve as critical ecological determinants of well-connected interaction networks between trees and their symbiotic fungi. Experimental warming and rainfall reduction led to shifts in taxonomic and functional composition of ectomycorrhizal fungal communities that corresponded with significant reductions in interaction network connectivity. We conclude that climate change scenarios that reduce soil moisture and decrease tree host performance have high potential to disrupt the interaction networks formed by trees and fungi in high-latitude forests.
Keywords: fungi, climate change, ectomycorrhiza, mycorrhizal interaction network, photosynthesis
Abstract
The interaction networks formed by ectomycorrhizal fungi (EMF) and their tree hosts, which are important to both forest recruitment and ecosystem carbon and nutrient retention, may be particularly susceptible to climate change at the boreal–temperate forest ecotone where environmental conditions are changing rapidly. Here, we quantified the compositional and functional trait responses of EMF communities and their interaction networks with two boreal (Pinus banksiana and Betula papyrifera) and two temperate (Pinus strobus and Quercus macrocarpa) hosts to a factorial combination of experimentally elevated temperatures and reduced rainfall in a long-term open-air field experiment. The study was conducted at the B4WarmED (Boreal Forest Warming at an Ecotone in Danger) experiment in Minnesota, USA, where infrared lamps and buried heating cables elevate temperatures (ambient, +3.1 °C) and rain-out shelters reduce growing season precipitation (ambient, ~30% reduction). EMF communities were characterized and interaction networks inferred from metabarcoding of fungal-colonized root tips. Warming and rainfall reduction significantly altered EMF community composition, leading to an increase in the relative abundance of EMF with contact-short distance exploration types. These compositional changes, which likely limited the capacity for mycelial connections between trees, corresponded with shifts from highly redundant EMF interaction networks under ambient conditions to less redundant (more specialized) networks. Further, the observed changes in EMF communities and interaction networks were correlated with changes in soil moisture and host photosynthesis. Collectively, these results indicate that the projected changes in climate will likely lead to significant shifts in the traits, structure, and integrity of EMF communities as well as their interaction networks in forest ecosystems at the boreal–temperate ecotone.
Ectomycorrhizal fungal communities consist of diverse assemblages that are capable of connecting multiple host trees belowground via shared colonization by the same fungal individual (1–3). This potential is particularly true in forests at the boreal–temperate ecotone, where high densities of boreal and temperate tree species cooccur and share many ectomycorrhizal fungal species (4). However, our understanding of the formation and function of mycelial networks among forest trees is significantly hampered by methodological challenges (5). For instance, it is extremely difficult to physically trace mycelial connections between hosts through soil, effectively impeding the ability to demonstrate the presence of the same fungal individual on different host individuals. Thus, researchers have largely relied on inference from colonization of shared fungal taxa among plant individuals growing in close physical proximity to construct interaction networks that model potential belowground mycelial connections among hosts (2, 6). In spite of these challenges, research investigating ectomycorrhizal fungal communities and their interaction networks with diverse host trees remains critical because of their roles in multiple ecological processes, from the individual to ecosystem level (7). For example, seedling recruitment of many tree species can be significantly improved when seedlings have access to shared mycelial networks created by ectomycorrhizal fungi (8–11). The presence of shared mycelial networks between trees can also potentially suppress invasive plant species colonization and foster greater resilience to ecosystem disturbance (12). Furthermore, considerable amounts of carbon (C) and nitrogen (N) move through and are stored in the ectomycorrhizal fungal biomass constituting shared mycelial networks, making them a critical component of forest C and N cycles (13–17).
The response and resilience to climate change of ectomycorrhizal fungi and the interaction networks they form among diverse forest trees remain highly uncertain (18–21). Forests at the boreal–temperate ecotone are predicted to experience increased evapotranspiration from warming, leading to chronically lowered soil moisture, which may be further exacerbated by longer periods between precipitation events (22). These changes are expected to have particularly strong effects on tree populations positioned near the extremes of their latitudinal ranges (23, 24), with cascading belowground effects on their associated ectomycorrhizal fungal communities (4, 25, 26). The resilience of ectomycorrhizal fungal communities and interaction networks to climate change likely have particularly important implications for tree range contraction and expansion (27, 28). For instance, if ectomycorrhizal fungi are not resilient to warmer and/or drier conditions, their disruption may exacerbate negative effects on plant populations that are already stressed at the lower extremes of their latitudinal range and lead to rapid range contraction (29, 30). In addition, if temperate tree hosts rely on established mycelial networks for their recruitment (31, 32), then the disruption of boreal ectomycorrhizal fungal community structure may also impede expansion of tree populations past their upper latitudinal ranges. Conversely, if ectomycorrhizal fungal communities and interaction networks are resilient to climate change, they may act as a buffer and ameliorate further negative effects by maintaining nutrient and water supply to hosts under unfavorable conditions (33–35).
The stability of interaction networks to disturbances can depend on their emergent properties (36). Two key network properties hypothesized to promote stability are network generalization and nestedness (37, 38). Highly generalized networks feature members that interact with many species across trophic levels (37), which is thought to buffer the network to member losses. This network property is commonly estimated in mutualistic bipartite networks with a network specialization metric (H2’), where networks that highly generalized will have low values compared to more specialized networks that will have values closer to one (39). Related to this network-level specialization metric, the selectivity index (d’) estimates the level of specialization versus generalization of each member in the network (39). Nestedness, which can be estimated with the metric “weighted nestedness based on overlap and decreasing fill” (weighted NODF) (40), characterizes the structure of associations in bipartite networks. Highly nested networks are composed of both generalist and specialist species that exclusively associate with generalists from the opposite trophic level. Additionally, highly nested networks rarely have specialist-specialist associations. Theoretically, bipartite networks with low nestedness (i.e., where specialist-specialist associations are common) have a higher risk of destabilization because of high dependence on these specializations (36). Ectomycorrhizal interaction networks are typically complex and characterized by a high degree of generalization, where dozens of fungal taxa colonize multiple host trees (41). Intriguingly, unlike other types of mutualistic networks, however, ectomycorrhizal interaction networks typically have antinested structure (i.e., lower nestedness than expected by chance). The reason remains unclear, but low nestedness may result from both ectomycorrhizal plant and fungal specialists being rare and thus having low occurrences of generalist–specialist interactions. Alternatively, the disproportionate richness of fungi relative to host plants may make it difficult to detect nestedness in ectomycorrhizal associations (2). While there is some support for these hypotheses in other mutualistic networks, particularly those formed by animal–plant mutualisms, our understanding of how changes to ectomycorrhizal interaction network complexity and structure in response to both warming and reduced rainfall in situ remains a major knowledge gap. It might be expected that warming and reduced rainfall would alter network structure if C allocation to ectomycorrhizal fungi is reduced and negatively affects fungal biomass production, thus altering the physical connections among plants and fungi in the networks.
In addition to network-level properties, the functional traits that govern the response of individual ectomycorrhizal fungi to climate change may be critical to resilience of tree-fungal interaction networks at the boreal–temperate ecotone. The extramatrical mycelium (EMM) of ectomycorrhizal fungi forages, extracts, and translocates resources from soil as well as colonizes newly formed roots, establishes the physical mycelial connections between different tree individuals, and represents an organ that is highly variable in terms of its functional traits (17). The exploration distance and hydrophobicity of EMM are consistently reported as important traits governing ectomycorrhizal fungal responses to climate change (25, 42, 43). Exploring greater distance and volume of the soil comes with a C cost that must be paid by host trees (44). If host tree productivity is reduced under warmer and/or drier conditions, then it is expected that ectomycorrhizal fungi employing these exploration strategies will be replaced by taxa with shorter distance exploration strategies (25). This shift, which would limit the potential for the same fungal individual to connect different tree individuals, could notably the alter the structure of interaction networks. The hydrophobicity of the EMM is another trait that may be important to ectomycorrhizal fungi under water limiting conditions. Specifically, EMM with hydrophilic properties can facilitate the uptake of dissolved nutrients under conditions where water is plentiful, however, under reduced rainfall, this same trait may make EMM susceptible to water loss compared to those taxa that produce hydrophobic mycelial structures (e.g., rhizomorphs) (45). Since these traits dictate the extent, longevity, and connectivity (via root colonization) of EMM, shifts in trait frequency and abundance may ultimately lead to changes in interaction network structure and functioning.
In this study, our objective was to examine the responses of ectomycorrhizal fungal communities and their interaction networks with forest trees to experimental warming and reduced rainfall to better understand and predict future changes at the boreal–temperate ecotone. We used DNA metabarcoding and bipartite network analysis to characterize ectomycorrhizal fungal communities and their interaction networks with two boreal (Pinus banksiana and Betula papyrifera) and two temperate (Pinus strobus and Quercus macrocarpa) tree hosts grown under a factorial combination of experimentally elevated above- and below-ground temperatures (ambient, +3.1 °C above ambient) and reduced rainfall (ambient, ~30% rainfall reduction) treatments replicated across three plots each at two sites in northern Minnesota, USA over a 4-y period (four host species × two sites × two warming treatment levels × two rain reduction treatment levels × three replicate plots; Total N = 96). In particular, we tested the following four hypotheses. (H1) Warming and reduced rainfall will alter ectomycorrhizal fungal community structure. (H2) Warming and rainfall reduction will alter the functional trait composition of ectomycorrhizal fungal communities, favoring taxa with contact-short distance exploration types and those with hydrophobic mycelia. (H3) The alteration of taxonomic and functional diversity will correspond to reduced generalization and nestedness of ectomycorrhizal interaction networks due to decreases in the relative abundances of generalist fungal taxa possessing relatively extensive EMM. (H4) Changes in both ectomycorrhizal fungal community structure and interaction network properties will be greatest in the combined warming and reduced rainfall treatment, consistent with, or perhaps driven by, shifts in tree host species physiological sensitivity to warming in contrasting soil moisture conditions (46).
Results
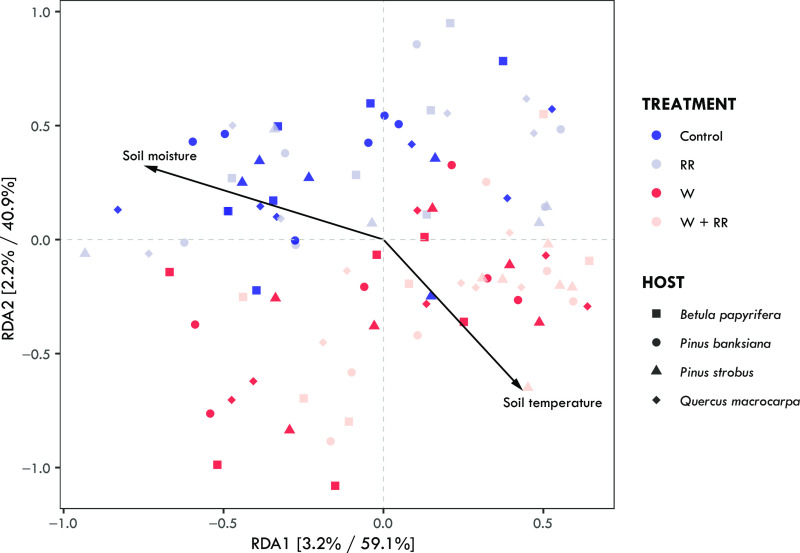
Our results provide mixed support for H1. While the climate change treatments did not significantly alter alpha diversity of the ectomycorrhizal communities (SI Appendix, Fig. S5 and Table S6), they did cause significant compositional changes. Ectomycorrhizal fungal community composition differed significantly with warming (PERMANOVA; Warming: F1,93 = 2.60; P = 0.001; SI Appendix, Table S7), but not with reduced rainfall (PERMANOVA; Reduced Rainfall: F1,93 = 1.33; P = 0.117; SI Appendix, Table S7), although there was a significant warming and reduced rainfall treatment interaction (PERMANOVA; Warming × Reduced Rainfall: F1,93 = 1.55; P = 0.015; SI Appendix, Table S7). The ambient (control) communities were dominated by basidiomycete ectomycorrhizal fungi, averaging 94.2% relative abundance across all hosts, and by OTUs from the genera Tomentella, Russula, and Sebacina (Fig. 1). Shifts in ectomycorrhizal fungal community composition in response to warming and reduced rainfall were driven primarily by increased relative abundances of ascomycete ectomycorrhizal fungal OTUs, such as Tuber and Wilcoxina as well as OTUs from the basidiomycete genera Inocybe, Thelephora, Hebeloma, Laccaria, and Clavulina (Fig. 1). Additionally, the Pinus-specialist Suilloid genera, Rhizopogon and Suillus, had considerable increases in relative abundance with the warming and reduced rainfall treatments on P. banksiana and P. strobus, respectively (SI Appendix, Fig. S7). Ectomycorrhizal fungal community composition also differed significantly among the four tree host species (PERMANOVA; Host: F3,93 = 3.26; P = 0.001; SI Appendix, Table S7) and a redundancy analysis revealed that a significant proportion of the variation in ectomycorrhizal fungal community composition was explained by mean daily soil moisture and temperature during the growing season (Fig. 2).
Fig. 1.
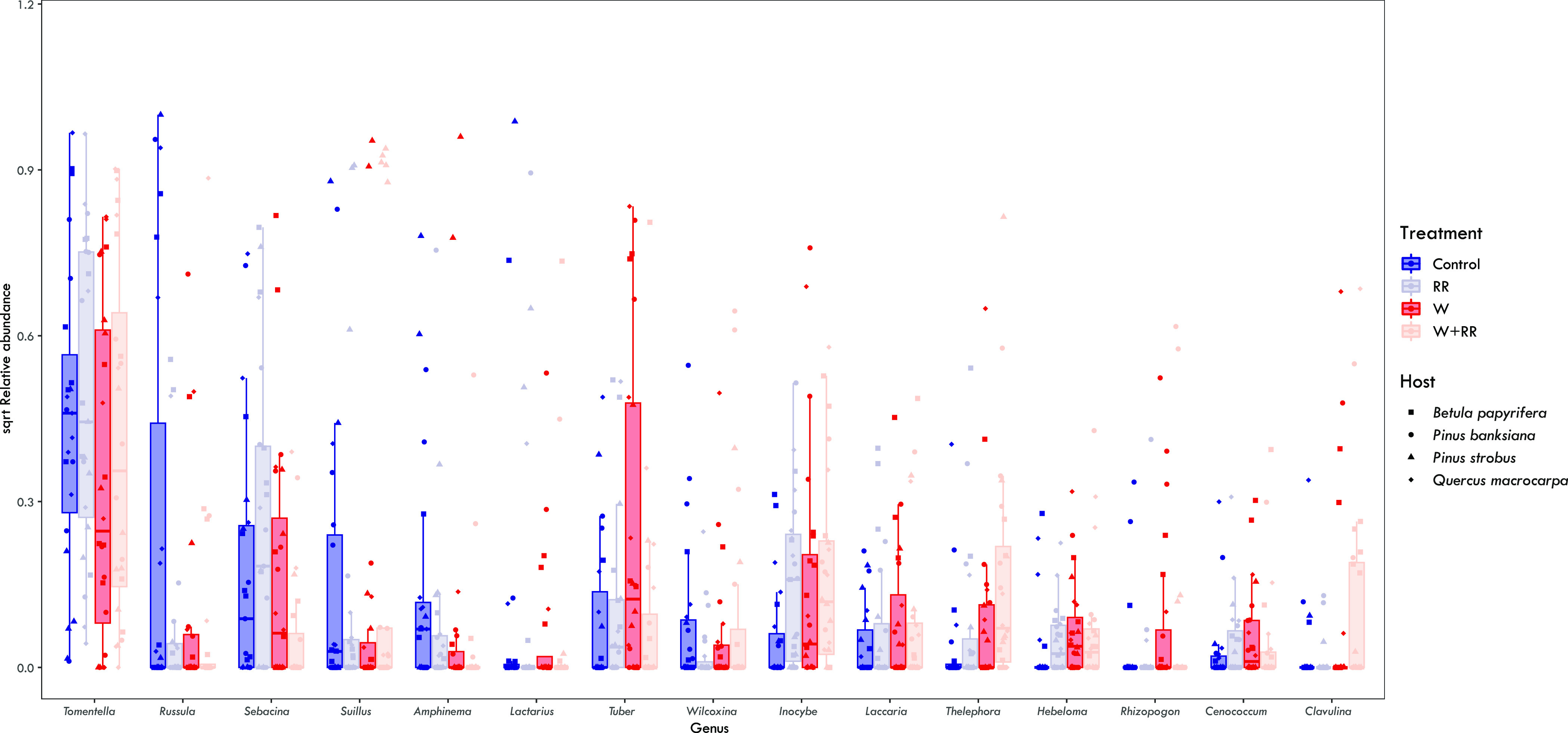
The relative abundances (data are square-root transformed) of top 15 ectomycorrhizal fungal genera across the warming and reduced rainfall treatments. Boxplots represent the median (line in box), 25 percentile (lower hinge) and 75 percentile (upper hinge) ranges, and whiskers represent the range. Points represent the relative abundance of each genus per sample (N = 94) and are color coded by treatment and shape is coded to each host species.
Fig. 2.
Redundancy analysis biplot based on Bray–Curtis dissimilarity matrix of ectomycorrhizal fungal communities associated with Betula papyrifera (square), Pinus banksiana (circle), Pinus strobus (triangle), and Quercus macrocarpa (diamond), and constrained to measured daily mean soil moisture and temperature from ambient (Control), warming (W) and reduced rainfall (RR) and combined (W+RR) treatments. Point colors correspond to treatment, and shape corresponds to the associated tree host. The relative contribution of each axis to the total inertia in the data, as well as to the constrained space only, respectively, are indicated in percent at the axis titles.
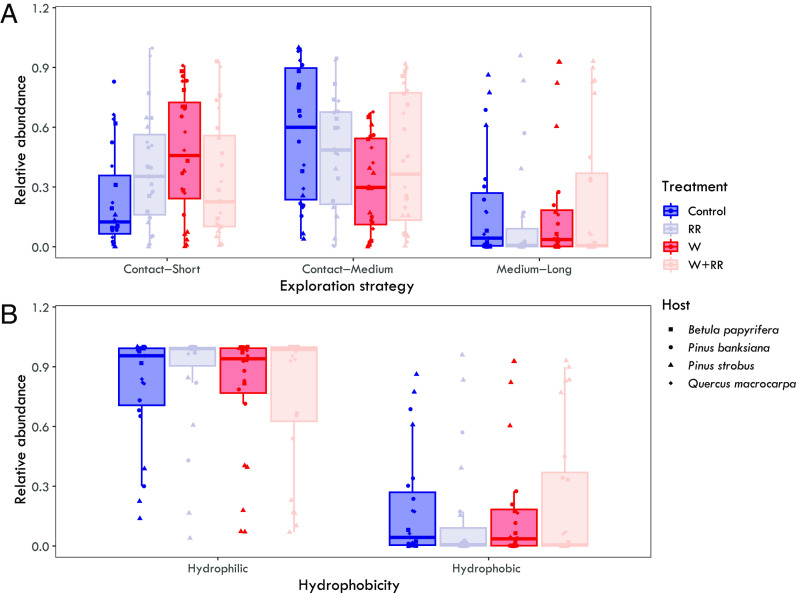
Changes in ectomycorrhizal fungal community structure in response to warming and reduced rainfall as well as to host species also corresponded with significant changes in the relative abundances of ectomycorrhizal fungal exploration types (SI Appendix, Table S8 and Fig. 3A). Supporting H2, ‘Contact-Short’ exploration strategists were significantly affected by the interactive effects of the warming and rainfall reduction treatments (W × RR: F1,15 = 6.39; P = 0.023), where the relative abundance was significantly higher in warmed, ambient rainfall plots compared to ambient-ambient plots (Fig. 3A), but not in warmed conditions under reduced rainfall. Further supporting H2, ‘Contact-Medium’ exploration strategists were also affected by the effects of warming (Warming: F1,15 = 4.49; P = 0.051). ‘Contact-Medium’ exploration strategists had higher relative abundance in the ambient plots compared to the warmed, ambient rainfall and warmed, reduced rainfall plots (Fig. 3A). The relative abundance of ‘Medium-Long’ exploration strategists did not, however, differ significantly between ambient and the other climate change treatments (SI Appendix, Table S8). Host species also affected the relative abundance of ‘Contact-Short’ (Host: F3,58 = 9.92; P < 0.0001), ‘Contact-Medium’ (Host: F3,58 = 3.58; P = 0.019), and ‘Medium-Long’ (Host: F3,58 = 37.78; P < 0.0001) strategies, with P. strobus ectomycorrhizal fungal communities featuring a higher relative abundance of the ‘Medium-Long’ associated with Suilloid taxa. There was no significant difference in the relative abundance of hydrophobic taxa among treatments (SI Appendix, Table S8 and Fig. 3B). The relative abundances of hydrophilic and hydrophobic taxa, however, differed significantly among the hosts (SI Appendix, Table S8), with a higher relative abundance of hydrophilic taxa associated with B. papyrifera and Q. macrocarpa and more hydrophobic taxa with P. banksiana and P. strobus (SI Appendix, Fig. S6).
Fig. 3.
The relative abundance of exploration types (A) and hydrophobicity (B) in ectomycorrhizal fungal communities associated with Betula papyrifera (square), Pinus banksiana (circle), Pinus strobus (triangle), and Quercus macrocarpa (diamond) exposed to ambient (Control), warming (W), and reduced rainfall (RR), and combined (W+RR) treatments. Boxplots represent the median (line in box), 25 percentile (lower hinge), and 75 percentile (upper hinge) ranges, and whiskers represent the range. Points represent the relative abundance of each strategy per sample (N = 94) and are color coded by treatment and shape is coded to each host species.
Growing season mean photosynthetic and stomatal conductance rates varied significantly among the four host species and also depended on the warming treatment (Host × Warming: Photosynthesis: F3,58 = 6.65; P = 0.0006; Conductance: P = 0.003; F3,58 = 4.01 P = 0.0114; SI Appendix, Table S5). Under both ambient temperatures and the warming treatment, P. strobus had significantly lower photosynthetic rates and stomatal conductance than the other three host species (SI Appendix, Fig. S2). Under ambient temperatures, B. papyrifera had significantly higher photosynthetic rates than Q. macrocarpa, but the two were not significantly different under warming (SI Appendix, Fig. S2). Further, the photosynthetic rates and stomatal conductance were lower for B. papyrifera, P. banksiana, P. strobus under warming, but higher for Q. macrocarpa (SI Appendix, Fig. S2). Unlike warming, the reduced rainfall treatment significantly lowered the photosynthetic rates of all host species (Reduced rainfall: F1,15 = 4.79; P = 0.045; SI Appendix, Fig. S2 and Table S5), which all showed a positive relationship between growing season average net photosynthetic rates and average soil moisture (SI Appendix, Fig. S3; P < 0.01). Finally, tree host height, mean stem diameter, and allometric stem mass were all significantly influenced by an interaction between the warming and rainfall reduction treatment, with increases with warming under ambient rainfall and decreases with warming under reduced rainfall (SI Appendix, Fig. S3 and Tables S2 and S5; P < 0.05).
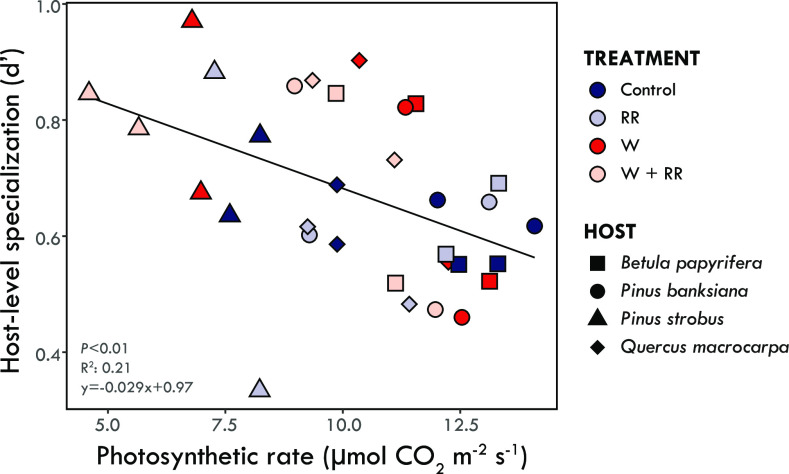
Estimates of ectomycorrhizal interaction network properties were significantly different from null models for each index and network in the study (SI Appendix, Table S9). Specialization (H2’) was significantly higher than null model values in every network. Conversely, nestedness of the interaction networks (weighted NODF) was significantly lower than null model values (SI Appendix, Table S9), consistent with previous studies showing an antinestedness structure in ectomycorrhizal symbioses (i.e., lower weighted NODF values compared to null models) (2, 47). Supporting H3, warming and reduced rainfall treatments caused significant and consistent changes to interaction network complexity and structure (SI Appendix, Fig. S8 and Table S9). Specifically, the network specialization (H2’) index increased with the warming and reduced rainfall treatments and was highest in the combined treatment at both sites. H2’ was also negatively correlated with mean growing season soil moisture (P = 0.049; R2 = 0.50; Fig. 4A), indicating that specialization was highest at low soil moisture. Conversely, the nestedness of the interaction networks (weighted NODF) was lowest in the combined treatment at both sites (SI Appendix, Table S9) and positively correlated with mean growing season soil moisture (P = 0.034; R2 = 0.56; Fig. 4B). Finally, tree host species (=node)–level specialization (d’) was negatively related to growing season mean photosynthetic rates, indicating that hosts with reduced photosynthetic capacity were colonized by more specialized ectomycorrhizal fungal communities (P = 0.008; R2 = 0.21; Fig. 5), supporting H4.
Fig. 4.
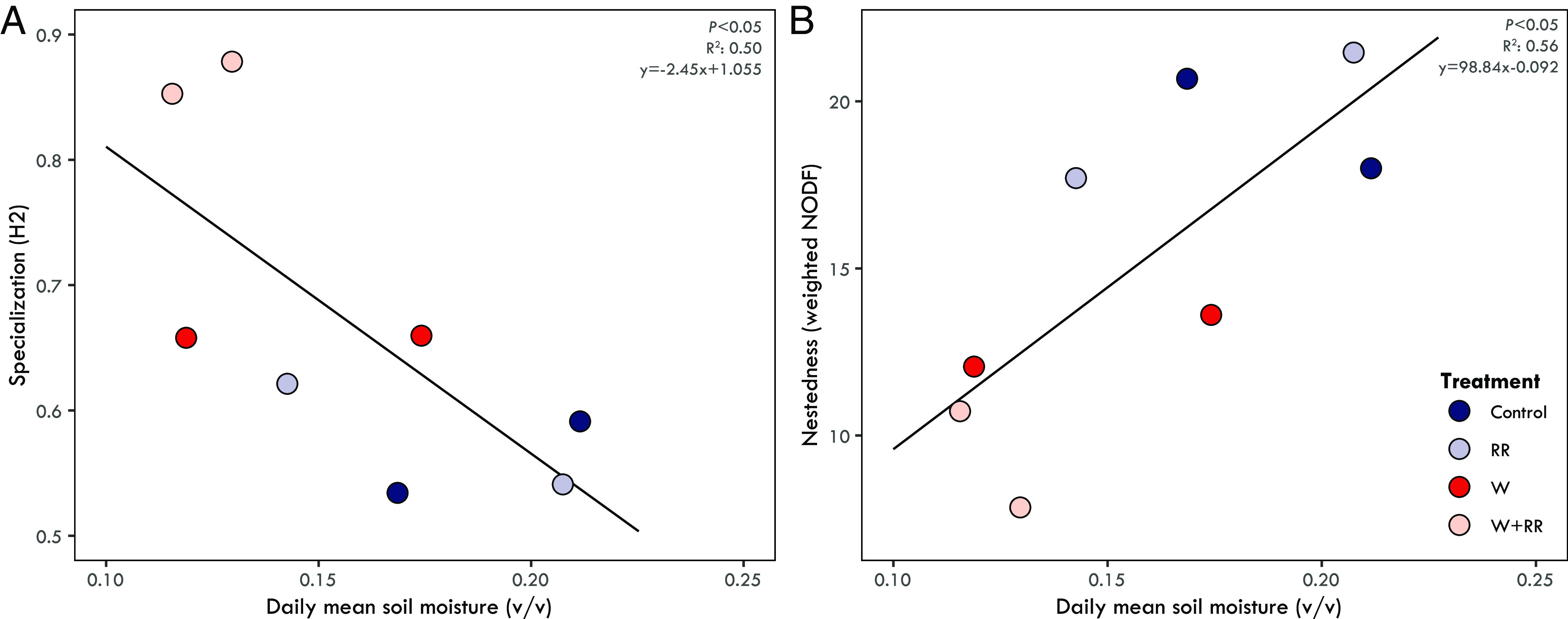
The relationship between interaction network specialization (A) and nestedness (B) and daily mean soil moisture during the growing season. Each point represents a bipartite network associated with each site and treatment [color coded: ambient temperature & ambient precipitation (Control) = dark blue; ambient temperature & reduced rainfall (RR) = light blue; warmed & ambient precipitation (W) = red, warmed & reduced rainfall (W+RR) = pink]. Network specialization (H2’) had a significant negative relationship with soil moisture (Linear regression: P < 0.05; R2: 0.50; y = −2.45x + 1.055). Network nestedness (weighted NODF) was positively related to soil moisture (Linear regression: P < 0.05; R2: 0.56; y = 98.84x − 0.092).
Fig. 5.
The relationship between host-level specialization (d’) and photosynthetic rate (Anet). Each point represents a growing season mean photosynthetic rate (µmol CO2 m-2 s−1) and species-level specialization index (d’) for each tree host species [coded by shape: Betula papyrifera (square); Pinus banksiana (circle); Pinus strobus (triangle); Quercus macrocarpa (diamond)] under each of the climate change treatments [color coded: ambient temperature & ambient precipitation (Control) = dark blue; ambient temperature & reduced rainfall (RR) = light blue; warmed & ambient precipitation (W) = red, warmed & reduced rainfall (W+RR) = pink] at each site (N = 32). Host-level specialization was negatively related to host photosynthetic rate (Linear regression: P < 0.01; R2: 0.21; y = −0.029x + 0.97); Linear regression with outlier removed (line not shown): P < 0.001; R2: 0.33; y = −0.034x + 1.03).
Discussion
Despite the widely recognized importance of ectomycorrhizal symbioses in forest ecosystems, how climate change will alter ectomycorrhizal fungal communities and their traits remains poorly understood, with the response and resilience to climate change of ectomycorrhizal interaction networks having gone largely unexamined to date. Our findings suggest that the effects of warming and reduced rainfall not only will lead to significant changes in ectomycorrhizal fungal community structure, but also will contribute to a disruption in the structure of ectomycorrhizal interaction networks at the boreal–temperate ecotone. Specifically, both warming and reduced rainfall significantly decreased the level of network generality inferred from the bipartite network analyses, resulting in less redundancy in the networks. This lowered redundancy was strongly correlated with reductions in soil moisture caused by the warming and reduced rainfall treatments and was most pronounced in the combined treatment (Fig. 4A). Since adequate water is important for the growth and survival of ectomycorrhizal fungi (48), we suspect water limitation in particular may directly contribute to shifts in ectomycorrhizal community composition and the concordant changes in interaction network structure. Additionally, reduced soil moisture reduced host productivity for three of the four hosts, which could have led to indirect negative effects on ectomycorrhizal fungal communities and interaction network structure through reduced C allocation (25, 49). Other studies have shown that ectomycorrhizal fungal biomass production is dependent on the amount of host C allocation (50) and that when water is limiting and photosynthesis is correspondingly reduced, EMM biomass production also declines (51, 52).
It appears that shifts in ectomycorrhizal fungal community composition were likely a primary factor undergirding the observed changes in interaction network structure in the warming and reduced rainfall treatments. Under ambient conditions, the ectomycorrhizal fungal communities of all the hosts studied were dominated by Tomentella, Russula, and Sebacina species. These genera are among the most common in forests globally (53) and share a suite of traits that may contribute to their dominance and the presence of highly redundant bipartite interaction networks. First, they have wide host ranges and colonize tree host species primarily via EMM and not from spores (54, 55). The host generality of these taxa is a prerequisite to the formation of mycelial networks between different tree individuals (41, 56) and their high abundance in ectomycorrhizal fungal communities suggests there is a high potential for the formation of mycelial connections between different tree individuals of the same or different species in the forests where they occur. Second, while there is variation in exploration type morphology within and among these genera, the species belonging to each have hydrophilic EMM that can be relatively extensive [i.e., medium distance exploration types; (57)]. The extent of the EMM is an important feature not only for exploring the soil for nutrients, but presumably for colonizing fine roots and forming mycelial connections among different hosts (17, 55, 58). Since the hyphae of these taxa are hydrophilic, they may be more susceptible to water loss under reduced osmotic potential (45). This may explain their negative response to the warmed and reduced rainfall treatments, which had significantly reduced soil moisture. Tradeoffs between traits related to stress tolerance (e.g., moisture niche) and competitive ability (e.g., growth rate) have been documented among free-living fungi, where community dominants tend to have narrower niche breadth (59). While the application of this relationship to symbiotic fungi is complicated by dependence on host C provisions, it is reasonable to expect similar tradeoffs among ectomycorrhizal fungi. Finally, there is evidence suggesting that these dominant ectomycorrhizal genera are important in accessing organic N (60–62), facilitating soil N retention (63, 64), and augmenting host nutrition (65, 66). Together, the dominance of these taxa and the low level of interaction network specialization found in ambient plots indicates that these taxa are likely key components of shared mycelial networks in these high latitude forests.
Under warming and reduced rainfall, the ectomycorrhizal fungal communities shifted in dominance toward members of the Ascomycota as well as specific basidiomycete genera such as Inocybe, Thelephora, Hebeloma, Laccaria, and Clavulina (Fig. 1). Apart from Thelephora, all these EM fungi possess contact-short distance exploration strategies and their increased relative abundances with warming are consistent with findings of previous studies conducted at the B4WarmED sites (4, 25, 26) as well as other climate change experiments (67–70). Many of these taxa also possess traits consistent with a life history strategy that allocates resources to maximize reproduction and dispersal at the cost of an extensive EMM and colonizing roots almost exclusively from spores (54, 71, 72). Further, these fungi tend to be found in low abundance in the active EMF communities (i.e., colonized roots) of mature forests, but have resistant spores that lead to their accumulation and domination of spore banks (73). The colonization of roots from spores often results in numerous small genets that turn over quickly (12, 74). Further, their ability to access and mobilize nutrients to support their hosts from organic pools is limited (54, 75–78). While many are generalists in terms of host associations, their spatially limited and short-lived EMM may limit their capacity to connect tree hosts via shared mycelial networks in both space and over time (74, 79).
Ascomycete ectomycorrhizal fungi are common in both disturbed and early successional soils (80–83). They often possess traits likely favored in these habitats, including low-biomass short-distance exploration strategies (57), production of resistant propagules that dominate spore banks (84), frequent asexual spore production (85), and small genets that frequently turnover (86). However, some ectomycorrhizal ascomycetes are notably stress tolerant, with Cenococcum geophilum being perhaps the most notable (87–90), and may provide benefits to host nutrition during periods of water stress (65, 91–93). At the same time, variation in tolerance to water stress and nutritional benefits to hosts under water stress has been found even among ectomycorrhizal fungal taxa in the same genus, making generalizations at higher taxonomic groupings challenging (65, 94). Further, there may be limits to host benefits provided by ectomycorrhizal fungi if water stress becomes too extreme. For instance, in a warming and rainfall reduction experiment conducted in a semi-arid shrubland system, host nutrition declined significantly compared to the control despite the dominance of ascomycete ectomycorrhizal fungi (95). In our system, and in other temperate and boreal forest ecosystems, it remains unclear if ascomycete ectomycorrhizal fungi are involved in maintaining host nutrition during periods of water stress, colonizing host roots from resistant spore banks when other members fall out of the community, or are less carbon costly to stressed host plants. Both laboratory- and field-based experiments parsing these possibilities will be a key next step in better linking ectomycorrhizal fungal community structure to functioning under shifting environmental conditions.
Interestingly, in addition to the increase in contact-short distance exploration types with warming and reduced rainfall, we did also observe a notable increase in Rhizopogon spp. and Suillus americanus (SI Appendix, Fig. S7). These Suilloid taxa produce rhizomorphs, which are hydrophobic long-distance exploration structures that likely require significant host C investment to sustain (44). Since Suillus and Rhizopogon almost exclusively associate with Pinaceae hosts, the increase in their abundance under altered environmental conditions contributed to the increased network-level specialization metrics here. Their increased abundance, however, is surprising because Suilloid taxa are generally thought of as C-costly taxa, and we found that both pine hosts exhibited significant reductions in photosynthetic capacity with warming and reduced rainfall. Indeed, Fernandez et al. (25) showed that ectomycorrhizal fungal taxa that produce longer distance exploration strategies were positively correlated with photosynthetic capacity in Abies balsamea and Betula papyrifera in adjacent closed canopy B4WarmED plots. In this study, we observed a similar response except for Pinus-specific Suilloid taxa. Our Suilloid results are, however, consistent with a greenhouse study that grew Pinus pinaster seedlings under varying water availability and found that increasing drought conditions favored certain Suillus and Rhizopogon species. It is possible that due to their hydrophobic rhizomorphs, these taxa may facilitate greater water transport under reduced soil moisture (96). Alternatively, Bruns et al. (73) proposed that Suilloid ectomycorrhizal fungi may have adapted the ability to extract more C from their hosts compared to generalist taxa. In a scenario where competition with typically dominant ectomycorrhizal fungi is reduced by increased climate change–related stress, Suilloid ectomycorrhizal fungi may be able to extract relatively high amounts of C without necessarily increasing nutrient returns, effectively becoming parasitic under these conditions.
While our study contributes to a mounting body of literature suggesting that mycorrhizal fungi are sensitive to changes in climate, we acknowledge several limitations that should be considered. First, the presence of mycelial connections between different tree individuals by the same fungal individual were not physically assessed, instead they were inferred based on sequence read abundance from metabarcoding of fine roots. This approach, while effective identifying shared taxa, cannot detect physical connections between plants via ectomycorrhizal fungi. As such, this study should be considered a first look at the potential response of ectomycorrhizal mycelial network structure to climate change, with future experiments aimed at validating the physical network responses using approaches that integrate metabarcoding, isotopic labeling, and visualization techniques (i.e., minirhizotron imaging, mesocosms) or quantify taxon-specific EMM (i.e., ingrowth bags) (17). Second, while we attempted to capture host representatives from both temperate and boreal latitudinal ranges as well as major phylogenies (broadleaf versus conifer), there were other ectomycorrhizal host species in the plots contributing to ectomycorrhizal fungal community composition that were not sampled. This could have led to sampling bias if those other tree host ectomycorrhizal fungal communities did not respond in a similar manner. That said, based on previous work from the B4WarmED experiment both above- (24, 46) and below-ground (4, 25, 26), we expect similar negative responses in terms of host photosynthetic performance and changes in ectomycorrhizal fungal community composition. Third, while our results contribute to a mounting body of work suggesting that changes to host productivity under warming and drought stress are critical drivers of changes in ectomycorrhizal fungal communities, our analyses did not include root responses (i.e., production; specific root length), which may be equally important. Future studies should include root measures to explicitly test above- and below-ground host responses and linkages to ectomycorrhizal communities and interaction networks. Finally, because the tree hosts in the experiment are not mature trees and have relatively small root systems, it is plausible that they may be more susceptible to water stress than adults, and result in stronger ectomycorrhizal responses to the climate change treatments.
Conclusions
In this study, we have documented important changes in both ectomycorrhizal fungal community composition and interaction network structure associated with cooccurring temperate and boreal tree hosts in response to one of the most realistic tests of potential changes in temperature and rainfall. In particular, the climate change–induced effects on ectomycorrhizal fungal composition, which favored ectomycorrhizal fungal taxa with limited mycelial growth and shorter turnover times, likely disrupts the formation and fungal of shared mycelial networks among diverse forest trees. We hypothesize that this disruption will exacerbate negative effects on tree host performance and distribution, particularly those adapted to cooler and wetter climates (as documented at this site, 24). Since this study was positioned near the latitudinal boundaries of the tree hosts, however, it may not necessarily reflect their response at mid-range latitudes and future effort should be placed on further characterizing responses of ectomycorrhizal interaction networks to climate change in a variety of forest systems. Given the emerging recognition that plant-fungal networks are integral properties of terrestrial ecosystems (97–99), it is imperative that future research aims to understand how resilient they are to changing climate as well as their influence on ecosystem processes.
Methods
Sites & Experimental Design.
The study was conducted in the B4WarmED experiment (100, 101), which is located at two sites in northern Minnesota, USA, at the Cloquet Forestry Center (Cloquet, MN: 46°40’46” N, 92°31’12” W, 382 m a.s.l., 4.8 °C mean annual temperature, 783 mm mean annual precipitation) and the Hubachek Wilderness Research Center (Ely, MN: 47°56’46” N, 91°45’29” W, 415 m a.s.l., 2.6 °C mean annual temperature, 726-mm mean annual precipitation). Soil pH from the top 0 to 5 cm of the profile had a mean value of 5.40 and 5.44 for Cloquet and Ely, respectively (SI Appendix, Table S1). Each site includes 36 circular 3-m diameter plots exposed to different levels of simultaneous above and belowground warming (ambient, historical targets of +1.7 °C, and +3.4 °C, N = 12 for each treatment). Plots are located in either a closed or open canopy condition (plots with a mature forest overstory dominated by Populus tremuloides, or no overstory; N = 18 in each condition). In the open canopy condition, half of the plots are exposed to an additional rain reduction treatment (ambient, dry; N = 9 for each treatment) in a full factorial design (100, 101). In 2012, 11 tree species that cooccur at the ecotone were planted in each plot in a randomized fashion, including six native angiosperms (Acer rubrum, A. saccharum, Betula papyrifera, Populus tremuloides, Quercus macrocarpa and Q. rubra), one naturalized angiosperm (Rhamnus cathartica), and four native gymnosperms (Abies balsamea, Picea glauca, Pinus banksiana, and Pinus strobus). Some of the original plants did not survive that first growing season and were replanted the following year (SI Appendix, Supplemental File 1). Unlike our previous ectomycorrhizal-related research at B4WarmED, which was conducted in the closed canopy plots (25), this study was carried out in the open canopy plots where the rain reduction treatments were present in the highest warming treatment levels (ambient, +3.4 °C) for a total of 24 plots sampled (two sites × two warming treatment levels × two rainfall reduction treatment levels × three replicate plots for each treatment combination).
Warming was accomplished via infrared lamp heaters aboveground and soil heating cables belowground (detailed in ref. 100). Briefly, lamps and cables were turned on annually from early spring to late autumn (ca. 8 mo per y) via a feedback control that acted concurrently and independently at the plot scale to maintain a fixed temperature differential from ambient conditions above- and belowground. The warming treatment led to a realized increase of 3.1 °C above ambient plant and soil temperatures. The rainfall reduction treatment included two levels and was installed in half of the plots within each block; ambient and an average of ca. 30% of growing season precipitation removed (24). The rainfall manipulation relies on custom-made 20 m2 heavy duty tarps on a furling system to be deployed only during individual rainfall events (see ref. 101 for further details). Manual rain gauges above the vegetation within the plots and time-domain reflectometry probes monitor soil moisture on an hourly basis from 0 to 20-cm upper soil profile throughout the entire season within each plot, allowing estimates of the efficacy of the rainfall removal. Soil temperatures were measured every 10 s then averaged in 15-min intervals and logged for the duration of the experiment within each plot using two sealed thermocouples (type T) installed at the depth of 10 cm. Soil moisture was monitored hourly within each plot from 0 to 20-cm depth using time delay reflectometer (TDR) probes (Campbell Scientific, Logan, UT, USA) and expressed as volumetric water content. Since ectomycorrhizal fungi are most active during the late summer and autumn months (102), we calculated the daily mean growing season (June to October) soil temperature and moisture for each plot to be used in all downstream analyses. Soil water content was ca. 15% higher at the Cloquet site compared to the soils at the Ely site during the growing season, likely due to differences in sand content (SI Appendix, Table S1). Compared to ambient conditions, the warming treatment reduced average soil moisture by 17% and 30% at Cloquet and Ely sites, while the reduced rainfall treatment reduced soil moisture by 2% and 15%, respectively. Together, the combined warming and reduced rainfall treatment reduced soil moisture by 39% and 32% at the two sites, respectively (SI Appendix, Fig. S1B and Table S4).
To calculate net N mineralization and nitrification rates, soil inorganic nitrogen pools (NH4+ and NO3) were measured over four consecutive time periods in 2013 (November, 2012–April, 2013, May–June, 2013; July–August, 2013, and September–October, 2013). Two 2.5-cm-diameter soil cores were obtained from each plot (9 cm depth) at the beginning of each period, sieved (2 mm), homogenized, shaken with 2 M KCl for 1 h, filtered, and the extracts analyzed for NH4+ and NO3−. One additional 5-cm diameter by 9-cm deep PVC core was installed in each plot, the tops capped, and left to incubate until the end of the period, when it was processed in the same way as the initial samples. Net N mineralization and nitrification rates were calculated as the difference in the inorganic nitrogen pools at the end and the beginning of each incubation period, with rates summed over all incubation periods during the year to obtain an annual rate. Neither net mineralization nor net nitrification rates were significantly affected by warming (NH4: F1,19 = 0.75; P = 0.39, NO3: F1,19 = 2.47; P = 0.13), reduced rainfall (NH4: F1,19 = 0.11; P = 0.74, NO3: F1,19 = 0.195; P = 0.66), or their combination (NH4: F1,19 = 1.03; P = 0.32, NO3: F1,19 = 0.79; P = 0.38) (SI Appendix, Table S4).
Ectomycorrhizal Fungal Community Sampling.
From the 11 tree species planted in the plots, we sampled the ectomycorrhizal fungal communities of four hosts from both major tree lineages (angiosperm and gymnosperm) from each forest biome (boreal and temperate) for inclusion in our study; the boreal angiosperm Betula papyrifera, the boreal gymnosperm Pinus banksiana, the temperate angiosperm Quercus macrocarpa, and the temperate gymnosperm Pinus strobus. In October 2016, saplings of the four tree species were harvested from the plots (four host species × two sites × two warming treatment levels × two rain reduction treatment levels × three replicate plots; N = 96) and whole intact root systems from each replicate were then processed by rinsing of adhering soil. For each replicate, the entirety of the extracted fine root system was sampled and transferred on ice to the laboratory within 4 h to be prepared for DNA extraction. In the lab, each root sample was carefully rewashed and dried at 40 °C for 48 h. Samples were then gently individually crushed inside of folded paper to separate the fine roots colonized by ectomycorrhizal fungi from the larger noncolonized coarse roots. From the fine root pool, 20 mg from each sample were placed in screw-cap tubes and homogenized for 1 min via bead beating (BioSpec Products, Bartlesville, OK, USA). Total genomic DNA from each of the root homogenate samples was extracted using the chloroform extraction method detailed in Kennedy et al. (6). ITS1 rDNA subunit was PCR amplified using a barcoded fungal-specific ITS1F-ITS2 primer pair under cycling conditions detailed in Fernandez et al. (25). Amplicons from each sample were cleaned and normalized using Charm ‘Just-a-Plate’ kits (Charm, San Diego, CA, USA). The samples were then pooled into a single library and sequenced at the University of Minnesota Genomics Center using 250-bp paired-end V2 MiSeq Illumina chemistry (Illumina, San Diego, CA, USA).
Bioinformatics.
We used the FAST pipeline (v1.102) to process the high-throughput sequence data using the paired-end read option (https://github.com/ZeweiSong/FAST). This included the 96 root samples, a mock community, two negative extraction controls, and a PCR negative control for a total of 100 samples. To account for any sequences in the negative controls, we subtracted the total reads for each operational taxonomic unit (OTU, grouped at 97% and identified by USEARCH-based matching to the UNITE Database v. 08.22.16) from each sample (a total of 481 reads were found in all negative controls). Two samples failed to yield any sequence reads and were removed from the dataset. Based on two contaminant OTUs found in the mock community, both with <9 sequence reads, we set all OTUs with <8 sequence reads to zero (103). Ectomycorrhizal fungal OTUs were parsed from other fungal guilds using FUNGuild (104). From the 3,689,386 sequence reads passing the quality filtering steps, 2,086,830 sequence reads could be assigned to the ectomycorrhizal guild with FUNGuild. Using criteria detailed in Fernandez et al. (25) we were able to manually assign 35 additional unassigned OTUs (due to missing genus taxonomy required by FUNGuild) as ectomycorrhizal, which accounted for an additional 928,830 sequence reads. In total, 241 OTUs were assigned to the ectomycorrhizal guild and comprised 3,015,660 of the total 3,689,386 sequence reads across all samples (82%). From the remaining sequence reads, 367,232 (10%) belonged to 293 nonectomycorrhizal OTUs (i.e., saprotrophs, pathotrophs) and 306,943 (8%) belonged to 324 OTUs that were unable to be identified to guild. Raw sequence read files are available in NCBI short read archive (# PRJNA561610) (105).
Host Photosynthesis and Growth.
Photosynthetic rates (Anet) at light saturating conditions (1,200 μmol m−2 s−1 photosynthetically active radiation) and leaf diffusive conductance (gs) were measured in situ at both sites across all treatments during two to five independent campaigns conducted from June to September in all years (2012 to 2016) using Li-Cor 6400xt portable photosynthesis systems (Li-Cor, Lincoln, NE, USA). We calculated mean photosynthetic rate and mean conductance for each host at the plot level across all campaigns and years to be used in downstream analyses. Finally, host height, stem diameter, and allometric stem mass measurements from the final year of the study were measured for each host plant.
Statistics.
All statistical analyses were run in R (version 4.1.0). To test for effects of the warming, reduced rainfall, on soil properties we used two-way linear mixed-models with block nested in site and set as a random factor using the lme function in the ‘nlme’ package. To test for the effects of host species, warming, and rainfall reduction on plant performance metrics, we used three-way linear mixed-models with plot nested in block, and block nested in site as a random effect. To evaluate the effects of warming and reduced rainfall treatments as well as host on ectomycorrhizal fungal alpha-diversity, we analyzed observed OTU richness with a three-way linear mixed-models, with plot nested in block, and block nested in site as nested random factors. To examine the effects of warming, reduced rainfall, and host on ectomycorrhizal fungal community composition, we used a factorial permutational multivariate ANOVA (PERMANOVA) using the adonis2 function in the ‘vegan’ package (version 2.5 to 7). Prior to examining treatment effects on ectomycorrhizal fungal community composition, OTU sequence reads were Hellinger transformed using the decostand function in the ‘vegan’ package (version 2.5 to 7). Pair-wise distances were then calculated based on Bray-Curtis dissimilarity. The PERMANOVA model included all main effects and interactions set at 999 permutations. Additionally, we used redundancy analysis (RDA) to visualize and relate how ectomycorrhizal fungal community composition responded to changes in soil temperature and soil moisture.
To further assess ectomycorrhizal fungal community responses to warming and reduced rainfall, we compared mean relative abundances of the following ectomycorrhizal fungal functional trait groupings: exploration type (Contact-Short, Contact-Medium, or Medium-Long) and mycelial hydrophobicity (Hydrophilic or Hydrophobic), with three-way linear mixed-models, with plot nested in block, and block nested in site as nested random factors. When main effects were significant, post hoc Dunnett’s tests were used to compare the relative abundance of each specific functional grouping in each treatment compared to the control (ambient temperature and precipitation) with the ‘emmeans’ package (version 1.6.0). Exploration types and hydrophobicity assignments for individual OTUs were based on genus-level identities following Agerer (57) and Lilleskov et al. (106) (SI Appendix, Table S3). Since the exploration types of some ectomycorrhizal fungal genera can vary in extent among species and under different environmental conditions (57, 104), we used groupings based on the range for each genus (Contact-Short; Contact-Medium; Medium-Long).
Network Analysis.
To model interaction network complexity and structure between ectomycorrhizal fungi and the four tree hosts, we calculated network indices for each 2-mode weighted network using the ‘bipartite’ package (version 2.16). Each network was constructed from incidence matrices from ectomycorrhizal fungal OTU sequence reads pooled, normalized (percent relative abundance), and rounded to the nearest integer for each combination of plant host, treatment, and site (N = 8). We calculated network specialization (H2’) for each of the eight networks (39); networks with a high generalization have relatively low H2’ compared to more specialized networks. To estimate network nestedness, we calculated the quantitative metric weighted nestedness based on overlap and decreasing fill (a.k.a. weighted NODF) (40). The significance of each of the indices was assessed by comparing observed values to null models (N = 1,000) constructed with r2dtable and vaznull algorithms (SI Appendix, Table S9). Calculated network indices were used in linear regression analyses to understand their relationship to mean growing season soil moisture. Bipartite network visualizations were generated using the LGL layout algorithm in ‘igraph’ package (version 1.2.6).
In addition to the network-level indices, we also calculated node-level indices for the plant hosts to understand their influence on interaction network structure under the experimental treatments. We were specifically interested in how the productivity of each host plant affected the generality (vs. specificity) of their ectomycorrhizal fungal communities (i.e., the level of shared symbionts) in relation to warming and reduced rainfall. To do this, we calculated the selectivity index (d’) (39) for each host in each network and then took those values and regressed them on growing season mean photosynthetic rates. Collectively, this analysis allowed for a direct assessment of the relationship between ectomycorrhizal host productivity and the ectomycorrhizal symbiont generality.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (CSV)
Acknowledgments
We would like to thank A. Certano, K. Uy, H. Upin and Z. Smith for laboratory and field assistance. Funding was provided by the U.S. Department of Energy, Office of Science, and Office of Biological and Environmental Research (award number DE-FG02-07ER644456); Minnesota Agricultural Experiment Station (MN-42-030 and MN-42-060); the Minnesota Department of Natural Resources; College of Food, Agricultural, and Natural Resources Sciences and Wilderness Research Foundation, University of Minnesota; NSF Biological Integration Institute program (NSF-DBI 2021898).
Author contributions
C.W.F., L.M., P.B.R., and P.G.K. designed research; C.W.F., L.M., A.S., R.B., S.E.H., R.A.M., P.B.R., and P.G.K. performed research; C.W.F. analyzed data; and C.W.F., L.M., A.S., R.B., S.E.H., R.A.M., P.B.R., and P.G.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Raw sequence read files are available in NCBI short read archive (# PRJNA561610) (105). All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Newman E. I., “Mycorrhizal links between plants: Their functioning and ecological significance” in Advances in Ecological Research, Begon M., Fitter A. H., Ford E. D., Macfadyen A., Eds. (Academic Press, 1988), pp. 243–270. [Google Scholar]
- 2.Bahram M., Harend H., Tedersoo L., Network perspectives of ectomycorrhizal associations. Fungal Ecol. 7, 70–77 (2014). [Google Scholar]
- 3.Taudiere A., et al. , Beyond ectomycorrhizal bipartite networks: projected networks demonstrate contrasted patterns between early- and late-successional plants in Corsica. Front. Plant Sci. 6, 881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mucha J., et al. , Effect of simulated climate warming on the ectomycorrhizal fungal community of boreal and temperate host species growing near their shared ecotonal range limits. Microb. Ecol. 75, 348–363 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karst J., Jones M. D., Hoeksema J. D., Positive citation bias and overinterpreted results lead to misinformation on common mycorrhizal networks in forests. Nat. Ecol. Evol. 7, 501–511 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Kennedy P. G., Izzo A. D., Bruns T. D., There is high potential for the formation of common mycorrhizal networks between understorey and canopy trees in a mixed evergreen forest. J. Ecol. 91, 1071–1080 (2003). [Google Scholar]
- 7.Horton T. R., Ed., Mycorrhizal Networks (Springer, Netherlands, 2015), vol. 224. [Google Scholar]
- 8.Nara K., Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 169, 169–178 (2006). [DOI] [PubMed] [Google Scholar]
- 9.McGuire K. L., Common ectomycorrhizal networks may maintain monodominance in a tropical rain forest. Ecology 88, 567–574 (2007). [DOI] [PubMed] [Google Scholar]
- 10.van Der Heijden M. G. A., Horton T. R., Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 97, 1139–1150 (2009). [Google Scholar]
- 11.Booth M. G., Hoeksema J. D., Mycorrhizal networks counteract competitive effects of canopy trees on seedling survival. Ecology 91, 2294–2302 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Nara K., Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol. 169, 169–178 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Ekblad A., et al. , The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: Role in carbon cycling. Plant Soil 366, 1–27 (2013). [Google Scholar]
- 14.Clemmensen K. E., et al. , Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Fernandez C. W., Kennedy P. G., Moving beyond the black-box: Fungal traits, community structure, and carbon sequestration in forest soils. New Phytol. 205, 1378–1380 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z., Yuan Y., Liu Q., Yin H., Plant nitrogen acquisition from inorganic and organic sources via root and mycelia pathways in ectomycorrhizal alpine forests. Soil Biol. Biochem. 136, 107517 (2019). [Google Scholar]
- 17.Fernandez C. W., The advancing mycelial frontier of ectomycorrhizal fungi. New Phytol. 230, 1296–1299 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Simard S. W., et al. , Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 26, 39–60 (2012). [Google Scholar]
- 19.Pickles B. J., Egger K. N., Massicotte H. B., Green D. S., Ectomycorrhizas and climate change. Fungal Ecol. 5, 73–84 (2012). [Google Scholar]
- 20.Bennett A. E., Classen A. T., Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 101, e02978 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Baldrian P., López-Mondéjar R., Kohout P., Forest microbiome and global change. Nat. Rev. Microbiol. 21, 487–501 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Price D. T., et al. , Anticipating the consequences of climate change for Canada’s boreal forest ecosystems. Environ. Rev. 21, 322–365 (2013). [Google Scholar]
- 23.Reich P. B., et al. , Geographic range predicts photosynthetic and growth response to warming in co-occurring tree species. Nat. Clim. Change 5, 148–152 (2015). [Google Scholar]
- 24.Reich P. B., et al. , Even modest climate change may lead to major transitions in boreal forests. Nature 608, 540–545 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Fernandez C. W., et al. , Ectomycorrhizal fungal response to warming is linked to poor host performance at the boreal-temperate ecotone. Global Change Biol. 23, 1598–1609 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Van Nuland M. E., et al. , Warming and disturbance alter soil microbiome diversity and function in a northern forest ecotone. FEMS Microbiol. Ecol. 96, fiaa108 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Lankau R. A., Zhu K., Ordonez A., Mycorrhizal strategies of tree species correlate with trailing range edge responses to current and past climate change. Ecology 96, 1451–1458 (2015). [Google Scholar]
- 28.Pither J., Pickles B. J., Simard S. W., Ordonez A., Williams J. W., Below-ground biotic interactions moderated the postglacial range dynamics of trees. New Phytol. 220, 1148–1160 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Evans P., Brown C. D., The boreal–temperate forest ecotone response to climate change. Environ. Rev. 25, 423–431 (2017). [Google Scholar]
- 30.Steidinger B. S., et al. , Ectomycorrhizal fungal diversity predicted to substantially decline due to climate changes in North American Pinaceae forests. J. Biogeogr. 47, 772–782 (2020). [Google Scholar]
- 31.Nuñez M. A., Dickie I. A., Invasive belowground mutualists of woody plants. Biol. Invasions 16, 645–661 (2014). [Google Scholar]
- 32.Pec G. J., Simard S. W., Cahill J. F., Karst J., The effects of ectomycorrhizal fungal networks on seedling establishment are contingent on species and severity of overstorey mortality. Mycorrhiza 30, 173–183 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Lehto T., Effect of drought on Picea sitchensis seedlings inoculated with mycorrhizal fungi. Scandinavian J. Forest Res. 7, 177–182 (1992). [Google Scholar]
- 34.Alvarez M., et al. , Ectomycorrhizal fungi enhance nitrogen and phosphorus nutrition of Nothofagus dombeyi under drought conditions by regulating assimilative enzyme activities. Physiol. Plant. 136, 426–436 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Kipfer T., Wohlgemuth T., van der Heijden M. G. A., Ghazoul J., Egli S., Growth response of drought-stressed pinus sylvestris seedlings to single- and multi-species inoculation with ectomycorrhizal fungi. PLOS One 7, e35275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landi P., Minoarivelo H. O., Brännström Å., Hui C., Dieckmann U., “Complexity and stability of adaptive ecological networks: A survey of the theory in community ecology” in Systems Analysis Approach for Complex Global Challenges, Mensah P., Katerere D., Hachigonta S., Roodt A., Eds. (Springer International Publishing, 2018), pp. 209–248. [Google Scholar]
- 37.Waser N. M., Chittka L., Price M. V., Williams N. M., Ollerton J., Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060 (1996). [Google Scholar]
- 38.Hoiss B., Krauss J., Steffan-Dewenter I., Interactive effects of elevation, species richness and extreme climatic events on plant–pollinator networks. Global Change Biol. 21, 4086–4097 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Blüthgen N., Menzel F., Blüthgen N., Measuring specialization in species interaction networks. BMC Ecology 6, 9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida-Neto M., Ulrich W., A straightforward computational approach for measuring nestedness using quantitative matrices. Environ. Modell. Software 26, 173–178 (2011). [Google Scholar]
- 41.Molina R., Horton T. R., “Mycorrhiza specificity: Its role in the development and function of common mycelial networks” in Mycorrhizal Networks, Ecological Studies, Horton T. R., Ed. (Springer, Netherlands, 2015), pp. 1–39. [Google Scholar]
- 42.Deslippe J. R., Simard S. W., Below-ground carbon transfer among Betula nana may increase with warming in Arctic tundra. New Phytol. 192, 689–698 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Morgado L. N., et al. , Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Global Change Biol. 21, 959–972 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weigt R. B., Raidl S., Verma R., Agerer R., Exploration type-specific standard values of extramatrical mycelium – a step towards quantifying ectomycorrhizal space occupation and biomass in natural soil. Mycol. Progress 11, 287–297 (2012). [Google Scholar]
- 45.Unestam T., Sun Y.-P., Extramatrical structures of hydrophobic and hydrophilic ectomycorrhizal fungi. Mycorrhiza 5, 301–311 (1995). [Google Scholar]
- 46.Reich P. B., et al. , Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 562, 263–267 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Toju H., Guimarães P. R. Jr., Olesen J. M., Thompson J. N., Below-ground plant–fungus network topology is not congruent with above-ground plant–animal network topology. Sci. Adv. 1, e1500291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coleman M. D., Bledsoe C. S., Lopushinsky W., Pure culture response of ectomycorrhizal fungi to imposed water stress. Can. J. Bot. 67, 29–39 (1989). [Google Scholar]
- 49.Sapes G., Demaree P., Lekberg Y., Sala A., Plant carbohydrate depletion impairs water relations and spreads via ectomycorrhizal networks. New Phytol. 229, 3172–3183 (2021). [DOI] [PubMed] [Google Scholar]
- 50.Hobbie E. A., Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies. Ecology 87, 563–569 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Castaño C., et al. , Seasonal dynamics of the ectomycorrhizal fungus Lactarius vinosus are altered by changes in soil moisture and temperature. Soil Biol. Biochem. 115, 253–260 (2017). [Google Scholar]
- 52.Hagenbo A., et al. , Production and turnover of mycorrhizal soil mycelium relate to variation in drought conditions in mediterranean pinus pinaster, pinus sylvestris and quercus ilex forests. New Phytol. 230, 1609–1622 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Tedersoo L., et al. , Global diversity and geography of soil fungi. Science 346, 1256688 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Nara K., Spores of ectomycorrhizal fungi: Ecological strategies for germination and dormancy. New Phytol. 181, 245–248 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Peay K. G., Kennedy P. G., Bruns T. D., Rethinking ectomycorrhizal succession: are root density and hyphal exploration types drivers of spatial and temporal zonation? Fungal Ecol. 4, 233–240 (2011). [Google Scholar]
- 56.Kennedy P. G., Walker J. K. M., Bogar L. M., “Interspecific mycorrhizal networks and non-networking hosts: Exploring the ecology of the host genus alnus” in Mycorrhizal Networks, Ecological Studies, Horton T. R., Ed. (Springer, Netherlands, 2015), pp. 227–254. [Google Scholar]
- 57.Agerer R., Exploration types of ectomycorrhizae. Mycorrhiza 11, 107–114 (2001). [Google Scholar]
- 58.Anderson I. C., Cairney J. W. G., Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 31, 388–406 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Maynard D. S., et al. , Consistent trade-offs in fungal trait expression across broad spatial scales. Nat. Microbiol. 4, 846–853 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Bödeker I. T. M., Nygren C. M. R., Taylor A. F. S., Olson Å., Lindahl B. D., ClassII peroxidase-encoding genes are present in a phylogenetically wide range of ectomycorrhizal fungi. ISME J. 3, 1387–1395 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Cline L. C., Huggins J. A., Hobbie S. E., Kennedy P. G., Organic nitrogen addition suppresses fungal richness and alters community composition in temperate forest soils. Soil Biol. Biochem. 125, 222–230 (2018). [Google Scholar]
- 62.Fernandez C. W., See C. R., Kennedy P. G., Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytol. 226, 569–582 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Hasselquist N. J., Högberg P., Dosage and duration effects of nitrogen additions on ectomycorrhizal sporocarp production and functioning: An example from two N-limited boreal forests. Ecol. Evol. 4, 3015–3026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallander H., Ekblad A., “The importance of ectomycorrhizal networks for nutrient retention and carbon sequestration in forest ecosystems” in Mycorrhizal Networks, Ecological Studies, Horton T. R., Ed. (Springer, Netherlands, 2015), pp. 69–90. [Google Scholar]
- 65.Pena R., Polle A., Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J. 8, 321–330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pena R., Tejedor J., Zeller B., Dannenmann M., Polle A., Interspecific temporal and spatial differences in the acquisition of litter-derived nitrogen by ectomycorrhizal fungal assemblages. New Phytol. 199, 520–528 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Geml J., et al. , Long-term warming alters richness and composition of taxonomic and functional groups of arctic fungi. FEMS Microbiol. Ecol. 91, fiv095 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Treseder K. K., Marusenko Y., Romero-Olivares A. L., Maltz M. R., Experimental warming alters potential function of the fungal community in boreal forest. Global Change Biol. 22, 3395–3404 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Rosenstock N., Ellström M., Oddsdottir E., Sigurdsson B. D., Wallander H., Carbon sequestration and community composition of ectomycorrhizal fungi across a geothermal warming gradient in an Icelandic spruce forest. Fungal Ecol. 40, 32–42 (2019). [Google Scholar]
- 70.Mrak T., et al. , Different belowground responses to elevated ozone and soil water deficit in three European oak species (Quercus ilex, Q. pubescens and Q. robur). Sci. Total Environ. 651, 1310–1320 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Gherbi H., Delaruelle C., Selosse M.-A., Martin F., High genetic diversity in a population of the ectomycorrhizal basidiomycete Laccaria amethystina in a 150-year-old beech forest. Mol. Ecol. 8, 2003–2013 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Cripps C. L., Ectomycorrhizal fungi above and below ground in a small, isolated aspen stand: A simple system reveals fungal fruiting strategies and an edge effect. Fungi For. Ecosyst. Syst. Diversity Ecol. 89, 249–265 (2004). [Google Scholar]
- 73.Bruns T. D., Bidartondo M. I., Taylor D. L., Host specificity in ectomycorrhizal communities: What do the exceptions tell us? Integr. Comp. Biol. 42, 352–359 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Wadud Md. A., Nara K., Lian C., Ishida T. A., Hogetsu T., Genet dynamics and ecological functions of the pioneer ectomycorrhizal fungi laccaria amethystina and laccaria laccata in a volcanic desert on mount fuji. Mycorrhiza 24, 551–563 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Abuzinadah R. A., Read D. J., The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. New Phytol. 103, 481–493 (1986). [Google Scholar]
- 76.Abuzinadah R. A., Read D. J., The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. New Phytol. 112, 61–68 (1989). [Google Scholar]
- 77.Finlay R. D., Frostegård Å., Sonnerfeldt A.-M., Utilization of organic and inorganic nitrogen sources by ectomycorrhizal fungi in pure culture and in symbiosis with Pinus contorta Dougl. ex Loud. New Phytol. 120, 105–115 (1992). [Google Scholar]
- 78.Horton T. R. “Spore dispersal in ectomycorrhizal fungi at fine and regional scales” in Biogeography of Mycorrhizal Symbiosis, Ecological Studies, Tedersoo L., Ed. (Springer International Publishing, 2017), pp. 61–78. [Google Scholar]
- 79.Fiore-Donno A.-M., Martin F., Populations of ectomycorrhizal Laccaria amethystina and Xerocomus spp. show contrasting colonization patterns in a mixed forest. New Phytol. 152, 533–542 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Mikola P., Studies on the ectendotrophic mycorrhiza of Pine. Acta Forestalia Fennica 79, 7160 (1966), (June 16, 2023). [Google Scholar]
- 81.Ursic M., Peterson R. L., Husband B., Relative abundance of mycorrhizal fungi and frequency of root rot on Pinus strobus seedlings in a southern Ontario nursery. Can. J. For. Res. 27, 54–62 (1997). [Google Scholar]
- 82.Frank J. L., et al. , Rodent dispersal of fungal spores promotes seedling establishment away from mycorrhizal networks on Quercus garryana. Botany 87, 821–829 (2009). [Google Scholar]
- 83.Taschen E., et al. , Whose truffle is this? Distribution patterns of ectomycorrhizal fungal diversity in Tuber melanosporum brûlés developed in multi-host Mediterranean plant communities. Environ. Microbiol. 17, 2747–2761 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Taylor D. L., Bruns T. D., Community structure of ectomycorrhizal fungi in a Pinus muricata forest: Minimal overlap between the mature forest and resistant propagule communities. Mol. Ecol. 8, 1837–1850 (1999). [DOI] [PubMed] [Google Scholar]
- 85.Healy R. A., et al. , High diversity and widespread occurrence of mitotic spore mats in ectomycorrhizal Pezizales. Mol. Ecol. 22, 1717–1732 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Molinier V., et al. , Fine-scale genetic structure of natural Tuber aestivum sites in southern Germany. Mycorrhiza 26, 895–907 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Jany J.-L., Martin F., Garbaye J., Respiration activity of ectomycorrhizas from Cenococcum geophilum and Lactarius sp. in relation to soil water potential in five beech forests. Plant Soil 255, 487–494 (2003). [Google Scholar]
- 88.Hasselquist N., Germino M. J., McGonigle T., Smith W. K., Variability of Cenococcum colonization and its ecophysiological significance for young conifers at alpine–treeline. New Phytol. 165, 867–873 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Kerner R., et al. , Comprehensive proteome analysis in Cenococcum geophilum Fr. As a tool to discover drought-related proteins. J. Proteomics 75, 3707–3719 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Fernandez C. W., Koide R. T., The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol. 6, 479–486 (2013). [Google Scholar]
- 91.Nardinia A., Salleo S., Tyree M. T., Vertovec M., Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann. For. Sci. 57, 305–312 (2000). [Google Scholar]
- 92.Gehring C. A., Sthultz C. M., Flores-Rentería L., Whipple A. V., Whitham T. G., Tree genetics defines fungal partner communities that may confer drought tolerance. Proc. Natl. Acad. Sci. U.S.A. 114, 11169–11174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Querejeta J. I., et al. , Lower relative abundance of ectomycorrhizal fungi under a warmer and drier climate is linked to enhanced soil organic matter decomposition. New Phytol. 232, 1399–1413 (2021). [DOI] [PubMed] [Google Scholar]
- 94.Navarro-Ródenas A., Lozano-Carrillo M. C., Pérez-Gilabert M., Morte A., Effect of water stress on in vitro mycelium cultures of two mycorrhizal desert truffles. Mycorrhiza 21, 247–253 (2011). [DOI] [PubMed] [Google Scholar]
- 95.León-Sánchez L., et al. , Poor plant performance under simulated climate change is linked to mycorrhizal responses in a semi-arid shrubland. J. Ecol. 106, 960–976 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brownlee C., Duddridge J. A., Malibari A., Read D. J., The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport. Plant Soil 71, 433–443 (1983). [Google Scholar]
- 97.Simard S. W., Durall D. M., Mycorrhizal networks: A review of their extent, function, and importance. Can. J. Bot. 82, 1140–1165 (2004). [Google Scholar]
- 98.Tedersoo L., Bahram M., Zobel M., How mycorrhizal associations drive plant population and community biology. Science 367, eaba1223 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Alaux P.-L., Zhang Y., Gilbert L., Johnson D., Can common mycorrhizal fungal networks be managed to enhance ecosystem functionality? Plants People Planet 3, 433–444 (2021). [Google Scholar]
- 100.Rich R. L., et al. , Design and performance of combined infrared canopy and belowground warming in the B4WarmED (Boreal Forest Warming at an Ecotone in Danger) experiment. Global Change Biol. 21, 2334–2348 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Stefanski A., Bermudez R., Sendall K. M., Montgomery R. A., Reich P. B., Surprising lack of sensitivity of biochemical limitation of photosynthesis of nine tree species to open-air experimental warming and reduced rainfall in a southern boreal forest. Global Change Biol. 26, 746–759 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Wallander H., Nilsson L. O., Hagerberg D., Bååth E., Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 151, 753–760 (2001). [DOI] [PubMed] [Google Scholar]
- 103.Nguyen N. H., Smith D., Peay K., Kennedy P., Parsing ecological signal from noise in next generation amplicon sequencing. New Phytol. 205, 1389–1393 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Nguyen N. H., et al. , FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248 (2016). [Google Scholar]
- 105.Fernandez C. W., et al. , Warming and Drying Effects of Ectomycorrhizal Fungal Communities at the Boreal-Temperate Ecotone. NCBI Short Read Archive. https://www.ncbi.nlm.nih.gov/bioproject/561610. Deposited 22 August 2019.
- 106.Lilleskov E. A., Hobbie E. A., Horton T. R., Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 4, 174–183 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (CSV)
Data Availability Statement
Raw sequence read files are available in NCBI short read archive (# PRJNA561610) (105). All study data are included in the article and/or supporting information.