Abstract
Background:
The objective was to determine if abdominal fat is related to poor muscle health.
Methods:
This cross-sectional study included 428 males and 534 females with appendicular lean mass (ALM, kg) from dual energy x-ray absorptiometry (DXA), grip strength (kg), and upper extremity muscle “quality” (grip strength/arm lean mass) measured (1996-2001) in the Framingham Offspring Study. Sex-specific linear regressions associated adiposity measures [waist circumference (WC, cm) and visceral adipose tissue (VAT, cm3), and subcutaneous adipose tissue (SAT, cm3)] as Z-scores with each measure of muscle, adjusting for covariates. Models were further stratified by body mass index (BMI, <30, ≥30 kg/m2).
Results:
Mean (±SD) age was 60 ± 9 years and BMI was 28.9 ± 4.6 kg/m2 (men) and 27.7 ± 5.8 kg/m2, (women). In men, the BMI-stratified analyses showed higher WC was associated with higher ALM (P<0.0001 each) but with lower muscle quality (P<0.02) in both BMI groups. Higher SAT was also associated with higher ALM (P=0.0002) and lower muscle quality (P=0.0002) in men with BMI<30, but not in obese men. In women, higher WC, SAT, and VAT were each associated with higher ALM but lower muscle quality, particularly in obese women. Higher SAT (P=0.05) and VAT (P=0.04) were associated with higher quadriceps strength in women with BMI<30 kg/m2 but not in obese women.
Conclusions:
Higher abdominal fat may be associated with greater lean mass but poorer muscle quality, particularly in obese women. This suggests that adipose tissue may have endocrine influences on muscle, which should be confirmed in longitudinal studies.
Keywords: muscle, epidemiology, sarcopenia, obesity
Introduction
Globally, the number of older adults is projected to grow to nearly 1.5 billion by 2050 (1). As the aging demographic shifts, conditions that affect older adults are of growing public health concerns. Fifteen percent of Americans aged 60 years and older, and half of Americans aged 80 years and older have sarcopenia, (2, 3) a disease characterized by muscle weakness, low muscle quantity/quality, and impaired physical function. (4) Sarcopenia contributes to reduced mobility, disability, institutionalization, and mortality (5, 6) with substantial associated healthcare costs. (7) Despite the gravity of this condition, the exact etiology of sarcopenia is not fully understood.
Aging is associated with an accumulation of body fat that parallels loss of muscle mass and strength. Both aging and obesity are characterized by low levels of inflammation, an independent risk factor for loss of muscle mass, strength, (8-11) and physical function (12). Adipose tissue secretes adipocytokines, a family of hormones and cytokines that are catabolic to muscle. (11, 13-15) Higher fat mass is also associated with lower circulating adiponectin, which may have positive effects on muscle through its anti-inflammatory properties (16, 17) or its influence on muscle glucose metabolism. (18-20) These metabolic changes are determined not only by fat mass, but also by fat distribution. Indeed, abdominal adiposity is more strongly predictive of cardio-metabolic disease, morbidity, and mortality versus whole body estimates of fat mass.(21) Even when considering the impact of abdominal fat, the visceral and subcutaneous compartments may have separate, independent systemic endocrine effects.(22, 23) Visceral adipose tissue (VAT) is particularly metabolically active and releases adipocytokines such as adiponectin, leptin, resistin, and interleukin-6 (IL-6).(24) There is, however, strong evidence that subcutaneous adipose tissue (SAT) also secretes adipocytokines directly into circulation.(25) While obesity and abdominal obesity are considered to be risk factors for disability (26-29) and poorer physical function (30), whether abdominal adiposity is an independent determinant of decline in muscle health is not known, nor is the physiologic mechanism for this hypothesized link. Yet few prior clinical studies have examined the interrelations of fat, adipocytokines, and muscle health. A longitudinal study of older adults reported that greater DXA-derived total fat mass was associated with lower muscle quality, and accelerated loss of lean mass over 7 years, which was not explained by higher levels of adipocytokines (31). These previous studies tended to include very old healthy adults, and used DXA-estimates of fat depots instead of measures from computed tomography (CT), which can accurately distinguish between SAT and VAT (24, 32), particularly in obese women.(33)
Given the gaps in literature, the primary objective of this study was to determine if abdominal fat is associated with poor muscle health. The secondary objective was to determine whether inflammatory markers may lie on the mechanistic pathway for the impact of abdominal fat on muscle. We hypothesized that higher waist circumference, VAT, and SAT would each be associated with lower lean mass, muscle strength, and upper extremity muscle quality, and that these associations would be at least partly mediated by markers of inflammation [IL-6, monocyte chemoattractant protein-1 (MCP-1), resistin, and adiponectin].
Methods
Study Design and Sample
This cross-sectional study included 962 members of the Framingham Study Offspring Cohort, which was composed of the adult children, and their spouses, of those enrolled in the population-based Framingham Study Original Cohort. The Offspring Cohort began in 1971 to investigate familial risk factors for cardiovascular disease within the cohort. The study enrolled 5,124 men and women (age range 5–70 y), and they have been examined at approximately 4-8 year intervals.(34) Measurements for the present study were ascertained in 1998-2001.
Inclusion and Exclusion Criteria
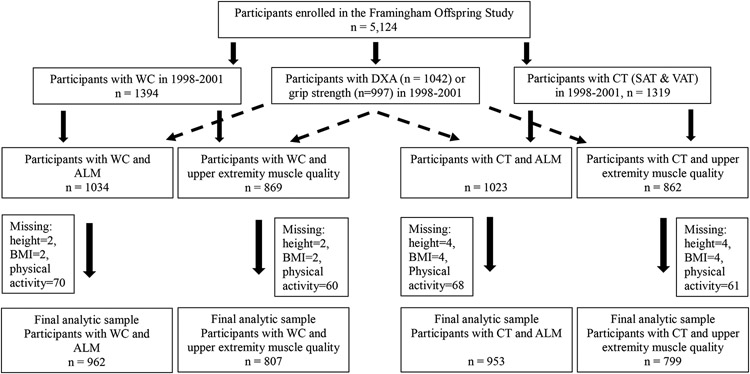
Inclusion criteria for this analysis included documentation of at least one measure of abdominal adiposity (waist circumference or CT fat mass) and one lean mass or muscle strength measure taken during the examinations in 1998-2001. Of the Offspring Cohort members who survived and participated in examinations in 1998-2001, 1,394 individuals with waist circumference measures, 1,042 individuals with whole body DXA scans, 997 individuals with grip strength measures, and 1,319 individuals who participated in the Multi-Detector Computer Tomography (MDCT) study and had abdominal CT scans were considered for the current study (Figure 1). Individuals who participated in the MDCT study included men aged ≥35 years and non-pregnant, women aged ≥40 years. Due to MDCT scanner constraints, those who weighed >160kg were excluded.(35) Twenty-nine males and 40 females were excluded due to missing covariate information. The final analytic sample included 962 participants (428 males and 534 females).
Fig 1. Flow chart showing total number of participants enrolled in the Framingham Offspring Study and the final number of participants included in the analyses.
Notes. WC= Waist circumference, DXA= dual energy x-ray absorptiometry, CT= computed tomography, SAT= subcutaneous adipose tissue, VAT= visceral adipose tissue, ALM= appendicular lean mass, BMI= body mass index.
Abdominal Adiposity Measures
We included three measures of abdominal adiposity: waist circumference; VAT; and SAT measured in the abdominal area. Waist circumference (inches) is considered the most reliable surrogate of visceral adiposity (36) and was measured by a trained professional applying an anthropometric tape at the level of the umbilicus, recording the reading at mid-inspiration with the participant breathing normally and rounding to the nearest 0.25 inches.(37) VAT (cm3) and SAT (cm3) volumes were calculated from CT scans of the abdomen as previously published.(38) Twenty-five consecutive 5 mm-thick slices using an 8-slice multidetector CT scanner (LightSpeed Ultra; General Electric, Milwaukee, WI) were obtained with participants lying in a supine position. A three-dimensional (3D) workstation tool (Aquarius 3D Workstation; TeraRecon Inc, San Mateo, CA) was used to evaluate CT slices for abdominal adipose tissue quantity and density. Trained technicians outlined the abdominal muscular wall manually, identifying a designated region of interest. Using a radiographic pixel threshold between −195 and −45 HU with center attenuation of −120 HU, SAT and VAT regions were automatically identified. Mean SAT and VAT volumes in cm3 were recorded as was attenuation in HU.
Lean mass and muscle strength
Measures of lean mass
Appendicular lean mass (ALM, kg) was calculated from whole body DXA scans obtained from a Lunar DPX-L (Lunar Radiation Corp. Madison, WI) in the fast mode with participants in standard positioning per recommendations of the manufacturer.(39) ALM was calculated as the sum of the lean mass of the arms and the legs.
Measures of muscle strength
Grip strength (kg) was measured using an adjustable Jamar isometric hand-held dynamometer. Participants squeezed the dynamometer maximally for 3 seconds. A total of six trials were done with three trials on each hand. The maximum value, regardless of the hand from which it was taken, was used for analysis. (39)
Quadriceps strength (kg) was measured using a Nicholas hand-held isometric dynamometer (test-retest reliability >85%).(40) The right leg was preferentially assessed, but in the case that the right leg could not be measured, the left was measured in its place. A single tester was used to perform all measurements. To test, participants were to be seated with hands in lap, back against the chair, and knee at 60o flexion with the foot flat on the floor. The dynamometer was positioned perpendicular to the leg along the anterior tibial surface 6 cm above the lateral malleolus. The participant was instructed to extend his/her leg maximally for 3 seconds. This procedure was repeated and the higher of the two measurements was used for analysis.
Upper extremity muscle quality
Upper extremity “muscle quality,” or strength per kg of lean mass (41) was calculated as baseline grip strength (kg) divided by lean mass (kg) of the “arms region”, (entirety of upper extremities).
Circulating markers of inflammation
We assessed 4 adipocytokines as potential mediators: IL-6, MCP-1, resistin, and adiponectin. Serum IL-6, MCP-1, resistin (R&D Systems), and adiponectin (Quantikine) were measured in duplicate by ELISA from fasting blood samples collected and stored at −80°C.(42) Intra-assay coefficients of variation (% CV) for are 3.1±2.2 (IL-6), 3.8±3.3 (MCP-1), 9.0±2.0 (resistin), and 5.8±0.6 (adiponectin).(43) Standard quality control evaluations were performed and all intra-assay coefficients of variation were <10%. The details of the assays and measurements have been described in previous studies.(42, 44)
Covariables
Covariable information was collected in 1998-2001 and included sex, age, height, weight, body mass index (BMI), physical activity, smoking, and menopausal status in females. Height in inches (in) was measured with shoes removed, rounding to the nearest quarter inch using a stadiometer. Weight in pounds (lbs.) was measured with participants only wearing light clothing using a standardized balance beam scale. BMI was calculated by dividing the weight in kg by the square of height in meters (kg/m2). Physical activity was assessed based on the Physical Activity Scale for the Elderly (PASE) score, a questionnaire used for self-reporting of physical activity over the past 7 days (13). The smoking status of the participants was assessed via questionnaire as current versus noncurrent smokers. Women’s menopausal status was categorized into two groups: postmenopausal women (defined as women who reported no menstrual periods for at least 1 year or were currently using hormone replacement therapy) versus premenopausal women.
Statistical Analyses
Baseline measurements differed between males and females, therefore, sex-stratified analyses were performed. Additionally, due to high Pearson’s correlation coefficients between BMI and abdominal adiposity measures, models were stratified by BMI <30 kg/m2 and ≥30 kg/m2. Abdominal adiposity measures (waist circumference, VAT, and SAT) were first converted into corresponding z-scores and then modeled as continuous variables. In primary analyses, sex-specific multivariable linear regression was used to calculate regression coefficients (β) for the differences in muscle mass, muscle strength, and muscle quality associated with a 1 SD higher WC, VAT and SAT within each strata of BMI. All models were adjusted for age, height, physical activity, current smoking, and menopause status in women only. We also calculated Pearson’s correlation coefficients of BMI with each of the abdominal adiposity measures.
In secondary analyses, final sex- and BMI- stratified models were further adjusted for the inflammation markers, one marker at a time. If the added biomarker variable was significantly associated with the dependent variable in the regression model (regression coefficient P-value less than 0.05) and changed the main effect of WC, VAT or SAT by ≥10% compared to the model without the biomarker, we concluded that the association of WC, VAT or SAT with the dependent variable was explained, at least in part, by the added biomarker. (45)
A nominal 2-sided P value of 0.05 was considered statistically significant for the analyses. All analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary NC).
Results
Baseline characteristics are shown in Table 1. Mean age of the participants was 60 years. Men weighed more, had higher BMI, physical activity, waist circumference and VAT compared with women. As expected men also had higher lean muscle mass and muscle strength measures while women had higher SAT values compared with men. Muscle quality values were similar in men versus women.
Table 1.
Baseline characteristics (mean ± standard deviation) of 549 males and 624 females from the Framingham Offspring Cohort in 1998-2001.
| Sample characteristics | Male | Female |
|---|---|---|
| Age (years) | 60 ± 9 | 60 ± 9 |
| BMI (kg/m2) | 28.9 ± 4.6 | 27.7 ± 5.8 |
| Height (m) | 1.75 ± 0.07 | 1.61 ± 0.06 |
| Weight (kg) | 88 ± 15 | 72 ± 16 |
| Physical activity index | 38.4 ± 6.5 | 37.3 ± 5.5 |
| Current smokers, n (%) | 56 (10.3) | 58 (9.3) |
| Post-menopausal women, n (%) | - | 561 (89.9) |
| Waist Circumference (inches) | 41 ± 4 | 38 ± 6 |
| SAT (cm3) | 2698 ± 1108 | 3298 ± 1459 |
| VAT (cm3) | 2677 ± 1119 | 1624 ± 856 |
| Appendicular Lean Mass (kg) | 24.7 ± 3.2 | 16 ± 2.3 |
| Upper extremity muscle qualitya | 6.3 ± 1.4 | 6.3 ± 2 |
| Quadriceps strength (kg) | 23.2 ± 6.5 | 18.9 ± 5.2 |
| Grip strength (kg) | 44.2 ± 9.8 | 26 ± 7.7 |
Notes. BMI= Body Mass Index, SAT= Subcutaneous adipose tissue, VAT= Visceral adipose tissue.
Upper extremity muscle quality calculated as kg of grip strength per kg of arms lean mass
In the initial sex-stratified analysis, each standard deviation higher measure of adiposity (WC, SAT or VAT) was associated with higher ALM and lower muscle quality (kg grip strength per kg of arms lean mass) in men and in women (each P<0.0001, Table 2). No significant associations were observed for muscle strength.
Table 2.
Adjusted sex-specific associations of adiposity measures (z-scores) with appendicular lean mass (kg), upper extremity muscle quality (kg of grip strength per kg of arms lean mass), and grip strength (kg) in the Framingham Offspring Cohort in 1998-2001.
| Appendicular lean mass (kg) | Upper extremity muscle Quality | |||||||
|---|---|---|---|---|---|---|---|---|
| N | βa | SE | P value | N | βa | SE | P value | |
| Males | ||||||||
| WC Z score | 428 | 1.666 | 0.117 | <0.0001 | 352 | −0.638 | 0.070 | <0.0001 |
| SAT Z score | 425 | 1.144 | 0.124 | <0.0001 | 349 | −0.518 | 0.071 | <0.0001 |
| VAT Z score | 425 | 0.950 | 0.124 | <0.0001 | 349 | −0.346 | 0.072 | <0.0001 |
| Females | ||||||||
| WC Z score | 534 | 1.312 | 0.074 | <0.0001 | 455 | −0.973 | 0.090 | <0.0001 |
| SAT Z score | 528 | 1.046 | 0.079 | <0.0001 | 450 | −0.947 | 0.088 | <0.0001 |
| VAT Z score | 528 | 0.911 | 0.081 | <0.0001 | 450 | −0.751 | 0.089 | <0.0001 |
| Quadriceps Strength (Kg) | Grip Strength (Kg) | |||||||
| Males | ||||||||
| WC Z score | 438 | 0.386 | 0.303 | 0.20 | 424 | 0.005 | 0.407 | 0.99 |
| SAT Z score | 434 | 0.428 | 0.309 | 0.17 | 420 | −0.495 | 0.409 | 0.22 |
| VAT Z score | 434 | 0.162 | 0.311 | 0.60 | 420 | 0.420 | 0.421 | 0.32 |
| Females | ||||||||
| WC Z score | 533 | 0.158 | 0.229 | 0.49 | 485 | 0.443 | 0.327 | 0.175 |
| SAT Z score | 527 | 0.213 | 0.224 | 0.34 | 480 | 0.160 | 0.331 | 0.628 |
| VAT Z score | 527 | 0.206 | 0.225 | 0.36 | 480 | 0.301 | 0.322 | 0.350 |
Notes. WC= Waist Circumference, SA= Subcutaneous adipose tissue, VAT= Visceral adipose tissue. Statistically significant results are p ≤ 0.05 and are presented in bold.
Beta coefficient adjusted for age, height, physical activity index (PAI), current smoking and menopausal status (in women),
BMI was highly correlated with waist circumference (correlation coefficient (r): 0.91 in men, 0.89 in women), SAT (r: 0.74 in men, 0.81 in women), and VAT (r: 0.63 in men, 0.69 in women), with P<0.001 for all correlations. These high correlations supported stratification of men and women by BMI (<30 kg/m2 and ≥30 kg/m2). As shown in the BMI stratified results in Table 3, in men, regardless of their BMI category, higher WC was associated with higher lean mass but lower muscle quality. Higher SAT was associated with higher lean mass and lower muscle quality in men with BMI<30, but not in obese men (Table 3). No associations were observed between VAT and either quadriceps strength or grip strength (Table 4).
Table 3.
Adjusted sex-specific associations of adiposity measures (z-scores) with appendicular lean mass (kg) and upper extremity muscle quality (kg of grip strength per kg of arms lean mass) stratified by BMI in the Framingham Offspring Cohort in 1998-2001.
| Appendicular lean mass (kg) | Upper extremity muscle quality | |||||||
|---|---|---|---|---|---|---|---|---|
| BMI<30 kg/m2 | N | βa | SE | P value | N | βa | SE | P value |
| Males | ||||||||
| WC Z score | 305 | 1.148 | 0.205 | <0.0001 | 255 | −0.658 | 0.123 | <0.0001 |
| SAT Z score | 303 | 0.602 | 0.193 | 0.002 | 253 | −0.450 | 0.118 | 0.0002 |
| VAT Z score | 303 | 0.291 | 0.165 | 0.08 | 253 | −0.119 | 0.102 | 0.24 |
| Females | ||||||||
| WC Z score | 394 | 0.659 | 0.121 | <0.0001 | 342 | −0.681 | 0.160 | <0.0001 |
| SAT Z score | 388 | 0.387 | 0.114 | 0.0008 | 336 | −0.718 | 0.144 | <0.0001 |
| VAT Z score | 388 | 0.370 | 0.110 | 0.0009 | 336 | −0.441 | 0.143 | 0.002 |
| BMI≥30 kg/m2 | ||||||||
| Males | ||||||||
| WC Z score | 123 | 1.556 | 0.334 | <0.0001 | 97 | −0.422 | 0.185 | 0.02 |
| SAT Z score | 122 | 0.099 | 0.268 | 0.71 | 96 | −0.173 | 0.144 | 0.23 |
| VAT Z score | 122 | 0.260 | 0.253 | 0.30 | 96 | −0.103 | 0.146 | 0.48 |
| Females | ||||||||
| WC Z score | 140 | 1.424 | 0.202 | <0.0001 | 113 | −0.999 | 0.209 | <0.0001 |
| SAT Z score | 140 | 0.680 | 0.183 | 0.0003 | 114 | −0.810 | 0.179 | <0.0001 |
| VAT Z score | 140 | 0.404 | 0.157 | 0.01 | 114 | −0.509 | 0.143 | 0.0006 |
Notes. BMI= Body Mass Index, WC= Waist Circumference, SA= Subcutaneous adipose tissue, VAT= Visceral adipose tissue. Statistically significant results are p ≤ 0.05 and are presented in bold.
Models for WC, SAT, and VAT were analyzed separately where each model was adjusted for age, height, physical activity index (PAI), current smoking and menopausal status (in women)
Table 4.
Adjusted sex-specific association of adiposity measures (z-scores) with measures of muscle strength stratified by BMI in the Framingham Offspring Cohort in 1998-2001.
| Quadriceps strength (kg) | Grip strength (kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| BMI<30 kg/m2 | N | βa | SE | P value | N | βa | SE | P value |
| Males | ||||||||
| WC Z score | 305 | −0.093 | 0.584 | 0.87 | 299 | −0.212 | 0.778 | 0.79 |
| SAT Z score | 303 | −0.485 | 0.536 | 0.37 | 297 | −1.141 | 0.720 | 0.11 |
| VAT Z score | 303 | −0.171 | 0.446 | 0.70 | 297 | 0.586 | 0.596 | 0.32 |
| Females | ||||||||
| WC Z score | 391 | 0.831 | 0.420 | 0.04 | 356 | 0.500 | 0.574 | 0.38 |
| SAT Z score | 385 | 0.770 | 0.380 | 0.04 | 350 | −0.109 | 0.527 | 0.83 |
| VAT Z score | 385 | 0.574 | 0.365 | 0.11 | 350 | 0.619 | 0.509 | 0.22 |
| BMI≥30 kg/m2 | ||||||||
| Males | ||||||||
| WC Z score | 133 | −0.026 | 0.743 | 0.97 | 125 | −1.238 | 1.000 | 0.21 |
| SAT Z score | 131 | 0.641 | 0.662 | 0.33 | 123 | −1.560 | 0.841 | 0.07 |
| VAT Z score | 131 | −0.187 | 0.672 | 0.78 | 123 | −0.276 | 0.955 | 0.77 |
| Females | ||||||||
| WC Z score | 142 | −0.879 | 0.550 | 0.11 | 129 | −0.800 | 0.902 | 0.38 |
| SAT Z score | 142 | −0.560 | 0.491 | 0.26 | 130 | −1.390 | 0.830 | 0.10 |
| VAT Z score | 142 | −0.456 | 0.415 | 0.27 | 130 | −0.752 | 0.645 | 0.24 |
Notes. BMI= Body Mass Index, WC= Waist Circumference, SA= Subcutaneous adipose tissue, VAT= Visceral adipose tissue. Statistically significant results are p ≤ 0.05 and are presented in bold.
Models for WC, SAT, and VAT were analyzed separately where each model was adjusted for age, height, physical activity index (PAI), current smoking and menopausal status (in women alone)
For women, as shown in Table 3, higher WC, SAT, and VAT were each associated with higher lean mass but lower muscle quality although the magnitude of associations was stronger in obese women. In women with BMI<30 kg/m2 but not in obese women, higher WC and SAT were each associated with higher quadriceps strength [β (SE): BMI<30 kg/m2, WC= 0.832 (0.420); P= 0.05 and SAT= 0.770 (0.381); P= 0.04). Similar associations were observed for VAT but the associations did not reach statistical significance (P=0.11, Table 4). No statistically significant associations between adipose measures and grip strength were observed.
Mediation by adipocytokines
In men with BMI<30 kg/m2, association of higher SAT with higher ALM was partly mediated by adiponectin [β (SE) for SAT: BMI<30 kg/m2, 0.420 (0.222); P=0.06] such that the magnitude of the association was lower compared to the fully adjusted model without adiponectin [β (SE) for SAT: BMI<30 kg/m2, 0.602 (0.193); P=0.006]. Other biomarkers did not have a significant impact on the magnitude of association for any of the adiposity measures with any of the outcomes in men or women with BMI<30 kg/m2 (data not shown). Inflammatory biomarkers did not have a significant impact on the magnitude of association for any of the adiposity measures with any of the outcomes in men or women with BMI≥30 kg/m2 (data not shown).
Discussion
These results suggest that in both men and women, higher adiposity is associated with higher muscle mass as measured by appendicular lean mass but lower muscle quality captured by the amount of muscle strength per unit of lean mass. These findings persisted across the two BMI groups (BMI<30 kg/m2 and ≥30 kg/m2). Abdominal adiposity was not consistently associated with measures of muscle strength that did not account for the force per unit of lean mass. In men with BMI<30 kg/m2, association of higher SAT with higher ALM was partly explained by adiponectin while no such contribution by inflammatory biomarkers was observed in women.
In 2,623 men and women aged 70-79 from Health ABC study, a cross-sectional analysis reported that those at the high and low extremes of percentage of body fat (from DXA) had lower levels of leg muscle quality (calculated as the ratio of strength to muscle mass for both upper and lower extremities) than those in the midrange (41). This effect was small, but of similar magnitude to that of age itself in its effect on muscle quality, in that the negative effect of a kg fat was similar to that of a year of age. While the current study did not examine total body fat, results from abdominal body fat showed similar inverse association such that in men, one unit higher z-score for SAT was associated with 1kg (SD: 0.12) higher ALM and with 0.51 kg (0.07) lower muscle strength per kg of arms lean mass (muscle quality). Similarly, in women, one unit higher z-score for SAT was associated with 1kg (SD: 0.08) higher ALM and with 0.94 kg (0.09) lower muscle strength per kg of lean mass. The association in women was largely driven by obese women. Findings from the current study are also consistent with the results from a longitudinal study from Health ABC, which reported that every SD greater DXA-derived fat mass was related to 1.3 kg more leg lean mass at baseline in men and 1.5 kg in women (p < .01). However, over 7 years of follow-up, greater fat mass was associated with associated with lower muscle quality, and it predicted accelerated loss of lean mass over 7 years, which was not explained by higher levels of adipocytokines (31).
A few prior studies suggest that abdominal obesity measured by waist circumference is associated with increased functional limitations and disability (27, 29). A meta-analysis of 50 prospective, longitudinal studies of older persons aged 65± years reported that BMI of 30 kg/m2 and above was associated with 60% higher odds of functional decline [pooled OR (95%CI) = 1.60 (1.43-1.80). However, results from the current study showed no significant association of any of the adiposity measures with grip strength in any of the BMI groups. Associations of adiposity measures with quadriceps strength were inconsistent such that higher WC, SAT, and VAT were associated with higher quadriceps strength only in normal and overweight women but not in obese women or in men. Associations for VAT did not reach statistical significance.
Age-related declines in muscle mass and muscle quality as reported in previous studies (41, 46) could be due to advanced age of these participants compared to the participants of the current study. Additionally, discrepant findings could be due to decreased proportion of type II fibers, increased connective tissue, fatty infiltration, and altered muscle metabolism. In obesity, upregulation of MCP-1 is associated with macrophage accumulation and activation in adipose tissues, and insulin resistance. (47) Significantly raised MCP-1 levels and IL-6 levels in obese and sarcopenic obese older adults support the theory of chronic inflammation due to excess adiposity (15, 47). Resistin is released by adipocytes during adipocyte differentiation and has been known to mediate insulinotropic action and fat accumulation potentially at the expense of muscle strength. (11, 48) Lower adiponectin levels are found in inactive obese individuals and shows an inverse relationship with muscle strength, particularly in older adults. (11) In spite of the strong rationale for mediation of the association of adiposity measures and muscle measures by inflammatory markers, no such mediation was observed for IL-6, MCP-1, and resistin in the current study. However, in men with BMI<30 kg/m2, the association of higher SAT with higher ALM was partly mediated by adiponectin, while no such mediation by inflammatory biomarkers was observed in obese men or in women. Given the weak correlation between adiponectin and SAT in men (r = −0.04, P = 0.41), this unexpected association in men with BMI in the normal or overweight range could be due to random chance given the multiple tests that were performed for four inflammation markers.
This present study is one of the first to examine association of specific abdominal fat depots with muscle characteristics. Use of an established population-based cohort with large numbers of men and women across a wide age range is a strength of this study. The Framingham Study Offspring cohort is well-characterized, enabling us to examine several measures of muscle and physical function while accounting for potential confounding variables. This study has some limitations. The cross-sectional design precludes causal inferences linking abdominal fat depots with lean mass and muscle quality and identifying adipocytokines as a physiologic mechanism. CT scans were collected as a part of the MDCT study, which could not include extremely obese individuals due to weight limits for the table attached to the CT scanner. The results of this study are predominantly generalizable to males and females of European ancestry. Given the observational nature of this study, the results may be affected by residual confounding.
Conclusions
This study suggests that higher abdominal adiposity is associated with higher lean mass but lower muscle quality in men and women. These associations persisted across the two BMI groups. The magnitude of associations was stronger in obese women. These results suggest that although larger amounts of body fat may confer greater lean mass, the strength of the muscle per unit of lean mass is adversely associated with fat depots regardless of location. While these findings raise the possibility that adipose tissue can have endocrine influences on muscle function, future studies should determine the mechanisms that may link adipose tissue and muscle quality. Longitudinal studies are needed to confirm findings from this cross-sectional study.
Funding
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Numbers R03AG053679 and R01 AG051728; the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number R01 AR041398); the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract number HHSN268201500001I); the National Institute on Aging support of the Boston Claude D. Pepper Center Older American Independence Centers (grant number 1P30AG031679 to SS); and the MSTAR Training Grant (grant number NIA 5T35AG038027-09 (MPI) to RR).
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the official views of the National Institutes of Health.
Competing interests:
Dr. Sahni reports institutional grants from Dairy Management Inc., Solarea Bio Inc., has reviewed grants for the American Egg Board’s Egg Nutrition Center and National Dairy Council, and she serves on a scientific advisory board for the Institute for the Advancement of Foods and Nutrition Sciences (IAFNS, unpaid position ended July 2022). Dr. Kiel has received grant funding to his institution from Amgen, Solarea Bio Inc., and Radius Health. He serves on the scientific advisory boards of Pfizer and Solarea Bio Inc. Dr. Hannan has received institutional grant funding from Amgen. Dr. Raghupathy and Dr. McLean have no relevant financial or non-financial interests to disclose related to this current work.
Abbreviations
- ALM
Appendicular lean mass
- DXA
Dual energy x-ray absorptiometry
- WC
Waist circumference
- VAT
Visceral adipose tissue
- SAT
Subcutaneous adipose tissue
- BMI
Body Mass Index
- IL-6
Interleukin-6
- CT
Computed tomography
- MCP-1
Monocyte chemoattractant protein-1
- MDCT
Multi-Detector Computer Tomography
- 3D
Three-dimensional
- PASE
Physical Activity Scale for the Elderly
- r
Correlation coefficient
Footnotes
Research involving Human Participants and Informed Consent: This study was performed in accordance with the Declaration of Helsinki. All study participants gave informed consent for the parent Framingham Heart Study, which was approved by the IRB at Boston University. The current study utilized previously collected data and the protocol was approved by the Institutional Review Board (or Ethics Committee) of Advarra (Protocol #Pro00044485).
Availability of data and materials: Data described in the manuscript, code book, and analytic code will be made available upon request pending application to and approval by the Framingham Heart Study.
References
- 1.United Nations, Department of Economic and Social Affairs, Division. P. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). 2019. 2019. Report No.: 978-92-1-148325-3. [Google Scholar]
- 2.Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab. 2010;35(5):707–12. [DOI] [PubMed] [Google Scholar]
- 3.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020;68(7):1410–8. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–96. [DOI] [PubMed] [Google Scholar]
- 6.Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J Am Geriatr Soc. 2020;68(7):1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. [DOI] [PubMed] [Google Scholar]
- 8.Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–54. [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–32. [DOI] [PubMed] [Google Scholar]
- 11.Bucci L, Yani SL, Fabbri C, Bijlsma AY, Maier AB, Meskers CG, et al. Circulating levels of adipokines and IGF-1 are associated with skeletal muscle strength of young and old healthy subjects. Biogerontology. 2013;14(3):261–72. [DOI] [PubMed] [Google Scholar]
- 12.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64(4):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. [DOI] [PubMed] [Google Scholar]
- 14.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12(6):887–8. [DOI] [PubMed] [Google Scholar]
- 15.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102(3):919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96(5):1723–32. [PubMed] [Google Scholar]
- 17.Chiarugi P, Fiaschi T. Adiponectin in health and diseases: from metabolic syndrome to tissue regeneration. Expert Opin Ther Targets. 2010;14(2):193–206. [DOI] [PubMed] [Google Scholar]
- 18.Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51–9. [DOI] [PubMed] [Google Scholar]
- 19.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, et al. Plasma adiponectin concentration is associated with skeletal muscle insulin receptor tyrosine phosphorylation, and low plasma concentration precedes a decrease in whole-body insulin sensitivity in humans. Diabetes. 2002;51(6):1884–8. [DOI] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. [DOI] [PubMed] [Google Scholar]
- 21.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–41. [DOI] [PubMed] [Google Scholar]
- 23.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 24.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karastergiou K, Fried SK. Multiple adipose depots increase cardiovascular risk via local and systemic effects. Curr Atheroscler Rep. 2013;15(10):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrucci L, Alley D. Obesity, disability, and mortality: a puzzling link. Arch Intern Med. 2007;167(8):750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houston DK, Stevens J, Cai J. Abdominal fat distribution and functional limitations and disability in a biracial cohort: the Atherosclerosis Risk in Communities Study. Int J Obes (Lond). 2005;29(12):1457–63. [DOI] [PubMed] [Google Scholar]
- 28.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc. 2011;59(5):781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes (Lond). 2006;30(2):364–73. [DOI] [PubMed] [Google Scholar]
- 30.Murphy RA, Reinders I, Register TC, Ayonayon HN, Newman AB, Satterfield S, et al. Associations of BMI and adipose tissue area and density with incident mobility limitation and poor performance in older adults. Am J Clin Nutr. 2014;99(5):1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66(8):888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Chen YE, Eitzman DT. Imaging body fat: techniques and cardiometabolic implications. Arterioscler Thromb Vasc Biol. 2014;34(10):2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, et al. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring). 2010;18(11):2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. [DOI] [PubMed] [Google Scholar]
- 35.Onuma OK, Pencina K, Qazi S, Massaro JM, D'Agostino RB Sr., Chuang ML, et al. Relation of Risk Factors and Abdominal Aortic Calcium to Progression of Coronary Artery Calcium (from the Framingham Heart Study). Am J Cardiol. 2017;119(10):1584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28(8):1018–25. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Troy LM, Rogers GT, Fox CS, McKeown NM, Meigs JB, et al. Longitudinal association between dairy consumption and changes of body weight and waist circumference: the Framingham Heart Study. Int J Obes (Lond). 2014;38(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JJ, Pedley A, Hoffmann U, Massaro JM, Keaney JF Jr., Vasan RS, et al. Cross-Sectional Associations of Computed Tomography (CT)-Derived Adipose Tissue Density and Adipokines: The Framingham Heart Study. J Am Heart Assoc. 2016;5(3):e002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53(3):M214–21. [DOI] [PubMed] [Google Scholar]
- 40.Piao C, Yoshimoto N, Shitama H, Makino K, Wada F, Hachisuka K. Validity and reliability of the measurement of the quardriceps femoris muscle strength with a hand-held dynamometer on the affected side in hemiplegic patients. J UOEH. 2004;26(1):1–11. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–30. [DOI] [PubMed] [Google Scholar]
- 42.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51(6):1651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frankel DS, Vasan RS, D'Agostino RB Sr., Benjamin EJ, Levy D, Wang TJ, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53(9):754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr., et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109(5):613–9. [DOI] [PubMed] [Google Scholar]
- 45.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286(10):1230–1. [DOI] [PubMed] [Google Scholar]
- 47.Lim JP, Leung BP, Ding YY, Tay L, Ismail NH, Yeo A, et al. Monocyte chemoattractant protein-1: a proinflammatory cytokine elevated in sarcopenic obesity. Clin Interv Aging. 2015;10:605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. [DOI] [PubMed] [Google Scholar]