Abstract
As cases of coronavirus 2019 (COVID-19) keep rising, reported deaths are increasing. Public health measures have been implemented with mixed efficacy. As vaccines are becoming more widely available and accessible globally, treating critically ill COVID-19 patients remains an issue with only dexamethasone found to be therapeutically effective to date. However, trials studying the efficacy of IL-6 inhibitors, namely tocilizumab have been underway with promising results. This paper is a narrative review that aims to review the current evidence provided by randomized clinical trials (RCT) for the use of tocilizumab in COVID-19. Electronic database searches were carried out in Medline, PubMed, Embase, Google Scholar, and ongoing clinical trial registries with the period set from January 1, 2020 to February 20, 2021. Prepublication manuscripts were found using the pre-print repository medRxiv. Keywords included “COVID-19,”“coronavirus,”“SARS-CoV-2,”“sepsis,”“pneumonia,”“cytokine storm,”“cytokine release syndrome,”“IL-6 inhibitors,” and “tocilizumab,” as exact phrases, and a combination of subject headings according to databases syntax. Only trials with a clear and well-defined methodology, at least 100 patients recruited, and which have had results published either after peer review or in pre-print were included. In hospitalized patients with severe COVID-19, who are hypoxic and have a CRP ≥ 75 mg/L, the current evidence favors the use of a combination of tocilizumab and corticosteroids to reduce mortality, among other clinical benefits. There is also overwhelming evidence of the good safety profile of tocilizumab with only few cases of neutropenia reported with a decrease in infection rates. Tocilizumab is currently thought to work through the inhibition of IL-6 receptors (IL-6R), preventing downstream activation of pro-inflammatory reactions and cytokine release syndrome.
Keywords: COVID-19, tocilizumab, randomized clinical trial, review, IL-6 inhibitors, mortality, clinical outcomes
Introduction
The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of a high burden of mortality and morbidity globally due to the exponential rise in cases of COVID-19 pneumonia. 1 Initially, before the development of vaccines, public health measures such as social distancing, surgical mask-wearing, and lockdown restrictions were the only effective strategy to control the disease. 2 Several therapeutic agents have been studied and tried to evaluate their efficacy in reducing mortality rates and improving outcomes in hospitalized patients. 3 One of these agents includes interleukin (IL)-6 inhibitors, medications commonly used for autoimmune diseases such as rheumatoid arthritis (RA). 4 The theory behind their potential benefit in COVID-19 is the role of cytokine dysregulation in serious COVID-19 cases with IL-6 the key driver of this hyperinflammation. 5 Additionally, elevated levels of IL-6 have been found to be predictive of the likelihood of mechanical ventilation. 6 Most notably trials evaluating the use of tocilizumab have been conducted with more underway and that include other agents such as sarilumab and siltuximab. 7
This paper is a narrative review that aims to evaluate the available evidence gathered from the biggest randomized clinical trials (RCTs) with the highest quality of evidence regarding the use of tocilizumab in COVID-19 patients, focusing on the strengths and limitations of the studies and highlighting the potential methodological flaws and biases.
Methods
Electronic database searches were carried out in Medline, PubMed, Embase, Google Scholar, and ongoing clinical trial registries (clinicaltrials.gov), with the period set from January 1, 2020 to February 20, 2021. MedRxiv was used to identify unpublished and ongoing studies, within the same set period. The search process was focused on clinical trials where tocilizumab was evaluated in the treatment of SARS-CoV-2, COVID-19, sepsis, and/or cytokine storm. Keywords included “COVID-19,”“coronavirus,”“SARS-CoV-2,”“sepsis,”“pneumonia,”“cytokine storm,”“cytokine release syndrome,”“IL-6 inhibitors,” and “tocilizumab,” as exact phrases, and a combination of subject headings according to databases syntax. The references listed in each identified article were also screened and manually searched. No language restrictions were imposed in any of the searches. Clinical trials were chosen based on their magnitude and quality. Only trials which recruited at least 100 patients AND have had results published (either after peer review or in pre-print) were included. The trials also needed to have a clear and well-defined methodology. Trials that showed discrepancies between the information found in the published manuscript and what was listed on the clinical trial registry were generally excluded (e.g. recruitment method, primary endpoints, statistical approach…). Additionally, the COVID-NMA website, 8 which performs living mapping of registered trials, was used to cross-reference with our search results and add any relevant trials or information to this review.
Discussion
The evidence
Tocilizumab has become a drug of interest in the treatment of COVID-19 pneumonia after several observational studies have shown decreased mortality with its use in severely ill COVID-19 patients. However, the studies had a limited sample, were not controlled for confounding, or had missing data regarding other concomitant treatments administered and relevant markers for COVID-19 such as PaO2:FiO2, among other limitations.9–12 In a systematic review regarding the use of tocilizumab in COVID-19, Cortegiani et al. 13 evaluated retrospective and prospective observational pre-clinical and clinical studies. However, no randomized trials were included in their review. The authors found that most studies had a small sample size and a high or moderate risk of bias, mostly due to confounding. There were also variations in dosing (single or double), and drug availability which might have impacted both sample sizes and study designs. Additionally, the risk of secondary bacterial infection was unclear as some studies found a higher rate of infection in those treated with tocilizumab with associated neutropenia and thrombocytopenia. The authors conclude that there is insufficient evidence concerning the clinical efficacy and safety of tocilizumab in patients with COVID-19, and its use should be limited to the experimental setting. 13 Since then, multiple randomized trials have been underway. It is worth noting that the proportion of overall corticosteroid use in these trials increased over time, especially after the release of the RECOVERY Trial results on the use of dexamethasone in severe COVID-19 (Figure 1). 14
Figure 1.
Timeline of RCTs evaluating the use of tocilizumab in COVID-19 patients. The dates set refer to the last date at which a patient was recruited, except for June 22, 2020, which refers to the earliest date when results of the RECOVERY Trial concerning Dexamethasone were made public. Whether a study was able to meet its primary endpoint or failed to do so is highlighted by blue and red circles, respectively.
An open-label, prospective, multicenter RCT enrolled 126 patients with COVID-19 pneumonia and PaO2:FiO2 ratio between 200- and 300-mmHg to receive tocilizumab (two 8 mg/kg doses with a max dose of 800 mg given 12 h apart) or standard of care in 24 hospitals in Italy. 15 Patients in the control arm could receive tocilizumab as rescue therapy if they clinically deteriorated. The study found no benefit on disease progression/clinical deterioration compared with standard of care; however, the safety profile of tocilizumab was demonstrated with no increase of infection between the two groups. 15 While none of the primary endpoints were met, the study had several limitations. Its open-label nature introduces a significant risk of bias since many retrospective studies showed a clinical benefit of tocilizumab, investigators could have been biased in the assessment of the primary endpoint and administering rescue therapy to the control group. The trial also failed to achieve 80% power as the sample size was much smaller than calculated (126 vs 398). 15 Selection bias is another issue as the study excluded patients with a more severe disease, preventing analysis of the efficacy of tocilizumab in advanced cases of COVID-19 pneumonia.
The BACC trial, a double-blind, placebo-controlled, multicenter trial enrolled 243 hospitalized patients who fulfilled at least two of the following criteria: fever (>38°C), pulmonary infiltrates, or requirement for supplemental oxygen to maintain a saturation >92%, and at least one of the following laboratory criteria also had to be met: a C-reactive protein (CRP) level >50 mg/L, a ferritin level >500 ng/mL, a d-dimer level >1000 ng/mL, or a lactate dehydrogenase (LDH) level >250 U/L. 16 The intervention group received tocilizumab 8 mg/kg of body weight with a maximal dose of 800 mg. The study failed to reach statistical significance in its primary outcomes (death or intubation) but again demonstrated the relative safety of tocilizumab with less infections in the intervention group though with an increased rate of neutropenia. The study was appropriately powered, had a diverse population (45% of the patients were Hispanic or Latino), and was randomized and double-blinded which significantly reduced the risk of bias. However, as management for COVID-19 pneumonia was/is still evolving, the standard of care changed progressively as some patients also received concomitant remdesivir with strategies to delay intubation also being recommended, potentially biasing the results in favor of the control group. 16
The COVACTA trial, a double-blind, placebo controlled, multicenter RCT enrolled 438 hospitalized patients with bilateral pulmonary infiltrates and hypoxemia. Tocilizumab was administered in a single 8 mg/kg dose (maximal dose of 800 mg). To note, 36% of patients in the tocilizumab group received dexamethasone, compared to 55% in the control group. Clinical status and mortality at 28 days were not statistically different between the two groups. 17 However, the study found a possible benefit in the time to discharge and in the duration of ICU stay, with no difference in reported adverse events between the two groups. 17 The main limitation of this study is the lack of patient stratification by clinical signs of hyperinflammation as anti-IL-6 would most likely be more beneficial early on during the cytokine storm, and in those who develop severe inflammatory dysregulation, thus failing to address the benefit of tocilizumab in this subset of patients. 18 Corticosteroid use was also unequal between the two groups, with higher administration of steroids to the control group possibly due to clinical deterioration among patients in that group, which could have biased the results in favor of the control group. 17
Similar findings were seen with the CORIMUNO-TOCI-1 trial, an open-label, multicenter RCT involving hospitalized patients COVID-19 with moderate or severe pneumonia requiring at least 3 L/min of oxygen but without ventilation or admission to the ICU. 19 While no difference in day 28 mortality was found, there appeared to be a benefit in reducing the need for mechanical and noninvasive ventilation or death by day 14. There was also no increase in adverse or serious adverse events between the two groups, again demonstrating the safety profile of tocilizumab. 19 About 61% in the control group received dexamethasone versus 33% of patients in the tocilizumab group potentially masking the true clinical benefits of tocilizumab. Additionally, the study is underpowered with a small sample size (131), likely exaggerating the perceived benefits seen and confidence intervals obtained for the secondary outcomes.
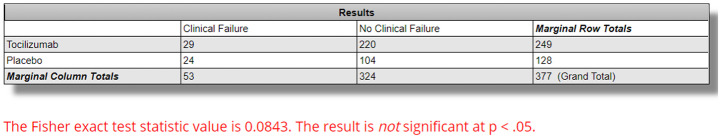
The EMPACTA trial, a double-blind, placebo-controlled, multicenter trial involving 389 hospitalized patients with PCR confirmed COVID-19 who were hypoxemic (O2 Saturation <94%) but were not on noninvasive or invasive ventilation. The intervention group received one or two doses of tocilizumab (8 mg/kg of body weight intravenously). 20 The study included sites enrolling high-risk and minority populations, with the primary outcomes defined as mechanical ventilation or death by day 28. 20 While the study showed statistical significance in reducing the likelihood of the composite outcome of progression to mechanical ventilation or death by day 28 (p = 0.04), there was no statistical difference in overall clinical failure between the two groups (12.0% in the tocilizumab group vs 19.3% in the placebo group, p = 0.0843 using exact Fisher Exact Test) (Figure 2). 20 Again, no difference in adverse events was seen. In contrast to previous studies, steroid use was almost equal between both groups as 55.4% of the tocilizumab group and 67.2% of the control group received dexamethasone. 20 The major strength of this study is its adequate power, blind nature, and focus on high-risk and racial and ethnic minority groups that are usually underrepresented in other studies.
Figure 2.
Fisher Exact Test for clinical failure between tocilizumab and placebo groups in the EMPACTA trial. Calculated using https://www.socscistatistics.com/tests/fisher/default2.aspx. Clinical failure occurred in 12.0% in the tocilizumab group (29/249) versus 19.3% in the placebo group (24/128) with p value set at less than 0.05.
The REMAP-CAP trial, an international, multifactorial adaptive platform, open-label trial investigated the use of tocilizumab (tocilizumab 8 mg/kg), sarilumab (400 mg once) or standard of care in critically ill COVID-19 patients. About 88% of patients who received IL-6 antagonists received tocilizumab. 21 As the standard of care changed to include corticosteroid use, 88% of patients also received steroids. The primary outcomes, respiratory and cardiovascular organ support–free days and days free of organ support to day 21 were met, with the median number of organ support–free days being 10 in the tocilizumab group and 0 in the control group, and the effects greater among patients with the highest CRP levels. 21 Hospital mortality was also significantly reduced: 28% for tocilizumab, 22.2% for sarilumab, 35.8% for control. The Number Needed to Treat (NNT) is 12.8 and 7.4 for tocilizumab and sarilumab, respectively.i,ii The median adjusted odds ratios for in-hospital survival were 1.64 (95% credible interval, 1.14–2.35) for tocilizumab and 2.01 (95% credible interval, 1.18–4.71) for sarilumab as compared with control. 21 Using Fisher Exact Test, the reduction in mortality between the tocilizumab group and the control group was statistically significant (p = 0.0278) (Figure 3). A statistically significant reduction was also found in 90-day survival, respiratory support free days, cardiovascular support free days, time to ICU discharge, and progression to invasive ventilation, ECMO or death. 21 There was also no significant difference in serious adverse events between the three groups. The study has many strengths as it is randomized, multi-centered, and importantly, all patients that were randomized were included in the analysis. The international trial steering committee was also blinded. The pragmatic, international design of the study allows for the generalization of results to the critically ill COVID-19 patient subpopulation. While the study’s open-label nature could introduce some bias, it is unlikely to affect mortality outcomes. However, as only a preliminary report is available, long-term outcomes of patients is still unknown with many still hospitalized. 21 Additionally, only a small number of patients received sarilumab, but this would not affect results for tocilizumab however larger trials studying sarilumab are required. Concerning hospital mortality, while the NNT for tocilizumab is adequate (12.8), the fragility index is weak (3) and indicates poor robustness of these results (Figure 4). 22
Figure 3.
Fisher Exact Test for mortality difference between tocilizumab and control groups in the REMAP-CAP trial. Calculated using https://www.socscistatistics.com/tests/fisher/default2.aspx.
Figure 4.
Fragility index for hospital mortality results between tocilizumab and control groups in the REMAP-CAP trial. Calculated using https://clincalc.com/Stats/FragilityIndex.aspx.
Contrary to the REMAP-CAP trial, Veiga et al. reported increased mortality in a randomized trial from Brazil that compared tocilizumab with standard care in 129 patients with COVID-19. 23 Aside from the lack of powering of the study, the trial was stopped early potentially overestimating the size of the effects (wide confidence interval of 1.59–43.2 obtained). 24 The fragility index of death at 15 days results is also very weak (2) (Figure 5). In a letter to the Editor concerning this trial, the authors conclude that the harm reported by Veiga et al.23,25 is likely an outlier considering all the previous studies showing tocilizumab’s safety.
Figure 5.
Fragility index for death at 15 days results between tocilizumab and control groups in the Veiga et al. RCT. Calculated using https://clincalc.com/Stats/FragilityIndex.aspx.
Finally, the RECOVERY trial, a multicenter, open-label, pragmatic adaptive trial enrolled 4116 patients in the assessment of tocilizumab, with 14% receiving invasive mechanical ventilation, 41% receiving non-invasive respiratory support, and 45% receiving no respiratory support other than oxygen. About 82% of all patients received corticosteroids and the median CRP was 143 mg/dL. 26 The primary outcome defined as 28 days mortality was met with 29% of the tocilizumab group and 33% of the usual care group dying within 28 days (rate ratio 0.86; 95% CI 0.77–0.96; p = 0.007), with an NNT of 25. iii This mortality benefit was seen only in those receiving systemic corticosteroids (27% vs 33%; Risk Ratio 0.80; 95% CI 0.70–0.90). 26 Patients allocated to tocilizumab were also more likely to be discharged from hospital alive within 28 days (54% vs 47%; rate ratio 1.22; 95% CI 1.12–1.34; p < 0.0001), and less likely to reach the composite endpoint of invasive mechanical ventilation or death (33% vs 38%; risk ratio 0.85; 95% CI 0.78–0.93; p = 0.0005). 26 While no significant effect of tocilizumab on subsequent receipt of non-invasive respiratory support, invasive mechanical ventilation, rate of successful cessation of invasive mechanical ventilation was found, there was a reduction in the use of hemodialysis or hemofiltration (5% vs 7%, risk ratio 0.75, 95% CI 0.59–0.96, p = 0.02). 26 The RECOVERY trial’s major strengths are the number of patients recruited (largest trial assessing tocilizumab) and the inclusive criteria of patients with varying respiratory support requirements facilitating generalizability of results to the population. Importantly, the trial compared the effects of combining steroids with tocilizumab versus tocilizumab alone. The validity of the results was also greater as the benefits of the primary outcome were consistent across all of the prespecified patient subgroups. 26 Additionally, the fragility index of the mortality benefits results is adequate (17) and well above the often cited median (8) (Figure 6). 22 As with the REMAP-CAP trial, only a preliminary report is available and information on the primary outcome is available for 92% of patients. 17% of patients in the tocilizumab group did not receive this treatment with the reasons unavailable. Long-term outcome is unavailable as many patients are hospitalized for more than 28 days but pre-planned analyses at 6 months will be done. Additionally, it is unknown if patients with a CRP < 75 mg/dL would benefit from this treatment. 26
Figure 6.
Fragility index for hospital mortality benefits results between tocilizumab and control groups in the RECOVERY trial. Calculated using https://clincalc.com/Stats/FragilityIndex.aspx.
Proposed mechanism of action of tocilizumab
Tocilizumab is a monoclonal antibody that acts as an antagonist of the IL-6 receptor (IL-6R). Production of endogenous IL-6 is induced by inflammatory stimuli and mediates a variety of immunological responses that could stimulate a hyperinflammatory state, which might lead to increased alveolar-capillary blood-gas exchange dysfunction resulting in acute respiratory distress syndrome (ARDS). IL-1β and TNFα, in particular, are the main activators of IL-6 expression. 27 Under normal circumstances, the levels of IL-6 are very low, and are increased when there is an infection or insult. Excessive release of IL-6 can cause a cytokine storm, with levels of IL-6 found to be proportional to the severity of the cytokine release syndrome (CRS). 28 Targeting IL-6 has become a point of interest in the treatment of COVID-19, as it has been found to be the most significant predictor of disease progression and mortality in patients. 29 Though the exact mechanism of action of tocilizumab is still unclear, the main hypothesis relies on tocilizumab preventing IL-6 from binding to its receptor and promoting the CRS sometimes seen in severe COVID-19 which leads to life-threatening multiorgan damage. 13 In short, the IL-6/IL-6R complex binds to the signal transducer glycoprotein 130 (gp130) and the downstream signal is mediated by JAK/STAT3. iv However, IL-6R exists in two forms: membrane-bound (mIL-6R) and soluble form (sIL-6R). IL-6 binds to mIL-6R, which is predominantly expressed on immune cells, and activates the cis-signaling pathway that promotes lymphocytes activation and differentiation. When IL-6 binds to sIL-6R, which is found virtually on all endothelial cells, the trans-signaling pathway is activated leading to increased vascular leakage and permeability, recruitment of neutrophils and monocytes and importantly, an increase in systemic cytokines production that leads to a cytokine storm.30,31 Tocilizumab is able to bind to mIL-6R and sIL-6R inhibiting both the cis and trans-signaling pathways, potentially treating or preventing the cytokine storm (Figure 7). 13
Figure 7.
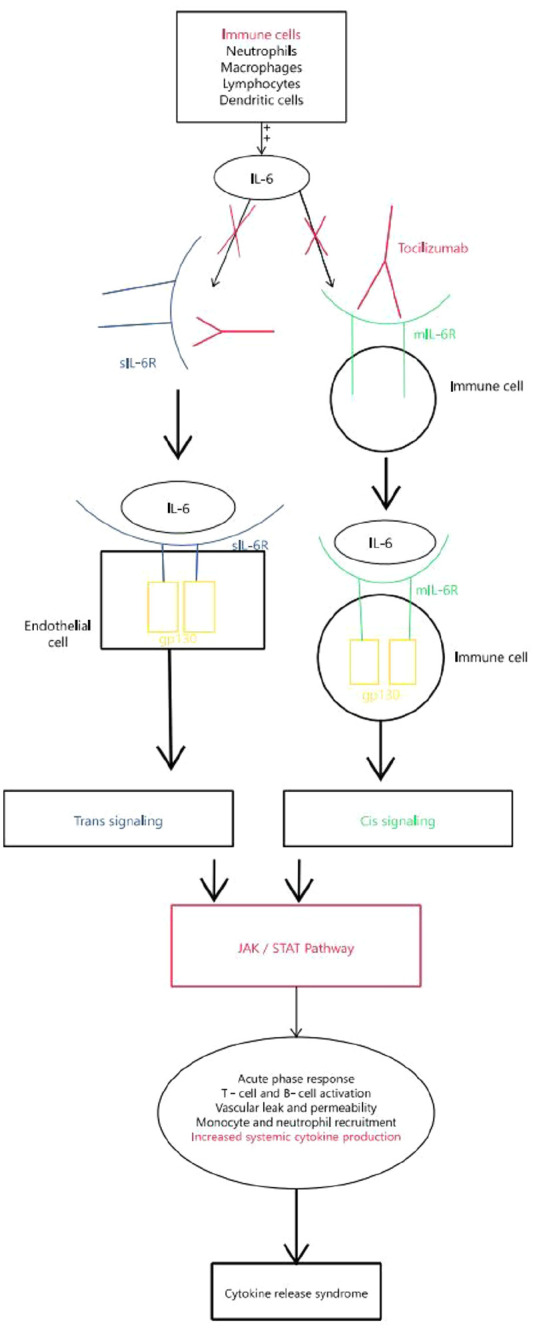
Overview of the IL-6 associated pathways leading to the cytokine release syndrome (CRS) seen in severe COVID-19.
gp130: glycoprotein 130; IL: interleukin; JAK/STAT: Janus kinases/signal transducer and activator of transcription; mIL-6R: membrane bound IL-6 receptor; sIL-6R: soluble IL-6 receptor.
Limitations
This review does not include every RCT but focuses on the bigger trials published. Additionally, the RECOVERY trial is still in preprint and has not yet been peer-reviewed. This review is limited mostly to tocilizumab while other drugs IL-6 inhibitors are under study such as sarilumab. Only preliminary reports from the REMAP-CAP and RECOVERY trial are currently available, with the RECOVERY trial not yet peer-reviewed. Additionally, we do not discuss other drugs that act on IL-6 such as JAK-1/2 inhibitors. Finally, more data will likely be published in the future that could potentially reinforce or refute the evidence found in this paper. Concerning the action of tocilizumab, the exact mechanism is still unclear, and the role of IL-6 in COVID-19 and the cytokine storm is still under study with more IL-6 inhibitors being evaluated.
Summary of findings
Early trials using tocilizumab monotherapy showed no clinical benefit. With systemic corticosteroids becoming part of standard care in severe COVID-19, 32 the combined use of tocilizumab and corticosteroids in later trials showed a decrease in mortality and hospitalization among other clinical benefits as seen in the REMAP-CAP and RECOVERY trials (Table 1). The RECOVERY trial in particular showed mortality benefit with tocilizumab only in patients who received concomitant corticosteroids. Additionally, several multicenter trials showed a good safety profile for tocilizumab and even though neutropenia was seen, the rate of infection was lower compared to the control groups. While REMAP-CAP showed the greatest benefits in patients with the highest CRP, and the RECOVERY trial recruited only patients with a CRP > 75 mg/dL, the true predictive nature of CRP still requires further investigation. Nonetheless, in hospitalized patients with severe COVID-19, who are hypoxic and have a CRP ≥ 75 mg/L, the current evidence suggests that the combination of tocilizumab and corticosteroids reduces mortality. Finally, the mechanism of action of tocilizumab is still unclear but is thought to involve binding to mIL-6R and sIL-6R preventing activation of IL-6 signaling pathways that promote pro-inflammatory reactions and favor the occurrence of CRS and cytokine storm.
Table 1.
Summary of findings of the randomized clinical trials that assessed the use of tocilizumab in COVID-19.
| Study | Type | Final Sample | Inclusion criteria | Primary endpoint | Corticosteroiduse | Findings |
|---|---|---|---|---|---|---|
| Salvaraniet al. 15 | Prospective,open label, multicenter, RCT | 123 patients (60 tocilizumab, 63control).Mean age 60. 61% male. |
PCR positive. Acute respiratory failure with PaO2/FiO2 200–300 mmHg, fever > 38°C during the last 2 days, and/or CRP of 10 mg/dL or greater and/or CRP level increased to at least twice the admission measurement |
Clinical worseningwithin 14 daysdefined as: 1. Admission to ICU with mechanical ventilation. 2. Death. 3. PaO2/FiO2 ratio less than 150 mmHg. |
4% of all patients | Primary endpoint not met. No evidence of adverse events Increase in neutropenia with decrease in infection incidence |
| BACC | Double-blind,placebo controlled,multicenter RCT | 243 patients (161 tocilizumab, 81placebo). 58% male. Mean age 59.8 years 45% Hispanicor Latino |
≥2 of the following: fever (>38°C), pulmonaryinfiltrates, or requirement for supplemental oxygen to maintain O2 Sat > 92% and ≥ 1 of the following laboratory criteria: CRP > 50 mg/L, ferritin > 500 ng/mL, d-dimer level > 1000 ng/mL, or a LDH > 250 U/L | Intubation ordeath | 10% of allpatients | Primary endpoint not met. No evidence of adverse events. Increase in neutropenia with decrease in infection incidence |
| COVACTA | Double-blind,placebo controlled, multicenter RCT | 438 patients (294 tocilizumab, 144 placebo). 70% male. Mean age 60. 56% white |
PCR positive. Bilateral chest infiltrates. O2 Sat ≤ 93% or PaO2/FiO2 ≤ 300 mmHg |
Status at day 28 on an ordinal scale ranging from 1 (discharged or ready for discharge) to 7 (death) | 42 % of all patients(55% in control and 36% in tocilizumabgroup) | Primary endpoint not met. Signal of benefit in time to discharge and duration of stay in ICU. No difference in adverse events. Decrease in infection incidence |
| CORIMUNO-TOCI-1 | Open-label, multicenter RCT | 131 patients (64 tocilizumab, 67 usual care). 68% male. Mean age 64 |
PCR positive or CT chest suggestive. Moderate, severe, or critical pneumonia (O2 > 3 L/min, WHO Clinical Progression Scale [WHO-CPS] score ≥ 5 |
1. Proportion of patients dead or needing noninvasive or mechanical ventilation on day 4 (>5 on the WHO-CPS). 2. Survival with no need for noninvasive or mechanical ventilation at day 14. |
48% of all patients (61% in control and33% in tocilizumab group) | No difference on day 28 mortality. Signal of benefit in reducing the need for mechanical and noninvasive ventilation or death by day 14. No increase in adverse or serious adverse events. Increase in neutropenia with decrease in infection incidence |
| EMPACTA | Double-blind, placebo controlled, multicenter RCT | 377 patients (249 tocilizumab, 128 placebo). 59% male. Mean age 56. 56.0% Hispanic or Latino, 14.9% Black, 12.7% American Indian or Alaska Native, 12.7% non-Hispanic White |
PCR positive. O2 Sat < 94% on room air |
Mechanical ventilation (invasive or extracorporeal membrane oxygenation). Death by day 28. |
83% of all patients (80% in tocilizumab, 87.5% in placebo) 55% received dexamethasone in tocilizumab group and 67% in placebo | Statistical significance in reducing the likelihood of progression to mechanical ventilation or death by day 28 (p = 0.04) BUT No statistical difference in overall clinical failure between the two groups (p = 0.0843). No increase in adverse or serious adverse events. Fewer serious infections. |
| REMAP-CAP | International, multifactorial adaptive platform, open-label trial | 865 patients (353 tocilizumab, 48 sarilumab, 402 control). 73% male. Mean age 61. 72% White, 17% Asian, 4% Black. |
ICU patients with suspected/confirmed COVID-19. Receiving respiratory support (invasive or noninvasive mechanical ventilation) or cardiovascular organ support (intravenous infusion of any vasopressor or inotrope) |
Number of respiratory and cardiovascular organ support–free days up to day 21. | 88% of all patients (85.5% in tocilizumab group, 95.8% in sarilumab, 86% in control) | Patients in IL-6 inhibitors group experienced more days free of organ support, with a median of 10 days. Effects greater with highest CRP. 6% hospital mortality reduction with tocilizumab p = 0.0278, OR 1.64 (95% credible interval, 1.14–2.35); Low fragility index (3); NNT 12.8. Statistically significant reduction was also found in 90-day survival, respiratory support free days, cardiovascular support free days, time to ICU discharge, and progression to invasive ventilation, ECMO or death. |
| Veiga et al. 23 | Multicenter, randomized, open label, parallel group, superiority RCT | 129 patients (65 tocilizumab, 64 control). | PCR positive. Pulmonary infiltrates. Supplemental O2 to maintain Sat > 93% or had been receiving mechanical ventilation for less than 24 h before analysis. ≥ of the following: D dimer > 1000 ng/mL, CRP >5 mg/dL, ferritin > 300 μg/L, or LDH > upper limit of normal. |
Clinical status measured at 15 days using a seven-level ordinal scale, was analyzed as a composite of death or mechanical ventilation | 86 % of all patients (83.6% in tocilizumab group, 88.7% in control) | Trial stopped early due to increased mortality found with tocilizumab (OR 6.42, 95% CI 1.59–43.2), weak fragility index (2). No difference in incidence of adverse events |
| RECOVERY | Randomized, controlled, open-label, platform RCT | 4116 patients (2022 tocilizumab, 2094 control). | Clinically suspected or laboratory confirmed SARS-CoV-2 infection | All-cause mortality | 82% of all patients | Mortality benefit (rate ratio 0.86; 95% CI 0.77–0.96; p = 0.007), NNT of 25, good fragility index (17). Only seen in those receiving systemic corticosteroids (27% vs 33%; risk ratio 0.80; 95% CI 0.70–0.90). Patients also more likely to be discharged from hospital alive within 28 days (54% vs 47%; rate ratio 1.22; 95% CI 1.12–1.34; p < 0.0001), and less likely to reach the composite endpoint of invasive mechanical ventilation or death (33% vs 38%; risk ratio 0.85; 95% CI 0.78–0.93; p = 0.0005)/ Reduction in the use of hemodialysis or hemofiltration (5% vs 7%, risk ratio 0.75, 95% CI 0.59–0.96, p = 0.02). |
Author biographies
Walid Alam received his bachelor’s degree (BS) in Medical Laboratory Sciences from the University of Balamand’s Faculty of Health Sciences in 2015, and his medical degree (MD) from the University of Balamand’s Faculty of Medicine in 2019. He worked as a house physician in the Emergency Department at the American University of Beirut Medical Center. He has also spent over a year as a postdoctoral research fellow in the Department of Internal Medicine, in the Divisions of Oncology and Infectious Diseases, where he worked on several projects. He was also involved in revising the Lebanese national guidelines for the management of MDR- and XDR-TB in Lebanon in coordination with the World Health Organization (WHO) and the Ministry of Health. Additionally, Dr. Alam recently joined the American University of Beirut’s Global Health Institute (GHI), an institute that aims to address global health challenges affecting the Middle East and North Africa (MENA) region. He is serving as a Subject Matter Expert to develop an Infectious Diseases Certificate Program for members of the Syrian refugee communities and host communities in Lebanon who are interested in becoming Community Health Workers.
Abdul Rahman Bizri, MD, MSc, DLSHTM, FRCP, is a consultant in Internal Medicine and Infectious Diseases at the American University of Beirut Medical Center (AUBMC), the Assistant Chairperson for Clinical Affairs, and the Director of Quality and Compliance Program at the Department of Internal Medicine. He is a member of the Steering Committee and Affiliate of the Conflict Medicine Program-Global Health Institute at AUB. He has served as the chairperson of the Infection Control Program and Infection control Committee at AUBMC till 2005 and established many infection-control programs in various teaching medical centers both private and governmental. Dr. Bizri has more than 85 publications on antibiotic resistance, and the changing epidemiology of infectious diseases mainly Salmonella, rabies, viral hepatitis, HIV/AIDS, invasive mycosis, microbiology of war related infections, immunization, refugee medicine, and COVID-19. He also serves as a WHO expert and advisor on infectious and transmissible diseases. He is a founding member and former president of the Lebanese AIDS Society and a founding member and former president of the Lebanese Society of Infectious Diseases and Clinical Microbiology. He is also a member of the Executive Committee of the Arab Board in Internal Medicine and the past chairperson of the committee to establish the Arab Board of Infectious Diseases. He is currently the Chairperson for the National Certification Committee and the Chairperson of the National Committee on COVID-19 Vaccine Preparedness and Deployment in Lebanon. Additionally, he is a member of the EPI expert group on immunization practices, the National Committee for Communicable Diseases, the National Expert Group on the National Response to contain the COVID-19 Pandemic, the COVID-19 Task Force for the Lebanese Society for Infectious Diseases, and the AUBMC COVID-19 Task Force.
Absolute Risk Reduction (ARR) = (Control event rate) − (Experimental event rate)Tocilizumab ARR = 0.358 − 0.28 = 0.078Tocilizumab NNT = 1/ARR = 1/0.078 = 12.8
Sarilumab ARR = 0.358 − 0.222 = 0.136Sarilumab NNT = 1/ARR = 1/0.136 = 7.4
Absolute Risk Reduction (ARR) = (Control event rate) − (Experimental event rate) ARR = 0.33 − 0.29 = 0.04 NNT = 1/ARR = 1/0.04 = 25
JAK: Janus kinase STAT3: signal transducer and activator of transcription 3.
Footnotes
Author contributions: Dr. Walid Alam was responsible for the inception of the project, data collection, data analysis, production of tables and figures, and writing the manuscript. Dr. Abdul Rahman Bizri contributed to data analysis, writing the manuscript, and the final revision.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Romagnoli S, Peris A, De Gaudio AR, et al. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol Rev 2020; 100(4): 1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotfi M, Hamblin MR, Rezaei N.COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta 2020; 508: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MJ, Hanif M, Haider MA, et al. Treatment options for COVID-19: a review. Front Med (Lausanne) 2020; 7: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Q, Guo M, Zheng Y, et al. Current evidence of interleukin-6 signaling inhibitors in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol 2020; 11: 615972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atal S, Fatima Z.IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut Med 2020; 34(4): 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020; 146(1): 128–136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Price CC.Overview on the use of IL-6 agents in the treatment of patients with cytokine release syndrome (CRS) and pneumonitis related to COVID-19 disease. Expert Opin Investig Drugs 2020; 29(12): 1407–1412. [DOI] [PubMed] [Google Scholar]
- 8.COVID-NMA. Living evidence synthesis, pharmacologic treatments, tocilizumab vs placebo/standard of care. https://covid-nma.com/living_data/index.php?treatment1=Tocilizumab (accessed 20 February 2021).
- 9.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2021; 181(1): 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison AR, Johnson JM, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun 2020; 114: 102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117(20): 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020; 19(7): 102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortegiani A, Ippolito M, Greco M, et al. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology 2021; 27(1): 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horby P, Lim WS, Emberson JR, et al.; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384(8): 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383(24): 2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021; 384: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furlow B.COVACTA trial raises questions about tocilizumab’s benefit in COVID-19. Lancet Rheumatol 2020; 2(10): e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181(1): 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama C, Mohan SV.Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon AC, Mouncey PR, Al-Beidh F, et al.; REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021; 384(16): 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh M, Srinathan SK, McAuley DF, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a fragility index. J Clin Epidemiol 2014; 67(6): 622–628. [DOI] [PubMed] [Google Scholar]
- 23.Veiga VC, Prats JAGG, Farias DLC, et al.; Coalition Covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021; 372: n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Briel M, Glasziou P, et al. Problems of stopping trials early. BMJ 2012; 344: e3863. [DOI] [PubMed] [Google Scholar]
- 25.McCreary EK, Meyer NJ.Covid-19 controversies: the tocilizumab chapter. BMJ 2021; 372: n244. [DOI] [PubMed] [Google Scholar]
- 26.Horby PW, Pessoa-Amorim G, Peto L, et al.; RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet 2021; 397: 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter CA, Jones SA.IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16(5): 448–457. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Li L, Shen A, et al. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig 2020; 40(6): 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santa Cruz A, Mendes-Frias A, Oliveira AI, et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol 2021; 12: 613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zegeye MM, Lindkvist M, Fälker K, et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun Signal 2018; 16(1): 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368(6490): 473–474. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Corticosteroids for COVID-19, https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed 20 February 2021).