Abstract
Catheter ablation is nowadays considered the treatment of choice for numerous cardiac arrhythmias in different clinical scenarios. Fluoroscopy has traditionally been the primary imaging modality for catheter ablation, providing real-time visualization of catheter navigation. However, its limitations, such as inadequate soft tissue visualization and exposure to ionizing radiation, have prompted the integration of alternative imaging modalities. Over the years, advancements in imaging techniques have played a pivotal role in enhancing the safety, efficacy, and efficiency of catheter ablation procedures. This manuscript aims to explore the utility of imaging, including electroanatomical mapping, cardiac computed tomography, echocardiography, cardiac magnetic resonance, and nuclear cardiology exams, in helping electrophysiology procedures. These techniques enable accurate anatomical guidance, identification of critical structures and substrates, and real-time monitoring of complications, ultimately enhancing procedural safety and success rates. Incorporating advanced imaging technologies into routine clinical practice has the potential to further improve clinical outcomes of catheter ablation procedures and pave the way for more personalized and precise ablation therapies in the future.
Keywords: Imaging, Cardiac computed tomography, Cardiac magnetic resonance, Echocardiography, Electroanatomical mapping, Single-photon-emission computed tomography, Positron emission tomography
Introduction
Supraventricular tachycardia catheter ablation (CA) procedures, in general, are considered low risk interventions and have a high success rate. Electroanatomic mapping (EAM) systems and imaging are both considered not essential to improve safety or outcomes in this setting. However, some re-entrant atrial tachycardias and atrial fibrillation ablation procedures could significantly benefit from using these technologies.
Ventricular arrhythmia (VA) ablation procedures are complex, associated to a non-negligible rate of complications and require expertise and advanced electrophysiologist’s skills. They have been commonly performed in high volume centres by highly experienced operators. The VA ablation therapy has evolved tremendously in the last 25 years, largely associated to a big progress in the understanding of the important role that the fibrotic tissue plays in the development of sustained ventricular tachycardia.1
Along with great improvement in catheter ablation technologies, cardiac imaging has undergone a great evolution. Different imaging modalities are nowadays considered essential tools to ensure procedure safety, assist in pre-procedure planification and help in catheter ablation guidance. Its usefulness has also been recognized and recommended to identify the VA substrate and improving outcomes in VA consensus document and guidelines.2,3 Cardiac imaging implementation in the standard VA management and treatment workup has importantly contributed to the widespread of these complex ablation procedures and to offer VA ablation earlier in the evolution of the disease.
The role of different imaging modalities in substrate identification, procedure planning and guidance for both, and atrial and ventricular arrhythmias, will be summarized in this issue.
Cardiac imaging for atrial arrhythmias procedures
Electroanatomical mapping
Traditionally, electrophysiological studies and ablation of atrial arrhythmias (AA) have been performed under fluoroscopic guidance, with the consequent4 exposure to radiation. The EAM using electroanatomical navigation systems has made it possible to reduce the need for fluoroscopy in atrioventricular nodal re-entrant tachycardia, atrioventricular re-entrant tachycardia, atrial flutter, and atrial fibrillation (AF) ablation procedures5–8 without compromising either efficacy or safety.6–8
The EAM permits recording bipolar, unipolar, or multipolar voltages; local activation times, or identifying certain characteristics of the electrograms (EGMs), such as the presence of Complex Fractionated Atrial EGMs, or rotors in AF. Additional information, such as conduction velocity, activation direction, and propagation can be evaluated. Far beyond anatomically based procedures in AF [pulmonary vein isolation (PVI)], functional atrial mapping has gained special relevance, allowing to identify potential—and amenable to ablation—arrhythmogenic substrate outside the PV. An international position paper has recently reviewed all available AF mapping technologies.9 Cardiac magnetic resonance (CMR) imaging, cardiac computed tomography (CT), and intracardiac echocardiography (ICE) can be integrated into EAM systems during AF and other complex AA ablation procedures, as Figure 1 shows. This may result in better procedural outcomes and efficiency, and less fluoroscopy utilization.10–14 The CMR atrial tissue fibrosis has been independently associated with the likelihood of recurrent AF,15 although a CMR fibrosis-guided AF ablation appears to be not superior to a pure PVI-based strategy.15 Conversely, macro-re-entrant AA after AF ablation may benefit from this CMR-guided strategy.16 Nonetheless, large discrepancies between CMR fibrosis and low voltage zones (LVZ) may still be found.17 Atrial anatomy assessment is best obtained from CT images due to their great spatial resolution. The CT-EAM fusion may improve the efficacy of AF ablation procedures, although it still remains a matter of debate.18 Still, CT can define the cardiac anatomy, included uncommon variants and the atrial wall thickness (WT);19 and may improve procedural safety: detection of thrombi,20 oesophageal position21 coronary disease, etc.
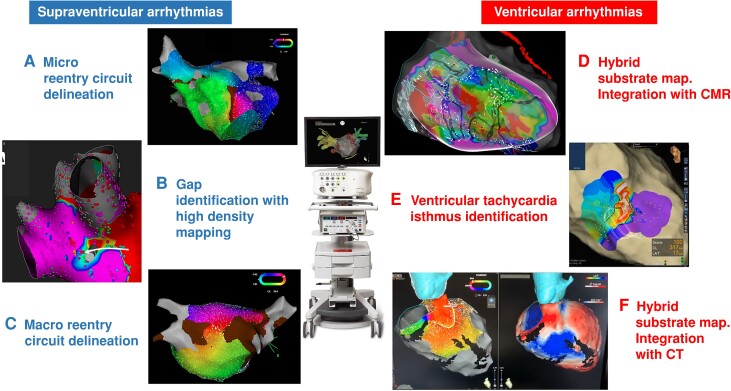
Figure 1.
Usefulness of electroanatomical mapping (EAM) for catheter ablation of atrial and ventricular arrhythmias: A) delineation of a micro-re-entry circuit in the left atrial anterior wall; B) identification of a gap in the ablation line of the right pulmonary arteries; C) delineation of a macro-re-entry circuit of a roof-dependent left atrial flutter; D) integration of EAM with cardiac magnetic resonance in a patient with a scar-related ventricular tachycardia; E) protected VT isthmus identification; F) integration of EAM showing an activation map of a VT with anteroseptal exit with the pre-procedural CT containing myocardial wall thickness and lipomatous metaplasia information. CMR, cardiac magnetic resonance; CT, cardiac computed tomography.
Atrial fibrosis promotes heterogeneous slow conduction, increasing AF vulnerability and the likelihood of AF recurrence.22 The PVI clinical outcomes in persistent AF may be improved when adding a voltage-guided substrate modification approach targeting LVZ.23 Yet, the presence of LVZ can predict AF recurrences, while there is a bad correlation between the extent of LVZ and the indexed left atrial volume.24 Moreover, the presence of any deceleration zone in sinus rhythm is also an independent predictor of AF recurrence, not always correlated to the presence of LVZ.25,26
Echocardiography
Ultrasound (US) imaging has been increasingly used for electrophysiology (EP) procedures. Pre-, peri-, and post-procedural imaging is critical to improve the procedural safety and success; as a matter of fact, each interventional stage can potentially benefit from real-time visualization of the underlying anatomy, thereby avoiding any anatomical assumptions that may be incorrect and potentially dangerous.
Conventionally performed via a palpation-based approach, vascular access is the procedural step associated with the highest number of complications, with an incidence ranging between 1% and 13%.27–30 The US guidance has been demonstrated to significantly decrease (up to 65%) the risk of major vascular complications compared to an anatomical landmark-based approach. Similarly, this approach may successfully reduce the risk of minor complications (e.g. groin haematoma and inadvertent arterial puncture), thereby promoting a beneficial effect on comorbidities, hospitalization duration, and healthcare expenditure.31 The US-guided access may be especially beneficial for EP procedures requiring a large bore access into the femoral vein [e.g. single-shot devices and percutaneous left atrial appendage (LAA) occlusion]32–34; additionally, vascular US has been also adopted to guide vascular closure device deployment.35,36
Transseptal access is another critical step of left-sided EP procedures and can be associated with serious complications (e.g. cardiac tamponade, aortic puncture, and systemic embolism). Transoesophageal echocardiography (TEE) is commonly used to guide septal puncture (Figure 2). The main limitation of TEE is that it relies on a second operator to allow active and cooperative septal visualization optimization while the main operator is focused on manoeuvring the apparatus for transseptal access. The ICE is a valid alternative to TEE for transseptal access, as well as for a wide variety of other uses, as it allows direct, real-time visualization of many cardiac structures that are critical for EP procedures. The ICE currently plays a central role for flourless ablation procedures.37 One of the most important features of ICE is continuous monitoring and early detection of peri-procedural complications, including steam pops, thrombus formation, and pericardial effusion/tamponade (Figure 2). Another advantage of this technology is the lack of need for general anaesthesia or an additional operator for TEE manoeuvring. The ICE catheter can also be advanced into the left atrium and is also currently used for LAA occlusion guidance; this approach has been demonstrated to reduce procedural duration and patient turnover.38,39 Additionally, novel 3D-ICE probes feature direct anatomical visualization in multiplane/multislice and 3D modes.40,41
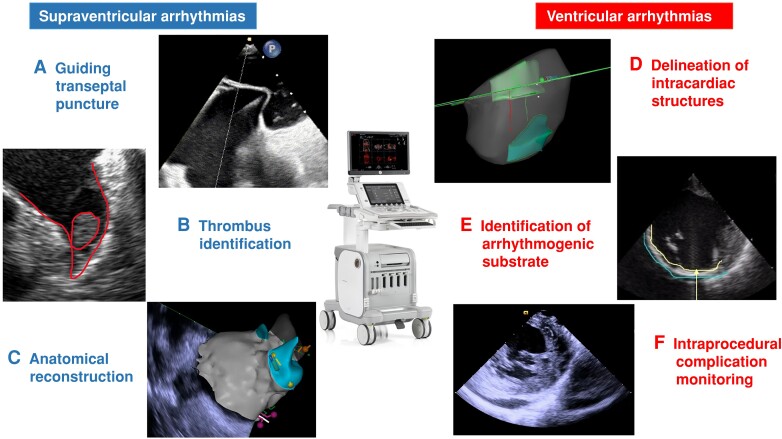
Figure 2.
Usefulness of ultrasounds for catheter ablation of atrial and ventricular arrhythmias: A) transseptal puncture guided by transoesophageal echocardiography; B) intracardiac thrombus visualization in the left atrial appendage; C) left atrial reconstruction using Carto Sound Module; D) papillary muscle reconstruction using Carto Sound Module; E) shot axis view in a patient with an inferior myocardial infarction); F) short axis view in a patient with pericardial effusion during a VT ablation.
Computed tomography
Computed tomography has undergone significant technical evolution since its inception in the 1970s. This imaging modality has become an increasingly important tool for the non-invasive evaluation of cardiovascular anatomy and function. The evolution of cardiac CT has resulted in improvements in spatial and temporal resolution, image quality, motion artefact reduction, radiation dose reduction, and advanced post-processing techniques. These advancements have made cardiac CT an increasingly valuable tool for helping CA procedures.
Due to its great spatial resolution, cardiac CT provides detailed anatomical information of the atrium, including the size, shape, location of vessels, and their relationship with extracardiac structures (Figure 3). In the setting of PVI, the integration of CT images into the EAM42,43 allows visualization of the fossa ovalis44 anatomy and identification of unexpected anatomical PV variants,45 facilitating procedural planning. This information can be particularly useful during cryoballoon ablation, as PV ostium shape and orientation can predict PV occlusion during the procedure, identifying unfavourable anatomies in which a point-by-point strategy might be more appropriate.46
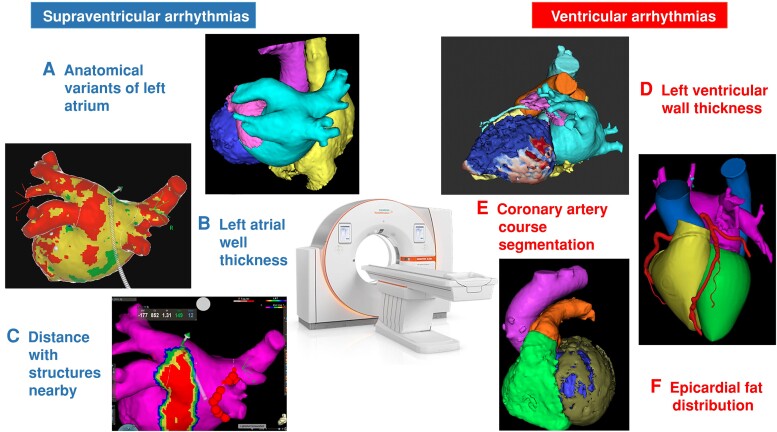
Figure 3.
Usefulness of cardiac computed tomography (CT) for catheter ablation of atrial and ventricular arrhythmias: A) pre-procedural CT showing a common ostium of the inferior PVs; B) post-processed CT showing a color-coded map of the left atrial WT; C) isodistance map of the esophagus projected in the CT reconstruction of the left atrium; D) three-dimensional reconstruction of the left ventricle WT map (and channels) in a patient with an inferior infarction; E) coronary arteries course; F) left ventricular epicardial fat distribution. PVs, pulmonary veins; WT, wall thickness.
Cardiac CT also allows to obtain information of the left atrial WT, a major determinant of lesion transmurality.19,47 Left atrial WT-guided titration of radiofrequency delivery for paroxysmal and persistent AF ablation has been proved to allow for highly efficient and effective procedures.47 Moreover, CT information can be used to increase procedure safety, allowing 3D visualization of the left atrium’s relationship with the oesophagus,48,49 the left superior pulmonary veins’ relationship with the bronchi,50 and the distance between the right upper PV and the right pericardiophrenic artery, an indirect marker of the phrenic nerve course.51
All this information allows us to move towards a personalized ablation strategy based on the patient’s specific anatomy, preventing injury to sensible extracardiac structures during ablation. Cardiac CT also plays a major role in post-procedural handling, as it is the gold standard for verification of different complication diagnoses, such as the atrio-oesophageal fistula52 or PV stenosis. Although initially designed for atrial fibrillation ablation, CT-image integration into the EAM may also improve the efficacy of CA in other complex atrial arrhythmic substrates. The use of CT scans may help to identify the origin of atrial ectopic focus.53 Moreover, cardiac CT provides detailed information to properly select the course of the linear ablation lesions in patients with left54 or right55 atrial macro-re-entrant circuits. Conversely, left atrial WT maps have shown to be helpful in visualizing previous linear ablations and gaps, facilitating activation map interpretation in left atrial flutters.
Cardiac magnetic resonance
Pre-ablation cavotricuspid isthmus (CTI) visualization has gained significant attention in recent years with the development of the CMR-EP system.56 This innovative technique allows for high-resolution visualization of the anatomy, substrate, and ablation lesions without the need for fluoroscopy, making it particularly useful in procedures requiring detailed anatomical visualization like CTI ablation.56 This can lead to a reduction in radiation exposure for both the patient and the operator.
The use of real-time CMR, if translated to AF procedures, could be immensely beneficial. The prevalence and health burden of AF are much higher than atrial flutter, and there is a need to further improve procedural management of AF.
The use of CMR in the management of AF has a lot of benefits. For instance, it has been previously demonstrated that CMR can be used to assess underlying disease tissue or atrial fibrosis, as Figure 4 shows.57 These increasing levels of atrial fibrosis were independently linked to hard clinical outcomes like major cerebrovascular and cardiovascular events.58 Also, the extent of disease in the left atrium has been shown to be a significant predictor of recurrence after CA. This was proved in the multicenter DECAAF I study, where patients were divided into four different groups of atrial fibrosis with high discrepancies in outcomes depending on the quantity of fibrosis.15
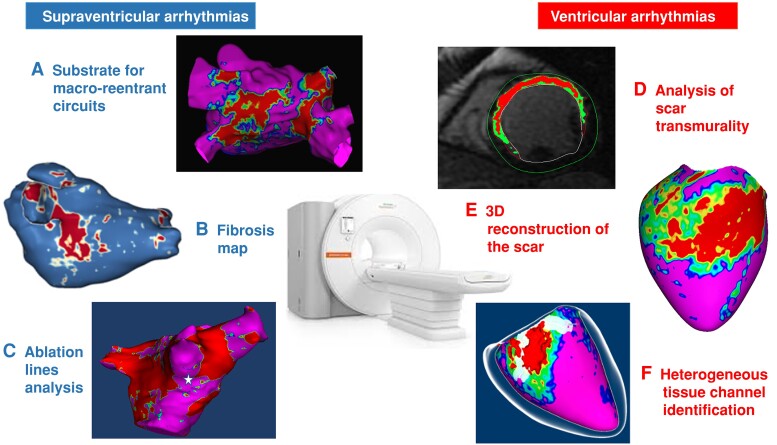
Figure 4.
Usefulness of cardiac magnetic resonance (CMR) for catheter ablation of atrial and ventricular arrhythmias: A) pre-procedural CMR showing the fibrosis distribution in the left atrium in a patient with atypical left atrial flutter; B) post-processed CMR showing fibrosis distribution in a patient with atrial fibrillation; C) pre-procedural CMR showing the iatrogenic fibrosis due to a previous ablation in a patient who underwent a redo ablation procedure for atrial fibrillation; D) short axis view in a patient with an anterior myocardial infarction; E) pixel signal intensity map 3D reconstruction in a patient with an inferior infarction; F) heterogeneous tissue channel delineation.
A sub-analysis of this study by Akoum et al.59 showed that covering more fibrosis by ablation lesions can lead to fewer recurrences after the procedure. The DECAAF II trial was designed to prospectively test this hypothesis. So, patients were randomly assigned to receive either PVI-only or PVI plus fibrosis-guided ablation. However, the study results showed no significant differences between the two arms.60
Nonetheless, the application of CMR remains valuable, especially pre- and post-procedures. As mentioned earlier, the evaluation of baseline left atrial fibrosis provides valuable prognostic information for patients after the procedure. Additionally, the evaluation of the distance between the atrium and the oesophagus is a critical determinant of oesophageal injury due to CA.61,62 Therefore, getting an image before the ablation can guide us on efficacy and safety outcomes. On the other hand, post-procedural CMR can also help guide redo ablations. Studies have shown that using CMR to guide redo ablations can lead to shorter procedural time and lower chances of recurrence.63
Positron emission tomography-scan and single-photon-emission computed tomography-scan
Nuclear cardiology exams (NCEs), namely single-photon-emission computed tomography (SPECT) and positron emission tomography (PET), constitute relevant imaging tools for cardiovascular diseases. To date, the main clinical applications of NCEs involve myocardial ischaemia, ranging from coronary artery disease (CAD) to coronary microvascular dysfunction.64 In detail, while stress/rest SPECT can identify ischaemic and necrotic areas, specific tracers in PET scan allow investigation of myocardial metabolism (18F-FDG-PET), and quantitative measurement of myocardial perfusion (H215O-PET or 13N-PET).64
To date, the clinical role of NCEs in cardiac arrhythmias is still limited. In fact, CMR and CT scan constitute the gold standard for substrate characterization and morpho-functional assessment of most myocardial diseases.3 In selected cases, however, NCEs can provide significant information to guide diagnosis, prognostic assessment, and treatment strategies for patients with arrhythmias.
Among supraventricular arrhythmias, NCEs have been mainly applied to AF. For instance, 99mTc-MIBI SPECT perfusion imaging has been proposed to identify CAD as a substrate for unexplained AF.65 In the setting of AF, however, the diagnostic value of SPECT has recently shown a low predictive value for CAD.66 In fact, fast and irregular heartbeat may frequently account for poor image quality and subsequent inaccuracy in assessing ischaemia-induced regional wall motion abnormalities.67 Instead, quantitative data from perfusion PET have shown that, even in the absence of epicardial CAD, myocardial blood flow and coronary flow reserve are abnormal in patients with persistent AF.68 These data suggest that coronary microvascular dysfunction may be the consequence rather than the cause of AF,68 and deserve further investigation by future studies.
NCEs have been employed also to characterize supraventricular arrhythmogenic substrates before CA. In one study,69 SPECT was used in combination with other imaging techniques to identify atrial cardiomyopathy in young patients undergoing CA of atrial tachycardia. More recently, hybrid 99mTc-Pyrophosphate SPECT/CT has been proposed to detect latent inflammatory processes in patients with AF and biopsy-proven myocarditis.70 Overall, these data indicate that an improved detection of atrial scars and inflammation may help identifying non-responders to CA, even in the absence of significant left atrial dilation.3 Finally, a role for I-123-Metaiodobenzylguanidine SPECT imaging has been suggested to investigate dysautonomic manifestations in patients with AF undergoing CA.71 Again, the clinical value of NCEs in defining suitable ablation targets and in predicting procedural outcomes is still to be proved.
Cardiac imaging for ventricular arrhythmias procedures
Electroanatomical mapping
The advent of 3D-EAM systems in the late 1990s clearly marked a major historical turning point for cardiac EP, particularly with respect to CA of VA in patients with structural heart disease.72
The 3D-EAM systems combine three important72 features: (i) real-time visualization of catheters without the use of X-rays, (ii) 3D display of the virtual anatomy of heart chambers in relation to local EGM data, and (iii) fusion with non-invasive images of the heart (ICE, CT scan, CMR…). The 3D-EAM systems, which are very effective to reduce patient and staff exposure to fluoroscopy,73 are currently widely used in daily practice for all types of CA procedures74 in particular in over 90% of all ventricular tachycardia (VT) ablation procedures.75,76
With respect to VT ablation, one of the key innovative features of 3D-EAM systems was, for the first time, the complete virtual visualization of VT circuits through complete endocardial activation maps of the ventricles,77,78 thus, allowing an accurate characterization of critical VT isthmuses, including not only their dimensions but also the elements that form their lateral boundaries (see Supplementary material online, Video).
The presence of slow conduction zones related to myocardial scars of various aetiologies (post-infarction, post-myocarditis…) is the pathophysiological substrate for macro-re-entry, which is the predominant mechanism of VT in patients with structural heart disease. Such scars harbour local abnormal ventricular activities (so-called LAVAs) in terms of voltage, duration, and morphology (i.e. split/fractioned EGM, late potentials…). Cross-correlation studies with CMR,79,80 histology,81 and VT mapping79 have shown the accuracy of bipolar/unipolar voltage mapping in unmasking scars, with unipolar voltage mapping being more appropriate to detect intramural scars82 and possibly diffuse fibrosis that cannot be easily imaged by CMR.83
Because many VTs are poorly tolerated, which impedes VT mapping,84 substrate-based VT ablation that circumvents this issue, by targeting LAVAs, has early become very popular.75 LAVAs related to VT circuits85 can be directly86 or indirectly87 identified during 3D-EAM. Areas harbouring slow conduction can also be unveiled by pace-mapping88 or by pacing with double ventricular extrastimuli.87
In patients with structural heart disease, a recent meta-analysis of the literature89 showed that substrate-based ablation (aimed at modifying the substrate) was associated with better outcomes than ‘standard’ ablation (aimed at ablating stable VT) for the combined endpoint of VA recurrence and all-cause mortality.
Merging 3D-EAM maps with other cardiac imaging modalities is very useful when planning VT ablation procedures. Thus, ICE is a key exam to visualize papillary muscles that can hardly be outlined by 3D-EAM systems. The CT scan and CMR90 are also important tools for the identification of ventricular arrhythmogenic substrate in patients with structural heart disease, with VT channels meandering through scars highlighted by a dedicated software applied to CMR.91
Echocardiography
The role of ultrasound assessment in VAs procedures is wide and includes the following tools: transthoracic echocardiography, TEE, and ICE.
Transthoracic echocardiography is useful in pre-procedural planning to evaluate the patient risk of VAs and to rule out left ventricle thrombus (contrast echocardiography may be considered in this case).92 Global function of the left ventricle should be assessed with left ventricular ejection fraction (LVEF). The LVEF is a parameter integrated in the current risk stratification for pre-procedural mechanical support, besides its role as a known marker of VAs risk.93 However, the absolute number of sudden cardiac death (SCD) victims is higher in the group of patients with LVEF >50%.94 For this reason, a mildly reduced or normal LVEF should not be used to rule out VAs. If a patient is planned for a VT ablation, regional function evaluation by transthoracic echocardiography is useful to guide towards the scar area, in case of a suspected scar-dependent VT.2 On the other hand, if a patient is evaluated for a premature ventricular contraction (PVC) ablation, transthoracic echocardiography can predict the recovery of LVEF in case of suspected PVC-induced cardiomyopathy. In a seminal study by Penela et al.,95 an LV end-diastolic diameter >63 mm identified patients who will not normalize LVEF after PVC ablation.
Transthoracic echocardiography or TEE or ICE should be always available in the EP lab during VA ablation to rule out pericardial puncture/bleeding.2 Indeed, PVC ablation is associated with a 2% complication rate, with pericardial effusion being the most frequent (40% of total complications).96 Furthermore, during epicardial procedures with unintended puncture of the right ventricle can occur in up to 17% of cases.2
The TEE or ICE can be used to guide transseptal puncture. Being the LV with an inferior and anterior structure compared to the septum, the preferred position for the transseptal access should be the antero-inferior portion of interatrial septum.
Finally, ICE is recommended to localize the ostia of the coronary arteries prior to ablation in the sinuses of Valsalva.2 It is also beneficial to identify and target the papillary muscles with ablation and to assess for catheter stability.97 In particular, ICE allows a direct visualization of the papillary muscle during mapping and helps to correctly identify the anatomical site of the arrhythmogenic focus; during CA, it confirms contact between the ablation catheter and the target, avoiding collateral damage to surrounding anatomical structures (mitral valve chordae and leaflets).98 It may be useful, during the procedure, as an adjuvant technique to identify wall segments with wall thinning, wall motion abnormalities, and segments with increased echogenicity, and also to identify intracardiac thrombi.2
Computed tomography
Several studies have demonstrated the potential of cardiac CT for pre-procedural planning in VT ablation. Wall thickness assessment using cardiac CT has recently emerged as an alternative cardiac imaging method to characterize arrhythmogenic substrate before VT ablation procedures,99 showing a good correlation with low voltage areas and LAVAs.100 Ghannam et al.101 described that WT correctly identify the ablation targets in a population of post-infarction patients who underwent VT ablation. However, compared to CMR, cardiac CT presents a lower contrast-to-noise ratio, a characteristic that reduces the cardiac CT capability for scar characterization. As consequences, CT may not accurately identify non-transmural areas of myocardial scar, especially in cases where the scar is in the sub-endocardium in ischaemic patients.90 In patients with non-ischaemic cardiomyopathy, who typically do not show myocardial thinning in scarred areas, the usefulness of CT may be also inferior compared to CMR.
The other side of the coin is the high spatial resolution of CT. While CMR spatial resolution usually ranges from 1.4 to 2 mm, CT spatial resolution is typically close to 0.5 mm. This results in better identification of the coronary arteries, phrenic nerve, and epicardial fat distribution102,103 (Figure 3). Yamashita et al.104 previously showed how the integration of cardiac CT during epicardial VT ablation can increase the safety of the procedure avoiding radiofrequency delivery in the very proximity of these structures. Furthermore, cardiac CT can easily identify lipomatous metaplasia, which facilitates the propensity of re-entry VT circuits in healed myocardial infarction.105 Moreover, CT identifies intracardiac thrombus, which can be present in up to 11% of patients referred for scar-related VT-CA, as recently reported.106 Finally, cardiac CT can be useful in the setting of PVC ablation. First, it can help to identify the site of origin in patients with outflow-tract PVCs by analysing the presence of anatomical modifications due to chronic overload.107 Moreover, CT imaging integration into the EAM can be useful for aiding ablation procedures of PVC originating from complex intracardiac structures, such as the papillary muscles108 for the aortic cusps.
The use of cardiac CT has shown to be a valuable tool in assisting CA of VA and can be easily integrated into the procedure workflow. However, it is important to carefully consider the potential limitations of the technique, such as limited soft tissue contrast and the risk of radiation exposure, particularly in patients requiring multiple scans.
Cardiac magnetic resonance
Fibrotic tissue has been recognized to be the main substrate for VA, is present in various degrees even in the case of a focal origin, and supports re-entry circuits. Contrast-enhanced CMR has proved its capacity to identify with high precision this scarred tissue, which confers an increased arrhythmia susceptibility.1 Scar identification and quantification have demonstrated to help identify those patients at higher risk for VA in both ischaemic and non-ischaemic cardiomyopathies in various clinical scenarios, in a significant number of studies.109–111
The identification of myocardial scar and its distribution pattern through the myocardial WT has been shown to be of help to focus mapping and ablation on the area of interest and also to decide the endocardial approach vs. epicardial approach for free wall VTs or the right access vs. left access for septal scar-related VTs.112
The CMR also permits to differentiate between dense non-excitable scar and border zone fibrotic tissue surrounding the scar or creating channels through the scar or between the scar and an anatomical obstacle like a valve annulus. These channels can be identified using dedicated software for CMR post-processing.91 The capacity of CMR to identify these channels is superior to that of the CT, the latter failing to detect the presence of arrhythmogenic substrate in one-third of patients with subendocardial myocardial infarction. However, the performance of CT improves in the presence of transmural scars.90 A significant number of patients undergoing VT ablation have implantable cardioverter-defibrillator (ICD) that can cause image artefacts in CMR. In those patients, wideband CMR sequences have shown to decrease these artefacts while maintaining similar accuracy for substrate characterization.113
The presence of channels and the border zone channel mass has been shown to be the strongest determinant of VA occurrence after a myocardial infarction after adjustment for other variables related with the LV function and scar.114 These channels also distinguish patients at higher risk of VTs during follow-up in other clinical situations like cardiac resynchronization therapy and even in non-ischaemic cardiomyopathy.109,111,115
The 3D reconstruction of the information obtained with the CMR displaying the heart anatomy, the scar, and the 3D structure of the conducting channels can be imported into the navigation system and integrated with the EAM. The CMR scar and channels have been shown to have a good correlation with the low voltage areas and channels identified with EAM.80,116 The CMR allows to recognize the substrate architecture and distribution along the WT, otherwise, neither visible nor mappable with standard mapping and ablation catheters that only obtain direct information from the endocardial or epicardial surface.79,80,116 The use of the information provided by the CMR once integrated into the navigation system has demonstrated to help performing more efficient procedures and obtain better outcomes. As compared with the standard VT ablation guided solely by the EAM, CMR-guided VT ablation is feasible and safe, significantly reduces the procedural, fluoroscopy, and radiofrequency times, and is associated to a higher non-inducibility rate and lower VT recurrence.117,118
Positron emission tomography-scan and single-photon-emission computed tomography-scan
In patients with VA, the applications of NCEs are wider and encompass ischaemic and non-ischaemic diseases. In patients with mid-to-high pre-test probability of CAD and stress-induced ectopies from the LV, SPECT can predict epicardial vessel stenosis, even in the absence of LV systolic dysfunction.119 Nonetheless, NCEs are not currently recommended by the ESC in the diagnostic workup of CAD-related VA.3 Instead, FDG-PET has a recognized role in diagnosing both cardiac and extracardiac sarcoidosis.3 In addition, FDG-PET scan has been proved clinically helpful in lymphocytic myocarditis with VA, in particular when CMR is contraindicated or unsuitable due to ICD-related artefacts.120 Finally, recent studies suggested that FDG-PET is capable of identifying even the ‘hot-phases’ of primary cardiomyopathies of the dilated and arrhythmogenic spectrum.121
Beyond their diagnostic value, NCEs have shown a prognostic role in CAD,65,122 as well as in other clinical scenarios. For instance, 99mTc-MIBI myocardial perfusion SPECT has been found useful to quantify LV scarring, and predicts outcomes in response to cardiac resynchronization therapy.122 Consistently, the beneficial effects of biventricular pacing on septal metabolism can be proved by PET.123
In patients with inflammatory cardiomyopathy, the anteroseptal localization of FDG-PET abnormalities, more commonly found in cardiac sarcoidosis rather than classic lymphocytic myocarditis, is capable of predicting worse arrhythmic outcomes.120 In inflammatory cardiomyopathy, the documentation of active myocarditis by multimodal workup including FDG-PET has shown to predict major VA recurrences even after CA.124
In light of their diagnostic role, NCEs have been investigated also to guide treatment strategies, such as CA of VA. For instance, SPECT-CT fusion imaging has been found time-sparing and useful to characterize LV substrate and scars.125 In addition, areas with perfusion/innervation mismatch on SPECT scans could identify sites of LAVA on EAM to guide CA procedures.126 Due to the lack of strong evidence, however, NCEs are not mentioned by the last ESC guidelines among the imaging tools recommended before CA procedures in patients with VA.2,3 Exception is made for PET-CT scan in patients with inflammatory heart diseases like myocarditis or cardiac sarcoidosis,3 who will likely benefit also from PET-CMR fusion imaging in the near future.127 Finally, in the absence of artefacts from right ventricular pacing,128 FDG-PET may find application in following-up ICD carriers with myocarditis, to allow disease restaging and guide the withdrawal of immunosuppressive therapies.120
Future directions
The future of cardiac imaging in arrhythmia and EP will be shaped by advances in technology and a greater understanding of the underlying arrhythmia mechanisms. Progress in these directions will be intertwined, leading to improved patient outcomes and increased efficiency of healthcare delivery.
A clearly charted direction would be the use of 3D imaging technologies, which are expected to become widespread, improving our ability to acquire unappreciated structural and disease-induced remodelling detail. Native T1 mapping and extracellular volume acquisition are making inroads, allowing to better characterize diffuse fibrosis.129 Furthermore, fusion imaging, the integration of multiple imaging modalities, will enrich our ability to assess disease-modified heart structure/function. Indeed, hybrid PET/CMR scanners have already become commercially available130; the trend of fusion imaging will continue to grow.
Development of real-time imaging technologies will allow feedback during EP procedures. For instance, ICE, a high-resolution visualization of cardiac structures, enables integration of real-time images with EAM. Novel developments, such as electromechanical wave imaging, a high-frame rate ultrasound technique,131 have made initial advances in non-invasively mapping the electromechanical activation of arrhythmias. Furthermore, while commercial systems for electrocardiographic imaging already exist, they do not yet have the spatial resolution necessary to clearly delineate targets for ablation; improved approaches are likely to be developed in near future.
The most rapid advances in technology will likely be made by artificial intelligence (AI). In addition to its widening use in electrocardiogram (ECG) analysis,132 AI has also been used in segmentation, scar/fibrosis assessment, and clinical parameter extraction.133 We are witnessing the first application of deep learning on raw CMRs and the use of multi-modality deep learning to predict risk of arrhythmia and time to SCD.134 Future AI advances will enable identification of additional imaging patterns and biomarkers that are associated with specific types of arrhythmias.
Finally, digital twin technology is poised to play important role in personalized treatment planning and in prognostication of patients’ disease trajectory. Heart digital twins (mechanistically-based personalized computational models of patients’ hearts) have already made novel contributions to uncovering arrhythmogenic mechanisms135 and to the guidance of atrial136 and VAs.137 The benefit of the digital twin technology is that treatment can be tailored based on the patient’s response to therapy. The AI is also being combined with digital twins,138 helping broaden clinical data inclusion in the models. In the future, we will witness the creation of continuously-adjustable heart digital twins based on patients’ tracked data.
Supplementary Material
Contributor Information
Antonio Berruezo, Arrhythmia Unit, Teknon Medical Centre, Carrer de Vilana, 12, 08022 Barcelona, Spain.
Diego Penela, Arrhythmia Unit, Humanitas Research Hospital, Via Alessandro Manzoni, 56, 20089 Rozzano Milan, Italy.
Beatriz Jáuregui, Arrhythmia Unit - Miguel Servet University Hospital, P.º de Isabel la Católica, 1-3, 50009 Zaragoza, Spain.
Carlo de Asmundis, Heart Rhythm Management Centre, Universitair Ziekenhuis Brussel-Vrije Universiteit Brussel, Blvd Géneral Jacques 137, 1050 Ixelles, Brussels, Belgium.
Giovanni Peretto, Arrhythmia Unit, Ospedale San Raffaele Hospital, Via Olgettina, 60, 20132 Milan, Italy.
Nassir Marrouche, Department of Cardiology, Tulane University School of Medicine, 1430 Tulane Ave, New Orleans, LA 70112, USA.
Natalia Trayanova, Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21218, USA; Department of Applied Math and Statistics, Johns Hopkins University, Baltimore, MD 21218, USA; Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, MD 21218, USA.
Christian de Chillou, INSERM IADI U1254, University Hospital Nancy, University of Lorraine, 29 Av. du Maréchal de Lattre de Tassigny, 54000 Nancy, France.
Supplementary material
Supplementary material is available at Europace online.
References
- 1. Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TKet al. . Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri Net al. . 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019;21:1143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 4. Estner HL, Bongiorni MG, Chen J, Dagres N, Hernandez-Madrid A, Blomström-Lundqvist Cet al. . Use of fluoroscopy in clinical electrophysiology in Europe: results of the European Heart Rhythm Association survey. Europace 2015;17:1149–52. [DOI] [PubMed] [Google Scholar]
- 5. Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJet al. . Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J 2006;27:1223–9. [DOI] [PubMed] [Google Scholar]
- 6. Casella M, Dello Russo A, Pelargonio G, Del Greco M, Zingarini G, Piacenti Met al. . Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NO-PARTY multicentre randomized trial. Europace 2016;18:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G, Wang Y, Proietti R, Wang X, Ouyang F, Ma CSet al. . Zero-fluoroscopy approach for ablation of supraventricular tachycardia using the Ensite NavX system: a multicenter experience. BMC Cardiovasc Disord 2020;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falasconi G, Penela D, Soto-Iglesias D, Jáuregui B, Chauca A, Antonio RSet al. . A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:629–39. [DOI] [PubMed] [Google Scholar]
- 9. de Groot NMS, Shah D, Boyle PM, Anter E, Clifford GD, Deisenhofer Iet al. . Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology working group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. Europace 2022;24:313–30. [DOI] [PubMed] [Google Scholar]
- 10. Scaglione M, Caponi D, Di Donna P, Riccardi R, Bocchiardo M, Azzaro Get al. . Typical atrial flutter ablation outcome: correlation with isthmus anatomy using intracardiac echo 3D reconstruction. Europace 2004;6:407–17. [DOI] [PubMed] [Google Scholar]
- 11. Kistler PM, Rajappan K, Jahngir M, Earley MJ, Harris S, Abrams Det al. . The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006;17:1093–101. [DOI] [PubMed] [Google Scholar]
- 12. Bertaglia E, Della Bella P, Tondo C, Proclemer A, Bottoni N, De Ponti Ret al. . Image integration increases efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMerge Italian Registry. Europace 2009;11:1004–10. [DOI] [PubMed] [Google Scholar]
- 13. Caponi D, Corleto A, Scaglione M, Blandino A, Biasco L, Cristoforetti Yet al. . Ablation of atrial fibrillation: does the addition of three-dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: a randomized comparison of Carto-Merge vs. Carto-XP three-dimensional mapping ablation in patients with paroxysmal and persistent atrial fibrillation. Europace 2010;12:1098–104. [DOI] [PubMed] [Google Scholar]
- 14. Sommer P, Bertagnolli L, Kircher S, Arya A, Bollmann A, Richter Set al. . Safety profile of near-zero fluoroscopy atrial fibrillation ablation with non-fluoroscopic catheter visualization: experience from 1000 consecutive procedures. Europace 2018;20:1952–8. [DOI] [PubMed] [Google Scholar]
- 15. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski Fet al. . Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 16. Fochler F, Yamaguchi T, Kheirkahan M, Kholmovski EG, Morris AK, Marrouche NF. Late gadolinium enhancement magnetic resonance imaging guided treatment of post-atrial fibrillation ablation recurrent arrhythmia. Circ Arrhythm Electrophysiol 2019;12:e007174. [DOI] [PubMed] [Google Scholar]
- 17. Eichenlaub M, Mueller-Edenborn B, Minners J, Figueras I, Ventura RM, Forcada BRet al. . Comparison of various late gadolinium enhancement magnetic resonance imaging methods with high-definition voltage and activation mapping for detection of atrial cardiomyopathy. Europace 2022;24:1102–11. [DOI] [PubMed] [Google Scholar]
- 18. Mammadli A, Demirtola AI, Diker E. Impact of image integration on clinical and procedural outcomes of radiofrequency catheter ablation of atrial fibrillation: a meta-analysis of randomized controlled trials. J Arrhythmia 2021;37:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teres C, Soto-Iglesias D, Penela D, Jáuregui B, Ordoñez A, Chauca Aet al. . Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘Ablate by-LAW’ single-centre study—a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 20. Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging 2013;6:185–94. [DOI] [PubMed] [Google Scholar]
- 21. Teres C, Soto-Iglesias D, Penela D, Falasconi G, Viveros D, Meca-Santamaria Jet al. . Relationship between the posterior atrial wall and the esophagus: esophageal position and temperature measurement during atrial fibrillation ablation (AWESOME-AF). A randomized controlled trial. J Interv Card Electrophysiol 2022;65:651–61. [DOI] [PubMed] [Google Scholar]
- 22. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto Ket al. . Left atrial low-voltage areas predict atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. Int J Cardiol 2018;257:97–101. [DOI] [PubMed] [Google Scholar]
- 23. Junarta J, Siddiqui MU, Riley JM, Dikdan SJ, Patel A, Frisch DR. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace 2022;24:1585–98. [DOI] [PubMed] [Google Scholar]
- 24. Bergonti M, Spera FR, Ferrero TG, Nsahlai M, Bonomi A, Tijskens Met al. . Characterization of atrial substrate to predict the success of pulmonary vein isolation: the prospective, multicenter MASH-AF II (Multipolar Atrial Substrate High Density Mapping in Atrial Fibrillation) study. J Am Heart Assoc 2023;12:e027795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohguchi S, Inden Y, Yanagisawa S, Fujita R, Yasuda K, Katagiri Ket al. . Regional left atrial conduction velocity in the anterior wall is associated with clinical recurrence of atrial fibrillation after catheter ablation: efficacy in combination with the ipsilateral low voltage area. BMC Cardiovasc Disord 2022;22:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuo M-J, Ton AN-K, Lo L-W, Lin Y-J, Chang S-L, Hu Y-Fet al. . Abnormal conduction zone detected by isochronal late activation mapping accurately identifies the potential atrial substrate and predicts the atrial fibrillation ablation outcome after pulmonary vein isolation. Circ Arrhythm Electrophysiol 2023;16:e011149. [DOI] [PubMed] [Google Scholar]
- 27. Benali K, Khairy P, Hammache N, Petzl A, Da Costa A, Verma Aet al. . Procedure-related complications of catheter ablation for atrial fibrillation. J Am Coll Cardiol 2023;81:2089–99. [DOI] [PubMed] [Google Scholar]
- 28. Hussain SK, Eddy MM, Moorman L, Malhotra R, Darby AE, Bilchick Ket al. . Major complications and mortality within 30 days of an electrophysiological procedure at an academic medical center: implications for developing national standards. J Cardiovasc Electrophysiol 2015;26:527–31. [DOI] [PubMed] [Google Scholar]
- 29. Della Rocca DG, Di Biase L, Mohanty S, Trivedi C, Gianni C, Romero Jet al. . Targeting non-pulmonary vein triggers in persistent atrial fibrillation: results from a prospective, multicentre, observational registry. Europace 2021;23:1939–49. [DOI] [PubMed] [Google Scholar]
- 30. Pannone L, Mouram S, Della Rocca DG, Sorgente A, Monaco C, Del Monte Aet al. . Hybrid atrial fibrillation ablation: long-term outcomes from a single-centre 10-year experience. Europace 2023;25:euad114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang TKM, Wang MTM, Martin A. Meta-analysis of ultrasound-guided vs conventional vascular access for cardiac electrophysiology procedures. J Arrhythmia 2019;35:858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Del Monte A, Almorad A, Pannone L, Della Rocca DG, Bisignani A, Monaco Cet al. . Pulmonary vein isolation with the radiofrequency balloon catheter: a single centre prospective study. Europace 2023;25:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Della Rocca DG, Del Monte A, Bala G, Pannone L, Ströker E, Monaco Cet al. . Transient inferior ST-segment elevation and ventricular fibrillation after cavotricuspid isthmus pulsed-field ablation. JACC Clin Electrophysiol 2023;9:704–6. [DOI] [PubMed] [Google Scholar]
- 34. Del Monte A, Chierchia GB, Della Rocca DG, Pannone L, Sorgente A, Bala Get al. . Posterior wall isolation via a multi-electrode radiofrequency balloon catheter: feasibility, technical considerations, endoscopic findings and comparison with cryoballoon technologies. J Interv Card Electrophysiol 2023. doi: 10.1007/s10840-023-01549-1 (Online ahead of print). [DOI] [PubMed] [Google Scholar]
- 35. Mohanty S, Trivedi C, Beheiry S, Al-Ahmad A, Horton R, Della Rocca DGet al. . Venous access-site closure with vascular closure device vs. manual compression in patients undergoing catheter ablation or left atrial appendage occlusion under uninterrupted anticoagulation: a multicentre experience on efficacy and complications. Europace 2019;21:1048–54. [DOI] [PubMed] [Google Scholar]
- 36. Del Prete A, Della Rocca DG, Calcagno S, Di Pietro R, Del Prete G, Biondi-Zoccai Get al. . Perclose Proglide™ for vascular closure. Future Cardiol 2021;17:269–82. [DOI] [PubMed] [Google Scholar]
- 37. Canpolat U, Faggioni M, Della Rocca DG, Chen Q, Ayhan H, Vu AAet al. . State of fluoroless procedures in cardiac electrophysiology practice. J Innov Card Rhythm Manag 2020;11:4018–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berti S, Paradossi U, Meucci F, Trianni G, Tzikas A, Rezzaghi Met al. . Periprocedural intracardiac echocardiography for left atrial appendage closure: a dual-center experience. JACC Cardiovasc Interv 2014;7:1036–44. [DOI] [PubMed] [Google Scholar]
- 39. Gianni C, Horton RP, Della Rocca DG, Mohanty S, Al-Ahmad A, Bassiouny MAet al. . Intracardiac echocardiography- versus transesophageal echocardiography-guided left atrial appendage occlusion with Watchman FLX. J Cardiovasc Electrophysiol 2021;32:2781–4. [DOI] [PubMed] [Google Scholar]
- 40. Della Rocca DG, Gianni C, Magnocavallo M, Mohanty S, Al-Ahmad A, Tschopp DRet al. . 3-Dimensional intracardiac echocardiography-guided percutaneous closure of a residual leak via radiofrequency applications after LAAO. JACC Clin Electrophysiol 2022;8:1609–12. [DOI] [PubMed] [Google Scholar]
- 41. Messele LF, Khan MZ, Darden D, Agarwal S, Krishan S, Pasupula DKet al. . Outcomes of percutaneous left atrial appendage occlusion device implantation in atrial fibrillation patients based on underlying stroke risk. Europace 2023;25:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tops LF, Schalij MJ, den Uijl DW, Abraham TP, Calkins H, Bax JJ. Image integration in catheter ablation of atrial fibrillation. Europace 2008;10:iii48–56. [DOI] [PubMed] [Google Scholar]
- 43. Niinuma H, George RT, Arbab-Zadeh A, Lima JAC, Henrikson CA. Imaging of pulmonary veins during catheter ablation for atrial fibrillation: the role of multi-slice computed tomography. Europace 2008;10:iii14–21. [DOI] [PubMed] [Google Scholar]
- 44. Graham LN, Melton IC, MacDonald S, Crozier IG. Value of CT localization of the fossa ovalis prior to transseptal left heart catheterization for left atrial ablation. Europace 2007;9:417–23. [DOI] [PubMed] [Google Scholar]
- 45. Marazzi R, De Ponti R, Lumia D, Fugazzola C, Salerno-Uriarte JA. Common trunk of the inferior pulmonary veins: an unexpected anatomical variant detected before ablation by multi-slice computed tomography. Europace 2007;9:121. [DOI] [PubMed] [Google Scholar]
- 46. Sorgente A, Chierchia GB, de Asmundis C, Sarkozy A, Namdar M, Capulzini Let al. . Pulmonary vein ostium shape and orientation as possible predictors of occlusion in patients with drug-refractory paroxysmal atrial fibrillation undergoing cryoballoon ablation. Europace 2011;13:205–12. [DOI] [PubMed] [Google Scholar]
- 47. Falasconi G, Penela D, Soto-Iglesias D, Francia P, Teres C, Saglietto Aet al. . Personalized pulmonary vein antrum isolation guided by left atrial wall thickness for persistent atrial fibrillation. Europace 2023;25:euad118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y-G, Yang M, Li Y, Wang Q, Yu L, Sun J. Spatial relationship between left atrial roof or superior pulmonary veins and bronchi or pulmonary arteries by dual-source computed tomography: implication for preventing injury of bronchi and pulmonary arteries during atrial fibrillation ablation. Europace 2011;13:809–14. [DOI] [PubMed] [Google Scholar]
- 49. Gavin AR, Singleton CB, McGavigan AD. Assessment of oesophageal position by direct visualization with luminal contrast compared with segmentation from pre-acquired computed tomography scan-implications for ablation strategy. Europace 2014;16:1304–8. [DOI] [PubMed] [Google Scholar]
- 50. Canpolat U, Aytemir K, Hızal M, Hazırolan T, Yorgun H, Sahiner Let al. . Imaging before cryoablation of atrial fibrillation: is phrenic nerve palsy predictable? Europace 2014;16:505–10. [DOI] [PubMed] [Google Scholar]
- 51. Schley P, Gülker H, Horlitz M. Atrio-oesophageal fistula following circumferential pulmonary vein ablation: verification of diagnosis with multislice computed tomography. Europace 2006;8:189–90. [DOI] [PubMed] [Google Scholar]
- 52. Pérez-Castellano N, Villacastín J, Moreno J, Macaya C. Pivotal role of integrated electroanatomic mapping with three-dimensional multislice computed tomography scan in the ablation of a left atrial ectopic focus. Europace 2007;9:119–20. [DOI] [PubMed] [Google Scholar]
- 53. Cho Y, Lee W, Park E-A, Oh I-Y, Choi E-K, Seo J-Wet al. . The anatomical characteristics of three different endocardial lines in the left atrium: evaluation by computed tomography prior to mitral isthmus block attempt. Europace 2012;14:1104–11. [DOI] [PubMed] [Google Scholar]
- 54. Klemm HU, Weber TF, Johnsen C, Begemann PGC, Meinertz T, Ventura R. Anatomical variations of the right coronary artery may be a source of difficult block and conduction recurrence in catheter ablation of common-type atrial flutter. Europace 2010;12:1608–15. [DOI] [PubMed] [Google Scholar]
- 55. Falasconi G, Penela D, Soto-Iglesias D, Terés C, Jáuregui B, Martí-Almor Jet al. . Multidetector computed tomography identification of previous ablation lines: insights for left atrial flutter ablation. Heart Rhythm 2022;19:1753–4. [DOI] [PubMed] [Google Scholar]
- 56. Chubb H, Harrison JL, Weiss S, Krueger S, Koken P, Bloch LØet al. . Development, preclinical validation, and clinical translation of a cardiac magnetic resonance–electrophysiology system with active catheter tracking for ablation of cardiac arrhythmia. JACC Clin Electrophysiol 2017;3:89–103. [DOI] [PubMed] [Google Scholar]
- 57. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish ENet al. . Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. King JB, Azadani PN, Suksaranjit P, Bress AP, Witt DM, Han FTet al. . Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol 2017;70:1311–21. [DOI] [PubMed] [Google Scholar]
- 59. Akoum N, Wilber D, Hindricks G, Jais P, Cates J, Marchlinski Fet al. . MRI assessment of ablation-induced scarring in atrial fibrillation: analysis from the DECAAF study. J Cardiovasc Electrophysiol 2015;26:473–80. [DOI] [PubMed] [Google Scholar]
- 60. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. . Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khoshknab M, Kuo L, Zghaib T, Arkles J, Santangeli P, Marchlinski FEet al. . Esophageal luminal temperature rise during atrial fibrillation ablation is associated with lower radiofrequency electrode distance and baseline impedance. J Cardiovasc Electrophysiol 2021;32:1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ayoub T, El Hajjar AH, Singh Sidhu GD, Bhatnagar A, Zhang Y, Mekhael Met al. . Esophageal temperature during atrial fibrillation ablation poorly predicts esophageal injury: an observational study. Heart Rhythm O2 2021;2:570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Quinto L, Cozzari J, Benito E, Alarcón F, Bisbal F, Trotta Oet al. . Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case–control study. Europace 2020;22:1805–11. [DOI] [PubMed] [Google Scholar]
- 64. Schofield R, Menezes L, Underwood SR. Nuclear cardiology: state of the art. Heart 2021;107:954–61. [DOI] [PubMed] [Google Scholar]
- 65. Askew JW, Miller TD, Hodge DO, Gibbons RJ. The value of myocardial perfusion single-photon emission computed tomography in screening asymptomatic patients with atrial fibrillation for coronary artery disease. J Am Coll Cardiol 2007;50:1080–5. [DOI] [PubMed] [Google Scholar]
- 66. Smit MD, Tio RA, Slart RHJA, Zijlstra F, Van Gelder IC. Myocardial perfusion imaging does not adequately assess the risk of coronary artery disease in patients with atrial fibrillation. Europace 2010;12:643–8. [DOI] [PubMed] [Google Scholar]
- 67. Wichter T. Impaired myocardial perfusion in atrial fibrillation cause or effect? Europace 2010;12:611–3. [DOI] [PubMed] [Google Scholar]
- 68. Range FT, Schäfers M, Acil T, Schäfers KP, Kies P, Paul Met al. . Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur Heart J 2007;28:2223–30. [DOI] [PubMed] [Google Scholar]
- 69. Ju W, Li M, Wang DW, Yang B, Shao Y, Wang Jet al. . Idiopathic isolated fibrotic atrial cardiomyopathy underlies unexplained scar-related atrial tachycardia in younger patients. Europace 2018;20:1657–65. [DOI] [PubMed] [Google Scholar]
- 70. Ilyushenkova J, Sazonova S, Zavadovsky K, Batalov R, Rogovskaya Y, Anfinogenova Yet al. . Diagnostic efficacy of cardiac scintigraphy with 99mTc-Pyrophosphate for latent myocardial inflammation in patients with atrial fibrillation. Cardiol Res Pract 2020;2020:5983751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lemery R, Ben-Haim S, Wells G, Ruddy TD. I-123-Metaiodobenzylguanidine imaging in patients with atrial fibrillation undergoing cardiac mapping and ablation of autonomic ganglia. Heart Rhythm 2017;14:128–32. [DOI] [PubMed] [Google Scholar]
- 72. Knackstedt C, Schauerte P, Kirchhof P. Electro-anatomic mapping systems in arrhythmias. Europace 2008;10:iii28–34. [DOI] [PubMed] [Google Scholar]
- 73. Heidbuchel H, Wittkampf FHM, Vano E, Ernst S, Schilling R, Picano Eet al. . Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 2014;16:946–64. [DOI] [PubMed] [Google Scholar]
- 74. Marinskis G, Bongiorni MG, Dagres N, Lewalter T, Pison L, Blomstrom-Lundqvist Cet al. . X-ray exposure hazards for physicians performing ablation procedures and device implantation: results of the European Heart Rhythm Association survey. Europace 2013;15:444–6. [DOI] [PubMed] [Google Scholar]
- 75. Dagres N, Cantu F, Geelen P, Lewalter T, Proclemer A, Blomstrom-Lundqvist C. Current practice of ventricular tachycardia ablation in patients with implantable cardioverter-defibrillators. Europace 2012;14:135–7. [DOI] [PubMed] [Google Scholar]
- 76. Tilz RR, Lenarczyk R, Scherr D, Haugaa KH, Iliodromitis K, Pürerfellner Het al. . Management of ventricular tachycardia in the ablation era: results of the European Heart Rhythm Association survey. Europace 2018;20:209–13. [DOI] [PubMed] [Google Scholar]
- 77. de Chillou C, Lacroix D, Klug D, Magnin-Poull I, Marquié C, Messier Met al. . Isthmus characteristics of reentrant ventricular tachycardia after myocardial infarction. Circulation 2002;105:726–31. [DOI] [PubMed] [Google Scholar]
- 78. Miljoen H, State S, de Chillou C, Magnin-Poull I, Dotto P, Andronache Met al. . Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 2005;7:516–24. [DOI] [PubMed] [Google Scholar]
- 79. Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CFB, Siebelink H-MJ, Kroft LJM, Bax JJet al. . Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J 2011;32:104–14. [DOI] [PubMed] [Google Scholar]
- 80. Codreanu A, Odille F, Aliot E, Marie P-Y, Magnin-Poull I, Andronache Met al. . Electroanatomic characterization of post-infarct scars. J Am Coll Cardiol 2008;52:839–42. [DOI] [PubMed] [Google Scholar]
- 81. Glashan CA, Androulakis AFA, Tao Q, Glashan RN, Wisse LJ, Ebert Met al. . Whole human heart histology to validate electroanatomical voltage mapping in patients with non-ischaemic cardiomyopathy and ventricular tachycardia. Eur Heart J 2018;39:2867–75. [DOI] [PubMed] [Google Scholar]
- 82. Bazan V, Frankel DS, Santangeli P, Garcia FC, Tschabrunn CM, Marchlinski FE. Three-dimensional myocardial scar characterization from the endocardium: usefulness of endocardial unipolar electroanatomic mapping. J Cardiovasc Electrophysiol 2019;30:427–37. [DOI] [PubMed] [Google Scholar]
- 83. Muser D, Nucifora G, Castro SA, Enriquez A, Chahal CAA, Magnani Set al. . Myocardial substrate characterization by CMR T1 mapping in patients with NICM and no LGE undergoing catheter ablation of VT. JACC Clin Electrophysiol 2021;7:831–40. [DOI] [PubMed] [Google Scholar]
- 84. Della Bella P, Baratto F. Evolving patterns of ventricular tachycardia modifying our mapping techniques. Europace 2012;14:ii1–2. [DOI] [PubMed] [Google Scholar]
- 85. Battaglia A, Odille F, Magnin-Poull I, Sellal J-M, Hoyland P, Hooks D Vet al. . An efficient algorithm based on electrograms characteristics to identify ventricular tachycardia isthmus entrance in post-infarct patients. Europace 2020;22:109–16. [DOI] [PubMed] [Google Scholar]
- 86. Acosta J, Penela D, Andreu D, Cabrera M, Carlosena A, Vassanelli Fet al. . Multielectrode vs. point-by-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace 2018;20:512–9. [DOI] [PubMed] [Google Scholar]
- 87. Acosta J, Andreu D, Penela D, Cabrera M, Carlosena A, Korshunov Vet al. . Elucidation of hidden slow conduction by double ventricular extrastimuli: a method for further arrhythmic substrate identification in ventricular tachycardia ablation procedures. Europace 2018;20:337–46. [DOI] [PubMed] [Google Scholar]
- 88. de Chillou C, Groben L, Magnin-Poull I, Andronache M, Abbas M, Zhang Net al. . Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm 2014;11:175–81. [DOI] [PubMed] [Google Scholar]
- 89. Briceño DF, Romero J, Villablanca PA, Londoño A, Diaz JC, Maraj Iet al. . Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace 2018;20:104–15. [DOI] [PubMed] [Google Scholar]
- 90. Jáuregui B, Soto-Iglesias D, Zucchelli G, Penela D, Ordóñez A, Terés Cet al. . Arrhythmogenic substrate detection in chronic ischaemic patients undergoing ventricular tachycardia ablation using multidetector cardiac computed tomography: compared evaluation with cardiac magnetic resonance. Europace 2021;23:82–90. [DOI] [PubMed] [Google Scholar]
- 91. Andreu D, Ortiz-Pérez JT, Fernández-Armenta J, Guiu E, Acosta J, Prat-González Set al. . 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace 2015;17:938–45. [DOI] [PubMed] [Google Scholar]
- 92. Siebelink H-MJ, Scholte AJHA, Van de Veire NR, Holman ER, Nucifora G, van der Wall EEet al. . Value of contrast echocardiography for left ventricular thrombus detection postinfarction and impact on antithrombotic therapy. Coron Artery Dis 2009;20:462–6. [DOI] [PubMed] [Google Scholar]
- 93. Pannone L, Falasconi G, Cianfanelli L, Baldetti L, Moroni F, Spoladore Ret al. . Sudden cardiac death in patients with heart disease and preserved systolic function: current options for risk stratification. J Clin Med 2021;10:1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani Ret al. . Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death study. J Am Coll Cardiol 2006;47:1161–6. [DOI] [PubMed] [Google Scholar]
- 95. Penela D, Fernández-Armenta J, Aguinaga L, Tercedor L, Ordoñez A, Bisbal Fet al. . Clinical recognition of pure premature ventricular complex-induced cardiomyopathy at presentation. Heart Rhythm 2017;14:1864–70. [DOI] [PubMed] [Google Scholar]
- 96. Wang J-S, Shen Y-G, Yin R-P, Thapa S, Peng Y-P, Ji K-Tet al. . The safety of catheter ablation for premature ventricular contractions in patients without structural heart disease. BMC Cardiovasc Disord 2018;18:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vergara P, Scarfò I, Esposito A, Colantoni C, Palmisano A, Altizio Set al. . Characterization of the electrophysiological substrate in patients with Barlow’s disease. J Cardiovasc Electrophysiol 2021;32:3179–86. [DOI] [PubMed] [Google Scholar]
- 98. Vergara P, Altizio S, Falasconi G, Pannone L, Gulletta S, Della Bella P. Electrophysiological substrate in patients with Barlow’s disease. Arrhythmia Electrophysiol Rev 2021;10:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bourier F, Martin R, Martin CA, Takigawa M, Kitamura T, Frontera Aet al. . Is it feasible to offer ‘targeted ablation’ of ventricular tachycardia circuits with better understanding of isthmus anatomy and conduction characteristics? Europace 2019;21:i27–33. [DOI] [PubMed] [Google Scholar]
- 100. Komatsu Y, Cochet H, Jadidi A, Sacher F, Shah A, Derval Net al. . Regional myocardial wall thinning at multidetector computed tomography correlates to arrhythmogenic substrate in postinfarction ventricular tachycardia: assessment of structural and electrical substrate. Circ Arrhythm Electrophysiol 2013;6:342–50. [DOI] [PubMed] [Google Scholar]
- 101. Ghannam M, Cochet H, Jais P, Sermesant M, Patel S, Siontis KCet al. . Correlation between computer tomography-derived scar topography and critical ablation sites in postinfarction ventricular tachycardia. J Cardiovasc Electrophysiol 2018;29:438–45. [DOI] [PubMed] [Google Scholar]
- 102. Sramko M, Hoogendoorn JC, Glashan CA, Zeppenfeld K. Advancement in cardiac imaging for treatment of ventricular arrhythmias in structural heart disease. Europace 2019;21:383–403. [DOI] [PubMed] [Google Scholar]
- 103. Piers SRD, van Huls van Taxis CFB, Tao Q, van der Geest RJ, Askar SF, Siebelink H-MJet al. . Epicardial substrate mapping for ventricular tachycardia ablation in patients with non-ischaemic cardiomyopathy: a new algorithm to differentiate between scar and viable myocardium developed by simultaneous integration of computed tomography and contrast-enhanced magnetic resonance imaging. Eur Heart J 2013;34:586–96. [DOI] [PubMed] [Google Scholar]
- 104. Yamashita S, Sacher F, Mahida S, Berte B, Lim HS, Komatsu Yet al. . Role of high-resolution image integration to visualize left phrenic nerve and coronary arteries during epicardial ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2015;8:371–80. [DOI] [PubMed] [Google Scholar]
- 105. Xu L, Zahid S, Khoshknab M, Moss J, Berger RD, Chrispin Jet al. . Lipomatous metaplasia prolongs repolarization and increases repolarization dispersion within post-infarct ventricular tachycardia circuit cites. Europace 2023;25:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bonnin T, Roumegou P, Sridi S, Mahida S, Bustin A, Duchateau Jet al. . Prevalence and risk factors of cardiac thrombus prior to ventricular tachycardia catheter ablation in structural heart disease. Europace 2023;25:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Korshunov V, Penela D, Linhart M, Acosta J, Martinez M, Soto-Iglesias Det al. . Prediction of premature ventricular complex origin in left vs. right ventricular outflow tract: a novel anatomical imaging approach. Europace 2019;21:147–53. [DOI] [PubMed] [Google Scholar]
- 108. Rivera S, de la Paz Ricapito M, Tomas L, Parodi J, Molina GB, Banega Ret al. . Results of cryoenergy and radiofrequency-based catheter ablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration. Circ Arrhythm Electrophysiol 2016;9:e003874. [DOI] [PubMed] [Google Scholar]
- 109. Thomsen AF, Bertelsen L, Jøns C, Jabbari R, Lønborg J, Kyhl Ket al. . Scar border zone mass and presence of border zone channels assessed with cardiac magnetic resonance imaging are associated with ventricular arrhythmia in patients with ST-segment elevation myocardial infarction. Europace 2023;25:978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fernandez-Armenta J, Berruezo A, Mont L, Sitges M, Andreu D, Silva Eet al. . Use of myocardial scar characterization to predict ventricular arrhythmia in cardiac resynchronization therapy. Europace 2012;14:1578–86. [DOI] [PubMed] [Google Scholar]
- 111. Leonardi S, Raineri C, De Ferrari GM, Ghio S, Scelsi L, Pasotti Met al. . Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur Heart J 2009;30:2003–10. [DOI] [PubMed] [Google Scholar]
- 112. Andreu D, Ortiz-Pérez JT, Boussy T, Fernández-Armenta J, de Caralt TM, Perea RJet al. . Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the approach needed for ablation. Eur Heart J 2014;35:1316–26. [DOI] [PubMed] [Google Scholar]
- 113. Roca-Luque I, Van Breukelen A, Alarcon F, Garre P, Tolosana JM, Borras Ret al. . Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace 2020;22:598–606. [DOI] [PubMed] [Google Scholar]
- 114. Jáuregui B, Soto-Iglesias D, Penela D, Acosta J, Fernández-Armenta J, Linhart Met al. . Cardiovascular magnetic resonance determinants of ventricular arrhythmic events after myocardial infarction. Europace 2022;24:938–47. [DOI] [PubMed] [Google Scholar]
- 115. Linhart M, Doltra A, Acosta J, Borràs R, Jáuregui B, Fernández-Armenta Jet al. . Ventricular arrhythmia risk is associated with myocardial scar but not with response to cardiac resynchronization therapy. Europace 2020;22:1391–400. [DOI] [PubMed] [Google Scholar]
- 116. Fernández-Armenta J, Berruezo A, Andreu D, Camara O, Silva E, Serra Let al. . Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2013;6:528–37. [DOI] [PubMed] [Google Scholar]
- 117. Andreu D, Penela D, Acosta J, Fernández-Armenta J, Perea RJ, Soto-Iglesias Det al. . Cardiac magnetic resonance–aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm 2017;14:1121–8. [DOI] [PubMed] [Google Scholar]
- 118. Soto-Iglesias D, Penela D, Jáuregui B, Acosta J, Fernández-Armenta J, Linhart Met al. . Cardiac magnetic resonance-guided ventricular tachycardia substrate ablation. JACC Clin Electrophysiol 2020;6:436–47. [DOI] [PubMed] [Google Scholar]
- 119. Bière L, Mezdad T-H, Dupuis J-M, Vervueren L, Rakotonirina H, Prunier Fet al. . Long-term prognostic significance of right bundle-branch morphology ventricular ectopy induced during stress test in patients with intermediate to high probability of coronary artery disease. Europace 2018;20:528–34. [DOI] [PubMed] [Google Scholar]
- 120. Peretto G, Busnardo E, Ferro P, Palmisano A, Vignale D, Esposito Aet al. . Clinical applications of FDG-PET scan in arrhythmic myocarditis. JACC Cardiovasc Imaging 2022;15:1771–80. [DOI] [PubMed] [Google Scholar]
- 121. Peretto G, Sommariva E, Di Resta C, Rabino M, Villatore A, Lazzeroni Det al. . Myocardial inflammation as a manifestation of genetic cardiomyopathies: from bedside to the bench. Biomolecules 2023;13:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Morishima I, Okumura K, Tsuboi H, Morita Y, Takagi K, Yoshida Ret al. . Impact of basal inferolateral scar burden determined by automatic analysis of 99mTc-MIBI myocardial perfusion SPECT on the long-term prognosis of cardiac resynchronization therapy. Europace 2017;19:573–80. [DOI] [PubMed] [Google Scholar]
- 123. Neri G, Zanco P, Zanon F, Buchberger R. Effect of biventricular pacing on metabolism and perfusion in patients affected by dilated cardiomyopathy and left bundle branch block: evaluation by positron emission tomography. Europace 2003;5:111–5. [DOI] [PubMed] [Google Scholar]
- 124. Peretto G, Sala S, Basso C, Rizzo S, Radinovic A, Frontera Aet al. . Inflammation as a predictor of recurrent ventricular tachycardia after ablation in patients with myocarditis. J Am Coll Cardiol 2020;76:1644–56. [DOI] [PubMed] [Google Scholar]
- 125. Friehling M, Menon PG, Ludwig DR, Schwartzman D. Single-photon emission computed tomographic-multidetector computed tomographic fusion image integration: a potential aid to left ventricular substrate ablation. Europace 2014;16:1860–3. [DOI] [PubMed] [Google Scholar]
- 126. Gimelli A, Menichetti F, Soldati E, Liga R, Scelza N, Zucchelli Get al. . Predictors of ventricular ablation’s success: viability, innervation, or mismatch? J Nucl Cardiol 2021;28:175–83. [DOI] [PubMed] [Google Scholar]
- 127. Palmisano A, Vignale D, Peretto G, Busnardo E, Calcagno C, Campochiaro Cet al. . Hybrid FDG-PET/MR or FDG-PET/CT to detect disease activity in patients with persisting arrhythmias after myocarditis. JACC Cardiovasc Imaging 2021;14:288–92. [DOI] [PubMed] [Google Scholar]
- 128. Preumont N, Jansens J-L, Berkenboom G, van de Borne P, Stoupel E, Goldman S. Effects of right ventricular pacing on regional myocardial glucose metabolism. Europace 2005;7:584–91. [DOI] [PubMed] [Google Scholar]
- 129. Kammerlander AA, Marzluf BA, Zotter-Tufaro C, Aschauer S, Duca F, Bachmann Aet al. . T1 mapping by CMR imaging: from histological validation to clinical implication. JACC Cardiovasc Imaging 2016;9:14–23. [DOI] [PubMed] [Google Scholar]
- 130. Nensa F, Bamberg F, Rischpler C, Menezes L, Poeppel TD, la Fougère Cet al. . Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol 2018;28:4086–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Grubb CS, Melki L, Wang DY, Peacock J, Dizon J, Iyer Vet al. . Noninvasive localization of cardiac arrhythmias using electromechanical wave imaging. Sci Transl Med 2020;12:eaax6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJet al. . An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–7. [DOI] [PubMed] [Google Scholar]
- 133. Popescu DM, Abramson HG, Yu R, Lai C, Shade JK, Wu KCet al. . Anatomically informed deep learning on contrast-enhanced cardiac magnetic resonance imaging for scar segmentation and clinical feature extraction. Cardiovasc Digit Health J 2022;3:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Popescu DM, Shade JK, Lai C, Aronis KN, Ouyang D, Moorthy MVet al. . Arrhythmic sudden death survival prediction using deep learning analysis of scarring in the heart. Nat Cardiovasc Res 2022;1:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sung E, Prakosa A, Zhou S, Berger RD, Chrispin J, Nazarian Set al. . Fat infiltration in the infarcted heart as a paradigm for ventricular arrhythmias. Nat Cardiovasc Res 2022;1:933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WHet al. . Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga Het al. . Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng 2018;2:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shade JK, Prakosa A, Popescu DM, Yu R, Okada DR, Chrispin Jet al. . Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci Adv 2021;7:eabi8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.