Abstract
Aims
Cardiac pacing represents a key element in the field of electrophysiology and the treatment of conduction diseases. Since the first issue published in 1999, EP Europace has significantly contributed to the development and dissemination of the research in this area.
Methods
In the last 25 years, there has been a continuous improvement of technologies and a great expansion of clinical indications making the field of cardiac pacing a fertile ground for research still today. Pacemaker technology has rapidly evolved, from the first external devices with limited longevity, passing through conventional transvenous pacemakers to leadless devices. Constant innovations in pacemaker size, longevity, pacing mode, algorithms, and remote monitoring highlight that the fascinating and exciting journey of cardiac pacing is not over yet.
Conclusion
The aim of the present review is to provide the current ‘state of the art’ on cardiac pacing highlighting the most important contributions from the Journal in the field.
Keywords: Pacemaker, Cardiac pacing, CIED, Leadless pacing, State of the art
Introduction
Cardiac pacing represents a key element in the field of electrophysiology and the treatment of conduction diseases. Since the first issue published in 1999, EP Europace has significantly contributed to the development and dissemination of the research in this area.1 In the last 25 years, there has been a continuous improvement of technologies and a great expansion of clinical indications making the field of cardiac pacing a fertile ground for research still today. Several issues have been an object of debate and subject of extensive research. Starting from 1999, more than 1 300 papers focused on several different aspects of cardiac pacing have been published in the Journal. The aim of the present review is to provide the current ‘state of the art’ on cardiac pacing highlighting the most important contributions from the Journal in the field.
History of cardiac pacing: a long and fascinating journey
The history of cardiac pacing is a long and fascinating journey with distant origins.2,3 In the late 1700, the Italian physician Luigi Galvani published his first experimental findings describing the effect of an electric current on the muscles of dead frogs’ legs and heart laying the ground for modern cardiac electrophysiology.2 Early attempts to artificially pace the human heart began in the 1930s with the pioneering experiences of the Australian anaesthetist Mark Lidweel and the American physiologist Albert S. Hyman in the setting of cardiac resuscitation.4 Albert S. Hyman first reported a “resuscitation of the stopped heart by intracardial therapy” by the “experimental use of an artificial pacemaker” coining the term we still used today.2,5 In the 1950s and the early 1960s, the historical experiences of Wilfred Bigelow, John Callaghan, Jack Hopps, and Paul M. Zoll later paved the way for the development of the pacing technology and the clinical application of pacemakers to treat cardiac arrhythmias.2
The first fully implantable pacemaker was performed in Stockholm in 1958 by the cardiac surgeon Åke Senning, using a device built by the medical engineer Rune Elmqvist. The device was successfully implanted in Arne Larsonn, a 43-year-old patient who suffered from Stokes–Admans attacks secondary a myocarditis. The first device weighed 180 g (compared to 20–50 g of modern pacemakers), and the pulse generator failed within a few hours from the implant being replaced the same day.2,3 Arne Larsonn underwent 26 pacemaker replacements and died at age 86 from malignant skin cancer, a sign of the success of this technology.2 The implementation of permanent cardiac pacing in clinical practice was firstly aimed at treating Morgagni–Adams–Stokes syndrome or bradyarrhythmic cardiac arrest, and only later was cardiac pacing specifically designed to treat different forms of bradyarrhythmia. After the initial breakthrough experience, the advances in pacemaker technology with the parallel increase of the clinical indications for pacing led to continuous improvements in the field.6 The technology has rapidly evolved, from the first external devices with limited longevity, passing through conventional transvenous pacemakers (TV-PPM) to leadless devices.7 Constant innovations in pacemaker size, longevity, pacing mode, algorithms, and remote monitoring highlight that this fascinating and exciting journey is not over yet.6,8–11
Epidemiology of pacemaker implantations
In the last decades, the use of pacemakers has dramatically increased.6,12–15 From an epidemiological perspective, the ageing population and the improving survival among patients with heart diseases who potentially need a pacemaker led to a significant increase in implantation rates.16 Recent estimates reported that the number of patients undergoing pacemaker implantation has steadily increased up to an annual implant rate of 1 million devices.16
Patients aged 65 and over are rapidly growing, counting today more than 8% of people worldwide with future predictions estimating an even higher percentage in 2050.17 Cardiac rhythm disturbances and the degeneration of the cardiac conduction system are significantly more prevalent in elderly patients with approximately 80% of pacemaker implants occurring in patients older than 65 years old.6,13,18,19 In parallel, the most recent European Society of Cardiology (ESC) guidelines have expanded the indications for pacemaker implantation leading to a substantial increase in pacemaker use in different clinical settings.6
Nevertheless, a precise estimate of pacemaker implants is of difficult analysis since most of the data available derive from retrospective studies or real-world registries with their intrinsic typical limitations.12 A previous analysis of claims files from the Health Care Finance Administration for Medicare beneficiaries from 1990 to 1999 reported that rates of implantation of cardiac devices increased from 3.26 implantations per 1 000 beneficiaries in 1990 to 4.64 implantations per 1 000 beneficiaries in 1999, representing an increase of 42% in 10 years.20
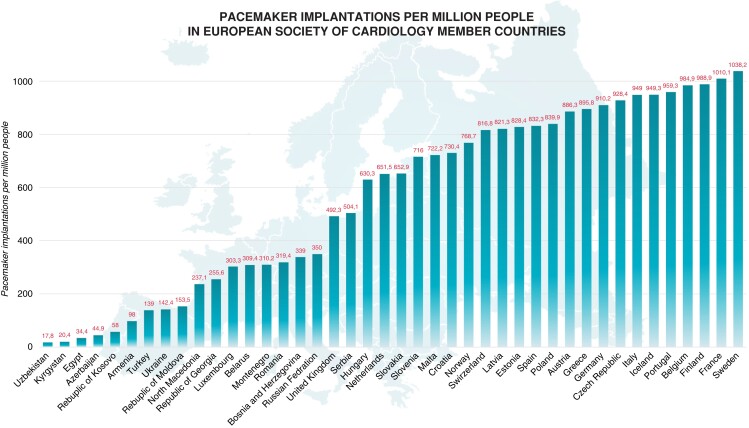
According to the latest ESC cardiovascular disease statistics,21 there was a median of 652.2 (IQR 267.5–874.7) pacemaker implants per million inhabitants of ESC member countries (Figure 1). A significant variability of implant rates has been reported among countries, ranging from <50 pacemaker implantations per million people in Azerbaijan, Egypt, Kyrgyzstan, and Uzbekistan to >1 000 implantations per million people in France and Sweden.21 The 2020 survey of the ESC member countries reported that the median of hospitals implanting pacemakers per million people was 2.8 (IQR 1.7–4.4) with low performance in middle-income compared with high-income countries (<1 hospital per million people in Egypt, Kyrgyzstan, and Uzbekistan compared with >7 hospitals per million people in Belgium, Cyprus, Germany, and Switzerland) highlighting important geographical differences.21
Figure 1.
Pacemaker implantations per million people in European Society Cardiology member countries. Adapted from Timmis A. et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. 2022.
Modes and indications of cardiac pacing
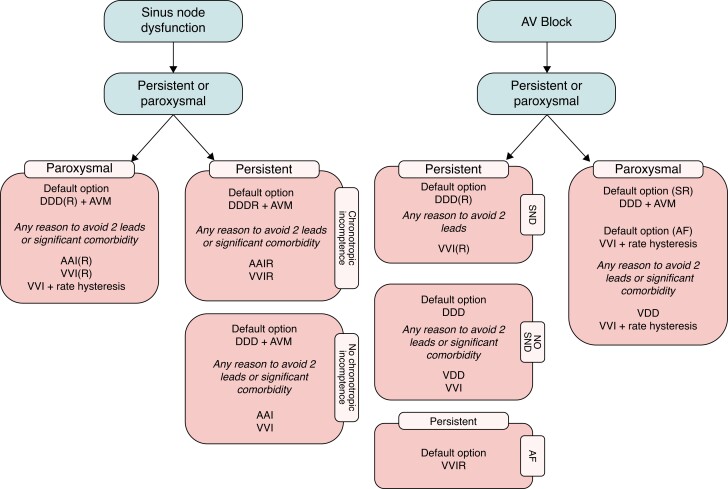
In general, the type and mode of cardiac pacing are determined by the specific nature of the conduction system disease [sinus node dysfunction (SND), atrioventricular (AV) block, bundle branch block, etc.].6 Usually, cardiac pacing is indicated in case of high-degree AV block or when the bradyarrhythmias are symptomatic.6Figure 2 summarizes the optimal pacing mode in SND and AV block. A complete and detailed overview of types, modes, and indications for cardiac pacing has been recently reported in the latest 2021 ESC guidelines.6
Figure 2.
Optimal pacing mode in sinus node dysfunction and atrio-ventricular block. Adapted from ref.6 AF, atrial fibrillation; AV, atrioventricular; AVM, atrioventricular management [i.e. AV delay programming (avoiding values > 230 ms) or specific algorithms to avoid/reduce unnecessary ventricular pacing]; CRT, cardiac resynchronization therapy; SND, sinus node dysfunction; SR, sinus rhythm. (R) indicates that the programming of such a pacing mode is preferred only in the case of chronotropic incompetence. Reasons to avoid two leads include young age and limited venous access.
Optimal pacing mode and algorithm selection to avoid right ventricular pacing: current evidence and future directions
Preservation of the physiologic cardiac activation from the atria to the ventricles is key to mimic the natural electromechanical coupling of the heart.22,23 Though enabling AV synchrony, DDD/R mode is burdened by about 24% incidence of persistent atrial fibrillation (AF) at 2 years in DDDR pacemaker recipients,24 and by a 12% prevalence of heart failure (HF).25 The cause of AF, left ventricular dysfunction, and HF is probably multifactorial and is until now incompletely understood,22,24–26 but to a certain extent, it is related to suboptimal AV coupling and the amount of right ventricular stimulation (RVp). The association of RVp > 30% with HF and AF development, hospitalizations, and death across multiple trials and clinical settings set the premises for the RVP minimization (RVpm) strategy, which prevents the unfavourable drawbacks of electro-mechanical dyssynchrony induced by RVp.22 To preserve the physiological ventricular activation, algorithms to minimize RVp were developed,27 whose functioning ranges from AV delay hysteresis with automatic search of intrinsic conduction (thereby determining 1:1 AV conduction) to automatic mode switching from DDD to AAI or ADI (which implies tolerance on non-conducted P waves). Table 1 shows the key aspects of RVpm vs. maintenance of AV sequential stimulation in major clinical studies.24,28–34 An excellent review on the functioning of RVpm algorithms across manufacturers by Jankelson et al.27 highlights that AV delay search up to 450 ms provides the same extent of RVp reduction as ADI(AAI)/DDD switching algorithms in SND patients with AV block 1st, endpoints as AF burden, atrial volume, and LV function being similar in a randomized comparison,35 while the latter may be more effective in patients with intermittent AV block 2nd–3rd.32 Unwanted side effects of RVpm algorithms rarely consist of ventricular tachyarrhythmias determined by long pauses, whereas they most commonly are related to the occurrence of very long PR intervals, which may cause pacemaker-mediated tachycardia on one side or, in the worst of cases, AV uncoupling by an inefficient preload coupled to increased atrial pressure/stretching and sometimes functional mitral regurgitation (Figure 3). In fact, though earliest studies in SND patients with normal AV conduction proved that RVpm decreases persistent AF compared to customary DDD pacing, no survival benefit occurred.22 The broad population of pacemaker recipients is instead likely to have AV conduction shifting from normal to markedly prolonged (>300 ms) up to transient/permanent AV block owing to advanced age and changing medical conditions; thus, a trade-off between preserving the intrinsic cardiac activation and ensuring the optimal AV coupling becomes necessary.22,27,36 The ANSWER study32 used the RVpm strategy with a feature to pace also in the event of persistently long PR intervals in a mixed population (42% of intermittent AV block patients): a significant reduction of secondary endpoints (cardiac death/HF hospitalization and cardiovascular hospitalizations) occurred in the RVpm arm, hinting that RVpm is worthwhile but should allow physiological (<300 ms) AV intervals.32 The delicate balance of targeting these two endpoints came evident in several trials, which pinpointed a long PR interval as a risk marker for AF and HF in pacemaker recipients and implantable cardioverter–defibrillator (ICD) candidates.22, 25,37,38 Indeed, an increased incidence of AF at long term by the RVpm strategy occurred in SND patients with a baseline PR > 180 ms compared to maintenance of AV coupling by DDDR pacing in the DANPACE trial, which also found no difference in AF occurrence and burden based on the amount of RVp, while no difference was observed in terms of mortality, HF, AF, and stroke in the long-term between AAIR and DDDR pacing.22,37,38
Table 1.
Key aspects of RVpm vs. maintenance of AV sequential stimulation in major clinical studies. Adapted from Biffi M. et al., Expert Rev Med Devices 2021; 18:161–177
| Study, year | Number of patients | Comparison | Mortality | HF events | AF | Main findings |
|---|---|---|---|---|---|---|
| MOST, 1998 28 | n = 2 010 (SND population) | DDD vs. VVI | = | ↓ | ↓ | Cum %VP associated to RVPIC |
| DAVID, 2002 29 | n = 506 (ICD recipients) | DDDR-70 vs. VVI-40 | ↑ | ↑ | ‘Unnecessary’ atrial and RV pacing are detrimental | |
| SAVE-PACE, 2007 30 | n = 1 065 (SND population) | DDD + RVpm vs. DDD | = | = | ↓ | RVpm algorithm ↓ AF onset |
| DANPACE, 2011 31 | n = 1 415 (SND population) | DDD/R vs. AAI/R | = | = | ↓ | AF is related to prolonged AV interval rather than to Cum %VP |
| ANSWER, 2015 32 | n = 632 (mixed population of PM recipients) | DDDR + RVpm vs. DDDR pacing | ↓ | ↓ | = | Secondary endpoints; primary endpoint similar |
| MINERVA, 2019 24 | n = 1 166 (SND population) | DDDR vs. DDDR + RVpm Baseline PR ≤ 180 ms vs. ≥180 ms | ↑↓ | AF is related to prolonged AV interval rather than to Cum %VP. | ||
| CARE HF, 2009 33 | n = 813 (CRT recipients) | CRT vs. OPT | ↓ | ↓ | Long PR is detrimental in HF patients | |
| REAL CRT, 2020 34 | n = 82 (mixed population with EF ≥ 35% and PR ≥ 220 ms) | CRT vs. DDD + RVpm | ↓ | AF is related to prolonged AV interval rather than to Cum %VP. |
AF, atrial fibrillation; AVB, atrioventricular block; CRT, cardiac resynchronization therapy; Cum %VP, cumulative percentage ventricular pacing; DDD-70, dual-chamber rate response pacing at 70 bpm; HBP, His bundle pacing; HF, heart failure; OPT, optimal pharmacologic therapy; PM, pacemaker; RVpm, right ventricular pacing minimization; RVPIC, RV pacing-induced cardiomyopathy; SND, sinus node disease; VVI-40, ventricular back-up pacing at 40 bpm.
The name of the studies are indicated as bold.
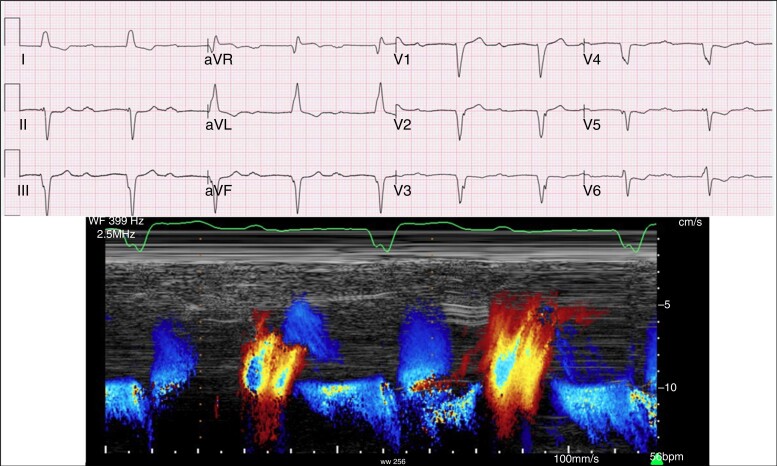
Figure 3.
Example of a too-long PR interval enabled by the RVpm strategy, with severe mitral regurgitation at a PR interval = 560 ms.
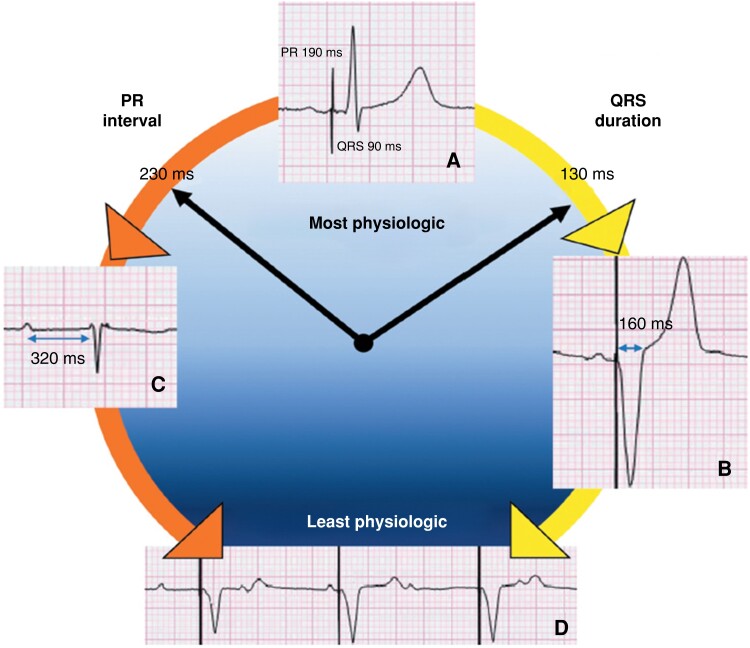
In the Minerva trial,24 the effect of RVpm on AF incidence was observed only in patients with a PR ≤ 180 ms, confirming that AV coupling as enabled by DDDR pacing is clinically warranted. The concept of ‘physiological’ AV interval remains difficult and should be evaluated at an individual level to avoid both the risk of a too-short (Figure 4) or a too-long PR interval (Figure 5). While there is consensus that a PR > 300 ms may cause symptoms because of a suboptimal preload,22,27,36,37 a PR > 230 ms marked the boundary of a non-physiologic PR interval in HF patients, who benefit from cardiac resynchronization therapy (CRT) irrespective of QRS duration and morphology.22 Moreover, CRT reduced new AF onset compared to RVpm in pacemaker recipients with a PR ≥ 220 ms.34 Thus, the knowledge of AV coupling as a ‘vulnerable’ physiological cornerstone has to be incorporated in the strategy of RVpm39 and dictates for careful pacemaker programming: RVpm is strongly recommended in patients with normal AV conduction but should be tailored to avoid very long PR intervals that promote an unfavourable ventricular filling (Figure 6). The broad adoption of conduction system pacing (CSP) will make the RVpm strategy easier in patients requiring >20% RVp owing to the possibility to achieve a physiological PR interval at no trade-off for RVp-induced cardiomyopathy (Figure 5).9,40
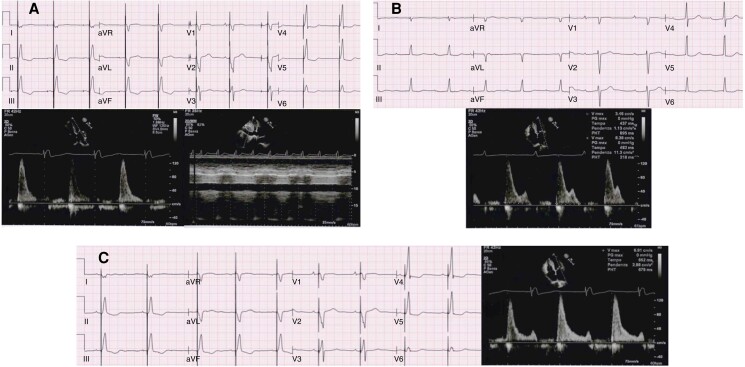
Figure 4.
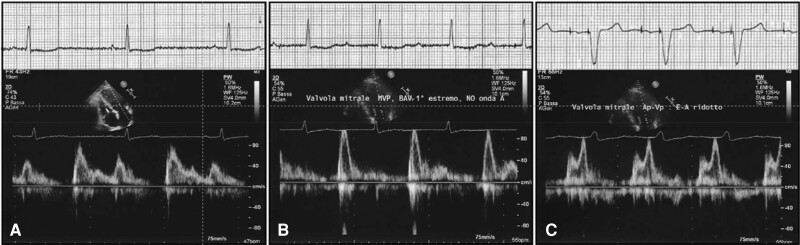
Example of a too-short PR interval in an 80-year patient with normal systolic LV function and AV block 1st/intermittent 2:1 AV block, presenting with liver congestion and swelling ankles. (A) Absence of atrial systole and restrictive filling pattern, inferior vena cava unresponsive to breathing while being DDD paced (paced AV delay 180 ms, sensed AV delay 130 ms). (B) With RVpm and lower-rate 40-bpm atrial systole occurs at a variable diastolic filling time owing to unstable PR intervals 340–400 ms. (C) At a sensed AV delay 180 m, a consistent diastolic filling time with still truncated A wave and restrictive pattern is observed, unmasking the difficulty to achieve an optimal AV coupling in aged patients.
Figure 5.
Example right ventricular pacing–induced cardiomyopathy in a SND disease patient with marked bradycardia and borderline PR interval, despite RVpm: see diastolic left ventricular filling pattern during sinus bradycardia (A). Atrial stimulation with RVpm results in an abnormally prolonged PR interval with E/A overlap and decreased LV preload (B): the patient was visited for swelling ankles and shortness of breath 6 months after implant. Tailored programming to maintain atrioventricular coupling (C) unveiled slightly abnormal diastolic LV function (E/A ∼ 0.7): 8 months later, the patient was hospitalized with HF and worsened LV ejection fraction at 36% due to RV stimulation. Adapted from Biffi M. et al., Expert Rev Med Devices 2021; 18:161–177.
Figure 6.
Representation of the most physiologic pacing settings as learnt from the history of cardiac stimulation. Normal atrial activation and physiologic conduction to the ventricles, as enabled by selective His bundle pacing, are preferred to ensure the best cardiac performance (A). Atrioventricular coupling with a relatively short QRS duration (130–160) by either right ventricular or biventricular pacing is a less physiologic alternative in complete heart block (B), while minimization of ventricular stimulation is a viable alternative until the 230–260 PR range when intrinsic conduction is persistent for the majority of time (C). Progressively increasing paced QRS duration (right arrow) or lengthening of the intrinsic PR interval (left arrow) promotes non-physiologic pacing and worsens cardiac function mimicking VVI stimulation, that is the least physiologic setting (D). Adapted from Biffi M. et al., Expert Rev Med Devices 2021; 18:161–177.
While several ongoing trials are comparing CSP with CRT in HF patients, the randomized Physio Vp-AF study of CSP vs. RVpm will evaluate the occurrence of persistent AF in patients with SND and a baseline PR ≥ 180 ms or with intermittent AV block 1st and 2nd, adding further knowledge to the therapeutic challenge of RVpm vs. AV coupling optimization.41
Cardiac implantable electronic device–related complications and malfunctions: evaluation, troubleshooting, and practical management
Cardiac implantable electronic device (CIED)–related complications are not uncommon occurring in ≅10% of patients within 6 months of the implant.42–45 Most of these complications are related to the presence of transvenous leads and subcutaneous pockets. Cardiac perforation occurs in 0.4–1.2% of patients undergoing CIED implants.42,46,47 In a multi-centre series from the United Kingdom examining 10 631 CIED procedures, the rate of perforation was 0.5%.48 Overall, 98.6% of perforation presented beyond 24 h from the time of implant. The most common presenting symptom was chest pain occurring in 46% of patients. Lead electrical abnormalities were present in 86%. Tamponade was present in 17% of patients with oral anticoagulant being a risk factor. Pericardiocentesis was required in 98.6% of patients while one patient required surgical repair. In this series, all cases required lead revision. A conservative approach in certain cases (i.e. cases with normal lead parameters and a small effusion or an effusion that is drained without recurrence) might be prudent. However, these patients should be followed closely due to a higher risk of developing a significant effusion that requires an intervention. In a multi-centre series including 48 perforations (22 managed conservatively and 26 with lead revision), conservative management was associated with a higher rate of complications specifically recurrent/worsening pericardial effusion requiring drainage.49
Lead-related complications are also not uncommonly encountered with CIED implants.50 In the Danish registry, lead-related re-intervention was needed in 2.4% of patients within 6 months of implantation.42 In the FOLLOWPACE study, lead-related complications (excluding perforation) occurred in 5.5% of patients within 2 months of implantation.51 These lead-related complications remain common, so much so that the TV pacing and ICD leads are often referred to as the ‘Achilles heel’ of CIEDs.52
CIED infection occurs in less than 1% of new implants and is higher with generator exchange (1.5–4%) and device upgrades (2%).42,53 The risk of infection seems to increase with larger generators (Cardiac Resyncchronisation Therapy-Pacemake [CRT-D] and ICD > pacemaker PR interval [PPM] and Cardiac Resynchronisation Therapy-Defibrillator [CRT-P]), more complex procedures, and non-denovo CIEDs.54 CIED infection is associated with significant mortality, morbidity, and healthcare expenditure.55 Hence, prevention is paramount.
An antibacterial envelop was shown to reduce the risk of CIED infection in high-risk subgroups.56,57 This therapy should be considered in those patients considered at high risk of infection. Prevention of pocket haematoma is also important to reduce CIED infection. The presence of haematoma increases infection risk. In an analysis from the SIMPLE trial, the rate of peri-operative haematoma was 2.2%.58 The risk of infection in those patients was 10.6%. In this analysis, bridging with heparin and LMWH was associated with a 2.65-fold higher rate of haematoma formation.
Leadless cardiac pacing
Leadless pacemakers are emerging as an alternative to traditional TV-PPM. The Micra transcatheter pacing system (TPS) (Medtronic) has been studied extensively in clinical trials.59–61 The Micra investigational development exemption (IDE) study enrolled 726 patients.59 Implant success rate was >99%, and notably, no macro-dislodgment or infections were reported in this study. However, the rate of pericardial effusion was 1.5%. The Micra post-approval registry (PAR), an FDA mandated study, enrolled more than 1 800 patients to monitor the performance of this technology in a real-world setting.60 The results of this study mimicked the Micra IDE results. The implant success rate was 99.1%. There was a low rate of dislodgment 0.05% and no infection requiring device removal. The rate of pericardial effusion was lower in this study (0.44% meeting major complication definition and 0.77% total pericardial effusion) as compared to the IDE. The Micra TPS clinical trials included a pre-specified comparison cohort of patients implanted with TV-PPM. Up to 63% reduction in major complications with leadless pacemaker (LP) as compared to TV-PPM was noted in these two clinical trials.
The Centers for Medicare and Medicaid Services issued a national coverage determination for LP.61 All Medicare patients receiving LP are automatically enrolled in a Continuous Evidence Development (CED) study. The Micra CED study compared outcomes of Medicare beneficiaries receiving a Micra device vs. those receiving a single-chamber TV-PPM. This study enrolled 5 764 patients implanted with Micra LP and 9 662 patient TV-PPM. There was no difference in 30-day complications between the two groups. The LP cohort had a higher rate of pericardial effusion as compared to the TV-PPM cohort (0.8% vs. 0.4%, P < 0.001) but a lower rate of device-related complications (1.4 vs. 2.6%, P < 0.001). When this cohort was followed for 2 years, LP were associated with 31% reduction in major complication mainly driven by 38% reduction in need for reintervention.47 This reduction in complications and need for reintervention was also seen in high-risk subgroups (patients on dialysis, diabetics, etc.).62
The AVEIR LP (ABBOTT) is the modified version of the prior ABBOTT LP (Nanostim). Unlike Micra TPS which is a tine-based fixation device, AVEIR has a helix-based fixation mechanism. The LEADLESS II Phase 2 trial showed that the implant success rate was 98%.63 Major complications occurred in 4% during follow-up including pericardial effusion in (1.5%), dislodgment (1%), and groin complications (1%). Recently, the result of a clinical trial testing the efficacy and safety of a dual-chamber AVEIR LP was published.64 The implant success rate was 98.3%. The rate of intra-procedural dislodgment was 1.7% and during follow-up (1.7%). The rate of pericardial effusion was 0.7%.
While the rate of perforation seems to be improving, some concerns remain regarding the severity of perforation with LPs. A score to predict patients’ risk of cardiac perforation has been developed and validated using the Micra TPS clinical trials data.65 Patients could be divided into low risk (0.4% perforation rate), intermediate risk (≅2% risk), and high risk (≅4.5%). This score could potentially be used to counsel patients regarding their risk and device choice.
The WiSE-CRT system is currently the only leadless system able to provide left ventricular pacing. It is currently still in clinical trials in the U.S. and has not received FDA approval yet. It currently has a role in failed CS upgrades and possibly non-responders to traditional CRT.66 The original data show a significant implant complication rate due to a high rate of arterial access complications. The transeptal approach is currently used for implantation and might be a safer option in experienced hands. Experience with a totally leadless CRT using the WiSE-CRT system has been published with encouraging results.67
The EHRA/HRS/LAHRS/APHRS issued a practical consideration document regarding LP.68 Endorsing the ESC guidelines, its use is recommended in patients with upper extremity access limitation and possibly as an alternative to traditional TV-PPM.6
Lead extraction
What’s new concerning infections of CIED?
Device-related infection is a severe complication to CIED therapy. In the Danish pacemaker and ICD register that included 97 750 consecutive patients, the device-related infection incidence during device lifetime was 1.19% (1.12–1.26) for pacemaker, 1.91% (1.71–2.13) for ICD, 2.18% (1.78–2.64) for CRT-P, and 3.35% (2.92–3.83) for CRT-D.69
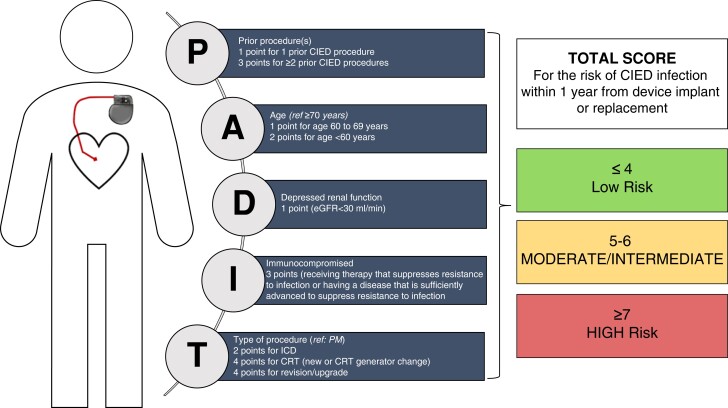
Detection of the subgroup of patients at increased risk of CIED infection is crucial, in order to take preventive measures. The PADIT infection risk score is composed of age, procedure type, renal insufficiency, immunocompromised status, and number of previous procedures (Figure 7).55 In a US data set of 54 042 index procedures among 51 623 patients with 574 infections, a one-unit increase in the PADIT score was associated with a relative 28% increase in infection risk. This score could be used in clinical practice to identify patients who may benefit from targeted interventions to reduce infection risk during implant, upgrade, or revision.70
Figure 7.
The PADIT risk score. From ref.55 PADIT, Prevention of Arrythmia Device Prevention Trial.
As mentioned above, an antibiotic-eluting absorbable envelope (TyRXTM, Medtronic, Minneapolis, USA) has been developed to reduce the infection rate.55 One hundred and forty-four patients undergoing CIED implantation who received the antibacterial envelope were compared with a matched cohort of 382 CIED patients from a Swedish centre. The envelope group had a higher PADIT score, 5.9 ± 3.1 vs. 3.9 ± 3.0 (P < 0.0001). For the primary endpoint, no local infections occurred in the envelope group, compared with 2.6% in the control group (P = 0.04), with a more pronounced difference in the patients with a high (>7 points) PADIT score, 0 vs. 9.9% (P = 0.01). This study confirms the clinical efficacy and the interest of using an antibacterial envelope in the prevention of local CIED infection in patients with a higher risk guided by the PADIT score.57
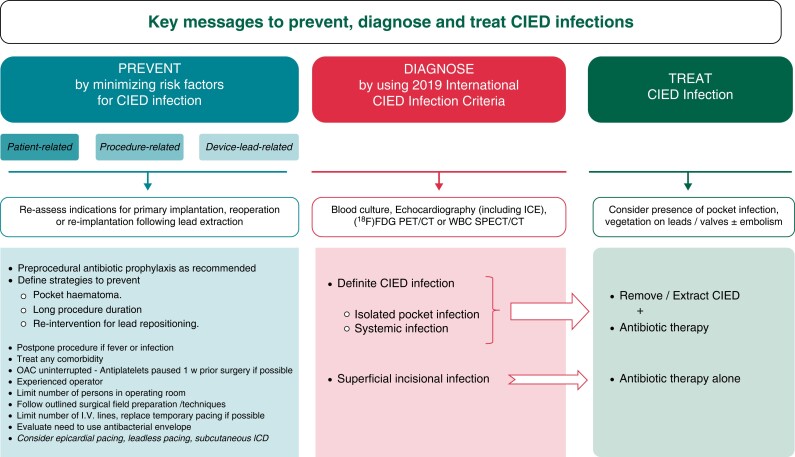
An international consensus document on how to prevent, diagnose, and treat CIED infections has been recently released.71 This document gives guidance on the use of these antibacterial envelopes, but also on novel device alternatives, novel oral anticoagulants, prolonged antibiotics post-implantation, and definitions on minimum-quality requirements for centres, operators, and volumes. Many important insights are developed and delivered about all these crucial topics (Figure 8).71
Figure 8.
Summary of key messages for prevention, diagnosis, and management of CIED infections. From ref.71 CIED, cardiac implantable electronic device; [18F] FDG PET/CT, fluorodeoxyglucose positron emission tomography–computed tomography; ICD, implantable cardiac defibrillator; ICE, intracardiac echocardiography; OAC, oral anticoagulation; w, week; WBC SPECT/CT, white blood cell single-photon emission computed tomography–computed tomography.
Lead extraction indications and tools
Indications for lead extractions are well summarized in the 2018 EHRA expert consensus document.72 They are divided into two groups:
Infection indications (pocket infection/erosion, lead/valvular endocarditis, bacteriaemia…)
Non-infection indications (lead failure, abandoned lead, venous access issues, access to magnetic resonance imaging (MRI), chronic pain, recall…)
Lead removal includes a wide spectrum of tools and techniques, ranging from simple manual traction to multiple procedures and combined approaches that are also explained in this same document: superior approach, inferior/femoral approach, simple traction, locking stylets, mechanical non-powered telescoping sheaths, powered sheaths, snares, baskets, compression coils, occlusion balloons, etc.72
The ELECTRa study73 is, still currently, the largest prospective registry on transvenous lead extractions (TLE), which included a total of 3 555 consecutive patients of whom 3 510 underwent TLE at 73 centres in 19 European countries and confirmed the safety and efficacy of the current practice of TLE. Complete clinical and radiological success rates were 96.7% (95% CI 96.1–97.3%) and 95.7% (95% CI 95.2–96.2%), respectively. The primary endpoint of the in-hospital procedure-related major complication rate was 1.7% (95% CI 1.3–2.1%) (58/3510 patients) including a mortality of 0.5% (95% CI 0.3–0.8%) (17/3510 patients).73 TLE was associated in this registry with a higher success rate with lower all-cause complication and mortality rates in high volume compared with low-volume centres. The later paved the way for qualifications and training of operators, procedural volume, environment, and anaesthesia considerations.72
Longer dwelling time often requires the use of powered/mechanical sheaths for TLE. The PROMET study74 collected data on a total of 2 205 patients (age 66.0 ± 15.7 years) with 3 849 leads targeted for extraction in six European lead extraction centres. The median lead dwell time was 74 months. Clinical success was obtained in 97.0% of procedures, and complete extraction was achieved for 96.5% of leads. Major complications occurred in 22/2 205 procedures (1%), with a peri-operative or procedure-related mortality rate of 4/2 205 (0.18%), and minor complications in 3.1% of procedures. This study suggests that rotational TLE tools and techniques obtain similar results and can be proposed as an alternative to the laser methods.74
Very recently, single-centre data from 166 consecutive patients that underwent TLE requiring advanced techniques (245 leads in total, dwelling time 9.4 ± 6.3 years) have been analysed and reported.75 In this cohort, laser sheaths were used in 64.9%, powered mechanical sheaths in 35.1% of the procedures as primary extraction tools. The efficacy and safety of laser and mechanical sheaths were similar; however, in the subgroup of crossover procedures, mechanical tools had better performance regarding clinical success.75
TLE is sometimes a difficult and risky procedure requiring tool diversity and staff experience that are key for improving outcomes in the most complicated cases.
Cardiac pacing in special populations
Pacing after transcatheter aortic valve implantation
Since the beginning of transcatheter aortic valve implantation (TAVI) nearly 20 years ago, injury to the conduction system necessitating pacemaker implantation showed up as a significant problem that initially involved nearly 25% of patients and is currently closer to 10%.6, 76 The recent ESC guidelines dedicated a full chapter to the controversy of when pacing following TAVI is indicated.6 The risk of fainting in an old fragile patient dictates an aggressive approach, while short-term as well as potential long-term complications with pacemaker implantation in this population are not negligible.77,78
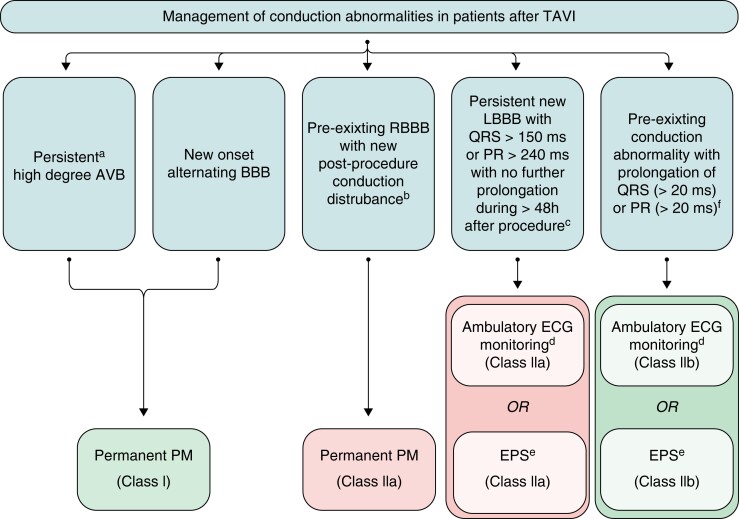
There are multiple publications on preprocedural and post-procedural risk factors and predictors of permanent pacemaker implantation following TAVI.26,79–85 While a lack of any conduction disturbance following TAVI carries a very low risk of development of advanced AV block and development of complete AV block that does not resolve over 24–48 h necessitates permanent pacemaker, there are many intermediate situations of an injury to the conduction system that need specific approaches including prolonged monitoring and electrophysiological conduction studies. A guideline-recommended6 approach is illustrated in Figure 9.
Figure 9.
Management of conduction abnormalities after TAVI. From ref.6 AF, atrial fibrillation; AV, atrioventricular; AVB, atrioventricular block; BBB, bundle branch block; ECG, electrocardiogram; EPS, electrophysiology study; HV, His-ventricular interval; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; PM, pacemaker; RBBB, right bundle branch block; TAVI, transcatheter aortic valve implantation. a24–48-h post-procedure. bTransient high-degree AVB, PR prolongation, or axis change. cHigh-risk parameters for high-degree AV block in patients with new-onset LBBB include AF, prolonged PR interval and LVEF < 40%. dAmbulatory continuous ECG monitoring for 7–30 days. eEPS with HV ≥ 70 ms may be considered positive for permanent pacing. fWith no further prolongation of QRS or PR during 48-h observation.
Pacing following cardiac surgery
Atrioventricular block occurs in 1–8% of cardiac operations (more common following valve operations than after coronary artery bypass) while SND may also occur in fewer operations as well as following heart transplantation.6,86,87 Due to the potential reversibility of post-operative block, the ideal timing for permanent pacemaker implantation has been a matter of debate over many years.88 The current ESC guidelines6 recommend a waiting period of at least 5 days with potential shortening if there is CAVB with low or no escape, with a low chance of recovery or in cases of valvular surgery with early AVB that never recovers over a 48-h observation period. In cases of endocarditis, when AVB occurs during surgery, high-risk parameter for persistent AVB exist (Staphylococcus aureus, intra-cardiac abscess, tricuspid involvement or previous valvular surgery). In these cases, permanent pacing should be installed immediately during surgery using epicardial approach.89
Tricuspid surgery forms a special group as traditional pacing involves crossing of the tricuspid valve. A mechanical tricuspid valve cannot be crossed by a pacing lead. Epicardial pacing is preferred over a lead crossing a repaired or bioprosthetic valve. When preexisting leads exist, removal and epicardial implantation are preferred over sawing the lead near the valve although the latter is not entirely contraindicated. Ventricular pacing with a preexisting bioprosthetic tricuspid valve is preferably done via coronary sinus or epicardially.90
For further information on pacing following heart transplantation, the reader is referred to chapter 8.2.3 in the ESC pacing guidelines.6
Pacing in congenital heart conditions
While a detailed discussion of this complex topic is beyond the scope of this publication, several principles were emphasized in the recent European guidelines for pacing.6 Overall, all indications in this group are based on expert opinion as there are no randomized controlled trials. An important principle is not to implant endovascular leads in the presence of intracardiac shunts. Other conditions with limited venous access may necessitate epicardial pacing. The most common aetiology of AV block in congenital heart diseases is post-operative block. Whereas in children, post-operative block usually resolves within 7–10 days (which sets the optimal time to wait before permanent pacemaker implantation), such information is scarce in adults. While patients with post-operative high-degree AV block should be paced (LOR = 1), those who had complete AV block in the perioperative period which recovered later but remained with bifascicular block may be considered for pacing (LOR = IIB). In situations of high risk for pacing in the presence of complex congenital heart disease, permanent epicardial leads should be implanted during cardiac operation.
The second important congenital situation is congenital AV block. Patients with congenital AV block should be paced if any risk factor of the following exists: symptoms, pauses > 3× the cycle length of the escape rhythm, broad QRS escape long QT complex ventricular ectopy, and daytime mean rate < 50. Some experts believe that any congenital AV block should be paced to reduce the likelihood of potentially lethal arrhythmias (IIB recommendation).
Pacing in hypertrophic cardiomyopathy
While RV apical pacing has been shown in several trials to modestly reduce outflow tract gradients, pacing for this indication is rarely justified.91 It may be considered for this purpose in patients who have another indication for pacing, in symptomatic patients who are drug refractory and cannot undergo any intervention (surgery or septal reduction) or in those undergoing septal myectomy or septal ablation with resultant AV block.
Rare diseases
For a detailed discussion of pacing in rare situations such as neuromuscular diseases,92,93 genetic cardiomyopathies (mainly Lamin AC),94–96 infiltrative, metabolic, and inflammatory disease, including the decision among pacemaker and ICD implantation, please refer to the guideline document.6
CIED management in complex clinical scenario
MRI environment
The management of implantable devices in an MRI environment has long been discussed in the literature97–100 and is best covered recently by an EHRA document on magnetic interference.101 The potential effects of MRI on CIEDs include triggering of asynchronous pacing thus resulting in atrial or ventricular arrhythmias, heating of the heart tissue surrounding leads resulting in change in electrical parameters, and reprogramming of the device including power on reset and signs of battery depletion. Oversensing is most common and may result in pacing inhibition and/or inappropriate ICD therapies if not pre-programmed. Overall, the incidence of significant complications with appropriate programming is very low and clear recommendations exist about preparation and pre-programming of patients with devices before MRI. All these recommendations refer to patients several weeks or more following implantation; recent implantation is considered a relative contraindication.
Abandoned leads, epicardial leads, and adapters are considered contraindications to MRI due to the lack of information and theoretical arguments but have been shown by small series not to cause any troubles.102 The current wide availability of MRI conditional devices makes the procedure much simpler and safe. Nevertheless, the availability of device-competent staff and emergency routines in the MRI suite are still necessary.
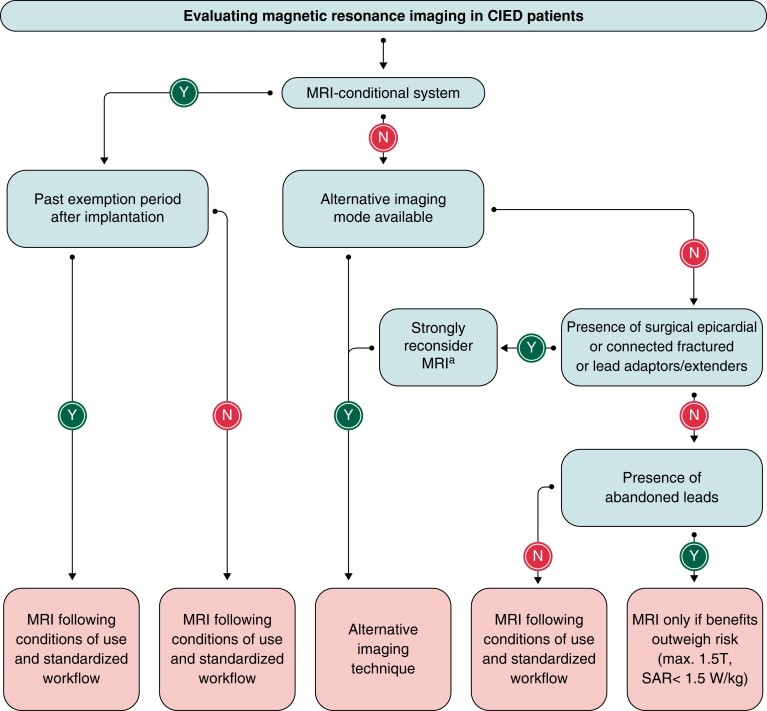
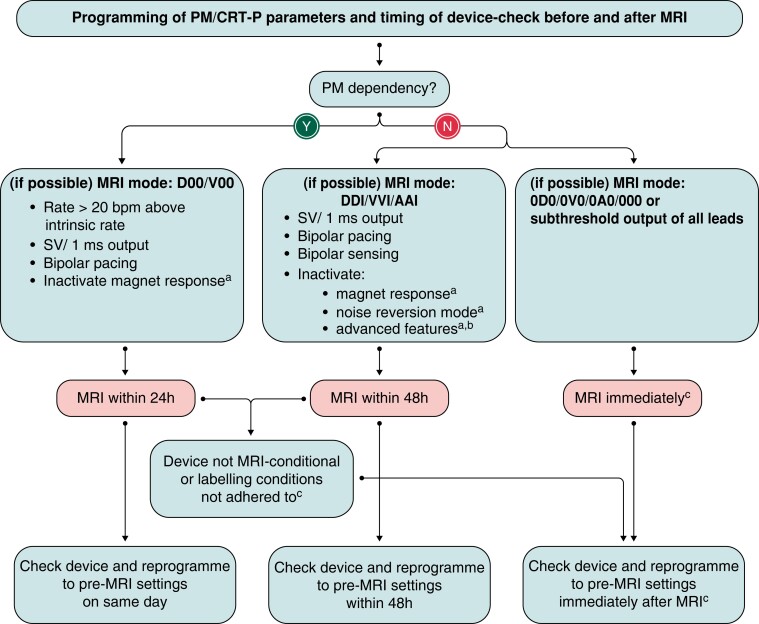
Figures 10 and 11 summarize the EHRA approach to MRI with devices.101 Further details on specific programming and follow-up are available in the EHRA document.101
Figure 10.
Flowchart for evaluating MRI in CIED patients. From ref.101 MRI, magnetic resonance imaging; SAR, specific absorption rate. aConsider only if there is no imaging alternative, and the results of the test is crucial for applying life-saving therapies for the patients.
Figure 11.
Programming of device parameters and timing of device check before and after MRI. From ref.101 AF, atrial fibrillation; MRI, magnetic resonance imaging. aIf available. bRate hysteresis; atrial anti-tachycardia pacing; premature ventricular complex and premature atrial contraction triggered pacing; AF therapies–rate smoothing; overdrive pacing; conducted AF response. cIn CIED with automatic MRI mode activation, the scan may be performed electively after the pre-scan follow-up and reprogramming after the intervention may not be necessary.
Perioperative management of implantable devices
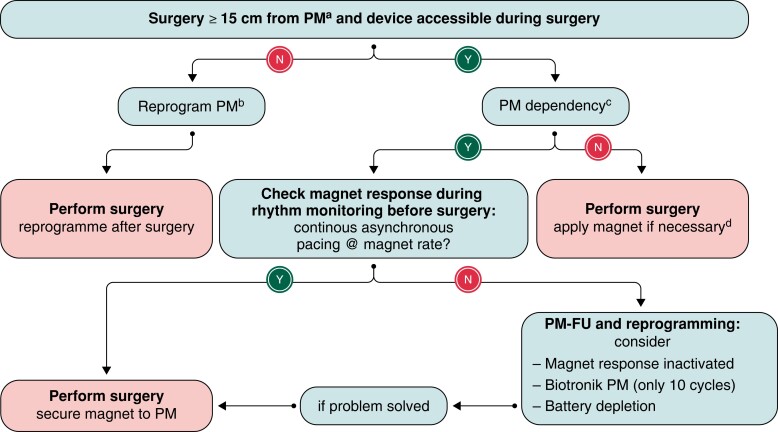
This topic is also thoroughly reviewed in EHRA consensus paper on electromagnetic interference (EMI) with practical recommendations, some of which are innovative.101 The principal risk of surgery is EMI caused by cautery (mainly unipolar), and it is relevant mostly in procedures performed above the umbilicus. Notably, magnet use during surgery, which used to be discouraged in the past due to illusive reprogramming of devices with open reed switch, is now recommended if needed during surgery. Safe taping of the magnet over the device is recommended. Magnets are used to prevent oversensing inhibition of pacemakers in dependent patients or detection of the cautery by defibrillators resulting in inappropriate therapy delivery. Magnet can be used if the operative field is not too close (15 cm) to the device. When the field is close, then, reprogramming of the device is necessary before surgery if the patient is pacemaker dependent or has an ICD. Figure 12 illustrates the EHRA-recommended approach to management of devices during surgery.101
Figure 12.
Algorithm for perioperative management of PM (including CRT-P) during surgery. From ref.101aReprogramming/magnet application is optional, if surgery is performed below the iliac crest and no full-body return electrodes are used; basynchronous mode (D00/V00/A00); rate response may be inactivated to avoid rapid pacing with patient mobilization or respiratory monitoring (if the PM has a minute-ventilation sensor); cabsence of intrinsic escape rhythm or heart rate, 50-bpm causing symptoms; dasystole or haemodynamically relevant bradycardia during electrocautery.
Radiotherapy in the presence of implantable devices
Over the years, 2–7% of patients with CIEDS undergoing therapeutic radiation developed some kind of device malfunction.101,103,104 The risk of malfunction is related to the location and cumulative dose of irradiation, mainly at the generator site, type of energy (proton beam more dangerous), and modes of shielding.105 Until recently, there was wide variation in the approach to patients with CIEDS undergoing radiation therapy106 and the EHRA document meant to set more uniform standards.101
The main effect of radiation on CIEDs is in damaging device memories, causing temporary or permanent programming change, and rarely EMI during the irradiation session. The damage is cumulative and may develop late rather than early in the course of radiation sessions. Operations to remove the pacemaker to a different place are very rarely necessary these days and mainly done if the CIED interferes with effective energy delivery to the tumour site.
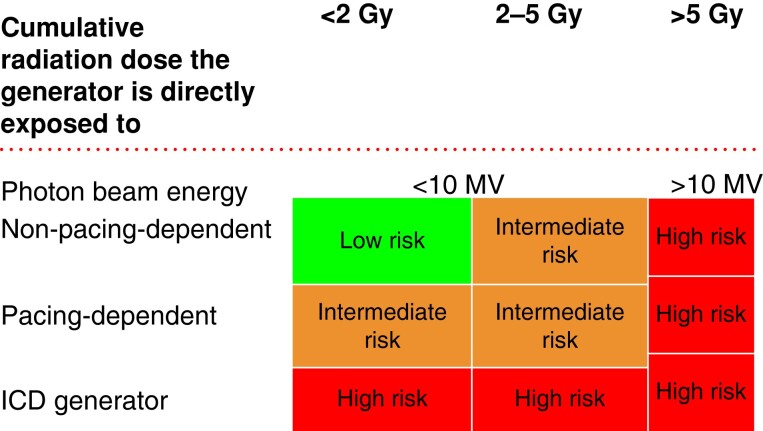
Risk stratification is needed prior to radiation therapy. This is based on the radiation dose for the device, type of energy (photon beam?), pacemaker dependency, and the presence of an ICD (Figure 13). All patients with CIEDS have to be monitored at least vocally during the session, and a code cart should be available. CIED-trained professionals should be available in the hospital.
Figure 13.
Risk stratification for CIED malfunction. From ref.101
In the low-risk group, the device has to be interrogated prior to and after completion of all radiotherapy fractions. In the intermediate-risk group devices, interrogation should take place as above but also in the middle of the period. ECG monitoring is mandatory in any case of suspicion of device malfunction during sessions of radiation. Remote monitoring is also valuable.
In the high-risk group, remote monitoring or weekly interrogation is recommended. All other aspects are unchanged and ECG monitoring is also mandatory during session. Most cases do not have to be reprogrammed for the irradiation. For more detailed discussion of this topic, please refer to EHRA document.101
Future perspectives in cardiac pacing
The evolution of cardiac pacing has progressed through several eras that include the development of the first implantable permanent pacemakers, dual-chamber pacing, advanced programming, cardiac resynchronization therapy, remote monitoring, leadless pacing, and finally physiologic pacing. Physiologic pacing has evolved rapidly through a variety of anatomic targets including the His bundle, the left bundle branches, and now the right bundle branch.107 Innovation in cardiac pacing is more intense and diverse than ever before. The development of physiologic pacing has been one of the most notable advances in pacing in the several decades. While tremendous progress has been made, the field of physiologic pacing remains in its infancy. Left bundle branch area pacing is now the dominant and most reproducible form of physiologic pacing,108 but how that is optimally combined with other pacing technologies is largely unknown. We still don’t have pacemaker generators designed to deliver physiologic pacing nor do we know what combination of physiologic and resynchronization technologies result in optimal treatment (and prevention) of HF.109 While the longevity of pacemaker batteries has improved over the years, pacemaker battery innovation has been characterized by relatively small, incremental steps in battery chemistry. Rechargeable pacemakers would avoid many of the challenges associated with the need for repeated generator replacements. While the external application of electromagnetic induction currents to recharge pacemakers was reported in 1965,110 rechargeable technologies have not entered clinical practice. However, the future of pacing will likely include not one but several rechargeable battery technologies. Advances in both external charge technologies and self-recharging devices have the potential to accelerate the development and the utility of these systems. Self-recharging devices that harvest in vivo biomechanical energy including through the use of triboelectric nanogenerators have exciting possibilities.111 The development of leadless pacing has also been a notable advance in pacing. Within a decade, leadless pacing has evolved from single-chamber VVI/R devices, to single-chamber VDD pacing, and now dual-chamber pacing. Modular dual-chamber leadless pacing has entered clinical trials, and the early results are very promising with 97% of patients achieving ≥70% atrioventricular synchrony.64 Totally leadless cardiac resynchronization therapy has been demonstrated to be effective with leadless RV pacemakers paired with leadless ultrasound-based endocardial left ventricular pacing.67 Integration of leadless technologies across indications and across device platforms will continue to evolve. As more and heterogeneous pacing technologies enter clinical practice, selection of pacing systems for specific patients will also become more complex. Personalization of pacing therapy will be more important than ever. The COVID pandemic highlighted the value of remote and virtual care. Future advances in pacing will also include further adaptions that facilitate more patient-centred care that is more convenient and accessible. Technologies for remote programming are being developed and have the potential to change care dramatically, potentially removing the need for most in-person visits.112 Personalized approaches to pacing will evolve in the future, especially as machine learning and artificial intelligence are applied predictive analytics. For example, AI-assisted analysis of ECGs may help identify patients who would benefit from permanent pacing before they develop symptoms from conduction disorders. Such techniques have already been used to predict who requires pacemaker implantation after TAVI.113 Improved pacemaker diagnostics and their analysis will also allow for improved personalized care. One notable example is the ability of device diagnostics to identify patients who may have sleep apnoea.114 Personalized medicine has been a challenge for medical therapy, but device therapy may be able to deliver on this promise more effectively, particularly due to synergies in innovation in how we identify who needs pacing and when (i.e. AI-based prediction using ECGs), how we provide pacing (i.e. remote analysis and programming), and how we use the information we gather from pacing (i.e. identification of sleep apnoea).
Contributor Information
Pascal Defaye, Cardiology Department, University Hospital and Grenoble Alpes University, CS 10217, Grenoble Cedex 9, Grenoble 38043, France.
Mauro Biffi, Cardiology Unit, Cardiac Thoracic and Vascular Department, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Mikhael El-Chami, Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta, Georgia, USA.
Serge Boveda, Clinique Pasteur, Heart Rhythm Department, Toulouse, France.
Michael Glikson, Cardiology Department, Jesselson Integrated Heart Center Shaare Zedek Medical Center and Hebrew University Faculty of Medicine, Jerusalem, Israel.
Jonathan Piccini, Duke University, Duke Clinical Research Institute, Durham, NC, USA.
Marco Vitolo, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico Di Modena, Modena, Italy; Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, Modena, Italy.
Funding
No funding was received for this work.
References
- 1. Sutton R. Introducing Europace. Europace 1999;1:1. [Google Scholar]
- 2. Ward C, Henderson S, Metcalfe NH. A short history on pacemakers. Int J Cardiol 2013;169:244–8. [DOI] [PubMed] [Google Scholar]
- 3. Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol 2006;8:17–81. [PMC free article] [PubMed] [Google Scholar]
- 4. Ball CM, Featherstone PJ. The early history of cardiac pacing. Anaesth Intensive Care 2019;47:320–1. [DOI] [PubMed] [Google Scholar]
- 5. Hyman AS. Resuscitation of the stopped heart by intracardial therapy: ii. Experimental use of an artificial pacemaker. Arch Intern Med 1932;50:283–305. [Google Scholar]
- 6. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 7. Boveda S, Lenarczyk R, Haugaa KH, Iliodromitis K, Finlay M, Lane Det al. Use of leadless pacemakers in Europe: results of the European Heart Rhythm Association survey. Europace 2018;20:555–9. [DOI] [PubMed] [Google Scholar]
- 8. Ferrick AM, Raj SR, Deneke T, Kojodjojo P, Lopez-Cabanillas N, Abe Het al. 2023 HRS/EHRA/APHRS/LAHRS expert consensus statement on practical management of the remote device clinic. Europace 2023;25:euad123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burri H, Jastrzebski M, Cano Ó, Čurila K, de Pooter J, Huang Wet al. EHRA Clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace 2023;25:1208–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burri H, Starck C, Auricchio A, Biffi M, Burri M, D’Avila Aet al. EHRA Expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), and the Latin-American Heart Rhythm Society (LAHRS). Europace 2021;23:983–1008. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed FZ, Sammut-Powell C, Kwok CS, Tay T, Motwani M, Martin GPet al. Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2022;24:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med 2008;23:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zecchin M, Torre M, Carrani E, Sampaolo L, Ciminello E, Ortis Bet al. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: an analysis by age groups. Eur J Intern Med 2021;84:38–45. [DOI] [PubMed] [Google Scholar]
- 14. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SEet al. European Society of Cardiology: Cardiovascular Disease Statistics 2019 (executive summary). Eur Heart J Qual Care Clin Outcomes 2020;6:7–9. [DOI] [PubMed] [Google Scholar]
- 15. Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 2014;17:69–77. [DOI] [PubMed] [Google Scholar]
- 16. Aktaa S, Abdin A, Arbelo E, Burri H, Vernooy K, Blomström-Lundqvist Cet al. European Society of Cardiology Quality Indicators for the care and outcomes of cardiac pacing: developed by the Working roup for Cardiac Pacing Quality Indicators in collaboration with the European Heart Rhythm Association of the European Society of Cardiology. Europace 2022;24:165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He W, Goodkind D, Kowal P. An aging world: 2015. International Population Reports, P95/16-1. U.S. Census Bureau. Washington, DC: U.S. Government Publishing Office; 2016. [Google Scholar]
- 18. Boriani G, Valenti AC, Vitolo M. Clinical implications of assessing frailty in elderly patients treated with permanent cardiac pacing. J Cardiovasc Med (Hagerstown) 2022;23:87–90. [DOI] [PubMed] [Google Scholar]
- 19. Savelieva I, Fumagalli S, Kenny RA, Anker S, Benetos A, Boriani Get al. EHRA Expert consensus document on the management of arrhythmias in frailty syndrome, endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2023;25:1249–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cabell CH, Heidenreich PA, Chu VH, Moore CM, Stryjewski ME, Corey GRet al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J 2004;147:582–6. [DOI] [PubMed] [Google Scholar]
- 21. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt Det al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J 2022;43:716–99. [DOI] [PubMed] [Google Scholar]
- 22. Biffi M, Capobianco C, Spadotto A, Bartoli L, Sorrentino S, Minguzzi Aet al. Pacing devices to treat bradycardia: current status and future perspectives. Expert Rev Med Devices 2021;18:161–77. [DOI] [PubMed] [Google Scholar]
- 23. Arnold M, Richards M, D’Onofrio A, Faulknier B, Gulizia M, Thakur Ret al. Avoiding unnecessary ventricular pacing is associated with reduced incidence of heart failure hospitalizations and persistent atrial fibrillation in pacemaker patients. Europace 2023;25:euad065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boriani G, Pieragnoli P, Botto GL, Puererfellner H, Mont L, Ziacchi Met al. Effect of PR interval and pacing mode on persistent atrial fibrillation incidence in dual chamber pacemaker patients: a sub-study of the international randomized MINERVA trial. Europace 2019;21:636–44. [DOI] [PubMed] [Google Scholar]
- 25. Somma V, Ha FJ, Palmer S, Mohamed U, Agarwal S. Pacing-induced cardiomyopathy: a systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023;20:282–90. [DOI] [PubMed] [Google Scholar]
- 26. Tsushima T, Al-Kindi S, Palma Dallan LA, Fares A, Yoon SH, Wheat HLet al. Clinical impact of right ventricular pacing burden in patients with post-transcatheter aortic valve replacement permanent pacemaker implantation. Europace 2023;25:1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jankelson L, Bordachar P, Strik M, Ploux S, Chinitz L. Reducing right ventricular pacing burden: algorithms, benefits, and risks. Europace 2019;21:539–47. [DOI] [PubMed] [Google Scholar]
- 28. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee Ret al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854–62. [DOI] [PubMed] [Google Scholar]
- 29. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia Het al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA 2002;288:3115–23. [DOI] [PubMed] [Google Scholar]
- 30. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick Det al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 2007;357:1000–8. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard Det al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J 2011;32:686–96. [DOI] [PubMed] [Google Scholar]
- 32. Stockburger M, Boveda S, Moreno J, Da Costa A, Hatala R, Brachmann Jet al. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J 2015;36:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen Aet al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail 2009;11:699–705. [DOI] [PubMed] [Google Scholar]
- 34. Botto GL, Iuliano A, Occhetta E, Belotti G, Russo G, Campari Met al. A randomized controlled trial of cardiac resynchronization therapy in patients with prolonged atrioventricular interval: the REAL-CRT pilot study. Europace 2020;22:299–305. [DOI] [PubMed] [Google Scholar]
- 35. Calvi V, Pisanò EC, Brieda M, Melissano D, Castaldi B, Guastaferro Cet al. Atrioventricular interval extension is highly efficient in preventing unnecessary right ventricular pacing in sinus node disease: a randomized cross-over study versus dual- to atrial single-chamber mode switch. JACC Clin Electrophysiol 2017;3:482–90. [DOI] [PubMed] [Google Scholar]
- 36. Antonio RS, Benito EM, Tolosana JM, Emilce Trucco M, Mont L. Presyncopal episodes after implantation of dual-chamber pacemaker programmed in SafeR pacing mode. Europace 2017;19:807. [DOI] [PubMed] [Google Scholar]
- 37. Nielsen JC, Thomsen PE, Højberg S, Møller M, Riahi S, Dalsgaard Det al. Atrial fibrillation in patients with sick sinus syndrome: the association with PQ-interval and percentage of ventricular pacing. Europace 2012;14:682–9. [DOI] [PubMed] [Google Scholar]
- 38. Brandt NH, Kirkfeldt RE, Nielsen JC, Mortensen LS, Jensen GVH, Johansen JBet al. Single lead atrial vs. dual chamber pacing in sick sinus syndrome: extended register-based follow-up in the DANPACE trial. Europace 2017;19:1981–7. [DOI] [PubMed] [Google Scholar]
- 39. Salden F, Kutyifa V, Stockburger M, Prinzen FW, Vernooy K. Atrioventricular dromotropathy: evidence for a distinctive entity in heart failure with prolonged PR interval? Europace 2018;20:1067–77. [DOI] [PubMed] [Google Scholar]
- 40. Zanon F, Ellenbogen KA, Dandamudi G, Sharma PS, Huang W, Lustgarten DLet al. Permanent His-bundle pacing: a systematic literature review and meta-analysis. Europace 2018;20:1819–26. [DOI] [PubMed] [Google Scholar]
- 41. Pastore G, Bertini M, Bonanno C, Coluccia G, Dell'Era G, De Mattia Let al. The PhysioVP-AF study, a randomized controlled trial to assess the clinical benefit of physiological ventricular pacing vs. managed ventricular pacing for persistent atrial fibrillation prevention in patients with prolonged atrioventricular conduction: design and rationale. Europace 2023;25:euad082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kirkfeldt RE, Johansen JB, Nohr EA, Jorgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Biffi M, Ammendola E, Menardi E, Parisi Q, Narducci ML, De Filippo Pet al. Real-life outcome of implantable cardioverter-defibrillator and cardiac resynchronization defibrillator replacement/upgrade in a contemporary population: observations from the multicentre DECODE registry. Europace 2019;21:1527–36. [DOI] [PubMed] [Google Scholar]
- 44. König S, Svetlosak M, Grabowski M, Duncker D, Nagy VK, Bogdan Set al. Utilization and perception of same-day discharge in electrophysiological procedures and device implantations: an EHRA survey. Europace 2021;23:149–56. [DOI] [PubMed] [Google Scholar]
- 45. Frausing M, Kronborg MB, Johansen JB, Nielsen JC. Avoiding implant complications in cardiac implantable electronic devices: what works? Europace 2021;23:163–73. [DOI] [PubMed] [Google Scholar]
- 46. Mahapatra S, Bybee KA, Bunch TJ, Espinosa RE, Sinak LJ, McGoon MDet al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005;2:907–11. [DOI] [PubMed] [Google Scholar]
- 47. El-Chami MF, Bockstedt L, Longacre C, Higuera L, Stromberg K, Crossley Get al. Leadless vs. transvenous single-chamber ventricular pacing in the Micra CED study: 2-year follow-up. Eur Heart J 2022;43:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Waddingham PH, Elliott J, Bates A, Bilham J, Muthumala A, Honarbakhsh Set al. Iatrogenic cardiac perforation due to pacemaker and defibrillator leads: a contemporary multicentre experience. Europace 2022;24:1824–33. [DOI] [PubMed] [Google Scholar]
- 49. Acha M R, Rafael A, Keaney JJ, Elitzur Y, Danon A, Shauer Aet al. The management of cardiac implantable electronic device lead perforations: a multicentre study. Europace 2019;21:937–43. [DOI] [PubMed] [Google Scholar]
- 50. Palmisano P, Facchin D, Ziacchi M, Nigro G, Nicosia A, Bongiorni MGet al. Rate and nature of complications with leadless transcatheter pacemakers compared with transvenous pacemakers: results from an Italian multicentre large population analysis. Europace 2023;25:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PAet al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012;9:728–35. [DOI] [PubMed] [Google Scholar]
- 52. Swerdlow CD, Kalahasty G, Ellenbogen KA. Implantable cardiac defibrillator lead failure and management. J Am Coll Cardiol 2016;67:1358–68. [DOI] [PubMed] [Google Scholar]
- 53. Han HC, Hawkins NM, Pearman CM, Birnie DH, Krahn AD. Epidemiology of cardiac implantable electronic device infections: incidence and risk factors. Europace 2021;23:iv3–iv10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blomstrom-Lundqvist C, Ostrowska B. Prevention of cardiac implantable electronic device infections: guidelines and conventional prophylaxis. Europace 2021;23:iv11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boriani G, Vitolo M, Wright DJ, Biffi M, Brown B, Tarakji KGet al. Infections associated with cardiac electronic implantable devices: economic perspectives and impact of the TYRX™ antibacterial envelope. Europace 2021;23:iv33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss Eet al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 57. Chaudhry U, Borgquist R, Smith JG, Mörtsell D. Efficacy of the antibacterial envelope to prevent cardiac implantable electronic device infection in a high-risk population. Europace 2022;24:1973–80. [DOI] [PubMed] [Google Scholar]
- 58. Masiero S, Connolly SJ, Birnie D, Neuzner J, Hohnloser SH, Vinolas Xet al. Wound haematoma following defibrillator implantation: incidence and predictors in the Shockless Implant Evaluation (SIMPLE) trial. Europace 2017;19:1002–6. [DOI] [PubMed] [Google Scholar]
- 59. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang Set al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 60. El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JPet al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–7. [DOI] [PubMed] [Google Scholar]
- 61. Piccini JP, El-Chami M, Wherry K, Crossley GH, Kowal RC, Stromberg Ket al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs transvenous single-chamber ventricular pacemaker. JAMA Cardiol 2021;6:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boveda S, Higuera L, Longacre C, Wolff C, Wherry K, Stromberg Ket al. Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups. Europace 2023;25:1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reddy VY, Exner DV, Doshi R, Tomassoni G, Bunch TJ, Estes NAMet al. Primary results on safety and efficacy from the LEADLESS II-phase 2 worldwide clinical trial. JACC Clin Electrophysiol 2022;8:115–7. [DOI] [PubMed] [Google Scholar]
- 64. Knops RE, Reddy VY, Ip JE, Doshi R, Exner DV, Defaye Pet al. A dual-chamber leadless pacemaker. N Engl J Med 2023;388:2360–70. [DOI] [PubMed] [Google Scholar]
- 65. Piccini JP, Cunnane R, Steffel J, El-Chami MF, Reynolds D, Roberts PRet al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: experience with the Micra transcatheter pacemaker. Europace 2022;24:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sidhu BS, Sieniewicz B, Gould J, Elliott MK, Mehta VS, Betts TRet al. Leadless left ventricular endocardial pacing for CRT upgrades in previously failed and high-risk patients in comparison with coronary sinus CRT upgrades. Europace 2021;23:1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carabelli A, Jabeur M, Jacon P, Rinaldi CA, Leclercq C, Rovaris Get al. European Experience with a first totally leadless cardiac resynchronization therapy pacemaker system. Europace 2021;23:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boersma LV, El-Chami M, Steinwender C, Lambiase P, Murgatroyd F, Mela Tet al. Practical considerations, indications, and future perspectives for leadless and extravascular cardiac implantable electronic devices: a position paper by EHRA/HRS/LAHRS/APHRS. Europace 2022;24:1691–708. [DOI] [PubMed] [Google Scholar]
- 69. Olsen T, Jørgensen OD, Nielsen JC, Thøgersen AM, Philbert BT, Johansen JB. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018). Eur Heart J 2019;40:1862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ahmed FZ, Blomström-Lundqvist C, Bloom H, Cooper C, Ellis C, Goette Aet al. Use of healthcare claims to validate the Prevention of Arrhythmia Device Infection Trial cardiac implantable electronic device infection risk score. Europace 2021;23:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MGet al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020;41:2012–32. [DOI] [PubMed] [Google Scholar]
- 72. Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy Let al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace 2018;20:1217. [DOI] [PubMed] [Google Scholar]
- 73. Bongiorni MG, Kennergren C, Butter C, Deharo JC, Kutarski A, Rinaldi CAet al. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur Heart J 2017;38:2995–3005. [DOI] [PubMed] [Google Scholar]
- 74. Starck CT, Gonzalez E, Al-Razzo O, Mazzone P, Delnoy PP, Breitenstein Aet al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: a multicentre retrospective study on advanced mechanical lead extraction techniques. Europace 2020;22:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zsigmond EJ, Saghy L, Benak A, Miklos M, Makai A, Hegedus Zet al. A head-to-head comparison of laser vs. powered mechanical sheaths as first choice and second line extraction tools. Europace 2023;25:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ravaux JM, Di Mauro M, Vernooy K, Kats S, Mariani S, Ronco Det al. Permanent pacemaker implantation following transcatheter aortic valve implantation using self-expandable, balloon-expandable, or mechanically expandable devices: a network meta-analysis. Europace 2021;23:1998–2009. [DOI] [PubMed] [Google Scholar]
- 77. Schwerg M, Baldenhofer G, Dreger H, Bondke H, Stangl K, Laule Met al. Complete atrioventricular block after TAVI: when is pacemaker implantation safe? Pacing Clin Electrophysiol 2013;36:898–903. [DOI] [PubMed] [Google Scholar]
- 78. Urena M, Webb JG, Tamburino C, Munoz-Garcia AJ, Cheema A, Dager AEet al. Permanent pacemaker implantation after transcatheter aortic valve implantation: impact on late clinical outcomes and left ventricular function. Circulation 2014;129:1233–43. [DOI] [PubMed] [Google Scholar]
- 79. Natarajan MK, Sheth TN, Wijeysundera HC, Chavarria J, Rodes-Cabau J, Velianou JLet al. Remote ECG monitoring to reduce complications following transcatheter aortic valve implantations: the Redirect TAVI study. Europace 2022;24:1475–83. [DOI] [PubMed] [Google Scholar]
- 80. Schoechlin S, Jalil F, Blum T, Ruile P, Hein M, Nührenberg TGet al. Need for pacemaker implantation in patients with normal QRS duration immediately after transcatheter aortic valve implantation. Europace 2019;21:1851–6. [DOI] [PubMed] [Google Scholar]
- 81. Nijenhuis VJ, Van Dijk VF, Chaldoupi SM, Balt JC, Ten Berg JM. Severe conduction defects requiring permanent pacemaker implantation in patients with a new-onset left bundle branch block after transcatheter aortic valve implantation. Europace 2017;19:1015–21. [DOI] [PubMed] [Google Scholar]
- 82. Badertscher P, Knecht S, Zeljković I, Sticherling C, de Asmundis C, Conte Get al. Management of conduction disorders after transcatheter aortic valve implantation: results of the EHRA survey. Europace 2022;24:1179–85. [DOI] [PubMed] [Google Scholar]
- 83. Zito A, Princi G, Lombardi M, D’Amario D, Vergallo R, Aurigemma Cet al. Long-term clinical impact of permanent pacemaker implantation in patients undergoing transcatheter aortic valve implantation: a systematic review and meta-analysis. Europace 2022;24:1127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Muntané-Carol G, Urena M, Nombela-Franco L, Amat-Santos I, Kleiman N, Munoz-Garcia Aet al. Arrhythmic burden in patients with new-onset persistent left bundle branch block after transcatheter aortic valve replacement: 2-year results of the MARE study. Europace 2020;23:254–63. [DOI] [PubMed] [Google Scholar]
- 85. Muntané-Carol G, del Val D, Junquera L, Faroux L, Delarochellière R, Paradis JMet al. Timing and evolution of advanced conduction disturbances in patients with right bundle branch block undergoing transcatheter aortic valve replacement. Europace 2020;22:1537–46. [DOI] [PubMed] [Google Scholar]
- 86. Waddingham PH, Behar JM, Roberts N, Dhillon G, Graham AJ, Hunter RJet al. Post-operative cardiac implantable electronic devices in patients undergoing cardiac surgery: a contemporary experience. Europace 2021;23:104–12. [DOI] [PubMed] [Google Scholar]
- 87. Wiggins NB, Chong DT, Houghtaling PL, Hussein AA, Saliba W, Sabik JFet al. Incidence, indications, risk factors, and survival of patients undergoing cardiac implantable electronic device implantation after open heart surgery. Europace 2017;19:1335–42. [DOI] [PubMed] [Google Scholar]
- 88. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OAet al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 89. Hill TE, Kiehl EL, Shrestha NK, Gordon SM, Pettersson GB, Mohan Cet al. Predictors of permanent pacemaker requirement after cardiac surgery for infective endocarditis. Eur Heart J Acute Cardiovasc Care 2021;10:329–34. [DOI] [PubMed] [Google Scholar]
- 90. Noheria A, van Zyl M, Scott LR, Srivathsan K, Madhavan M, Asirvatham SJet al. Single-site ventricular pacing via the coronary sinus in patients with tricuspid valve disease. Europace 2018;20:636–42. [DOI] [PubMed] [Google Scholar]
- 91. Kappenberger L, Linde C, Daubert C, McKenna W, Meisel E, Sadoul Net al. Pacing in hypertrophic obstructive cardiomyopathy. A randomized crossover study. PIC Study Group. Eur Heart J 1997;18:1249–56. [DOI] [PubMed] [Google Scholar]
- 92. Joosten IBT, Janssen CEW, Horlings CGC, den Uijl D, Evertz R, van Engelen BGMet al. An evaluation of 24h Holter monitoring in patients with myotonic dystrophy type 1. Europace 2023;25:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Joosten IBT, van Lohuizen R, den Uijl DW, Evertz R, de Greef BTA, van Engelen BGMet al. Electrocardiographic predictors of infrahissian conduction disturbances in myotonic dystrophy type 1. Europace 2021;23:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rootwelt-Norberg C, Skjølsvik ET, Chivulescu M, Bogsrud MP, Ribe MP, Aabel EWet al. Disease progression rate is a strong predictor of ventricular arrhythmias in patients with cardiac laminopathies: a primary prevention cohort study. Europace 2023;25:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Boriani G, Biagini E, Ziacchi M, Malavasi VL, Vitolo M, Talarico Met al. Cardiolaminopathies from bench to bedside: challenges in clinical decision-making with focus on arrhythmia-related outcomes. Nucleus 2018;9:442–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Auricchio A, Demarchi A, Özkartal T, Campanale D, Caputo ML, di Valentino Met al. Role of genetic testing in young patients with idiopathic atrioventricular conduction disease. Europace 2023;25:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Roguin A, Schwitter J, Vahlhaus C, Lombardi M, Brugada J, Vardas Pet al. Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices. Europace 2008;10:336–46. [DOI] [PubMed] [Google Scholar]
- 98. Munawar DA, Chan JEZ, Emami M, Kadhim K, Khokhar K, O’Shea Cet al. Magnetic resonance imaging in non-conditional pacemakers and implantable cardioverter-defibrillators: a systematic review and meta-analysis. Europace 2020;22:288–98. [DOI] [PubMed] [Google Scholar]
- 99. Fluschnik N, Tahir E, Erley J, Müllerleile K, Metzner A, Wenzel JPet al. 3 Tesla magnetic resonance imaging in patients with cardiac implantable electronic devices: a single centre experience. Europace 2023;25:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Seewöster T, Löbe S, Hilbert S, Bollmann A, Sommer P, Lindemann Fet al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: best practice and real-world experience. Europace 2019;21:1220–8. [DOI] [PubMed] [Google Scholar]
- 101. Stuhlinger M, Burri H, Vernooy K, Garcia R, Lenarczyk R, Sultan Aet al. EHRA Consensus on prevention and management of interference due to medical procedures in patients with cardiac implantable electronic devices. Europace 2022;24:1512–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Horwood L, Attili A, Luba F, Ibrahim EH, Parmar H, Stojanovska Jet al. Magnetic resonance imaging in patients with cardiac implanted electronic devices: focus on contraindications to magnetic resonance imaging protocols. Europace 2017;19:812–7. [DOI] [PubMed] [Google Scholar]
- 103. Zaremba T, Jakobsen AR, Sogaard M, Thogersen AM, Riahi S. Radiotherapy in patients with pacemakers and implantable cardioverter defibrillators: a literature review. Europace 2016;18:479–91. [DOI] [PubMed] [Google Scholar]
- 104. Malavasi VL, Imberti JF, Tosetti A, Romiti GF, Vitolo M, Zecchin Met al. A systematic review and meta-analysis on oncological radiotherapy in patients with a cardiac implantable electronic device: prevalence and predictors of device malfunction in 3121 patients. Eur J Clin Invest 2023;53:e13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Azraai M, D’Souza D, Lin Y-H, Nadurata V. Current clinical practice in patients with cardiac implantable electronic devices undergoing radiotherapy: a literature review. Europace 2021;24:362–74. [DOI] [PubMed] [Google Scholar]
- 106. Lenarczyk R, Potpara TS, Haugaa KH, Deharo JC, Hernandez-Madrid A, Del Carmen Exposito Pineda Met al. Approach to cardio-oncologic patients with special focus on patients with cardiac implantable electronic devices planned for radiotherapy: results of the European Heart Rhythm Association survey. Europace 2017;19:1579–84. [DOI] [PubMed] [Google Scholar]
- 107. Jastrzebski M, Kielbasa G, Moskal P, Bednarek A, Rajzer M, Curila Ket al. Right bundle branch pacing: criteria, characteristics, and outcomes. Heart Rhythm 2023;20:492–500. [DOI] [PubMed] [Google Scholar]
- 108. Keene D, Anselme F, Burri H, Perez OC, Curila K, Derndorfer Met al. Conduction system pacing, a European survey: insights from clinical practice. Europace 2023;25:euad019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jastrzebski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LMet al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm 2022;19:13–21. [DOI] [PubMed] [Google Scholar]
- 110. Furman S, Raddi WJ, Escher DJ, Denize A, Schwedel JB, Hurwitt ES. Rechargeable pacemaker for direct myocardial implantation. Arch Surg 1965;91:796–800. [DOI] [PubMed] [Google Scholar]
- 111. Ryu H, Park HM, Kim MK, Kim B, Myoung HS, Kim TYet al. Self-rechargeable cardiac pacemaker system with triboelectric nanogenerators. Nat Commun 2021;12:4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ploux S, Strik M, Demoniere F, Rakotoarimanana D, Zemmoura A, Deplagne Aet al. Remote interrogation and reprogramming of cardiac implantable electronic devices using a custom multivendor solution. Heart Rhythm 2023;20:547–51. [DOI] [PubMed] [Google Scholar]
- 113. Truong VT, Beyerbach D, Mazur W, Wigle M, Bateman E, Pallerla Aet al. Machine learning method for predicting pacemaker implantation following transcatheter aortic valve replacement. Pacing Clin Electrophysiol 2021;44:334–40. [DOI] [PubMed] [Google Scholar]
- 114. Leggio M, Lombardi M, Caldarone E, D'Emidio S, Severi P, Armeni Met al. Pacemaker-detected severe sleep apnoea predicts new-onset atrial fibrillation. Europace 2018;20:2046–7. [DOI] [PubMed] [Google Scholar]