Abstract
Approximately half of Medicare beneficiaries are enrolled in Medicare Advantage (MA), a private plan alternative to traditional Medicare (TM). Yet little is known about diagnosed dementia rates among MA enrollees, limiting population estimates. All (100%) claims of Medicare beneficiaries using encounter data for MA and claims for TM for the years 2015 to 2018 were used to quantify diagnosed dementia prevalence and incidence rates in MA, compare rates to TM, and provide estimates for the entire Medicare population and for different racial/ethnic populations. In 2017, dementia incidence and prevalence among MA beneficiaries were 2.54% (95% confidence interval [CI]: 2.53 to 2.55) and 7.04% (95% CI: 7.03 to 7.06). Comparison to TM adjusted for sociodemographic and health differences among beneficiaries in MA and TM; the prevalence of diagnosed dementia among beneficiaries in MA was lower (7.1%; 95% CI: 7.12 to 7.13) than in TM (8.7%; 95% CI: 8.71 to 8.72). The diagnosed dementia incidence rate was also lower in MA (2.50%; 95% CI: 2.50 to 2.50) compared to TM (2.99%; 95% CI: 2.99 to 2.99). There were lower rates in MA compared to TM for men and women and White, Black, Hispanic, Asian, American Indian/Alaska Native persons. Diagnosed dementia prevalence and incidence for the entire Medicare population was 7.9% (95% CI: 7.91 to 7.93) and 2.8% (95% CI: 2.77 to 2.78). Lower diagnosed dementia rates in MA compared to TM may exacerbate racial/ethnic disparities in diagnosed dementia. Rates tracked over time will provide understanding of the impact on dementia diagnosis of 2020 MA risk adjustment for dementia.
Keywords: Alzheimer's disease, dementia, diagnosis, Medicare, Medicare Advantage
1. INTRODUCTION
For persons with dementia, receiving a diagnosis supports access to pharmaceutical and nonpharmaceutical interventions to better manage symptoms and maintain quality of life. 1 , 2 Diagnosis helps families plan for financial and caregiving needs. 3 Moreover, given the large social and economic costs associated with dementia, population estimates of diagnosed dementia help plan for healthcare needs and expenditures for the entire population. 3 , 4 As the primary purchaser and regulator of healthcare for older adults in the United States, Medicare policies can directly impact dementia diagnosis rates.
Eligible persons can receive Medicare benefits from traditional Medicare (TM) or through Medicare Advantage (MA) plans. In 2022 approximately half of all Medicare beneficiaries and larger percentages of racial and ethnic minorities were enrolled in MA plans. 5 Unlike TM, MA plans receive capitation payments per beneficiary per month from the federal government, which creates incentives for these private plans to restrict the use of healthcare services while encouraging the use of preventive services. Many papers have documented the differences in quality and healthcare use between MA and TM beneficiaries. 6 For example, MA beneficiaries are more likely to report having had an annual wellness visit and structured cognitive assessment than TM beneficiaries. 7 Moreover, MA plans have higher incentives compared to TM to document enrollee diagnoses as Centers for Medicare and Medicaid Services (CMS) risk‐adjusts payments to MA plans based on plan enrollee diagnoses. While dementia diagnosis was not used for risk adjustment during our sample period, it was reintroduced in 2020, with likely impacts on the diagnosis of dementia. 8 Relatively little is known about dementia diagnosis rates for MA beneficiaries and how these compare with TM beneficiaries. Moreover, there is limited information on diagnosed dementia prevalence and incidence for all individuals above the age of 65 and particularly for racial/ethnic minority persons.
Medicare beneficiaries’ healthcare claims data are a valuable resource for understanding the diagnosis of dementia in the older US population and the health care received by those diagnosed with dementia. Large samples facilitate precise estimates for the entire population, as well as for different groups of racial and ethnic minorities. We employ validated algorithms to enable accurate identification of dementia diagnoses in claims data. 9 While researchers have used claims for TM beneficiaries to examine dementia diagnoses, data for all MA beneficiaries first became available to the researchers in 2019 with the CMS's release of MA encounter data for year 2015. However, the encounter data, which include diagnosis and healthcare use records for MA beneficiaries are collected differently than conceptually equivalent claims for TM beneficiaries. Unlike TM, Medicare Advantage Organizations (MAOs), not the providers, submit claims to the CMS.
As more than half of the Medicare population is projected to be enrolled in MA plans in the near future, it is crucial to estimate diagnosed dementia rates in this population. 5 In particular, it is important to have accurate estimates for racial and ethnic minorities as larger percentages enroll in MA. Moreover, differences in diagnosis rates may suggest differences in care provision that may point to opportunities to improve dementia care and advance health equity. In this study we used MA encounter data through 2018 (latest available during study period) to quantify dementia diagnosis rates for the MA population. We analyzed differences in the prevalence and incidence of dementia diagnoses by insurance type adjusting for sociodemographic and health differences in the beneficiary populations in MA and TM. Additionally, given the limited analyses of the newly released MA encounter data, we assess whether differences are attributable to how data are provided to CMS for the encounter data files. Finally, we pooled data sources to quantify diagnosed dementia prevalence and incidence for the entire population of Medicare beneficiaries and separately for men and women and different racial/ethnic populations.
RESEARCH IN CONTEXT
Systematic Review: The Medicare Advantage (MA) population is growing rapidly. Yet little is known about diagnosed dementia rates among MA enrollees, limiting population estimates.
Interpretation: Diagnosed dementia prevalence and incidence were lower in MA compared to traditional Medicare (TM) after adjustments for sociodemographic and health differences among beneficiaries in MA and TM. Differences between MA and TM in diagnosed dementia was highest for Black persons among all racial/ethnic groups. Differences in care may explain lower adjusted diagnosed dementia rates in MA compared to TM and may exacerbate racial/ethnic disparities given that a disproportionate number of racial and ethnic minorities enroll in MA.
Future Directions: A critical next step is to investigate how payment systems to MA plans affect private plan incentives to provide services that may lead to the detection and diagnosis of dementia.
2. METHODS
2.1. Data
This retrospective study used claims for 100% of Medicare beneficiaries enrolled in TM and encounter data for 100% of Medicare beneficiaries enrolled in MA plans from 2015 to 2018 (latest year of encounter data available at time of study). These data include all claims from Part A (hospital stay), Part B (outpatient), Part C (encounter), and Part D (prescription drugs). While TM data have been used extensively, encounter files containing claims for MA beneficiaries were first made available to researchers by CMS in 2019. Details about encounter data are available at CMS. 10 Briefly, encounter data include two types of records: (1) an encounter data record and (2) chart review records. An encounter data record indicates that a service was provided to MA plan enrollees. However, there can be multiple records associated with the same service. Chart review records allow MAOs to add and delete diagnoses that are different from the initially submitted encounter data records. Evidence indicates that MAOs primarily use chart reviews to add more diagnoses to initial encounter records, and a greater use of chart reviews is associated with higher payments to the plans. 11 , 12 We also found that for dementia diagnoses, approximately 20% of the claims were from chart review records (Appendix Table A1). As the MA encounter data files are relatively new to the researchers, we also used Optum's de‐identified Clinformatics Data Mart (CDM) database for the years 2015 to 2018 to assess the quality of dementia diagnosis found in MA encounter data. CDM data include all claims for patients who are provided health insurance by a large insurance company and include procedure and diagnosis codes and dates of service. The CDM collects data directly from providers within the insurance company. On the other hand, encounter data go through additional steps for inclusion in MA encounter files. Providers first submit claims to the MAOs for payments based on their internal systems. The MAOs then submit these data to CMS for risk adjustment. CMS finally performs additional checks on the data for completion; any errors or problems can cause CMS to reject submitted claims. These additional steps in the submission process can potentially affect the quality of MA encounter data. Therefore, we use claims for MA enrollees from the same insurer in the CDM and in the MA encounter data to validate the quality of the MA encounter files. We compare rates of diagnosed dementia from the CDM data to the MA encounter data from the same large insurance company (Appendix Table A2 and Appendix Figure A1 and Figure A2).
FIGURE A1.
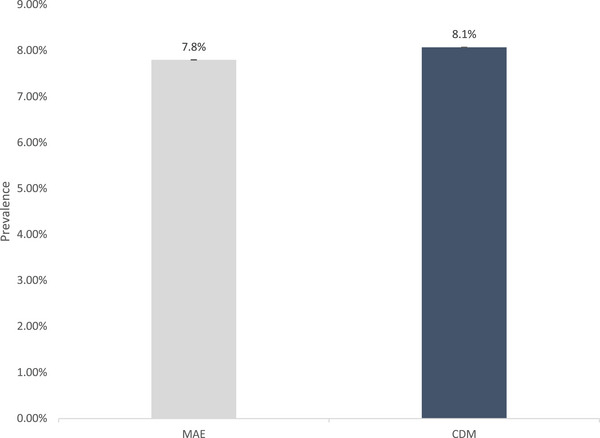
Dementia Prevalence in MA Beneficiaries from Encounter and CDM data. CDM are Medicare Advantage beneficiaries in the CDM data. MAE are estimates in the MA Encounter data from the same large insurance company represented in the CDM data for year 2016.
FIGURE A2.
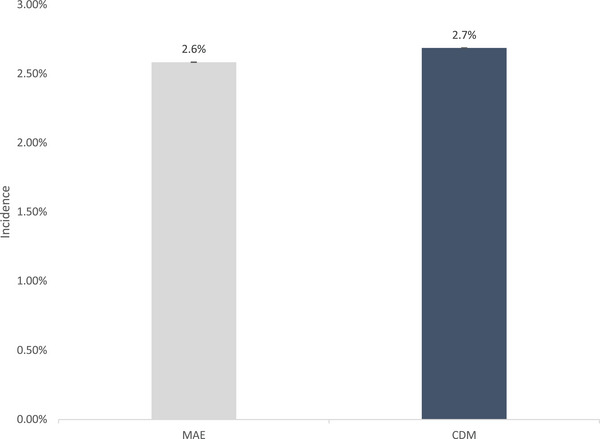
Dementia Incidence in MA Beneficiaries from Encounter and CDM data. CDM are Medicare Advantage beneficiaries in the CDM data. MAE are estimates in the MA Encounter data from the same large insurance company represented in the CDM data for year 2016.
2.2. Study population
We selected beneficiaries aged 65 or older with Part D who were continuously enrolled in TM or MA for at least 2 years. The pooled sample for 2016 to 2017 included 32,032,275 (53% of total) beneficiaries in TM and 28,689,510 (47% of total) beneficiaries in MA.
Dementia is defined using the following codes from the International Classification of Diseases, Ninth and Tenth Revisions: 331.0, 331.11, 331.19, 331.2, 331.7, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 294.0, 294.10, 294.11, 294.20, 294.21, 294.8, 797, 331.82, F01.50, F01.51, F02.80, F02.81, F03.90, F03.91, F05, G13.8, G30.0, G30.1, G30.8, G30.9, G31.01, G31.09, G31.1,G31.2, G31.83, G94, and R41.81. Diagnosis codes are identified in the inpatient, outpatient, home health care, skilled nursing facility, and carrier settings. We also included Part D claims for treatment of dementia symptoms with donepezil, galantamine, rivastigmine, or memantine. Dementia symptoms such as amnesia, aphasia, mild cognitive impairment, and apraxia and agnosia were identified with the following ICD‐9 and ICD‐10 codes: 780.93, 784.3, 331.83, 784.69, R41.1, R41.2, R41.3, R47.01, R48.1, R48.2, R48.8, and G318.4. Claims from Part D and codes for dementia symptoms are used only in combination with dementia diagnosis codes and at a different point in time for verification based on a published algorithm and described below. 9 While the Chronic Conditions Warehouse (CCW) algorithm only uses dementia codes, our algorithm uses claims for drugs prescribed for dementia symptoms and codes for dementia symptoms along with dementia diagnosis codes to identify dementia. Moreover, the CCW algorithm only looks for a second diagnosis claim in the hospital outpatient or carrier setting, while our algorithm verifies a diagnosis in any setting with a second diagnosis, a dementia symptom, a dementia drug claim, or death. Prior work found this algorithm improved the detection of dementia using claims data, particularly among minority populations. 9
2.3. Outcomes
The main outcomes of interest were the percentage of the population with a dementia diagnosis in 2016 and 2017 in MA and TM and the percentage of the population with incident dementia diagnosis in 2016 and 2017 in MA and TM. We additionally estimated overall dementia prevalence and incidence for the entire Medicare population. A technical document (see supplemental materials) provides documentation and code for replicability and research use. The package allows a researcher to replicate the algorithm generating these outcomes on any claims data or electronic health records with information on diagnoses, diagnosis date, and death date by patient. This version of the package was written and tested on SAS Enterprise Guide 7.1 and should run with any compatible version of SAS. It outputs yearly datasets with variables for the dates of a verified dementia in that year and the first valid dates of dementia across all years.
2.3.1. Dementia prevalence
We used a second code within 1 year of the first diagnosis to verify the diagnosis. We required one dementia diagnosis code and a second dementia diagnosis code or one dementia symptom code or drug code within 1 year or death within 1 year after diagnosis. To measure dementia prevalence, we calculated the percentage of the total population with a verified dementia diagnosis in that year. We calculated these separately by insurance type and by gender and race/ethnicity. Our analysis sample consisted of beneficiaries who were continuously enrolled in either TM or MA at least throughout the verification period. For example, to get estimates for diagnosed dementia prevalence in TM in the year 2017, we required that the beneficiaries were continuously enrolled in TM in 2017 and 2018 allowing for beneficiary death.
2.3.2. Dementia incidence
To measure dementia incidence, we required that the at‐risk population of beneficiaries had no verified dementia diagnosis 1 year prior to the year of interest. We required verification of the incident dementia diagnosis as described in the preceding paragraph. We calculated these separately by insurance type and by gender and race/ethnicity. Our analysis sample consisted of beneficiaries in TM or MA who were continuously enrolled in either TM or MA in at least the year of interest, the year before, and the year after allowing for patient death. For example, to calculate dementia incidence in 2017 for MA, our analysis sample consisted of MA beneficiaries that were continuously enrolled in MA in the year 2016, 2017, and 2018.
2.4. Statistical analysis
We report diagnosed dementia prevalence and incidence in MA and TM for the years 2016 and 2017. We estimated separate models for each year to examine dementia incidence and prevalence by insurance type, adjusting for the sociodemographic and health characteristics of the beneficiaries. For beneficiaries in TM and MA, we estimated models controlling for race, 5‐year age groups (< 70, 70 to 74, 75 to 79, 80+), sex, Charlson Comorbidity Index (CCI), dual‐eligibility status, and Part D premium low‐income subsidy (LIS) status. We generated a prediction for each unique combination of patient characteristics. To facilitate comparisons between diagnosis rates in TM and MA that are not driven by the observable differences in TM and MA beneficiaries, we weighted MA estimates to match the underlying characteristics of the TM sample in that year. Unadjusted dementia prevalence and incidence rates by insurance type are included in Table 1. To calculate dementia prevalence and incidence by sex and race/ethnicity, we weighted each subgroup in MA to match the underlying characteristics of that subgroup in TM in that year. To calculate dementia prevalence and incidence estimates for the full population, we weight the unadjusted MA estimates by the proportion of the population in MA plans and the unadjusted TM estimates by the proportion of the population in TM. Subpopulation analyses are age adjusted. That is, for rates of females compared to males, we adjusted the age distribution of females to match the age distribution of males. For rates within racial/ethnic subgroups, we adjusted the age distribution within each subgroup to match the age distribution of the White population.
TABLE 1.
Characteristics of beneficiaries diagnosed with ADRD in traditional Medicare (TM) and Medicare Advantage (MA).
| 2016 | 2017 | |||
|---|---|---|---|---|
| TM | MA | TM | MA | |
| N | 1,436,180 | 951,649 | 1,409,848 | 1,053,203 |
| Sex | ||||
| Female | 67.68 | 64.71 | 67.25 | 64.47 |
| Age | ||||
| Mean Age | 82.9 | 82.1 | 82.8 | 82.1 |
| < 70 | 5.83 | 5.72 | 5.81 | 5.56 |
| 70 to 74 | 11.31 | 12.61 | 11.53 | 12.67 |
| 75 to 79 | 16.72 | 18.60 | 17.07 | 18.94 |
| 80+ | 66.14 | 63.07 | 65.58 | 62.83 |
| Race | ||||
| White | 79.95 | 67.91 | 79.78 | 67.91 |
| Black | 9.60 | 11.60 | 9.32 | 11.78 |
| Hispanic | 6.35 | 16.23 | 6.44 | 15.90 |
| Asian | 2.78 | 3.16 | 2.99 | 3.23 |
| AIAN | 0.37 | 0.15 | 0.38 | 0.16 |
| Missing/Other | 0.96 | 0.95 | 1.09 | 1.02 |
| Other | ||||
| Dual | 34.48 | 23.58 | 33.98 | 23.49 |
| LIS | 2.35 | 3.19 | 2.30 | 3.20 |
| CCI | 4.22 | 4.42 | 4.30 | 4.53 |
| Unadjusted rates | ||||
| Incidence (%) | 3.12 | 2.54 | 2.99 | 2.54 |
| Prevalence (%) | 9.03 | 6.93 | 8.74 | 7.04 |
Note. This table presents the characteristics of TM and MA beneficiaries with a verified dementia claim in 2016 and 2017. Sample is 100% of TM beneficiaries and 100% MA beneficiaries. These beneficiaries were continuously enrolled in year t and year t+1. Dementia in year t is verified with dementia diagnosis code or dementia symptom code or drug code or death in year t+1.
3. RESULTS
3.1. Sample characteristics
Among 2,463,051 Medicare beneficiaries with a verified dementia diagnosis in 2017 (Table 1), 1,053,203 (42.8%) were enrolled in MA and 1,409,848 (57.2%) were in TM (Table 1). Around 65% beneficiaries in MA and 67% beneficiaries in TM are female. Beneficiaries above the age of 80 accounted for around 63% of the MA population and 66% of the TM population. Of the beneficiaries with a dementia diagnosis, 67.9% were White, 11.8% were Black, and 15.9% were Hispanic in MA compared to 79.8% White, 9.3% Black, and 6.4% Hispanic in TM. Of the beneficiaries in MA, 23% were eligible for both Medicare and Medicaid compared to 34% of TM beneficiaries. Around 3% of MA beneficiaries and 2% of TM beneficiaries were eligible for Part D premium LIS. The CCI was 4.53 for MA beneficiaries and 4.30 for TM beneficiaries. Unadjusted dementia diagnosis prevalence and incidence in MA was 7.04% and 2.54% compared to 8.74% and 2.99% in TM.
3.2. Diagnosed dementia prevalence by insurance type
Based on models that adjust for sociodemographic and health characteristics of beneficiaries and weighted to match the underlying characteristics of TM sample in that year, the prevalence of diagnosed dementia in the MA population in 2016 and 2017 was 7.2% (95% CI: 7.21 to 7.22) and 7.1% (95% CI: 7.12 to 7.13), respectively (Figure 1A). Compared to MA, the prevalence of diagnosed dementia was higher in TM in both 2016 (9.0%; 95 CI: 9.03 to 9.04) and 2017 (8.7%; 95 CI: 8.71 to 8.72). Figure 1B shows diagnosed dementia prevalence by sex and race/ethnicity for MA and TM beneficiaries for the year 2017. These estimates are from models that adjust for beneficiary characteristics and weighted to match the subgroup characteristics of the TM subgroup in that year. The prevalence of diagnosed dementia in MA compared to TM was lower in females (8.1% vs 9.1%) and in males (5.8% vs 6.7%). Prevalence of diagnosed dementia was also lower in MA compared to TM across racial/ethnic populations. The rates were lower in MA compared to TM among White (7.0% vs 8.4%), Black (9.0% vs 12.9%), Hispanic (8.8% vs 11.4%), Asian (7.4% vs 8.7%), and American Indian/Alaska Native (8.5% vs 9.7%) persons.
FIGURE 1.
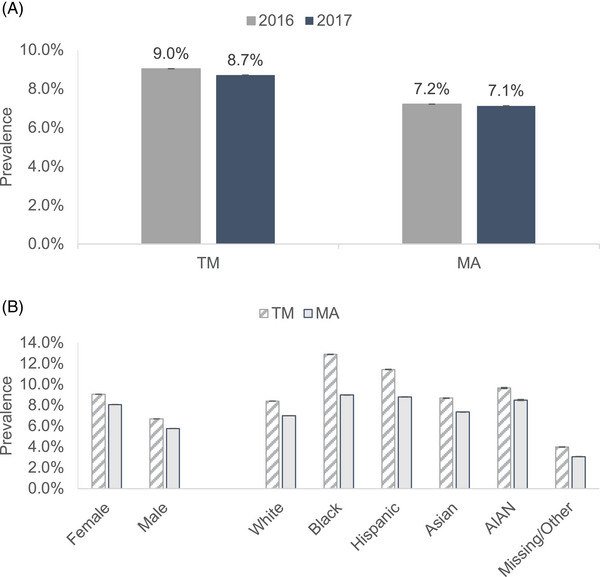
(A) Dementia prevalence in traditional Medicare (TM) and Medicare Advantage (MA) in 2016 and 2017. (B) Dementia prevalence by sex and race/ethnicity in TM and MA in 2017.
3.3. Diagnosed dementia incidence by insurance type
Estimates of diagnosed incident dementia are among beneficiaries without a dementia diagnosis in the previous year and from models that adjust for sociodemographic and health characteristics of beneficiaries and weighted to match the underlying characteristics of TM sample in that year. Among beneficiaries without a dementia diagnosis in the previous year, 2.5% of MA beneficiaries had an incident diagnosis of dementia in 2016 and in 2017 (Figure 2A). Compared to MA, diagnosed dementia incidence rates were higher among TM beneficiaries where 3.1% and 3.0% of TM beneficiaries had an incident dementia diagnosis in 2016 and 2017, respectively. Figure 2B shows diagnosed dementia incidence by sex and race/ethnicity for MA and TM beneficiaries in 2017. Compared to TM, the diagnosed dementia incidence was lower in females in MA (2.7% vs 3.2%) and in males in MA (2.2% vs 2.6%). A similar pattern was observed across race/ethnic groups. Diagnosed dementia incidence rates were lower in MA compared to TM for White (2.4% vs 2.9%), Black (2.9% vs 4.1%), Hispanic (2.7% vs 3.7%), Asian (2.3% vs 3.1%), and American Indian/Alaska Native (3.1% vs 3.7%) persons.
FIGURE 2.
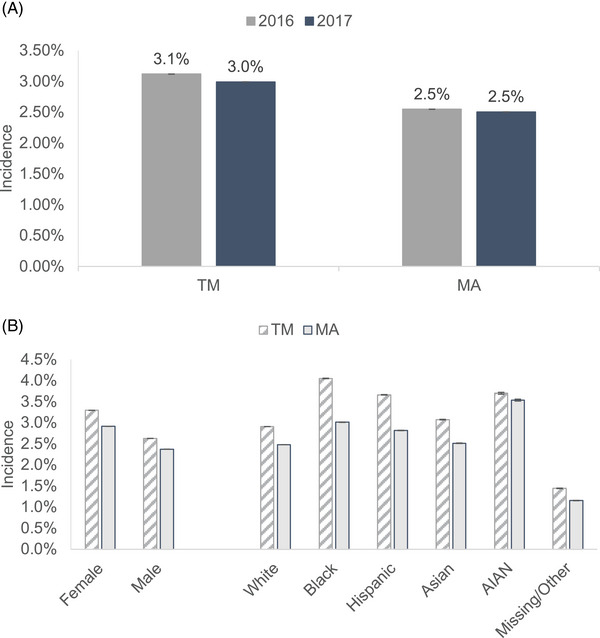
(A) Dementia incidence in traditional Medicare (TM) and Medicare Advantage (MA) in 2016 and 2017. (B) Dementia incidence by sex and race/ethnicity in TM and MA in 2017.
3.4. Diagnosed dementia prevalence and incidence rates for the full population
Table 2 presents overall diagnosed dementia prevalence and incidence rates for the entire Medicare population for the year 2017. Diagnosed dementia prevalence in the entire Medicare population was 7.92% (95% CI: 7.91 to 7.93). Diagnosed dementia prevalence rates were higher in the older population, with rates around 13% for the 80 to 84 age group, 21% for the 85 to 89 age group, and 31% for the 90+ age group. Compared to males, age‐adjusted diagnosed dementia prevalence was higher for females (6.5% vs 7.6%). Age‐adjusted diagnosed dementia prevalence was higher in Black (10.9%), Hispanic (10.0%), and American Indian/Alaska Native (9.67%) persons compared to White persons (7.7%). Compared to White persons (7.7%), diagnosed dementia prevalence was lower among Asian persons (7.2%).
TABLE 2.
Dementia prevalence and incidence rates for all Medicare beneficiaries.
| Prevalence | Incidence | |||
|---|---|---|---|---|
| N | % | N | % | |
| Overall | 2,463,051 | 7.92 (7.91 to 7.93) | 702,829 | 2.77 (2.77 to 2.78) |
| Age group | ||||
| 65 to 69 | 140,504 | 1.84 (1.83 to 1.85) | 40,191 | 0.80 (0.79 to 0.80) |
| 70 to 74 | 296,055 | 3.37 (3.36 to 3.38) | 102,316 | 1.29 (1.28 to 1.29) |
| 75 to 79 | 440,099 | 6.96 (6.94 to 6.98) | 136,942 | 2.42 (2.41 to 2.43) |
| 80 to 84 | 539,178 | 13.07 (13.04 to 13.10) | 152,151 | 4.34 (4.32 to 4.36) |
| 85 to 89 | 545,801 | 21.03 (20.98 to 21.08) | 143,972 | 7.05 (7.02 to 7.09) |
| 90+ | 501,414 | 31.02 (30.95 to 31.09) | 127,257 | 11.14 (11.08 to 11.19) |
| Sex | ||||
| Male (ref) | 835,929 | 6.52 (6.51 to 6.54) | 259,381 | 2.47 (2.46 to 2.48) |
| Female | 1,627,122 | 7.60 (7.59 to 7.61) | 443,448 | 2.65 (2.64 to 2.66) |
| Race | ||||
| White (ref) | 1,839,921 | 7.70 (7.69 to 7.71) | 536,764 | 2.73 (2.72 to 2.73) |
| Black | 255,458 | 10.85 (10.81 to 10.89) | 66,172 | 3.65 (3.62 to 3.67) |
| Hispanic | 258,196 | 9.97 (9.93 to 10.00) | 68,032 | 3.31 (3.29 to 3.34) |
| Asian | 76,222 | 7.19 (7.14 to 7.24) | 21,983 | 2.55 (2.51 to 2.58) |
| American Indian/Alaska Native | 7079 | 9.67 (9.46 to 9.88) | 2195 | 3.74 (3.59 to 3.89) |
| Missing/Other | 26,175 | 7.47 (7.41 to 7.53) | 7683 | 2.59 (2.55 to 2.63) |
Note. This table presents dementia prevalence (Columns 1 and 2) and incidence (Columns 3 and 4) rates for all Medicare beneficiaries for the year 2017. These beneficiaries were continuously enrolled in year t and year t+1. Dementia in year t is verified with dementia diagnosis code or dementia symptom code or drug code or death in year t+1. Rates by sex and race were age‐adjusted to reference group.
Diagnosed dementia incidence rates for the full population are presented in Column 2 of Table 2. Overall, 2.8% of all Medicare beneficiaries had an incident dementia diagnosis in 2017. These rates were higher among the older population with a rate of 7.1% for beneficiaries in the 85 to 89 age group and a rate of 11.1% for beneficiaries above the age of 90. Age‐adjusted diagnosed dementia incidence was higher in females compared to males enrolled in Medicare (2.7% vs 2.5%). Compared to White persons (2.7%), age‐adjusted diagnosed dementia incidence was higher among Black (3.7%), Hispanic (3.3%), and American Indian/Alaska Native (3.7%) persons. Asian persons had lower diagnosed dementia incidence compared to White persons (2.6% vs 2.7%).
4. DISCUSSION
To our knowledge, this is the first study to quantify diagnosed dementia rates among all beneficiaries in MA. A second contribution is the comparison of diagnosed dementia incidence and prevalence in MA and TM adjusted for differences in the sociodemographic and health differences in the beneficiary populations. Additionally, we combined MA encounter data and Medicare claims data to provide new estimates of diagnosed dementia prevalence and incidence for the entire Medicare population and separately for men and women and for different racial/ethnic populations.
The incidence and prevalence of diagnosed dementia were 2.5% and 7.1%, respectively, among beneficiaries in MA. Our estimated dementia prevalence in MA was slightly higher than estimates from studies that estimated prevalence using data from few MA plans whose beneficiaries were not representative of the entire MA population. 13 , 14 Dementia prevalence estimates from these studies ranged between 5.5% and 6.5%.
We found that (adjusted for different patient populations) dementia prevalence in MA was 7.1% and 8.7% in TM in 2017. We also found that dementia incidence was lower in MA compared to TM (2.5% vs 3.1%). Across sex and race/ethnic groups, we also found that dementia prevalence and incidence were lower in MA compared to TM. Differences in diagnosis rates among Black and Hispanic persons in MA compared to TM were particularly striking. While differences in patient populations in MA and TM are well known and have been shown to be associated with differences in care, 15 the differences in diagnosed dementia reported here adjusted for these and may point to how payment systems to MA plans create incentives for private plans to restrict the use of healthcare services and specialist referral services that when rendered may lead to the detection and diagnosis of dementia.
We found that diagnosed dementia prevalence was 7.9% and dementia incidence was 2.8% in the entire Medicare population for the year 2017. Recently published results from a nationally representative sample of older adults from the Health and Retirement Study and using the Harmonized Cognitive Assessment Protocol (HCAP) found that dementia prevalence was 10% in 2016. 16 Diagnosed dementia among older Americans is about two percentage points less than this nationally representative estimate of dementia prevalence, suggesting 20% of the population may be undiagnosed. A strength of this study is quantifying incidence rates of diagnosed dementia in the population and diagnosed incidence and prevalence rates for different racial and ethnic populations, who are not well represented in surveys. Compared to HCAP estimates, our estimates were lower (but within 95% CIs) for Black (10.85%; 95% CI: 10.81 to 10.89 vs. 15%; 95% CI: 10 to 19) and Hispanic (9.97%; 95% CI: 9.93 to 10.0 vs 10%; 95% CI: 7 to 13) persons. No estimates for Asian and American Indian/Alaska Native persons are available based on HCAP. We reported that 7.19% of Asian persons had a dementia diagnosis and 9.67% of American Indian/Alaska Native persons had a dementia diagnosis. Racial and ethnic minorities disproportionately enroll in MA plans relative to TM, which makes quantifying diagnosed dementia in MA increasingly important for understanding population‐level estimates among racial/ethnic minority populations.
This study is also important for tracking diagnosis rates over time. CMS reintroduced Alzheimer's disease and related dementias (ADRD) to MA risk adjustment in 2020. An increase in reimbursement might incentivize plans to better detect and record dementia diagnosis but may also lead to unintended diagnosing practices. The estimates of diagnosed dementia incidence and prevalence in the MA population in this paper and compared to TM provide a benchmark to compare how diagnosis rates evolve after the policy change. Differences in the benefits available in MA and TM are growing and may impact the prevalence of diagnosed dementia through changes in who enrolls in MA and/or switches in or out of an MA plan. The passage of the Creating High‐Quality Results and Outcomes Necessary to Improve Chronic Care (CHRONIC) removed benefit uniformity (i.e., plans can design disease specific benefits) for some long‐term services and supports. Additionally, as of 2020, plans can offer Special Supplemental Benefits for the Chronically Ill (SSBCI), and the benefits do not have to be primarily health‐related if the item or service can reasonably improve or maintain health or function of the enrollee.
This study has limitations. First, our goal was to measure diagnosed dementia prevalence and incidence in the US population and for different racial/ethnic populations. The estimate is not a measure of persons living with dementia as some persons are undiagnosed (about 20% by our comparison with HCAP estimates). Second, controls for observable differences between the beneficiaries in MA and TM does not account for differences in the patient populations that may be unobserved and related to dementia diagnosis. Third, beneficiaries in MA plans may switch to TM, with higher rates of switching for racial and ethnic minority groups. 17 We quantified dementia prevalence and incidence by race and ethnicity for beneficiaries in MA 2017 and did not restrict them to MA in 2018. Although the change in the magnitude of estimates in this sample compared to continuous enrollment was small, we found evidence that switching had a differential impact on estimates by race/ethnicity. For example, incidence estimates for Whites increased by 0.07 percentage points and by 0.15 percentage points for Blacks. Fourth, Medicare encounter data are relatively new compared to TM claims data, and healthcare service use is collected and reported in a different way than in TM data and thus may impact the differences in diagnosis rates we reported. We assessed this issue by comparing MA encounter data with Optum's de‐identified CDM database for the years 2015 to 2018. In our analysis, we compared the dementia diagnosis rates of MA beneficiaries in the CDM data to the dementia diagnosis rates in the MA encounter data from the same large insurance company represented in the CDM data for the year 2016. That is, we used two data sources to compare dementia diagnosis for the same beneficiaries. Summary statistics for the two samples are shown in Appendix Table A2. Diagnosed dementia prevalence is 7.8% based on encounter data and 8.1% based on CDM data (Appendix Figure A1). Diagnosed dementia incidence is 2.6% based on encounter data and 2.7% based on CDM data (Appendix Figure A2). The results suggest that MA encounter data provide reliable estimates of diagnosed dementia among MA beneficiaries.
5. CONCLUSION
The MA population has grown rapidly over the last decade and includes a disproportionate share of older minorities. Yet, until recently, without CMS's release of MA encounter data, there was no national data to quantify diagnosed dementia among MA beneficiaries and for populations of racial/ethnic minorities, in comparison to beneficiaries in TM, and to quantify and track trends overtime for the entire population of older Americans. The lower adjusted diagnosed dementia rates in MA compared to TM may be attributable to differences in care. Rates should be tracked over time to understand the impact on diagnosis of the 2020 risk adjustment for dementia and how expanded coverage for health and non‐health benefits in MA plans impact switching between MA and TM among persons living with dementia.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Consent was not necessary.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors have nothing to report. Funding support was through National Institute on Aging P30AG066589, “Center for Advancing Sociodemographic and Economic Study of Alzheimer's Disease and Related Dementias (CeASES‐ADRD),” National Institute on Aging P30AG043073, “Resource Center for Minority Aging Research,” and National Institute on Aging, R01AG055401, “Effectiveness of therapeutics and health care services in reduction of health disparities in Alzheimer's disease.”
1.
TABLE A1.
Distribution of dementia claims in encounter records.
| All | Carrier | Inpatient | HHA | Outpatient | SNF | |
|---|---|---|---|---|---|---|
| N (% of all total) | 24,618,225 | 75.68% | 5.43% | 5.41% | 10.50% | 2.98% |
| Original claim | 80.06% | 79.07% | 64.54% | 90.71% | 87.19% | 88.83% |
| Chart review | 12.24% | 11.94% | 23.97% | 8.65% | 10.60% | 10.57% |
| Linked | 7.71% | 8.99% | 11.49% | 0.64% | 2.21% | 0.60% |
Note. This table presents the distribution of dementia claims in encounter records. Carrier, Inpatient, home health agency (HHA), outpatient, and skilled nursing facilities (SNFs) are the encounter data files. Original claims are claims in the encounter data. Chart review refers to claims submitted by MAO to add or delete a diagnosis. Linked refers to chart review records that are linked to original claims.
TABLE A2.
Summary statistics for Medicare Advantage beneficiaries using encounter and Clinformatics Data Mart (CDM) data.
| MAE | CDM | |
|---|---|---|
| N | 218,464 | 230,332 |
| Sex | ||
| Female | 65.56 | 64.67 |
| Age | ||
| <70 | 5.61 | 4.74 |
| 70 to 74 | 12.24 | 11.02 |
| 75 to 79 | 17.90 | 16.73 |
| 80+ | 64.25 | 67.51 |
| Race | ||
| White | 71.28 | 67.85 |
| Black | 13.39 | 12.35 |
| Hispanic | 11.44 | 10.10 |
| Asian | 2.82 | 2.60 |
| AIAN | 0.16 | |
| Missing/Other | 0.91 | 7.10 |
| Other | ||
| Dual or LIS | 31.10 | 32.15 |
| CCI | 4.69 | 4.13 |
| Unadjusted rates | ||
| Incidence (%) | 2.76 | 2.24 |
| Prevalence (%) | 7.88 | 7.01 |
Note. This table presents the characteristics of Medicare Advantage beneficiaries in the CDM data and characteristics from MA encounter for MA beneficiaries insured by the same large insurance company represented in the CDM data in 2016. These beneficiaries were continuously enrolled in year t and year t+1. Dementia in year t is verified with a dementia diagnosis code or dementia symptom code or drug code or death in year t+1.
Haye S, Thunell J, Joyce G, et al. Estimates of diagnosed dementia prevalence and incidence among diverse beneficiaries in traditional Medicare and Medicare Advantage. Alzheimer's Dement. 2023;15:e12472. 10.1002/dad2.12472
REFERENCES
- 1. Dubois B, Padovani A, Scheltens P, Rossi A, Dell'Agnello G. Timely diagnosis for Alzheimer's disease: a literature review on benefits and challenges. J Alzheimers Dis. 2015;49(3):617‐631. doi: 10.3233/JAD-150692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson L, Tang E, Taylor JP. Dementia: timely diagnosis and early intervention. BMJ. 2015;350:h3029. doi: 10.1136/bmj.h3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Association A . 2022 Alzheimer's Disease Facts and Figures. [DOI] [PubMed]
- 4. Zissimopoulos J, Crimmins E, StClair P. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2015;18(1):25‐39. doi: 10.1515/fhep-2014-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobson GA, Blumenthal D. Medicare advantage enrollment growth: implications for the US Health Care System. JAMA. 2022;327(24):2393. doi: 10.1001/jama.2022.8288 [DOI] [PubMed] [Google Scholar]
- 6. Park S, White L, Fishman P, Larson EB, Coe NB. Health care utilization, care satisfaction, and health status for medicare advantage and traditional medicare beneficiaries with and without Alzheimer disease and related dementias. JAMA Netw Open. 2020;3(3):e201809. doi: 10.1001/jamanetworkopen.2020.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacobson M, Thunell J, Zissimopoulos J. Cognitive Assessment At Medicare's Annual Wellness Visit In Fee‐For‐Service And Medicare Advantage Plans: study examines the use of Medicare's annual wellness visit and receipt of cognitive assessment among Medicare beneficiaries enrolled in fee‐for‐service Medicare or Medicare Advantage. Health Aff (Millwood). 2020;39(11):1935‐1942. doi: 10.1377/hlthaff.2019.01795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Advance Notice of Methodological Changes for Calendar Year (CY) 2020 for the Medicare Advantage (MA) CMS‐HCC Risk Adjustment Model. December 20, 2018.
- 9. Thunell J, Ferido P, Zissimopoulos J. Measuring Alzheimer's disease and other dementias in diverse populations using medicare claims data. Akushevich I, ed. J Alzheimers Dis. 2019;72(1):29‐33. doi: 10.3233/JAD-190310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chronic Conditions Data Warehouse User Documentation. Accessed December 5, 2022. https://www2.ccwdata.org/web/guest/user‐documentation
- 11. Billions in Estimated Medicare Advantage Payments from Chart Reviews Raise Concerns. Accessed December 5, 2022. https://oig.hhs.gov/oei/reports/oei‐03‐17‐00470.pdf
- 12. Meyers DJ, Trivedi AN. Medicare advantage chart reviews are associated with billions in additional payments for some plans. Med Care. 2021;59(2):96‐100. doi: 10.1097/MLR.0000000000001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer's disease and related dementias in Medicare Advantage plans. Alzheimers Dement Diagn Assess Dis Monit. 2020;12(1):e12048. doi: 10.1002/dad2.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen HQ, Borson S, Khang P, et al. Dementia diagnosis and utilization patterns in a racially diverse population within an integrated health care delivery system. Alzheimers Dement Transl Res Clin Interv. 2022;8(1):e12279. doi: 10.1002/trc2.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curto V, Einav L, Finkelstein A, Levin J, Bhattacharya J. Health care spending and utilization in public and private medicare. Am Econ J Appl Econ. 2019;11(2):302‐332. doi: 10.1257/app.20170295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79(12):1242‐1249. doi: 10.1001/jamaneurol.2022.3543. Published online October 24, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martino SC, Mathews M, Damberg CL, et al. Rates of disenrollment from medicare advantage plans are higher for racial/ethnic minority beneficiaries. Med Care. 2021;59(9):778‐784. doi: 10.1097/MLR.0000000000001574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


