Abstract
Objectives:
Head and neck squamous cell carcinoma (HNSCC) causes severe pain and opioids, the mainstay of pain management, may have immunomodulatory effects. We evaluated the effect of opioids on immunotherapy efficacy in recurrent/metastatic (R/M) HNSCC patients.
Materials and Methods:
In a retrospective study of 66 R/M HNSCC patients from 2015–2020, opioid dosage, calculated as mean morphine milligram equivalent per day, was assessed on the day of anti-PD-1 monoclonal antibody (mAb) treatment and most recent prior visit. Intratumoral T cells were evaluated by single cell RNAseq and immunohistochemistry prior to treatment. Univariable and multivariable Cox proportional hazards and logistic regression models were used to estimate the association between opioid usage, progression-free survival (PFS), overall survival (OS), disease control rate.
Results:
Patients were 79% male, 35% oropharynx, 35% oral cavity, 40% locoregional recurrence, and 56% platinum failure. Higher opioid dosage by continuous variable was significantly associated with lower PFS (p=0.016) and OS (p<0.001). In multivariable analysis, including platinum failure status and PD-L1, higher opioids were associated with lower OS. Opioid usage by categorical variable was associated with significantly lower intratumoral CD8+ T cells. Opioid receptor, OPRM1, expression was identified in intratumoral and circulating T cells.
Conclusions:
In our study cohort of anti-PD-1 mAb treatment in R/M HNSCC patients, higher opioids were associated with significantly lower PFS and OS and lower CD8+ T cells in the tumor microenvironment. To our knowledge, this is the first analysis in R/M HNSCC patients and further research into the clinical and biologic effect of opioids is warranted.
Keywords: head and neck cancer, opioids, immunotherapy, T cells, survival
1.0. Introduction
Head and neck squamous cell carcinoma (HNSCC) represents a heterogeneous group of tumors that most commonly originate in the oral cavity, oropharynx, hypopharynx, and larynx. Traditional risk factors typically include alcohol use and tobacco exposure1; however, approximately 25% of oropharyngeal SCC cases worldwide are attributable to human papilloma virus (HPV) infection, with an estimated 65% in the United States2. Currently, the main treatments for HNSCC include surgery, radiotherapy, chemotherapy, and immunotherapy, alone or in combination1. Despite advances in prevention, diagnosis, and treatment strategies, 5-year survival rate for locally advanced disease has remained 50%, and it has not significantly improved in the past 10 years3. Recurrent and/or metastatic (R/M) HNSCC patients, regardless of HPV status, have poor prognosis with a median overall survival of about a year4. In 2016, immunotherapy with immune checkpoint inhibitors (ICI), pembrolizumab and nivolumab, became standard of care for the treatment of R/M HNSCC patients that failed platinum-based therapy, and then subsequently in 2019, pembrolizumab monotherapy or chemotherapy plus pembrolizumab based on PD-L1 status became standard frontline therapy5. While anti-PD-1 monoclonal antibody (mAb) therapy showed no improvement in progression-free survival, there was significantly better overall survival5, as observed in other solid tumor types6. Still, unfortunately only a minority of patients benefit from anti-PD-1 mAb therapy7.
HNSCC patients experience pain from a variety of sources, related to both the cancer itself as well as associated with diagnostic and therapeutic interventions used to treat the cancer8. The areas of the head and neck are highly vulnerable to pain due to dense innervation of sensory and sympathetic nerves sourcing many anatomical structures in a small region9–11. Tumor site and stage are considered risk factors for cancer pain prior to treatment12. Furthermore, pain related to cancer treatment was the third-most important issue identified by HNSCC patients13, present in about 70% of HNSCC patients after treatment and often persisting for more than 6 months14. Opioid treatment is the mainstay for pain control, being used both for background and breakthrough cancer pain episodes8, 15. There is a higher prevalence and quantity of opioid use in HNSCC patients compared to lung or colon cancer16. With the increase in opioid consumption, it is important to understand the impact of these drugs on treatment strategies, including ICIs.
Opioids have many non-analgesic effects, including direct and indirect effects on anti-tumor immunity. Direct effects on immune cells are thought to manifest via the mu opioid receptor (OPRM1). Activated human T-cells can express OPRM1 RNA as well as the functional OPRM1 protein17; expression levels are modulated by extracellular signaling mediators (e.g., IL-4, IL-6, TNFα, IFNγ)18, 19. Morphine exposure to peripheral blood mononuclear cells from patients with gastric cancer in vitro decreased the percentage of CD8+ T cells and increased CD4+CD25+FoxP3+ regulatory T cells20 suggesting an anti-inflammatory shift in circulating immunity. Indirect effects are thought to manifest via the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis. Prolonged use of opioids increases activity in the HPA axis and SNS, resulting in sustained release of immunosuppressive glucocorticoids and subsequent reduction in T cell proliferation and natural killer (NK) cell cytotoxicity21, 22. Clinically, NK cell infiltration into breast cancer tissue is decreased in women who received more perioperative systemic opioids23. Although opioids affect different aspects of immune function in cancer, their effect on immunotherapy treatment in HNSCC remains unknown.
Evidence from clinical studies and preclinical models suggests that opioids can influence anti-tumor immunity; however, actual data derived from cancer populations are still inconclusive24. Given that only a minority of patients will respond to ICIs, there is an urgent need to improve the anti-tumor immune response and increase the rate of responders to ICI. We hypothesize that immunotherapy efficacy is impaired by immunosuppressive actions of exogenous opioids given for pain management. We performed a retrospective analysis of ICI efficacy, opioid intake, and the tumor immune landscape in R/M HNSCC patients who received anti-PD-1 mAb therapy in the first line or platinum failure setting. Lastly, we leveraged publicly available single cell RNA sequencing data from HNSCC patients and healthy donors to explore the differential expression of OPRM1 on immune cells subpopulations.
2.0. Methods
2.1. Study Design.
This is a retrospective analysis of opioid intake and its effect on the efficacy of treatment with anti-PD-1 mAb monotherapy in R/M HNSCC patients who were treated at the UPMC Hillman Cancer Center between 2015 and 2020 that had consented to the UPMC Hillman tissue banking protocol (HCC 99–069). Clinical data were obtained via chart review, including baseline demographic and clinical characteristics such as age at diagnosis, sex, race, HPV status for oropharyngeal tumors, treatments received, data on response by RECIST 1.1, progression free survival (PFS) and overall survival (OS). Disease control rate (DCR) was defined as complete response (CR), partial response (PR), or stable disease (SD) by RECIST 1.1. In addition, the usage of opioids prior to treatment and on the “day of treatment” were obtained based on the assumption that patients’ prescriptions directly reflect opioid usage. To obtain opioid prescription information, chart review was performed and all opioid prescriptions from the “day of treatment” and the most recent visit prior to “day of treatment” were recorded. The opioids used were acetaminophen-hydrocodone, oxycodone, fentanyl, acetaminophen-oxycodone, tramadol, acetaminophen-codeine, and oxycodone-paracetamol. Morphine Milligram Equivalency (MME) was calculated using a calculator generated by the Center for Disease Control (CDC) to convert different opioid medication doses to an equianalgesic morphine dose. Neutrophil to Lymphocyte ratio (NLR) was calculated based on values on the “day of treatment” with the first dosage of anti-PD-1 mAb therapy.
2.2. Immune profiling in patient tissues.
Thirty six out of 66 patients in the current cohort had previously undergone analysis for intratumor CD8+ T cells (CD8)25 as well as Foxp3+ T cells (Treg). To conduct these analyses, antigen retrieval was done on formalin-fixed paraffin-embedded sections from patient samples. Tissue sections were then stained with Pan cytokeratin eFluor 570 (Life Technologies), CD8 Alexa Fluor 647 (Biolegend), Foxp3 Alexa Fluor 488 (Life Technologies), and DAPI, and mounted with ProLong Diamond Antifade Mountant (Life Technologies). These sections were then imaged with an Olympus IX83 microscope with analysis via ImageJ software. PD-L1 analysis was run on a Thermo Scientific Lab Vision 480 Autostainer using the anti-PD-L1 clone SP142, with positive control run on tonsillar tissue. Tissue sections were scanned by Aperio. Finally, PD-L1 Immunohistochemistry (IHC) scoring was calculated using the combined positive score (CPS).
2.3. Bioinformatic analysis of publicly available RNA sequencing data.
For sourcing and processing of publicly available data, filtered single cell data including gene counts and sample annotations were procured and transformed as previously described26. Further manipulations were conducted in R. Scaling normalization of raw gene counts using the scran package’s computeSumFactors function.27 Top variable genes were ascertained using the scran package’s modelGeneVar and getTopHVGs functions. These genes were then used as the subset of features for principal component analysis using the scran package’s runPCA, and the base values were used to build a UMAP from runUMAP.28 For comparison to the clusters given in the original dataset, new labels were obtained by clustering cells using the nearest neighbor algorithm in scran, buildSNNGraph, and clusters were identified using the igraph function cluster_louvain29. In total, 130,849 cells passed quality control measures and were assessed in the final dataset. Ten different immune cell subtypes were obtained and parsed from the annotated genes, which included CD19+ B cells, CD14+ monocytes, CD16+ myeloid derived suppressor cells, CD8+ cytotoxic T cells, dendritic cells, mast cells, cytotoxic NK cells, plasma dendritic cells, CD4+FOXP3− T conventional cells and CD4+FOXP3+ T regulatory cells. Unsupervised clustering was used to visualize only CD3+ T cell types based on canonical gene expression patterns. A UMAP was generated to visualize distribution of samples by health status. For differential gene expression analysis, pseudo-bulk RNAseq matrices were created from single cell data representing the entire dataset and each annotated cell type. Each attribute was then modeled as a design matrix in DESeq2, and contrasts were used to find the differential expression between conditions in the full matrix and within each cell type classification. In addition, a Wald test was performed between specific groups to target the specific comparisons.
2.4. Statistics.
All statistical analysis was performed using R (version 4.1.2) and RStudio (version 1.1.456) software. For the descriptive analysis, we calculated frequency and percentage for categorical variables; mean (SD) for normally distributed continuous variables and median (IQR) for non-normally distributed continuous variables. Opioid dose was treated as both continuous variables in the univariable analysis. To estimate the association between disease control rate (DCR) and opioid dose, we used univariable and multivariable logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs). To estimate the association between PFS, OS, DCR and opioids as well as other covariates including age, sex, tumor site, platinum failure, type of recurrence, smoking, PD-L1 status and NLR, univariable and multivariable Cox proportional hazard models were fitted. Variables with a P-value of <0.2 in univariable analysis were included in the multivariable model, as well as other clinically significant covariates.
3.0. Results
3.1. Demographics and clinical characteristics
Data was available on 66 recurrent/metastatic HNSCC patients treated with anti-PD-1 mAb therapy. Baseline characteristics are shown in Table 1. The median age was 64 (range 40–81), most patients were male (79%), white (94%), and the most common primary sites were oral cavity (35%) and oropharynx (35%). Seventy percent of the oropharyngeal SCC patients were positive for HPV. The majority of patients had locoregional recurrence only (40%), compared to distant only disease (24%) or both (36%). The most common indication for anti-PD-1 mAb therapy was in the platinum failure setting (56%) with the remainder of patients receiving therapy in the first line R/M setting. For those patients with tissue available for PD-L1 analysis (n=45), 60% had a PD-L1 CPS ≥ 1.
Table 1.
R/M HNSCC Patient Characteristics
| Category | N (%) |
|---|---|
|
| |
| Age | Median 62.5 (IQR 57.3, 70) |
|
| |
| Sex | Female: 14 (21) |
| Male: 52 (79) | |
|
| |
| Race | White: 62 (94) |
| Black: 4 (6) | |
|
| |
| Smoking | Yes: 41 (62) |
| No: 25 (38) | |
|
| |
| Primary Site | Oral Cavity: 23 (35) |
| Oropharynx: 23 (35) | |
| HPV1 (+): 16/23 (70) | |
| Larynx/Hypopharynx: 13(20) | |
| Other: 7 (10) | |
|
| |
| Type of Recurrence | Locoregional only: 26 (40) |
| Distant only: 16 (24) | |
| Locoregional + Distant: 24 (36) | |
|
| |
| Indication for Anti-PD-1 | Platinum Failure: 37 (56%) |
| Frontline: 29 (44%) | |
|
| |
| PD-L1 by CPS2 | Positive: 27 (41) |
| Negative: 18 (27) | |
| Unknown: 21 (32) | |
Human Papilloma virus (HPV) by p16 or HPV in situ hybridization (ISH) for oropharynx primary
PD-L1 expression by combined positive score (CPS). Positive defined as ≥1
3.2. Opioid usage
Sixty three percent of patients (42/66) were prescribed opioids prior to ICI treatment and on the day of ICI treatment. The pretreatment timepoint was defined as the most recent visit prior to the start date of ICI treatment. The median time from pretreatment visit to the day of treatment was 29 days. Opioid prescriptions were converted to morphine milligram equivalency (MME) for each patient. Figure 1 illustrates a frequency distribution to tabulate the number of values using a bin width of 50 MME and to compare the proportion of patients within each bin that received the corresponding MME amount per day at the pretreatment and day of treatment timepoints. The median opioid dosages for pretreatment and day of treatment timepoint were 30 MME per day and 33.75 MME per day, respectively. For further statistical analysis, opioid usage by continuous variable was converted to mean opioid dose per 10 MME across both pretreatment and day of timepoints.
Figure 1:
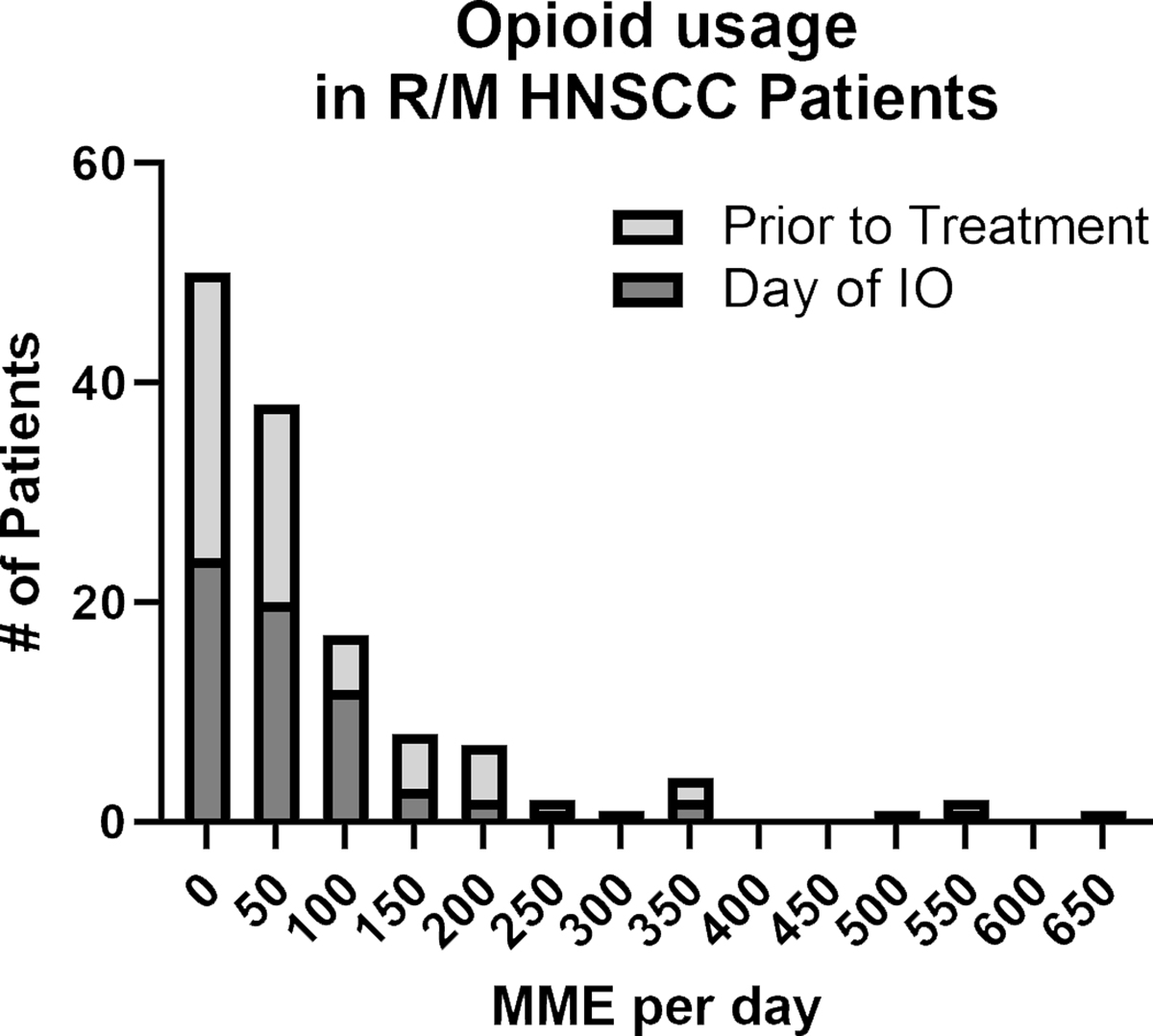
Stacked bar frequency distribution of opioid usage in R/M HNSCC patients prior to anti-PD-L1 treatment and on the day of treatment. Opioid usage was calculated as morphine milligram equivalency amount per day based on opioid prescriptions identified by chart review.
3.3. Impact of opioid usage on immunotherapy efficacy
In our cohort, the response rate was 17% (11/66) with 3 complete responses and the remainder partial responses, with 20% (13/60) achieving stable disease, for a disease control rate (DCR) of 36%. The median PFS and OS were 2.8 months and 9.73 months, respectively. Univariate analysis showed that only platinum failure was significantly associated with DCR (Supplemental Table 1). Higher opioid doses by continuous variable were significantly associated with lower PFS (HR=1.03, 95% CI(1.01, 1.06), p=0.016) and lower OS (HR=1.05, 95% CI(1.02, 1.08), p<0.001; Table 2). Platinum failure and increased NLR were also associated with survival (Table 2); however, PD-L1 was not (Supplemental Table 1). In multivariate analysis, including platinum failure status, higher opioid doses were associated with lower OS (HR=1.04, 95% CI(1.01, 1.08), p=0.013) but not PFS in both multivariate models that included PD-L1 expression (Table 3) and did not include PD-L1 expression (Supplemental Table 2). Because of a significant correlation between NLR and platinum failure status, all three variables were not included together in the same multivariate model. In the model including NLR with or without PD-L1, higher opioids were similarly associated with OS (HR = 1.04, 9% CI(1.00,1.07), p=0.028) but not PFS. (Supplemental table 3 and 4). There was no correlation between opioid dosage and PD-L1 or NLR. Lastly, there was no difference in baseline characteristics among patients who received different doses of pretreatment opioids, including in NLR and percent that were platinum failure.
Table 2.
Significant Variables Identified in Univariate Survival Analysis
| Variable* | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| NLR | 1.07 | 1.03,1.10 | <0.001 | 1.04 | 1.01,1.08 | 0.019 |
| Platinum Failure (Yes vs. No) | 2.66 | 1.50,4.74 | <0.001 | 3.03 | 1.54,5.97 | 0.001 |
| Opioid dosage | 1.03 | 1.01,1.06 | 0.016 | 1.05 | 1.02,1.08 | <0.001 |
For non-significant variables tested, see Suppl Table 1
Table 3.
Multivariate model that included PD-L1 for OS and PFS.
| Variable | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| PD-L1 (Yes vs. No) | 0.66 | 0.34,1.29 | 0.200 | 0.54 | 0.25,1.16 | 0.110 |
| Platinum Failure (Yes vs. No) | 2.47 | 1.20,5.08 | 0.014 | 3.89 | 1.67,9.06 | 0.002 |
| Opioid dosage | 1.02 | 0.99,1.06 | 0.200 | 1.04 | 1.01,1.08 | 0.013 |
3.4. Impact of opioid usage on tumor immune landscape prior to treatment
Thirty-four patients in this patient cohort had undergone tumor microenvironment analysis previously for intratumoral CD8+ T cells and CD4+FoxP3+ Tregs as part of a prior published analysis generated by Zandberg and colleagues25. Seventy percent of patients (24/34) were prescribed opioids at the time of tumor resection. We sought to determine if opioid usage impacted the immune cell presence in the tumor microenvironment using immunohistochemical evaluation (Figure 2A). Analysis of opioid dosage as a categorical variable (yes/no) showed that those patients on opioids had significantly less CD8+ T cells (β = −0.2, 95% CI (−0.37, −0.03), p=0.019) in the tumor microenvironment compared to patients not taking any opioids (Figure 2B). There were no statistical differences in the number of FoxP3+ Tregs (β = 0.0, 95% CI (−0.07, 0.07), p > 0.90) or in the ratio of CD8+ cells to Treg (CD8/Treg) (β = −2.2, 95% CI (−6.6, 2.3), p = 0.30, Figure 2B).
Figure 2:
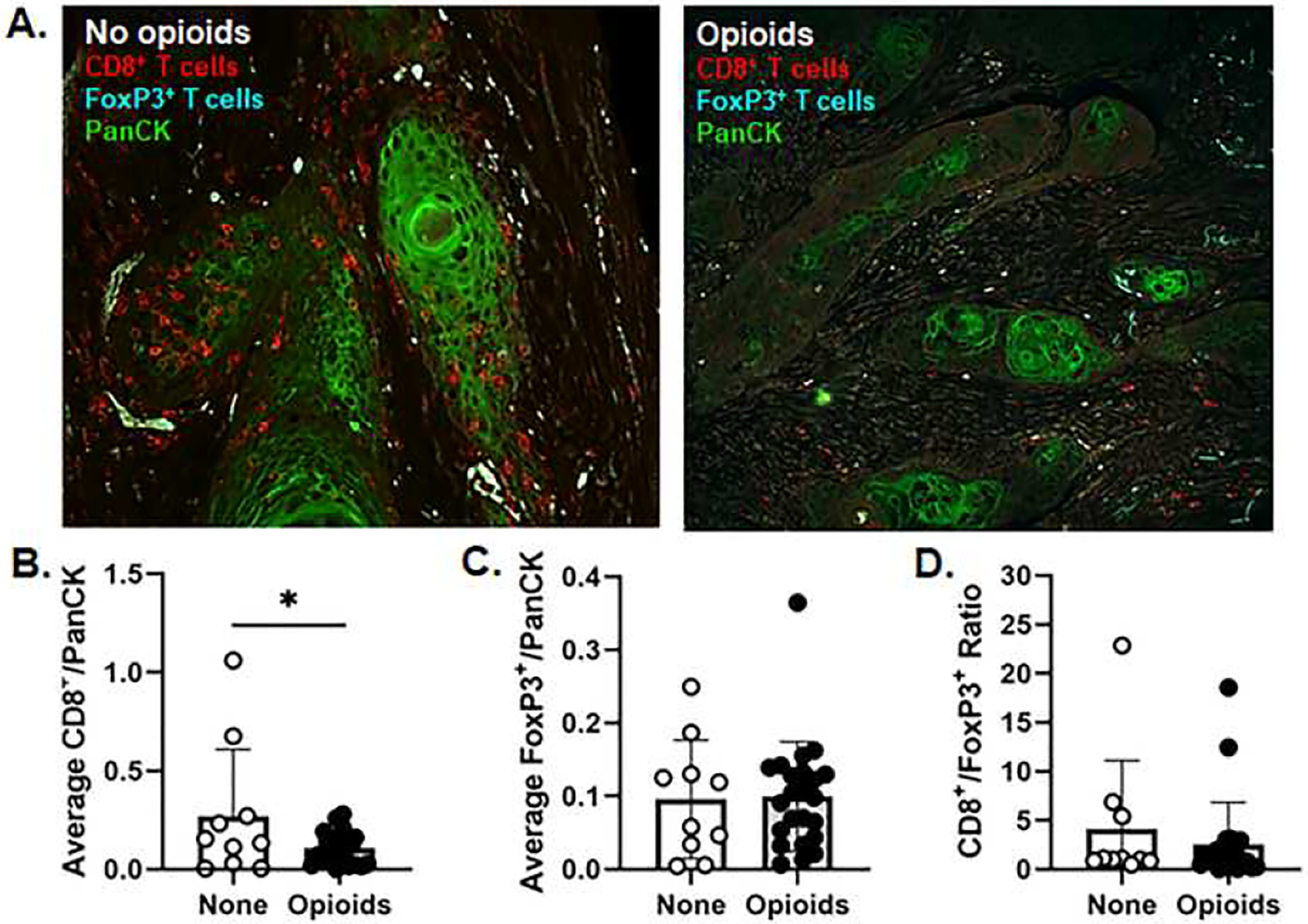
A) Representative images of pan cytokeratin (PanCK, green), anti-CD8 (red), and anti-FoxP3 (cyan) immunostaining in formalin-fixed paraffin-embedded tumor sections from HNSCC patients prescribed no opioids (Left) or opioids (right) prior to treatment (20x magnification). Average CD8+ T cell (B) and FoxP3+ T cell (C) counts normalized to the PanCK+ area as well as the ratio of CD8+ T cells to FoxP3+ cells (D) were evaluated rom HNSCC patients taking no opioids (open circles) or opioids (black circles) prior to treatment. *p<0.05 Error bars indicate standard deviation.
3.5. OPRM1 expression in tumor infiltrating immune cells from HNSCC patients.
Our retrospective analysis suggests that opioid usage prior to treatment was associated with a more immunosuppressive tumor landscape (i.e., reduction in CD8+ T cells in the tumor). We hypothesize opioid signaling in the tumor microenvironment can directly influence T cell activity via opioid receptor, OPRM1. OPRM1 expression has been identified on lymphocyte populations in clinical studies and preclinical models24; however, OPRM1 gene expression in tumor infiltrating immune cells from HNSCC patients is currently unknown. Using a recently publicly available single cell RNAseq dataset generated by Cillo and colleagues26, we assessed the differential expression of OPRM1 gene in CD3+ tumor infiltrating lymphocytes (TIL) and peripheral blood mononuclear cells (PBMCs) isolated from 26 HNSCC patients (18 HPV- HNSCC, 8 HPV+ HNSCC) compared to healthy donors (i.e. tonsil tissue from 4 sleep apnea patients; PBMCs from 6 healthy donors). Evaluation of OPRM1 gene expression across all cells found a significant increase in expression on TIL from HNSCC patients compared to PBMCs (padj=0.00182, wald test). Further evaluation within immune cell subtype clustering revealed that OPRM1 expression is primarily present in T lymphocytes found in the tissues and blood (Figure 3A). When immune cells from PBMCs and tissue infiltrating immune cells were pooled, there was no significant difference in OPRM1 expression in CD3+ T cell subtypes between HD and HNSCC patients (Figure 3B), However, within HNSCC patient samples, we observed a significant increase in OPRM1 expression in tumor infiltrating CD8+ T cells (padj=0.0018) and FoxP3+ Treg cell (p=0.043) compared to circulating CD8+ and FoxP3+ T cells from HNSCC blood (Figure 3C).
Figure 3.
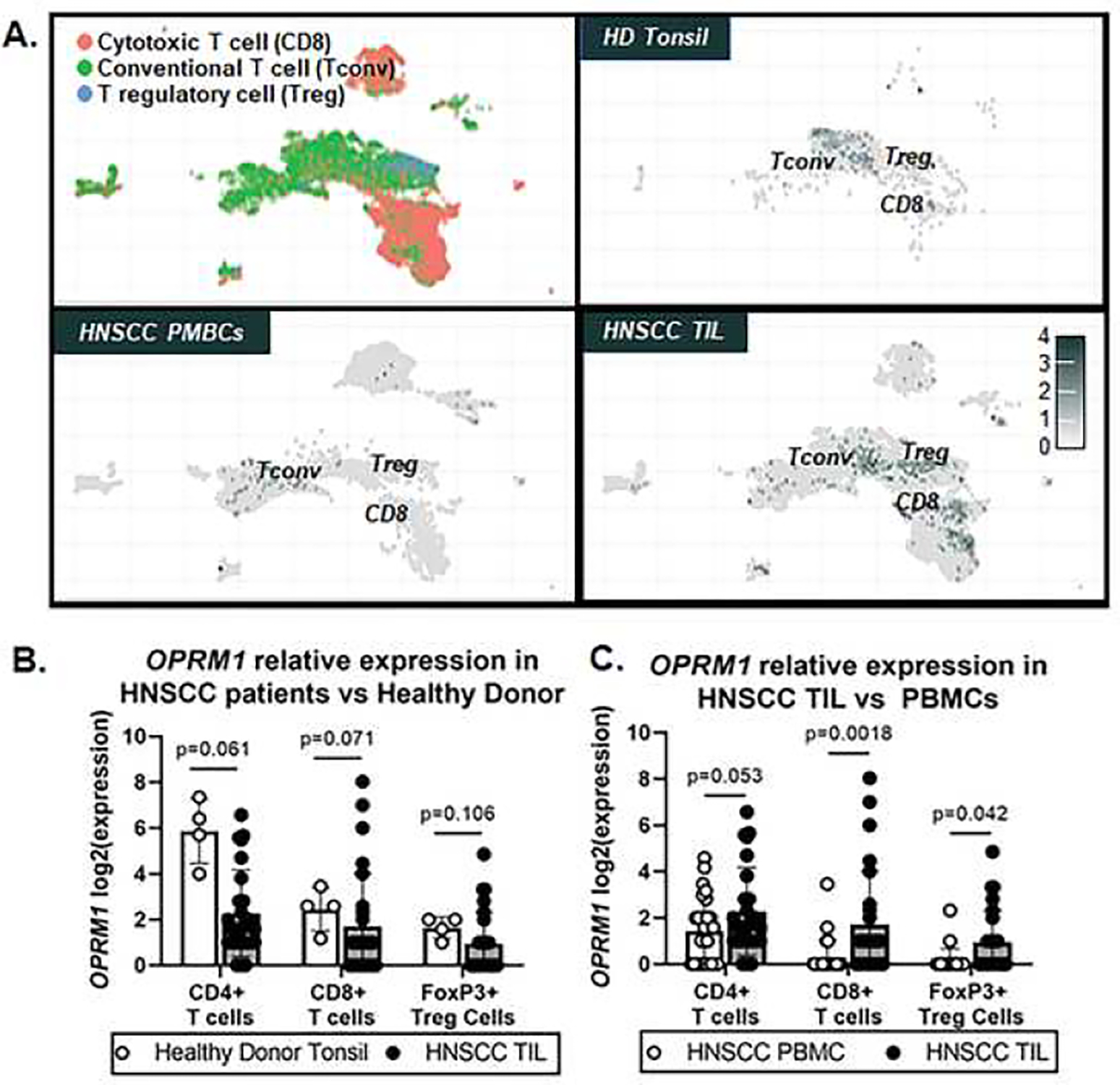
Publicly available scRNAseq data was leveraged to assess differential gene expression changes in OPRM1. A) Distinct UMAPS of CD3+ T cell subtypes (i.e. CD8 cytotoxic, CD4 conventional, FoxP3 regulatory) were generated and used to show OPRM1 gene expression represented as a heat map in samples from healthy donor (HD) and HNSCC patients. Tonsil/Tumor and peripheral blood mononuclear cell (PBMC) samples are differentially plotted. Relative expression is represented as Log2(expression). Quantitative analysis of OPRM1 Log2(expression) in T cell subsets using the Wald Test between (B) HNSCC tumor-infiltrating cells (TIL) and health donor tonsil as well as (C) HNSCC TIL versus HNSCC PBMCs. *p<0.05.
4.0. Discussion
This study retrospectively assessed the relationship between pretreatment opioids and the efficacy of anti-PD-1 mAb therapy in R/M HNSCC, as well as the relationship between opioid use and the tumor immune microenvironment. Our study showed that higher opioid doses pretreatment were associated with significantly lower PFS and OS in univariate analyses, and independently associated with lower OS in multivariate analysis. Patients that were on opioids also had significantly lower CD8+ T cells in the tumor microenvironment compared to those that were not.
Pain is experienced by up to 80% of HNSCC patients8, 30 and in our R/M HNSCC patient cohort, 63% of patients were actively taking opioids for pain management prior to and at the time treatment. Given the likely opioid consumption in HNSCC patients, it is imperative to better understand the influence of these drugs on therapeutic strategies to treat cancer. Meta-analysis on opioid-sparing surgical studies report an association between improved recurrence-free and/or OS with regional/neuraxial anesthesia compared with systemic opioids in non-HNC cancers (i.e. prostate, colorectal)31. Additionally, opioid use has been previously associated with worse outcomes with anti-PD-1 mAb treatment in advanced cancer patients. A recent systematic review by Cani and colleagues found 13 clinical analyses that evaluated combined ICI-opioid treatments. Although the evidence was retrospective and heterogeneous, all reports found a negative correlation in terms of overall survival, progression-free survival or time-to-treatment failure between opioids and ICIs32. Specifically, Taniguchi and colleagues found that regular opioid usage during nivolumab treatment was associated with decreased PFS in NSCLC patients33. Additionally, Iglesias-Santamaria and colleagues also showed that concomitant use of opioids had a detrimental effect on the efficacy of anti-PD-1 mAb therapy in patients with advanced solid tumors34. Our study also showed lower efficacy of anti-PD-1 mAb therapy with increased opioid usage and to our knowledge is the first evaluation specifically in R/M HNSCC patients.
Opioids have been observed to have immunosuppressive effects, such as reducing T cell function35, 36, downregulation of MHC class II37, and increasing the number of immunosuppressive regulatory FoxP3+ T cells38. For example, a single morphine injection (10 mg/kg) in rodents decreased basal MHC class II protein expression on B lymphocytes through the hypothalamic-pituitary-adrenal (HPA) axis and opioid-induced corticosterone release from the adrenal gland39. Morphine is the first-line opioid employed to manage cancer-related pain. Morphine’s effects are typically mediated by specific opioid receptors (μ, δ, and κ) either in the central or peripheral nervous system. Outside the nervous system, opioid receptors are also found in tumor cells as well as lymphocytes, indicating opioids’ potential to directly influence tumor cell proliferation and metastatic potential40, 41. Our data indicates that opioid use prior to ICI treatment effected the immune microenvironment in the tumor. We did importantly identify a significant reduction in CD8+ T cell presence in patients using opioids. We did not find any difference in FoxP3+ T cell presence in patients using opioids compared to no opioid use, however, this may have been due to the time of signal assessment; others have demonstrated that Tregs are associated with IO failure but may not be predictive of IO response at resection of the primary tumor in colorectal cancer42. Given FoxP3+ T cells’ capacity to repress anti-tumor immunity, their role becomes crucial for patients with immunotherapy. More data is needed on the effect of opioids on the tumor immune microenvironment in cancer patients. Previously, one study in breast cancer showed a reduction in NK cells in the tumor with systemic perioperative opioids compared with local injection23. To our knowledge, this is the first study to evaluate differences in the tumor immune microenvironment by systemic opioid usage in anti-PD-1 treated patients.
It is not known if systemic opioid use for analgesia can directly influence T cell activity. The functional implications of opioid receptors on immune cells continues to be disputed likely because OPRM1 expression in T-cells is non-constitutive and activation dependent35. In immune cells, opioid receptor activation is thought to trigger interactions that interfere with immune cell activation pathways involving cAMP and MAPK, ultimately suppressing function35, 36, 43. Our retrospective analyses of scRNAseq data from HNSCC patients found an increase in OPRM1 gene expression on CD8+ T cells in patient tumor tissue compared to circulating CD8+ T cells in the blood. While opioid usage was not considered in this dataset, these data suggest that opioid use could drive additional suppression of CD8+ T cells in the tumor. This is consistent the previously published work by Liang and colleagues who observed that at least 12 months of chronic morphine use results in an increase in OPRM1 mRNA expression in T and B lymphocytes compared to healthy subjects37. However, the evaluation of OPRM1 expression in immune cells was performed using scRNAseq data collected from surgical HNSCC patients that were not considered R/M at the time of evaluation. It is possible that OPRM1 expression in T cell populations could be different in TIL from R/M patients. Further investigation is needed to examine the relationship between opioid use and OPRM1 functional expression and mechanisms of interaction in tumor-associated T cells in R/M HNSCC.
Our study has several limitations. First, the interplay and biologic effect of pain and opioids on tumor progression and outcomes independent of treatment is complex with conflicting data as to whether opioid usage is associated with worse outcomes in advanced cancer patients44. While we cannot fully rule out that those that had pain and required higher opioids had biologically more aggressive disease, it is notable that we found no differences in baseline characteristics, including in NLR and platinum failure status by opioid usage. Further, higher opioids were associated with decreased CD8+ T cells in the tumor microenvironment, an important biologic correlation for the hypothesis that opioids may directly affect the efficacy of anti-PD-1 mAb therapy; decreased CD8+ T cells in the tumor immune microenvironment are an established predictor of reduced efficacy with immunotherapy25. Additional weaknesses include our lower sample size and that the study is retrospective and thus it is possible that opioid prescriptions may not reflect actual usage. However, fluctuations (e.g., increase or decrease in dosage from the visit prior to and at immunotherapy treatment, SD=111.5 MME) were observed in 66.7% of patients and would indicate active monitoring for pain management during the treatment period. We acknowledge that our findings are hypothesis generating and future studies are needed with prospective diagnostic documentation.
In conclusion, our study represents the first evaluation of the effect of opioids on the efficacy of anti-PD-1 mAb therapy in R/M HNSCC patients. We observed that higher opioids were associated with significantly lower PFS and OS and lower CD8+ T cells in the tumor microenvironment. Although research has identified possible mechanisms of how opioids can affect immunity, appropriate in vitro and in vivo studies are needed to elucidate the detailed mechanism in which the opioids could affect ICI efficacy. The clinical potential is that treatment of cancer pain with non-opioid based approaches, when possible, could improve the immune response and the therapeutic benefit of immune checkpoint inhibitors. Further research into the clinical and biologic effect of opioids in immunotherapy treated patients is warranted.
Supplementary Material
Highlights:
High opioid use in R/M HNSCC was associated with reduced immunotherapeutic efficacy
High opioid use was associated with decreased CD8 T cells in the R/M HNSCC tissue.
Tumor infiltrating CD8 T cells from HNSCC patients express opioid receptor, OPRM1
Study of the impact of opioids on the immune system and immunotherapy are needed
Acknowledgements:
We thank the UPMC Hillman Cancer Center Bioinformatics Core for their efforts on the bioinformatic aspect of this manuscript.
Funding Statement:
This work was supported by a grant from the Virginia Kaufman Foundation and the Clinical and Translational Science Institute at the University of Pittsburgh (NNS, MLN). This project also used the UPMC Hillman Cancer Center Bioinformatics Core shared resource, which is supported in part by award P30CA047904.
Footnotes
Disclosure Statement:
RL Ferris consults for Aduro Biotech Inc., Bain Capital Life Sciences, Iovance Biotherapeutics Inc., Nanobiotix, Ono Pharmaceutical Co. Ltd., Torque Therapeutics Inc., and TTMS; is on the advisory board for Amgen, AstraZeneca/MedImmune, Bristol-Myers Squibb, EMB Serono, GlaxoSmithKline, Lilly, MacroGenics, Merck, Numab Therapeutics AG, Pfizer, PPD, Regeneron Pharmaceuticals Inc., and Tesaro; receives clinical trial support from AstraZeneca/MedImmune, Bristol-Myers Squibb, and Merck; and receives research funding from Astra-Zeneca/MedImmune, Bristol-Myers Squibb, Tesaro, TTMS, and VentiRx Pharmaceuticals.
GM Delgoffe consults for and/or is on the scientific advisory board of BlueSphere Bio, Century Therapeutics, Novasenta, Pieris Pharmaceuticals and Western Oncolytics/Kalivir; has grants from bluebird bio, Novasenta, Pfizer, Pieris Pharmaceuticals, TCR2 and Western Oncolytics/Kalivir; and owns stock in Novasenta.
DP Zandberg is on the advisory board of Blueprint Medicines and Prelude Therapeutics, consults for Macrogenics, and received institutional support for role as PI from Merck, BMS, AstraZeneca, GlaxoSmithKline, Aduro, Astellas, Macrogenics, Lilly, Bicara, Checkmate Pharma, and Novasenta.
All other authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 Bibliography
- 1.Cramer JD, Burtness B, Le QT and Ferris RL. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol. 2019;16:669–683. [DOI] [PubMed] [Google Scholar]
- 2.Brennan S, Baird AM, O’Regan E and Sheils O. The Role of Human Papilloma Virus in Dictating Outcomes in Head and Neck Squamous Cell Carcinoma. Front Mol Biosci. 2021;8:677900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin Y, Zheng X, Gao W, Wang B and Wu Y. Tumor microenvironment and immune-related therapies of head and neck squamous cell carcinoma. Mol Ther Oncolytics. 2021;20:342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise-Draper TM, Bahig H, Karivedu V and Burtness B. Current Therapy for Metastatic Head and Neck Cancer: Evidence, Opportunities, and Challenges. Am Soc Clin Oncol Educ Book. 2022;42:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Borcoman E, Marret G and Le Tourneau C. Paradigm Change in First-Line Treatment of Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, ., Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F and Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleh K, Eid R, Haddad FG, Khalife-Saleh N and Kourie HR. New developments in the management of head and neck cancer - impact of pembrolizumab. Ther Clin Risk Manag. 2018;14:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bossi P, Ghiani M, Argenone A and Depenni R. Is pain part of a systemic syndrome in head and neck cancer? Support Care Cancer. 2020;28:451–459. [DOI] [PubMed] [Google Scholar]
- 9.Bicanic I, Hladnik A, Dzaja D and Petanjek Z. The Anatomy of Orofacial Innervation. Acta Clin Croat. 2019;58:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haggard P and de Boer L. Oral somatosensory awareness. Neurosci Biobehav Rev. 2014;47:469–84. [DOI] [PubMed] [Google Scholar]
- 11.Moayedi Y, Duenas-Bianchi LF and Lumpkin EA. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci Rep. 2018;8:9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasco MA, Cordero J and Dundar Y. Chronic Pain Management in Head and Neck Oncology. Otolaryngol Clin North Am. 2020;53:865–875. [DOI] [PubMed] [Google Scholar]
- 13.Cramer JD, Johnson JT and Nilsen ML. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol Head Neck Surg. 2018;159:853–858. [DOI] [PubMed] [Google Scholar]
- 14.Mirabile A, Airoldi M, Ripamonti C, Bolner A, Murphy B, Russi E, Numico G, Licitra L and Bossi P. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit Rev Oncol Hematol. 2016;99:100–6. [DOI] [PubMed] [Google Scholar]
- 15.Mercadante S, Masedu F, Valenti M and Aielli F. Breakthrough pain in patients with head & neck cancer. A secondary analysis of IOPS MS study. Oral Oncol. 2019;95:87–90. [DOI] [PubMed] [Google Scholar]
- 16.Sethi RKV, Panth N, Puram SV and Varvares MA. Opioid Prescription Patterns Among Patients With Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg. 2018;144:382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borner C, Stumm R, Hollt V and Kraus J. Comparative analysis of mu-opioid receptor expression in immune and neuronal cells. J Neuroimmunol. 2007;188:56–63. [DOI] [PubMed] [Google Scholar]
- 18.Kraus J Regulation of mu-opioid receptors by cytokines. Front Biosci (Schol Ed). 2009;1:164–70. [DOI] [PubMed] [Google Scholar]
- 19.Langsdorf EF, Mao X and Chang SL. A role for reactive oxygen species in endotoxin-induced elevation of MOR expression in the nervous and immune systems. J Neuroimmunol. 2011;236:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou M, Zhou NB, Li H, Wang BS, Wang XQ, Wang XW, Wang KG and Xue FS. Morphine and ketamine inhibit immune function of gastric cancer patients by increasing percentage of CD4(+)CD25(+)Foxp3(+) regulatory T cells in vitro. J Surg Res. 2016;203:306–12. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Flores R and Weber RJ. Differential effects of buprenorphine and morphine on immune and neuroendocrine functions following acute administration in the rat mesencephalon periaqueductal gray. Immunopharmacology. 2000;48:145–56. [DOI] [PubMed] [Google Scholar]
- 22.Saurer TB, Ijames SG and Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J Neuroimmunol. 2006;177:18–26. [DOI] [PubMed] [Google Scholar]
- 23.Desmond F, McCormack J, Mulligan N, Stokes M and Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311–9. [PubMed] [Google Scholar]
- 24.Boland JW, McWilliams K, Ahmedzai SH and Pockley AG. Effects of opioids on immunologic parameters that are relevant to anti-tumour immune potential in patients with cancer: a systematic literature review. Br J Cancer. 2014;111:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandberg DP, Menk AV, Velez M, Normolle D, DePeaux K, Liu A, Ferris RL and Delgoffe GM. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J Immunother Cancer. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, Oesterreich S, Chen W, Lafyatis R, Bruno TC, Ferris RL and Vignali DAA. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity. 2020;52:183–199 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lun AT, Bach K and Marioni JC. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 2016;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy DJ, Campbell KR, Lun AT and Wills QF. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics. 2017;33:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csardi G and Nepusz T. The igraph software package for complex network research. InterJournal, complex systems. 2006;1695:1–9. [Google Scholar]
- 30.Reyes-Gibby CC, Anderson KO, Merriman KW, Todd KH, Shete SS and Hanna EY. Survival patterns in squamous cell carcinoma of the head and neck: pain as an independent prognostic factor for survival. J Pain. 2014;15:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng M, Chen W, Hou W, Li L, Ding M and Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016;7:15262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cani M, Bironzo P, Garetto F, Buffoni L and Cotogni P. Immune Checkpoint Inhibitors and Opioids in Patients with Solid Tumours: Is Their Association Safe? A Systematic Literature Review. Healthcare (Basel). 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniguchi Y, Tamiya A, Matsuda Y, Adachi Y, Enomoto T, Azuma K, Kouno S, Tokoro A and Atagi S. Opioids impair Nivolumab outcomes: a retrospective propensity score analysis in non-small-cell lung cancer. BMJ Support Palliat Care. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias-Santamaria A Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol. 2020;22:1481–1490. [DOI] [PubMed] [Google Scholar]
- 35.Borner C, Kraus J, Bedini A, Schraven B and Hollt V. T-cell receptor/CD28-mediated activation of human T lymphocytes induces expression of functional mu-opioid receptors. Mol Pharmacol. 2008;74:496–504. [DOI] [PubMed] [Google Scholar]
- 36.Borner C, Warnick B, Smida M, Hartig R, Lindquist JA, Schraven B, Hollt V and Kraus J. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183:882–9. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, Liu R, Chen C, Ji F and Li T. Opioid System Modulates the Immune Function: A Review. Transl Perioper Pain Med. 2016;1:5–13. [PMC free article] [PubMed] [Google Scholar]
- 38.Erfani N, Khademi B, Haghshenas MR, Mojtahedi Z, Khademi B and Ghaderi A. Intracellular CTLA4 and regulatory T cells in patients with laryngeal squamous cell carcinoma. Immunol Invest. 2013;42:81–90. [DOI] [PubMed] [Google Scholar]
- 39.Nugent AL, Houghtling RA and Bayer BM. Morphine suppresses MHC-II expression on circulating B lymphocytes via activation of the HPA. J Neuroimmune Pharmacol. 2011;6:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cata JP, Sood AK and Eltzschig HK. Anesthetic Drugs and Cancer Progression. Anesthesiology. 2020;133:698–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H, Zhang H, Weng ML, Zhang J, Jiang N, Cata JP, Ma D, Chen WK and Miao CH. Morphine promotes tumorigenesis and cetuximab resistance via EGFR signaling activation in human colorectal cancer. J Cell Physiol. 2021;236:4445–4454. [DOI] [PubMed] [Google Scholar]
- 42.Aristin Revilla S, Kranenburg O and Coffer PJ. Colorectal Cancer-Infiltrating Regulatory T Cells: Functional Heterogeneity, Metabolic Adaptation, and Therapeutic Targeting. Front Immunol. 2022;13:903564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefano GB and Kream R. Endogenous opiates, opioids, and immune function: evolutionary brokerage of defensive behaviors. Semin Cancer Biol. 2008;18:190–8. [DOI] [PubMed] [Google Scholar]
- 44.Ramirez MF, Gorur A and Cata JP. Opioids and cancer prognosis: A summary of the clinical evidence. Neurosci Lett. 2021;746:135661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


