Abstract
Arthritis is a common degenerative disease of joints, which has become a public health problem affecting human health, but its pathogenesis is complex and cannot be eradicated. Coptis chinensis (CC) has a variety of active ingredients, is a natural antibacterial and anti-inflammatory drug. In which, berberine is its main effective ingredient, and has good therapeutic effects on rheumatoid arthritis (RA), osteoarthritis (OA), gouty arthritis (GA). RA, OA and GA are the three most common types of arthritis, but the relevant pathogenesis is not clear. Therefore, molecular mechanism and prevention and treatment of arthritis are the key issues to be paid attention to in clinical practice. In general, berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC play the role in treating arthritis by regulating Wnt1/β-catenin and PI3K/AKT/mTOR signaling pathways. In this review, active ingredients, targets and mechanism of CC in the treatment of arthritis were expounded, and we have further explained the potential role of AHR, CAV1, CRP, CXCL2, IRF1, SPP1, and IL-17 signaling pathway in the treatment of arthritis, and to provide a new idea for the clinical treatment of arthritis by CC.
Keywords: Coptis chinensis, arthritis, berberine, Wnt1/β-catenin signaling pathway, PI3K/Akt/mTOR signaling pathway
Graphical Abstract
1 Introduction
Arthritis is a series of inflammatory diseases occurring in human joints or surrounding tissues, and it can lead to joint disability in serious cases. The incidence of arthritis is increasing year by year. There are more than 355 million arthritis patients worldwide, and the number is still increasing (Jia, 2021). Rheumatoid arthritis (RA) and osteoarthritis (OA) have the highest incidence (Wang, 2020; She, 2020), and the pathogenesis of them is complex. RA is a chronic autoimmune disease caused by synovial joint inflammation, which gradually leads to joint damage, cartilage degradation, disability, with a high disability rate in the later stage of the disease (Bird et al., 2022). OA is the most common type of arthritis associated with age and occurs most often in the elderly (Roškar and Hafner-Bratkovič, 2022). The causes of RA, gouty arthritis (GA) and OA are varied, mainly caused by the combined effects of congenital genetic factors and acquired environmental factors, and the related molecular mechanisms are complicated. The prevention and treatment of these three types of arthritis are the focus of research. Currently, arthritis cannot be cured clinically, and joint function can only be maintained through drug therapy. However, long-term treatment with single drug or combined immunosuppressive drugs have great limitations and cause adverse reactions. Therefore, it has important significance to explore the pathogenesis of arthritis and develop natural drugs to treat arthritis.
Coptis chinensis (CC) is the dried rhizome of the Ranunculaceae plant Coptis chinensis Franch., Coptis deltoidea C.Y.Cheng et Hsiao, or Coptis teeta Wall (National Pharmacopoeia Commission, 2020), which has antibacterial, anti-inflammatory, antioxidant, anti-tumor, antiarrhythmic and other pharmacological effects (Gai et al., 2018). CC is commonly used in clinical treatment of cardiovascular and cerebrovascular diseases, diabetes, cancer and other diseases (Fu et al., 2021). CC contains more than 130 chemical components, mainly including alkaloids, coumarins, organic acids, and flavonoids (Zhou et al., 2020). CC has good therapeutic effect on RA, OA, GA (Yue et al., 2019; Huang et al., 2021; Zhang et al., 2022; Elkomy et al., 2022). The alkaloid berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride show significant antibacterial and anti-inflammatory effects. Studies have shown that berberine can effectively treat RA, OA and GA, mainly by reducing the level of inflammatory factors, regulating intestinal flora, promoting uric acid excretion, and improving the inflammatory response damage of joints and their surrounding tissues (Fan et al., 2021; Xu and Li, 2021). At the molecular level, CC can improve arthritis by regulating Wnt1/β-catenin, PI3K/AKT/mTOR and NF-κB signaling pathways, inhibiting the expression of pro-inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), etc (Zhao et al., 2014; Zhou et al., 2019; Shen et al., 2020; Chen et al., 2023). Berberine, palmatine, coptisine and other components are the main components of CC in the treatment of arthritis. Interleukin-10 (IL-10), IL-1β, mitogen-activated protein kinase (MAPK), IL-6, matrix metalloproteinase-3 (MMP-3), TNF-α and other targets have been confirmed to play important roles in the treatment of arthritis by CC. The regulation of IL-17 signaling pathway in chondrocytes could inhibit the overexpression and activation of key proteins, such as IL-17RA, ACT1 and TRAF6, which could improve the occurrence of cartilage inflammation in OA. Research has shown that aryl hydrocarbon receptor (AHR), caveolin-1 (CAV1), c-reactive protein (CRP), C-X-C motif chemokine 2 (CXCL2), interferon regulatory factor-1 (IRF1), secreted phosphoprotein 1 (SPP1) and other targets are key targets in the pathogenesis of arthritis (Chen et al., 2022; Wang et al., 2022; Sakthiswary et al., 2022; Zhu et al., 2022; Li et al., 2023; Yang et al., 2023), but role of them in the treatment of arthritis by CC has not been verified and further clarification is needed.
Studies have shown that chemical drugs used in the treatment of arthritis have different degrees of toxicity and side effects. CC, as a natural Chinese herbal medicine with low toxicity of antibacterial and anti-inflammatory, is feasible to develop as a drug for treating arthritis. However, there are few studies on the treatment of arthritis by CC. Therefore, it is of great significance to clarify the mechanism of CC in the treatment of arthritis, which can provide new research directions for clinical drug development. We have elaborated the active ingredients, targets and mechanism in the treatment of RA, OA and GA by CC, revealed the potential targets and related pathways of CC in the treatment of arthritis, and provided new insights into the study of the molecular mechanism of CC in treating arthritis. In this review, the chemical components, targets and pathways of CC in the treatment of arthritis were discussed in detail, the molecular mechanism of CC in treating arthritis was elaborated, and the potential therapeutic targets were analyzed, providing new ideas for clinical prevention and treatment of arthritis.
2 The pathogenesis of three types of arthritis
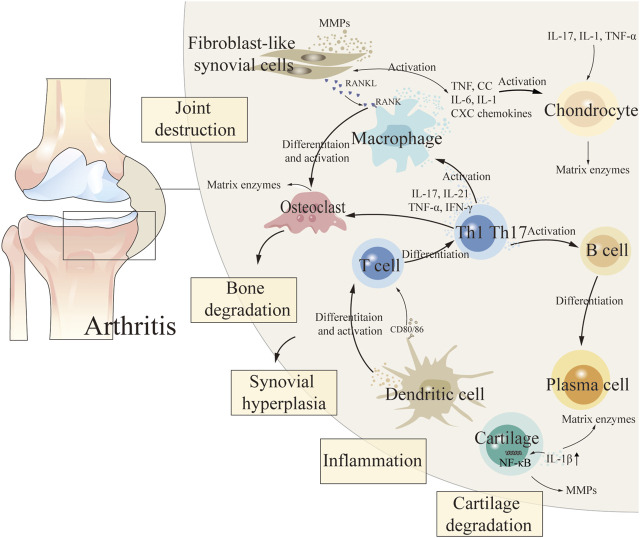
The etiology of arthritis is complex and relevant pathogenesis has not been clarified (Chen et al., 2021). During the pathogenesis of arthritis, fibroblast-like synovial cells (FLS), chondrocytes, intrinsic immune cells (dendritic cells and macrophages), and adaptive immune cells (T and B cells) in synovial tissue release a variety of cytokines that lead to persistent destruction of cartilage and subchondral bone, thereby exacerbating the degree of arthritis (So and Martinon, 2017; Xing, 2021; Xu, 2022) (Figure 1 demonstrated the pathogenesis of arthritis). Non-steroidal anti-inflammatory drugs, anti-rheumatism drugs, traditional Chinese medicine (TCM) compounds and other drugs are commonly used in clinical treatment of arthritis. But chemical therapy cannot cure arthritis, and it can only relieve joint function, and long-term use of these treatments can cause relatively significant toxic and side effects, causing liver, kidney and cardiovascular toxicity (Li, 2022; Yu, 2022). TCM has unique advantages which due to its characteristics of multiple components, low toxicity, few side effects and good curative effects in the treatment of RA and OA (Li et al., 2022; Meng et al., 2022). The effective components of TCM show great potential in the treatment of arthritis by inhibiting inflammatory response, alleviating oxidative stress, regulating chondrocyte metabolism and regulating related signaling pathways (Li et al., 2017).
FIGURE 1.
The pathogenesis of arthritis.
2.1 Rheumatoid arthritis
RA is an autoimmune inflammatory disease with systemic sequelae (Hyndman, 2017), the main symptoms of which are synovial inflammation, production of rheumatoid factors and antibodies against citrulline proteins, and destruction of cartilage and bone (McInnes and Schett, 2011). The pathogenesis of RA is confused and difficult curative ratio, which is mainly caused by excessive proliferation of synovial cells, increased levels of inflammatory factors and abnormal toll-like receptor signaling pathway (Wen, 2022). Inflammatory response is an important pathological process of RA. Abnormal secretion of proinflammatory cytokines, chemokines and proteases will disturb the balance of the body and lead to cartilage and bone damage (Fang et al., 2020). The pathological feature of RA is the infiltration of synovial inflammatory cells in multiple joints. Nuclear factor кB receptor activating factor (RANKL), prostaglandins and matrix metalloproteinases (MMPs) are induced by pro-inflammatory cytokines, including TNF-α, IL-6 and interleukin-1 (IL-1), causing joint pain and swelling (Wallach, 2016). At the molecular level, MAPK, TLR7/NF-кB and apoptosis signaling pathways are the main signaling pathways involved in regulating the invasion and abnormal behavior of RA-FLS cells (Zheng, 2012; Bottini and Firestein, 2013). Currently, the main treatment is to reduce inflammation and relieve pain (Fan et al., 2020). Modern studies of TCM have shown that a variety of monomer components of TCM have the efficacy of treating RA (Yao et al., 2023), including sinomenine, artemisinin, total glucosides of paeony and berberine (Ou et al., 2010; Wang et al., 2011; Xia and Li, 2017; Sharma et al., 2022).
2.2 Osteoarthritis
OA is the most common type of arthritis that causes joint pain and disability, and it has a high incidence (Li et al., 2022). The main characteristics of OA are cartilage degeneration, synovial hyperplasia, osteophyte formation and subchondral osteosclerosis, but the pathogenesis has not been clearly defined (Chen et al., 2022). The occurrence and development of OA is closely related to degradation of matrix and release of bioactive substances, which promote the release of MMPs and eventually lead to chondrolysis (Uthman et al., 2003). Inflammatory factors and receptors are involved in the occurrence and development of OA, which can cause degenerative changes of chondrocytes through MAPK/ERK, JAK2/STAT3, NF-κB, Wnt/β-catenin and PI3K/AKT signaling pathways (Hwang et al., 2005; Roemer et al., 2011; Min et al., 2017). Chondrocytes are the source and target of pro-inflammatory cytokines in OA. Pro-inflammatory cytokine interleukin-1 is an important inflammatory mediator secreted by early OA and a key inflammatory cytokine involved in the pathogenesis of OA (Li et al., 2019; Yang et al., 2021). IL-1β mainly affects the metabolism of articular cartilage extracellular matrix and chondrocytes, and plays an important role in the pathogenesis of OA by inducing excessive release of inflammatory mediators cycloooxygenase-2 (COX-2) and iNOS, and overexpression of cartilage MMPs. IL-1β, TNF-α and IL-6 are three highly expressed inflammatory cytokines in OA joints, which are actively produced by chondrocytes, synovial cells, macrophages and osteoblasts, and can be used as indicators of the progression of OA (Zhou et al., 2016a).
2.3 Gouty arthritis
GA is an inflammatory reactive disease that causes joint pain due to the dysfunction of purine metabolism and uric acid (UA) excretion in the body (AbdullGaffar et al., 2020). The pathogenesis of GA is related to the inflammatory response caused by the deposition of monosodium urate (MSU) around the joint, which stimulates the synovial membrane to produce pathological reactions such as synovial vasodilation and leukocyte exudation, which mainly involve the mediation of MAPK and NF-κB signaling pathways and the activation of TNF-α, IL-1 and other inflammatory cytokines (Choe et al., 2014; Terkeltaub, 2017; Lv, 2020; Liu et al., 2022).
3 Chemical components and mechanism of Coptis chinensis in the treatment of arthritis
3.1 The chemical components of Coptis chinensis in treating arthritis
CC has obvious inhibitory effect on acute and chronic inflammatory reactions (Park et al., 2018). CC contains a variety of anti-inflammatory active ingredients, such as berberine, palmatine, coptisine, etc. (Hu et al., 2013; Wang et al., 2014; Zhou et al., 2015; Zhou et al., 2016; Zhou et al., 2017; Qiu et al., 2018; Sujitha et al., 2018; Wang et al., 2019; Wu et al., 2019; Zhou et al., 2019; Zhou et al., 2020; Shen et al., 2020; Yu et al., 2020; Fan et al., 2021; Cheng et al., 2022; Asila et al., 2022; Li et al., 2022; Kou et al., 2022) (Table 1), which can achieve anti-inflammatory effects mainly by inhibiting the activity of key proteins in the inflammatory signaling pathway and blocking the transmission of inflammatory signals (Hu and Mo, 2017; Geng, 2018). Berberine has a significant anti-inflammatory activity and can treat a variety of arthritis, especially RA and OA (Hu et al., 2010; Zhou et al., 2016c).
TABLE 1.
Main chemical components of CC in treating arthritis and related pharmacological data.
| Compounds | Classifications | Pathways/Targets | Animal/Cell | Effective dose | References |
|---|---|---|---|---|---|
| Berberine | OA | Caspase-3, ADAMTS5, MMP-13 | Chondrocytes | 10 μg/mL | Zhou et al. (2017a) |
| TLR4/NF-κB signaling | SD rats/Primary articular chondrocytes | 200 μM | Zhou et al. (2020b) | ||
| 75 μM | |||||
| AMPK signaling | AMPKα1 global knockout (KO) mice/Chondrocytes | 1.1 mg/mL | Li et al. (2022b) | ||
| 5, 25 μM | |||||
| NF-κB pathway | SD rats/RAW 264.7 RCCs | 120 μM | Kou et al. (2022) | ||
| 40 μM | |||||
| p38/MAPK | SD rats/Primary articular chondrocytes | 200 μM | Zhou et al. (2015) | ||
| 75 μM | |||||
| RA | PI3K/AKT, Wnt1/β-catenin, AMPK/lipogenesis | SD rats | 200 mg/kg | Shen et al. (2020) | |
| p38/ERK MAPK pathway | Primary FLS-RA | 12.5, 25 μM | Wang et al. (2019a) | ||
| ASK1/p38 signaling | RAW 264.7 | 25, 50, 75 µM | Sujitha et al. (2018) | ||
| AA-SM cells | |||||
| Septic arthritis | NF-κB/JNK-RANKL axis | Adult male mice | 50, 100, 200 mg/kg | Asila et al. (2022) | |
| Adjuvant-induced arthritis (AIA) | AMPK/HIF-1α pathway | SD rats/Peritoneal macrophages | 80 mg/kg | Yu et al. (2020) | |
| 10 μM | |||||
| AMPK/NF-кB pathway | SD rats | 80 mg/kg | Zhou et al. (2019) | ||
| Type II collagen-induced arthritis | VEGF, p-JNK, p-p38 | SD rats | 200 mg/kg | Wang et al. (2014a) | |
| GA | COX-2, NALP3, TGF-β | Acute gouty arthritis (AGA) patients | 0.4 g/time | Fan et al. (2021) | |
| Palmatine | GA | NF-κB/NLRP3, Nrf2 pathways | KM male mice/THP-1 cells | 100 mg/kg | Cheng et al. (2022a) |
| 80 μM | |||||
| OA | Wnt/β-catenin, Hedgehog pathways | New Zealand rabbits/Primary chondrocytes | 100 mg/L | Zhou et al. (2016b) | |
| 10, 25, 50, 100 mg/L | |||||
| Coptisine | GA | Caspase-1 | Male Kun Ming mice/RAW264.7 | 2.91, 5.79, 11.61 mg/kg | Wu et al. (2019) |
| 1, 10, 30 μM | |||||
| Jatrorrhizine | Collagen-induced arthritis (CIA) | Anti-CII, IgG1 | SD rats | 8 mg/kg | Hu et al. (2013) |
| Magnoflorine | 8.7 mg/kg | ||||
| Jatrorrhizine hydrochloride | RA | MAPK, ERK, p38 | SD rats | 50 mg/kg | Qiu et al. (2018) |
3.1.1 Berberine
Berberine is the main active ingredient in CC that plays an anti-inflammatory and antibacterial role. It can effectively treat a variety of arthritis by down-regulating the production and expression of various inflammatory mediators and inhibiting the activation of inflammatory pathways (He et al., 2018). Berberine has a strong anti-rheumatoid effect and can slow the progression of RA by targeting mitochondrial oxidative phosphorylation (Fan et al., 2018; Elkomy et al., 2022) confirmed that berberine could effectively inhibit RA inflammation. By inhibiting autophagy of FLS cells, berberine induces RA-FLSs cycle arrest in G0/G1 phase, induces RA-FLSs cell death, inhibits the expression of vascular endothelial growth factor, regulates the level of anti-inflammatory factors, and achieves the purpose of treating RA (Wang et al., 2014; Huang et al., 2021). Wang (2011) verified that berberine induced apoptosis of RA-FLSs mainly through the mechanism of up-regulating the expression of pro-apoptotic protein apoptosis regulator BAX (Bax), inhibiting the expression of anti-apoptotic proteins apoptosis regulator Bcl-2 (Bcl-2) and Bcl-xl, and promoting the activation of caspase-3, caspase-9 and PARP. (Wang et al., 2017) found that berberine could reduce the expression level of interleukin-17 (IL-17) and IL-6, promote the expression of IL-10 and transforming growth factor-β (TGF-β) in serum, and improve the clinical symptoms of RA. Berberine can significantly suppress the activation of p-ERK, p-p38 and p-JNK, reduce the destruction of inflammatory cells on joint tissues, and exert anti-RA activity (Wang et al., 2014). During the treatment of RA, berberine reduced the expression levels of TNF-α, IL-17, interferon-γ (IFN-γ), MMPs and RAR-related orphan receptor γt (RORγt) (Sharma et al., 2022). Studies have shown that berberine treats RA by specifically inhibiting T cells, involving the balance between Treg and Th17 cells, providing a potential target for berberine in the treatment of arthritis (Li et al., 2017; Vita et al., 2021).
(Xie et al., 2018) found that berberine could increase the enzymatic antioxidant levels, such as SOD, glutathione peroxidase, catalase and glutathione-S-transferase in osteoporosis rats, which is helpful to prevent osteoporosis. (Huang et al., 2021) found that berberine promoted the proliferation and activity of IL-1β-induced inflammatory degenerative chondrocytes by inhibiting cell inflammatory response and activating TGF-β/Smad2/3 signaling pathway, and reduced the degree of OA development. Berberine is a potential therapeutic drug for OA. MMPs play a significant role in OA-induced articular cartilage damage (Dean et al., 1989; Hu et al., 2011) showed that berberine could inhibit the expression of matrix metalloproteinase-1 (MMP-1), MMP-3 and matrix metalloproteinase-13 (MMP-13) and effectively treat OA. Connective tissue growth factor (CCN2) is abundantly expressed response. Berberine inhibits CCN2 to produce IL-1β by down-regulating ROS-mediated NF-κB signaling pathway in fibroblast synovial cells, regulates cartilage damage and alleviates OA (Liu et al., 2015). Berberine can inhibit the expression of NO, prostaglandin E2 (PGE2), iNOS, COX-2, MMP-3 and MMP-13 induced by IL-1β, downregulate the expression of inflammatory mediators and reduce the inflammatory response in chondrocytes (Zhou et al., 2016).
By inhibiting the NOD-like receptor thermal protein domain 3 (NLRP3)/Toll-like receptor signaling pathway, berberine downregulated the expression levels of IL-2, IL-6 and TNF-α, and alleviated the degree of ankle swelling in GA mice (Jian et al., 2020). Berberine reduces the expression levels of NLRP3, TNF-α and IL-1β and the level of intracellular reactive oxygen species, thereby reducing MSU crystal-induced inflammation in rats (Liu et al., 2016). Berberine improves the acute symptoms of GA by inhibiting the activity of joint elastase and thereby inhibiting the infiltration of joint synovium neutrophils (Dinesh and Rasool, 2017). The increase of serum UA level is the key to AGA attack. Berberine can dilate blood vessels, improve blood flow and increase the expression of human urate transporter, thus increasing blood UA excretion and reducing UA level in the body. In addition, berberine can also improve insulin resistance and inhibit UA synthesis (Jin et al., 2019; Rondanelli et al., 2020). IL-1β is considered to be the initiating factor of AGA inflammatory response (Li et al., 2019), PGE2 has a strong inflammatory effect and is involved in the whole process of GA inflammatory response, and COX-2 is a key enzyme in the synthesis of PGE2 in the body (Liu et al., 2019). Fan et al. (Fan et al., 2021) showed that assisted treatment of AGA of berberine could significantly inhibit the expressions of inflammatory factors IL-1β, COX-2, nucleotide-binding oligomerization domain-like receptor 3 (NALP3) and TGF-β, reduce the levels of CRP, ESR and UA, and effectively relieve the symptoms of AGA.
3.1.2 Palmatine
Palmatine has been proved to have antipyretic, antibacterial and anti-inflammatory activities, it has been used as an anti-inflammatory agent in clinical practice (Pathan et al., 2015; Zhou et al., 2017). Palmatine has a good effect in the treatment of OA, it can effectively inhibit the expression of MMP-1, MMP-3 and matrix metalloproteinase-9 (MMP-9) induced by IL-1β by blocking Wnt1/β-catenin and Hedgehog signaling pathways, and improve OA (Zhou, 2014). Palmatine can inhibit expression of IL-1β and MMPs, it promotes the expression of cyclopamine which is inhibitor of the Hedgehog signaling pathway and suppress Wnt/β-atenin signaling pathway, to exert protective effect on OA and possess potential antalgic effect (Zhou et al., 2016). Research has shown that palmatine can improve joint swelling and significantly inhibit the expression of IL-1β, IL-6, IL-18, TNF-α in joint tissue, block the infiltration of inflammatory cells into the synovium and joint cavity, to achieve the therapeutic effect of GA (Cheng et al., 2022).
3.1.3 Other ingredients
Alkaloids in CC are the main anti-inflammatory active ingredients. Besides berberine and palmatine, coptisine, jarrorhizine hydrochloride and magnoflorine also play important roles in the anti-inflammatory effect of CC. The expression level of C-X-C motif chemokine 12 (CXCL12) in synovium of patients with OA was significantly increased, and C-X-C chemokine receptor 4 (CXCR4) was its receptor (Wei et al., 2010; Bragg et al., 2019). Coptisine, as a CXCR4 antagonist, inhibits the overexpression of ADAMTS4,5 in chondrocytes induced by CXCL12, improving cartilage degradation and subchondral bone damage (Yang et al., 2023). Coptisine inhibits the activation of NLRP3 inflammatory bodies by blocking caspase-1, which can be used to treat GA associated with NLRP3 inflammatory bodies (Wu et al., 2019). Jarrorhizine hydrochloride can significantly inhibit the expression levels of IL-1β, IL-6, IL-8, matrix metalloproteinase-2 (MMP-2), and MMP-3, suppress the proliferation, migration, and secretion of synovial cells, prevent bone destruction, and thus improve the severity of RA (Qiu et al., 2018). Magnoflorine has significant anti-inflammatory effect, which may improve RA by promoting the synthesis of proteoglycans in chondrocytes (Yue et al., 2013; Gui et al., 2015). Researches have shown that magnoflorine reduces IL-1β-induced inflammatory cytokine levels and inhibits inflammatory responses in AIA rats by regulating the PI3K/AKT/NF-κB signaling pathway (Shen et al., 2022). In a traumatic osteoarthritis model, magnoflorine can promote the proliferation, chondrogenesis and migration of cartilage progenitor cells by activating the chondrogenic signaling pathway, thereby directly reducing articular cartilage degeneration (Cai et al., 2020). By inhibiting the expression of TNF-α, IL-6, IL-1β, MCP-1, iNOS and IFN-β, magnoflorine improved the degree of joint destruction and macrophage infiltration in synovial tissue of CIA mice, and achieved the purpose of treating arthritis by inhibiting the activation of NF-κB and MAPK signaling pathways (Wang et al., 2023a).
3.2 The mechanism of Coptis chinensis in treating arthritis
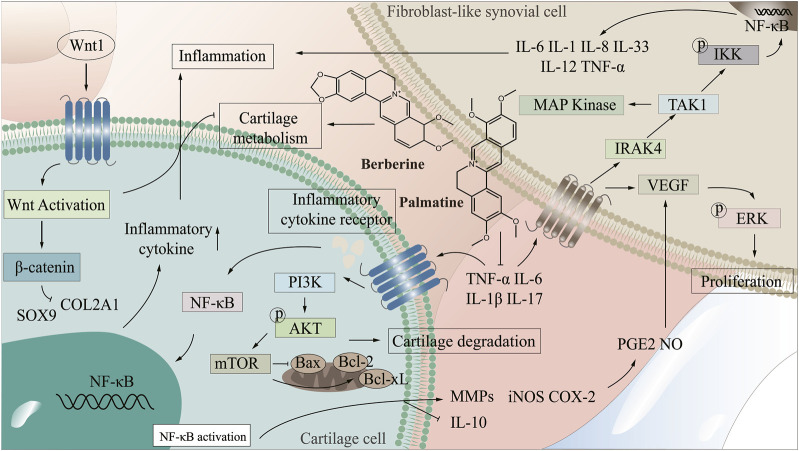
There are many uncomfortable symptoms in the clinical treatment of arthritis, and the development of natural drugs is greatly on demand. CC, as an antibacterial and anti-inflammatory Chinese medicine, presents an excellent potential for treating arthritis. By improving the targeting of CC to arthritic damaged tissues and enhancing the bioavailability of CC in treatment, it is promising to realize the development of novel natural medicines with enhanced curative effect and low side effects. Therefore, it is extremely important to clarify the mechanism of CC in treating arthritis. The NF-κB signaling pathway is one of the crucial pathways in the pathogenesis of arthritis, abnormal activation of which will lead to synovial inflammation, chondrocyte apoptosis and destruction (Qing et al., 2014; Wu, 2021; Zeng, 2022). PI3K/AKT/mTOR signaling pathway is a central regulator of cell growth, proliferation and cell cycle, and plays a significant role in chondrocyte degeneration (Xu et al., 2014). Wnt/β-catenin signaling pathway affects bone modeling and bone remodeling, especially the differentiation of osteoblasts, which may be a potential target for treating bone diseases (Wang et al., 2014). In the treatment of arthritis, CC can reduce the levels of IL-1β, TNF-α, IL-6, COX-2, NALP3 and TGF-β, regulate NF-κB and PI3K/AKT/mTOR signaling pathways, which promote the proliferation of articular chondrocytes, inhibit apoptosis, and enhance cell healing ability, thereby improving bone and joint, inhibiting bone destruction and reducing inflammation in joints and surrounding tissues (Fan et al., 2021; Jie et al., 2022; Liu et al., 2023), (Figure 2 demonstrated the mechanism of CC in the treatment of arthritis).
FIGURE 2.
The mechanism of CC in the treatment of arthritis.
3.2.1 Wnt1/β-catenin signaling pathway
Wnt1/β-catenin signaling pathway plays a key role in cell proliferation, differentiation and autoimmune regulation (Cici et al., 2019). Wnt1/β-catenin signaling pathway produces a significant role in tissue repair and joint homeostasis by regulating the activity of synovial cells, osteoblasts and chondrocytes in joint tissue, and it can cause a variety of arthritis when abnormal (Mei et al., 2019; Alharbi et al., 2022). FLS are important factors in osteoremodeling in arthritis, and Wnt1/β-catenin signaling pathway exert marked effects in the survival of FLS cells (Dinesh et al., 2020). Studies have shown that abnormal Wnt1/β-catenin signaling pathway is the main mechanism of RA (Miao et al., 2013). In RA, Wnt1/β-catenin pathway signal transduction results in polymorphic changes of osteocytes/chondrocytes, causing bone erosion and cartilage degradation (Sujitha et al., 2020). Wnt1 is mainly expressed in synovial cells, after the activation of Wnt signaling pathway, the expression of β-catenin increases, which promotes the secretion of inflammatory factors. RA-FLS is activated and induces RA, Cai et al. (2022) confirmed that blocking Wnt1/β-catenin signaling pathway and inhibiting TNF-induced migration, invasion and inflammation of RA-FLS cells can effectively alleviate adjuvant arthritis (AA). Some miRNAs can be used as inhibitors of Wnt1/β-catenin signaling pathway to further prevent RA (Miao et al., 2014; Sujitha et al., 2020) showed that berberine could inhibit Wnt1/β-catenin signal transduction through miR-23a activation, thereby improving RA. Wnt1/β-catenin signaling pathway controls bone and joint development and is closely related to the pathogenesis and progression of OA (Ahmad et al., 2020). IL-1β-induced chondrocyte degeneration may be accompanied by activation of Wnt/β-catenin pathway, which exerts an important effect in the degeneration and destruction of OA articular cartilage (Su et al., 2012). Sry-box transcription factor 9 (SOX9) has the function of promoting cartilage anabolism, and abnormal expression may lead to OA (Carmon et al., 2023; Alahdal et al., 2021) showed that activation of Wnt1/β-catenin pathway inhibited the expression of SOX9 and collagen type II, and impaired the cartilage differentiation and regeneration of MSCs in OA patients. (Li et al., 2022) demonstrated that MiR-376c-3p from Adipose mesenchymal stem cell (ADSC) derived exosomes regulated the Wnt1/β-catenin signaling pathway by targeting WNT3 or WNT9a, improving chondrocyte degradation and synovial fibrosis induced by OA. Lietman et al. (2018) found that regulation of Wnt pathway could improve OA symptoms in surgery-induced mouse OA model. Mei et al. (2019) confirmed that inhibition of Wnt1/β-catenin pathway signal transduction and regulation of β-catenin stability in macrophages could effectively improve GA. Berberine can induce Dvl-1 inhibitor-CYLD to inhibit the expression of FZD4, LRP5 and Dvl-1, regulate the Wnt1/β-catenin signaling pathway in adjuvant arthritis FLS cells, and reduce the expression level of intracellular β-catenin, thus improving arthritis (Shen et al., 2020). Palmatine inhibits the progression of OA by regulating the Wnt1/β-catenin signaling pathway (Xuan et al., 2019). Therefore, the Wnt1/β-catenin signaling pathway can be used as a potential target for treating various types of arthritis (Zhou, 2014; Shang et al., 2021).
3.2.2 PI3K/AKT/mTOR signaling pathway
PI3K/AKT/mTOR signaling pathway is mainly mediated by growth factor signal transduction to lipid metabolism, protein synthesis and cell proliferation and survival, and other physiological processes, and it is related to inflammation, autoimmune diseases and hematological malignancies, affecting cell proliferation, differentiation, metastasis and apoptosis (Foster et al., 2012; Abeyrathna and Su, 2015; Liu et al., 2021). The PI3K/AKT/mTOR signaling pathway is crucial for the normal metabolism of joint tissues and is closely related to the occurrence and development of OA and RA (Sun et al., 2020; Zhou et al., 2023; Zhang et al., 2001) found that the spontaneous and induced activation of AKT and the level of pAKT in RA patients were higher than those in OA patients. (Dinesh and Rasool, 2019). demonstrated that PI3K/AKT signaling pathway could be used as a key target for RA treatment, and inhibition of abnormal activation of PI3K/AKT signaling pathway played a key role in the prevention and treatment of RA (Ansari et al., 2022; Hashiramoto et al., 2007) confirmed that abnormal PI3K/AKT signaling pathway can lead to RA synovial overgrowth and joint destruction. Abnormal activation of PI3K/AKT signaling pathway can increase the expression level of anti-apoptotic genes in synovial cells of RA patients, and then leads to the exacerbation of RA disease (Harris et al., 2009; Smith et al., 2010). Wang (Wang, 2020) found that downregulation of PI3K/AKT pathway and inhibition of over-activation of AKT could effectively improve RA. Studies have shown that activation of PI3K/AKT signaling pathway leads to accelerated proliferation of FLS cells in AA and aggravation of the course of arthritis (Dinesh and Rasool, 2019). Abnormal activation of PI3K/AKT/mTOR signaling pathway will destroy the normal function of cartilage and subchondral bone (Sun et al., 2020). As immune cells, synovial macrophages are closely related to the occurrence and development of OA. Activated macrophages are regulated by the PI3K/AKT signaling pathway, and their activation status is highly correlated with the severity of OA (Zhang et al., 2020). Inhibition of PI3K/AKT/mTOR signaling pathway activates autophagy, promotes anabolism and inhibits catabolism of OA chondrocytes, and effectively treats OA (Wang, 2022). Studies have shown that quercetin regulates PI3K/AKT signaling pathway to improve arthritis by binding to and inhibiting PI3K in mouse epidermal cells to inhibit AKT phosphorylation (Khan et al., 2019). Berberine delays the progression of osteoporosis, RA and OA by regulating the PI3K/AKT signaling pathway (Wong et al., 2020). Therefore, the key proteins in PI3K/AKT signaling pathway can be used as potential targets of CC in the treatment of arthritis for in-depth study.
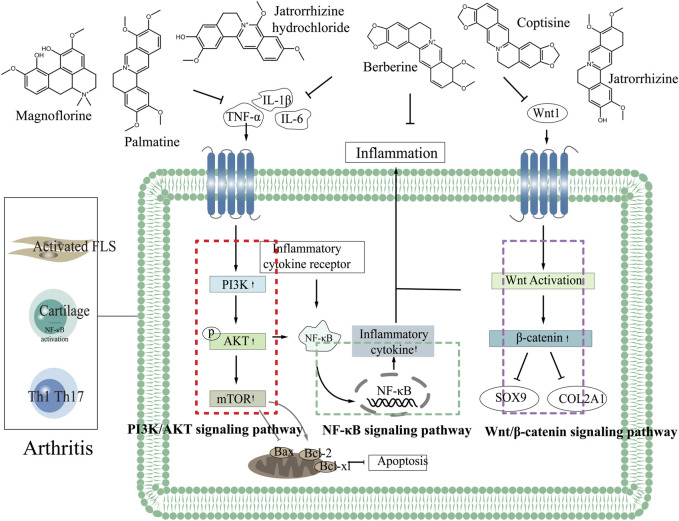
By combining the pathogenesis of three types of arthritis with the related targets and pathways of CC in treating arthritis, we elucidated the mechanism, providing a new idea for the development of CC as a candidate drug for treating arthritis. Most of the components in CC in treating arthritis are alkaloids. Six components including berberine in CC have good effects in the treatment of arthritis, which can inhibit the expression of TNF-α, IL-6, IL-1β through PI3K/AKT, Wnt1/β-catenin and NF-κB signaling pathways, thereby reducing the inflammatory response and achieving the purpose of treating arthritis (Figure 3 demonstrated the chemical constituents and the pathways involved in the treatment of arthritis by CC).
FIGURE 3.
The chemical constituents and the pathways involved in the treatment of arthritis by CC.
4 Deep exploration based on potential therapeutic targets for arthritis
In this review, we have summarized the targets involved in the pathogenesis of arthritis, elaborated potential therapeutic targets, and provided a new idea for the exploration of the mechanism of CC in the treatment of arthritis. Studies have shown that CC can treat a variety of arthritis, and it is better for OA, RA and GA. CC has the characteristics of multiple components, multiple targets, and multiple pathways in the treatment of arthritis. Currently, IL-10, IL-1β, IL-6, MMP-3, TNF-α and other targets have been verified to play a role in treating arthritis by CC (Table 2) (Sun et al., 2011; Yuan et al., 2014; Kienhorst et al., 2015; Huang et al., 2019; Thomas, 2020; Che et al., 2021; Li et al., 2021; Wu et al., 2021; Arra et al., 2022; Zhang et al., 2022; Cao et al., 2022; Du et al., 2022; Pacifici, 2022; Wirth et al., 2022; Xiao et al., 2022; Yang et al., 2022; Sun et al., 2023a; Barbarroja et al., 2023; Li et al., 2023; Brix et al., 2023; Wang et al., 2023b; Wang et al., 2023; Ghosh et al., 2023; González-Chávez et al., 2023; Hecquet et al., 2023; Jahantigh et al., 2023; Ji et al., 2023; La Bella et al., 2023; Leung et al., 2023; Lin et al., 2023; Luo et al., 2023; Zeng et al., 2023; Zhang et al., 2023). However, the types of arthritis treated with these targets are not completely clear. Targets, β2-adrenergic receptor (ADRB2), AHR, CRP, IRF1, prostaglandin G/H synthase 1 (PTGS1), SPP1 and other targets, exert key effects in the pathogenesis of arthritis, but role of them in the treatment of arthritis with CC has not been confirmed (Table 3) (Xu et al., 2004; Koskela et al., 2012; Ko et al., 2013; Li et al., 2013; Xue et al., 2014; Song et al., 2016; Trzybulska et al., 2018; Bonelli et al., 2019; Chu et al., 2019; Lin et al., 2019; Sun et al., 2020; Orecchini et al., 2020; Yang et al., 2020; Nalesso et al., 2021; Tang et al., 2021; Xu and Xu, 2021; Zou et al., 2021; Balasundaram et al., 2022; Cai et al., 2022; Liu et al., 2022; Wang et al., 2022; Zhang et al., 2022; Zhang et al., 2022; Han et al., 2022; Man et al., 2022; Nguyen et al., 2022; Qian et al., 2022; Tavallaee et al., 2022; Zhuang et al., 2022; Afnan et al., 2023; Baggio et al., 2023; Sun et al., 2023b; Zhou et al., 2023; Fan et al., 2023; Huang et al., 2023; Ning et al., 2023; Qin et al., 2023; Skougaard et al., 2023; Tu et al., 2023; Xie et al., 2023; Yan et al., 2023; Yu et al., 2023; Zhao et al., 2023). We have elaborated on the role of targets such as MMP-3, IL-1β, MAPK, IL-6, ADRB2, AHR, CRP, CAV1, CXCL2, SPP1 and other targets in treating arthritis, explored the potential targets and mechanisms of CC in treating arthritis, analyzed the feasibility of CC as an anti-arthritis drug, and provided a theoretical basis for subsequent research.
TABLE 2.
Verified targets involved in the treatment of arthritis by CC.
TABLE 3.
Targets in the pathogenesis of arthritis.
| Target | Protein ID | Arthritis type | Expression in arthritis | Literature |
|---|---|---|---|---|
| ADRB2 | Beta-2 adrenergic receptor | OA, RA | Upregulated | Xu et al. (2004), Sun et al. (2020b) |
| AHR a | Aryl hydrocarbon receptor | RA, CIA, OA | Downregulated | Zhuang et al. (2022), Huang et al. (2023) |
| Bax | Apoptosis regulator BAX | OA, RA, CIA, psoriatic arthritis (PsA), AIA | Downregulated | Shen et al. (2022), Zhou et al. (2023a), Baggio et al. (2023) |
| Bcl-2 | Apoptosis regulator Bcl-2 | Upregulated | ||
| BCL2L1 | Bcl-2-like protein 1 | OA, RA | Downregulated | Koskela et al. (2012), Yang et al. (2020) |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 | RA | Upregulated | Balasundaram et al. (2022) |
| CAV1 a | Caveolin-1 | CIA, RA, AA | Upregulated | Song et al. (2016), Trzybulska et al. (2018), Zou et al. (2021) |
| CCND1 | G1/S-specific cyclin-D1 | OA | Upregulated | Man et al. (2022) |
| COL3A1 | Collagen alpha-1(III) chain | OA | Upregulated | Han et al. (2022) |
| CRP a | C-reactive protein | RA, AIA, PsA | Upregulated | Afnan et al. (2023), Skougaard et al. (2023), Yu et al. (2023) |
| CDKN1A | Cyclin-dependent kinase inhibitor 1 | RA, OA, CIA | Downregulated | Chu et al. (2019), Liu et al. (2022b), Fan et al. (2023) |
| CXCL2 a | C-X-C motif chemokine 2 | RA, CIA, PsA | Upregulated | Zhang et al. (2022c), Jie et al. (2022), Nguyen et al. (2022) |
| HMOX1 | Heme oxygenase 1 | OA | Downregulated | Nalesso et al. (2021) |
| IRF1 a | Interferon regulatory factor-1 | RA, OA | Upregulated | Bonelli et al. (2019), Zhao et al. (2023) |
| MMP-2 | Matrix metalloproteinase-2 | RA, OA, CIA, AIA | Upregulated | Ko et al. (2013), Li et al. (2013), Xue et al. (2014), Cai et al. (2022a) |
| MMP-9 | Matrix metalloproteinase-9 | |||
| NOS2 | Nitric oxide synthase | OA, GA, CIA | Upregulated | Orecchini et al. (2020), Zhang et al. (2022d), Zhou et al. (2023b) |
| PPARG | Peroxisome proliferator-activated receptor gamma | RA, OA | Downregulated | Tavallaee et al. (2022), Qin et al. (2023) |
| PTGS1 | Prostaglandin G/H synthase 1 | RA, OA | Upregulated | Qian et al. (2022), Tu et al. (2023) |
| PTGS2 | Prostaglandin G/H synthase 2 | |||
| SPP1 a | Secreted phosphoprotein 1 | RA, OA, CIA | Upregulated | Lin et al. (2019), Cai et al. (2022b), Du et al. (2022) |
| STAT1 | Signal transducer and activator of transcription 1-alpha/beta | RA, OA, CIA | Upregulated | Tang et al. (2021), Xu and Xu (2021), Sun et al. (2023b) |
| TGFB1 | Transforming growth factor beta-1 proprotein | RA, OA, GA, CIA | Upregulated | Wang et al. (2022b), Ning et al. (2023), Xie et al. (2023), Yan et al. (2023) |
Key targets in the pathogenesis of arthritis.
IL-6 plays an important role in the development of RA (Cheng et al., 2022), and it is associated with inflammatory response and cartilage loss in the pathogenesis of OA (Hou et al., 2020). IL-10 is an important anti-inflammatory and immunosuppressive cytokine that not only prevents the occurrence of arthritis, but also has an inhibitory effect on the development of arthritis (Charbonnier et al., 2010). IL-1β is related to the inflammation of synovium, which can affect the normal metabolism of chondrocytes, change the structure and function of osteocytes, promote the apoptosis of chondrocytes and the decomposition of cartilage matrix, and it plays a key role in the pathogenesis of arthritis (Bai, 2021). TNF-α is a pro-inflammatory cytokine secreted by membrane-forming FLS and mainly distributed in the joint space of RA, anti-TNF therapy is the preferred therapy for severe RA patients (Taylor et al., 2022). Activated ADRB2 in osteoblasts stimulates osteoclastogenesis and upregulates RANKL expression, thereby reducing bone formation and promoting bone resorption, leading to bone loss and osteoarthritis (Ma et al., 2011; Liang et al., 2018). AHR can be used as a key target for the treatment of RA (Xi et al., 2022), it has a variety of potential roles in the immune system. Various natural products can alleviate synovial inflammation and restore immune balance in RA patients by binding to AHR in fibroblast-like synovial cells and T cells (Stockinger et al., 2014; Hui and Dai, 2020). The expression of anti-apoptotic proteins Bcl-xl increased significantly in arthritis patients (Chen et al., 2016). CAV1 is a regulator of various cell signaling pathways. Reducing the expression of CAV1 can inhibit the expression of IL-1β-induced CCL2 mRNA and promote the apoptosis of RA-FLS (Li et al., 2017). CXCL2 promotes osteoclast formation and is associated with bone erosion in RA. Studies have shown that blocking expression of CXCL2 may be a means of treating RA (Wang et al., 2021). IRF1 promotes chondrogenesis of hADSCs by up-regulating HILPDA level, and it provides a new biomarker for the treatment of osteoarthritis (Zhao et al., 2023). As a proteolytic enzyme secreted by synovial fibroblasts, MMPs are involved in the pathogenesis of arthritis and play an important role in inflammatory response and joint destruction (Murphy and Nagase, 2008). The levels of MMP-2 and MMP-9 are elevated in the serum of RA patients, which can reflect the early inflammatory level of RA (Hu et al., 2011). MMP-3 plays a key role in the pathogenesis of RA and is one of the key indicators for the treatment of RA (Lerner et al., 2018) Studies have shown that when PTGS1 is overexpressed, the migration and invasion of OA synovial cells increase, and the apoptosis rate decreases (Wang et al., 2019). In collagen induced arthritis, SPP1 secreted by FLSs promotes the formation of osteoclast through PI3K/AKT signals. Regulating the expression of SPP1 gene in FLSs may be a potential method to treat RA bone injury in the joint microenvironment (Cai et al., 2022). Therefore, ADRB2, AHR, CRP, IRF1, PTGS1, SPP1 and other targets can be used as potential targets for CC in the treatment of arthritis, and it is of great significance to explore its role of CC in the treatment of arthritis.
5 Conclusion
TCM plays an important role in the treatment of arthritis due to its multi-component, multi-efficacy and multi-target characteristics. CC, as an antibacterial and anti-inflammatory Chinese medicine, has a good effect in treating arthritis. In this review, we have summarized the chemical constituents, targets and related pathways of CC in treating arthritis, discussed the mechanism of CC in the treatment of arthritis from the molecular level, clarified the potential targets, and provided reasonable directions for clinical treatment of arthritis. Berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC have the effect of treating arthritis, especially berberine can treat a variety of arthritis, such as RA, OA and GA. Berberine improves arthritis by reducing cell inflammation, improving chondrocyte function, promoting cartilage synthesis and repair, and promoting uric acid excretion. Palmatine can significantly block the Wnt1/β-catenin signaling pathway, protect chondrocytes and knee cartilage, and inhibit the progression of OA. At the molecular level, six components including berberine can improve RA, OA, GA and other types of arthritis by regulating PI3K/AKT, Wnt1/β-catenin and NF-кB signaling pathways. IL-10, IL-1β, IL-6, MMP-3, TNF-α, COX-2, TGF-β, Caspase-1, MAPK and other targets have been confirmed to play key roles in the treatment of arthritis by CC, and can be used as targets for clinical treatment of arthritis, providing scientific basis for the development of rational targeted drugs for the treatment of arthritis. AHR, CAV1, CRP, CXCL2, IRF1, SPP1 and other targets play important roles in the pathogenesis of arthritis and can be used as key targets for the treatment of arthritis. However, the role of them of CC in the treatment of arthritis remains to be further verified.
All in all, berberine, palmatine, coptisine, jatrorrhizine, magnoflorine and jatrorrhizine hydrochloride in CC can effectively treat arthritis, and have been proved. At the molecular level, CC plays a critical role in the treatment of arthritis by regulating NF-κB, Wnt1/β-catenin and PI3K/AKT/mTOR signaling pathway, inhibiting the expression of IL-6, IL-1β, MMP-3 and TNF-α. In this review, we have concluded with a summary and our insights on the chemical components, targets and pathways of CC in the treatment of arthritis, and discussed the relevant mechanism and potential targets, providing scientific basis for CC in the clinical treatment of arthritis.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant No. 81973699, No. 82274361).
Author contributions
ML: Conceptualization, methodology, writing–original draft, software. FT: Software, writing–review and editing. JG: Formal analysis. XL: Formal analysis. LM: Resources. MJ: Supervision, Project administration. JZ: Supervision, funding acquisition. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| CC | Coptis chinensis |
| RA | Rheumatoid arthritis |
| OA | Osteoarthritis |
| GA | Gouty arthritis |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-1 | Interleukin-1 |
| TNF-α | Tumor necrosis factor-α |
| iNOS | Inducible nitric oxide synthase |
| IL-10 | Interleukin-10 |
| IL-17 | Interleukin-17 |
| MAPK | Mitogen-activated protein kinase |
| MMP-1 | Matrix metalloproteinase-1 |
| MMP-2 | Matrix metalloproteinase-2 |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-9 | Matrix metalloproteinase-9 |
| MMP-13 | Matrix metalloproteinase-13 |
| MMPs | Matrix metalloproteinases |
| AHR | Aryl hydrocarbon receptor |
| CAV1 | Caveolin-1 |
| CRP | C-reactive protein |
| CXCL2 | C-X-C motif chemokine 2 |
| IRF1 | Interferon regulatory factor-1 |
| SPP1 | Secreted phosphoprotein 1 |
| TCM | Traditional Chinese medicine |
| COX-2 | Cycloooxygenase-2 |
| MSU | Monosodium urate |
| UA | Uric acid |
| FLS | Fibroblast-like synoviocytes |
| Bax | Apoptosis regulator BAX |
| Bcl-2 | Apoptosis regulator Bcl-2 |
| TGF-β | Transforming growth factor-β |
| IFN-γ | Interferon-γ |
| RORγt | RAR-related orphan receptor γt |
| CCN2 | Connective tissue growth factor |
| HIF-1α | Hypoxia inducible factor 1α |
| PGE2 | Prostaglandin E2 |
| NLRP3 | NOD-like receptor thermal protein domain 3 |
| AGA | Acute gouty arthritis |
| NALP3 | Nucleotide-binding oligomerization domain-like receptor 3 |
| CXCL12 | C-X-C motif chemokine 12 |
| CXCR4 | C-X-C chemokine receptor 4 |
| AA | Adjuvant arthritis |
| ADSC | Adipose mesenchymal stem cell |
| ADRB2 | β2-adrenergic receptor |
| PTGS1 | Prostaglandin G/H synthase 1 |
| SOX9 | Sry-box transcription factor 9 |
References
- AbdullGaffar B., Abdul Hameed B., Fodeh S., Sreeram R. (2020). Concomitant gouty and tuberculous granulomatous arthritis. Int. J. Surg. Pathol. 28, 288–289. 10.1177/1066896919865778 [DOI] [PubMed] [Google Scholar]
- Abeyrathna P., Su Y. (2015). The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 74, 38–48. 10.1016/j.vph.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afnan A., Saleem A., Akhtar M. F. (2023). Chrysin, a 5,7-dihydroxyflavone restrains inflammatory arthritis in rats via subsiding oxidative stress biomarkers and inflammatory cytokines. Inflammopharmacology 47, 1863–1878. 10.1007/s10787-023-01229-6 [DOI] [PubMed] [Google Scholar]
- Ahmad N., Ansari M. Y., Haqqi T. M. (2020). Role of iNOS in osteoarthritis: pathological and therapeutic aspects. J. Cell. Physiol. 235, 6366–6376. 10.1002/jcp.29607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahdal M., Huang R., Duan L., Zhiqin D., Hongwei O., Li W., et al. (2021). Indoleamine 2, 3 dioxygenase 1 impairs chondrogenic differentiation of mesenchymal stem cells in the joint of osteoarthritis mice model. Front. Immunol. 12, 781185. 10.3389/fimmu.2021.781185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi K. S., Afzal O., Altamimi A. S. A., Almalki W. H., Kazmi I., Al-Abbasi F. A., et al. (2022). Potential role of nutraceuticals via targeting a Wnt/β-catenin and NF-κB pathway in treatment of osteoarthritis. J. Food Biochem. 46 (2022), e14427. 10.1111/jfbc.14427 [DOI] [PubMed] [Google Scholar]
- Ansari B., Aschner M., Hussain Y., Efferth T., Khan H. (2022). Suppression of colorectal carcinogenesis by naringin. Phytomedicine 96 (2022), 153897. 10.1016/j.phymed.2021.153897 [DOI] [PubMed] [Google Scholar]
- Arra M., Swarnkar G., Alippe Y., Mbalaviele G., Abu-Amer Y. (2022). IκB-ζ signaling promotes chondrocyte inflammatory phenotype, senescence, and erosive joint pathology. Bone Res. 10, 12. 10.1038/s41413-021-00183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asila A., Liu J., Liu J., Liao J. (2022). Immunomodulatory effects of berberine on Staphylococcus aureus-induced septic arthritis through down-regulation of Th17 and Treg signaling pathways. Acta. Biochim. Pol. 69, 215–226. 10.18388/abp.2020_5948 [DOI] [PubMed] [Google Scholar]
- Baggio C., Luisetto R., Boscaro C., Scanu A., Ramonda R., Albiero M., et al. (2023). Leucocyte abnormalities in synovial fluid of degenerative and inflammatory arthropathies. Int. J. Mol. Sci. 24, 5450. 10.3390/ijms24065450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M. X. (2021). Study on the protective effect of Calcitonin on rat chondrocyte injury induced by IL-1β. Shandong Province: Shandong University. [Google Scholar]
- Balasundaram A., Udhaya Kumar S., George Priya Doss C. (2022). A computational model revealing the immune-related hub genes and key pathways involved in rheumatoid arthritis (RA). Adv. Protein Chem. Struct. Biol. 129, 247–273. 10.1016/bs.apcsb.2021.11.006 [DOI] [PubMed] [Google Scholar]
- Barbarroja N., López-Montilla M. D., Cuesta-López L., Pérez-Sánchez C., Ruiz-Ponce M., López-Medina C., et al. (2023). Characterization of the inflammatory proteome of synovial fluid from patients with psoriatic arthritis: potential treatment targets. Front. Immunol. 14 (2023), 1133435. 10.3389/fimmu.2023.1133435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A., Oakden-Rayner L., McMaster C., Smith L. A., Zeng M., Wechalekar M. D., et al. (2022). Artificial intelligence and the future of radiographic scoring in rheumatoid arthritis: a viewpoint. Arthritis Res. Ther. 24, 268. 10.1186/s13075-022-02972-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli M., Dalwigk K., Platzer A., Olmos Calvo I., Hayer S., Niederreiter B., et al. (2019). IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes: JAKinibs suppress the interferon response in RA-FLSs. Exp. Mol. Med. 51, 75. 10.1038/s12276-019-0267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini N., Firestein G. S. (2013). Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 9, 24–33. 10.1038/nrrheum.2012.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg R., Gilbert W., Elmansi A. M., Isales C. M., Hamrick M. W., Hill W. D., et al. (2019). Stromal cell-derived factor-1 as a potential therapeutic target for osteoarthritis and rheumatoid arthritis. Ther. Adv. Chronic. Dis. 10, 2040622319882531. 10.1177/2040622319882531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix N., Glerup M., Foell D., Kessel C., Wittkowski H., Berntson L., et al. (2023). Inflammatory biomarkers can differentiate acute lymphoblastic leukemia with arthropathy from juvenile idiopathic arthritis better than standard blood tests. J. Pediatr. 258, 113406. 10.1016/j.jpeds.2023.113406 [DOI] [PubMed] [Google Scholar]
- Cai L., Zhou M. Y., Hu S., Liu F. Y., Wang M. Q., Wang X. H., et al. (2022a). Umbelliferone inhibits migration, invasion and inflammation of rheumatoid arthritis fibroblast-like synoviocytes and relieves adjuvant-induced arthritis in rats by blockade of wnt/β-catenin signaling pathway. Am. J. Chin. Med. 50, 1945–1962. 10.1142/s0192415x22500835 [DOI] [PubMed] [Google Scholar]
- Cai X., Zheng Y., Ren F., Zhang S., Wu L., Yao Y. (2022b). Secretory phosphoprotein 1 secreted by fibroblast-like synoviocytes promotes osteoclasts formation via PI3K/AKT signaling in collagen-induced arthritis. Biomed. Pharmacother. 155 (2022), 113687. 10.1016/j.biopha.2022.113687 [DOI] [PubMed] [Google Scholar]
- Cai Z., Hong M., Xu L., Yang K., Li C., Sun T., et al. (2020). Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 126, 109733. 10.1016/j.biopha.2019.109733 [DOI] [PubMed] [Google Scholar]
- Cao D., Fan Q., Li Z., Chen M., Jiang Y., Lin R., et al. (2022). Transcriptomic profiling revealed the role of apigenin-4′-O-α-L-rhamnoside in inhibiting the activation of rheumatoid arthritis fibroblast-like synoviocytes via MAPK signaling pathway. Phytomedicine 102 (2022), 154201. 10.1016/j.phymed.2022.154201 [DOI] [PubMed] [Google Scholar]
- Carmon I., Zecharyahu L., Elayyan J., Meka S. R. K., Reich E., Kandel L., et al. (2023). HU308 mitigates osteoarthritis by stimulating sox9-related networks of carbohydrate metabolism. J. Bone Min. Res. 38, 154–170. 10.1002/jbmr.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnier L. M., Han W. G., Quentin J., Huizinga T. W. J., Zwerina J., Toes R. E. M., et al. (2010). Adoptive transfer of IL-10-secreting CD4+CD49b+ regulatory T cells suppresses ongoing arthritis. J. Autoimmun. 34, 390–399. 10.1016/j.jaut.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Che N., Sun X., Gu L., Wang X., Shi J., Sun Y., et al. (2021). Adiponectin enhances B-cell proliferation and differentiation via activation of akt1/STAT3 and exacerbates collagen-induced arthritis. Front. Immunol. 12, 626310. 10.3389/fimmu.2021.626310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Xie W. G., Zhou J. G. (2021). Difference and significance of thymidine kinase 1 expression in gouty arthritis, rheumatoid arthritis and osteoarthritis. J. Chengdu Med. Coll. 16, 730–733. 10.3969/j.issn.1674-2257.2021.06.011 [DOI] [Google Scholar]
- Chen H., Pan J., Wang J. D., Liao Q. M., Xia X. R. (2016). Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, induces apoptosis in rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 39, 39–46. 10.1007/s10753-015-0220-3 [DOI] [PubMed] [Google Scholar]
- Chen H., Zhao J., Hu J., Xiao X., Shi W., Yao Y., et al. (2022a). Identification of diagnostic biomarkers, immune infiltration characteristics, and potential compounds in rheumatoid arthritis. Biomed. Res. Int. 2022, 1926661. 10.1155/2022/1926661 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen J., Liu J., Chen S., Lai R., Zheng C., Lu J., et al. (2022b). Salinomycin alleviates osteoarthritis progression via inhibiting Wnt/β-catenin signaling. Int. Immunopharmacol. 112, 109225. 10.1016/j.intimp.2022.109225 [DOI] [PubMed] [Google Scholar]
- Chen L., Liu X., Wang X., Lu Z., Ye Y. (2023). Berberine alleviates acute lung injury in septic mice by modulating Treg/Th17 homeostasis and downregulating NF-κB signaling. Drug Des. devel. Ther. 17, 1139–1151. 10.2147/dddt.s401293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. J., Ma X. D., Ai G. X., Yu Q. X., Chen X. Y., Yan F., et al. (2022a). Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2 pathways. Drug Des. devel. Ther. 16, 2119–2132. 10.2147/dddt.s356307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Chen J., Rong X. (2022b). Mechanism of emodin in the treatment of rheumatoid arthritis. Evid. Based Complement. Altern. Med. 2022, 9482570. 10.1155/2022/9482570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J. Y., Jung H. Y., Park K. Y., Kim S. K. (2014). Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatol. Oxf. 53, 1043–1053. 10.1093/rheumatology/ket474 [DOI] [PubMed] [Google Scholar]
- Chu Y., Wang J., Zhou X. (2019). Mast cell chymase in synovial fluid of collagen-induced-arthritis rats regulates gelatinase release and promotes synovial fibroblasts proliferation via FAK/p21 signaling pathway. Biochem. Biophys. Res. Commun. 514, 336–343. 10.1016/j.bbrc.2019.04.121 [DOI] [PubMed] [Google Scholar]
- Cici D., Corrado A., Rotondo C., Cantatore F. P. (2019). Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int. J. Mol. Sci. 20, 5552. 10.3390/ijms20225552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F. (1989). Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J. Clin. Invest. 84, 678–685. 10.1172/JCI114215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinesh P., Kalaiselvan S., Sujitha S., Rasool M. (2020). MiR-145-5p mitigates dysregulated Wnt1/β-catenin signaling pathway in rheumatoid arthritis. Int. Immunopharmacol. 82, 106328. 10.1016/j.intimp.2020.106328 [DOI] [PubMed] [Google Scholar]
- Dinesh P., Rasool M. (2019). Berberine mitigates IL-21/IL-21R mediated autophagic influx in fibroblast-like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis 24, 644–661. 10.1007/s10495-019-01548-6 [DOI] [PubMed] [Google Scholar]
- Dinesh P., Rasool M. (2017). Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 44, 26–37. 10.1016/j.intimp.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Du J., Zheng L., Chen S., Wang N., Pu X., Yu D., et al. (2022). NFIL3 and its immunoregulatory role in rheumatoid arthritis patients. Front. Immunol. 13 (2022), 950144. 10.3389/fimmu.2022.950144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkomy M. H., Alruwaili N. K., Elmowafy M., Shalaby K., Zafar A., Ahmad N., et al. (2022). Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics 14, 563. 10.3390/pharmaceutics14030563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. D., Tan P. Y., Jin L., Qu Y., Yu Q. H. (2023). Bioinformatic identification and validation of autophagy-related genes in rheumatoid arthritis. Clin. Rheumatol. 42, 741–750. 10.1007/s10067-022-06399-2 [DOI] [PubMed] [Google Scholar]
- Fan X. L., Yang X. H., Zhou X. (2021). Clinical efficacy of berberine in treatment of acute gouty arthritis and its effect on serum levels of inflammation factors. J. Pract. Med. 37, 2413–2417+22. [DOI] [Google Scholar]
- Fan X. X., Leung E. L., Xie Y., Liu Z. Q., Zheng Y. F., Yao X. J., et al. (2018). Suppression of lipogenesis via reactive oxygen species-AMPK signaling for treating malignant and proliferative diseases. Antioxid. Redox. Signal. 28, 339–357. 10.1089/ars.2017.7090 [DOI] [PubMed] [Google Scholar]
- Fan X. X., Xu M. Z., Leung E. L. H., Jun C., Yuan Z., Liu L. (2020). ROS-responsive berberine polymeric micelles effectively suppressed the inflammation of rheumatoid arthritis by targeting mitochondria. Nano-Micro Lett. 12, 76. 10.1007/s40820-020-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q., Zhou C., Nandakumar K. S. (2020). Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat. Inflamm. 2020, 3830212. 10.1155/2020/3830212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. G., Blunt M. D., Carter E., Ward S. G. (2012). Inhibition of PI3K signaling spurs new therapeutic opportunities in inflammatory/autoimmune diseases and hematological malignancies. Pharmacol. Rev. 64, 1027–1054. 10.1124/pr.110.004051 [DOI] [PubMed] [Google Scholar]
- Fu L., Fu Q., Li J. (2021). Research progress on chemical constituents and pharmacological effects of Coptis chinensis. Acta. Chin. Med. Pharmacol. 49, 87–92. 10.19664/j.cnki.1002-2392.210044 [DOI] [Google Scholar]
- Gai X. H., Liu S. X., Ren T T. (2018). Research progress on chemical constituents and pharmacological effects of Coptis chinensis. Chin. Tradit. Herb. Drugs. 49, 4919–4927. 10.7501/j.issn.0253-2670.2018.20.032 [DOI] [Google Scholar]
- Geng Y. N. (2018). Berberine protects inflammatory injury of pancreatic beta cells induced by cytokines. Tianjin: Tianjin Medical University. [Google Scholar]
- Ghosh R., Dey R., Sawoo R., Bishayi B. (2023). Simultaneous neutralization of TGF-β and IL-6 attenuates Staphylococcus aureus-induced arthritic inflammation through differential modulation of splenic and synovial macrophages. Scand. J. Immunol. 97, e13252. 10.1111/sji.13252 [DOI] [PubMed] [Google Scholar]
- González-Chávez S. A., López-Loeza S. M., Acosta-Jiménez S., Cuevas-Martínez R., Pacheco-Silva C., Chaparro-Barrera E., et al. (2023). Low-intensity physical exercise decreases inflammation and joint damage in the preclinical phase of a rheumatoid arthritis murine model. Biomolecules 13, 488. 10.3390/biom13030488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y., Qiu X., Xu Y., Li D., Wang L. (2015). Bu-Shen-Ning-Xin decoction suppresses osteoclastogenesis via increasing dehydroepiandrosterone to prevent postmenopausal osteoporosis. Biosci. Trends 9, 169–181. 10.5582/bst.2015.01011 [DOI] [PubMed] [Google Scholar]
- Han Y., Wu J., Gong Z., Zhou Y., Li H., Chen Y., et al. (2022). Identification and development of the novel 7-genes diagnostic signature by integrating multi cohorts based on osteoarthritis. Hereditas 159, 10. 10.1186/s41065-022-00226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. J., Foster J. G., Ward S. G. (2009). PI3K isoforms as drug targets in inflammatory diseases: lessons from pharmacological and genetic strategies. Curr. Opin. Investig. Drugs. 10, 1151–1162. 10.1016/j.cct.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Hashiramoto A., Sakai C., Yoshida K., Tsumiyama K., Miura Y., Shiozawa K., et al. (2007). Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphatidylinositol 3-kinase/Akt. Arthritis Rheum. 56, 2170–2179. 10.1002/art.22727 [DOI] [PubMed] [Google Scholar]
- He X. Y., Shuai S. Q., Dang W. T. (2018). Research progress of berberine in the regulation of inflammation. J. Med. West Chin. 30, 1714–1717. 10.3969/j.issn.1672-3511.2018.11.035 [DOI] [Google Scholar]
- Hecquet S., Combier A., Steelandt A., Pons M., Wendling D., Molto A., et al. (2023). Characteristics of patients with difficult-to-treat rheumatoid arthritis in a French single-centre hospital. Rheumatology 2023, kead143. 10.1093/rheumatology/kead143 [DOI] [PubMed] [Google Scholar]
- Hou S. M., Chen P. C., Lin C. M., Fang M. L., Chi M. C., Liu J. F. (2020). CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res. Ther. 22, 251. 10.1186/s13075-020-02331-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. Y., Mo Z. X. (2017). Research progress on pharmacological actions and mechanism of berberine. Chin. J. Exp. Tradit. Med. Formulae. 23, 213–219. 10.13422/j.cnki.syfjx.2017200213 [DOI] [Google Scholar]
- Hu P. F., Chen W. P., Tang J. L., Bao J. p., Wu L. d. (2011a). Protective effects of berberine in an experimental rat osteoarthritis model. Phytother. Res. 25, 878–885. 10.1002/ptr.3359 [DOI] [PubMed] [Google Scholar]
- Hu W., Rong C., Chen F. H. (2011b). Research progress on the role of matrix metalloproteinases in the pathogenesis of rheumatoid arthritis. J. Anhui Med. 32, 671–672. 10.3969/j.issn.1000-0399.2011.05.046 [DOI] [Google Scholar]
- Hu Y., Hu Z., Wang S., Dong X., Xiao C., Jiang M., et al. (2013). Protective effects of Huang-Lian-Jie-Du-Tang and its component group on collagen-induced arthritis in rats. J. Ethnopharmacol. 150, 1137–1144. 10.1016/j.jep.2013.10.038 [DOI] [PubMed] [Google Scholar]
- Hu Z., Jiao Q., Ding J., Liu F., Liu R., Shan L., et al. (2010). Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Care Res. 63, 949–959. 10.1002/art.30202 [DOI] [PubMed] [Google Scholar]
- Huang C. C., Chiou C. H., Liu S. C., Hu S. L., Su C. M., Tsai C. H., et al. (2019). Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: implications for the treatment of rheumatoid arthritis. J. Pineal. Res. 66, e12560. 10.1111/jpi.12560 [DOI] [PubMed] [Google Scholar]
- Huang D. N., Wu F. F., Zhang A. H., Sun H., Wang X. J. (2021a). Efficacy of berberine in treatment of rheumatoid arthritis: from multiple targets to therapeutic potential. Pharmacol. Res. 169, 105667. 10.1016/j.phrs.2021.105667 [DOI] [PubMed] [Google Scholar]
- Huang T., Cheng L., Jiang Y., Zhang L., Qian L. (2023). Indole-3-pyruvic acid alleviates rheumatoid arthritis via the aryl hydrocarbon receptor pathway. Ann. Transl. Med. 11, 213. 10.21037/atm-23-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ma S. T., Yao J. (2021b). Efficiency limits of concentrating spectral-splitting hybrid photovoltaic-thermal (PV-T) solar collectors and systems. J. Shandong Med. 61, 28–32. 10.1038/s41377-021-00465-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Dai Y. (2020). Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 126, 469–474. 10.1111/bcpt.13372 [DOI] [PubMed] [Google Scholar]
- Hwang S. G., Yu S. S., Poo H., Chun J. S. (2005). c-Jun/activator protein-1 mediates interleukin-1beta-induced dedifferentiation but not cyclooxygenase-2 expression in articular chondrocytes. J. Biol. Chem. 280, 29780–29787. 10.1074/jbc.M411793200 [DOI] [PubMed] [Google Scholar]
- Hyndman I. J. (2017). Rheumatoid arthritis: past, present and future approaches to treating the disease. Int. J. Rheum. Dis. 20, 417–419. 10.1111/1756-185X.12823 [DOI] [PubMed] [Google Scholar]
- Jahantigh M., Abtahi Froushani S. M., Afzale Ahangaran N. (2023). Benefits of bone marrow-derived mesenchymal stem cells primed with estradiol in alleviating collagen-induced arthritis, Iran. J. Basic. Med. Sci. 26, 400–407. 10.22038/IJBMS.2023.68112.14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Du W., Che W., Wang L., Zhao L. (2023). Apigenin inhibits the progression of osteoarthritis by mediating macrophage polarization. Molecules 28, 2915. 10.3390/molecules28072915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. K. (2021). Effect of macrophage-specific Nfe2l1 deficiency on the occurrence and development of osteoarthritis and its related mechanisms. Liaoning Province: China Medical University. [Google Scholar]
- Jian R., Yang M., Zheng S. L. (2020). Regulatory effect of berberine on NLRP3/TLRs in mice with gouty arthritis. J. Chongqing Med. Univ. 45, 251–256. 10.13406/j.cnki.cyxb.002318 [DOI] [Google Scholar]
- Jie S. S., Sun H. J., Liu J. X. (2022). Huanglian jiedutang regulates inflammatory immunity to relieve rheumatoid arthritis. Chin. J. Exp. Tradit. Med. Formulae. 28, 28–33. 10.13422/j.cnki.syfjx.20221336 [DOI] [Google Scholar]
- Jin S. J., Wang G. W., Chun L. (2019). Effects of berberine combined with clomiphene on endothelial function, endocrine indexes and clinical outcomes in patients with polycystic ovary syndrome and infertility. J. Pract. Med. 30, 100–103. [DOI] [Google Scholar]
- Khan H., Sureda A., Belwal T., Çetinkaya S., Süntar İ., Tejada S., et al. (2019). Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 18, 647–657. 10.1016/j.autrev.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienhorst L. B., van Lochem E., Kievit W., Dalbeth N., Merriman M. E., Phipps-Green A., et al. (2015). Gout is a chronic inflammatory disease in which high levels of interleukin-8 (CXCL8), myeloid-related protein 8/myeloid-related protein 14 complex, and an altered proteome are associated with diabetes mellitus and cardiovascular disease. Arthritis Rheumatol. 67, 3303–3313. 10.1002/art.39318 [DOI] [PubMed] [Google Scholar]
- Ko J. Y., Choi Y. J., Jeong G. J., Im G. I. (2013). Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 34, 5359–5368. 10.1016/j.biomaterials.2013.03.066 [DOI] [PubMed] [Google Scholar]
- Koskela H. L., Eldfors S., Ellonen P., van Adrichem A. J., Kuusanmäki H., Andersson E. I., et al. (2012). Somatic STAT3 mutations in large granular lymphocytic leukemia. N. Engl. J. Med. 366, 1905–1913. 10.1056/NEJMoa1114885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou L., Huang H., Tang Y., Sun M., Li Y., Wu J., et al. (2022). Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: a trapping strategy. J. Control. Release. 347, 237–255. 10.1016/j.jconrel.2022.04.037 [DOI] [PubMed] [Google Scholar]
- La Bella S., Rinaldi M., Di Ludovico A., Di Donato G., Di Donato G., Salpietro V., et al. (2023). Genetic background and molecular mechanisms of juvenile idiopathic arthritis. Int. J. Mol. Sci. 24, 1846. 10.3390/ijms24031846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., Neidhöfer S., Reuter S., Matthias T. (2018). MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 32, 550–562. 10.1016/j.berh.2019.01.006 [DOI] [PubMed] [Google Scholar]
- Leung Y. Y., Kavanaugh A., Ritchlin C. T. (2023). Expert perspective: management of the psoriatic arthritis patient after failure of one tumor necrosis factor inhibitor. Arthritis Rheumatol. 75, 1312–1324. 10.1002/art.42498 [DOI] [PubMed] [Google Scholar]
- Li F., Xu Z., Xie Z. (2022c). Patient-derived functional organoids as a personalized approach for drug screening against hepatobiliary cancers. Apoptosis 17, 319–341. 10.1016/bs.acr.2022.01.011 [DOI] [PubMed] [Google Scholar]
- Li H. M., Dang W. T., Yang X. H. (2017b). Advances in anti-inflammatory mechanism of berberine. Chin. Med. Her. 14, 31–34. [Google Scholar]
- Li H., Peng Y., Wang X., Sun X., Yang F., Sun Y., et al. (2019a). Astragaloside inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Immunopharmacol. Immunotoxicol. 41, 497–503. 10.1080/08923973.2019.1637890 [DOI] [PubMed] [Google Scholar]
- Li H., Xie X. W., Zhao Y. L. (2022a). Research progress on mechanism of effective components of traditional Chinese medicine in preventing and treating osteoarthritis. Chin. Tradit. Herb. Drugs. 53, 7543–7552. 10.7501/j.issn.0253-2670.2022.23.026 [DOI] [Google Scholar]
- Li J., Wang Y., Chen D., Liu-Bryan R. (2022b). Oral administration of berberine limits post-traumatic osteoarthritis development and associated pain via AMP-activated protein kinase (AMPK) in mice. Osteoarthr. Cartil. 30, 160–171. 10.1016/j.joca.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang X., Guo D., Shi Y., Zhang S., Yang R., et al. (2023b). The mechanism of action of paeoniae radix rubra-angelicae sinensis radix drug pair in the treatment of rheumatoid arthritis through PI3K/AKT/NF-κB signaling pathway. Front. Pharmacol. 14 (2023), 1113810. 10.3389/fphar.2023.1113810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu H., Shi W., Yang J., Xu D. (2017a). Insights into the action mechanisms of traditional Chinese medicine in osteoarthritis. Evid. Based Complement. Altern. Med. 2017, 5190986. 10.1155/2017/5190986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu M., Fang G., Li K., Cui W., Li L., et al. (2021). MicroRNA-186-5p downregulation inhibits osteoarthritis development by targeting MAPK1. Mol. Med. Rep. 23, 253. 10.3892/mmr.2021.11892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jin Z., Lu X. (2017c). MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1. Mol. Cell. Biochem. 432, 123–130. 10.1007/s11010-017-3003-3 [DOI] [PubMed] [Google Scholar]
- Li S., Li L., Yan H., Jiang X., Hu W., Han N., et al. (2019b). Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep. 20, 4623–4633. 10.3892/mmr.2019.10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. N. (2022). Preparation of methotrexate-gold nano-targeted formulation and its treatment of rheumatoid arthritis. Jilin Province: Yanbian University. [Google Scholar]
- Li X., Sun H., Li H., Li D., Cai Z., Xu J., et al. (2023a). A single-cell RNA-sequencing analysis of distinct subsets of synovial macrophages in rheumatoid arthritis. DNA Cell. Biol. 42, 212–222. 10.1089/dna.2022.0509 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang S., Wang Y., Zhou C., Chen G., Shen W., et al. (2013). Inhibitory effect of the antimalarial agent artesunate on collagen-induced arthritis in rats through nuclear factor kappa B and mitogen-activated protein kinase signaling pathway. Transl. Res. 161, 89–98. 10.1016/j.trsl.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Liang H., Zeng Y., Feng Y., Wu H., Gong P., Yao Q. (2018). Selective β2-adrenoreceptor signaling regulates osteoclastogenesis via modulating RANKL production and neuropeptides expression in osteocytic MLO-Y4 cells. J. Cell. Biochem. 120, 7238–7247. 10.1002/jcb.27998 [DOI] [PubMed] [Google Scholar]
- Lietman C., Wu B., Lechner S., Shinar A., Sehgal M., Rossomacha E., et al. (2018). Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight 3, e96308. 10.1172/jci.insight.96308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Shen P., Huang Y., Han L., Ba X. (2023). Wutou decoction attenuates the synovial inflammation of collagen-induced arthritis rats via regulating macrophage M1/M2 type polarization. J. Ethnopharmacol. 301 (2023), 115802. 10.1016/j.jep.2022.115802 [DOI] [PubMed] [Google Scholar]
- Lin Z., Tian X. Y., Huang X. X., He L. L., Xu F. (2019). microRNA-186 inhibition of PI3K-AKT pathway via SPP1 inhibits chondrocyte apoptosis in mice with osteoarthritis. J. Cell. Physiol. 234, 6042–6053. 10.1002/jcp.27225 [DOI] [PubMed] [Google Scholar]
- Liu G., He G., Zhang J., Zhang Z., Wang L. (2022b). Identification of SCRG1 as a potential therapeutic target for human synovial inflammation. Front. Immunol. 13 (2022), 893301. 10.3389/fimmu.2022.893301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. X., Jie S. S., Chen B. (2023). Mechanisms of huanglian jiedu decoction in treating acute gouty arthritis based on NLRP3 inflammasome and TLR4/NF-κB signal pathway. Chin. J. Exp. Tradit. Med. Formulae. 10.13422/j.cnki.syfjx.20230802 [DOI] [Google Scholar]
- Liu L., Wang D., Liu M. Y., Yu H., Chen Q., Wu Y., et al. (2022a). The development from hyperuricemia to gout: key mechanisms and natural products for treatment. Acupunct. Herb. Med. 2, 25–32. 10.1097/hm9.0000000000000016 [DOI] [Google Scholar]
- Liu P., Chen Y., Wang B., Wang Z., Li C., Wang Y. (2019). Expression of microRNAs in the plasma of patients with acute gouty arthritis and the effects of colchicine and etoricoxib on the differential expression of microRNAs. Arch. Med. Sci. 159, 1047–1055. 10.5114/aoms.2018.75502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Lee H. P., Hung C. Y., Tsai C. H., Li T. M., Tang C. H. (2015). Berberine attenuates CCN2-induced IL-1βexpression and prevents cartilage degradation in a rat model of osteoarthritis. Toxico. Appl. Pharmacol. 289, 20–29. 10.1016/j.taap.2015.08.020 [DOI] [PubMed] [Google Scholar]
- Liu S., Ma H., Zhang H., Deng C., Xin P. (2021). Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 230, 108793. 10.1016/j.clim.2021.108793 [DOI] [PubMed] [Google Scholar]
- Liu Y. F., Wen C. Y., Chen Z., Wang Y., Huang Y., Tu S. H. (2016). Effects of berberine on NLRP3 and IL-1β expressions in monocytic THP-1 cells with monosodium urate crystals-induced inflammation. Biomed. Res. Int. 2016, 2503703. 10.1155/2016/2503703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Lei Y., Guo X., Zhu D., Zhang H., Guo Z., et al. (2023). CX-4945 inhibits fibroblast-like synoviocytes functions through the CK2-p53 axis to reduce rheumatoid arthritis disease severity. Int. Immunopharmacol. 119 (2023), 110163. 10.1016/j.intimp.2023.110163 [DOI] [PubMed] [Google Scholar]
- Lv S. (2020). Study on the mechanism of pulchinenoside b4 anti-gouty arthritis (GA) based on metabolomics. Jiangxi Province: Jiangxi University of Traditional Chinese Medicine. [Google Scholar]
- Ma Y., Nyman J. S., Tao H., Moss H. H., Yang X., Elefteriou F. (2011). β2-adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 152, 1412–1422. 10.1210/en.2010-0881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man G., Yang H., Shen K., Zhang D., Zhang J., Wu H., et al. (2022). Circular RNA RHOT1 regulates miR-142-5p/CCND1 to participate in chondrocyte autophagy and proliferation in osteoarthritis. J. Immunol. Res. 2022, 4370873. 10.1155/2022/4370873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. B., Schett G. (2011). The pathogenesis of rheumatoid arthritis. New Engl. J. Med. 365, 2205–2219. 10.1056/nejmra1004965 [DOI] [PubMed] [Google Scholar]
- Mei J., Zhou F., Qiao H., Tang T. (2019). Nerve modulation therapy in gouty arthritis: targeting increased sFRP2 expression in dorsal root ganglion regulates macrophage polarization and alleviates endothelial damage. Theranostics 9, 3707–3722. 10.7150/thno.33908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. Y., Jiang L. J., Qiu Q. (2022). Research advances on quality markers of Chinese materia medica for prevention and treatment of rheumatoid arthritis. Chin. Arch. Tradit. Chin. Med. 40, 48–53. 10.13193/j.issn.1673-7717.2022.04.009 [DOI] [Google Scholar]
- Miao C. G., Yang Y. Y., He X., Huang C., Huang Y., Qin D., et al. (2014). MicroRNA-152 modulates the canonical Wnt pathway activation by targeting DNA methyltransferase 1 in arthritic rat model. Biochimie 106, 149–156. 10.1016/j.biochi.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Miao C. G., Yang Y. Y., He X., Li X. f., Huang C., Huang Y., et al. (2013). Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell. Signal 25, 2069–2078. 10.1016/j.cellsig.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Min S., Wang C., Lu W., Xu Z., Shi D., Chen D., et al. (2017). Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-alpha in knee osteoarthritis patients. Clin. Rheumatol. 36, 2351–2358. 10.1007/s10067-017-3690-x [DOI] [PubMed] [Google Scholar]
- Murphy G., Nagase H. (2008). Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Rheumatology 4, 128–135. 10.1038/ncprheum0727 [DOI] [PubMed] [Google Scholar]
- Nalesso G., Thorup A. S., Eldridge S. E., De Palma A., Kaur A., Peddireddi K., et al. (2021). Calcium calmodulin kinase II activity is required for cartilage homeostasis in osteoarthritis. Sci. Rep. 11, 5682. 10.1038/s41598-021-82067-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pharmacopoeia Commission (2020). Pharmacopoeia of the people Republic of China. Beijing: Chemical Industry Press, 316. [Google Scholar]
- Nguyen C. T., Furuya H., Das D., Marusina A. I., Merleev A. A., Ravindran R., et al. (2022). Peripheral γδ T cells regulate neutrophil expansion and recruitment in experimental psoriatic arthritis. Arthritis Rheumatol. 74, 1524–1534. 10.1002/art.42124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X., Ni Y., Cao J., Zhang H. (2023). Liquiritigenin attenuated collagen-induced arthritis and cardiac complication via inflammation and fibrosis inhibition in mice. Chem. Pharm. Bull. (Tokyo). 71, 269–276. 10.1248/cpb.c22-00684 [DOI] [PubMed] [Google Scholar]
- Orecchini E., Mondanelli G., Orabona C., Volpi C., Adorisio S., Calvitti M., et al. (2020). Artocarpus tonkinensis extract inhibits LPS-triggered inflammation markers and suppresses RANKL-induced osteoclastogenesis in RAW264.7. Front. Pharmacol. 11, 593829. 10.3389/fphar.2020.593829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y., Li W., Li X., Lin Z., Li M. (2010). Sinomenine reduces invasion and migration ability in fibroblast-like synoviocytes cells co-cultured with activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, CD147. Rheumatol. Int. 31, 1479–1485. 10.1007/s00296-010-1506-2 [DOI] [PubMed] [Google Scholar]
- Pacifici M. (2022). Osteoarthritis and chronic pain: interleukin-6 as a common denominator and therapeutic target. Sci. Signal. 15, eadd3702. 10.1126/scisignal.add3702 [DOI] [PubMed] [Google Scholar]
- Park S. M., Min B. G., Jung J. Y., Jegal K. H., Lee C. W., Kim K. Y., et al. (2018). Combination of Pelargonium sidoides and Coptis chinensis root inhibitsnuclear factor kappa B-mediated inflammatory response in vitro and in vivo . BMC Compl. Altern. Med. 18, 20–32. 10.1186/s12906-018-2088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan N. B., Parvez A., Bader A., Shaheen U., Hadda T. B. (2015). Synthesis, characterization, crystal structure determination and biological screening of novel N-1 and C5 alkyl substituted scaffolds of pyrimidine. Eur. J. Med. Chem. 103, 594–599. 10.1016/j.ejmech.2013.12.036 [DOI] [PubMed] [Google Scholar]
- Qian Q., Gao Y., Xun G., Wang X., Ge J., Zhang H., et al. (2022). Synchronous investigation of the mechanism and substance basis of tripterygium glycosides tablets on anti-rheumatoid arthritis and hepatotoxicity. Appl. Biochem. Biotechnol. 194, 5333–5352. 10.1007/s12010-022-04011-6 [DOI] [PubMed] [Google Scholar]
- Qin C., Diaz-Gallo L. M., Tang B., Wang Y., Nguyen T. D., Harder A., et al. (2023). Repurposing antidiabetic drugs for rheumatoid arthritis: results from a two-sample mendelian randomization study. Eur. J. Epidemiol. 38, 809–819. 10.1007/s10654-023-01000-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y. F., Zhang Q. B., Zhou J. G., Jiang L. (2014). Changes in toll-like receptor (TLR)4-NFκB-IL1β signaling in male gout patients might be involved in the pathogenesis of primary gouty arthritis. Rheumatol. Int. 34, 213–220. 10.1007/s00296-013-2856-3 [DOI] [PubMed] [Google Scholar]
- Qiu H., Sun S., Ma X., Cui C., Chen G., Liu Z., et al. (2018). Jatrorrhizine hydrochloride suppresses proliferation, migration, and secretion of synoviocytes in vitro and ameliorates rat models of rheumatoid arthritis in vivo . Int. J. Mol. Sci. 19, 1514. 10.3390/ijms19051514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer F. W., Guermazi A., Felson D. T., Niu J., Nevitt M. C., Crema M. D., et al. (2011). Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann. Rheum. Dis. 70, 1804–1809. 10.1136/ard.2011.150243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M., Infantino V., Riva A., Petrangolini G., Faliva M. A., Peroni G., et al. (2020). Polycystic ovary syndrome management:a review of the possible amazing role of berberine. Arch. Gynecol. Obstet. 301, 53–60. 10.1007/s00404-020-05450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roškar S., Hafner-Bratkovič I. (2022). The role of inflammasomes in osteoarthritis and secondary joint degeneration diseases. Life (Basel) 12, 731. 10.3390/life12050731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakthiswary R., Uma Veshaaliini R., Chin K. Y., Das S., Sirasanagandla S. R. (2022). Pathomechanisms of bone loss in rheumatoid arthritis. Front. Med. (Lausanne). 9, 962969. 10.3389/fmed.2022.962969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X., Böker K. O., Taheri S., Hawellek T., Lehmann W., Schilling A. F. (2021). The interaction between microRNAs and the wnt/β-catenin signaling pathway in osteoarthritis. Int. J. Mol. Sci. 22, 9887. 10.3390/ijms22189887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Tirpude N. V., Bhardwaj N., Kumar D., Padwad Y. (2022). Berberis lycium fruit extract and its phytoconstituents berberine and rutin mitigate collagen-CFA-induced arthritis (CIA) via improving GSK3β/STAT/Akt/MAPKs/NF-κB signaling axis mediated oxi-inflammation and joint articulardamage in murine model. Inflammopharmacology 30, 655–666. 10.1007/s10787-022-00941-z [DOI] [PubMed] [Google Scholar]
- She P. (2020). The application of inflammatory-responsive polymers nanomedicines for treatment of arthritis. Jilin Province: Jilin University. [Google Scholar]
- Shen P., Jiao Y., Miao L., Chen J. H., Momtazi-Borojeni A. A. (2020). Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J. Cell. Mol. Med. 24, 12234–12245. 10.1111/jcmm.15803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Fan X., Qu Y., Tang M., Huang Y., Peng Y., et al. (2022). Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro . Phytomedicine 104, 154339. 10.1016/j.phymed.2022.154339 [DOI] [PubMed] [Google Scholar]
- Skougaard M., Ditlev S. B., Søndergaard M. F., Kristensen L. E. (2023). Cytokine signatures in psoriatic arthritis patients indicate different phenotypic traits comparing responders and non-responders of IL-17a and TNFα inhibitors. Int. J. Mol. Sci. 24, 6343. 10.3390/ijms24076343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Weedon H., Papangelis V., Walker J., Roberts-Thomson P. J., Ahern M. J. (2010). Apoptosis in the rheumatoid arthritis synovial membrane: modulation by disease-modifying anti-rheumatic drug treatment. Rheumatol. Oxf. 49, 862–875. 10.1093/rheumatology/kep467 [DOI] [PubMed] [Google Scholar]
- So A. K., Martinon F. (2017). Inflammation in gout: mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 13, 639–647. 10.1038/nrrheum.2017.155 [DOI] [PubMed] [Google Scholar]
- Song L. N., Kong X. D., Wang H. J., Zhan L. b. (2016). Establishment of a rat adjuvant arthritis-interstitial lung disease model. Biomed. Res. Int. 2016, 2970783. 10.1155/2016/2970783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B., Di Meglio P., Gialitakis M., Duarte J. H. (2014). The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432. 10.1146/annurev-immunol-032713-120245 [DOI] [PubMed] [Google Scholar]
- Su Y. X., Lin X. Y., Chen X. M. (2012). Effect of IL-1β on Wnt/β-catenin pathway in rat articular chondrocytes. Fujian J. Tradit. Chin. Med. 43, 46–48. 10.13260/j.cnki.jfjtcm.010478 [DOI] [Google Scholar]
- Sujitha S., Dinesh P., Rasool M. (2020). Berberine encapsulated PEG-coated liposomes attenuate Wnt1/β-catenin signaling in rheumatoid arthritis via miR-23a activation. Eur. J. Pharm. Biopharm. 149, 170–191. 10.1016/j.ejpb.2020.02.007 [DOI] [PubMed] [Google Scholar]
- Sujitha S., Dinesh P., Rasool M. (2018). Berberine modulates ASK1 signaling mediated through TLR4/TRAF2 via upregulation of miR-23a. Toxicol. Appl. Pharmacol. 359, 34–46. 10.1016/j.taap.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Sun J. L., Yan J. F., Li J., Wang W. R., Yu S. B., Zhang H. Y., et al. (2020b). Conditional deletion of Adrb2 in mesenchymal stem cells attenuates osteoarthritis-like defects in temporomandibular joint. Bone 133, 115229. 10.1016/j.bone.2020.115229 [DOI] [PubMed] [Google Scholar]
- Sun K., Luo J., Guo J., Yao X., Jing X., Guo F. (2020a). The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr. Cartil. 28, 400–409. 10.1016/j.joca.2020.02.027 [DOI] [PubMed] [Google Scholar]
- Sun L., Wang X., Kaplan D. L. (2011). A 3D cartilage - inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials 32, 5581–5589. 10.1016/j.biomaterials.2011.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Guo Y., Chang L., Zhang J. (2023b). Long noncoding RNA H19 synergizes with STAT1 to regulate SNX10 in rheumatoid arthritis. Mol. Immunol. 153, 106–118. 10.1016/j.molimm.2022.11.018 [DOI] [PubMed] [Google Scholar]
- Sun Y., Su S., Li M., Deng A. (2023a). Inhibition of miR-182-5p targets FGF9 to alleviate osteoarthritis. Anal. Cell. Pathol. (Amst). 2023, 5911546. 10.1155/2023/5911546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K. T., Lin C. C., Lin S. C., Wang J. H., Tsai S. W. (2021). Kurarinone attenuates collagen-induced arthritis in mice by inhibiting Th1/Th17 cell responses and oxidative stress. Int. J. Mol. Sci. 22, 4002. 10.3390/ijms22084002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavallaee G., Lively S., Rockel J. S., Ali S. A., Im M., Sarda C., et al. (2022). Contribution of MicroRNA-27b-3p to synovial fibrotic responses in knee osteoarthritis. Arthritis Rheumatol. 74, 1928–1942. 10.1002/art.42285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. C., Matucci Cerinic M., Alten R., Avouac J., Westhovens R. (2022). Managing inadequate response to initial anti-TNF therapy in rheumatoid arthritis: optimising treatment outcomes. Ther. Adv. Musculoskelet. Dis. 14, 1759720X221114101. 10.1177/1759720X221114101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkeltaub R. (2017). What makes gouty inflammation so variable. BMC Med. 15, 158. 10.1186/s12916-017-0922-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. L. (2020). Caring for patients in a new pandemic: the necessity and challenges of observational research. J. Clin. Invest. 130, 6225–6227. 10.1172/JCI143292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzybulska D., Olewicz-Gawlik A., Sikora J., Frydrychowicz M., Kolecka-Bednarczyk A., Kaczmarek M., et al. (2018). The effect of caveolin-1 knockdown on interleukin-1β-induced chemokine (C-C motif) ligand 2 expression in synovial fluid-derived fibroblast-like synoviocytes from patients with rheumatoid arthritis. Adv. Clin. Exp. Med. 27, 1491–1497. 10.17219/acem/75611 [DOI] [PubMed] [Google Scholar]
- Tu B., Fang R., Zhu Z., Chen G., Peng C., Ning R. (2023). Comprehensive analysis of arachidonic acid metabolism-related genes in diagnosis and synovial immune in osteoarthritis: based on bulk and single-cell RNA sequencing data. Inflamm. Res. 72, 955–970. 10.1007/s00011-023-01720-4 [DOI] [PubMed] [Google Scholar]
- Uthman I., Raynauld J. P., Haraoui B. (2003). Intra-articular therapy in osteoarthritis. Postgrad. Med. J. 79, 449–453. 10.1136/pmj.79.934.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita A. A., Aljobaily H., Lyons D. O., Pullen N. A. (2021). Berberine delays onset of Collagen-Induced arthritis through T Cell suppression. Int. J. Mol. Sci. 22, 3522. 10.3390/ijms22073522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. (2016). The cybernetics of TNF: old views and newer ones. Semin. Cell. Dev. Biol. 50, 105–114. 10.1016/j.semcdb.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Wang C., Wang F., Lin F., Duan X., Bi B. (2019b). Naproxen attenuates osteoarthritis progression through inhibiting the expression of prostaglandinl-endoperoxide synthase 1. J. Cell. Physiol. 234, 12771–12785. 10.1002/jcp.27897 [DOI] [PubMed] [Google Scholar]
- Wang D. D. (2020b). CP-25 down-regulates CXCR4-gβγ-PI3K/AKT mediated migration in fibroblast-like synoviocytes of rheumatoid arthritis by inhibiting GRK2 translocation. Anhui Province: Anhui Medical University. [DOI] [PubMed] [Google Scholar]
- Wang H., Tu S., Yang S., Shen P., Huang Y., Ba X., et al. (2019a). Berberine modulates LPA function to inhibit the proliferation and inflammation of FLS-RA via p38/ERK MAPK pathway mediated by LPA1. Altern. Med. 2019, 2580207. 10.1155/2019/2580207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. (2020a). Study on the pharmacodynamics and mechanism of compound Ruteng capsules in treating arthritis based on network pharmacology. Hubei Province: South-central University for Nationalities. [Google Scholar]
- Wang L., Ishihara S., Li J., Miller R. E., Malfait A. M. (2023b). Notch signaling is activated in knee-innervating dorsal root ganglia in experimental models of osteoarthritis joint pain. Arthritis Res. Ther. 25, 63. 10.1186/s13075-023-03039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li P., Zhou Y., Gu R., Lu G., Zhang C. (2023a). Magnoflorine ameliorates collagen-induced arthritis by suppressing the inflammation response via the NF-κB/MAPK signaling pathways. J. Inflamm. Res. 16, 2271–2296. 10.2147/jir.s406298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. A. (2022). CADM1 regulates chondrocyte anabolism and catabolism in osteoarthritis and its underlying molecular mechanism. Anhui Province: Anhui Medical University. [Google Scholar]
- Wang Q. T., Zhang L. L., Wu H. X., Wei W. (2011). The expression change of β-arrestins in fibroblast-like synoviocytes from rats with collagen-induced arthritis and the effect of total glucosides of paeony. J. Ethnopharmacol. 133, 511–516. 10.1016/j.jep.2010.10.022 [DOI] [PubMed] [Google Scholar]
- Wang Q., Yu X., Gong M. (2022a). Single-cell transcriptome analysis reveals the importance of IRF1/FSTL1 in synovial fibroblast subsets for the development of rheumatoid arthritis. Comput. Math. Methods Med. 2022, 1169614. 10.1155/2022/1169614 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X., He X., Zhang C. F., Guo C. R., Wang C. Z., Yuan C. S. (2017). Anti-arthritic effect of berberine on adjuvant-induced rheumatoid arthritis in rats. Biomed. Pharmacother. 89, 887–893. 10.1016/j.biopha.2017.02.099 [DOI] [PubMed] [Google Scholar]
- Wang X. H. (2011). Effects of berberine on human rheumatoid arthritis fibroblast-like synoviocytes and its underlying molecular. Shandong Province: Shandong University. [DOI] [PubMed] [Google Scholar]
- Wang X., Long H., Chen M., Zhou Z., Wu Q., Xu S., et al. (2022b). Modified Baihu decoction therapeutically remodels gut microbiota to inhibit acute gouty arthritis. Front. Physiol. 13 (2022), 1023453. 10.3389/fphys.2022.1023453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Sun L., He N., An Z., Yu R. (2021). Increased expression of CXCL2 in ACPA-positive rheumatoid arthritis and its role in osteoclastogenesis. Clin. Exp. Immunol. 203, 194–208. 10.1111/cei.13527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li Y. P., Paulson C., Shao J. Z., Zhang X., Wu M., et al. (2014b). Wnt and the Wnt signaling pathway in bone development and disease. Front. Bio (Landmark Ed. 19, 379–407. 10.2741/4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang X., Xu C., Yao Y., Wu C., Xu W., et al. (2023c). κNeoisoastilbin ameliorates acute gouty arthritis via suppression of the NF-B/NLRP3 pathway. Evid. Based Compl. Altern. Med. 2023, 1–8. 10.1155/2023/7629066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chen Z., Yang S., Wang Y., Huang Z., Gao J., et al. (2014a). Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation 37, 1789–1798. 10.1007/s10753-014-9909-y [DOI] [PubMed] [Google Scholar]
- Wei L., Kanbe K., Lee M., Wei X., Pei M., Sun X., et al. (2010). Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev. Biol. 341, 236–245. 10.1016/j.ydbio.2010.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z. H. (2022). Clinical research progress on pathogenesis of rheumatoid arthritis. Clin. Med. 42, 123–125. 10.19528/j.issn.1003-3548.2022.07.046 [DOI] [Google Scholar]
- Wirth T., Balandraud N., Boyer L., Lafforgue P., Pham T. (2022). Biomarkers in psoriatic arthritis: a meta-analysis and systematic review. Front. Immunol. 13 (2022), 1054539. 10.3389/fimmu.2022.1054539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. K., Chin K. Y., Ima-Nirwana S. (2020). Berberine and musculoskeletal disorders: the therapeutic potential and underlying molecular mechanisms. Phytomedicine 73, 152892. 10.1016/j.phymed.2019.152892 [DOI] [PubMed] [Google Scholar]
- Wu B. X. (2021). Experimental study of siRNA interference combined with BMSCs in the treatment of rheumatoid arthritis based on the NF-κB signaling pathway. Jiangsu Province: Yangzhou University. [Google Scholar]
- Wu J., Luo Y., Jiang Q., Li S., Huang W., Xiang L., et al. (2019). Coptisine from Coptis chinensis blocks NLRP3 inflammasome activation by inhibiting caspase-1. Pharmacol. Res. 147, 104348. 10.1016/j.phrs.2019.104348 [DOI] [PubMed] [Google Scholar]
- Wu X., Liu Y., Jin S., Wang M., Jiao Y., Yang B., et al. (2021). Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat. Commun. 12, 4977. 10.1038/s41467-021-25246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi X., Ye Q., Fan D., Cao X., Wang Q., Wang X., et al. (2022). Polycyclic aromatic hydrocarbons affect rheumatoid arthritis pathogenesis via aryl hydrocarbon receptor. Front. Immunol. 13, 797815. 10.3389/fimmu.2022.797815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T., Li J. X. (2017). Effect of artemisia annua combined with methotrexate on levels of IL-1, IL-10, and TNF-α in peripheral blood of patients with rheumatoid arthritis. J. Sichuan Med. 10, 53–55. 10.16252/j.cnki.issn1004-0501-2017.10.013 [DOI] [Google Scholar]
- Xiao J., Zhang G., Mai J., He Q., Chen W., Li J., et al. (2022). Bioinformatics analysis combined with experimental validation to explore the mechanism of XianLing GuBao capsule against osteoarthritis. J. Ethnopharmacol. 294 (2022), 115292. 10.1016/j.jep.2022.115292 [DOI] [PubMed] [Google Scholar]
- Xie H., Wang Q., Zhang X., Wang T., Hu W., Manicum T., et al. (2018). Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabetic osteoporosis. Biomed. Pharmacother. 108, 280–287. 10.1016/j.biopha.2018.08.131 [DOI] [PubMed] [Google Scholar]
- Xie L., Li Y., Tang W., Zhang Q., Luo C., Long X. (2023). Stattic alleviates pulmonary fibrosis in a mouse model of rheumatoid arthritis-relevant interstitial lung disease. Exp. Biol. Med. (Maywood) 2023, 153537022311579. 10.1177/15353702231157934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J. K. (2021). Polyamidoamine derivative-mediated miR-30a delivery in the treatment of rheumatoid arthritis and the mechanism analysis. Jilin Province: Jilin University. [Google Scholar]
- Xu B., Arlehag L., Rantapää-Dahlquist S. B., Lefvert A. K. (2004). beta2-adrenergic receptor gene single-nucleotide polymorphisms are associated with rheumatoid arthritis in northern Sweden. Scand. J. Rheumatol. 33, 395–398. 10.1080/03009740410010326 [DOI] [PubMed] [Google Scholar]
- Xu F., Li X. J. (2021). Study on the mechanism of single herb extract in the treatment of gouty arthritis. Rheum. Arthritis. 10, 68–71. 10.3969/j.issn.2095-4174.2021.12.019 [DOI] [Google Scholar]
- Xu H. G., Yu Y. F., Zheng Q., Zhang W., Wang C. D., Zhao X. Y., et al. (2014). Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone 66, 232–239. 10.1016/j.bone.2014.06.018 [DOI] [PubMed] [Google Scholar]
- Xu H., Xu B. (2021). Κbmsc-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-B p65 to chondrocytes. Mediat. Inflamm. 2021, 9972805. 10.1155/2021/9972805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. (2022). The anti-inflammatory effect and intracellular signling pathway mechanism study of Nootkatone (NTK) in Osteoarthritis. Shandong Province: Shandong University. [Google Scholar]
- Xuan F., Yano F., Mori D., Chijimatsu R., Maenohara Y., Nakamoto H., et al. (2019). Wnt/β-catenin signaling contributes to articular cartilage homeostasis through lubricin induction in the superficial zone. Arthritis Res. Ther. 21, 247. 10.1186/s13075-019-2041-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., McKelvey K., Shen K., Minhas N., March L., Park S. Y., et al. (2014). Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatol. Oxf. 53, 12270–12279. 10.1093/rheumatology/keu254 [DOI] [PubMed] [Google Scholar]
- Yan Y., Lu A., Dou Y., Zhang Z., Wang X. Y., Zhai L., et al. (2023). Nanomedicines reprogram synovial macrophages by scavenging nitric oxide and silencing CA9 in progressive osteoarthritis. Adv. Sci. (Weinh). 10, 2207490. 10.1002/advs.202207490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Ni B., Li C., Sun W., Wang Z., Wang H., et al. (2023a). circRNA_17725 promotes macrophage polarization towards M2 by targeting FAM46C to alleviate arthritis. Mediat. Inflamm. 2023 (2023), 6818524. 10.1155/2023/6818524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Lee H. E., Moon S. J., Ko K. M., Koh J. H., Seok J. K., et al. (2022). Direct binding to NLRP3 pyrin domain as a novel strategy to prevent NLRP3-driven inflammation and gouty arthritis. Arthritis Rheumatol. 72, 1192–1202. 10.1002/art.41245 [DOI] [PubMed] [Google Scholar]
- Yang K., Xie Q., Liao J., Zhao N., Liang J., Liu B., et al. (2023b). Shang-Ke-Huang-Shui and coptisine alleviate osteoarthritis in the knee of monosodium iodoacetate-induced rats through inhibiting CXCR4 signaling. J. Ethnopharmacol. 311, 116476. 10.1016/j.jep.2023.116476 [DOI] [PubMed] [Google Scholar]
- Yang Y., You X., Cohen J. D., Zhou H., He W., Li Z., et al. (2020). Sex differences in osteoarthritis pathogenesis: a comprehensive study based on bioinformatics. Med. Sci. Monit. 26, e923331. 10.12659/MSM.923331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Feng L., Huang J., Zhang X., Lin W., Wang B., et al. (2021). Asiatic acid protects articular cartilage through promoting chondrogenesis and inhibiting inflammation and hypertrophy in osteoarthritis. Eur. J. Pharmacol. 907, 174265. 10.1016/j.ejphar.2021.174265 [DOI] [PubMed] [Google Scholar]
- Yao C. J., Li Y. L., Xiong Q. (2023). Bioinformatics prediction of potential traditional Chinese medicines in treatment of rheumatoid arthritis. Acta. Chin. Med. 38, 152–160. 10.16368/j.issn.1674-8999.2023.01.027 [DOI] [Google Scholar]
- Yu J., Hu C., Dai Z., Xu J., Zhang L., Deng H., et al. (2023). Dipeptidyl peptidase 4 as a potential serum biomarker for disease activity and treatment response in rheumatoid arthritis. Int. Immunopharmacol. 119 (2023), 110203. 10.1016/j.intimp.2023.110203 [DOI] [PubMed] [Google Scholar]
- Yu Y., Cai W., Zhou J., Lu H., Wang Y., Song Y., et al. (2020). Anti-arthritis effect of berberine associated with regulating energy metabolism of macrophages through AMPK/HIF-1α pathway. Int. Immunopharmacol. 87, 106830. 10.1016/j.intimp.2020.106830 [DOI] [PubMed] [Google Scholar]
- Yu Z. L. (2022). Efficacy evaluation, action mechanism and pharmacodynamic material basis of Zhuang medicine Fenghegui against rheumatoid arthritis. Jiangxi Province: Jiangxi University of Traditional Chinese Med. [Google Scholar]
- Yuan X., Garrett-Sinha L. A., Sarkar D., Yang S. (2014). Deletion of IFT20 in early stage T lymphocyte differentiation inhibits the development of collagen-induced arthritis. Bone Res. 2, 14038. 10.1038/boneres.2014.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue M., Tao Y., Fang Y., Lian X., Zhang Q. (2019). The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. 33, 12311–12323. 10.1096/fj.201900425RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue R., Zhao L., Hu Y., Jiang P., Wang S., Xiang L., et al. (2013). Metabolomic study of collagen-induced arthritis in rats and the interventional effects of Huang-Lian-Jie-Du-Tang, a traditional Chinese medicine. Evid. Based Compl. Altern. Med. 2013, 439690. 10.1155/2013/439690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Shen C., Mo S., Ni C., Lin Y., Fang Y., et al. (2023). The effective treatment of purpurin on inflammation and adjuvant-induced arthritis. Molecules 28, 366. 10.3390/molecules28010366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z. (2022). The effect and mechanism of pelargonidin on osteoarthritis based on NF-κB signaling pathway. Jiangxi Province: Nanchang University. [Google Scholar]
- Zhang H., Cai D., Bai X. (2020). Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 28, 555–561. 10.1016/j.joca.2020.01.007 [DOI] [PubMed] [Google Scholar]
- Zhang H. G., Wang Y., Xie J. F., Liang X., Liu D., Yang P., et al. (2001). Regulation of tumor necrosis factor alpha-mediated apoptosis of rheumatoid arthritis synovial fibroblasts by the protein kinase Akt. Arthritis Rheum. 44, 1555–1567. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Huang K., Cai H. L. (2022a). Experimental study on the regulation of subchondral bone plate osteoprotegerin/receptor activator of nuclear factor Kappa-B ligand system by berberine to retard the development of osteoarthritis in rabbits, Chin. J. Orthop. Traumatol. 35, 464–469. 10.12200/j.issn.1003-0034.2022.05.011 [DOI] [PubMed] [Google Scholar]
- Zhang N., Zheng N., Luo D., Lin D., Que W., Wang H., et al. (2022b). Long non-coding RNA NR-133666 promotes the proliferation and migration of fibroblast-like synoviocytes through regulating the miR-133c/MAPK1 Axis. Front. Pharmacol. 13 (2022), 887330. 10.3389/fphar.2022.887330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Liu J., Sun Y., Zhou Q., Ding X., Chen X. (2023). Chinese herbal compound Huangqin Qingrechubi capsule reduces lipid metabolism disorder and inflammatory response in gouty arthritis via the LncRNA H19/APN/PI3K/AKT cascade. Pharm. Biol. 61, 541–555. 10.1080/13880209.2023.2191641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao W., Zhao Y., Zhao Z., Lv Z., Zhang Z., et al. (2022c). Inflammatory macrophages exacerbate neutrophil-driven joint damage through ADP/P2Y1 signaling in rheumatoid arthritis. Sci. Chin. Life Sci. 65, 953–968. 10.1007/s11427-020-1957-8 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li S., Jin P., Shang T., Sun R., Lu L., et al. (2022d). Dual functions of microRNA-17 in maintaining cartilage homeostasis and protection against osteoarthritis. Nat. Commun. 13, 2447. 10.1038/s41467-022-30119-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhang T., Xia C., Shi L., Wang S., Zheng X., et al. (2014). Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J. Cell. Mol. Med. 18, 283–292. 10.1111/jcmm.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang X., Nie K. (2023). IRF1 promotes the chondrogenesis of human adipose-derived stem cells through regulating HILPDA. Tissue Cell. 82 (2023), 102046. 10.1016/j.tice.2023.102046 [DOI] [PubMed] [Google Scholar]
- Zheng S. C. (2012). Serum levels and significances of Toll-like receptor 7 in rheumatoid arthritis. Jiangsu Province: Suzhou University. [Google Scholar]
- Zhou J. T., Li C. L., Tan L. H., Xu Y. F., Liu Y. H., Mo Z. Z., et al. (2017b). Inhibition of Helicobacter pylori and its associated urease by palmatine: investigation on the potential mechanism. PLoS One 12, e0168944. 10.1371/journal.pone.0168944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yu Y., Yang X., Wang Y., Song Y., Wang Q., et al. (2019). Berberine attenuates arthritis in adjuvant-induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF-кB pathway. Eur. J. Pharmacol. 852, 179–188. 10.1016/j.ejphar.2019.02.036 [DOI] [PubMed] [Google Scholar]
- Zhou K., Hu L., Liao W., Yin D., Rui F. (2016d). Coptisine prevented IL-β-induced expression of inflammatory mediators in chondrocytes. Inflammation 39, 1558–1565. 10.1007/s10753-016-0391-6 [DOI] [PubMed] [Google Scholar]
- Zhou M., Tan W., Hasimu H., Liu J., Gu Z., Zhao J. (2023a). Euphorbium total triterpenes improve Freund's complete adjuvant-induced arthritis through PI3K/AKT/Bax and NF-κB/NLRP3 signaling pathways. J. Ethnopharmacol. 306 (2023), 116146. 10.1016/j.jep.2023.116146 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Sun H. J., Liu S. M. (2023b). Effects of total saponins from Dioscorea nipponica makino on monosodium urate-induced M1-polarized macrophages through arachidonic acid signaling pathway: an in vitro study. Chin. J. Integr. Med. 29, 44–51. 10.1007/s11655-022-3721-6 [DOI] [PubMed] [Google Scholar]
- Zhou R., Xiang C. P., Zhang J. J., Hong-Jun Y. (2020a). Research progress on chemical compositions of Coptidis Rhizoma and pharmacological effects of berberine. Chin. J. Chin. Mat. Med. 45, 4561–4573. 10.19540/j.cnki.cjcmm.20200527.202 [DOI] [PubMed] [Google Scholar]
- Zhou X. D. (2014). Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro . Zhejiang Province: Zhejiang University. [Google Scholar]
- Zhou X., Lin X., Xiong Y., Jiang L., Li W., Li J., et al. (2016b). Chondroprotective effects of palmatine on osteoarthritis in vivo and in vitro: a possible mechanism of inhibiting the wnt/β-catenin and Hedgehog signaling pathways. Int. Immunopharmacol. 34, 129–138. 10.1016/j.intimp.2016.02.029 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Deng M., He B. (2016a). Research progress in the construction of rat induced osteoarthritis model. Int. J. Orthop. 37, 304–310. 10.3969/j.issn.1673-7083.2016.05.009 [DOI] [Google Scholar]
- Zhou Y., Liu S., Ming J., Li Y., Deng M., He B. (2017a). Sustained release effects of berberine-loaded chitosan microspheres on in vitro chondrocyte culture. Drug Dev. Ind. Pharm. 403, 1703–1714. 10.1080/03639045.2017.1339076 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liu S. Q., Yu L., He B., Wu S. H., Zhao Q., et al. (2015). Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 20, 1187–1199. 10.1007/s10495-015-1152-y [DOI] [PubMed] [Google Scholar]
- Zhou Y., Ming J., Deng M., Li Y., Li B., Li J., et al. (2020b). Berberine-mediated up-regulation of surfactant protein D facilitates cartilage repair by modulating immune responses via the inhibition of TLR4/NF-ĸB signaling. Pharmacol. Res. 155, 104690. 10.1016/j.phrs.2020.104690 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Tao H., Li Y., Deng M., He B., Xia S., et al. (2016c). Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur. J. Pharmacol. 789, 109–118. 10.1016/j.ejphar.2016.07.027 [DOI] [PubMed] [Google Scholar]
- Zhu H., Xiong X. G., Lu Y., Wu H. C., Zhang Z. H., Sun M. J. (2022). The mechanism of the anti-inflammatory effect of oldenlandia diffusa on arthritis model rats: a quantitative proteomic and network pharmacologic study. Ann. Transl. Med. 10, 1098. 10.21037/atm-22-3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H., Li B., Xie T., Xu C., Ren X., Jiang F., et al. (2022). Indole-3-aldehyde alleviates chondrocytes inflammation through the AhR-NF-κB signalling pathway. Int. Immunopharmacol. 113 (2022), 109314. 10.1016/j.intimp.2022.109314 [DOI] [PubMed] [Google Scholar]
- Zou B., Zheng J., Deng W., Tan Y., Jie L., Qu Y., et al. (2021). Kirenol inhibits RANKL-induced osteoclastogenesis and prevents ovariectomized-induced osteoporosis via suppressing the Ca2+-NFATc1 and Cav-1 signaling pathways. Phytomedicine 80, 153377. 10.1016/j.phymed.2020.153377 [DOI] [PubMed] [Google Scholar]