Abstract
Exosomes have emerged as a promising cell-free therapeutic approach. However, challenges in large-scale production, quality control, and heterogeneity must be overcome before they can be used clinically. Biomimetic exosomes containing key components of natural exosomes have been assembled through extrusion, artificial synthesis, and liposome fusion to address these limitations. These exosome-mimetics (EMs) possess similar morphology and function but provide higher yields, faster large-scale production, and similar size compared to conventional exosomes. This article provides an overview of the chemical and biological properties of various synthetic exosome systems, including nanovesicles (NVs), EMs, and hybrid exosomes. We highlight recent advances in the production and applications of nanobiotechnology and discuss the advantages, limitations, and potential clinical applications of programming assembly of exosome mimetics.
Keywords: Exosomes, Biomimetic exosomes, Exosome-mimetics (EMs), Hybrid exosomes, Drug delivery, Nanomedicine
Graphical abstract
Approaches for the development of biomimetic exosomes as nonotheranostic platform.
1. Introduction
Exosomes are small lipid bilayer vesicles with a diameter of 30–150 nm. They are formed from early endosomes by invagination of the cell membrane and subsequently budding into multivesicular bodies (MVBs) under the regulation of the transport complexes and related proteins. Once secreted into the extracellular space, exosomes are fused with cell membranes [1] and act as mediators of cellular signals, delivering their “cargo”, such as proteins, mRNAs, or miRNAs, to recipient cells, enabling intercellular information transmission without direct cell-to-cell contact. Some exosomes also play a role in regulating various cellular processes, including cell migration, proliferation, apoptosis, immune response, angiogenesis, stem cell division or differentiation, neovascularization, and cellular waste removal [2], making them the primary mechanism by which cells exert their paracrine effects. In contrast to cell therapy, cell-free exosome therapy offers a promising new approach that avoids immune rejection and tumorigenic risk associated with cell transplantation. Moreover, exosomes have the advantages of nanostructure effects, more stability, low immunogenicity, and biocompatibility, making them efficient carriers for targeted drug delivery.
As exosomes have a lipid bilayer structure similar to that of cell membranes, and the biological functions of exosomes depend on the anchor proteins in the exosomal membrane. Many exosome-specific or selective proteins are displayed on the exosome membrane, including tetraspanin proteins, such as CD9, CD63, CD81, and proteins involved in cell adhesion and transport, lipid rafts, and others. Two members of the endosomal sorting complex (ESCORT) pathway required for exosome transport, Alix and TSG101, are often used as cancer biomarkers [3]. Membrane proteins can be located on the exosome surface or within the membrane, which can be applied as cancer biomarkers [4]. Exosomal membrane proteins are involved in exosome-receptor cell interactions. Expression of relevant exosomal membrane proteins can also be directly adopted or modified to allow exosomes as drug delivery systems and therapeutic platforms, including in targeted therapeutic approaches [5].
Despite current significant advances in exosome-based cell-free therapy and in the field of nanotheranostics, exosomes or MSCs-derived exosomes as drug carriers still face many challenges in clinical translation, such as large-scale manufacture, quality control, and heterogeneity [[6], [7], [8]]. Previously, it was proposed that exosomes can be called “programmable exosome” after being synthesized or modified by various biotechnologies [9,10]. Here we introduce the biomimetic exosomes in this paper include exosome-like nanoparticles (NVs), exosomes mimetics (EMs), and hybrid exosomes (HEs), which were obtained by top-down, bottom-up and biohybrid synthetic approach, respectively [11]. Here, we propose the concept of programmable biomimetic exosomes actually refers to a kind of engineered biomimetic exosome, and such programmable biomimetic exosomes have similar size, biomorphology and characteristic membrane proteins resemble naturally occurring exosomes. In addition to targeting ability, programmable biomimetic exosomes can be modified by both chemical methods and genetic engineering methods. Programming assembly of exosomes using nanobiotechnology have overcome the limitations of natural exosomes and emerged as a potential solution to these challenges. This synthesis biotechnique enables the creation of “programmable” exosomes by controllably modifying the structure and function, allowing for rapid large-scale production with similar size, morphology, and membrane protein markers to natural exosomes. This review provides insight into the composition and functional properties of biomimetic exosomes and focuses on state-of-the-art technologies for their production, with highlights on the significant potential of programming assembly of biomimetic exosomes for therapeutic applications and the concerns and application prospects of biomimetic exosomes, which provide valuable suggestions for the future research and clinical application. The development of biomimetic exosomes through nanobiotechnology holds great promise for advanced drug delivery, combining the advantages of natural and synthetic nanoparticles. It is expected that these programming assembly technologies will promote biomimetic exosomes to be more widely used in the future.
2. Strategies to fabricate biomimetic exosomes
Exosomes are extracellular nanovesicles secreted by cells. There are two main approaches for generating biomimetic exosomes. The first is to enhance the natural secretion of cells through techniques such as increasing the number of cells and optimizing the ability of cells to secrete exosomes. The second is to skip the natural process of cellular exosome secretion by inducing cells to break down and form extracellular vesicles that resemble exosomes directly using extrusion and microfluidics techniques. The latter approach has several advantages, including the ability to producing large quantities of biocompatible artificial exosomes. Additionally, reconstituting the cell membrane to form NVs can significantly increase their practical application.
2.1. Optimized reconstitution of the cellular membrane into biomimetic exosomes
Self-assembly is a phenomenon in which structural units spontaneously form an ordered structure. This process is ubiquitous in cells and is the basic unit of life activities. Both exosomes and cell membranes have a bilayer structural backbone formed through the self-assembly of lipid molecules. S. Marrink first observed the assembly of randomly disordered lipid molecules into an ordered bilayer structure on the atomic scale using molecular dynamics simulations [12]. To generate suitable cell membranes for reassembly into nanostructures, hypotonic treatment, repeated freeze-thawing, and ultrasonic disruption followed by nanopore extrusion methods can be used. In this section, we will briefly introduce recent advances in this top-down strategy for synthesizing EMs or exosome-liposome hybrids for disease treatment. At last, we will propose future directions for the use of EM hybrid nanocarriers for precise targeted therapy.
2.1.1. Extrusion method
EMs can be obtained through the extrusion of cell membrane filters, with NV diameter regulated by adjusting the pore sizes of the filters (Fig. 1). Currently, the most used cell membrane filters are polycarbonate membrane filters. In the field of tumor therapy, EMs has been obtained by a continuous extrusion of monocytes or macrophages using filters with diminishing pore sizes. These EMs have shown more than 100 times higher productivity even though they have similar properties to natural exosomes [13]. Jeong et al. generated EMs potential for wound healing from live embryonic stem cells using filter extrusion [14]. They found that the yield of EMs increased significantly with the filter size decreasing from 10 μm to 5 μm and 1 μm. However, these EMs are highly heterogeneous due to the random assembly of the plasma membrane or cellular organelles during the cell extrusion process [15]. Moreover, the yield of EMs is also susceptible to the influence of donor cell types. Guo et al. used iron oxide nanoparticles to magnetically separate different subcellular origins and extruded endosomes to form vesicles using nonporous membranes, yielding a large number of EMs with high uniformity [16]. Kyong-Su Park et al. improved the existing extrusion technique by using ionic stress to separate cell membranes from cytoplasmic composition, followed by an ultrasound to purify EMs [17]. Although the extrusion method allows reconstituting cell membranes into smaller vesicles, the loss of the cell membrane on the filter membrane is a critical issue that cannot be ignored. A study also showed that using five freeze-thaw cycles increased the flexibility of the cell membrane, resulting in a 3-fold higher EM yield than the conventional method [18].
Fig. 1.
Establishment of the biofabrication of exosome-mimetic by extrusion methods. The cells were collected and sonicated followed by sequential extrusion through polycarbonate membranes to yield 50–200 nm nanovesicles.
It has also been reported that M1 macrophage EMs produced using 1 μm, 400 nm, and 200 nm pore size filters could repolarize the M2 tumor microenvironment into M1 macrophages and enhance antitumor efficacy [19]. EMs fabricated using this extrusion method can also be used as drug carriers. EMs loaded with siRNA can be easily uptake by recipient cells and induce functional knockdown in the targeted cells. siRNAs can be loaded into EMs by electroporation or by intracellularly expressing shRNA in the donor cells. In conclusion, these studies show that EMs produced by the extrusion method are promising as drug delivery systems.
Although exosome mimetics are highly productive and possess similar biological properties as natural exosomes, it cannot be ignored there is a huge disparity between naturally derived exosomes and exosome mimetics. The differences in these membrane proteins may lead to the alteration of biological properties. It has been found that the zeta potential and cytotoxicity were changed after intensive extrusion [9,10]. The production of natural exosomes is dependent on the cellular secretion mechanism loaded with specific cargo, while the exosome mimetics produced by extrusion method are mainly generated by pressurization of cells through filters with different pore sizes, and the cell membranes or cargos are randomly decomposed and reassemble into artificial exosomes during the extrusion process. As the whole process is random and uncontrollable, and the membrane protein distribution of artificial exosomes is also different from that of natural exosomes, which inevitably leads to different biological behaviors between exosome mimetics and natural exosomes [20]. It has also been pointed out that the extrusion method of producing exosome mimetics will change the structure of the plasma membrane, which will lead to drug leakage. Therefore, the production of exosome mimetics by extrusion method still needs further improvement to better meet the needs for clinical use.
2.1.2. Microfluidic devices
Microfluidic devices utilize channels with diameters between 10 and 200 μm to manipulate fluids at the microscale. These devices are characterized by miniaturization, high efficiency, automation, high purity, and scalability [21]. Many microfluidic devices have been developed for the production of artificial exosomes or lipid nanoparticles (LNP), including hydrodynamic flow, microvortices, chaotic mixing, and droplet formation [22]. Jo et al. successfully produced 100 nm diameter nanovesicles by using hydrophilic microfluidic arrays of 200 μm in length and 5 μm in width to squeeze cells. Such nanovesicles are similar to cell-secreted exosomes and can be used for macromolecular mRNA delivery [23]. Yoon et al. designed 500 nm-thick silicon nitride blades that can cleave live cells upon micro-channeling to cell membrane fragments, which subsequently self-assemble to form 100–300 nm diameter nanovesicles. These newly formed nanovesicles can transport encapsulated beads across the plasma membrane of the recipient cell and into the recipient cell [24].
Although the use of microfluidic devices in the production of artificial exosomes has not been well explored, several studies have successfully generated siRNA-loaded LNPs with diameters between 20 and 100 nm using a staggered herringbone microhybrid or similar microfluidic mixer [25,26], making it a novel approach with great application potentials. Microfluidic devices can generate nanovesicles of various sizes, but it is inevitable that other agents such as RNA and proteins inside the cells will also be bound to the nanovesicles, which will pose a challenge for the purification of nanovesicles [27].
We hope that microfluidic system with viscoelastic fluids, optics, and organic materials will lead to the development of automated and high-throughput microfluidic platforms, providing conventional devices for the commercial development of EMs.
2.1.3. Chemical induce blebbing approach
Methods such as extrusion can lead to a significant decrement of cell membranes, while microfluidic systems capable of producing EMs with high purity and low cost are often prohibitively complex, especially when multi-step processing is required. Alternatively, chemically induced methods can effectively avoid such situations and only require exposure to sulfhydryl-blocking reagent to induce blistering of cell membranes. As demonstrated by Dominique et al. this method can generate a large number of nanovesicles from murine T cell lymphoma EL4 cells [28]. Additionally, to facilitate the production of erythrocyte EVs, a large number of (10^13–10^14) EVs were purified from erythrocytes after treatment with calcium ion carriers, providing a strategy for obtaining EVs on a large scale. Chemically induced vesicle generation requires the addition of chemical surfactants, which can affect the nanovesicles and their contents, and the removal of chemical reagents and purification of nanovesicles was a major problem must be considered [29].
2.1.4. Nitrogen cavitation-based method
Exosome membrane proteins play an important role in recipient cells’ recognition, binding, and internalization of these vesicles, triggering the activation or inhibition of intercellular signaling pathways. Maintaining the structure and activity of these proteins is essential to produce EMs, as it distinguishes them from synthetic liposomes. The nitrogen cavitation method is promising for rapidly disrupting cell membranes while preserving their biological functions. Gao et al. utilized that method by dissolving nitrogen in the cytoplasm of HL60 cells under high pressure (350 psi) to form nitrogen bubbles in the cytoplasmic cell suspension, resulting in cell fragmentation. The desired vesicles were obtained via ultra-high-speed centrifugation, providing a controlled and effective approach that reduces the risk of genetic material transfer and avoids antigenic changes that may occur with chemical or other physical methods [30]. Similarly, the nitrogen cavitation method was used to prepare nanovesicles with a 100-fold higher yield from neutrophil cells. The strategy significantly reduced acute lung inflammation and showed great potential for the exploitation of personalized nanomedicine for specific diseases [31].
2.2. Bottom-up synthetic biology approach of biomimetic exosomes
Bottom-up synthetic biology provides a novel approach to creating nanomaterials from small molecules and gradually building up. This bottom-up idea has been widely applied to produce synthetic lipid-based nanovesicles, which exhibit better stability but limited biocompatibility. In contrast, natural exosomes have distinct advantages in biocompatibility and low immunogenicity. Therefore, combining synthetic lipid vesicles with exosomes can provide a mutually beneficial solution. Currently, approaches for bottom-up assembly include modifying liposomes with membrane proteins, embedding specific proteins into NPs, reassembling cellular lipid fractions, or creating fully synthetic exosome particles.
2.2.1. Decorated liposome with functional groups
To mimic the complexity of membranes on the surface of natural exosomes, various functional groups, including antibodies, proteins, and peptides, were conjugated to the surface of liposomal membranes through complex synthetic pathways. Molinaro et al. first developed a biomimetic vesicle that incorporates proteins from the cytoplasmic membrane into the phospholipid bilayer using a thin-layer evaporation method. The resulting nanovesicles showed tropism towards inflammatory endothelial cells [32]. Kexin Li et al. coupled a monoclonal antibody against the highly expressed surface receptor DEC205 to cationic lipids to allow the liposomes to target dendritic cells and reduce the toxicity of cationic liposomes to dendritic cells [33]. Yu et al. coupled a CD11c-specific monoclonal antibody to immunoliposomes to target Langerhans cells, and this synthetic vesicle promoted dendritic cell maturation and inhibited tumor growth [34]. Wang et al. successfully developed indocyanine green-loaded monoclonal antibody-coupled liposomes to treat H. pylori infection in vivo [35]. In addition to antibody coupling, surface modification of nanoliposomes using the transmembrane domains of cell membrane proteins has been achieved to enhance the tumor-targeting property. Zheng et al. produced triptolide-loaded liposomes with modified membrane proteins of Huh7 cells by extrusion, thus conferring the liposome tumor-targeting ability to effectively inhibit tumor growth in mice with in situ hepatocellular carcinoma [36]. However, the key membrane protein components mediating the targeting effect cannot be identified by current technology. MHC class I/peptide complexes and ligands involved in T-cell receptor interactions have also been coupled to vesicles for immunotherapy. Synthetic nanovesicles coated with biologically active APO2L/TRIAL greatly improved the ability to induce apoptosis and played a therapeutic role in antigen-induced T cell activation in the rheumatoid arthritis model [[37], [38], [39]]. In addition, co-assembling of anti-CD133 monoclonal antibody and low-density lipoprotein receptor-associated protein-1Angiopep-2 (An2) onto liposomes enhanced their targeting ability to LDL receptor-associated proteins on the blood-brain barrier (BBB) through An2, allowing the anti-CD133 monoclonal antibody to more efficiently target to the highly expressed CD133 on polymorphic glioblast cells. This dual targeting enabled liposomes to deliver temozolomide across the BBB for the treatment of polymorphic glial cancer stem cells [40]. This system provides a feasible approach for fabricating biomimetic exosomes with dual functions.
In addition to antibodies and proteins, some functional peptides have also been assembled into liposomes. Keum et al. coupled cell-penetrating peptides to liposomes using the thiol-maleimide reaction. The penetratin-conjugated liposomes were found to enhance permeation in vitro and in vivo, making them an excellent platform for drug delivery [41]. Huang et al. coupled epidermal growth factor receptor GE11 peptide to liposomes and then loaded them with both photosensitizer indocyanine green (ICG) and chemotherapy drug curcumin to achieve photothermal and chemotherapeutic effects on tumors [42]. Compared to native exosomes, decorated liposomes have certain advantages. Firstly, native exosomes are easily and rapidly cleared in vivo, which seriously affects their biological effects. In contrast, polyethylene glycolization of liposomes can significantly prolong the circulation time in vivo [43]. Secondly, it cannot be ignored that natural cell-derived exosomes have a low yield and may carry pathogens or disease-associated proteins, whereas liposomes are synthetic nano-vesicles with a more controlled yield and safety profile. Although natural exosomes have some targeting properties, they are still mainly metabolized by the liver and spleen. Decorated liposomes are shown better enriched in target cells [44]. Again, the drug loading capacity of decorated liposomes should be stronger than that of native exosomes.
Also the topology of proteins embedded in biological membranes is crucial for the function of these biomimetic exosomes, especially the ligand orientation for targeting of biomimetic exosomes. The orientation of reconstituted membrane proteins in artificial membranes depends on many parameters and is difficult to predict. Given the important carrier function that membrane proteins have, both functional studies and applications require effective control of protein orientation in the lipid bilayer.
Overall, the bottom-up strategy of assembling liposomes with exosome-like elements highly mimics the structure of cell membranes provides a simple way to better mimic the bioactive function of exosomes.
2.2.2. Synthetic biology-inspired biomimetic exosomes
In order to precisely mimic the functions of natural exosomes, researchers have turned to synthetic biology-based approaches. By using this technique, fully synthesized exosomes can be generated with all the functions of natural exosomes, including proteins, RNAs, and the most abundant lipid components. This approach starts from lipid microdroplets and involves the addition of microRNAs (miRNAs), proteins, and various lipid components, assembling them using a charge-mediated vesicle assembly technique to create synthetic extracellular vesicles (Fig. 2A). The resulting vesicles are structurally similar to natural exosomes but allow researchers to replicate, fine-tune, and refine the function of exosomes, making it a “programmable” exosome synthesis technique [45]. In a model of skin wound healing and a model of neoangiogenesis, these biomimetic exosomes were found to promote wound healing and neoangiogenesis. However, manipulating the complex composition of these biomimetic exosomes remains a challenge. To address this, Li et al. developed self-assembled supramolecular vesicles [46], where the negatively charged four-armed molecule TPE-BPA can spontaneously assemble into self-assembled neutral fluorescent vesicles by coordinating with eight positively charged CTAB molecules via ionic interactions with Fe2+. The TPE-BPA exhibiting a strong fluorescence emission in the aggregated state allows monitoring of vesicle transition by changes in fluorescence emission (Fig. 2B). Although these fully synthetic supramolecular vesicles lack the protein components on exosomes, they can be used to study exosome fusion and release behaviors [46].
Fig. 2.
Synthetic biology approaches in the development of biomimetic exosomes. (A) Schematic illustration of miRNA-containing lipid vesicles formed exclusively from artificial precursors generated by EV particle precursors at the periphery of water droplets and subsequently modified with surface proteins. Adapted from Ref. [45]with permission. Copyright © 2021 American Association for the Advancement of Science. (B) Novel supramolecular vesicles with reversible and controlled fusion and fission behaviour were fabricated, which undergo fusion upon oxidation of Fe2+ to Fe3+ and further fission upon reduction, and this biomimetic exosomes can be used as a nanocarrier for siRNA. Modified with permission of ref. [46]. Copyright © 2020 John Wiley & Sons, Inc.
The controlled encapsulation of different biomolecules achieved using synthetic biology techniques will have precise control over the biochemical and biophysical phenotypes of natural exosomes. This new technology can create synthetic vesicles with various properties beyond naturally occurring lipid carriers (e.g., EVs) with very similar functions. Furthermore, this technology also enables the synthesis of high-purity exosomes at a therapeutic scale, which is a breakthrough in overcoming some of the major limitations that currently hinder the therapeutic application of exosomes. Currently, the production of synthetic biology-inspired biomimetic exosomes is still in its infancy, and the standards and processes regarding the scale up of synthetic exosomes are not yet established. The production of synthetic exosomes mainly includes isolation, purification and analytical identification. From these aspects, establishing a standardized process will help to reduce the cost of synthetic exosomes.
2.3. Biohybrid exosomes
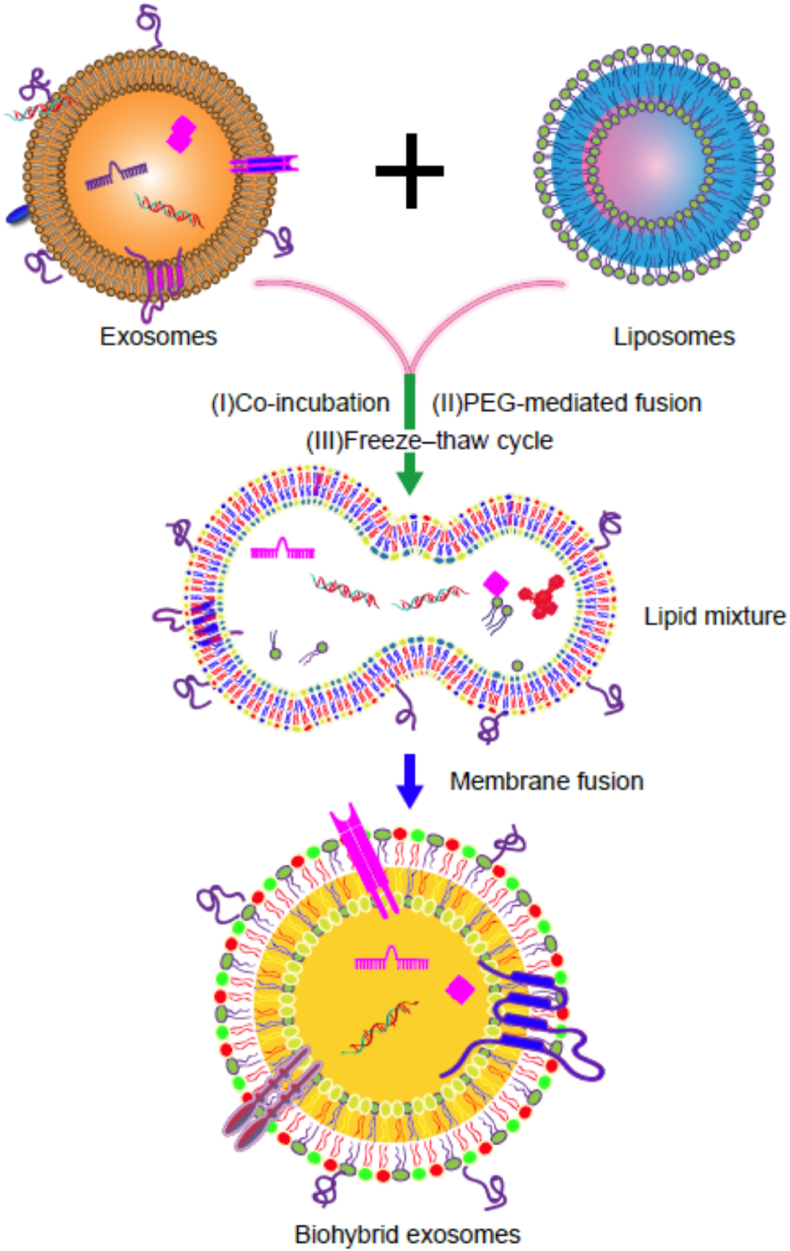
Membrane fusion is a natural biological process that occurs when two cell membranes combine. Fusing liposomes with exosomes has led to the development of membrane-fusogenic biomimetic particles. This approach takes advantage of liposome membranes for easy surface modification while effectively circumventing the deficiency of liposomes lacking endogenous functions. There are various strategies to fuse liposomes with exosomes, such as co-incubation and repeated freeze-thawing (Fig. 3). We have fused liposomes with chondrocyte-targeted exosomes by co-incubation, resulting in hybrid exosomes that successfully delivered CRISPR/Cas9 plasmids to chondrocytes to attenuate the progression of osteoarthritis [47]. Other groups have also obtained hybrid exosomes using repeated freeze-thawing. Sato et al. successfully obtained hybrid exosomes through repeated freezing in liquid nitrogen and thawing at room temperature. Their results suggested that the ice crystals formed during freezing could remove the hydrophilic surface from the cell membrane and potentially trigger changes in the structure and function of nanovesicles [47]. Lv et al. used the freeze-thawing procedure to generate hybrid nanoparticles of genetically engineered fibroblast-derived exosomes and heat-sensitive liposomes [48]. These hybrid nanoparticles exhibited better circulation and preferential accumulation in the tumor, releasing drugs under the influence of temperature [48]. Fusing liposomes with exosomes to obtain artificial exosomes is a novel approach to developing drug delivery systems. Liposomes do not carry genetic material, effectively reducing the risk of gene transfer. Similar to liposomes, cytoplasmic membrane from mature erythrocytes also has the advantages of high yield and low risk of gene transfer. Krivić et al. developed hybrid erythrocyte liposomes and conjugated anti-E. coli antibodies to the hybrid erythrocyte liposomes, achieving targeted delivery of antibiotics to Klebsiella aerogenes strains [49]. Additionally, co-extruded cell membranes from M2 and M1 macrophages can fabricate hybrid EMs, which inherit the anti-inflammatory properties of M2 macrophages and cytokine receptors from the M1 membrane. By loading black phosphorus nanosheets, this hybrid vesicle can target and accumulate at the inflamed knee joint under near-infrared (NIR) irradiation to eliminate inflammatory cells [50].
Fig. 3.
Scheme of the approach in synthesis bio-hybrid exosome-liposome. Freeze-thaw, incubation, and sonication were used for the synthesis.
The production of hybrid exosomes is still in the exploratory stage. The production of hybrid exosomes relies on the fusion between liposomes and exosomes. There are many methods to induce the fusion of liposomes and exosomes to form hybrid exosomes, but each method has its shortcomings. The co-incubation method has less effect on the contents and plasma membrane, but the fusion efficiency is very lower. The freeze-thaw method can promote the fusion of liposomes and exosomes with a high efficiency of 97.4%, but the freeze-thaw method may affect its contents and exosomal membrane [51]. Although, PEG8000 can enhance fusion efficiency, some unsafe problems of PEG have been noticed by researchers.
The choice of the method to improve the fusion efficiency of liposomes and exosomes still deserves our continued attention. Therefore, if we can establish detailed standards for the production of hybrid exosomes and determine the standard procedure for fusion, extraction and purification of hybrid exosomes, large-scale production of hybrid exosomes will be possible.
3. Improvements in biomimetic exosomes
3.1. Optimization of cellular conditions for exosome production
There are two scenarios for increasing the yield of biomimetic exosomes, which are based on modifications of natural exosomes. One method to increase the yield of biomimetic exosomes is to enhance the yield of natural exosomes, such as improving cell culture methods and optimizing exosome isolation techniques. Although increasing quantities of cells can increase the production of exosomes, it is challenging to achieve with conventional monolayer culture. Optimizing culture conditions can significantly increase exosome yield, but this dependent on the donor cell type and status.3D culture can maximize culture area to increase cell number, thereby promoting exosome yield. Cao et al. successfully obtained large-scale exosome production from 3D dynamic cell culture [52]. They cultured mesenchymal stem cells (MSCs) in a dynamic three-dimensional (3D) culture system after by attaching them to microcarrier beads. Application of this method has generated large amounts of exosomes with good manufacturing practice (GMP) grade for clinical osteoarthritis treatment [53]. Patel et al. also used a 3D printed biomaterial scaffold-perfusion bioreactor system to assess the dynamic culture response to extracellular vesicle production by endothelial cells (ECs) and found that this system can increase exosome production by more than 100-fold [54], while for other cells the yield may not be so high. For example, when mesenchymal stem cells (MSCs) cultured in 3D bioreactor, the production of exosomes in the supernatant was 2 times higher than in 2D culture [53]. And although 3D culture can significantly increase exosome production, it has also been found that the morphology and composition of exosomes can also be significantly altered.
In addition to changing the culture condition, some physical stimuli can also enhance the yield of exosomes. Fukuta et al. treated mouse melanoma and mouse fibroblasts with low-level electrical stimulation and found that low-level electrical treatment significantly increased the number of EV particles without cytotoxicity or interference in particle distribution [55]. Campbell et al. also demonstrated that electrical stimulation was effective in enhancing stem cell exosome production [56]. Serum starvation has also been shown to enhance exosome production in RPMI 8226, U266, and KM3 cells by 2.5-fold, 4.3-fold, and 3.8-fold, respectively [57]. Similarly, changes in temperature, pH, and hypoxia conditions also increased the production of exosomes [[58], [59], [60]]. Yan et al. found that mechanical stimulation may be essential for increased secretion of exosomes from umbilical cord MSCs. A rotary cell culture system significantly promoted exosome production at 36 rpm/min over 196 h [61]. Small molecule stimulation has also been found to enhance exosome production. Wang et al. investigated the effect of small molecule modulators on exosome secretion in MSCs and found that a combination of N-methyldopamine and norepinephrine increased exosome production by three-fold [62]. Cytochalasin, a fungal metabolite, can also promote exosome secretion by disrupting cytoskeletal integrity. Ashita Nair et al. showed that stimulated with 10 μg/mL cytochalasin-B increased exosome secretion by 3-fold [63]. Secretion of extracellular vesicles is regulated by calcium-dependent mechanisms. Thus, the alteration of Ca2+ concentration can affect exosome production. Savina et al. found that increasing intracellular Ca2+ stimulated exosome secretion [64]. Another study found that adiponectin, an adipocyte-secreted factor, stimulated the secretion of MSC exosomes by binding to T-cadherin [65]. Therefore, applying the 3D culture method in combination with external stimuli can expand cell number by optimizing the cellular microenvironment, thereby successfully increasing EV production and enhancing their therapeutic effects [66]. In addition, the 3D culture scaffold material can also encapsulate EVs to prevent their degradation, achieving a sustained and controlled release.
3.2. Increasing loading efficiency
Biomimetic exosomes have shown promise as drug delivery carriers. Enhancing their drug-loading efficiency is a crucial area of research. Currently, there are two universal encapsulation methods for loading drugs into biomimetic exosomes: pre-loading and post-loading. Pre-loading involves loading drugs into exosomes during biosynthesis, while post-loading refers to loading drugs into already-synthesized exosomes. Pre-loading is preferred for loading high molecular weight RNA, but its main drawback is the lack of control over the amount of drug delivered. Post-loading, on the other hand, is more efficient and includes several methods, such as co-incubation, sonication, lipofection, repeated freeze-thawing, extrusion, pH/temperature gradient, and calcium chloride (Fig. 4). Compared to pre-loading, post-loading provides greater control over the amount of drug delivered.
Fig. 4.
Drug loaded method for biomimetic exosomes. Drugs can be passively loaded in vitro by simple incubation, sonication, freeze-thaw electroporation, and extrusion, or they can be actively loaded using a pH gradient generated by ionophores or (NH4)2SO4.
Co-incubation involves co-culturing small molecule drugs with donor cells or synthetic EMs. Although the method is inexpensive and straightforward, it has a low encapsulation efficiency, and the loading amount is affected by the physicochemical properties of the drug [67]. Ultrasonication is another efficient method for drug loading, but it may cause damage to the exosome membrane, and there is also a risk of exosome aggregation under ultrasound [68]. Electroporation can also efficiently load drugs to synthetic EMs, but it also causes damage to exosome membranes. Zhou et al. constructed a dual-delivery exosome-based biological system using electroporation and successfully loaded galectin-9 siRNA with more satisfactory results in treating pancreatic ductal adenocarcinoma [69]. The repeated freeze-thaw method is simple and inexpensive, but it tends to lead to the inactivation of exosome membrane surface proteins [6]. The extrusion method is more efficient for generating EMs and drug loading, but it may cause structural reorganization of the membrane surface under external forces. pH/temperature gradient method is a method for drug loading by constructing pH/temperature differences between exosomes. Jeyaram et al. enhanced miRNA loading by constructing an extracellular vesicle membrane pH gradient without impairing the cellular uptake of extracellular vesicles and significant toxic reactions [70].
3.3. Surface engineering of biomimetic exosomes for increasing the targeting ability
Biomimetic exosomes can be further optimized for enhanced therapeutic efficacy and targeting ability through membrane surface modification and engineering. Genetic engineering approaches and chemical surface modification are the two main strategies for achieving this [71] (Fig. 5). Genetic engineering involves fusing targeting peptides with exosome surface molecules to build engineered exosomes with specific targeting properties. Chemical modification strategies include targeted peptides and protein modifications. For example, modification by peptides like cyclic Arg-Gly-Asp-D-Tyr-Lys (cRGDyK) and cyclic RGD (cRGD) can confer significant targeting ability of natural exosomes to tumors. Integrin α6β4-modified biomimetic exosomes showed improved adhesion properties and enhanced delivery of relevant therapeutic agents to cancer cells [72]. However, genetic engineering approaches carry a potential risk of gene transfer. Liang et al. designed CAP-Lamp2b plasmid to express chondrogenic targeting peptide by fusing Lamp2b to the surface of exosomes secreted by dendritic cells [73]. Then the purified CAP-exosomes were fused with liposomes to construct a hybrid CAP-exosome delivery system for genomic editing [47,74]. In addition to Lamp2b, other exosome membrane surface proteins, including CD63 and CD9, are also widely used as target ligands [75].
Fig. 5.
Schematic representation of surface functionalized biomimetic exosomes. (A) Genetic engineering approaches involve the expression of a gene encoding a targeting protein fused to an exosomal membrane protein in the donor cell, which then secretes this targeting protein on the biomimetic exosomes. (B) Targeting ligands on the surface of biomimetic exosomes can be inserted into the exosomes membrane by click chemistry in reaction with exosomal membrane proteins or by hydrophobic interaction with phospholipid bilayers using lipophilic or amphiphilic molecules. (C) Metabolic labeling, in which metabolite analogs are introduced into biomimetic exosomes in biosynthesis following cellular uptake of functional groups, such as azides, which allow subsequent bioorthogonal reactions of the targeting ligands. (D) The sortase A-mediated ligation has been shown to be applicable to modify the exosome to allow active loading of molecules onto the membrane surface of biomimetic exosomes.
Lipid surface modification can also be achieved using click chemistry. Zhang et al. utilized copper-free click chemistry and obtained functional neutrophil-derived exosomes, which retain the ability of neutrophils to converge toward inflammation and effectively alleviate the progression of rheumatoid arthritis [76]. Copper-catalyzed azide-alkyne cycloaddition is also a commonly used method for exosome chemical modification [77]. Insertion of amphiphilic molecules into exosome membranes is an easy way for chemical modification [78]. Kim et al. inserted aminoethylanisamide-polyethylene glycol into the surface of macrophage-derived exosome membranes to target the sigma receptor, which is highly expressed in tumor cells [79,80]. Although chemical modification of exosomes has fewer options compared to genetic engineering, it is worth exploring due to its rapidity and high yield.
3.4. Extending circulation time
Biomimetic exosomes have shown promise in treating various diseases, but pharmacokinetic studies have revealed a short circulating half-life in vivo, with some literature showing a half-time of approximately 2–30 min [81]. Such a short circulating time limits the amount of exogenous biomimetic exosomes that can reach the target organs and tissues to exert their intended biological effects. Circulating exogenous exosomes can be rapidly cleared by monocytes/macrophages or their associated reticuloendothelial system (liver, spleen, and lung). Therefore, various strategies have been developed to prolong the retention time of biomimetic exosomes in vivo by conferring surface modifications to exosomes to circumvent clearance by the immune system. One such strategy is to camouflage exosomes with cell surface molecules, such as CD24, CD44, CD47, PECAM-1, β2M, PD-L1, and DHMEQ, that can provide “don't eat me” signals [82]. CD47 is a highly glycosylated cell surface protein that, when functionalized, can lead to a longer half-life of exosomes. Studies have found that extracellular vesicles from MSCs overexpressing CD47 were still detectable after 120 min after tail vein injection, while natural extracellular vesicles disappeared in less than 30 min, suggesting that high CD47 expression can effectively prolong the half-life of EVs [83]. Another strategy is to conjugate albumin binding domains (ABDs) or albumin decoration to the exosomes. Human albumin has a half-life of several weeks, and conjugation of ABDs or albumin decoration has been widely used to extend the half-life of nanoparticles. For example, conjugation of ABDs into the extracellular loops of tetraspanins or directly fused ABDs with single-transmembrane EV-sorting domains by engineering approach prolonged the half-life of exosomes by more than 4.5 h after injecting into mice [84]. Moreover, modification of extracellular vesicles by coating with nanobody-PEG-lipids extended their half-life by more than 50 min [85]. Anchoring proline-alanine-serine (PAS) peptide sequences on the membrane surface also greatly prolonged the circulation time of cell membrane-derived nanotherapies in vivo [86]. Collectively, prolonging the circulation time of biomimetic exosomes could increase their uptake opportunity by receptor cells, significantly enhancing their therapeutic potential (Fig. 6).
Fig. 6.
Different modification strategies to enhance the function of biomimetic exosomes. (A)Increase the total yield of biomimetic exosomes.(B) Improve drug encapsulation efficiency.(C) Surface protein engineering improves targeting ability.(D) Increases the circulation time.
4. Biomimetic exosomes for drug delivery and theranostic applications
The application of biomimetic nanomedicine delivery systems improves drug pharmacokinetics, enhances therapeutic efficacy and mitigates toxic side effects [87]. Biomimetic exosomes emerged as a theranostic nanomedicine platform similar as exosomes, which can delivery drugs to overcome the disadvantages of natural exosomes and retain the advantages of natural exosomes. Various proteins, miRNAs, shRNAs, siRNAs, CRISPR/Cas9 systems, mRNAs and small molecules can be delivery by this new nanoplatform [74,[88], [89], [90]]. By delivering different drugs and reaching the target cells, the bioavailability of drugs can be effectively improved. It has been found that biomimetic exosomes can deliver docosahexaenoic acid (DHA) to tumor cells, which in turn promotes ferroptosis, thereby effectively treating cancer [91]. Biomimetic exosomes can also deliver siRNAs to play a therapeutic role, and another study found that encapsulation of CDK4 siRNA into biomimetic exosomes can significantly down-regulated CDK4 expression and inhibited tumor growth [92]. Biomimetic exosomes offers major advantages in facilitating loading CRISPR-Cas9 system as a novel delivery vehicle for gene editing therapeutics [93]. In another study, doxorubicin (DOX) delivered via biomimetic exosomes showed a significant reduction in the potential adverse effects, with no nephrotoxic side effects even at the highest dose of DOX, and animal studies showed that DOX delivered via biomimetic exosomes was effective in the treatment of breast cancer and inhibited tumor metastasis [94]. The above results strongly suggest that biomimetic exosomes are a therapeutic nanomedicine platform. We have summarized these therapeutic applications in a Table 1.
Table 1.
Examples of biomimetic exosomes for therapeutic interventions.
| Biomimetic nanovesicle | Source | Cargo | Preparation method | Application | Ref. |
|---|---|---|---|---|---|
| Exosome-mimic nanovesicles | CCR2-expressing M1 macrophages | Fe3O4 nanoparticles | Extrusion | Inhibit cancer metastasis | [91] |
| M1 macrophage | Docosahexaenoic acid (DHA) | Extrusion | Treatment of hepatocellular carcinoma | [95] | |
| MCF-10A cells | siRNA CDK4 | Extrusion | Cancer therapy | [92] | |
| Human macrophage(U937 cells) | Rapamycin | Extrusion | Therapy of hemangiomas | [96] | |
| CD47-overexpressing fibroblasts | GM-CSF, docetaxel | Freeze–thaw procedures | Chemoimmunotherapy, | [48] | |
| Human umbilical vein endothelial cells (HUVEC), murine mesenchymal stem cell line C3H (MSC), GFP expressing Mardin-Darby canine kidney (MDCK) |
Mthpc | PEG-mediated fusion | Biocamouflage of liposomes and drug delivery | [97] | |
| Macrophages | Doxorubicin | Extrusion | Breast cancer treatment | [98] | |
| U937 cells | Doxorubicin | Extrusion | Targeted delivery of chemotherapeutics | [99] | |
| Hybrid exosome-liposome | CD47-expressing tumor exosomes | miR497/triptolide | Sonicate and co-extrusion | Cancer therapy | [100] |
| Raw 264.7 cell, HER2-expressing CMS7 cell |
N.A. | Freeze–thaw cycles | In vitro cellular uptake | [101] | |
| HEK 293T cells | CRISPR–Cas9 plasmid | Incubation | CRISPR/Cas9 system | [93] | |
| Fibroblast cells (L-929 cells) | Nintedanib | Sonicate and co-extrusion | Anti-pulmonary fibrotic | [102] | |
| Dendritic cells were transfected with the CAP-Lamp2b | CRISPR–Cas9 plasmid | Incubation | Genomic editing for Osteoarthritis therapy | [47] | |
| M1 macrophage and M2 macrophages | Black phosphorus nanosheets | Extrusion | Rheumatoid arthritis treatment | [50] | |
| Macrophage (J774A.1 cells) | Doxorubicin, siRNA | Co-extrusion | Anti-cancer | [98,103] | |
| 4T1 cells | Doxorubicin | Extrusion | Breast cancer treatment | [94] | |
| MCF-7 cells | Trichostatin A | Freeze−thaw method | Breast cancer treatment | [104] | |
| Exosome-mimicking liposomes | Connexin 43 modified exosome-mimicking lipids | siRNA VEGF | Extrusion | Higher efficiency delivery of siRNA | [105] |
5. Conclusion and perspectives
Although exosomes are considered promising therapeutic agents, their large-scale preparation for clinical application remains an insurmountable obstruction. With the rapid development of synthetic biotechnology, biomimetic exosomes have emerged as a potential solution. Compared with natural exosomes, biomimetic exosomes have unique advantages in terms of yield and quality control, they offer more than 100 times yield than naturally secreted exosomes while preserving the biocompatibility of natural extracellular vesicles and exhibiting various new biological functions. Also as the synthesis process of exosomes is carried out in an artificial environment, which also helps to reduce the heterogeneity of exosomes. However, challenges and opportunities always accompany, including unclear mechanisms in complex biological environments, safety issues, and clinical quality control in clinical translation.
The current extrusion method is commonly utilized to prepare EMs. However, it presents challenges such as the loss of cell membranes during the preparation process, inadequate quality control, and uncertainty regarding the effect of extrusion on membrane proteins. Additionally, the encapsulation of contents during extrusion tends to be more random, making quantification of the newly generated EM content cannot difficult. Hybrid membrane fusion particles may also lose their characteristic properties due to the reassembly of membrane proteins. All these may lead to the heterogeneity of EMs. Meanwhile, the safety assessments of long-term stability and in vivo distribution of EMs need to be completed and require further exploration. We have compared the pros and cons of these strategies in Table 2.
Table 2.
Advantages and disadvantages of different strategies for preparing biomimetic exosomes.
| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Top-down strategies | Improved yield, partial retention of biological activity, a wide range of application | Passive encapsulation, no selective loading of drugs, more difficult and time-consuming for purification | [11,106] |
| Bottom-up strategies | Known composition, better homogeneity, more stability performance, | Limits in large-scale generation, high cost, challenging in decorated with multiple functional groups at the same time | [107] |
| Biohybrid | Larger-scale production | Limits in fusion efficiency, multiple freeze/thaw cycles damage exosomes structure | [108] |
However, there are still a number of technologies to overcome before biomimetic exosomes can be used in clinical practice. First, there is still no clear optimal procedure for synthesizing biomimetic exosomes. All existing methods for isolating, quantifying and characterizing biomimetic exosomes are based on definitive exosomes, making it difficult to distinguish between exosomes. Secondly, the heterogeneous composition of the donor cell source of biomimetic exosomes may also lead to recipient immunogenic effects. Even if the same donor cells of biomimetic exosomes are in different physiological states will affect the composition and therapeutic efficacy. Considering the above factors and concerns, a new set of quality control (QC) standards for biomimetic exosomes should be established so that clinical studies can be better promoted.
Looking back on this decade, the variety of top-down and bottom-up strategies for synthesizing exosomes in new directions is truly impressive. However, for future clinical translation, establishing quality control standards for the entire process of EMs, including membrane extrusion type and form, fusion, and modification, is still being explored. These challenges maintain the great potential of EMs in disease treatment. We hope that the advancement in the synthesis, storage, and modification of artificial exosomes will continue to be made, opening up the next step of development.
Ethics approval and consent to participate
Not applicable.
Author contributions
Y.J.L conceived the idea and drew figures; Y.M.Z supervised the project. X.X, L.M.X, C.N.W, J.X and Y.M.Z provided suggestions to this article; X.X, L.M.X and Y.J.L drafted the manuscript. All the authors listed have reviewed the final version of the manuscript and approved the final submission.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The research has received funding from the National Natural Science Foundation of China (82102607); Shandong Provincial Natural Science Foundation (ZR2023QH148); PhD Research Foundation of Affiliated Hospital of Jining Medical University (2022-BS-03, 2022-BS-04). This project was also partially supported by a Guangdong-Hong Kong Technology Cooperation Funding Scheme (TCFS) grant from Innovation and Technology Commission, Hong Kong SAR.
Contributor Information
Yuanmin Zhang, Email: yuanminzhang@mail.jnmc.edu.cn.
Yujie Liang, Email: liangyjie@126.com.
Data availability
No data was used for the research described in the article.
References
- 1.Han Q.F., Li W.J., Hu K.S., Gao J., Zhai W.L., Yang J.H., Zhang S.J. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol. Cancer. 2022;21(1):207. doi: 10.1186/s12943-022-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Huang J., Chen W., Li G., Li Z., Lei J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp. Mol. Med. 2022;54(9):1390–1400. doi: 10.1038/s12276-022-00855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Q., Su H., Li J., Lyon C., Tang W., Wan M., Hu T.Y. Clinical applications of exosome membrane proteins. Precision Clin. Med. 2020;3(1):54–66. doi: 10.1093/pcmedi/pbaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L., Xu X., Liang Y., Wen C., Ouyang K., Huang J., Xiao Y., Deng X., Xia J., Duan L. Osteoclast-targeted delivery of anti-miRNA oligonucleotides by red blood cell extracellular vesicles. J. Contr. Release : Off. J. Control. Release Soc. 2023;358:259–272. doi: 10.1016/j.jconrel.2023.04.043. [DOI] [PubMed] [Google Scholar]
- 6.Kimiz-Gebologlu I., Oncel S.S. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Contr. Release : Off. J. Control. Release Soc. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Hussen B.M., Faraj G.S.H., Rasul M.F., Hidayat H.J., Salihi A., Baniahmad A., Taheri M., Ghafouri-Frad S. Strategies to overcome the main challenges of the use of exosomes as drug carrier for cancer therapy. Cancer Cell Int. 2022;22(1):323. doi: 10.1186/s12935-022-02743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson S.W., Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J. Contr. Release : Off. J. Control. Release Soc. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Xu Q., Zi Z., Liu Z., Wan C., Crisman L., Shen J., Liu X. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell. 2020;55(6):784–801.e789. doi: 10.1016/j.devcel.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Z., Xiao K., Lin J., Liao Y., Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15(47) doi: 10.1002/smll.201903761. [DOI] [PubMed] [Google Scholar]
- 11.Li Y.J., Wu J.Y., Liu J., Xu W., Qiu X., Huang S., Hu X.B., Xiang D.X. Artificial exosomes for translational nanomedicine. J. Nanobiotechnol. 2021;19(1):242. doi: 10.1186/s12951-021-00986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrink S.J., Lindahl E., Edholm O., Mark A.E. Simulation of the spontaneous aggregation of phospholipids into bilayers. J. Am. Chem. Soc. 2001;123(35):8638–8639. doi: 10.1021/ja0159618. [DOI] [PubMed] [Google Scholar]
- 13.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J., Nilsson J., Lötvall J., Kim Y.K., Gho Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 14.Jeong D., Jo W., Yoon J., Kim J., Gianchandani S., Gho Y.S., Park J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials. 2014;35(34):9302–9310. doi: 10.1016/j.biomaterials.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Kang H., Kang M., Han C., Yi J., Kwon Y., Park J. Heterogeneous subcellular origin of exosome-mimetic nanovesicles engineered from cells. ACS Biomater. Sci. Eng. 2020;6(11):6063–6068. doi: 10.1021/acsbiomaterials.0c01157. [DOI] [PubMed] [Google Scholar]
- 16.Guo P., Busatto S., Huang J., Morad G., Moses M.A. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv. Funct. Mater. 2021;31(44) doi: 10.1002/adfm.202008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park K.S., Bergqvist M., Lässer C., Lötvall J. Targeting Myd88 using peptide-loaded mesenchymal stem cell membrane-derived synthetic vesicles to treat systemic inflammation. J. Nanobiotechnol. 2022;20(1):451. doi: 10.1186/s12951-022-01660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko K.W., Yoo Y.I., Kim J.Y., Choi B., Park S.B., Park W., Rhim W.K., Han D.K. Attenuation of tumor necrosis factor-α induced inflammation by umbilical cord-mesenchymal stem cell derived exosome-mimetic nanovesicles in endothelial cells. Tissue Eng. Regenerative Med. 2020;17(2):155–163. doi: 10.1007/s13770-019-00234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choo Y.W., Kang M., Kim H.Y., Han J., Kang S., Lee J.R., Jeong G.J., Kwon S.P., Song S.Y., Go S., et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12(9):8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 20.Li S.P., Lin Z.X., Jiang X.Y., Yu X.Y. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 2018;39(4):542–551. doi: 10.1038/aps.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havers M., Broman A., Lenshof A., Laurell T. Advancement and obstacles in microfluidics-based isolation of extracellular vesicles. Anal. Bioanal. Chem. 2023;415(7):1265–1285. doi: 10.1007/s00216-022-04362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y., Asghari M., Aslan M.K., Yilmaz A., Mateescu B., Stavrakis S., deMello A.J. Microfluidics for extracellular vesicle separation and mimetic synthesis: recent advances and future perspectives. Chem. Eng. J. 2021;404 [Google Scholar]
- 23.Jo W., Jeong D., Kim J., Cho S., Jang S.C., Han C., Kang J.Y., Gho Y.S., Park J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261–1269. doi: 10.1039/c3lc50993a. [DOI] [PubMed] [Google Scholar]
- 24.Yoon J., Jo W., Jeong D., Kim J., Jeong H., Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59:12–20. doi: 10.1016/j.biomaterials.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Chen D., Love K.T., Chen Y., Eltoukhy A.A., Kastrup C., Sahay G., Jeon A., Dong Y., Whitehead K.A., Anderson D.G. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012;134(16):6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 26.Zhigaltsev I.V., Belliveau N., Hafez I., Leung A.K., Huft J., Hansen C., Cullis P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir : ACS J. Surf. Colloids. 2012;28(7):3633–3640. doi: 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- 27.Thone M.N., Kwon Y.J. Extracellular blebs: artificially-induced extracellular vesicles for facile production and clinical translation. Methods (San Diego, Calif) 2020;177:135–145. doi: 10.1016/j.ymeth.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Ingato D., Edson J.A., Zakharian M., Kwon Y.J. Cancer cell-derived, drug-loaded nanovesicles induced by sulfhydryl-blocking for effective and safe cancer therapy. ACS Nano. 2018;12(9):9568–9577. doi: 10.1021/acsnano.8b05377. [DOI] [PubMed] [Google Scholar]
- 29.Joorabloo A., Liu T. Engineering exosome-based biomimetic nanovehicles for wound healing. J. Contr. Release : Off. J. Control. Release Soc. 2023;356:463–480. doi: 10.1016/j.jconrel.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Gao J., Chu D., Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Contr. Release : Off. J. Control. Release Soc. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J., Wang S., Dong X., Leanse L.G., Dai T., Wang Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020;3(1):680. doi: 10.1038/s42003-020-01410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molinaro R., Corbo C., Martinez J.O., Taraballi F., Evangelopoulos M., Minardi S., Yazdi I.K. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 2016;15(9):1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li K., Chang S., Wang Z., Zhao X., Chen D. A novel micro-emulsion and micelle assembling method to prepare DEC205 monoclonal antibody coupled cationic nanoliposomes for simulating exosomes to target dendritic cells. Int. J. Pharm. 2015;491(1–2):105–112. doi: 10.1016/j.ijpharm.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y., Wang H., Guo B., Wang B., Wan Z., Zhang Y., Sun L., Yang F. Microneedle-based two-step transdermal delivery of Langerhans cell-targeting immunoliposomes induces a Th1-biased immune response. Eur. J. Pharm. Biopharm. : Off. J. Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2022;177:68–80. doi: 10.1016/j.ejpb.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R., Song C., Gao A., Liu Q., Guan W., Mei J., Ma L., Cui D. Antibody-conjugated liposomes loaded with indocyanine green for oral targeted photoacoustic imaging-guided sonodynamic therapy of Helicobacter pylori infection. Acta Biomater. 2022;143:418–427. doi: 10.1016/j.actbio.2022.02.031. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y., Kong F., Liu S., Liu X., Pei D., Miao X. Membrane protein-chimeric liposome-mediated delivery of triptolide for targeted hepatocellular carcinoma therapy. Drug Deliv. 2021;28(1):2033–2043. doi: 10.1080/10717544.2021.1983072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Miguel D., Gallego-Lleyda A., Galan-Malo P., Rodriguez-Vigil C., Marzo I., Anel A., Martinez-Lostao L. Immunotherapy with liposome-bound TRAIL overcomes partial protection to soluble TRAIL-induced apoptosis offered by down-regulation of Bim in leukemic cells. Clin. Transl. Oncol.: Off. Publ. Fed. Spanish Oncol. Soc. Natl. Cancer Inst. Mexico. 2015;17(8):657–667. doi: 10.1007/s12094-015-1295-x. [DOI] [PubMed] [Google Scholar]
- 38.De Miguel D., Basáñez G., Sánchez D., Malo P.G., Marzo I., Larrad L., Naval J., Pardo J., Anel A., Martinez-Lostao L. Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol. Pharm. 2013;10(3):893–904. doi: 10.1021/mp300258c. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Lostao L., García-Alvarez F., Basáñez G., Alegre-Aguarón E., Desportes P., Larrad L., Naval J., Martínez-Lorenzo M.J., Anel A. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010;62(8):2272–2282. doi: 10.1002/art.27501. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.S., Shin D.H., Kim J.S. Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells. J. Contr. Release : Off. J. Control. Release Soc. 2018;269:245–257. doi: 10.1016/j.jconrel.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Keum T., Noh G., Seo J.E., Bashyal S., Sohn D.H., Lee S. Examination of effective buccal absorption of salmon calcitonin using cell-penetrating peptide-conjugated liposomal drug delivery system. Int. J. Nanomed. 2022;17:697–710. doi: 10.2147/IJN.S335774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X., Chen L., Zhang Y., Zhou S., Cai H.H., Li T., Jin H., Cai J., Zhou H., Pi J. GE11 peptide conjugated liposomes for EGFR-targeted and chemophotothermal combined anticancer therapy. Bioinorgan. Chem. Appl. 2021;2021 doi: 10.1155/2021/5534870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nosova A.S., Koloskova O.O., Nikonova A.A., Simonova V.A., Smirnov V.V., Kudlay D., Khaitov M.R. Diversity of PEGylation methods of liposomes and their influence on RNA delivery. MedChemComm. 2019;10(3):369–377. doi: 10.1039/c8md00515j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S., Li C., Yuan Y., Jiang L., Chen W., Jiang X. Dendritic lipopeptide liposomes decorated with dual-targeted proteins. Biomater. Sci. 2022;10(24):7032–7041. doi: 10.1039/d2bm00952h. [DOI] [PubMed] [Google Scholar]
- 45.Staufer O., Dietrich F., Rimal R., Schröter M., Fabritz S., Boehm H., Singh S., Möller M., Platzman I., Spatz J.P. Bottom-up assembly of biomedical relevant fully synthetic extracellular vesicles. Sci. Adv. 2021;7(36) doi: 10.1126/sciadv.abg6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Peng K., Li Y., Wang J., Huang J., Yan Y., Wang D., Tang B.Z. Exosome-mimetic supramolecular vesicles with reversible and controllable fusion and fission*. Angew Chem. Int. Ed. Engl. 2020;59(48):21510–21514. doi: 10.1002/anie.202010257. [DOI] [PubMed] [Google Scholar]
- 47.Liang Y., Xu X., Xu L., Iqbal Z., Ouyang K., Zhang H., Wen C., Duan L., Xia J. Chondrocyte-specific genomic editing enabled by hybrid exosomes for osteoarthritis treatment. Theranostics. 2022;12(11):4866–4878. doi: 10.7150/thno.69368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv Q., Cheng L., Lu Y., Zhang X., Wang Y., Deng J., Zhou J. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv. Sci. (Weinh) 2020;7(18) doi: 10.1002/advs.202000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krivić H., Himbert S., Sun R., Feigis M., Rheinstädter M.C. Erythro-PmBs: a selective polymyxin B delivery system using antibody-conjugated hybrid erythrocyte liposomes. ACS Infect. Dis. 2022;8(10):2059–2072. doi: 10.1021/acsinfecdis.2c00017. [DOI] [PubMed] [Google Scholar]
- 50.Zhao C., Song W., Ma J., Wang N. Macrophage-derived hybrid exosome-mimic nanovesicles loaded with black phosphorus for multimodal rheumatoid arthritis therapy. Biomater. Sci. 2022;10(23):6731–6739. doi: 10.1039/d2bm01274j. [DOI] [PubMed] [Google Scholar]
- 51.Kannavou M., Marazioti A., Stathopoulos G.T., Antimisiaris S.G. Engineered versus hybrid cellular vesicles as efficient drug delivery systems: a comparative study with brain targeted vesicles. Drug Delivery and Translational Res. 2021;11(2):547–565. doi: 10.1007/s13346-021-00900-1. [DOI] [PubMed] [Google Scholar]
- 52.Cao J., Wang B., Tang T., Lv L., Ding Z., Li Z., Hu R., Wei Q., Shen A., Fu Y., et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res. Ther. 2020;11(1):206. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan L., Li X., Xu X., Xu L., Wang D., Ouyang K., Liang Y. Large-scale preparation of synovial fluid mesenchymal stem cell-derived exosomes by 3D bioreactor culture. J. Vis. Exp. 2022;185 doi: 10.3791/62221. [DOI] [PubMed] [Google Scholar]
- 54.Patel D.B., Luthers C.R., Lerman M.J., Fisher J.P., Jay S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019;95:236–244. doi: 10.1016/j.actbio.2018.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuta T., Nishikawa A., Kogure K. Low level electricity increases the secretion of extracellular vesicles from cultured cells. Biochem. Biophys. Rep. 2020;21 doi: 10.1016/j.bbrep.2019.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell C.R., Berman A.E., Weintraub N.L., Tang Y.L. Electrical stimulation to optimize cardioprotective exosomes from cardiac stem cells. Med. Hypotheses. 2016;88:6–9. doi: 10.1016/j.mehy.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L., Wang H.X., Zhu X.J., Wu P.H., Chen W.Q., Zou P., Li Q.B., Chen Z.C. Serum deprivation elevates the levels of microvesicles with different size distributions and selectively enriched proteins in human myeloma cells in vitro. Acta Pharmacol. Sin. 2014;35(3):381–393. doi: 10.1038/aps.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otsuka K., Yamamoto Y., Ochiya T. Uncovering temperature-dependent extracellular vesicle secretion in breast cancer. J. Extracell. Vesicles. 2020;10(2) doi: 10.1002/jev2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorayappan K.D.P., Wanner R., Wallbillich J.J., Saini U., Zingarelli R., Suarez A.A., Cohn D.E., Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37(28):3806–3821. doi: 10.1038/s41388-018-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan L., Liu G., Wu X. Exosomes derived from umbilical cord mesenchymal stem cells in mechanical environment show improved osteochondral activity via upregulation of LncRNA H19. J. Orthop. Translat. 2021;26:111–120. doi: 10.1016/j.jot.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Bonacquisti E.E., Brown A.D., Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):660. doi: 10.3390/cells9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nair A., Bu J., Rawding P.A., Do S.C., Li H., Hong S. Cytochalasin B treatment and osmotic pressure enhance the production of extracellular vesicles (EVs) with improved drug loading capacity. Nanomaterials. 2021;12(1):3. doi: 10.3390/nano12010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savina A., Furlán M., Vidal M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 65.Nakamura Y., Kita S., Tanaka Y., Fukuda S., Obata Y., Okita T., Nishida H., Takahashi Y., Kawachi Y., Tsugawa-Shimizu Y., et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol. Ther. : the J. Am. Soc. Gene Therapy. 2020;28(10):2203–2219. doi: 10.1016/j.ymthe.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X., Xu L., Xia J., Wen C., Liang Y., Zhang Y. Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering. Acta Biomater. 2023:S1742–S7061. doi: 10.1016/j.actbio.2023.07.024. (23)00409-9. [DOI] [PubMed] [Google Scholar]
- 67.Mehryab F., Rabbani S., Shahhosseini S., Shekari F., Fatahi Y., Baharvand H., Haeri A. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62. doi: 10.1016/j.actbio.2020.06.036. [DOI] [PubMed] [Google Scholar]
- 68.Luan X., Sansanaphongpricha K., Myers I., Chen H., Yuan H., Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017;38(6):754–763. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou W., Zhou Y., Chen X., Ning T., Chen H., Guo Q., Zhang Y., Liu P., Zhang Y., Li C., et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 70.Jeyaram A., Lamichhane T.N., Wang S., Zou L., Dahal E., Kronstadt S.M., Levy D., Parajuli B., Knudsen D.R., Chao W., et al. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol. Ther. : the J. Am. Soc. Gene Therapy. 2020;28(3):975–985. doi: 10.1016/j.ymthe.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vázquez-Ríos A.J., Molina-Crespo Á., Bouzo B.L., López-López R., Moreno-Bueno G., de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019;17(1):85. doi: 10.1186/s12951-019-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang Y., Xu X., Li X., Xiong J., Li B., Duan L., Wang D., Xia J. Chondrocyte-targeted MicroRNA delivery by engineered exosomes toward a cell-free osteoarthritis therapy. ACS Appl. Mater. Interfaces. 2020;12(33):36938–36947. doi: 10.1021/acsami.0c10458. [DOI] [PubMed] [Google Scholar]
- 74.Duan L., Ouyang K., Wang J., Xu L., Xu X., Wen C., Xie Y., Liang Y., Xia J. Exosomes as targeted delivery platform of CRISPR/Cas9 for therapeutic genome editing. Chembiochem: a Eur. J. Chem. Biol. 2021;22(24):3360–3368. doi: 10.1002/cbic.202100359. [DOI] [PubMed] [Google Scholar]
- 75.Li Z., Zhou X., Wei M., Gao X., Zhao L., Shi R., Sun W., Duan Y., Yang G., Yuan L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019;19(1):19–28. doi: 10.1021/acs.nanolett.8b02689. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L., Qin Z., Sun H., Chen X., Dong J., Shen S., Zheng L., Gu N., Jiang Q. Nanoenzyme engineered neutrophil-derived exosomes attenuate joint injury in advanced rheumatoid arthritis via regulating inflammatory environment. Bioact. Mater. 2022;18:1–14. doi: 10.1016/j.bioactmat.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smyth T., Petrova K., Payton N.M., Persaud I., Redzic J.S., Graner M.W., Smith-Jones P., Anchordoquy T.J. Surface functionalization of exosomes using click chemistry. Bioconjugate Chem. 2014;25(10):1777–1784. doi: 10.1021/bc500291r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang Y., Iqbal Z., Lu J., Wang J., Zhang H., Chen X., Duan L., Xia J. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering, Mol. Ther. 2022;31(5):1207–1224. doi: 10.1016/j.ymthe.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim M.S., Haney M.J., Zhao Y., Yuan D., Deygen I., Klyachko N.L., Kabanov A.V., Batrakova E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018;14(1):195–204. doi: 10.1016/j.nano.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y., Hu Y., Wang Y., Li J., Liu F., Huang L. Nanoparticle delivery of pooled siRNA for effective treatment of non-small cell lung cancer. Mol. Pharm. 2012;9(8):2280–2289. doi: 10.1021/mp300152v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi Y., Nishikawa M., Shinotsuka H., Matsui Y., Ohara S., Imai T., Takakura Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013;165(2):77–84. doi: 10.1016/j.jbiotec.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Parada N., Romero-Trujillo A., Georges N., Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J. Adv. Res. 2021;31:61–74. doi: 10.1016/j.jare.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei Z., Chen Z., Zhao Y., Fan F., Xiong W., Song S., Yin Y., Hu J., Yang K., Yang L., et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.121000. [DOI] [PubMed] [Google Scholar]
- 84.Liang X., Niu Z., Galli V., Howe N., Zhao Y., Wiklander O.P.B., Zheng W., Wiklander R.J., Corso G., Davies C., et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles. 2022;11(7) doi: 10.1002/jev2.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kooijmans S.A.A., Fliervoet L.A.L., van der Meel R., Fens M., Heijnen H.F.G., van Bergen En, Henegouwen P.M.P., Vader P., Schiffelers R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Contr. Release : Off. J. Control. Release Soc. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Krishnamurthy S., Muthukumaran P., Jayakumar M.K.G., Lisse D., Masurkar N.D., Xu C., Chan J.M., Drum C.L. Surface protein engineering increases the circulation time of a cell membrane-based nanotherapeutic. Nanomed. Nanotechnol. Biol. Med. 2019;18:169–178. doi: 10.1016/j.nano.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y., Bi J., Huang J., Tang Y., Du S., Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020;15:6917–6934. doi: 10.2147/IJN.S264498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang Y., Iqbal Z., Wang J., Xu L., Xu X., Ouyang K., Zhang H., Lu J., Duan L., Xia J. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater. Sci. 2022;10(15):4095–4106. doi: 10.1039/d2bm00480a. [DOI] [PubMed] [Google Scholar]
- 89.Iqbal Z., Rehman K., Xia J., Shabbir M., Zaman M., Liang Y., Duan L. Biomaterial-assisted targeted and controlled delivery of CRISPR/Cas9 for precise gene editing. Biomater. Sci. 2023;11(11):3762–3783. doi: 10.1039/d2bm01636b. [DOI] [PubMed] [Google Scholar]
- 90.Duan L., Xu L., Xu X., Qin Z., Zhou X., Xiao Y., Liang Y., Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13(3):1387–1397. doi: 10.1039/d0nr07622h. [DOI] [PubMed] [Google Scholar]
- 91.Li P., Gao M., Hu Z., Xu T., Chen J., Ma Y., Li S., Gu Y. Synergistic ferroptosis and macrophage re-polarization using engineering exosome-mimic M1 nanovesicles for cancer metastasis suppression. Chem. Eng. J. 2021;409 [Google Scholar]
- 92.Yang Z., Xie J., Zhu J., Kang C., Chiang C., Wang X., Wang X., Kuang T., Chen F., Chen Z., et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J. Contr. Release. 2016;243:160–171. doi: 10.1016/j.jconrel.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Lin Y., Wu J., Gu W., Huang Y., Tong Z., Huang L., Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 2018;5(4) doi: 10.1002/advs.201700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gomes E.R., Souza F.R., Cassali G.D., Sabino A.P., Barros A.L.B., Oliveira M.C. Investigation of the antitumor activity and toxicity of tumor-derived exosomes fused with long-circulating and pH-sensitive liposomes containing doxorubicin. Pharmaceutics. 2022;14(11):2256. doi: 10.3390/pharmaceutics14112256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meng M., Zhang X., Li Q., Han J., Chen Y., Qiao H., Yang Y., Huang X. Engineering M1-derived nanovesicles loading with docosahexaenoic acid synergizes ferroptosis and immune activation for treating hepatocellular carcinoma. Cancer Nanotechnol. 2023;14(1):17. [Google Scholar]
- 96.Li H., Wang X., Guo X., Wan Q., Teng Y., Liu J. Development of rapamycin-encapsulated exosome-mimetic nanoparticles-in-PLGA microspheres for treatment of hemangiomas. Biomed. Pharmacother. 2022;148 doi: 10.1016/j.biopha.2022.112737. [DOI] [PubMed] [Google Scholar]
- 97.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12(7):6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 98.Rayamajhi S., Nguyen T.D.T., Marasini R., Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 99.Goh W.J., Zou S., Lee C.K., Ou Y.H., Wang J.W., Czarny B., Pastorin G. EXOPLEXs: chimeric drug delivery platform from the fusion of cell-derived nanovesicles and liposomes. Biomacromolecules. 2018;19(1):22–30. doi: 10.1021/acs.biomac.7b01176. [DOI] [PubMed] [Google Scholar]
- 100.Li L., He D., Guo Q., Zhang Z., Ru D., Wang L., Gong K., Liu F., Duan Y. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. 2022;20(1):50. doi: 10.1186/s12951-022-01264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato Y.T., Umezaki K., Sawada S., Mukai S-a, Sasaki Y., Harada N., Shiku H., Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016;6(1) doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun L., Fan M., Huang D., Li B., Xu R., Gao F., Chen Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120761. [DOI] [PubMed] [Google Scholar]
- 103.Evers M.J.W., van de Wakker S.I., de Groot E.M., de Jong O.G., Gitz-François JJJ, Seinen C.S., Sluijter J.P.G., Schiffelers R.M., Vader P. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Adv. Healthcare Mater. 2022;11(5) doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saha M., Ghosh S.S. Engineered hybrid nanosystem for homologous targeting of EMT induced triple negative breast cancer cells. ACS Appl. Bio Mater. 2023;6(2):681–693. doi: 10.1021/acsabm.2c00925. [DOI] [PubMed] [Google Scholar]
- 105.Lu M., Zhao X., Xing H., Liu H., Lang L., Yang T., Xun Z., Wang D., Ding P. Cell-free synthesis of connexin 43-integrated exosome-mimetic nanoparticles for siRNA delivery. Acta Biomater. 2019;96:517–536. doi: 10.1016/j.actbio.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Villarreal-Leal R.A., Cooke J.P., Corradetti B. Biomimetic and immunomodulatory therapeutics as an alternative to natural exosomes for vascular and cardiac applications. Nanomed. Nanotechnol. Biol. Med. 2021;35:102385. doi: 10.1016/j.nano.2021.102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.García-Manrique P., Gutiérrez G., Blanco-López M.C. Fully artificial exosomes: towards new theranostic biomaterials. Trends Biotechnol. 2018;36(1):10–14. doi: 10.1016/j.tibtech.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Cheng L., Zhang X., Tang J., Lv Q., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.