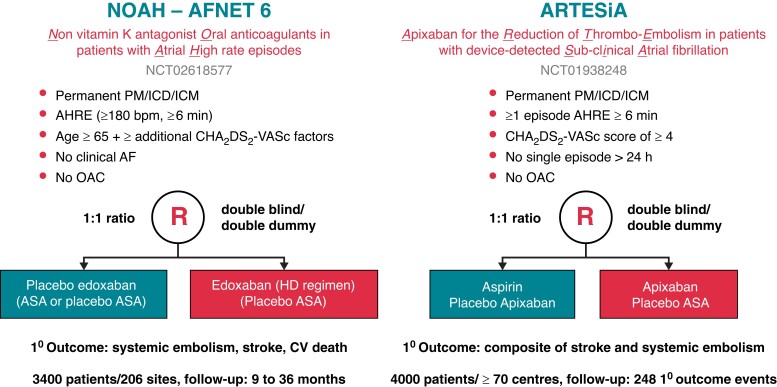
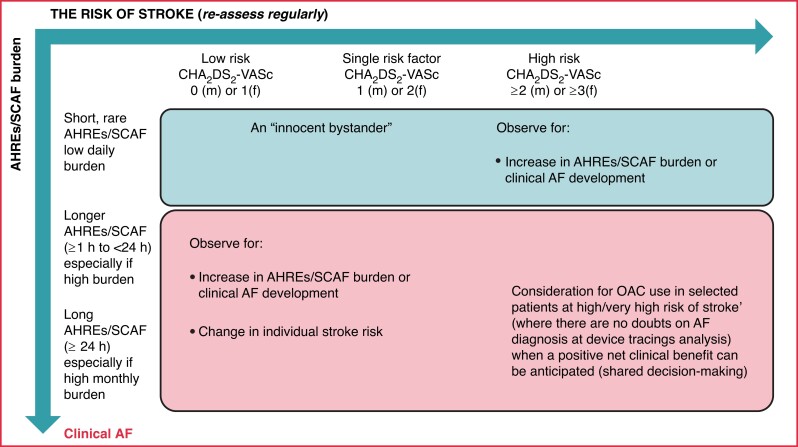
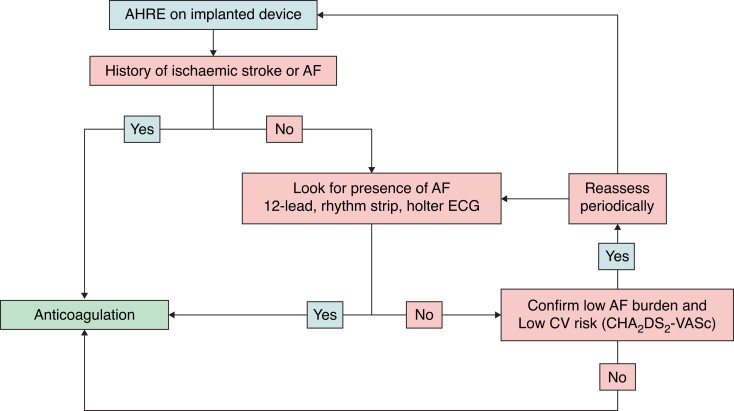
Abstract
Stroke prevention in patients with atrial fibrillation (AF) is one pillar of the management of this common arrhythmia. Substantial advances in the epidemiology and associated pathophysiology underlying AF-related stroke and thrombo-embolism are evident. Furthermore, the introduction of the non-vitamin K antagonist oral anticoagulants (also called direct oral anticoagulants) has clearly changed our approach to stroke prevention in AF, such that the default should be to offer oral anticoagulation for stroke prevention, unless the patient is at low risk. A strategy of early rhythm control is also beneficial in reducing strokes in selected patients with recent onset AF, when compared to rate control. Cardiovascular risk factor management, with optimization of comorbidities and attention to lifestyle factors, and the patient’s psychological morbidity are also essential. Finally, in selected patients with absolute contraindications to long-term oral anticoagulation, left atrial appendage occlusion or exclusion may be considered. The aim of this state-of-the-art review article is to provide an overview of the current status of AF-related stroke and prevention strategies. A holistic or integrated care approach to AF management is recommended to minimize the risk of stroke in patients with AF, based on the evidence-based Atrial fibrillation Better Care (ABC) pathway, as follows: A: Avoid stroke with Anticoagulation; B: Better patient-centred, symptom-directed decisions on rate or rhythm control; C: Cardiovascular risk factor and comorbidity optimization, including lifestyle changes.
Keywords: Atrial fibrillation, Stroke prevention, Rhythm control, Ablation, Anticoagulation, Bleeding risk, Pacemaker
Introduction
In the last decades, substantial progress has been made in relation to stroke prevention in patients with atrial fibrillation (AF). We have seen much progress in understanding the epidemiology and associated pathophysiology underlying AF-related stroke and thrombo-embolism. The introduction of the non-vitamin K antagonist oral anticoagulants (NOACs, also called direct oral anticoagulants, DOACs) has changed the landscape of stroke prevention in AF, such that the default should be to offer oral anticoagulation for stroke prevention, unless the patient is at low risk. Also, in selected patients with recent onset AF, a strategy of early rhythm control is beneficial in reducing strokes, compared to rate control. In addition, the importance of comorbidity and lifestyle management is increasingly recognized. Finally, in selected patients with absolute contraindications to long-term oral anticoagulation, the data for left atrial appendage occlusion (LAAO) or exclusion are increasingly compelling.
The aim of this state-of-the-art review article is to provide an overview of the current status of AF-related stroke and prevention strategies. Stroke prevention in patients with AF can be optimized with adherence to a holistic or integrated care approach to AF management, based on the evidence-based Atrial fibrillation Better Care (ABC) pathway, summarized as follows:1 A: Avoid stroke with Anticoagulation; B: Better patient-centred, symptom-directed decisions on rate or rhythm control; C: Cardiovascular risk factor and comorbidity optimization, including lifestyle changes.
Epidemiology and pathophysiology: a brief overview in relation to stroke
Epidemiology
Atrial fibrillation is the commonest cardiac arrhythmia globally, which is estimated to affect more than 46.3 million individual worldwide in 2016; indeed, due to the ageing population and increasing prevalence of cardiovascular risk factors, the prevalence of AF is expected to rise in the next 30–50 years.2,3 The Framingham Heart Study has shown that the prevalence of AF increased three-fold over the last 50 years.4
By 2050–60, the prevalence of AF is expected to reach 6–16 million in USA5,6 and ∼14 million in Europe.7,8 Although limited epidemiological data on AF are available in the Asia-Pacific region, given the increasing age and size of populations in this region, the burden of AF is expected to be even greater than in North America and Europe. It was estimated that by 2050, there will be ∼49 million men and 23 million women with AF in Asia.9 In the USA, the lifetime risk of AF was estimated as 36% and 30% in White males and females, respectively, and 21% and 22% in Black males and females, respectively.10 In Europe, the lifetime risk estimates of AF also reached about one in three in White individuals. Recent studies in Taiwan have revealed that the lifetime risk of AF was 16.9% and 14.6% in males and females, respectively.10
Hence, AF has become a worldwide public health problem and imposed major burden to the healthcare system. Indeed, recent analysis of the Global Burden of Diseases study 2019 indicated that the global disease burden of AF in term of incidences and mortality has increased by ∼1.1-fold and ∼1.4-fold from 1990 to 2019.11
One of the most important causes of increasing mortality and morbidity of AF is the occurrence of arterial thrombo-embolism and ischaemic stroke, as AF increases the risk of ischaemic stroke by five-fold, and is attributed as the aetiology in up to 25–30% of patients presented with acute ischaemic stroke. Moreover, stroke associated with AF is characterized by large and multiple infarcts involving different vascular territories.12
Nevertheless, there is a wide variability in stroke risk ranging from 0.5% per year to 9.3% per year between different AF patient populations.13 Therefore, assessment of stroke risk in AF patients is needed to determine the need for therapies, mainly oral anticoagulation to stroke prevention. Current clinical guidelines recommend the use of validated AF stroke risk scores, such as Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female) (CHA2DS2-VASc) score that comprising multiple clinical variables for risk stratification for the use of anticoagulation for stroke prevention in AF patients.14
The CHA2DS2-VASc score only includes the more common and validated clinical stroke risk factors, which have been extensively reviewed.15 Amonge these, the inclusion of female sex (Sc criterion) was considered more as a risk modifier rather than a risk factor per se. Indeed, the stroke risk in AF females patients was found to be age-dependent,16 and females with AF who are age ≥65 or report another non-sex stroke risk factor, have a higher stroke risk than males with the same non-sex stroke risk factors, hence being female is additive in terms of thromboembolic risk.17,18 This is important given the relative under-treatment of females,19 and should strokes occur in female AF patients, they tend to be more severe and disabling. The CHA2DS2-VASc score remains the best validated commonly used simple clinical stroke risk score,20 and the few validations of the CHA2DS2-VASc score without the Sc criterion (i.e. CHA2DS2-VA) have methodological issues.18
All simple clinical risk scores such as CHADS2 and CHA2DS2-VASc score have many limitations, as they are reductionist in nature and mere simplifications to aid decision-making. More complex clinical risk scores are evident [e.g. GARFIELD-AF (Global Anticoagulant Registry in the Field-Atrial Fibrillation), ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation)], as well as those adding biomarkers (e.g. ABC stroke score), but even then their c-indexes (a statistical measure of prediction) largely remain <0.7.21,22 Biomarkers (urine, blood, or imaging) always improve risk stratification compared to scores based on clinical factors, but many such biomarkers are non-specific, reflecting a sick patient or sick heart.22,23 Some scores were also derived from clinical trial cohorts, and the performance of these scores in real-world clinical practice is variable and where statistical significance is evident, this does necessarily not translate to practical application.24,25
Clinical risk scores in use are based on ‘static’ risk assessment, i.e. assessing the impact of a baseline risk on events occurring many years later, but in reality, the risk of stroke is dynamic, changing with ageing and incident comorbidities.26 There are increasing publications on the use of machine learning (ML) to account for the dynamic nature of the changing multi-morbidity risk factors, and when compared to clinical risk scores, or multi-morbid index, ML can further improve the stroke risk prediction in AF with c-indexes ∼0.9.27
Pathophysiology
In recent decades, there has been an increased understanding of the underlying pathogenesis of stroke in patients AF as summarized in detail elsewhere.12,28 In brief, hypercoagulability, atrial cardiomyopathy with endothelial damages, and reduced blood flow in the dilated atria as well as the left atrial appendage (LAA) without active contraction contribute to the pathological thrombus formation in the left atrium and thus systemic thrombo-embolism and stroke. Moreover, it has been increasingly recognized the role of atrial cardiomyopathies, due to a complex interplay of structural, architectural, contractile, and electrophysiological abnormalities, in contributing to the progression of AF as well as to the increased thrombo-embolic risk. Indeed, many different well-known risk factors for AF including aging, gender, smoking, alcohol consumption, obesity, diabetes, hypertension, left ventricular hypertrophy, valvular heart diseases, heart failure (HF), and myocardial infarction (MI) that cause atrial cardiomyopathy are also clinical variables that associated with stroke risk in AF.12
Recently, the 4S-AF classification scheme comprised of four domains [stroke risk (St), symptoms (Sy), severity of AF burden (Sb), and substrate (Su)] has been proposed to provide a comprehensive characterization, evaluation, and assessment of patients with AF.14 In the future, assessments of atrial structure and function using different imaging modalities should provide better insights into the possible thrombogenic mechanisms in individual patient and thus improve the risk prediction for stroke beyond current clinical stroke risk scores.29
Integrated care for atrial fibrillation
AF is the commonest sustained cardiac arrhythmia and is managed across the whole spectrum of healthcare professionals, ranging from general practitioners to internal medicine specialists to cardiologists.
While stroke prevention is central to the management of AF, this is only one pillar of the holistic or integrated care approach to AF management. This is important as there still remains a residual risk of adverse outcomes in AF patients despite oral anticoagulation, and while mortality in anticoagulated AF patients remains still high, only 1 in 10 deaths are related to stroke, while 7 in 10 are cardiovascular.30
Hence, we need a streamlined approach to ensure the pillars of AF care are delivered irrespective of which healthcare professional is managing the patient. Also, patients and their family or carers need to understand the priorities of management in a simple and practical manner. Hence, AF management guidelines have moved towards a more holistic or integrated care approach to management of AF.31
First, we need to confirm the diagnosis of the arrhythmia, followed by characterization and evaluation. As mentioned above, such characterization is based on the 4S-AF scheme,14 i.e. Stroke risk assessment (with the CHA2DS2-VASc score); Symptom severity [using the European Heart Rhythm Association (EHRA) score]; Severity of burden (whether spontaneously terminating or permanent); and Substrate (age, structural heart disease, comorbidities).
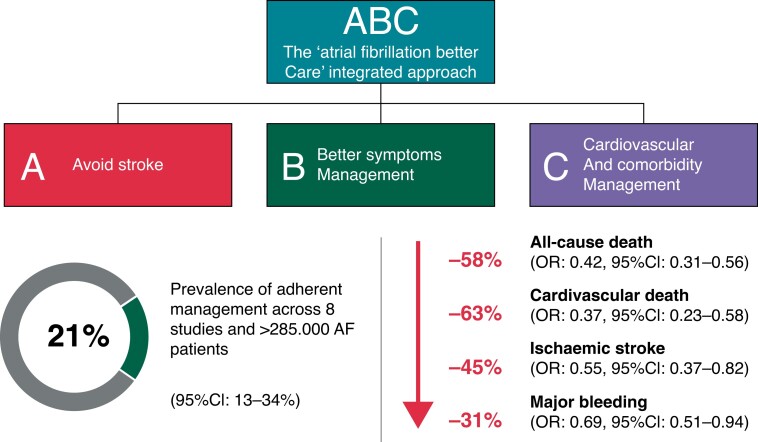
Following this, we treat the patient according to the ABC pathway.1 Adherence with such an approach has been shown in various studies including a clinical trial to be associated with improved clinical outcomes, including reductions in all-cause mortality, cardiovascular mortality, stroke, and major bleeding, as well as hospitalizations (Figure 1).32
Figure 1.
The ABC pathway.32 A: Avoid stroke with Anticoagulation, where the default is stroke prevention unless the patient is at low risk; B: Better symptom control, with patient-centred, symptom-directed decisions on rate or rhythm control; and C: Cardiovascular risk factor and comorbidity optimization, including attention to lifestyle changes, patient’s psychological morbidity, and consideration of patient values and preferences.
The evidence-based ABC pathway has been tested in numerous retrospective and prospective cohorts from different regions of the world,32 as well as post hoc analysis from adjudicated outcomes from clinical trials33,34 and the Mobile Atrial Fibrillation Application (mAFA)-II clinical trial. The latter was a prospective cluster randomized trial which showed a significant reduction in the primary outcome with the ABC pathway intervention using a mHealth App, compared to usual care:35 rates of the composite outcome of ‘ischaemic stroke/systemic thrombo-embolism, death, and re-hospitalization’ were lower with the mAFA intervention compared with usual care [1.9% vs. 6.0%; hazard ratio (HR): 0.39; 95% confidence interval (CI) 0.22–0.67; P < 0.001]. Rates of re-hospitalization were also lower with the mAFA intervention (1.2% vs. 4.5%; HR: 0.32; 95% CI: 0.17–0.60; P < 0.001). Notwithstanding the composite primary outcome, a post hoc win ratio analysis also shows the benefit of the mAFA intervention using the ABC pathway.36
Ongoing clinical trials are testing the impact of implementation of the ABC pathway in Europe [atrial fibrillation integrated approach in frail, multimorbidity and polymedicated older people (AFFIRMO)37] and in rural China [MIRACLE-AF (A New Model of Integrated Care of Older Patients With Atrial Fibrillation in Rural China); NCT04622514].
Avoid stroke and anticoagulation
Oral anticoagulation
Oral anticoagulant (OAC) therapy is the cornerstone of effective prevention of stroke and systemic embolism in patients with AF. Currently available OAC agents include vitamin K antagonists (VKAs) and NOACs also referred to as DOACs.
Vitamin K antagonists
The VKA family includes warfarin, acenocoumarol, phenprocoumon, phenindione, and fluindione.38 Overall, warfarin is the most frequently prescribed VKA in clinical practice, notwithstanding certain geographical variations such as, e.g. a widespread use of acenocoumarol in Spain and Germany or fluindione in France.39,40
The anticoagulant effect of VKAs is achieved indirectly, via inhibition of the vitamin K epoxide reductase complex subunit 1 resulting in altered functionality of vitamin K-dependent coagulation factors II, VII, IX, and X (and anticoagulant proteins C, S, and Z).41 Optimal anticoagulant effect of VKAs is usually achieved within 3–5 days of treatment initiation, depending on the individual patient pharmacogenetics, comorbidity, and co-medication.41
In addition to a slow onset and offset of their anticoagulant effect, VKAs have a narrow therapeutic interval and numerous drug–drug and drug–food interactions, requiring regular laboratory monitoring of anticoagulation effect and dose adjustments.14 Whereas the international normalized ratio (INR) value reflects instantaneous VKA anticoagulant effect intensity, the time in therapeutic range (TTR) reflects the quality of VKA management in a time interval and correlates well with thrombo-embolic and haemorrhagic event rates (an INR of 2–3 and TTR of >70% are recommended for adequate VKA therapy in patients with AF). In patients with AF, VKA therapy (mostly warfarin) reduced the risk of stroke by 64% and all-cause mortality by 26% compared with control or placebo.42
Non-vitamin K antagonist or direct oral anticoagulants
Oral direct inhibitors of coagulation Factor II (dabigatran) or activated factor X (rivaroxaban, apixaban, and edoxaban) have a rapid onset and offset of action, stable dose-related anticoagulant effect with less drug–drug interactions than VKAs and are used in fixed doses without routine laboratory monitoring of anticoagulant effect or food restrictions.43
In a meta-analysis44 of the respective landmark trials comparing the use of a NOAC vs. warfarin for the prevention of stroke and systemic embolism in patients with AF,45–48 the use of a NOAC was associated with statistically significant 19% reduction of the risk of stroke or systemic embolism (including a 51% reduction of haemorrhagic stroke risk and comparable ischaemic stroke risk reduction), a non-significant 14% reduction of the major bleeding risk [with significant 52% reduction in intracranial haemorrhage (ICH), and 25% increase in gastrointestinal (GI) bleeding], and a significant 10% reduction in all-cause mortality compared with warfarin. Whereas the impressive reduction of the ICH risk was consistent among all four NOACs, the risk of GI bleeding was significantly greater with dabigatran 150 mg twice daily,45 rivaroxaban 20 mg once daily,46 and edoxaban 60 mg once daily48 compared with warfarin. The effectiveness and safety of NOACs relative to VKAs has been broadly confirmed in numerous post-marketing observational studies.49
Non-adherence and non-persistence to OAC treatment increase the risk of both ischaemic and haemorrhagic complications and all-cause mortality.50 Although the persistence with any NOAC has been shown to be significantly higher than with VKAs [odds ratio (OR) 1.44, 95% CI 1.12–1.86], there is a considerable need for further improvement (in a recent meta-analysis of adherence and persistence to NOAC therapy among patients with AF, e.g. the overall proportion of patients with good adherence was 66%, and the proportion of persistence was 69%),51 and multiple patient-related, physicians-related, and healthcare system-related factors can influence individual adherence and persistence to OAC therapy.50
Despite a clear guidance on dose reduction criteria provided in the product information for each of the NOACs (Table 1), inappropriate under- or over-dosing is still not uncommon in clinical practice, especially for the elderly or other high-risk patients with AF.52 In a recent meta-analysis, inappropriate under-dosing has been shown to be associate with increased all-cause mortality (HR = 1.28, 95% CI 1.10–1.49; P = 0.006) and no effect on major bleeding (HR = 1.04, 95% CI 0.90–1.19; P = 0.625), while inappropriate overdosing was associated with significantly increased risk of major bleeding (HR = 1.41, 95% CI 1.07–1.85; P = 0.013).52 Hence, prescriber adherence to NOAC dosing guidelines is of key importance for achieving optimal clinical outcomes for patients with AF.
Table 1.
Dosing of NOAC for stroke prevention in AF43
| NOAC agent | Standard dose | Reduced dose | Dose reduction criteria |
|---|---|---|---|
| Apixaban | 5 mg twice daily | 2.5 mg twice daily | If two of three fulfilled:
|
| Dabigatran | 150 mg twice daily, 110 mg twice daily | Not applicable | No pre-specified dose reduction criteria in the RE-LY trial. Per SmPC: 110 mg twice daily if age > 80 years, concomitant verapamil, increased risk of GI bleeding |
| Edoxaban | 60 mg once daily | 30 mg once daily | If one of three fulfilled:
|
| Rivaroxaban | 20 mg once daily | 15 mg once daily | A CrCl of 15–49 mL/min |
NOAC, non-vitamin K antagonist oral anticoagulant; GI, gastrointestinal; CrCl, creatinine clearance; P-Gp, P-Glycoprotein; SmPC, Summary of Product Characteristic; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy.
Whereas routine laboratory monitoring of NOAC anticoagulant effect intensity is not needed, initial assessment (and then a regular re-assessment) of renal function is mandatory in patients with AF taking a NOAC, since all four NOACs are to some extent eliminated by the kidneys (dabigatran 80%, edoxaban 50%, rivaroxaban 35%, and apixaban 27%).43
Based on the high-quality randomized clinical trial (RCT)-based evidence and advantages of NOACs for long-term use, NOACs are recommended in preference to VKAs for stroke prevention in all NOAC-eligible patients with AF (Class I, level of evidence (LoE) A).14,53
(In)eligibility for non-vitamin K antagonist or direct oral anticoagulants
Pregnant women and patients with a prosthetic mechanical heart valve, moderate-to-severe mitral valve stenosis, or end-stage chronic kidney disease or on dialysis were not included in the landmark NOAC trials in AF.45–48
Pregnancy
NOACs are contraindicated in pregnant women, and proper contraceptive measures need to be undertaken in childbearing women before initiation of NOAC therapy.43
Patients with prosthetic mechanical heart valves
Available evidence does not support the use of NOACs in patients with prosthetic mechanical heart valves (Table 2). The RE-ALIGN (Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement) trial54 mostly included patients early after a prosthetic heart valve implantation (when the risk of early post-operative thrombotic and bleeding complications is the highest), enrolled patients with prosthetic heart valve in the mitral or aortic position (the former being more thrombogenic than the latter) and used dabigatran, which may be a poor alternative to VKAs in patients with mechanical heart valves since the tested dabigatran dosing regimens were insufficient to inhibit persistently high local mechanical valve-related thrombin levels, while further increase in the dabigatran dose would be associated with unacceptably high bleeding event rates.57
Table 2.
RCTs comparing a NOAC vs. warfarin in patients with mechanical prosthetic heart valves
| RCT | Study design | Study cohort | Main findings |
|---|---|---|---|
| RE-ALIGN54 | A Phase II dose-validation RCT comparing dabigatran at initial dose of 150, 220, or 300 mg twice daily (based on kidney function) and then adjusted to obtain a trough plasma level of ≥ 50 ng/mL vs. dose-adjusted warfarin with target INR 2.0–3.0 or 2.5–3.5 | Patients who underwent aortic or mitral valve replacement within the last 7 days (79% of patients) or ≥3 months earlier. n = 252 (terminated prematurely). | Increased rates of thromboembolic and bleeding complications with dabigatran, in comparison to warfarin, thus showing no benefit and an excess risk. Death or TE: HR 1.94 (95% CI, 0.64–5.86). Major bleeding: HR 1.76 (95% CI, 0.37–8.46). |
| PROACT Xa55 | A prospective, randomized, open-label trial with blinded end-point adjudication, comparing apixaban 5 mg twice daily vs. warfarin (target INR 2.0–3.0). The primary efficacy end point was the composite of valve thrombosis or valve-related thromboembolism. The primary safety end point was major bleeding defined as any episode of internal or external bleeding that caused death, hospitalization, or permanent injury or necessitated transfusion, pericardiocentesis, or reoperation. |
Patients with an On-X aortic valve implanted at least 3 months before enrolment. n = 863 (terminated owing to an excess of thromboembolic events in the apixaban group). |
Apixaban was less effective than warfarin and did not reach non-inferiority in the prevention of valve thrombosis or thromboembolism in patients with an On-X mechanical aortic valve. Major bleeding rates were 3.6%/patient-year with apixaban and 4.5%/patient-year with warfarin. |
| RIWA56 | A proof-of-concept, open-label, RCT assessing the incidence of thromboembolic and bleeding events of the rivaroxaban-based strategy (15 mg twice daily) in comparison to dose-adjusted warfarin. |
n = 44 patients with a prosthetic mechanical heart valve. A 90-day follow-up. |
Rivaroxaban 15 mg twice daily had TE and bleeding events similar to warfarin in patients with mechanical heart valves. |
RCT, randomized controlled trial; INR, international normalized ratio; TE, thromboembolic event; HR, hazard ratio; CI, confidence interval; NOAC, non-vitamin K antagonist oral anticoagulant; RIWA, Rivaroxaban vs. Warfarin in Patients With Metallic Prosthesis.
Although the major lessons from the RE-ALIGN trial [i.e. (i) avoid including patients too early after mechanic valve implantation, (ii) enrol patients with less thrombogenic valves in the aortic position, and (iii) use a factor Xa inhibitor and not dabigatran] were acknowledged in the design of subsequent PROACT Xa trial,55 apixaban was less effective than warfarin and did not reach non-inferiority in the prevention of valve thrombosis or thrombo-embolism in patients with a less thrombogenic On-X mechanical aortic valve (Table 2). Results of the small, proof-of-concept RIWA (Rivaroxaban vs. Warfarin in Patients With Metallic Prosthesis (RIWA) trial56 are promising, but a larger RCT is needed to evaluate the use of rivaroxaban in patients with mechanical prosthetic heart valves.
Patients with moderate-to-severe mitral stenosis
Whereas the retrospective observational data on the use of NOACs in patients with moderate-to-severe mitral stenosis were encouraging,58 in the recent INVICTUS (Investigation of Rheumatic AF Treatment Using Vitamin K Antagonists, Rivaroxaban or Aspirin Studies) RCT of n = 4531 patients with AF and rheumatic heart disease (mostly mitral valve stenosis, in 85% of patients),59 VKA therapy was associated with a lower rate of a composite of cardiovascular events or death than rivaroxaban therapy, without a higher rate of bleeding.
The ongoing non-inferiority open-label RCT, DAVID-MS (DAbigatran for Stroke PreVention in Atrial Fibrillation In MoDerate or Severe Mitral Stenosis)60 will enrol 686 patients with moderate or severe mitral stenosis in Hong Kong or China and randomize them to dabigatran (110 or 150 mg twice daily) or dose-adjusted VKA (target INR 2.0–3.0) for the prevention of the primary outcome of stroke or systemic embolism. Currently, the use of NOAC is not recommended in patients with AF and moderate-to-severe mitral valve stenosis.14,53
Patients with antiphospholipid syndrome
A recent systematic review and meta-analysis of four RCTs addressing the use of NOACs in patients with anti-phospholipid syndromes61 showed that the use of NOACs was associated with increased risk of subsequent arterial thrombotic events (OR 5.43; 95% CI, 1.87–15.75; P < 0.001, I2 = 0%), especially stroke, and comparable risks of subsequent VTE (OR 1.20; 95% CI, 0.31–4.55; P = 0.79, I2 = 0%) or major bleeding (OR 1.02; 95% CI, 0.42–2.47; P = 0.97; I2 = 0%) compared with VKAs. Hence, patients with anti-phospholipid syndromes should be treated with VKAs in preference to NOACs.43
Patients with end-stage CKD or on dialysis
Based on the lack of high-quality data resulting from the exclusion criteria in respective landmark trials of NOAC in AF, dabigatran (either 150 mg or 110 mg twice daily) use is not approved in patients with a creatinine clearance (CrCl) of <30 mL/min or on dialysis in Europe (dabigatran 75 mg twice daily is approved in patients with CrCl 15–29 mL/min in the USA), while the use of rivaroxaban, apixaban, and edoxaban is not approved in patients with a CrCl of <15 mL/min or on dialysis in Europe, and apixaban is approved in patients on dialysis in the USA.43 Indeed, the USA,53 but not European,14 AF guidelines provide a Class IIb recommendation that, in patients with AF and CrCl <15 mL/min or on dialysis, it might be reasonable to prescribe warfarin (INR 2.0–3.0) or apixaban for oral anticoagulation.
Results of the two small, largely under-powered RCTs (i.e. the RENAL-AF study,62 comparing apixaban 5 mg twice daily vs. adjusted-dose warfarin with target INR 2.0–3.0, which was stopped early because of slow enrolment after only 154 patients and AXADIA (Compare Apixaban and Vitamin K Antagonists in Patients With Atrial Fibrillation and End-Stage Kidney Disease) study,63 comparing apixaban 2.5 mg twice daily vs. adjusted-dose phenprocoumon with target INR 2.0–3.0, which enrolled 97 patients) showed similarly high rates of thrombo-embolic and bleeding events with apixaban and VKAs, suggesting that patients with AF on haemodialysis remain at high risk of cardiovascular events despite OAC. However, both RCTs provide reassuring pharmacokinetic evidence that apixaban in the tested doses does not accumulate in patients with AF on dialysis.
A small three-arm Valkyrie pilot trial64 (n = 132) compared rivaroxaban 10 mg once daily (with and without 2000 μg menaquinone-7 three times weekly) with VKA therapy (target INR 2.0–3.0) in patients with AF on dialysis. Compared with VKA, rivaroxaban (with or without menaquinone-7) reduced ischaemic event rate without increasing bleeding with no difference in mortality. Similar to the RENAL-AF trial, the TTR in patients on VKA was sub-optimal.
The ongoing larger RCTs of patients with AF and on dialysis will compare VKA therapy vs. no OAC [the AVKDIAL (Oral Anticoagulation in Haemodialysis Patients) (NCT02886962) and DANWARD (Danish Warfarin-Dialysis Study) (NCT03862859) trial], apixaban 2.5 mg twice daily vs. no OAC [the SACK (Stroke Prophylaxis With Apixaban in CKD5 Patients With Atrial Fibrillation) (NCT05679024) trial], and apixaban 5 mg twice daily (2.5 mg twice daily for selected patients), warfarin, and no OAC [the SAFE-D (Strategies for the Management of Atrial Fibrillation in patiEnts Receiving Dialysis) (NCT03987711) trial], thus better informing the net clinical effect of OAC in these high-risk patients and specific OAC choice(s).
Patients with bioprosthetic heart valves
Only a small proportion of patients with bioprosthetic heart valves were enrolled in the landmark NOAC trials, 191 patients in the ENGAGE-AF (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation) (0.9% of the total study population)65 and 120 patients in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial (0.7%).66 The effects of respective NOAC in these small subgroups were consistent to the main trial findings.
Subsequent dedicated trials (Table 3) in patients with AF undergoing surgical mitral or aortic valve replacement with a bioprosthetic valve showed non-inferiority of respective NOAC in comparison to VKAs for the pre-specified composite endpoint. A meta-analysis including data form the RIVER trial, a small Brazilian study of dabigatran vs. VKAs (n = 27), and subgroup analyses from ENGAGE-AF and ARISTOTLE trials, showed comparable rates of major bleeding (HR 0.61, 95% CI 0.34–1.09) or stroke or systemic embolism (HR 0.47, 95% CI 0.17–1.29) with NOAC vs. VKA, but the point estimates favoured NOACs.70
Table 3.
RCTs comparing a NOAC vs. VKAs in patients with AF and bioprosthetic heart valves
| RCT | Study design | Study cohort | Main findings |
|---|---|---|---|
| RIVER67 | A randomized trial comparing rivaroxaban 20 mg once daily with dose-adjusted warfarin (target INR 2.0–3.0). The primary outcome was a composite of death, major cardiovascular events (stroke, TIA, SE, valve thrombosis, or hospitalization for HF), or major bleeding at 12 months. | n = 1005 patients with AF and a bioprosthetic mitral valve surgically implanted at least 48 h before enrolment. | In patients with AF and a bioprosthetic mitral valve, rivaroxaban was non-inferior to warfarin with respect to the mean time until the primary outcome of death, major cardiovascular events, or major bleeding at 12 months. Death or TE: HR 0.65 (95% CI, 0.35–1.20). Major bleeding: HR 0.54 (95% CI, 0.21–1.35) |
| ATLANTIS (Stratum 1)68 | An international, randomized, open-label, superiority trial comparing apixaban 5 mg twice daily (2.5 mg twice daily if impaired renal function or concomitant antiplatelet therapy) to VKAs. The primary endpoint was the composite of death, MI, stroke or TIA, SE, intracardiac or bioprosthesis thrombosis, DVT or PE, and life-threatening, disabling, or major bleeding over 1-year follow-up. The primary safety endpoint was major, disabling, or life-threatening bleeding. |
n = 1500 patients with TAVI (n = 451 patients with AF). | After TAVI, apixaban was not superior to the standard of care (that is, VKA in the Stratum 1). Death or TE: HR 1.02 (95% CI, 0.68–1.05). Major bleeding: HR 0.92 (95% CI, 0.52–1.60). |
| ENVISAGE-TAVI AF69 | A multi-centre, prospective, randomized, open-label, adjudicator-masked trial comparing edoxaban 60 mg once daily (30 mg once daily if CrCl 15–50 mL/min, body weight ≤ 60 kg, or concomitant P-glycoprotein inhibitor medication) with VKAs. The primary efficacy outcome was a composite of adverse events consisting of death from any cause, MI, ischaemic stroke, SE, valve thrombosis, or major bleeding. The primary safety outcome was major bleeding. |
n = 1426 patients with AF as the indication for OAC after successful TAVR. | In patients with AF who underwent successful TAVR, edoxaban was non-inferior to VKAs for a composite primary outcome of adverse clinical events. The incidence of major bleeding was higher with edoxaban than with VKAs. Death or TE: HR 1.02 (95% CI, 0.76–1.39). Major bleeding: HR 1.40 (95% CI, 1.03–1.91). |
INR, international normalized ratio; TIA, transient ischaemic attack; SE, systemic embolism; HF, heart failure; AF, atrial fibrillation; TE, thromboembolic event; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; DVT, deep venous thrombosis; PE, pulmonary embolism; TAVI, transcatheter aortic valve implantation; CrCl, creatinine clearance; TAVR, transcatheter aortic valve replacement; RCT, randomized clinical trial; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; OAC, oral anticoagulant.
In patients with a long-term indication for OAC, current European Guidelines recommend OAC monotherapy for patients with surgical bioprosthetic valves (Class I, LoE C), with a Class IIa LoE B recommendation to consider NOAC after 3 months in patients with AF,14,71 and NOAC can be considered in preference to VKA in AF patients undergoing bioprosthetic mitral valve replacement (Class IIb).71 The US Guidelines recommend either a NOAC or VKA in patients with a bioprosthetic valve implanted >3 months prior (Class I, LoE A) and VKA in patients with new-onset AF <3 months after bioprosthetic valve implantation (Class IIa, LoE B).72 For patients with an indication for OAC and undergoing Transcatheter Aortic Valve Implantation (TAVI), lifelong OAC is recommended (Class I, LoE B) with no preference expressed for NOAC or VKA, consistent with the results of ENVISAGE-TAVI AF (Edoxaban versus Standard of Care and Their Effects on Clinical Outcomes in Patients Having Undergone Transcatheter Aortic Valve Implantation–Atrial Fibrillation) and ATLANTIS (Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischemic and Hemorrhagic Events After Trans-Aortic Valve Implantation for Aortic Stenosis) Stratum 1 trials.71,72
Ongoing research
A new family of OAC agents, direct inhibitors of factor XIa asundexian and milvexian, has recently entered the phase III of a comprehensive drug development programme for thromboprophylaxis across the spectrum of indications, including stroke prevention in AF.73 These next-generation OAC agents are expected to better preserve haemostasis, while exerting at least comparable efficacy and better safety in comparison to the current standard of care in patients with AF, as represented by the direct factor Xa inhibitor apixaban used as the comparator in the ongoing Phase III trials (i.e. NCT05643573 with asundexian and NCT05757869 with milvexian).
Bleeding risk
The risk of bleeding in patients with AF reflects the interaction of modifiable and non-modifiable bleeding risks. Various bleeding risk factors are recognized, and the more common ones have been used to formulate bleeding risk stratification scores, which have been recently reviewed.74 The HAS-BLED score remains the best validated commonly used simple clinical bleeding risk score.20
The appropriate use of structured bleeding risk assessment tools is to draw attention to the modifiable bleeding risk factors for mitigation and to identify the high bleeding risk patients for early review and follow-up. This is supported by the bleeding risk analysis from the mAFA trial, where the usual care clusters had a 1-year major bleeding rate of 4.3%, while the mAFA intervention clusters using the HAS-BLED score as part of the ABC pathway reported a major bleeding rate of 2.1% at 1 year. OAC use declined in usual care, from 58.8% to 34.4% at 1 year, while in the intervention arm, OAC use increased from 53.4% to 70.2%.
Intracranial haemorrhage represents the most severe form of OAC-related bleeding, which is more evident in Asians.75 The decision whether to restart OAC after an ICH requires difficult management decision-making,76 although if an OAC is started, a NOAC is the preferred option.
Left atrial appendage occlusion
Rationale for left atrial appendage occlusion
There are several situations where an alternative to OAC in patients with AF may be desirable. Firstly, the use of OAC is not without risk, and patients are exposed to higher rates of bleeding while taking these medications. Therefore, there are certain situations whereby this may be deemed an inappropriate treatment option by physicians and patients alike (e.g. recent ICH, intractable recurrent GI bleeding, end-stage renal failure).77 In addition, some patients may suffer from resistant stroke that occurs despite appropriate guideline-directed anticoagulation therapy. The commonly used strategy of switching or implementing higher doses of OAC in such patients is not supported by trial evidence. There is also an issue of compliance which may be suboptimal with these medications. In the landmark studies of DOACs, discontinuation rates were between 21% and 27%.45–48 This may be more significant with the use of VKA, especially in younger patients where lifelong treatment and monitoring may be viewed as imposing significant lifestyle restrictions. For such patients, there is a need for a non-pharmacological solution to stroke prevention.
Observational studies in patients with non-valvular AF suggest the LAA is the site for the great majority (∼90%) of thrombus formation.78,79 The benefit of LAA ligation during cardiac surgeries has been shown by several cohort studies,80 and recently published randomized controlled trial data have proven the efficacy of this intervention.81 However, as most patients with AF do not require cardiac surgery, this method provides limited clinical impact for the majority. Consequently, percutaneous LAAO was introduced as a potential solution to address some of these issues in the early 2000s.82
Clinical data supporting left atrial appendage occlusion
Three randomized trials, two controlled against dose-adjusted warfarin and one against DOACs,83–85 along with several meta-analyses86–88 have shown that LAAO treatment has compared well with OAC, both with warfarin and with DOAC therapy. There appears to be possibly a small signal of excess of ischaemic strokes with LAAO, but this is more than offset by a substantial reduction in non-procedure–related bleeding and mortality. As such, LAAO may result in net clinical benefit.89
In addition to the trial data, several registries have reported on the clinical value of LAAO therapy for a variety of indications90–94 including patients for whom there is no other safe pharmacological alternatives.91,93 This particular group of patients were excluded in the OAC vs. LAAO clinical trials. Thus far, there are no prospective controlled studies that have evaluated LAAO in patients with an absolute contraindication to anticoagulation. Current evidence is derived from registries and cohort studies. The EWOLUTION (Evaluating Real-Life Clinical Outcomes in Atrial Fibrillation Patients Receiving the Watchman Left Atrial Appendage Closure Technology) study was a prospective observational registry of LAAO involving a total of 1025 patients, where 72% had a documented contraindication to anticoagulation.36 At 2-year follow-up, the rates of stroke and major non-procedural bleeding were reduced by 83% and 46% compared to predicted rates based on the CHA2DS2-VASc and HAS-BLED scores, respectively. The ASAP (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) study enrolled AF patients who were ineligible for warfarin.37 The authors cited that haemorrhagic tendency was the most common (93%) reason for warfarin ineligibility and found that the rate of ischaemic stroke was 1.7% per year with LAAO compared to the expected 7.3% per year based on the CHADS2 score. More recently, a prospective study of 1088 patients, where 83% had contraindications to anticoagulation, found that LAAO with the Amulet device was associated with a 67% reduction in ischaemic stroke rates compared to predicted risk by CHA2DS2-VASc score.38
Only a single study has specifically investigated the use of LAAO in AF patients with resistant stroke despite OAC therapy. Data from the ACP multi-centre registry showed that LAAO was associated with a 65% risk reduction in annual rates of stroke or transient ischaemic attack (TIA) and a 100% risk reduction in annual rates of major bleeding, compared to predicted rates based on the CHA2DS2-VASc and the HAS-BLED scores, respectively.28 At present, there are no studies with direct comparison of LAAO to standard medical therapy in patients with resistant stroke. With regards to compliance, an observational study by Zhai et al.95 which included 338 (total n = 658; 51.4%) patients with non-compliance suggested that LAAO may be feasible for this indication due to low rates of procedural complications.39
Is left atrial appendage occlusion the only option for patients with contraindications to oral anticoagulation?
It is important to bear in mind that there are other alternatives, apart from LAAO, in patients who may be deemed unsuitable for anticoagulation with warfarin.
In a pre-specified analysis of the AVERROES [Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment] trial, the investigators demonstrated that NOAC therapy with apixaban was tolerated in patients who previously failed treatment with warfarin due to poor anticoagulation control (42%), patient refusal (37%), and bleeding on VKA (8%).96 The benefits of apixaban are confirmed in the long-term follow-up from this trial.97 Moreover, for patients who are unable to tolerate even the shortest period of anticoagulation, the implantation of most LAAO devices requires long-term antiplatelet therapy, which contributes to similar bleeding risks compared with OAC.98
The observational data have also allowed the assessment of LAAO treatment against treatment with DOAC therapy.99 Network meta-analysis of observational and trial data suggests that whilst LAAO may be marginally less effective than DOAC therapy at preventing ischaemic stroke, it is highly effective at reducing major and life-threatening bleeding. This advantage continues for the whole duration of treatment, suggesting that, as time passes post-implantation, this may become an increasingly important benefit when compared to lifelong DOAC therapy.100,101
Importance of shared decision-making with the patient
From a patient perspective, it is important to highlight that there are other factors involved beyond mere efficacy and safety when ultimately deciding on the optimal treatment option. This includes long-term quality of life, overall satisfaction, and perceived inconvenience from potential side effects or complications. As part of our holistic care for these patients, it is therefore imperative to facilitate a shared decision-making process. In fact, this has been required for financial reimbursement of LAAO in USA, as per the Centers for Medicare & Medicaid Services. In this setting, there is a case to respect patient autonomy, regardless of how unwise this decision may seem. Furthermore, the chance to avoid anticoagulation as afforded by LAAO may be desired by certain patients according to lifestyle preferences (e.g. participation in high-risk contact sports). Several shared decision-making tools have previously been evaluated for stroke prevention in AF, although their role in LAAO remains to be determined.
Among those patients who may seem suitable for OAC, there are some who refuse treatment (medication averse) with an OAC102,103 and many who fail to adhere to or persist with OAC therapy, including DOAC treatment even after a previous ischaemic stroke attributable to AF.104 In this regard, patients may be willing to be exposed to a greater initial risk if this is balanced by an improvement in quality of life and subsequent reduction in bleeding events. Furthermore, patients may have high levels of anxiety post-stroke,105 especially in those with AF who were already on anticoagulation therapy before these events and are discharged on the same treatment. In such patients with resistant stroke, there may be a role for LAA occlusion106 and even combination therapy for LAAO and OAC,107,108 although this warrants further investigation.
Ongoing trials studying left atrial appendage occlusion
There are now large-scale ongoing trials comparing LAAO therapy with DOACs. Other trials are specifically enrolling patients for whom OAC is contraindicated or difficult, such as those with previous intracerebral haemorrhage, advanced chronic kidney disease, or patients for whom previous treatment with anticoagulation has failed to offer protection against ischaemic stroke. The Dutch COMPARE-LAAO (Comparing Effectiveness and Safety of Left Atrial Appendage Occlusion for Non-valvular Atrial Fibrillation Patients at High Stroke Risk Unable to Use Oral Anticoagulation Therapy) RCT (NCT04676880) intends to study whether LAAO is superior to optimal medical therapy for patients contraindicated to the use of OAC. The ASAP TOO (Assessment of the WATCHMAN™ Device in Patients Unsuitable for Oral Anticoagulation) trial (NCT02928497), which was aiming to obtain a similar proof of concept, terminated prematurely owing to low enrolment in countries that already have reimbursement for LAAO. The STROKECLOSE (Prevention of stroke by left atrial appandage closure in atrial fibrilation stroke patients with interacerebral hemorrhage) trial (NCT02830152) is randomizing patients with a previous intracranial haemorrhage to LAAO or optimal medical therapy according to the treating physician but is also facing slow enrolment for similar reasons.
Left atrial appendage occlusion: the Guidelines’ view
AF guidelines for the application of LAAO treatment have been offered by the European Society of Cardiology14,109 and other professional societies.110–113 Several professional societies too have published consensus documents that expand on the detail available in society guidelines.114,115 All these documents adhere to the principle that when an OAC can be used, it should take precedence over an Left atrial appendage closure (LAAC) implantation. However, it is important to take a shared decision-making approach, in which the patient is counselled about relevant bleeding risks with OAC and procedural complications with LAAO. The present advice from the European Heart Rhythm Association illustrates this in detail.116
Better symptom management
Rate vs. rhythm control on stroke
There are two primary clinical approaches to the management of AF, as follows:
(1) Rate control: slowing the ventricular rate to a level which is physiologically appropriate. Advantages of the rate control approach include ease simplicity avoiding the potential toxicity of anti-arrhythmic drugs or the risks and discomfort associated with electrical cardioversion or invasive left atrial ablation for recurrences of AF.
(2) Rhythm control: restoration and long-term maintenance of sinus rhythm; anti-arrhythmic drugs (ion channel blockers) are predominantly used, but occasionally autonomic manipulation, e.g. with beta blockers may prove valuable.
Rate control remains an essential component of therapy even if the primary strategy is rhythm control (e.g. in the case of a recurrent arrhythmia). Of the two prime treatment strategies for AF, rhythm control is intuitively more attractive as it offers physiological rate control, normal atrial activation and contraction, the correct sequence of atrioventricular (AV) activation, regular ventricular rhythm, and normal intracardiac haemodynamics and AV valve function. Thus, restoration and effective maintenance of sinus rhythm and normal atrial function has been inferred to reduce AF-related risk of stroke by eliminating some of the Virchow’s triad elements that promote thrombosis within the atria (stasis, endothelial abnormality, and increased thrombogenic blood factors).
Despite these theoretical prerequisites, the ‘traditional’ rhythm control strategy using anti-arrhythmic drugs has not proven superior to rate control in the pivotal RCTs (Table 4)117–123 because of the limited choice of drugs, their relatively low efficacy, increased and often poorly predicted risk of pro-arrhythmia, as well as untargeted side effects, particularly in older patients with concomitant heart disease who represent the largest proportion of those at risk of AF-related stroke. Later non-randomized data from AF registries and subgroup analyses have also revealed no consistent clinically significant differences, apart from incidental individual endpoints, in outcome between the two treatment strategies (Table 4).126–129
Table 4.
Studies of rate vs. rhythm control in atrial fibrillation: outcome of stroke and thromboembolism
| Study | n | Follow-up, years | Inclusion criteria/stroke risk factors | Primary endpoint | Difference in primary endpoint RhyC vs. RC | Stroke/TE | Anticoagulation requirements |
|---|---|---|---|---|---|---|---|
| PIAF124 | 252 | 1 | Persistent AF, 85% with (moderate) risk factors | Symptom improvement | Symptoms improved in 70 vs. 76 patients RhyC vs. RC, P = 0.317 | Not reported | All patients received OAC during the entire study period (INR 2–3) |
| STAF121 | 100 | 1.6 | Persistent AF | Composite of ACM, cardiovascular events, CPR, TE | 5.54%/yr vs. 6.09%/yr RhyC vs. RC (P = 0.99), 18/19 primary events occurred during AF (P = 0.049) | 3.1%/yr vs. 0.6%/yr RhyC vs. RC RhyC: 2/5 ischaemic strokes occurred on INR <2, 3/5 on stable INR > 2 RC: 1 stroke and 1 TE occurred on INR > 2 bleeding: 5.8%/yr | OAC prescribed according to the ACCP guidelines (1998) Patents 65–75 years without clinical risk factors received aspirin 325 mg. Continuation of OAC > 4 weeks post-cardioversion—at the discretion of the treating physician |
| HOT CAFÉ122 | 205 | 1.7 | First episode of persistent AF | Composite of ACM, TE, bleeding | OR, 1.98 (CI, 0.28–22.3), P > 0.71 | 3 ischaemic strokes (2.9%; 2/3 occurred on day 3 post-cardioversion on stable OAC and TTR and were fatal; 1 stroke occurred during AF recurrence on aspirin) vs. 1 TE (1%) on OAC RhyC vs. RC. No major bleeding | OAC considered according to the ACCP guidelines (1998) |
| RACE119 | 522 | 2.3 | Persistent AF, post-cardioversion, 91% had one or more risk factors for stroke | Composite of CVM, hospitalizations for CHF, TE, bleeding, PM, AAD adverse effects | 22.6% vs. 17.2% RhyC vs. RC (n.s.) | Trend towards more TE in RhyC vs. RC (7.9% vs. 5.5%); in RhyC, 6 strokes after discontinuation of OAC, 23 strokes while INR < 2; 73% had AF at the time of stroke. Bleeding: 9 (3.4%) vs. 12 (4.7%) RhyC vs. RC 20/21 occurred on OAC, 17/20 on INR >3 | Only patients in whom OAC was not contraindicated |
| AFFIRM117 | 4060 | 3.5 | Age ≥ 65 years, hypertension, diabetes, impaired left ventricular systolic function, CHF, or a prior stroke or TIA, PAF, or PersAF | All-cause mortality | 23.8% vs. 21.3% RhyC vs. RC [HR, 1.15 (CI, 0.99–1.34), P = 0.08] | Trend towards more ischaemic strokes in RhyC: 7.1% vs. 5.5%, P = 0.79), 79% of strokes in RhyC were due to no OAC or INR <2 ICH: 1.3% vs. 1.1% (P = 0.73) RhyC vs. RC Post hoc analysis: OAC associated with lower ACM rates [HR 0.50 (CI, 0.37–0.69), P < 0.00001] Major bleeding (not CNS): 6.9% vs. 7.7% (P = 0.44) RhyC vs. RC | The goal for OAC (warfarin) was INR 2–3. In the RhyC group, continuous OAC was encouraged but could be stopped at the physician’s discretion if sinus rhythm had apparently been maintained for at least 4, and preferably 12, consecutive weeks with AADs. In the RC group, continuous anticoagulation was mandated by the protocol |
| AF-CHF118 | 1376 | 3.1 | CHF (LVEF ≤ 35%, NYHA Class II−IV), PAF or PersAF | Cardiovascular mortality | 27% vs. 25% RhyC vs. RC (HR,1.06 [CI, 0.86−1.30], P = 0.59) | 3% vs. 4% RhyC vs. RC [HR, 0.74 (CI, 0.40–1.35), P = 0.32]; fatal strokes: 9 (1%) vs. 11 2%). Post hoc analysis: OAC associated with lower CVM [HR 0.38 (CI 0.23–0.6), P = 0.0003] and ACM [HR 0.48 (CI 0.30–0.77), P = 0.0025]. Non-CNS major bleeding: 4.4% vs. 3.6% (P = 0.45) RhyC vs. RC | OAC was recommended for all patients, but was not part of inclusion criteria. At enrolment, 86% and 90% patients were on OAC in RhyC vs. RC; these increased to 88% and 92% at 1 year |
| PAF-2125 | 137 | 1.3 | Paroxysmal AF | Prevention of permanent AF | 37% vs. 21% RhyC vs. RC, risk reduced by 57% | 3 (4%) vs. 1 (1%) RhyC vs. RC | OAC recommended |
| J-RHYTHM123 | 823 | 1.6 | Paroxysmal AF CHADS2 0: 43.3% CHADS2 1: 34.8% CHADS2 2+: 21.9% | Composite of ACM, stroke, TE, major bleeding, CHF hospitalization, or physical/psychologic disability requiring changes in the treatment strategy | 15.3% vs. 22.0% RhyC vs. RC [HR, 0.664 (CI, 0.481–0.917), P = 0.0128] | Stroke: 2.1% vs. 2.7%, TE: 0.2% vs. 0.2% RhyC vs. RC. Major bleeding: 0.25% vs. 0.5% RhyC vs. RC | OAC (warfarin, INR 1.6–3) used if one of the risk factors was present (age >65 years, hypertension, diabetes, CHF, stroke/TIA/TE, LAD > 50 mm, FS < 25%, EF < 40% OAC continued irrespective of the rhythm |
| ORBIT-AF126 | 6988 | 1 | First detected AF or PAF, CHADS2 ≥2 | Composite of death, stroke, non-CNS embolism, and TIA | 4.8% vs. 6.86% RhyC vs. RC [HR 0.90 (CI 0.77–1.06), P = 0.2032] Increased CV hospitalizations in RhyC [HR, 1.24 (CI 1.10–1.39), P = 0.0003] | First stroke, non-CNS embolism, or TIA: 1.14% vs. 1.54% RhyC vs. RC [HR, 0.87 (CI 0.66–1.16), P = 0.3452] | Warfarin or dabigatran in 72% |
| RECORD-AF127 | 5171 | 1 | PAF or PersAF. Age ≥ 75 or 70 yr and older with ≥ 1 risk factor (treated hypertension, diabetes, previous stroke/TIA, EF ≤ 40% | Therapeutic success and clinical outcomes | Clinically significant event: 17.2% vs. 18.2% RhyC vs. RC (P = 0.352) CVM: 0.9% vs. 2.8% (P < 0.001) Increased hospitalizations for arrhythmic events (11.3% vs. 7.3%, P < 0.001) | 1/7% vs. 2.8% RhyC vs. RC (P = 0.008) | Managed by treating physiscians |
| IMPACT post hoc128 | 870, 99 in RhyC | 1 | Ambulatory AF patients | AF-related ED visits and CV hospitalizations | 18.2% vs. 12.1% RhyC vs. RC driven by ED visits; odds ratio for ED visits: 2.16 (CI 1.17–3.98), P = 0.0141 | 0.% in RC | Managed by GP |
| CASTLE-AF subanalysis129 | 210 | 3.76 | PAF, PersAF, CHF EF ≤35% CIED | ACM and CHF hospitalization | 38.3% vs. 44.7% RhyC vs. RC [HR, 0.99 (CI 0.62–1.60), P = 0.976] | Not reported | Guideline-directed OAC |
| CABANA130 | 2204 | 4 | PAF, PersAF. Age ≥ 65 yr or at least one risk factor for stroke CHA2DS2-VASc 0–1: 17.9% CHA2DS2-VASc 2: 25.6% CHA2DS2-VASc 3+: 56.5% | Composite of ACM, disabling stroke, serious bleeding, or cardiac arrest | 8.0% vs. 9.2% ablation vs. drug therapy [HR, 0.86 (CI, 0.65–1.15), P = 0.30] | Disabling stroke: 0.1% vs. 0.7% [HR, 0.42 (CI 0.11–1.62), P = 0.19]. Major bleeding: 3.2% vs. 3.3% ablation vs. drug therapy [HR, 0.98 (CI 0.62–1.56), P = 0.93] | OAC according to 2011 guidelines, risk stratification by CHA2DS2-VASc |
| CASTLE-AF131 | 363 | 3.1 | PAF, PersAF, CHF EF ≤35% CIED | ACM and CHF hospitalization | 28.5% vs. 44.6% ablation vs. drug therapy [HR, 0.62 (CI, 0.43–0.87), P = 0.007] | Cerebrovascular accidents in the ablation group vs. drug therapy: (2.8%) vs. 11 (6%) HR, 0.46 (CI 0.16–1.33), P = 0.15 | Guideline-directed OAC |
| CAMTAF and ARC-HF132 | 102 | 7.8 | PersAF, CHF CAMTAF: EF < 50% ARC-AF: EF ≤35%. Mean EF: 31 ± 11% | ACM | Intention-to-treat: ACM and ACM/CV hospitalization did not differ [HR 0.89 (CI 0.44–1.77), P = 0.731 and HR, 0.80 (CI 0.43–1.47), P = 0.467, respectively] On-treatment, both reduced by 57% and 52%, respectively, in the ablation group | 1 (1%) stroke in the ablation group | OAC according to AF guidelines |
| EAST-AFNET 4133 | 798 | 5.1 | CHF, NYHA Class ≥ II or EF <50% | Composite of CVM, stroke, or hospitalization for worsening of CHF or for acute coronary syndrome | 5.7% vs. 7.9% early rhythm control vs. usual care [HR, 0.74 (CI 0.56–0.97), P = 0.003] | 0.4% vs. 1% early rhythm control vs. usual care [HR, 0.46 (CI 0.20–1.05), P = 0.07] | OAC according to AF guidelines |
| Inter-mountain Atrial Fibrillation Registry134 | 37 908 | 2.9 | CHADS2 0: 35.7–38.7% CHADS2 1: 24.9–26.6% CHADS2 2: 16.5–18.2% CHADS2 ≥ 3: 19.5–19.9% | Long-term stroke rates | NA | Ablation: 1.4%, medical therapy: 3.5% in the matched controls without AF: 1.4%, (P = 0.0001 for trend) | VKA continuous use recommended if CHADS2 > 2 |
EF, ejection fraction; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female); OAC, oral anticoagulant; AF, atrial fibrillation; INR, international normalized ratio; TE, thromboembolic event; HR, hazard ratio; CI, confidence interval; OR, odds ratio; TTR, therapeutic range; PM, pacemaker; n.s., not significant; ICH, intracranial haemorrhage; TIA, transient ischaemic attack; LVEF, left ventricular EF; CHF, congestive heart failure; CV, cardiovascular; ED, emergency department; CIED, cardiac implanted electrical device; CASTLE-AF, Catheter Ablation for Atrial Fibrillation with Heart Failure; EAST-AFNET 4, Early Treatment of Atrial Fibrillation for Stroke Prevention Trial; NA, Not Available; RhyC, Rhythm Control; RC, Rate Control; ACM, All-Cause Mortality; ACCP, American College of Chest Physicians; CPR, Cardiopulmonary Resuscitation; CVM, Cardiovascular Mortality; AAD, anti-arrhythmic drugs; PAF, Paroxysmal Atrial Fibrillation; PersAF, Persistent Atrial Fibrillation; NYHA, New York Heart Association; LAD, Left Atrial Diameter; FS, Fractional Shortening; EF, Ejection Fraction; CNS, Central Nervous System.
Anti-arrhythmic drugs
A significant shortcoming of earlier studies was insufficient oral anticoagulation limited to VKA and imperfect TTR maintenance which may have compromised the potentially beneficial effect of effective rhythm control. There have been no uniformed mandatory protocols for anticoagulation, and in many trials, the decision whether to prescribe an anticoagulant and for how long was left at the discretion of a treating physician. Other downsides was inability to achieve a clear difference with respect to rhythm and rate status in the two arms as a significant proportion of patients in the rhythm control arm failed to maintain sinus rhythm, and many patients in the rate control arm were in sinus rhythm at the end of the study [e.g. in the Atrial Fibrillation Follow up Investigation of Rhythm Management (AFFIRM), 42.9%, 38.5%, and 34.6% at 1, 3, and 5 years, respectively]117 and a significant cross-over between the arms [e.g. in Atrial Fibrillation in Congestive Heart Failure (AF-CHF), 21% of patients crossed over from rhythm to rate control, primarily because of the inability to maintain sinus rhythm].118
The major studies were AFFIRM trial,117 RAte Control vs. Electrical Cardioversion (RACE),119 and AF-CHF trial.118 The largest of the trials, AFFIRM, compared two treatment strategies in 4060 patients with paroxysmal or persistent AF and one or more risk factors associated with a high risk of stroke and death (age ≥ 65 years, hypertension, diabetes, impaired left ventricular systolic function, congestive HF, or a prior stroke or TIA).117 The primary endpoint was all cause mortality, whilst the combined secondary endpoint consisted of death, disabling stroke or anoxic encephalopathy, major bleed, or cardiac arrest. During 3.5-year follow-up, 77 ischaemic strokes occurred in the rate control arm and 80 in the rhythm control arm (5.5% vs. 7.1%, P = 0.79). Most strokes in both arms occurred in patients who were either not taking warfarin or who had a sub-therapeutic INR. In the rhythm control arm, 22% of strokes occurred in patients whose INR was < 2, and more than one-half (57%) occurred in patients not taking warfarin. These stroke outcomes should be also considered in the context of the likely recurrence of AF, including asymptomatic, in patients with strong risk factors for stroke.
In the RACE I trial which included 522 patients with persistent AF after previous cardioversion, 91% of whom had at least one risk factor for stroke; there has been a trend in favour of rate control with regards to the composite primary end point of cardiovascular death, hospital admission for HF, thrombo-embolic complications, severe bleeding, pacemaker implantation, and severe adverse effects of therapy: 17.26% vs. 22.6% with rate control vs. rhythm control (absolute difference, 5.4%; 90% CI, −11% to 0.4%), thus fulfilling the criterion for non-inferiority (absolute difference, 10% or less) and approaching superiority to rhythm control.119 Thrombo-embolic events occurred in 35 patients, all of whom had risk factors for stroke, and were more frequent in the rhythm control, with six patients, all in the rhythm-control group, having the thrombo-embolic complications after discontinuation of OAC (five were in sinus rhythm), whilst 23 patients sustained an event while receiving sub-therapeutical anticoagulant therapy (INR < 2). The majority of patients (73%) with thrombo-embolic events had AF at the time of the event. The majority of bleeding events (17 of 20) occurred on INR > 3.
The AF-CHF trial compared rate and rhythm control strategies in 1376 patients with HFrEF (ejection fraction ≤ 35%, New York Heart Association (NYHA) Class II–IV) showed no benefit of rhythm control on top of optimal HF therapy in the primary endpoint of cardiovascular death as well as pre-specified secondary endpoints including total mortality, worsening HF, stroke, and hospitalization.118 The incidence of stroke was 3% in with rhythm control and 4% with rate control.
Subsequent ‘on-treatment’ AFFIRM and AF-CHF analyses employing the actual rhythm status have shown that the use of OACs (mainly warfarin) has had a significant beneficial effect on survival and halved the risk of all-cause death [HR, 0.50 (CI, 0.37–0.69), P < 0.00001].135,136 In AF-CHF, OACs were associated with a 62% reduction in risk in the primary endpoint of cardiovascular death [HR, 0.38 (CI, 0.23–0.65), P = 0.0003], consonant with proven protective effects in patients with AF and risk factors for stroke.136
Ablation
The outcomes of rate vs. rhythm control studies highlighted the significant survival benefit of oral anticoagulation, underscored the need for continuous oral anticoagulation irrespective of the rhythm status, and exposed the problem of sub-therapeutic INR as inadequate anticoagulation. They also revealed significant limitations of pharmacological management of sinus rhythm. Long-term maintenance of sinus rhythm has proven difficult to achieve in patients with persistent AF, and the method is time-consuming and expensive due to the costs of the anti-arrhythmic drugs and the increased need for hospitalization. In short, it has been suggested that if sinus rhythm could be achieved safely and effectively, sinus rhythm would confer a favourable outcome,135 and a raft of small-size, open label studies of left atrial ablation have consistently demonstrated a greater freedom from AF with ensuant significant improvement in symptoms compared with pharmacological rhythm (and rate) control.137 The results of pulmonary vein isolation have been excellent in younger patients with recent onset paroxysmal AF and no or little macroscopic left atrial substrate, with very low rates of serious peri-procedural complications, including thrombo-embolic stroke, but when ablation therapies have expanded to encompass less selective patient populations with long-standing persistent forms of AF, more advanced left atrial remodelling, complex underlying heart disease, and risk factors (including those for stroke), and the duration of follow-up has extended to more than 1 year with the associated late attrition of the short-term anti-arrhythmic effect, the difference in outcomes has become less striking, and the ease of attaining the sinus rhythm has eroded. Nonetheless, pulmonary vein isolation with additional substrate modification when feasible is considered a superior strategy when rhythm control is preferred.
However, no randomized study has yet shown an effect on hard endpoints such as cardiovascular death, stroke, or all-cause mortality. The limitations of rhythm control by ablation when applied to the typical patient with AF (older age, complex comorbidities, and risk factors) have been made evident in the Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial that compared catheter ablation and drug therapy (88.4% received anti-arrhythmic drugs) for paroxysmal or persistent AF in 2204 patients aged ≥ 65 years or with at least one risk factor for stroke.130 Over a median follow-up of 48.5 months, the primary composite endpoint of death, cardiac arrest, disabling stroke, or serious bleeding was neutral (HR, 0.86, 95% CI, 0.65–1.1, P = 0.30) as was the secondary point of all-cause mortality, despite a nearly halved risk of AF recurrence (HR, 0.52, 95% CI, 0.45–0.60, P < 0.001) in the ablation-treated group. There have also been significant reductions in cardiovascular hospitalization rates and greater improvement in symptoms and quality of life compared with medical therapy. Just over the quarter of patients crossed over to the ablation group. The study only reported the incidence of disabling strokes which was low, and the difference was not statistically significant: there were three (0.3%) events in the ablation arm and seven (0.6%) in the drug therapy arm. In the pre-specified treatment received analysis, the primary endpoint was lower in the ablation than drug therapy (HR 0.67, 95% CI, 0.50–0.89, P = 0.006).
The guideline recommendations are based on the intention-to-treat analysis and support the use of ablation as a second-line therapy in patients persistent AF and comorbidities with the main indication for symptom relief. In patients with paroxysmal AF or persistent AF without risk factors for recurrence, AF ablation may be considered AF catheter ablation can be used as first-line therapy (class of recommendation IIa and IIb, respectively).138 AF ablation should be considered in clinically eligible patients with congestive HF and impaired left ventricular systolic function, particularly when tachycardia-induced cardiomyopathy is likely. In the latter setting, improvement in NYHA functional class and left ventricular systolic function owing to established rhythm control by ablation has been evidenced in a series of small randomized clinical studies,132,139 subgroup analysis of the CABANA trial,130 and lately, larger RCTs [CASTLE-AF (CASTLE-AF: Catheter Ablation for Atrial Fibrillation with Heart Failure) and, to some extent, Early Rhythm-Control Therapy in Patients with Atrial Fibrillation (EAST-AFNET 4, Early Treatment of Atrial Fibrillation for Stroke Prevention Trial)].131,140 In the CASTLE-AF study in 363 patients with paroxysmal or persistent AF and HF with HFrEF and a cardiac implantable electronic device [implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator (CRT-D)] in whom anti-arrhythmic drug therapy failed or was poorly tolerated, ablation was associated with significantly lower rates of a composite endpoint of all-cause death and hospitalizations for worsening HF (28.5% vs. 44.6%; HR, 0.62; 95% CI, 0.43–0.87; P = 0.007) as well as a secondary endpoint of all-cause death (13.4% vs. 25.0%; HR, 0.53; 95% CI, 0.32–0.86; P = 0.01).131 Compared with medical therapy aimed at rhythm and/or rate control, patients in the ablation group were more likely to remain in sinus rhythm and had a greater improvement in left ventricular systolic function. However, in the general AF population, <10% met the criteria of the CASTLE-AF.141
Both the 2020 European Society of Cardiology (ESC) Guidelines on AF and 2019 update on American College of Cardiology (ACC)/American Heart Association (AHA)/HRS AF included ablation in selected patients with symptomatic AF and HFrEF (CASTLE-AF criteria) to potentially lower mortality and hospitalization for HF with some difference in the strength of recommendation (IIa14 vs. IIb class.142) The ESC Guidelines also made an emphasis on patient choice when considering ablation in patients with likely tachycardia-induced cardiomyopathy with an intent to lessen or revert left ventricular systolic dysfunction.14
However, none of the individual studies or meta-analyses has shown a reduction in thrombo-embolic events, not in the least because of numerically low event rates due to guideline-driven anticoagulation and better treatment of underlying heart disease. Although the guidelines and expert consensus documents allow for discontinuation of oral anticoagulation if rhythm control is achieved, risk of stroke is low, and this is patient preference,138 ablation does not have an indication for stroke prevention or reduction.
Effect of early rhythm control on stroke and other outcomes, including death, cardiac hospitalization, symptoms, and quality of life
Effect on stroke
One important benefit of rhythm control in AF is the reduction of the risk of stroke, which has been demonstrated in many studies. While some of these studies had the rate of stroke as a separate end point, most incorporated stroke as a part of a composite end point which included other adverse events such as mortality and congestive HF.
A large population-based observational study from Canada enrolled patients older than 65 years with AF and compared the rates of stroke or TIA among patients using rhythm (Class Ia, Ic, and III anti-arrhythmics), vs. rate control (beta blockers, calcium channel blockers, and digoxin) medications.143 It included 16 325 and 41 193 patients in the rhythm and rate control groups, respectively. Even though the rate of anticoagulation was similar in both groups, the rate of stroke/TIA incidence rate was lower in patients treated with rhythm control in comparison with rate control therapy (1.74 vs. 2.49, per 100 person-years, P < 0.001). This was the first large study showing a beneficial relationship between rhythm control and stroke reduction. Another landmark study was the CABANA study, which aimed to determine whether catheter ablation is more effective than conventional medical therapy for improving outcomes in AF.130 Conventional medical therapy was defined as pharmacological rate or rhythm control, and the primary end point was a composite of death, disabling stroke, serious bleeding, or cardiac arrest. The intention-to-treat analysis showed that there was no significant difference between the study groups in the primary outcome. However, the CABANA study was limited by the large number of patients who crossed over from the medical therapy to the ablation group. When per-protocol analysis was performed, patients who underwent ablation had a lower rate of the composite end point of death, disabling stroke, serious bleeding, or cardiac arrest at 12-month follow-up than those treated with medical therapy, with a corresponding HR was 0.73 (95% CI, 0.54–0.99), confirming the findings of prior studies.
As a result of the two above-mentioned studies and others,144,145 it became generally accepted that rhythm control is associated with a reduction in the risk of stroke in patients with AF. None of these studies however limited their patients to those who received early rhythm control. It was not until 2020 that the impact of early rhythm control on stroke reduction was fully appreciated when the EAST-AFNET 4 trial was published.3,133 In this randomized multi-centre study, patients who had AF diagnosed ≤1 year before enrolment were randomized to either early rhythm control or usual care. Early rhythm control included treatment with either anti-arrhythmic drugs or ablation. Usual care consisted of management of symptoms of AF. The study enrolled 2789 patients at 135 centres and was stopped for efficacy during an interim analysis after a median follow-up of 5.1 years per patient. Although not a primary end by itself, stroke occurred in 40/6813 (0.6%) in the early rhythm control group and 62/6856 (0.9%) in the usual care group with a corresponding HR was 0.65 (95% CI, 0.44–0.97).
Hence, the EAST-AFNET 4 study provides some support for early rhythm control to reduce the rate of stroke in selected patients with AF. Important limitations of the EAST study include the lack of data on the quality of adherence to OAC in the trial arms, the intervention group regularly self-recorded electrocardiogram (ECG) twice weekly, which could have improved the overall adherence to treatment, etc. A real-world analysis from the ESC EORP-AF registry found that early rhythm control was associated with a lower rate of major adverse events, but this difference was non-significant on multivariate analysis, being mediated by differences in baseline characteristics and clinical risk profile.146 Also, early rhythm control was associated with greater healthcare resource utilization, and clinical outcomes were no different to the ‘no rhythm control’ group who were fully adherent to the ABC pathway.146
One of the most important findings of these studies is that the reduction of stroke occurred independent of anticoagulation medications, which were used equally in both rhythm and rate control groups. Collectively, these data provide ample support for rhythm control as a stroke reduction strategy.
Effect on death and cardiac hospitalization
In addition to stroke, the effect of early rhythm control on other adverse outcomes such as mortality and HF has been studied. The primary outcome for the EAST-AFNET 4 trial mentioned above was a composite of death from cardiovascular causes, stroke, or cardiac hospitalization with worsening of HF or acute coronary syndrome (ACS).133 The primary outcome event occurred in 3.9 per 100 person-years in the rhythm control group and in 5.0 per 100 person-years in the usual care group (HR, 0.79; 96% CI, 0.66 to 0.94; P = 0.005). When each of the different components of the composite end point was looked at separately, death from cardiovascular causes occurred in 67/6915 (1.0%) in the early rhythm control group and 94/6988 (1.3%) in the usual care group (HR 0.72, 95% CI, 0.52–0.98). Similarly, hospitalization with worsening of HF occurred in 139/6620 (2.1%) in the early rhythm control group and 169/6558 (2.6%) in the usual care group (HR 0.81, CI 95%, 0.65–1.02).
Since the publication of EAST-AFNET 4 trial, many subsequent studies were conducted to further define the relationship between early rhythm control and clinical outcomes. Real-world evidence supports the benefits of early rhythm control on clinical outcomes, especially if intervention was early (<3 months147) and in younger patients with less structural heart disease. A meta-analysis by Zhu et al.148 analysed eight studies involving 447 202 AF patients, where 23.5% of participants underwent an early rhythm-control strategy. The primary outcome was a composite of death, stroke, admission to hospital for HF, or ACS. Early rhythm-control strategy was found to be superior to rate control and was associated with reductions in the primary composite outcome (HR = 0.88, 95% CI: 0.86–0.89) and secondary outcomes, including stroke or systemic embolism (HR = 0.78, 95% CI: 0.71–0.85), ischaemic stroke (HR = 0.81, 95% CI: 0.69–0.94), cardiovascular death (HR = 0.83, 95% CI: 0.70–0.99), HF hospitalization (HR = 0.90, 95% CI: 0.88–0.92), and ACS (HR = 0.86, 95% CI: 0.76–0.98).
Effect on symptoms, quality of life, and cost effectiveness
In addition to its impact on the outcomes of stroke, death, and cardiac hospitalization, the effect of rhythm control on softer outcomes such as symptoms, quality of life, and cost-effectiveness was also studied. Interestingly, the beneficial effect of rhythm control on these end points was less striking.
In the EAST-AFNET 4 study, quality of life was included as a secondary outcome and assessed using the European Quality of Life-5 Dimensions (EQ-5D) visual analogue scale and the 12-Item Short-Form General Health Survey (SF-12). AF-related symptoms and cognitive function were also analysed as secondary outcomes and assessed using the EHRA score, and Montreal Cognitive Assessment, respectively. At follow-up, most patients in both early rhythm control and usual care groups were free from AF-related symptoms, and the changes from baseline in EHRA and EQ-5D scores did not differ significantly between the two groups. Similarly, cognitive function was stable during the follow-up period and similar between both groups.
These findings were corroborated by Nakamaru et al.149 who used an outpatient-based multi-centre AF registry including 2070 patients diagnosed within 5 years. The patients had health-related quality of life data collected at baseline and 1 year after treatment. They used the Atrial Fibrillation Effect on Quality-of-Life-overall summary (AFEQT-OS) score, with higher scores reflecting better quality of life. They also divided the patients into two groups according to AF stage: early and late AF (AF duration ≤1 and >1 year, respectively). After 1 year of treatment, the positive changes in the AFEQT-OS score were similar in patients with rhythm or rate control and were not affected by the AF stage.
All the data discussed above demonstrating better outcomes with early rhythm control may create some concerns about the magnitude of the economic burden associated with early rhythm control in countries with aging populations and high prevalence of AF such as USA and Europe. To that end, a cost effectiveness analysis was conducted in a German sub-study of the EAST-AFNET 4 trial and included 1664 patients randomized to early rhythm control (832 patients) and usual care (832 patients).150 The outcomes included are cost of hospitalization and medication, as well time to primary outcome and years survived. The study showed that clinical benefits of early rhythm control can be achieved at reasonable additional costs. With a willingness-to-pay value of ≥€55 000 per year without a primary outcome or per additional life year, cost-effectiveness of early rhythm control was thought to be highly probable (≥95% or ≥80%, respectively).
In summary a large body of evidence generated over the past 5 years clearly demonstrated the superiority of early rhythm control in reducing stroke, death, and cardiac hospitalization compared to the usual care of rate and symptoms control. Interestingly, this superiority did not extend to quality of life, where early rhythm control and rate control were not significantly different. This is important because a secondary analysis of the EAST-AFNET 4 trial showed that asymptomatic patients derive the same benefit as symptomatic patients regarding the primary outcome of death from cardiovascular causes, stroke, or cardiac hospitalization.151 As a result, the decision to establish and maintain sinus rhythm should be made without considering the presence of AF-related symptoms. Finally, most of these studies discussed in this section did not include patients with long-standing AF, a population that may need to be studied separately.
Stroke prevention after catheter ablation
Irrespective of stroke risk factors, it is generally recommended to continue OAC for at least 2 months following an AF ablation in all patients.14,152 The recommendation is primarily based on the knowledge that catheter ablation transiently damages the endothelium, creating a sore surface, a nidus for thrombus formation, with the notion of an increased risk for thrombo-embolism irrespective of traditional risk score calculations.153
Beyond this time, the continuation at long-term of OAC therapy is governed primarily by the patient’s stroke risk as assessed by the CHA2DS2-VASc score and not on the apparent success or failure of the ablation procedure. These recommendations are currently defined as Class I with a level of evidence C, i.e. according to expert opinion, in the ESC AF Guidelines and the 2017 HRS/EHRA/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Society of Electrophysiology and Cardiac Stimulation (SOLAECE) AF ablation consensus document without further specification of any cut-offs for CHA2DS2-VASc score.14,152 A similar recommendation to guide decision-making on continued OAC therapy is given in the 2020 Canadian AF Guidelines, although using a divergent risk score.154
Several observational studies and registries have suggested that the risk of stroke after ‘successful’ AF ablation in a wide variety of patient risk profiles144,153,155 is low enough to justify discontinuation of OAC beyond the first 3 months post-ablation, even though data on OAC were frequently missing156 (Table 5). Studies have reported that an AF ablation strategy lowers the rate of stroke when compared to a medical approach158 and that the stroke risk post-AF ablation is similar to that observed in a general population without AF.134,165,166 In a large Danish National Ablation and Prescription Registry with 4050 first time AF ablation patients followed for 3.4 years, the incidence rates of thrombo-embolism with and without OAC were low 0.56 (95% CI 0.40–0.78) and 0.64 (95% CI 0.46–0.89).160 The corresponding figures for serious bleedings were 0.99 (95% CI 0.77–1.27) and 0.44 (95% CI 0.29–0.65), respectively. It was concluded that the thrombo-embolic risk was low, and the serious bleeding risk associated with OAC [HR 2.05 (95% CI 1.25–3.35)] seemed to outweigh the benefits of thrombo-embolic risk reduction.160 Another post-AF ablation registry reported that the incidence rates of thrombo-embolism beyond 3 months post-ablation were low and similar in those with vs. without OAC, regardless of stroke risk; 1.11 vs. 0.69 per 100 patient years (P = 0.11), suggesting that it may be safe to discontinue OAC post-ablation under monitoring.172 A single-centre study reported no thrombo-embolic events late after AF ablation in patients without AF recurrences and who discontinued warfarain.157 These findings are consistent with a retrospective three-centres study reporting that all thrombo-embolic events (4%) occurred in patients with AF relapses after ablation (P < 0.001), while there was no difference in embolic events between groups with or without OAC.163 A meta-analysis of 3 436 high-risk patients with CHADS2 or CHA2DS2-VASc scores ≥2 found no difference in cerebrovascular events nor systemic thrombo-embolisms between patients continuing OAC vs. discontinuing OAC 3 months post-ablation [risk ratio (RR) 0.9, 95% CI 0.4–1.7, P = 0.64 and RR 1.2, 95% CI 0.7–2.2, P = 0.54].168 Given the increased risk of major bleeding among those who continued OAC (RR 6.5, 95% CI 2.5–16.7, P = 0.0001), it was concluded that discontinuation of OAC 3 months after AF ablation appears to be safe.168
Table 5.
Prospective and retrospective trials on OAC post-AF ablation and risk of stroke and bleeding
| Study, public | Study type | Pat no | Group comparisons | Primary outcome | Comment |
|---|---|---|---|---|---|
| Oral, Circulaton 2006153 | Single centre FU 25 months | 755 AF ablation | 522 pts in SR, VKA discontinued in 79% of 256 pts wo stroke risk factors and 68% of 266 pts with ≥1 risk factor | No patients in whom OAC was discontinued had a TE during follow-up TE in 0.9% within 2 weeks of ablation and 6–10 months post-ablation in 2 patients (0.2%) | OAC discontinuation appears safe after successful ablation, in pts wo and with stroke risk factors |
| Themistoclakis, JACC 2010144 | Prospective multi-centre, cohorts, FU 28 months | 3355 | OAC Off 2692 pts ON 663pts | Stroke; 0.07% vs. 0.45%, P = 0.06 Bleeds; 0.04% vs. 2%, (P < 0.0001) | CHADS2 score ≥2; Off 13% vs. ON 37% Favoured discontinued OAC post-ablation |
| Yagishita, Circ J 2011157 | Single centre, FU 44 months | 524 AF ablation | VKA discontinued in 93% of 429 pts wo AF recurrence | No TE in pts wo AF recurrence 3% TE in pts with AF recurrence | Low TE events if SR post-ablation |
| Hunter, Heart 2012158 | Multi-centre registry, 7 centres vs. EuroHeart Survey vs. hypothetical cohort wo AF FU 3 years | 1273 vs. 5333 vs. matched hypothetical cohort | Post-AF ablation pts vs. AAD AF pts vs. general population | Stroke/TIA: 0.5% vs. 2.8% (P < 0.0001) vs. 0.4% per patient-year, ns. Stroke OFF OAC vs. expected annual rate: 0.7% vs. 1.9% (CHA2DS2-VASc = 2) | AF ablation strategy lower rates of stroke vs. patients treated medically, no different risk vs. general population. |
| Bunch, Heart Rhythm 2013134 | Prospective AF Study Registry, FU 3 years | 37 908 | 4212 AF ablation pts vs. 16 848 matched AF controls wo ablation vs. 16 848 wo AF | Stroke rate 1.4% in AF pts with ablation vs. 3.5% in AF controls vs. 1.4% control wo AF (P trend 0.0001) at 1 year | Stroke risk post-AF ablation similar to patients wo AF. Ablation favourably affects stroke risk in AF. |
| Riley, J Cardiovasc Electrophysiol 2014155 | Single centre, FU 12 months | 1990 patients | OAC off in 40–65% | Stroke rate/year in patients ‘off ‘OAC, stratified by CHADS2 score similar; score 0–0.28%; score 1–0.07%; score 2–0.50%; P = NS. 75% with stroke/TIA—documented AF. | Study under-powered for conclusion on stroke events. |
| Noseworthy, J Am Heart Assoc. 2015159 | National administrative claims database, 12 months | 6886 | High vs. low stroke risk groups | Stroke 1.4% in CHA2DS2-VASc ≥2 pts vs. 0.3% in CHA2DS2-VASc 0–1 pts. Cardioembolic risk increased if OAC discontinued in high-risk pts [HR 2.48 (95% CI 1.11–5.52), P < 0.05] but not low risk pts. | Rate of OAC discontinuation higher in low vs. high risk (82% vs. 62.5%) at 12 months (CHA2DS2-VASc 0–1 vs. ≥2, < 0.001) |
| Karasoy, European Heart Journal 2015160 | AF ablation & Prescription Registry FU 3.4 years | 4050 | First time AF ablation pts | Incidence rates of thromboembolism with vs. without OAC 0.56 (95% CI 0.40–0.78) vs. 0.64 (95% CI 0.46–0.89). Serious bleedings w vs. wo OAC 0.99 (95% CI 0.77–1.27) vs. 0.44 (95% CI 0.29–0.65) | Serious bleeding risk associated with OAC [HR 2.05 (95% CI 1.25–3.35)], outweigh benefits of thromboembolic risk reduction. |
| Zheng, Journal of Geriatric Cardiology 2015161 | Meta-analysis RCT AF ablation trials | 13 trials, 1952 pts | 1097 AF ablation pts, 855 AAD pts | No difference in ischaemic stroke/TIA in AF ablation patients 0.64% vs. AAD patients 0.23% (RD: 0.003, 95% CI: −0.006 to 0.012, P = 0.470) | Larger prospective randomized trial warranted. |
| Nührich, Clin Res Cardiol 2015162 | Registry, FU 489 days | 460 | Paroxysmal AF 83 high-risk pts (previous stroke) vs. 377 low-risk pts (no stroke) | Thromboembolism more often in high-risk vs. low-risk pts (4.3 vs. 0.3%, P = 0.05) | OAC discontinued 38.6% high-risk vs. 66.3% low-risk (P = 0.0001) Favours to continue OAC post-ablation in high risk groups |
| Gallo, J Cardiovasc Med 2016163 | Retrospective study 3 AF ablation centres FU 60 months | 1500 | AFA with VKA vs. AFA wo VKA vs. rate control with VKA | TE not differ between groups (1% vs. 1.4% vs. 2.2%; P = 0.45). Bleeding events greater in pts on VKA; AFA 1.8% and rate control 2.4% vs. those without,0%, P = 0.003). All TE (4%) occurred in AFA pts with AF relapses (P < 0.001). | Routine ECG monitoring essential after OAC discontinuation in pts with high TE risk |
| Själander, JAMA Cardiology 2016164 | Ablation Registry, 1 year | 1585 | Ischaemic stroke in pts with a CHA2DS2-VASc score ≥ 2 and discontinued vs. continued OAC post-ablation | Patients with a CHA2DS2-VASc score ≥ 2. Higher rate of ischaemic stroke, 1.6% vs. 0.3% per year if discontinued vs. continued OAC (P = 0.046). | Discontinuation of OAC post-ablation unsafe in high-risk patients |
| Saliba, Heart Rhythm 2017165 | Database of health maintenance organization, | 4741 | 969 Post-AF ablation pts vs. 3772 matched AF controls wo ablation | Stroke/TIA rate 2.10 vs. 3.26 per 100 person-years in ablation group vs. non-ablation group. HR stroke/TIA 0.61 (95% CI 0.48–0.79) in ablation group vs. non-ablation. Adjusted HRs stroke alone, 0.62 (95% CI 0.47–0.82) | No data available on OAC strategy. Predominantly high-risk AF ablation patients have lower risk of stroke/TIA than patients treated medically. |
| Srivatsa, Circ Arrhythm Electrophysiol. 2018166 | Retrospective State registry, FU 3.6 yr | 8338 | 4169 AF ablation pts vs. 4169 AF matched Controls | AF ablation lower ischaemic stroke 0.37% vs. 0.59% in controls, HR = 0.68 (P = 0.04; CI 0.47–0.97); haemorrhagic stroke 0.11% vs. 0.35%, HR = 0.36 (P = 0.001; CI: 0.20–0.64). | No data available on OAC AF ablation patients lower risk of stroke than matched AF controls |
| Joza, J Cardiovasc Electrophysiol. 2018167 | Population-based cohort, FU 5 years | 3667 | 1240 AF ablation pts vs. 2427 propensity score matched AF pts wo ablation | No difference for stroke (adjusted HR, 0.88; 95% CI, 0.63–1.21) or major bleeds (adjusted HR, 0.88; 95% CI, 0.73–1.06). No evidence that CA decreases stroke rsik or major bleeding when adjusting for OAC use over time | OAC post-ablation 61% vs. 68% at 5 years. Favours to continue OAC post-ablation |
| Atti, J Atr Fibrillation 2018168 | Meta-analysis | 9 observational trials, 3436 patients | CHA2DS2-VASc or CHADS2 score ≥2. 1815 continued OACs vs. 1621 discontinued OAC post-AF ablation | No difference in risk of cerebrovascular events (RR: 0.85, 95% CI: 0.42–1.70, P= 0.64) and systemic thromboembolism (RR: 1.21, 95% CI: 0.66–2.23, P = 0.54). Continuation of OACs—increased risk of major bleeding (RR: 6.50, 95% CI: 2.53–16.74, P = 0.0001). | Discontinued OAC 3 months after AF ablation appears to be safe |
| Romero, J Cardiovasc electrophysiol 2019169 | Systematic review, FU 39.6 months | 5 studies, 3956 pts | TE events in AF post-ablation pts, on-OAC vs. off-OAC. High vs. low-risk cohorts (CHA2DS2-VASc ≥ 2 vs. ≤1) | Continued OAC associated with lower risk of TE in high-risk cohort (RR 0.41, 95% CI 0.21–0.82, P = 0.01) with 59% RR reduction. ICH higher in ON-OAC group (RR, 5.78; 95% CI, 1.33–25.08; P = 0.02). No significant benefit in low-risk cohort ON-OAC | Continued OAC after AF ablation in CHA2DS2-VASc ≥ 2results in decreased TE risk and a favourable net clinical benefit despite increased ICH. Continued OAC offers no benefit in CHA2DS2-VASc ≤ 1 |
| Proietti, JCE 2019156 | Meta-analysis, 10 prospective and 6 retrospective cohorts | 16 trials, 25 177 patients | 13 166 pts off-OAC, 12 011 pts on-OAC. | No difference in TE after AF ablation in pts on-OAC vs. off-OAC (risk ratio, 0.66; CI 0.38, 1.15). | No information on AF recurrence rates in groups. No definitive conclusion on safety of OAC discontinuation after successful SF ablation |
| Freeman, Circ Arrhythm Electrophysiol 2019170 | ORBIT registry | 21 595 | 1087 AF ablation pts vs. 1087 propensity score matched AF cohort on AAD | Cardiovascular and neurological events—not differ between AF ablation vs. AAD with a CHA2DS2 VASc score ≥2/ ≥ 3 for men/women. 23% OAC discontinued after ablation. | No difference in adjusted rates of all-cause death in pts treated with AF ablation vs. AAD only |
| Packer, JAMA 2019130 | RCT FU 4 years | 2204 | 1108 AF ablation vs. 1096 drug pts | No difference in disabling stroke 0.1% vs. 0.7% [HR 0.42 (95% CI 0.11–1.62), P = 0.19] | OAC as recommended by guidelines. Favours to continue OAC post-ablation |
| Rong, Am J Cardiol 2020171 | Single centre, FU 29.2 months | 796 pts | Discontinued OAC 3 months post-ablation | Incidence of thrombo-embolism 1.62 vs. 0.33 per 100 patient-years in those with vs. without AF recurrence. AF recurrence the only independent predictor of thrombo-embolism [4.837 (1.498 to 15.621), P = 0.008]. | Discontinued OAC unsafe in AF recurrence pts with high stroke risk—high incidence rate of thromboembolism. |
| Yang, Europace 2020172 | AF ablation registry, FU 24 months | 4512 | 3149 pts discontinued OAC (Off-OAC group) 3 months post-ablation vs. 1363 On-OAC group | Incidence rates for thromboembolism 0.54 (95% CI 0.39–0.76) vs. 0.86 (95% CI 0.56–1.30) per 100 patient-years for Off-OAC vs. On-OAC groups. | May be safe to discontinue OAC post-ablation under monitoring. AF recurrence, history of stroke, and diabetes mellitus—increased risk. |
| Kim, Europace 2021173 | Korean National Health Insurance FU 51 days | 8145 | 1629 AF ablation pts, 3258 AF medical therapy pts, 3258 non AF pts propensity scored matched | Incidence rate ratio of ischaemic stroke higher in sustained AF recurrence post-ablation (0.87%) vs. in sinus rhythm (0.24%, P = 0.017; log rank P = 0.003), and higher in medical therapy (1.09%) group than on-AG group (0.34%). | AF ablation reduces risk of stroke and bleeding to the extent of non-AF population compared to AAD |
| Pothineni, J Cardiovasc Electrophysiol 2021174 | Single centre, FU 3 year | 196 patients | OAC discontinued in 33.7% pts depending on stroke risk, mean 7.4 months post-ablation. 15.8% restarted OAC for AF recurrence. | 21.9% reduction in time exposed to OAC. No thromboembolic or major bleeding events | ICM or CIED. Study under-powered for conclusion on stroke events |
| Maduray, Clinical and Applied Thrombosis/Hemostasis 2022175 | Systematic review and meta-analysis | 20 trials, 22 429 patients (13 505 off-OAC) | Continued vs. discontinued OAC post-AF ablation | Stratified CHA2DS2-VASc score ≥2 for thromboembolic events favoured OAC continuation (OR 1.86; 95% CI: 1.02–3.40; P = 0.04). | Findings support sustained OAC in patients with CHA2DS2-VASc score of ≥2. |
| Liang, J Cardiovasc Electrophysiol. 2018176 | Single centre FU 3.5 years | 400 | First AF ablation persistent AF pts | 43.0% free of AF recurrence 43.5% off OAT at FU Cardiovascular events in 0.49/100 patient years, major bleeding in 0.98/100 patient years | Discontinued OAC in closely monitored pts wo AF recurrence—low stroke rate. Older age and CAD only predictors of CVE but not AF recurrence nor CHA2DS2-VASc score. |
CVE, cardiovascular events; wo¸ without; TE, thromboembolism, ICH, intracranial haemorrhage, pts, patients; CIED, cardiac-implanted electrical device; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female); RCT, randomized clinical trial; VKA, vitamin K antagonist; AF, atrial fibrillation; TIA, transient ischaemic attack; NS, not significant; HR, hazard ratio; CI, confidence interval; RR, risk ratio; ICM, implantable cardiac monitor; CIED, cardiac implanted electrical device; OAC, oral anticoagulant; OR, odds ratio; SR, Sinus Rhythm; FU, Follow-Up; AAD, Anti-Arrhythmic Drugs.
Other more recent national health insurance data reported lower rates of ischaemic stroke post-AF ablation in those remaining in sinus rhythm (0.24%) than in those with sustained AF recurrences (0.87%) to the extent of non-AF patients (0.34%) after 51 months and lower than a matched AF groups with medical therapy (1.09%%).173
Although these studies seems to support the perception that the stroke risk after a successful AF ablation is low enough to justify discontinuation of OAC, it is in sharp contrast to other studies advocating a continuation of OAC post-ablation, particularly in high risk groups,162,164,167 In a population-based cohort of AF patients, there was no difference in stroke (adjusted HR, 0.88; 95% CI, 0.63–1.21) or major bleeds (adjusted HR, 0.88; 95% CI, 0.73–1.06) between post-AF ablation patients vs. matched AF controls adjusting for OAC use over time.167 Moreover, in a national administrative claims database of 6886 patients, OAC discontinuation 3 months after AF ablation was associated with increased risk of thrombo-embolic events among high-risk (HR 2.48, 95% CI 1.11–5.52, P < 0.05) but not lower-risk patients.159 A meta-analysis of AF ablation randomized trials reported no difference in ischaemic stroke/TIA in AF ablation patients, 0.64%, vs. AAD patients, 0.23% (risk differences: 0.003, 95% CI: −0.006 to 0.012, P = 0.470),161 which is similar to findings in study from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT) registry,170 although data on OAC therapy were lacking. The importance of continued OAC in high stroke risk AF patients was underlined in a single-centre study related to the high incidence of thrombo-embolism in patients with vs. without AF recurrences post-ablation (0.62 vs. 0.33 per 100 patient-years).171 AF recurrence was the only independent predictor of thrombo-embolism [4.837 (1.498–15.621), P = 0.008].171 In a similar study of persistent AF patients, older age [HR =1.23 (95% CI: 1.09–1.38), P = 0.001] and coronary artery disease [HR = 5.36 (95% CI: 1.19–24.08), P = 0.028] were the only predictors associated with cardiovascular events post-ablation, while AF recurrence or CHA2DS2-VASc score was not.176
In a systematic review of five AF ablation studies, continued OAC after AF ablation in high stroke risk patients (CHA2DS2-VASc c ≥ 2) was associated with decreased thrombo-embolic events and a favourable net clinical benefit despite increased intracranial bleedings.169 A more recent meta-analysis including 20 studies with 22 429 patients (13 505 off-OAC) stratified CHA2DS2-VASc score ≥2 examining thrombo-embolic events, also favoured OAC continuation (OR 1.86; 95% CI: 1.02–3.40; P = 0.04).175
Randomized trials to guide clinicians on whether ‘successful’ AF ablations are sufficiently protective against stroke to permit discontinuation of long-term use of OAC are currently lacking. Two randomized trials addressing the prognostic impact of rhythm control therapies in general AF populations, the ATHENA (A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause in patiENts with Atrial fibrillation/atrial flutter) trial, comparing dronedarone vs. placebo,177and the EAST trial, assessing the efficacy of early rhythm control vs. usual care,133 both demonstrated a favourable outcome for the rhythm control arm, including a reduction in stroke rate. This is in contrast to the findings in the CABANA trial, comparing AF ablation vs. anti-arrhythmic drug therapy, which failed to show a significant reduction in primary endpoint and ischaemic stroke by AF ablation, albeit not surprising given the high cross-over rates.130
Despite this lack of knowledge, 16% of centres discontinued OAC even in patients at high risk178 and in another survey a majority based their decision not only on stroke risk factors alone but also considering clinical results and patient preference.179 When assessing the risk for stroke after AF ablation, other factors apart from conventional stroke risk factors may influence the likelihood of stroke, including the time spent in AF (AF burden) post-ablation, the presence of left atrial fibrosis/cardiomyopathy, secondary effects of extensive left atrial ablation lesions, other disease states, and effect of any therapies that might affect the stroke risk.
While the definition of a ‘successful’ AF ablation procedure relates to the absence of AF recurrences post-ablation, it is complicated by the various definitions used and applied ECG monitoring technique. Freedom from AF for the discontinuation of OAC cannot rely on absence of symptoms alone, as evident by the 12–37% under-estimation of AF recurrences post-ablation180,181 and reports that almost 50% are asymptomatic AF recurrences.182 Moreover, short-term freedom from recurrent AF might not predict long-term success, as there is a progressive decline in efficacy.183–185 Both paroxysmal and persistent AF progress to more persistent forms with higher AF burden with time,186 and even though AF progression was greatly slowed by rhythm control in registry studies187 and randomized trials,188 it was not eliminated.
More persistent AF forms and high AF burden are associated with higher thrombo-embolic risks than paroxysmal.189 In a retrospective cohort study of paroxysmal AF patients, ≥ 11% cumulative burden of AF, assessed by 14-day continuous ECG monitoring, was associated with a higher risk of ischaemic stroke while off-OAC even after adjusting for known stroke risk factors.190
There is great controversy about what amount of AF leads to increased risk of stroke, and the question is which AF duration cut-off should define an AF recurrence for which OAC should be discontinued or reinitiated. It was recently demonstrated that there is a clinically relevant dose–response relationship between increasing AF burden in paroxysmal AF patients and increasing risks of ischaemic stroke and mortality at 1 and 3 years.191 The study showed that episodes of AF ≥24 h were associated with a 37% increase in the adjusted risk of ischaemic stroke, while durations < 23 h were not associated with significantly increased risk,191 in line with the ASSERT (Atrial Fibrillation Reduction Atrial Pacing Trial) trial suggesting that clinically meaningful risk emerges with AF durations >24 h.192 Another retrospective study including non-anticoagulated patients with implantable cardiovascular devices193 reported that the stroke risk crossed an actionable threshold defined as >1%/year in patients with a CHA2DS2-VASc score of 2 with AF >23.5 h, a CHA2DS2-VASc score 3–4 with AF >6 min, and patients with a CHA2DS2-VASc score ≥5 even with no AF.
So far, the role of continuous ECG in monitoring post-AF ablation has not been thoroughly discussed in the decision-making process about when to discontinue or reinitiate OAC. The randomized AF ablation trials using continuous ECG monitoring demonstrated that intermittent Holter monitoring post-ablation significantly underestimate both AF recurrences and AF burden.194–196 Given this knowledge, even regular and prolonged intermittent ECG monitoring for AF burden estimates post-ablation, would at this point in time not be advised in cases with preference to discontinue anticoagulation, even if at low risk.197
Even in the absence of AF recurrences or high AF burden post-ablation, one may question a mechanistic link between AF and stroke risk related to the reported lack of clear temporal relationships.198,199 Some strokes may thus not be caused by AF directly but rather serve as a marker for vascular mechanisms with which AF is frequently associated.200–203
Given the continuum of increasing age and frequently change in comorbidities with associated change in thrombo-embolic risk profile, stroke risk needs to be re-evaluated at each clinical review. Recent studies have shown that patients with a change in their risk profile are more likely to sustain strokes.204 Moreover, the extent of ablation lesions may also render patients more prone to an atrial cardiomyopathy state with a higher risk of stroke.
A strategy of ‘pill-in-the-pocket’ anticoagulation with NOACs triggered by AF episodes on continuous ECG monitoring devices was tested in two trials enrolling patients with non-permanent AF and low risk for stroke.205,206 The recurrence of AF defined as a 6 min episodes (total AF burden >6 h/day) or ≥1 h, respectively, triggered re-initiation of NOAC, which decreased OAC utilization by 75% and 94%, respectively. No thrombo-embolic events were observed during the 12 months follow-up, although studies were not powered to assess the safety of subsequent stroke risk.
Even though observational studies reported that the risk of stroke or transient ischeamic attack (TIA) among patients who discontinued OAC after ‘successful’ AF ablation was as low as 0.7% per year, the studies were limited by a lack of information about stroke risks and medical comorbidities and were all non-randomized with associated limitations. Moreover, given the recent reports of a favourable net clinical benefit of continued OAC post-ablation in AF patients with CHA2DS2-VASc scores ≥2, it is currently questionable to discontinue OAC in AF patients with moderate or high stroke risk.
It therefore seems reasonable to advice against discontinuation of OAC after a successful ablation in patients with CHA2DS2-VASc score ≥ 2.
Only large RCTs can provide definitive answers on whether OAC can be safely discontinued in different subsets of patients. Although several ongoing trials (Table 6) may guide us for a better decision-making regarding OAC on long-term post-ablation, two of the trials rely mainly on the occurrence of silent emboli detected on magnetic resonance imaging.207,208 Even though silent cerebral emboli may be clinically important given the association between AF and increased risk of dementia210,211 and future risk of stroke,212,213 it is yet unclear whether AF ablation can prevent such silent emboli and thereby even clinical strokes in such patients.
Table 6.
Ongoing randomized control trials evaluating strategies for prevention of stroke or silent embolism following AF ablation
| Trial Acronym | No. of patients, follow-up | Inclusion criteria | Primary endpoint | Treatment arms |
|---|---|---|---|---|
| Schrickel, ODIn-AF (NCT02067182)207 | 564, 1 year | Paroxysmal or persistent AF CHA2DS2-VASc score ≥ 2 Sinus rhythm and no clinical AF recurrence after 3 months blanking and 3 months observation after ablation (72 h Holter) | New silent cerebral embolism or stroke on MR at 12 months vs. baseline MR | Dabigatran vs. discontinued OAC |
| Verma, OCEAN trial (NCT02168829)208 | 1572, 3 years | AF, ≥1 stroke risk factor without recurrent AF ≥ 1 year post-ablation on serial 24 h Holter. | Composite stroke, systemic embolism, or silent stroke on brain MR. | Rivaroxaban vs. ASA |
| Wazni, Am Heart J 2022, OPTION (NCT03795298)209 | 1600, 3 years | AF, AF ablation, CHA2DS2-VASc ≥2 men or ≥3 women | Composite stroke, systemic embolism or all-cause death, non-procedural major bleeding or clinically relevant non-major bleeding | WATCHMAN FLX vs. OAC |
AF, atrial fibrillation; MR, magnetic resonance imaging; OAC, oral anticoagulation; OCEAN, Optimal Anti-Coagulation for Enhanced-Risk Patients Post-Catheter Ablation for Atrial Fibrillation; ODIn-AF, Prevention of Silent Cerebral Thromboembolism by Oral Anticoagulation With Dabigatran After PVI for Atrial Fibrillation; OPTION, Comparison of Anticoagulation With Left Atrial Appendage Closure After AF Ablation; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female); ASA, acetylsalicylic acid.
Comorbidities and lifestyle changes
Comorbidity, cardiovascular risk factors, and unhealthy lifestyle behaviours may cause alterations in myocardial function and structure, thus facilitating the occurrence of AF which, in turn, may result in additional AF-related electrical and structural remodelling of atrial and ventricular myocardium.214,215 This multiple factor-related progression of abnormal atrial (and ventricular) substrate translates into poorer outcomes with rhythm control strategies, as well as a greater risk of AF-related morbidity and mortality.214
In 2019, there were 0.32 million [95% uncertainty interval (UI) 0.27 to 0.36] deaths from AF globally, and these age-standardized deaths were mostly attributable to high systolic blood pressure (34.0%; 95% UI, 27.3 to 41.0), high body mass index (20.2%; 95% UI, 11.2 to 31.2), alcohol use (7.4%; 95% UI, 5.8 to 9.0), smoking (4.3%; 95% UI, 2.9 to 5.9), and high-sodium diet (4.2%; 95% UI, 0.8 to 10.5).216 These findings underscore an urgent need for widespread implementation of sustainable strategies and interventions addressing modifiable risk factors in patients with AF.
Indeed, AF rarely comes truly alone. Reportedly, nearly 50% of patients with low risk profile at the time of first-onset AF were subsequently diagnosed with a clinically overt disease (mostly hypertension) in the next few years, most commonly within 6 months after first-diagnosed AF,204 which highlights the importance of periodical risk profile re-assessment in patients with incident AF, as recommended in the latest ESC AF Guidelines.14
The risk of major cardiovascular adverse events (MACEs) including morality in patients with AF increases proportionally to increasing burden of comorbidities217–220 and/or clustering of unhealthy lifestyle behaviours.221 Patients with AF have a greater risk of multi-morbidity (i.e. the presence ≥ 2 concomitant chronic comorbidities) in comparison to individuals without AF.218,222 A recent systematic review and meta-analysis of reports from 54 countries revealed a global prevalence of multi-morbidity of 37.2% (95% CI, 34.9–39.4) among adults and 51.0% (95% CI, 44.1–58.0) among individuals ≥ 60 years of age.223 The prevalence of multi-morbidity in contemporary AF cohorts, however, is nearly 2.5-fold higher, ranging from 80%219,222,224 to >90%.225
Patients with AF may have variable clinical phenotypes regarding concomitant comorbidities and unhealthy lifestyle behaviours. Whereas the risk of MACE was significantly higher in both patients with non-cardiovascular comorbidities and those with cardiovascular risk factors/comorbidities in comparison to low-risk patients, the risk of MACE also was significantly higher in patients with cardiovascular risk factors/comorbidities than in those with non-cardiovascular comorbidities in a large registry-based AF cohort.220
The risk of potentially deleterious consequences of the complex circulus vicious resulting in AF substrate development and progression can be effectively reduced by timely identification and optimal management of comorbidities, modifiable cardiovascular risk factors and unhealthy lifestyle in patients with AF, as promoted in recent AF guidelines.14,226
In addition to numerous observational studies, increasing number of RCTs has examined the effects of comorbidity/unhealthy lifestyle behaviours management in patients with AF (Tables 7 and 8). Notably, most of the earlier RCTs were focused on a single comorbidity or an isolated component of lifestyle behaviours (Table 7). Some of these studies reported neutral effect most likely owing to such selective approach not accounting for clinical complexity and clustering of risk factors in participating patients. Indeed, most of the RCT of interventions addressing multiple modifiable risk factors yielded positive findings in terms of reducing AF symptoms, AF burden, or increasing the success of rhythm control strategies (Table 2).
Table 7.
Comorbidity and risk factor overview and RCTs focusing on a single comorbidity or unhealthy lifestyle behaviour (positive studies are highlighted in italics)
| Risk factor | Impact on AF | |||
|---|---|---|---|---|
| Hypertension |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| SMAC-AF 237 | Patients undergoing ablation for AF:
|
Target systolic BP <120 mmHg |
AF recurrence post-ablation
|
|
| Pokushalov et al.238 |
Patients undergoing PVI with resistant hypertension:
|
Renal artery denervation |
AF freedom post-ablation
|
|
| ERADICATE-AF239 |
Patients undergoing PVI with resistant hypertension:
|
Renal artery denervation |
AF freedom post-ablation
|
|
| Diabetes mellitus |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| Deshmukh et al.247 |
Patients with diabetes undergoing ablation for AF:
|
Metformin vs. no metformin |
AF freedom post-ablation
|
|
| Obesity |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| No RCT focusing solely on the weight reduction (see Table 2 for RCTs exploring a structured intervention) | ||||
| Sleep disordered breathing |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| Hunt et al. 266 | Patients with OSA and AF undergoing PVI
|
CPAP |
AF recurrence post-ablation
|
|
| Caples et al.267 | Patients with OSA and AF undergoing electrical cardioversion
|
CPAP |
AF recurrence after successful electrical cardioversion
AF recurred in 25% of patients in each group |
|
| Traaen et al. 268 | Patients with OSA and non-permanent AF implanted a loop recorder
|
CPAP |
AF burden
No difference between the groups |
|
| Physical activity |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| Hegbom et al.279 |
Patients with permanent AF
|
Exercise training programme consisting of 24 training sessions with aerobic exercise and muscle strengthening |
Health-related QoL, symptoms
|
|
| Osbak et al.280 |
Patients with permanent AF (n = 45)
|
A 12-week aerobic exercise training. |
Exercise capacity, QoL
Significantly increased exercise capacity and 6MWT, decreased resting pulse rate and improved QoL in active patients. Cardiac output and natriuretic peptides were unchanged in both groups |
|
| Kato et al.281 |
Patients undergoing ablation for AF
|
A 30 min 2–3 times weekly moderate intensity, 60 min exercise endurance programme |
AF recurrence post-ablation
|
|
| Malmo et al.282 |
Patients with non-permanent AF implanted with a loop recorder to record AF burden
|
Aerobic interval training |
Time in AF, symptoms, cardiovascular health, and QoL
Reduced time in AF, significant improvement in symptoms, O2 peak, left atrial and ventricular function, and QoL compared with the control group |
|
| Skielboe et al. 283 | Patients with non-permanent AF (n = 75)
|
Comparison of low vs. high intensity exercise in reduction of AF burden |
Reduction in AF burden
No significant difference in AF burden reduction. No evidence of an increased risk was found for high-intensity compared with low-intensity exercise |
|
| Alcohol |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| Voskoboinik et al.288 |
Patients with non-permanent AF drinking ≥ 10 drinks per week
|
Abstinence from alcohol |
Freedom from AF recurrence after a 2-week ‘blanking period’ and total AF burden during 6 months of follow-up
|
|
| Coffeine |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| No RCT focusing on the effects of coffee consumption in AF patients | ||||
| Cigarette smoking |
|
|||
| RCT | Study population | Intervention | Outcomes | |
| No RCT focusing on the effects of smoking cessation in AF patients | ||||
AF, atrial fibrillation; OAC, oral anticoagulant; RCT, randomized clinical trial; BP, blood pressure; HR, hazard ratio; CI, confidence interval; PVI, pulmonary vein denervation; RAD, renal artery denervation; DM, diabetes mellitus; BMI, body mass index; SDB, sleep disordered breathing; OSA, obstructive sleep apnoea; CPAP, continuous positive airway pressure; QoL, quality of life; HRQoL, health-related QoL.
Table 8.
RCTs of interventions addressing multiple risk factors
| Study | Cohort size (n) | Intervention | Follow-up | Main findings |
|---|---|---|---|---|
| Abed et al.297 | 150 | Participation in a physician-led multiple risk factor modification clinic managing weight loss, OSA, hypertension, tobacco, alcohol, and glycaemic control. | 15 months | Intervention groups had lower AF symptom burden scores (11.8 vs. 2.6 points; P < 0.001) and fewer AF episodes (2.5 vs. no change; P = 0.01) and total duration (692-min decline vs. 419-min increase; P = 0.002). |
| Rienstra et al.298 RACE 3 | 245 | Risk factor–driven upstream therapy with MRAs, statins, ACE inhibitors or ARBs, and cardiac rehabilitation (physical activity, dietary restrictions, counselling) in patients with early persistent AF and heart failure. | 1 year | Sinus rhythm at 1 year after cardioversion by 7-day Holter monitoring occurred in 75% of the intervention and 63% of the conventional group (OR, 1.765; P = 0.021). |
| Gessler et al.299 SORT-AF | 133 | Weight-loss, dietary changes, a 6-month exercise programme in symptomatic non-permanent AF patients with a BMI 30–40 kg/m2 implanted with a loop recorder and undergoing catheter ablation for AF. | 1 year | AF burden reduction
|
OSA, obstructive sleep apnoea; AF, atrial fibrillation; MRA, mineralocorticoid receptor antagonist; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; RCT, randomized controlled trial; OR, odds ratio.
Overall, available evidence clearly supports active efforts to identify and address comorbidity, risk factors, and unhealthy lifestyle behaviours in patients with AF, suggesting that multi-disciplinary structured approaches addressing multiple risk factors (rather that selectively focusing on a single risk factors) are more effective in reducing AF burden and improving outcome in AF patients.300,301 Since patients with AF may first come to attention of physicians of various specialties, simple pathways for integrated holistic care for AF patients, such as the ABC pathway recommended by the ESC AF Gudelines,14 are essential to their optimal management.
Notably, the long-term adherence to structured multi-disciplinary interventions addressing risk factors may be challenging.302 More data are needed to inform optimization of the structure and targets of integrated treatment strategies, especially in clinically complex multi-morbid patients with AF, in whom the use of artificial intelligence303 could inform more clinically useful targeted approach(es) instead of a ‘treat all’ strategy which may not be feasible or sustainable. The ongoing research, including the 2020 EU Horizon AFFIRMO37 and EHRA-PATHS (Addressing multimorbidity in elderly atrial fibrillation patients through interdisciplinary, patient-centred, systematic care pathways)304 Research Projects will provide more data regarding the optimization of management of patients with AF in clinical practice.
Special circumstances with regards to stroke prevention in atrial fibrillation
Atrial fibrillation and coronary artery stenting
Antithrombotic therapy to prevent bleeding and ischaemic events is changeling in patients with AF who require antiplatelet therapy for percutaneous coronary intervention (PCI) and/or ACS.305–308 All published NOAC AF PCI studies [PIONEER-AF (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention) PCI trial, RE-DUAL (Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) PCI trial, AUGUSTUS (Open-Label, 2×2 Factorial, Randomized, Controlled Clinical Trial to Evaluate the Safety of Apixaban vs Vitamin K Antagonist and Aspirin vs Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention) trial, ENTRUST (Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) AF PCI trial] used safety parameters as primary endpoints.305–308 Bleeding endpoints were typically defined as major bleeding or clinically relevant non-major bleeding.305–308 Secondary efficacy endpoints included all-cause death, cardiovascular death, trial-defined MACE, MI, stroke, and stent thrombosis (ST). In addition to the four randomized controlled trials, several meta-analyses were presented to discuss this in more detail using larger retrospective datasets.309–313 Overall, regimens of NOACs plus a P2Y12-inhibitor were associated with lower bleeding risk compared with VKAs plus dual antiplatelet therapy. Moreover, regimens that stopped aspirin in the early phase after stenting (<30 days) caused less intracranial bleeding, while preserving efficacy. It was shown that bleeding events immediately after PCI were related to the puncture site and different from organ bleeding during follow-up. Thus, the access site is of importance to reduce the bleeding rates with lowest rate after puncture of the radial artery.308
At present, it remains unclear if the use of ticagrelor or prasugrel as more potent P2Y12-inhibitor reduces the ischaemic risks in this setting. Importantly, a recent sub-analysis of the ENTRUST-AF PCI study could demonstrate that in patients with AF who underwent PCI, the edoxaban-based regimen, as compared with VKA-based regimen, provides consistent safety and similar efficacy for ischaemic events in patients with AF regardless of their clinical presentation with ACS or chronic coronary syndrome (CCS).311 Furthermore, it was shown that the CHA2DS2-VASc score above 4 was helpful to predict the occurrence of ST in AF patients after PCI and stenting.314 Interestingly, the pattern of AF was also identified in a substudy to have an impact on outcome and ACS during follow-up.314 This finding is in line with other studies showing that patients with low AF burden (first manifestation; new-onset AF) or paroxysmal AF had more frequent ACS during follow-up than patients with non-paroxysmal AF.314–316 This finding may need further investigation to validate these results.
Overall, the 2020 ESC guidelines on diagnosis and management of AF recommend early cessation (≤1 week) of aspirin and continuation of DAT with a NOAC and a P2Y12 inhibitor (preferably clopidogrel) for up to 12 months in AF patients with ACS.14,317 The NOAC practical guide also suggests to stop clopidogrel after 6 months in patients with CCS and to continue with monotherapy using a NOAC.318 Nevertheless, the molecular interaction among endothelium, stent struts, and platelet activation in patients with irregular blood flow due to AF warrants further investigation. Biomarkers might be helpful to identify certain subcohorts.319
The elderly, frail, and multi-morbid
In AF, older age has always represented an important and prominent clinical factor. Indeed, both prevalence and incidence progressively rise with age,14 influencing significantly the clinical management.320,321 In particular, older age has been described consistently as a significant barrier to the prescription of OAC drugs, linked to the perceived high risk of bleeding and bleeding-predisposing factors (i.e. risk of falls, ability to comply with drugs prescription, dementia).320 Moreover, older age is described frequently as a significant predictor of OAC non-adherence in clinical practice.322 Recent analyses coming from the USA, focusing on patients ≥65 years old with high thrombo-embolic risk, indeed revealed the fact that despite a significant OAC uptake over time, there is still a substantial under prescription, particularly in oldest-old and in patients with chronic conditions.323,324
In the last years, despite these data still underlining the importance of ‘chronological’ age, there has been a progressive interest in studying and understanding the relationship between some ‘geriatric’ syndromes and AF, such as multi-morbidity, polypharmacy, and frailty, which all appeared to influence significantly clinical management and risk of adverse outcomes.225,325–328 The presence of all these syndromes/phenomena entails the so-called ‘clinical complexity’, which substantially affects all clinical aspects regarding the management and the natural history of AF patients.219
In Table 9, we summarize the main results from some of the larger studies published regarding the influence of geriatric syndromes on OAC prescription.
Table 9.
Relationship between multi-morbidity, polypharmacy and frailty with OAC prescription in AF
| Study | Year | Location | Patients | Epidemiology, n (%) | OAC prescription, n (%) | Impact on OAC prescription |
|---|---|---|---|---|---|---|
| Multi-morbidity | ||||||
| Proietti et al.218 | 2019 | Italy | 24 040 | CCI 0–3 19,745 (82.1) CCI ≥4 4295 (17.9) | 9646 (40.1) at baseline | Continuous CCI was inversely associated with OAC prescription at baseline (OR 0.91, 95% CI 0.89–0.92), as well as CCI ≥4 (OR 0.65, 95% CI 0.60–0.70) compared to CCI 0–3 |
| Dalgaard et al.329 | 2020 | USA | 34 174 | 0–2 CMs 13 194 (38.6) 3–5 CMs 17 331 (50.7) ≥ 6 CMs 3649 (10.7) | 29 239 (85.6) at discharge NOACs 20 480 (59.9) | At discharge compared to patients with 0–2 CMs, those with ≥6 CMs had lower odds of receiving OAC (OR 0.72, 95% CI 0.60–0.86), with a non-significant trend for those with 3–5 CMs (OR 0.93, 95% CI 0.82–1.05) Regarding the prescription of NOACs, a progressively higher number of CMs was inversely associated with the prescription of NOACs vs. VKAs (OR 0.72, 95% CI 0.67–0.78 and OR 0.59, 95% CI 0.50–0.69, respectively for 3–5 CMs and ≥6 CMs compared to 0–2 CMs) |
| Koziel et al.224 | 2021 | Balkans | 2712 | ≥2 CMs 2263 (83.4) | 1965 (72.4) NOACs 338 (12.5) | Patients with multi-morbidity (≥2 CMs) received less likely OAC than those without (62.1% vs. 74.5%, P < 0.001) No difference was found regarding NOACs prescription (P = 0.107) |
| Rasmussen et al.330 | 2022 | Denmark | 48 995 | 0–1 CMs 18 950 (38.7) 2–3 CMs 20 723 (42.3) 4–5 CMs 7190 (14.7) ≥ 6 CMs 2132 (4.3) | 38 068 (77.7) NOACs 20 699 (54.4) | Compared to patients with 0–1 CMs, increasing number of CMs was inversely associated with OAC prescription (2–3 CMs OR 0.79, 95% CI 0.75–0.83; 4–5 CMs OR 0.54, 95% CI 0.51–0.58; ≥ 6 CMs OR 0.38, 95% CI 0.35–0.42) |
| Polypharmacy | ||||||
| Mazzone et al.331 | 2016 | Italy | 305 | ≥5 drugs 84 (27.5) | 170 (55.7) | At hospital discharge presence of polypharmacy was associated with a higher risk of OAC non-prescription (OR 2.07, 95% CI 1.10–3.86) |
| Mongkhon et al.332 | 2020 | UK | 9845 | ≥5 drugs 2244 (22.8) | 3801 (38.6) NOACs 465 (12.0)a | In a large multivariate analysis, polypharmacy was inversely associated with OAC prescription (OR 0.62, 95% 0.51–0.75) No impact of polypharmacy was found on NOACs prescription |
| Koziel et al.224 | 2021 | Balkans | 2712 | ≥5 drugs 1505 (55.5) | 1965 (72.4) NOACs 338 (12.5) | Patients with polypharmacy (≥5 drugs) received less likely OAC than those without (59.9% vs. 82.5%, P < 0.001) No difference was found regarding NOACs prescription (P = 0.865) |
| Frailty | ||||||
| Gugganig et al.333 | 2019 | Swiss | 2369 | robust 681 (28.7) pre-frail 1436 (60.7) frail 252 (10.6) |
2141 (90.4) VKAs 936 (39.5) NOACs 1205 (50.9) | Frail patients were more likely prescribed with VKAs than pre-frail and robust ones (52.0% vs. 43.1% vs. 27.2%), while NOACs were less likely prescribed (36.1% vs. 48.6% vs. 61.1%) |
| Campitelli et al.334 | 2021 | Canada | 36 466 | robust 5703 (15.6) pre-frail 12 985 (35.6) frail 17 778 (48.8) |
18 514 (50.8) NOACs 9328 (50.4)a | Adjusted analyses showed that both being pre-frail and frail were inversely associated with OAC prescription (RR 0.97, 95% CI 0.94–1.00 and RR 0.95, 95% CI 0.92–0.98, respectively) |
| Wilkinson et al.335 | 2021 | UK | 61 177 | robust 6443 (10.5) mildly frail 20 352 (33.3) moderately frail 20 315 (33.2) severely frail 14 067 (23.0) |
30 916 (53.1)b NOACs 7329 (23.7)a | Increasing frailty was found to be associated with a higher likelihood of being prescribed with OAC, compared with being robust (OR 1.84, 95% CI 1.72–1.96, OR 2.34, 95% CI 1.18–2.50, OR 2.51, 95% CI 2.33–2.71, respectively for mild, moderate, and severe frailty) |
| Proietti et al.336 | 2022 | Europe | 10 177 | robust 1939 (19.1) pre-frail 6066 (59.6) frail 2172 (21.3) |
8676 (85.2) NOACs 3638 (35.7) | Compared to robust patients, frail ones were less likely to receive OAC (OR 0.70, 95% CI 0.55–0.89), while pre-frail were more likely to receive (OR 1.21, 95% CI 1.01–1.44) Compared to no OAC treatment, frail patients were less likely to receive both VKAs (OR 0.73, 95% CI 0.56–0.94) and NOACs (OR 0.54, 95% CI 0.41–0.70) than robust ones |
CCI, Charlson comorbidity index; CI, confidence interval; CMs, comorbidities; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulant; OR, odds ratio; RR, risk ratio; VKAs, vitamin K Antagonists; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female); AF, atrial fibrillation.
Among prescribed ones.
Among eligible patients for CHA2DS2-VASc ≥2.
Multi-morbidity, intended as the presence of several different chronic clinical conditions, appears to be a strong determinant and barrier to OAC prescription. An increasing burden of multi-morbidity expressed by the Charlson Comorbidity Index (CCI) was found inversely associated with OAC prescription, as well as ‘high’ multi-morbidity was associated with a lower likelihood of being prescribed with OAC.218 In another study, a very high burden of comorbidities (≥6) was associated with a 30% lower likelihood of being prescribed with OAC, while a progressively higher number of comorbidities was inversely associated with the chance of a patient of being prescribed with NOACs.329 Few data are available regarding the differential effectiveness and safety of OAC in AF patients with multi-morbidity, also appearing significantly more challenging. Indeed, in a series of sub-analyses stemming from NOACs Phase III trials, multi-morbidity does not seem to affect the effectiveness of both apixaban and edoxaban compared to warfarin, but some differences appear in safety outcomes,337,338 with apixaban appearing more favourable in terms of major bleeding risk in patients with a low burden of comorbidities337 and edoxaban being more favourable in terms of GI bleeding risk in patients with a high burden of comorbidities.338 On the contrary, in two very large claim-based and propensity score-matched analyses exploring the interaction between NOACs, VKAs, and multi-morbidity, all data strongly underline how apixaban seems to have a better effectiveness and safety profile compared to warfarin, dabigatran, and rivaroxaban in multi-morbid AF patients.339,340
Polypharmacy is also a significant barrier to OAC prescription, despite the high risk of events associated with its presence in AF patients325 (Table 9). In a UK nationwide study from a primary care setting in AF patients with cognitive impairment, polypharmacy represented a strong predictor of OAC non-prescription even in a large multi-variate analysis including several different clinical characteristics.332
Data regarding effectiveness and safety of OAC according to polypharmacy are controversial. In general, all NOACs are considered more favourable than warfarin even in patients reporting polypharmacy,341–343 notwithstanding while some studies suggest no difference between the various NOACs,344 others show conflicting data regarding possible differences between the various drugs.341,345
Regarding frailty, the evidence appears slightly more conflicting regarding the impact on OAC prescription (Table 9). While in some studies, frailty was reported as significantly associated with OAC under-prescription,334,336 or VKAs preferential prescription,333,336 in others a progressively higher degree of frailty was associated with a higher likelihood of being prescribed with OAC.335 A recent extensive systematic review and meta-analysis, while confirming the high prevalence of frailty among AF patients (∼40%) and its detrimental impact on the risk of adverse outcomes, was inconclusive regarding the likelihood of OAC prescription according to frailty levels.327 Indeed, while overall no difference was found in OAC prescription, as well as in NOACs vs. VKAs prescription, comparing the various possible degrees of frailty (robust, pre-frail, and frail), in some subgroups frail patients are significantly less prescribed with OAC than robust ones.327 Conversely, in population-based studies and in those focusing only on patients with high thrombo-embolic risk, frail patients were more likely to be prescribed with OAC than robust ones.327
Regarding the impact of OAC in frail AF patients, which appears to be still debated,346,347 data seem to be reassuring regarding the beneficial effect of OAC in frail AF patients,336,348 even though uncertainties remain regarding patients with a very high level of frailty for which in some studies was reported no difference in risk of outcomes between OAC treated and not treated patients.336 Looking at the potential differences between NOACs and VKAs, while data coming from NOACs Phase III trials seem to underline no major differences in terms of effectiveness (with only small advantages regarding safety),349 only a few real-life studies are available so far, generally underlying that in frail AF patients dabigatran, rivaroxaban, and apixaban have a beneficial effect on effectiveness outcomes, with apixaban showing the better profile in terms of safety when compared with VKAs.350–352 Furthermore, data regarding the comparison between the various NOACs seem to indicate that apixaban would be a more favourable clinical profile, particularly regarding the risk of major bleeding and other secondary bleeding outcomes.350,351
Atrial high-rate episode on cardiac-implanted electrical device and subclinical atrial fibrillation
Cardiac implanted electrical devices (CIEDs) with an atrial lead or with the capability of rhythm discrimination by means of specific algorithms (i.e. implantable cardiac monitors) allow continuous monitoring of the cardiac rhythm, with an extended ability to appropriately detect any atrial tachyarrhythmias, including AF.353 The atrial tachyarrhythmias detected by CIED have been reported in the literature as atrial high-rate episodes (AHREs),353–355 and their characterization and management have been extensively discussed in Guidelines.14 A key characteristic of AHREs episodes is that they are recorded exclusively through continuous monitoring with CIEDs and include various atrial arrhythmias such as AF, atrial flutter, and atrial tachycardias, often with the transition from regular to irregular rhythm in the same patient, with recordings that can be stored in the device memory, as intra-cavitary electrograms (EGMs).
A careful analysis of EGM tracings is recommended for diagnostic confirmation of the arrhythmia, excluding artefacts or noise.14,353 AHREs have been variably defined or specified but are currently defined by most as episodes of at least 5 min of atrial tachyarrhythmias with an atrial rate ≥ 175 b.p.m. and three criteria have to be fulfilled for a diagnosis of AHRE: no history of prior AF, lack of symptoms attributable to AF, and absence of AF on a 12-lead ECG recording. The term subclinical AF identifies AHRE confirmed to be an atrial tachyarrhythmia by visually adjudicated intra-cardiac EGMs. However, although not completely identical, the terms AHRE and subclinical AF are often used interchangeably in the literature.14 The term ‘AF burden’ has been often used to indicate the overall time spent in AF during a specified period of time (usually 24 h).356,357
The prevalence of AHREs among patients implanted with CIEDs is variable, depending on underlying heart disease, periods of observation, clinical profile, co-morbidities, and a previous history of atrial tachyarrhythmias. In the ASSERT study, subclinical atrial tachyarrhythmias with at least 6 min duration were detected within 3 months in around 10% of patients implanted with a CIED. During a follow-up period of 2.5 years, additional subclinical atrial tachyarrhythmias occurred in around 25% of patients, and around 16% of those who had subclinical atrial tachyarrhythmias developed a symptomatic ‘clinical’ AF.202 An analysis of all the data from the literature reveal that AHREs with a duration >5–6 min are common in patients implanted with CIEDs, with an incidence ranging between 10% and 68%,353,357 recently estimated in a meta-analysis to be around 28%, but with substantial heterogeneity among the different reports in the literature.356
In practice, the key questions on AHRE and subclinical AF are related to the threshold of detected AF duration or of daily AF burden which is significantly associated with stroke/systemic embolism and the risk/benefit ratio of OACs in this specific setting.358 As known, OACs are strongly recommended by consensus guidelines359,360 in patients presenting clinical AF when the CHA2DS2-VASc excludes a low-risk profile, irrespectively of symptoms,14,361 but according to current knowledge, the favourable risk/benefit ratio of anticoagulants in clinical AF cannot be directly transferred to AHREs.
The association between AHRE/subclinical AF of variable time duration and stroke/systemic thrombo-embolism has been evaluated by several observational studies.362–364
As shown, the risk of stroke/thrombo-embolism associated with AHRE is not negligible, and in a recent meta-analysis that excluded patients with prior clinical AF, patients with AHREs showed a 2.13-fold higher risk of thrombo-embolic events.365 Since this risk is actually lower than the 4.8-fold increase in the risk of stroke reported for clinical AF, two randomized controlled trials [ARTESiA and NOAH-AFNET6 (Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes)—Figure 2] are ongoing to evaluate anticoagulants in terms of risk-benefit ratio in this specific setting.362,363 Currently, AHRE episodes < 5 min in duration are not considered to be associated with a substantial risk of stroke.353,362
Figure 2.
ARTESiA and NOAH-AFNET 6 randomized controlled clinical trials. PM, pacemaker; ICD, implantable cardioverter defibrillator; ICM, implantable cardiac monitor, AHRE, atrial high rate episodes; AF, atrial fibrillation; OAC, oral anticoagulation; R, randomization; ASA, aspirin; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female).
AF burden and AHRE duration show a dynamic pattern, with a tendency to progression along with time and transition from burdens in the range of minutes or a few hours to 12–23 h and even more than 23 h, particularly in patients with a higher risk for stroke.366 AHREs with a duration > 23–24 h are associated with a significantly increased risk of stroke,192 and therefore in these cases, long-term anticoagulation becomes an important clinical consideration.14,367,368
Currently, while waiting for evidence-based recommendations, patient-tailored decision-making on the need for anticoagulation is required in patients with AHREs/subclinical AF, particularly in frail patients,369 taking into account that CIED-detected AHREs may occur with a marked temporal dissociation with regard to stroke events, thus suggesting that they may be actually a marker, rather than a risk factor for stroke.370 Indeed, there is an important heterogeneity in the perception of the thrombo-embolic risk associated with AHREs of different durations with variable thresholds of AHRE/AF burden used as a cut-off to start an OAC.371
As suggested by the guidelines, in patients with AHREs, there is a need for individualized decision-making, taking into account risk stratification for previous stroke, stroke risk factors using CHA2DS2-VASc in combination with the amount of detected AF burden associated co-morbidities, and predicted risk of bleeding, thus leading to a prediction of the expected risk-benefit ratio of treatment with anticoagulants.14 The result should be an integrated assessment with AHRE having a variable role, from an ‘innocent bystander’ to an important and evolutive finding, associated with a substantial risk of stroke/thrombo-embolism (Figure 3). Use of OACs, preferentially NOACs, may be justified in selected patients, such as patients with longer durations of AHRE/subclinical AF (in the range of several hours or ≥24 h), and with an estimated high/very high individual risk of stroke, accounting for a favourable anticipated net clinical benefit, to be shared with the patient, after appropriate information and considering patient’s preferences (Figure 4).14
Figure 3.
Proposed approach to patients with CIED-detected AHREs according to the 2020 ESC Guidelines14 (with permission). AF, atrial fibrillation; AHRE, atrial high-rate episode; OAC, oral anticoagulant; SCAF, subclinical atrial fibrillation; CIED, cardiac-implanted electrical device; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65–74 years, Sex category (female).
Figure 4.
Decision process for considering anticoagulation for patient with AHREs. ECG, electrocardiogram; AF, atrial fibrillation; CV, cardiovascular risk; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, Vascular disease, Age 65_74 years, Sex category (female).
Hence, it is appropriate to perform a tighter clinical follow-up, also using remote monitoring of the CIED,372 targeted to detect the development of clinical AF, to monitor the evolution of AHRE/AF burden and specifically the transition to AHRE lasting more than 24 h, as well as the onset or worsening of HF, or any clinical change that might suggest an important worsening in clinical conditions.373–377
Digital health
In the last years, there has been a great expansion of applications and trials of digital health solutions, particularly related to the mobile health (mHealth) field.378 Use of mHealth solutions has been applied both to AF screening strategies and to clinical management and monitoring.378,379
In the recent years, the field of AF screening strategies has seen a big development. The evidence that large proportion of AF patients can present with an asymptomatic status and that no major difference exists in terms of baseline thrombo-embolic risk and risk of major adverse events over long-term observation361 clearly highlighted the need for structured screening programmes to identify asymptomatic AF patients. Indeed, several data underlined how screening strategies have a significant yield of AF diagnosis, irrespective of the screening method and that very often these patients with asymptomatic AF have a high risk of stroke and thrombo-embolic events and are deemed to be prescribed with OAC drugs.379,380 In this context, the use of simple and widespread digital technology solutions using photoplethysmography (PPG) appeared to be promising tools to be used in implementing large-scale screening programmes.
Several studies have been performed to verify whether the use of digital mHealth solutions would be feasible tools to identify asymptomatic AF patients (Table 10). In the Huawei Heart Study, Guo et al.384 demonstrated that a programme using a wristband/wristwatch device was able, in the context of a structured screening programme, to identify 87% of patients with AF among those flagged with an irregular heart rhythm, with >90% positive predictive value (PPV). Similar data were showed by the Apple Heart Study, published in 2019, with ∼84% of PPV. More recently, Rizas et al.390 demonstrated that the use of PPG through a smartphone camera to identify asymptomatic AF patients granted more than twice the likelihood (OR 2.12, 95% CI 1.19–3.76) of identifying AF patients eligible to receive OAC than common usual care.
Table 10.
Studies involving digital health solutions for AF screening
| Study | Year | Design | n | Age | Study cohort | Country | Type of device | Monitoring time | % AF |
|---|---|---|---|---|---|---|---|---|---|
| Nemati et al.381 | 2016 | RSA | 36 | NA | Hospitalized | USA | Wristwatch | 3.5–8.5 min | 33 |
| Yan et al.382 | 2018 | PSA | 217 | 70.3 | Hospitalized | China | Smartphone camera | 20 s × 3 | 34.6 |
| Brasier et al.383 | 2019 | PSA | 592 | 78 | Hospitalized | Germany/Switzerland | Smartphone camera | 5 min | 41.9 |
| Guo et al.384 | 2019 | PSA | 187 912 | 34.7 | Outpatient | China | Wristband/Wristwatch | 60 s every 10 min for 14 days | 87 |
| Perez et al.385 | 2019 | mPSA | 419 297 | 41 | General | USA | Wristwatch | 3 min | 0.52 |
| Verbrugge et al.386 | 2019 | PSA | 12 328 | 49 | General | Belgium | Smartphone camera | 7 days | 0.01 |
| Zhang et al.387 | 2019 | PSA | 361 | 50 | Outpatient | China | Wristband/Wristwatch | 45 s every 10 min for 14 days | 8.6 |
| Chen et al.388 | 2020 | PR | 401 | NA | Hospitalized/Outpatient | China | Wristband | 3 min | 37 |
| Lubitz et al.389 | 2022 | PSA | 1057 | NA | General ≥22 years | USA | Wristband | 122 days | 32.2 |
| Rizas et al.390 | 2022 | RCT | 5551 | NA | General ≥65 years | Germany | Smartphone camera | 6 min | 1.33 |
AF, atrial fibrillation; mPSA, multi-centre prospective single arm; NA, not available; PSA, prospective single arm; RCT, randomized clinical trial.
The main issue of using digital mHealth tools and screening strategies is the ability of reducing the risk of stroke in the long-term observation. General evidence provided by an analysis of available studies underlines that despite substantial data indicating that screening would be likely to obtain a significant risk reduction in stroke and other adverse outcomes, solid proof is still lacking due to several methodological issues.379 Several studies, including the Heartline study which will enrol ≥65 years old subjects and will evaluate if the use of a PPG-based smartwatch AF detection in conjunction with an engagement/adherence module, will elucidate the actual ability of screening programmes to reduce risk of stroke.379,391
Furthermore, search for AF after an ischaemic stroke was traditionally based on use of Holter recordings, also of prolonged duration,392 or on implantable loop recorders,392,393 but more recently also digital tools such as smartwatches and smartphones (also called ‘wearables’), usually proposed with a direct-to-consumer approach,394,395 are currently implemented in daily practice. However, even if a wider use of digital tools is emerging, some issues related to organization of care, data management, digital literacy, and reimbursement are still open,396–400 and more studies are needed.
Going over the issue of screening, which still remains crucial in the clinical management of AF, use of digital tools, i.e. web- or mobile-based applications seems to be useful also in the improvement of engagement, quality of life, and clinical management of AF patients.401 For example, in the second phase of the mAFA II, the use of a mobile-based app used to deliver the ‘ABC’ pathway reduced the risk of a composite outcome of ischaemic stroke/systemic thrombo-embolism/all-cause death and hospitalization [HR 0.39, 95% CI 0.22–0.67] over 1-year follow-up observation.384 Current ongoing programmes, particularly the ‘AFFIRMO’ Programme, will provide more evidence about the implementation of AF clinical management and reduction of ischaemic stroke and other adverse outcomes risk through the use of digital health tools.37
Conclusions
As this state-of-the-art review illustrates, substantial advances in the field of stroke prevention in AF are evident over the last years. Advances in our understanding of the epidemiology and pathophysiology of stroke risk as well as refinements in stroke risk stratification are evident. While oral anticoagulation remains the mainstay, particularly with the NOACs, the emerging role of LAAO for selected patients with absolute contraindications to long-term anticoagulation is clear. In addition, the impact of early rhythm control in reducing stroke risk when used in selected patients with recent onset AF is supported by clinical trial evidence. Finally, a holistic or integrated care management approach based on the ABC pathway is fully supported by clinical trial evidence as well as retrospective and prospective cohorts, to be associated with improved clinical outcomes.
Contributor Information
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
Marco Proietti, Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy; Division of Subacute Care, IRCCS Istituti Clinici Scientifici Maugeri, Milan, Italy.
Tatjana Potpara, School of Medicine, Belgrade University, Belgrade, Serbia; Cardiology Clinic, University Clinical Centre of Serbia, Belgrade, Serbia.
Moussa Mansour, Massachusetts General Hospital, Boston, MA, USA.
Irina Savelieva, Clinical Academic Group, Molecular and Clinical Sciences Institute, St. George’s University of London, Cranmer Terrace London SW17 0RE, UK.
Hung Fat Tse, Cardiology Division, Department of Medicine, School of Clinical Medicine, LKS Faculty of Medicine, University of Hong Kong, Hong Kong, China.
Andreas Goette, Medizinische Klinik II: Kardiologie und Intensivmedizin, St. Vincenz-Krankenhaus Paderborn, Am Busdorf 2, 33098 Paderborn, Germany.
A John Camm, Clinical Academic Group, Molecular and Clinical Sciences Institute, St. George’s University of London, Cranmer Terrace London SW17 0RE, UK.
Carina Blomstrom-Lundqvist, Department of Cardiology, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Dhiraj Gupta, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Cardiology, Liverpool Heart & Chest Hospital, Liverpool, United Kingdom.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, via del Pozzo 71, 41125 Modena, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 2.Dong XJ, Wang BB, Hou FF, Jiao Y, Li HW, Lv SP et al. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace. 2023;25:793–803. [DOI] [PMC free article] [PubMed]
- 3. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LMet al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DDet al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WPet al. Secular trends in incidence of atrial fibrillation in olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 6. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JVet al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 7. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman Aet al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Carlo A, Bellino L, Consoli D, Mori F, Zaninelli A, Baldereschi Met al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI project. Europace 2019;21:1468–75. [DOI] [PubMed] [Google Scholar]
- 9. Wong CX, Brown A, Tse HF, Albert CM, Kalman JM, Marwick THet al. Epidemiology of atrial fibrillation: the Australian and Asia-Pacific perspective. Heart Lung Circ 2017;26:870–9. [DOI] [PubMed] [Google Scholar]
- 10. Chao TF, Liu CJ, Tuan TC, Chen TJ, Hsieh MH, Lip GYHet al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest 2018;153:453–66. [DOI] [PubMed] [Google Scholar]
- 11. Jiao M, Liu C, Liu Y, Wang Y, Gao Q, Ma A. Estimates of the global, regional, and national burden of atrial fibrillation in older adults from 1990 to 2019: insights from the global burden of disease study 2019. Front Public Health 2023;11:1137230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lip GY, Fauchier L, Freedman SB, Van Gelder I, Natale A, Gianni Cet al. Atrial fibrillation. Nat Rev Dis Primers 2016;2:16016. [DOI] [PubMed] [Google Scholar]
- 13. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CLet al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation 2023;147:e93–e621. [DOI] [PubMed] [Google Scholar]
- 14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 15. Lip GYH, Gue Y, Zhang J, Chao TF, Calkins H, Potpara T. Stroke prevention in atrial fibrillation. Trends Cardiovasc Med 2022;32:501–10. [DOI] [PubMed] [Google Scholar]
- 16. Marzona I, Proietti M, Farcomeni A, Romiti GF, Romanazzi I, Raparelli V. et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: A systematic review and meta-analysis of 993,600 patients. Int J Cardiol 2018;269:182–91. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen PB, Skjoth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA(2)DS(2)-VA score rather than CHA(2)DS(2)-VASc? Circulation 2018;137:832–40. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen PB, Overvad TF. Female sex as a risk modifier for stroke risk in atrial fibrillation: using CHA2DS2-VASc versus CHA2DS2-VA for stroke risk stratification in atrial fibrillation: a note of caution. Thromb Haemost 2020;120:894–8. [DOI] [PubMed] [Google Scholar]
- 19. Pilcher SM, Alamneh EA, Chalmers L, Bereznicki LR. The tasmanian atrial fibrillation study (TAFS): differences in stroke prevention according to sex. Ann Pharmacother 2020;54:837–45. [DOI] [PubMed] [Google Scholar]
- 20. Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee Ret al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemost 2018;118:2171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EMet al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J 2016;37:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rivera-Caravaca JM, Marin F, Vilchez JA, Galvez J, Esteve-Pastor MA, Vicente Vet al. Refining stroke and bleeding prediction in atrial fibrillation by adding consecutive biomarkers to clinical risk scores. Stroke 2019;50:1372–9. [DOI] [PubMed] [Google Scholar]
- 23. Camelo-Castillo A, Rivera-Caravaca JM, Marin F, Vicente V, Lip GYH, Roldan V. Predicting adverse events beyond stroke and bleeding with the ABC-stroke and ABC-bleeding scores in patients with atrial fibrillation: the murcia AF project. Thromb Haemost 2020;120:1200–7. [DOI] [PubMed] [Google Scholar]
- 24. Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdes M, Marin Fet al. Long-term bleeding risk prediction in ‘real world’ patients with atrial fibrillation: comparison of the HAS-BLED and ABC-bleeding risk scores. The murcia atrial fibrillation project. Thromb Haemost 2017;117:1848–58. [DOI] [PubMed] [Google Scholar]
- 25. Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, Valdes M, Vicente V, Lip GYHet al. Long-term stroke risk prediction in patients with atrial fibrillation: comparison of the ABC-stroke and CHA(2)DS(2)-VASc scores. J Am Heart Assoc 2017;6:e006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Domek M, Gumprecht J, Mazurek M, Chao TF, Lip GYH. Should we judge stroke risk by static or dynamic risk scores? A focus on the dynamic nature of stroke and bleeding risks in patients with atrial fibrillation. J Cardiovasc Pharmacol 2019;74:491–8. [DOI] [PubMed] [Google Scholar]
- 27. Lip GYH, Genaidy A, Tran G, Marroquin P, Estes C, Sloop S. Improving stroke risk prediction in the general population: a comparative assessment of common clinical rules, a new multimorbid index, and machine-learning-based algorithms. Thromb Haemost 2022;122:142–50. [DOI] [PubMed] [Google Scholar]
- 28. Ding WY, Gupta D, Lip GYH. Atrial fibrillation and the prothrombotic state: revisiting virchow's triad in 2020. Heart 2020;106:1463–8. [DOI] [PubMed] [Google Scholar]
- 29. López-Galvez R, Rivera-Caravaca JM, Roldán V, Orenes-Piñero E, Esteve-Pastor MA, López-García Cet al. Imaging in atrial fibrillation: a way to assess atrial fibrosis and remodeling to assist decision-making. Am Heart J 2023;258:1–16. [DOI] [PubMed] [Google Scholar]
- 30. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JLet al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YHet al. 2021 focused update consensus guidelines of the Asia Pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 2022;122:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli Det al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2022;122:406–14. [DOI] [PubMed] [Google Scholar]
- 33. Patel SM, Palazzolo MG, Murphy SA, Antman EM, Braunwald E, Lanz HJet al. Evaluation of the atrial fibrillation better care pathway in the ENGAGE AF-TIMI 48 trial. Europace 2022;24:1730–8. [DOI] [PubMed] [Google Scholar]
- 34. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive management with the ABC (atrial fibrillation better care) pathway in clinically complex patients with atrial fibrillation: a post hoc ancillary analysis from the AFFIRM trial. J Am Heart Assoc 2020;9:e014932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang Wet al. mAF-App II Trial Investigators . . Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–34. [DOI] [PubMed] [Google Scholar]
- 36. Romiti GF, Guo Y, Corica B, Proietti M, Zhang H, Lip GYHet al. Mobile health-technology-integrated care for atrial fibrillation: a win ratio analysis from the mAFA-II randomized clinical trial. Thromb Haemost. (EPUB ahead of print: 2023 May 29) [DOI] [PubMed] [Google Scholar]
- 37. Johnsen SP, Proietti M, Maggioni AP, Lip GYH. A multinational European network to implement integrated care in elderly multimorbid atrial fibrillation patients: the AFFIRMO consortium. Eur Heart J 2022;43:2916–8. [DOI] [PubMed] [Google Scholar]
- 38. De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann Fet al. Vitamin K antagonists in heart disease: current status and perspectives (section III). Position paper of the ESC working group on thrombosis–task force on anticoagulants in heart disease. Thromb Haemost 2013;110:1087–107. [DOI] [PubMed] [Google Scholar]
- 39. Potpara TS. Comparing non-vitamin K antagonist oral anticoagulants (NOACs) to different coumadins: the win-win scenarios. Thromb Haemost 2018;118:803–5. [DOI] [PubMed] [Google Scholar]
- 40. Le Heuzey JY, Ammentorp B, Darius H, De Caterina R, Schilling RJ, Schmitt Jet al. Differences among western European countries in anticoagulation management of atrial fibrillation. Data from the PREFER IN AF registry. Thromb Haemost 2014;111:833–41. [DOI] [PubMed] [Google Scholar]
- 41. Weitz JI, Harenberg J. New developments in anticoagulants: past, present and future. Thromb Haemost 2017;117:1283–8. [DOI] [PubMed] [Google Scholar]
- 42. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 43. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KGet al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MDet al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 45. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh Aet al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 46. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke Wet al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 47. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna Met al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 48. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JLet al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 49. Deitelzweig S, Bergrath E, di Fusco M, Kang A, Savone M, Cappelleri JCet al. Real-world evidence comparing oral anticoagulants in non-valvular atrial fibrillation: a systematic review and network meta-analysis. Future Cardiol 2022;18:393–405. [DOI] [PubMed] [Google Scholar]
- 50. Farinha JM, Jones ID, Lip GYH. Optimizing adherence and persistence to non-vitamin K antagonist oral anticoagulant therapy in atrial fibrillation. Eur Heart J Suppl 2022;24:A42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SSet al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2020;13:e005969. [DOI] [PubMed] [Google Scholar]
- 52. Caso V, de Groot JR, Sanmartin Fernandez M, Segura T, Blomstrom-Lundqvist C, Hargroves Det al. Outcomes and drivers of inappropriate dosing of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation: a systematic review and meta-analysis. Heart 2023;109:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JCet al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 54. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJet al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14. [DOI] [PubMed] [Google Scholar]
- 55. Wang TY, Svensson LG, Wen J, Vekstein A, Gerdisch M, Rao VUet al. Apixaban or warfarin in patients with an on-X mechanical aortic valve. NEJM Evidence 2023;2:EVIDoa2300067. [DOI] [PubMed] [Google Scholar]
- 56. Duraes AR, de Souza Lima Bitar Y, Schonhofen IS, Travassos KSO, Pereira LV, Filho JALet al. Rivaroxaban versus warfarin in patients with mechanical heart valves: open-label, proof-of-concept trial-the RIWA study. Am J Cardiovasc Drugs 2021;21:363–71. [DOI] [PubMed] [Google Scholar]
- 57. Fanaroff AC, Vora AN, Lopes RD. Non-vitamin K antagonist oral anticoagulants in patients with valvular heart disease. Eur Heart J Suppl 2022;24:A19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim JY, Kim SH, Myong JP, Kim YR, Kim TS, Kim JHet al. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol 2019;73:1123–31. [DOI] [PubMed] [Google Scholar]
- 59. Connolly SJ, Karthikeyan G, Ntsekhe M, Haileamlak A, El Sayed A, El Ghamrawy Aet al. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med 2022;387:978–88. [DOI] [PubMed] [Google Scholar]
- 60. Zhou M, Chan EW, Hai JJ, Wong CK, Lau YM, Huang Det al. Protocol, rationale and design of DAbigatran for stroke PreVention in atrial fibrillation in MoDerate or severe mitral stenosis (DAVID-MS): a randomised, open-label study. BMJ Open 2020;10:e038194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khairani CD, Bejjani A, Piazza G, Jimenez D, Monreal M, Chatterjee Set al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol 2023;81:16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pokorney SD, Chertow GM, Al-Khalidi HR, Gallup D, Dignacco P, Mussina Ket al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation 2022;146:1735–45. [DOI] [PubMed] [Google Scholar]
- 63. Reinecke H, Engelbertz C, Bauersachs R, Breithardt G, Echterhoff HH, Gerss Jet al. A randomized controlled trial comparing apixaban with the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation 2023;147:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Vriese AS, Caluwe R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol 2021;32:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Caterina R, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MFet al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 2017;69:1372–82. [DOI] [PubMed] [Google Scholar]
- 66. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna Met al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 2015;132:624–32. [DOI] [PubMed] [Google Scholar]
- 67. Guimaraes HP, Lopes RD, de Barros ESPGM, Liporace IL, Sampaio RO, Tarasoutchi Fet al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med 2020;383:2117–26. [DOI] [PubMed] [Google Scholar]
- 68. Collet JP, Van Belle E, Thiele H, Berti S, Lhermusier T, Manigold Tet al. Apixaban vs. standard of care after transcatheter aortic valve implantation: the ATLANTIS trial. Eur Heart J 2022;43:2783–97. [DOI] [PubMed] [Google Scholar]
- 69. Van Mieghem NM, Unverdorben M, Hengstenberg C, Mollmann H, Mehran R, Lopez-Otero Det al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med 2021;385:2150–60. [DOI] [PubMed] [Google Scholar]
- 70. Yokoyama Y, Briasoulis A, Ueyama H, Mori M, Iwagami M, Misumida Net al. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and bioprosthetic valves: a meta-analysis. J Thorac Cardiovasc Surg 2023;165:2052–9 e4. [DOI] [PubMed] [Google Scholar]
- 71. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs Jet al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 72. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile Fet al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2021;143:e35–71. [DOI] [PubMed] [Google Scholar]
- 73. Harrington J, Piccini JP, Alexander JH, Granger CB, Patel MR. Clinical evaluation of factor XIa inhibitor drugs: JACC review topic of the week. J Am Coll Cardiol 2023;81:771–9. [DOI] [PubMed] [Google Scholar]
- 74. Gorog DA, Gue YX, Chao TF, Fauchier L, Ferreiro JL, Huber Ket al. Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: executive summary of a European and Asia-Pacific expert consensus paper. Thromb Haemost 2022;122:1625–52. [DOI] [PubMed] [Google Scholar]
- 75. Kim HK, Tantry US, Smith SC Jr, Jeong MH, Park SJ, Kim MHet al. The east Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost 2021;121:422–32. [DOI] [PubMed] [Google Scholar]
- 76. Ivany E, Lotto RR, Lip GYH, Lane DA. Managing uncertainty: physicians’ decision making for stroke prevention for patients with atrial fibrillation and intracerebral hemorrhage. Thromb Haemost 2022;122:1603–11. [DOI] [PubMed] [Google Scholar]
- 77. Schulman S, Beyth RJ, Kearon C, Levine MN. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133:257s–98s. [DOI] [PubMed] [Google Scholar]
- 78. Aberg H. Atrial fibrillation. I. A study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand 1969;185:373–9. [PubMed] [Google Scholar]
- 79. Mahajan R, Brooks AG, Sullivan T, Lim HS, Alasady M, Abed HSet al. Importance of the underlying substrate in determining thrombus location in atrial fibrillation: implications for left atrial appendage closure. Heart 2012;98:1120–6. [DOI] [PubMed] [Google Scholar]
- 80. Tsai YC, Phan K, Munkholm-Larsen S, Tian DH, La Meir M, Yan TD. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg 2015;47:847–54. [DOI] [PubMed] [Google Scholar]
- 81. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma Met al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. [DOI] [PubMed] [Google Scholar]
- 82. Nakai T, Lesh MD, Gerstenfeld EP, Virmani R, Jones R, Lee RJ. Percutaneous left atrial appendage occlusion (PLAATO) for preventing cardioembolism: first experience in canine model. Circulation 2002;105:2217–22. [DOI] [PubMed] [Google Scholar]
- 83. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber Ket al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation 2013;127:720–9. [DOI] [PubMed] [Google Scholar]
- 84. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SKet al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 85. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala Pet al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. [DOI] [PubMed] [Google Scholar]
- 86. Panikker S, Lord J, Jarman JW, Armstrong S, Jones DG, Haldar Set al. Outcomes and costs of left atrial appendage closure from randomized controlled trial and real-world experience relative to oral anticoagulation. Eur Heart J 2016;37:3470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Turagam MK, Osmancik P, Neuzil P, Dukkipati SR, Reddy VY. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation: a meta-analysis of randomized trials. J Am Coll Cardiol 2020;76:2795–7. [DOI] [PubMed] [Google Scholar]
- 88. Reddy VY, Möbius-Winkler S, Miller MA, Neuzil P, Schuler G, Wiebe Jet al. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J Am Coll Cardiol 2013;61:2551–6. [DOI] [PubMed] [Google Scholar]
- 89. Brouwer TF, Whang W, Kuroki K, Halperin JL, Reddy VY. Net clinical benefit of left atrial appendage closure versus warfarin in patients with atrial fibrillation: a pooled analysis of the randomized PROTECT-AF and PREVAIL studies. J Am Heart Assoc 2019;8:e013525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hildick-Smith D, Landmesser U, Camm AJ, Diener HC, Paul V, Schmidt Bet al. Left atrial appendage occlusion with the amplatzer™ amulet™ device: full results of the prospective global observational study. Eur Heart J 2020;41:2894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tzikas A, Freixa X, Llull L, Gafoor S, Shakir S, Omran Het al. Patients with intracranial bleeding and atrial fibrillation treated with left atrial appendage occlusion: results from the amplatzer cardiac plug registry. Int J Cardiol 2017;236:232–6. [DOI] [PubMed] [Google Scholar]
- 92. Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll Jet al. Amplatzer amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (amulet IDE): a randomized, controlled trial. Circulation 2021;144:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt Bet al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: final 2-year outcome data of the EWOLUTION trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol 2019;12:e006841. [DOI] [PubMed] [Google Scholar]
- 94. Cruz-González I, Ince H, Kische S, Schmitz T, Schmidt B, Gori Tet al. Left atrial appendage occlusion in patients older than 85 years. Safety and efficacy in the EWOLUTION registry. Rev Esp Cardiol (Engl Ed) 2020;73:21–7. [DOI] [PubMed] [Google Scholar]
- 95. Zhai Z, Tang M, Su X, Chu H, Huang W, Zeng J. et al. Experience of left atrial appendage occlusion with the WATCHMAN device in Chinese patients. Anatol J Cardiol 2019;21:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coppens M, Synhorst D, Eikelboom JW, Yusuf S, Shestakovska O, Connolly SJ. Efficacy and safety of apixaban compared with aspirin in patients who previously tried but failed treatment with vitamin K antagonists: results from the AVERROES trial. Eur Heart J 2014;35:1856–63. [DOI] [PubMed] [Google Scholar]
- 97. Benz AP, Eikelboom JW, Yusuf S, Hohnloser SH, Kahl A, Beresh Het al. Long-term treatment with apixaban in patients with atrial fibrillation: outcomes during the open-label extension following AVERROES. Thromb Haemost 2021;121:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GYet al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 2012;11:225–31. [DOI] [PubMed] [Google Scholar]
- 99. Nielsen-Kudsk JE, Korsholm K, Damgaard D, Valentin JB, Diener HC, Camm AJet al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv 2021;14:69–78. [DOI] [PubMed] [Google Scholar]
- 100. Hanif H, Belley-Cote EP, Alotaibi A, Dvirnik N, Neupane B, Beyene Jet al. Left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation: a systematic review and network meta-analysis of randomized controlled trials. J Cardiovasc Surg (Torino) 2018;59:128–39. [DOI] [PubMed] [Google Scholar]
- 101. Sahay S, Nombela-Franco L, Rodes-Cabau J, Jimenez-Quevedo P, Salinas P, Biagioni Cet al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart 2017;103:139–47. [DOI] [PubMed] [Google Scholar]
- 102. Krittayaphong R, Phrommintikul A, Ngamjanyaporn P, Siriwattana K, Kanjanarutjawiwat W, Chantrarat Tet al. Rate of anticoagulant use, and factors associated with not prescribing anticoagulant in older Thai adults with non-valvular atrial fibrillation: a multicenter registry. J Geriatr Cardiol 2019;16:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball Det al. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost 2014;111:465–73. [DOI] [PubMed] [Google Scholar]
- 104. Glader EL, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke 2010;41:397–401. [DOI] [PubMed] [Google Scholar]
- 105. Kapoor A, Si K, Yu AYX, Lanctot KL, Herrmann N, Murray BJet al. Younger age and depressive symptoms predict high risk of generalized anxiety after stroke and transient ischemic attack. Stroke 2019;50:2359–63. [DOI] [PubMed] [Google Scholar]
- 106. Cruz-González I, González-Ferreiro R, Freixa X, Gafoor S, Shakir S, Omran Het al. Left atrial appendage occlusion for stroke despite oral anticoagulation (resistant stroke). results from the amplatzer cardiac plug registry. Rev Esp Cardiol (Engl Ed) 2020;73:28–34. [DOI] [PubMed] [Google Scholar]
- 107. Masjuan J, Salido L, DeFelipe A, Hernández-Antolín R, Fernández-Golfín C, Cruz-Culebras Aet al. Oral anticoagulation and left atrial appendage closure: a new strategy for recurrent cardioembolic stroke. Eur J Neurol 2019;26:816–20. [DOI] [PubMed] [Google Scholar]
- 108. Freixa X, Cruz-González I, Regueiro A, Nombela-Franco L, Estévez-Loureiro R, Ruiz-Salmerón Ret al. Left atrial appendage occlusion as adjunctive therapy to anticoagulation for stroke recurrence. J Invasive Cardiol 2019;31:212–6. [PubMed] [Google Scholar]
- 109. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 110. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman Bet al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–201. [DOI] [PubMed] [Google Scholar]
- 111. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YHet al. 2021 focused update of the 2017 consensus guidelines of the Asia pacific heart rhythm society (APHRS) on stroke prevention in atrial fibrillation. J Arrhythm 2021;37:1389–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell Cet al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018;27:1209–66. [DOI] [PubMed] [Google Scholar]
- 113. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CCet al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847–948. [DOI] [PubMed] [Google Scholar]
- 114. Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo Cet al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace 2014;16:1397–416. [DOI] [PubMed] [Google Scholar]
- 115. Tzikas A, Holmes DR Jr, Gafoor S, Ruiz CE, Blomstrom-Lundqvist C, Diener HCet al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017;19:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYHet al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. Europace 2020;22:184. [DOI] [PubMed] [Google Scholar]
- 117. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EBet al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. [DOI] [PubMed] [Google Scholar]
- 118. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KLet al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–77. [DOI] [PubMed] [Google Scholar]
- 119. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma Tet al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834–40. [DOI] [PubMed] [Google Scholar]
- 120. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation–pharmacological intervention in atrial fibrillation (PIAF): a randomised trial. Lancet 2000;356:1789–94. [DOI] [PubMed] [Google Scholar]
- 121. Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus Set al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the strategies of treatment of atrial fibrillation (STAF) study. J Am Coll Cardiol 2003;41:1690–6. [DOI] [PubMed] [Google Scholar]
- 122. Opolski G, Torbicki A, Kosior DA, Szulc M, Wozakowska-Kaplon B, Kolodziej Pet al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish how to treat chronic atrial fibrillation (HOT CAFE) study. Chest 2004;126:476–86. [DOI] [PubMed] [Google Scholar]
- 123. Ogawa S, Yamashita T, Yamazaki T, Aizawa Y, Atarashi H, Inoue Het al. Optimal treatment strategy for patients with paroxysmal atrial fibrillation: J-RHYTHM study. Circ J 2009;73:242–8. [DOI] [PubMed] [Google Scholar]
- 124. Grönefeld GC, Lilienthal J, Kuck KH, Hohnloser SH. Impact of rate versus rhythm control on quality of life in patients with persistent atrial fibrillation. Results from a prospective randomized study. Eur Heart J 2003;24:1430–6. [DOI] [PubMed] [Google Scholar]
- 125. Brignole M, Menozzi C, Gasparini M, Bongiorni MG, Botto GL, Ometto Ret al. An evaluation of the strategy of maintenance of sinus rhythm by antiarrhythmic drug therapy after ablation and pacing therapy in patients with paroxysmal atrial fibrillation. Eur Heart J 2002;23:892–900. [DOI] [PubMed] [Google Scholar]
- 126. Noheria A, Shrader P, Piccini JP, Fonarow GC, Kowey PR, Mahaffey KWet al. Rhythm control versus rate control and clinical outcomes in patients with atrial fibrillation: results from the ORBIT-AF registry. JACC Clin Electrophysiol 2016;2:221–9. [DOI] [PubMed] [Google Scholar]
- 127. Camm AJ, Breithardt G, Crijns H, Dorian P, Kowey P, Le Heuzey JYet al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (registry on cardiac rhythm disorders assessing the control of atrial fibrillation). J Am Coll Cardiol 2011;58:493–501. [DOI] [PubMed] [Google Scholar]
- 128. Govindapillai A, Cox JL, Thabane L, Doucette S, Xie F, MacKillop JHet al. Rhythm control vs rate control in a contemporary ambulatory atrial fibrillation cohort: post hoc analysis of the IMPACT-AF trial. CJC Open 2022;4:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhao Y, Krupadev V, Dagher L, Mahnkopf C, Sohns C, Sehner Set al. Pharmacological rhythm versus rate control in patients with atrial fibrillation and heart failure: the CASTLE-AF trial. J Interv Card Electrophysiol 2021;61:609–15. [DOI] [PubMed] [Google Scholar]
- 130. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JEet al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 132. Zakeri R, Ahluwalia N, Tindale A, Omar F, Packer M, Khan Het al. Long-term outcomes following catheter ablation versus medical therapy in patients with persistent atrial fibrillation and heart failure with reduced ejection fraction. Eur J Heart Fail 2023;25:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 134. Bunch TJ, May HT, Bair TL, Weiss JP, Crandall BG, Osborn JSet al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm 2013;10:1272–7. [DOI] [PubMed] [Google Scholar]
- 135. Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HLet al. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation 2004;109:1509–13. [DOI] [PubMed] [Google Scholar]
- 136. Talajic M, Khairy P, Levesque S, Connolly SJ, Dorian P, Dubuc Met al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol 2010;55:1796–802. [DOI] [PubMed] [Google Scholar]
- 137. Chen S, Pürerfellner H, Ouyang F, Kiuchi MG, Meyer C, Martinek Met al. Catheter ablation vs. antiarrhythmic drugs as ‘first-line’ initial therapy for atrial fibrillation: a pooled analysis of randomized data. Europace 2021;23:1950–60. [DOI] [PubMed] [Google Scholar]
- 138. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen S, Pürerfellner H, Meyer C, Acou WJ, Schratter A, Ling Zet al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2020;41:2863–73. [DOI] [PubMed] [Google Scholar]
- 140. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt Get al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021;144:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah NDet al. Generalizability of the CASTLE-AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm 2020;17:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JCet al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 143. Tsadok MA, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Humphries KHet al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation 2012;126:2680–7. [DOI] [PubMed] [Google Scholar]
- 144. Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo Aet al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 2010;55:735–43. [DOI] [PubMed] [Google Scholar]
- 145. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JSet al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:839–45. [DOI] [PubMed] [Google Scholar]
- 146. Proietti M, Vitolo M, Harrison SL, Lane DA, Fauchier L, Marin Fet al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the ESC-EHRA EORP-AF long-term general registry. Clin Res Cardiol 2022;111:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chao TF, Chan YH, Chiang CE, Tuan TC, Liao JN, Chen TJet al. Early rhythm control and the risks of ischemic stroke, heart failure, mortality, and adverse events when performed early (< 3 months): a nationwide cohort study of newly diagnosed patients with atrial fibrillation. Thromb Haemost 2022;122:1899–910. [DOI] [PubMed] [Google Scholar]
- 148. Zhu W, Wu Z, Dong Y, Lip GYH, Liu C. Effectiveness of early rhythm control in improving clinical outcomes in patients with atrial fibrillation: a systematic review and meta-analysis. BMC Med 2022;20:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nakamaru R, Ikemura N, Spertus JA, Kimura T, Katsumata Y, Fujisawa Tet al. Rate versus rhythm control in patients with newly diagnosed atrial fibrillation: effects of the treatment timing on health status outcomes. Am Heart J 2022;254:156–65. [DOI] [PubMed] [Google Scholar]
- 150. Gottschalk S, Kany S, Konig HH, Crijns HJ, Vardas P, Camm AJet al. Cost-effectiveness of early rhythm control vs. usual care in atrial fibrillation care: an analysis based on data from the EAST-AFNET 4 trial. Europace 2023;25:euad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns Het al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J 2022;43:1219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran Set al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006;114:759–65. [DOI] [PubMed] [Google Scholar]
- 154. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema Cet al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–92. [DOI] [PubMed] [Google Scholar]
- 155. Riley MP, Zado E, Hutchinson MD, Lin D, Bala R, Garcia FCet al. Risk of stroke or transient ischemic attack after atrial fibrillation ablation with oral anticoagulant use guided by ECG monitoring and pulse assessment. J Cardiovasc Electrophysiol 2014;25:591–6. [DOI] [PubMed] [Google Scholar]
- 156. Proietti R, AlTurki A, Di Biase L, China P, Forleo G, Corrado Aet al. Anticoagulation after catheter ablation of atrial fibrillation: an unnecessary evil? A systematic review and meta-analysis. J Cardiovasc Electrophysiol 2019;30:468–78. [DOI] [PubMed] [Google Scholar]
- 157. Yagishita A, Takahashi Y, Takahashi A, Fujii A, Kusa S, Fujino Tet al. Incidence of late thromboembolic events after catheter ablation of atrial fibrillation. Circ J 2011;75:2343–9. [DOI] [PubMed] [Google Scholar]
- 158. Hunter RJ, McCready J, Diab I, Page SP, Finlay M, Richmond Let al. Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 2012;98:48–53. [DOI] [PubMed] [Google Scholar]
- 159. Noseworthy PA, Yao X, Deshmukh AJ, Van Houten H, Sangaralingham LR, Siontis KCet al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc 2015;4:e002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Karasoy D, Gislason GH, Hansen J, Johannessen A, Køber L, Hvidtfeldt Met al. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J 2015;36:307–15. [DOI] [PubMed] [Google Scholar]
- 161. Zheng Y-R, Chen Z-Y, Ye L-F, Wang L-H. Long-term stroke rates after catheter ablation or antiarrhythmic drug therapy for atrial fibrillation: a meta-analysis of randomized trials. J Geriatr Cardiol 2015;12:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nuhrich JM, Kuck KH, Andresen D, Steven D, Spitzer SG, Hoffmann Eet al. Oral anticoagulation is frequently discontinued after ablation of paroxysmal atrial fibrillation despite previous stroke: data from the German ablation registry. Clin Res Cardiol 2015;104:463–70. [DOI] [PubMed] [Google Scholar]
- 163. Gallo C, Battaglia A, Anselmino M, Bianchi F, Grossi S, Nangeroni Get al. Long-term events following atrial fibrillation rate control or transcatheter ablation: a multicenter observational study. J Cardiovasc Med (Hagerstown) 2016;17:187–93. [DOI] [PubMed] [Google Scholar]
- 164. Själander S, Holmqvist F, Smith JG, Platonov PG, Kesek M, Svensson PJet al. Assessment of use vs discontinuation of oral anticoagulation after pulmonary vein isolation in patients with atrial fibrillation. JAMA Cardiology 2017;2:146–52. [DOI] [PubMed] [Google Scholar]
- 165. Saliba W, Schliamser JE, Lavi I, Barnett-Griness O, Gronich N, Rennert G. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: a propensity score–matched analysis. Heart Rhythm 2017;14:635–42. [DOI] [PubMed] [Google Scholar]
- 166. Srivatsa UN, Danielsen B, Amsterdam EA, Pezeshkian N, Yang Y, Nordsieck Eet al. CAABL-AF (California study of ablation for atrial fibrillation): mortality and stroke, 2005 to 2013. Circ Arrhythm Electrophysiol 2018;11:e005739. [DOI] [PubMed] [Google Scholar]
- 167. Joza J, Samuel M, Jackevicius CA, Behlouli H, Jia J, Koh Met al. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol 2018;29:1355–62. [DOI] [PubMed] [Google Scholar]
- 168. Atti V, Turagam MK, Viles-Gonzalez JF, Lakkireddy D. Anticoagulation after catheter ablation of atrial fibrillation: is it time to discontinue in select patient population? J Atr Fibrillation 2018;11:2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Romero J, Cerrud-Rodriguez RC, Diaz JC, Rodriguez D, Arshad S, Alviz Iet al. Oral anticoagulation after catheter ablation of atrial fibrillation and the associated risk of thromboembolic events and intracranial hemorrhage: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2019;30:1250–7. [DOI] [PubMed] [Google Scholar]
- 170. Freeman JV, Shrader P, Pieper KS, Allen LA, Chan PS, Fonarow GCet al. Outcomes and anticoagulation use after catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2019;12:e007612. [DOI] [PubMed] [Google Scholar]
- 171. Rong B, Han W, Lin M, Hao L, Zhang K, Chen Tet al. Thromboembolic risk of cessation of oral anticoagulation post catheter ablation in patients with and without atrial fibrillation recurrence. Am J Cardiol 2020;137:55–62. [DOI] [PubMed] [Google Scholar]
- 172. Yang WY, Du X, Jiang C, He L, Fawzy AM, Wang Let al. The safety of discontinuation of oral anticoagulation therapy after apparently successful atrial fibrillation ablation: a report from the Chinese atrial fibrillation registry study. Europace 2020;22:90–9. [DOI] [PubMed] [Google Scholar]
- 173. Kim M, Yu HT, Kim J, Kim TH, Uhm JS, Joung Bet al. Atrial fibrillation and the risk of ischaemic strokes or intracranial haemorrhages: comparisons of the catheter ablation, medical therapy, and non-atrial fibrillation population. Europace 2021;23:529–38. [DOI] [PubMed] [Google Scholar]
- 174. Pothineni NVK, Amankwah N, Santangeli P, Schaller RD, Supple GE, Deo Ret al. Continuous rhythm monitoring-guided anticoagulation after atrial fibrillation ablation. J Cardiovasc Electrophysiol 2021;32:345–53. [DOI] [PubMed] [Google Scholar]
- 175. Maduray K, Moneruzzaman M, Changwe GJ, Zhong J. Benefits and risks associated with long-term oral anticoagulation after successful atrial fibrillation catheter ablation: systematic review and meta-analysis. Clin Appl Thromb Hemost 2022;28:10760296221118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Liang JJ, Elafros MA, Mullen MT, Muser D, Hayashi T, Enriquez Aet al. Anticoagulation use and clinical outcomes after catheter ablation in patients with persistent and longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:823–32. [DOI] [PubMed] [Google Scholar]
- 177. Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RLet al. Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation 2009;120:1174–80. [DOI] [PubMed] [Google Scholar]
- 178. Lip GY, Proclemer A, Dagres N, Bongiorni MG, Lewalter T, Blomstrom-Lundqvist C. Periprocedural anticoagulation therapy for devices and atrial fibrillation ablation. Europace 2012;14:741–4. [DOI] [PubMed] [Google Scholar]
- 179. Chen J, Todd DM, Hocini M, Larsen TB, Bongiorni MG, Blomstrom-Lundqvist C. Current periprocedural management of ablation for atrial fibrillation in Europe: results of the European Heart Rhythm Association survey. Europace 2014;16:378–81. [DOI] [PubMed] [Google Scholar]
- 180. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes Aet al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (discern af): a prospective, multicenter study. JAMA Intern Med 2013;173:149–56. [DOI] [PubMed] [Google Scholar]
- 181. Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li J-H, Carbucicchio Cet al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 2005;112:307–13. [DOI] [PubMed] [Google Scholar]
- 182. Pontoppidan J, Cosedis-Nielsen J, Hvitfeldt Poulsen S, Steen Hansen P. Symptomatic and asymptomatic atrial fibrillation after pulmonary vein ablation and the impact on quality of life. Pacing Clin Electrophysiol 2009;32:717–26. [DOI] [PubMed] [Google Scholar]
- 183. Gaita F, Scaglione M, Battaglia A, Matta M, Gallo C, Galatà Met al. Very long-term outcome following transcatheter ablation of atrial fibrillation. Are results maintained after 10 years of follow up? Europace 2018;20:443–50. [DOI] [PubMed] [Google Scholar]
- 184. Tilz RR, Heeger C-H, Wick A, Saguner AM, Metzner A, Rillig Aet al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the Hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2018;11:e005250. [DOI] [PubMed] [Google Scholar]
- 185. Bertaglia E, Senatore G, De Michieli L, De Simone A, Amellone C, Ferretto Set al. Twelve-year follow-up of catheter ablation for atrial fibrillation: a prospective, multicenter, randomized study. Heart Rhythm 2017;14:486–92. [DOI] [PubMed] [Google Scholar]
- 186. De With RR, Marcos EG, Dudink E, Spronk HM, Crijns H, Rienstra Met al. Atrial fibrillation progression risk factors and associated cardiovascular outcome in well-phenotyped patients: data from the AF-RISK study. Europace 2020;22:352–60. [DOI] [PubMed] [Google Scholar]
- 187. Zhang Y-Y, Qiu C, Davis PJ, Jhaveri M, Prystowsky EN, Kowey Pet al. Predictors of progression of recently diagnosed atrial fibrillation in REgistry on cardiac rhythm DisORDers assessing the control of atrial fibrillation (RecordAF); United States cohort. Am J Cardiol 2013;112:79–84. [DOI] [PubMed] [Google Scholar]
- 188. Kuck KH, Lebedev DS, Mikhaylov EN, Romanov A, Gellér L, Kalējs Oet al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021;23:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders Pet al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 190. Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SHet al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol 2018;3:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Chew DS, Li Z, Steinberg BA, O'Brien EC, Pritchard J, Bunch TJet al. Arrhythmic burden and the risk of cardiovascular outcomes in patients with paroxysmal atrial fibrillation and cardiac implanted electronic devices. Circ Arrhythm Electrophysiol 2022;15:e010304. [DOI] [PubMed] [Google Scholar]
- 192. Van Gelder IC, Healey JS, Crijns HJGM, Wang J, Hohnloser SH, Gold MRet al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–44. [DOI] [PubMed] [Google Scholar]
- 193. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation 2019;140:1639–46. [DOI] [PubMed] [Google Scholar]
- 194. Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck Get al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation—the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 196. Aguilar M, Macle L, Deyell MW, Yao R, Hawkins NM, Khairy Pet al. Influence of monitoring strategy on assessment of ablation success and postablation atrial fibrillation burden assessment: implications for practice and clinical trial design. Circulation 2022;145:21–30. [DOI] [PubMed] [Google Scholar]
- 197. Charitos EI, Ziegler PD, Stierle U, Robinson DR, Graf B, Sievers HHet al. Atrial fibrillation burden estimates derived from intermittent rhythm monitoring are unreliable estimates of the true atrial fibrillation burden. Pacing Clin Electrophysiol 2014;37:1210–8. [DOI] [PubMed] [Google Scholar]
- 198. Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler Jet al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8:1416–23. [DOI] [PubMed] [Google Scholar]
- 199. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto Cet al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 200. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker Cet al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]
- 201. Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak Ret al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the atrial diagnostics ancillary study of the mode selection trial (MOST). Circulation 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]
- 202. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci Aet al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 203. Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CTet al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol 2015;8:1040–7. [DOI] [PubMed] [Google Scholar]
- 204. Chao T-F, Liao J-N, Tuan T-C, Lin Y-J, Chang S-L, Lo L-Wet al. Incident co-morbidities in patients with atrial fibrillation initially with a CHA2DS2-VASc score of 0 (males) or 1 (females): implications for reassessment of stroke risk in initially ‘low-risk’ patients. Thromb Haemost 2019;119:1162–70. [DOI] [PubMed] [Google Scholar]
- 205. Waks JW, Passman RS, Matos J, Reynolds M, Thosani A, Mela Tet al. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: results from the tailored anticoagulation for non-continuous atrial fibrillation (TACTIC-AF) pilot study. Heart Rhythm 2018;15:1601–7. [DOI] [PubMed] [Google Scholar]
- 206. Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein Ret al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the rhythm evaluation for anticoagulation with continuous monitoring (REACT.COM) pilot study. J Cardiovasc Electrophysiol 2016;27:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Schrickel JW, Linhart M, Bansch D, Thomas D, Nickenig G. Rationale and design of the ODIn-AF trial: randomized evaluation of the prevention of silent cerebral thromboembolism by oral anticoagulation with dabigatran after pulmonary vein isolation for atrial fibrillation. Clin Res Cardiol 2016;105:95–105. [DOI] [PubMed] [Google Scholar]
- 208. Verma A, Ha ACT, Kirchhof P, Hindricks G, Healey JS, Hill MDet al. The optimal anti-coagulation for enhanced-risk patients post-catheter ablation for atrial fibrillation (OCEAN) trial. Am Heart J 2018;197:124–32. [DOI] [PubMed] [Google Scholar]
- 209. Wazni OM, Boersma L, Healey JS, Mansour M, Tondo C, Phillips Ket al. Comparison of anticoagulation with left atrial appendage closure after atrial fibrillation ablation: rationale and design of the OPTION randomized trial. Am Heart J 2022;251:35–42. [DOI] [PubMed] [Google Scholar]
- 210. Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis Get al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol 2019;73:989–99. [DOI] [PubMed] [Google Scholar]
- 211. Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke 2011;42:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Melkas S, Sibolt G, Oksala NKJ, Putaala J, Pohjasvaara T, Kaste Met al. Extensive white matter changes predict stroke recurrence up to 5 years after a first-ever ischemic stroke. Cerebrovascular Diseases 2012;34:191–8. [DOI] [PubMed] [Google Scholar]
- 213. Oksala NKJ, Oksala A, Pohjasvaara T, Vataja R, Kaste M, Karhunen PJet al. Age related white matter changes predict stroke death in long term follow-up. J Neurol Neurosurg Psychiatry 2009;80:762–6. [DOI] [PubMed] [Google Scholar]
- 214. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chen YC, Voskoboinik A, Gerche A, Marwick TH, McMullen JR. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 2021;77:2846–64. [DOI] [PubMed] [Google Scholar]
- 216. Dong XJ, Wang BB, Hou FF, Jiao Y, Li HW, Lv SPet al. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace 2023;25:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Proietti M, Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Roldan Rabadan I, Muniz Jet al. Relationship between multimorbidity and outcomes in atrial fibrillation. Exp Gerontol 2021;153:111482. [DOI] [PubMed] [Google Scholar]
- 218. Proietti M, Marzona I, Vannini T, Tettamanti M, Fortino I, Merlino Let al. Long-term relationship between atrial fibrillation, multimorbidity and oral anticoagulant drug use. Mayo Clin Proc 2019;94:2427–36. [DOI] [PubMed] [Google Scholar]
- 219. Romiti GF, Proietti M, Vitolo M, Bonini N, Fawzy AM, Ding WYet al. Clinical complexity and impact of the ABC (atrial fibrillation better care) pathway in patients with atrial fibrillation: a report from the ESC-EHRA EURObservational research programme in AF general long-term registry. BMC Med 2022;20:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Proietti M, Vitolo M, Harrison SL, Lane DA, Fauchier L, Marin Fet al. Impact of clinical phenotypes on management and outcomes in European atrial fibrillation patients: a report from the ESC-EHRA EURObservational research programme in AF (EORP-AF) general long-term registry. BMC Med 2021;19:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Lee SR, Choi EK, Park SH, Lee SW, Han KD, Oh Set al. Clustering of unhealthy lifestyle and the risk of adverse events in patients with atrial fibrillation. Front Cardiovasc Med 2022;9:885016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Jani BD, Nicholl BI, McQueenie R, Connelly DT, Hanlon P, Gallacher KIet al. Multimorbidity and co-morbidity in atrial fibrillation and effects on survival: findings from UK biobank cohort. Europace 2018;20:f329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Chowdhury SR, Chandra Das D, Sunna TC, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine 2023;57:101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Koziel M, Simovic S, Pavlovic N, Kocijancic A, Paparisto V, Music Let al. Impact of multimorbidity and polypharmacy on the management of patients with atrial fibrillation: insights from the BALKAN-AF survey. Ann Med 2021;53:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Wu J, Nadarajah R, Nakao YM, Nakao K, Wilkinson C, Mamas MAet al. Temporal trends and patterns in atrial fibrillation incidence: a population-based study of 3.4 million individuals. Lancet Reg Health Eur 2022;17:100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JAet al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation 2020;141:e750–72. [DOI] [PubMed] [Google Scholar]
- 227. Liatakis I, Manta E, Tsioufis C. Hypertension and atrial fibrillation: epidemiological data, pathogenesis, and therapeutic implications. Am J Hypertens 2019;32:725–6. [DOI] [PubMed] [Google Scholar]
- 228. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639–54. [DOI] [PubMed] [Google Scholar]
- 229. Dzeshka MS, Shahid F, Shantsila A, Lip GYH. Hypertension and atrial fibrillation: an intimate association of epidemiology, pathophysiology, and outcomes. Am J Hypertens 2017;30:733–55. [DOI] [PubMed] [Google Scholar]
- 230. Lee SR, Park CS, Choi EK, Ahn HJ, Han KD, Oh Set al. Hypertension burden and the risk of new-onset atrial fibrillation: a nationwide population-based study. Hypertension 2021;77:919–28. [DOI] [PubMed] [Google Scholar]
- 231. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJet al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 232. Potpara TS, Stankovic GR, Beleslin BD, Polovina MM, Marinkovic JM, Ostojic MCet al. A 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation: implications of arrhythmia progression on prognosis: the Belgrade atrial fibrillation study. Chest 2012;141:339–47. [DOI] [PubMed] [Google Scholar]
- 233. Aspberg S, Chang Y, Atterman A, Bottai M, Go AS, Singer DE. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J 2016;37:3203–10. [DOI] [PubMed] [Google Scholar]
- 234. Ishii M, Ogawa H, Unoki T, An Y, Iguchi M, Masunaga Net al. Relationship of hypertension and systolic blood pressure with the risk of stroke or bleeding in patients with atrial fibrillation: the Fushimi AF registry. Am J Hypertens 2017;30:1073–82. [DOI] [PubMed] [Google Scholar]
- 235. Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JBet al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986–92. [DOI] [PubMed] [Google Scholar]
- 236. Harskamp RE, Lucassen WAM, Lopes RD, Himmelreich JCL, Parati G, Weert H. Risk of stroke and bleeding in relation to hypertension in anticoagulated patients with atrial fibrillation: a meta-analysis of randomised controlled trials. Acta Cardiol 2022;77:191–5. [DOI] [PubMed] [Google Scholar]
- 237. Parkash R, Wells GA, Sapp JL, Healey JS, Tardif JC, Greiss Iet al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation: a randomized, open-label clinical trial (SMAC-AF [substrate modification with aggressive blood pressure control]). Circulation 2017;135:1788–98. [DOI] [PubMed] [Google Scholar]
- 238. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov Aet al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–70. [DOI] [PubMed] [Google Scholar]
- 239. Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin Eet al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 2020;323:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade atrial fibrillation study. Int J Cardiol 2013;168:4744–9. [DOI] [PubMed] [Google Scholar]
- 242. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio Eet al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Chao TF, Leu HB, Huang CC, Chen JW, Chan WL, Lin SJet al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol 2012;156:199–202. [DOI] [PubMed] [Google Scholar]
- 244. Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CFet al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol 2014;13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Anselmino M, Matta M, D'Ascenzo F, Pappone C, Santinelli V, Bunch TJet al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace 2015;17:1518–25. [DOI] [PubMed] [Google Scholar]
- 246. Donnellan E, Aagaard P, Kanj M, Jaber W, Elshazly M, Hoosien Met al. Association between pre-ablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
- 247. Deshmukh A, Ghannam M, Liang J, Saeed M, Cunnane R, Ghanbari Het al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:1232–9. [DOI] [PubMed] [Google Scholar]
- 248. Chatterjee NA, Giulianini F, Geelhoed B, Lunetta KL, Misialek JR, Niemeijer MNet al. Genetic obesity and the risk of atrial fibrillation: causal estimates from Mendelian randomization. Circulation 2017;135:741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RSet al. Obesity and the risk of new-onset atrial fibrillation. Jama 2004;292:2471–7. [DOI] [PubMed] [Google Scholar]
- 250. Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp Met al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol 2015;1:139–52. [DOI] [PubMed] [Google Scholar]
- 251. Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DHet al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9:e004378. [DOI] [PubMed] [Google Scholar]
- 252. Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GCet al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J 2008;29:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Guglin M, Maradia K, Chen R, Curtis AB. Relation of obesity to recurrence rate and burden of atrial fibrillation. Am J Cardiol 2011;107:579–82. [DOI] [PubMed] [Google Scholar]
- 254. Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr, Dublin S, Jensen PNet al. Association of body mass index, diabetes, hypertension, and blood pressure levels with risk of permanent atrial fibrillation. J Gen Intern Med 2013;28:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey Det al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 256. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CXet al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 257. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan Ret al. PREVEntion and regReSsive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 258. Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CEet al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the look AHEAD randomized trial. Am Heart J 2015;170:770–7 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Youssef I, Kamran H, Yacoub M, Patel N, Goulbourne C, Kumar Set al. Obstructive sleep apnea as a risk factor for atrial fibrillation: a meta-analysis. J Sleep Disord Ther 2018;7:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Cadby G, McArdle N, Briffa T, Hillman DR, Simpson L, Knuiman Met al. Severity of OSA is an independent predictor of incident atrial fibrillation hospitalization in a large sleep-clinic cohort. Chest 2015;148:945–52. [DOI] [PubMed] [Google Scholar]
- 261. Monahan K, Brewster J, Wang L, Parvez B, Goyal S, Roden DMet al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol 2012;110:369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ng CY, Liu T, Shehata M, Stevens S, Chugh SS, Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am J Cardiol 2011;108:47–51. [DOI] [PubMed] [Google Scholar]
- 263. Qureshi WT, Nasir UB, Alqalyoobi S, O'Neal WT, Mawri S, Sabbagh Set al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol 2015;116:1767–73. [DOI] [PubMed] [Google Scholar]
- 264. Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KVet al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–94. [DOI] [PubMed] [Google Scholar]
- 265. Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GCet al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J 2015;169:647–54 e2. [DOI] [PubMed] [Google Scholar]
- 266. Hunt TE, Traaen GM, Aakeroy L, Bendz C, Overland B, Akre Het al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm 2022;19:1433–41. [DOI] [PubMed] [Google Scholar]
- 267. Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol 2019;278:133–6. [DOI] [PubMed] [Google Scholar]
- 268. Traaen GM, Aakeroy L, Hunt TE, Overland B, Bendz C, Sande LOet al. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2021;204:573–82. [DOI] [PubMed] [Google Scholar]
- 269. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008;118:800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes 2011;4:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LYet al. Physical activity, obesity, weight change, and risk of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol 2014;7:620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart 2015;101:1627–30. [DOI] [PubMed] [Google Scholar]
- 273. Azarbal F, Stefanick ML, Salmoirago-Blotcher E, Manson JE, Albert CM, LaMonte MJet al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc 2014;3:e001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Faselis C, Kokkinos P, Tsimploulis A, Pittaras A, Myers J, Lavie CJet al. Exercise capacity and atrial fibrillation risk in veterans: a cohort study. Mayo Clin Proc 2016;91:558–66. [DOI] [PubMed] [Google Scholar]
- 275. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan Ret al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 276. Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace 2009;11:1156–9. [DOI] [PubMed] [Google Scholar]
- 277. Andersen K, Farahmand B, Ahlbom A, Held C, Ljunghall S, Michaelsson Ket al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 2013;34:3624–31. [DOI] [PubMed] [Google Scholar]
- 278. Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko Jet al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA my heart study. J Am Coll Cardiol 2013;61:1177–82. [DOI] [PubMed] [Google Scholar]
- 279. Hegbom F, Stavem K, Sire S, Heldal M, Orning OM, Gjesdal K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol 2007;116:86–92. [DOI] [PubMed] [Google Scholar]
- 280. Osbak PS, Mourier M, Kjaer A, Henriksen JH, Kofoed KF, Jensen GB. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J 2011;162:1080–7. [DOI] [PubMed] [Google Scholar]
- 281. Kato M, Ogano M, Mori Y, Kochi K, Morimoto D, Kito Ket al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: a randomized controlled clinical trial. Eur J Prev Cardiol 2019;26:1931–40. [DOI] [PubMed] [Google Scholar]
- 282. Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll Oet al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation 2016;133:466–73. [DOI] [PubMed] [Google Scholar]
- 283. Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation—a randomized controlled trial. PLoS One 2017;12:e0170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MGet al. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016;5:e004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol 2014;64:281–9. [DOI] [PubMed] [Google Scholar]
- 286. Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp MEet al. Alcohol and incident atrial fibrillation—A systematic review and meta-analysis. Int J Cardiol 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 287. Gemes K, Malmo V, Laugsand LE, Loennechen JP, Ellekjaer H, Laszlo KDet al. Does moderate drinking increase the risk of atrial fibrillation? The Norwegian HUNT (Nord-Trondelag Health) study. J Am Heart Assoc 2017;6:e007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara Set al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–8. [DOI] [PubMed] [Google Scholar]
- 289. Cheng M, Hu Z, Lu X, Huang J, Gu D. Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies. Can J Cardiol 2014;30:448–54. [DOI] [PubMed] [Google Scholar]
- 290. Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart 2013;99:1383–9. [DOI] [PubMed] [Google Scholar]
- 291. Abdelfattah R, Kamran H, Lazar J, Kassotis J. Does caffeine consumption increase the risk of new-onset atrial fibrillation? Cardiology 2018;140:106–14. [DOI] [PubMed] [Google Scholar]
- 292. Mattioli AV, Bonatti S, Zennaro M, Mattioli G. The relationship between personality, socio-economic factors, acute life stress and the development, spontaneous conversion and recurrences of acute lone atrial fibrillation. Europace 2005;7:211–20. [DOI] [PubMed] [Google Scholar]
- 293. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose Met al. Smoking and incidence of atrial fibrillation: results from the atherosclerosis risk in communities (ARIC) study. Heart Rhythm 2011;8:1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: a meta-analysis of prospective studies. Int J Cardiol 2016;218:259–66. [DOI] [PubMed] [Google Scholar]
- 295. Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol 2018;25:1437–51. [DOI] [PubMed] [Google Scholar]
- 296. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Yet al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J Cardiovasc Electrophysiol 2018;29:699–706. [DOI] [PubMed] [Google Scholar]
- 297. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp MEet al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. Jama 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 298. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brugemann Jet al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J 2018;39:2987–96. [DOI] [PubMed] [Google Scholar]
- 299. Gessler N, Willems S, Steven D, Aberle J, Akbulak RO, Gosau Net al. Supervised obesity reduction trial for AF ablation patients: results from the SORT-AF trial. Europace 2021;23:1548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters Ret al. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. Routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J 2012;33:2692–9. [DOI] [PubMed] [Google Scholar]
- 301. Angaran P, Mariano Z, Dragan V, Zou L, Atzema CL, Mangat Iet al. The atrial fibrillation therapies after ER visit: outpatient care for patients with acute AF—the AFTER3 study. J Atr Fibrillation 2015;7:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Nguyen BO, Crijns H, Tijssen JGP, Geelhoed B, Hobbelt AH, Hemels MEWet al. Long-term outcome of targeted therapy of underlying conditions in patients with early persistent atrial fibrillation and heart failure: data of the RACE 3 trial. Europace 2022;24:910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Lip GYH, Tran G, Genaidy A, Marroquin P, Estes C. Revisiting the dynamic risk profile of cardiovascular/non-cardiovascular multimorbidity in incident atrial fibrillation patients and five cardiovascular/non-cardiovascular outcomes: a machine-learning approach. J Arrhythm 2021;37:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Heidbuchel H, Van Gelder IC, Desteghe L; EHRA-PATHS Investigators . ESC And EHRA lead a path towards integrated care for multimorbid atrial fibrillation patients: the horizon 2020 EHRA-PATHS project. Eur Heart J 2022;43:1450–2. [DOI] [PubMed] [Google Scholar]
- 305. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura Tet al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- 306. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose Pet al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- 307. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran Ret al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- 308. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo Get al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335–43. [DOI] [PubMed] [Google Scholar]
- 309. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx Pet al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67. [DOI] [PubMed] [Google Scholar]
- 310. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CPet al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol 2019;4:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Gargiulo G, Cannon CP, Gibson CM, Goette A, Lopes RD, Oldgren Jet al. Safety and efficacy of double vs. triple antithrombotic therapy in patients with atrial fibrillation with or without acute coronary syndrome undergoing percutaneous coronary intervention: a collaborative meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J Cardiovasc Pharmacother 2021;7:f50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Capodanno D, Di Maio M, Greco A, Bhatt DL, Gibson CM, Goette Aet al. Safety and efficacy of double antithrombotic therapy with non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. De Caterina R, Agewall S, Andreotti F, Angiolillo DJ, Bhatt DL, Byrne RAet al. Great debate: triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting should be limited to 1 week. Eur Heart J 2022;43:3512–27. [DOI] [PubMed] [Google Scholar]
- 314. Goette A, Eckardt L, Valgimigli M, Lewalter T, Laeis P, Reimitz PEet al. Clinical risk predictors in atrial fibrillation patients following successful coronary stenting: ENTRUST-AF PCI sub-analysis. Clin Res Cardiol 2021;110:831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Goette A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Kuck KHet al. Presenting pattern of atrial fibrillation and outcomes of early rhythm control therapy. J Am Coll Cardiol 2022;80:283–95. [DOI] [PubMed] [Google Scholar]
- 316. Goette A, Lip GYH, Jin J, Heidbuchel H, Cohen AA, Ezekowitz Met al. Differences in thromboembolic complications between paroxysmal and persistent atrial fibrillation patients following electrical cardioversion (from the ENSURE-AF study). Am J Cardiol 2020;131:27–32. [DOI] [PubMed] [Google Scholar]
- 317. Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RFet al. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation 2020;141:781–3. [DOI] [PubMed] [Google Scholar]
- 318. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe Let al. The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 2018;20:1231–42. [DOI] [PubMed] [Google Scholar]
- 319. Goette A, Hammwohner M, Bukowska A, Scalera F, Martens-Lobenhoffer J, Dobrev Det al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int J Cardiol 2012;154:141–6. [DOI] [PubMed] [Google Scholar]
- 320. Zathar Z, Karunatilleke A, Fawzy AM, Lip GYH. Atrial fibrillation in older people: concepts and controversies. Front Med 2019;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Volgman AS, Nair G, Lyubarova R, Merchant FM, Mason P, Curtis ABet al. Management of atrial fibrillation in patients 75 years and older: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:166–79. [DOI] [PubMed] [Google Scholar]
- 322. Gebreyohannes EA, Salter S, Chalmers L, Bereznicki L, Lee K. Non-adherence to thromboprophylaxis guidelines in atrial fibrillation: a narrative review of the extent of and factors in guideline non-adherence. Am J Cardiovasc Drugs 2021;21:419–33. [DOI] [PubMed] [Google Scholar]
- 323. Ko D, Lin KJ, Bessette LG, Lee SB, Walkey AJ, Cheng Set al. Trends in use of oral anticoagulants in older adults with newly diagnosed atrial fibrillation, 2010–2020. JAMA Netw Open 2022;5:e2242964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Munir MB, Hlavacek P, Keshishian A, Guo JD, Mallampati R, Ferri Met al. Oral anticoagulant underutilization among elderly patients with atrial fibrillation: insights from the United States medicare database. J Interv Card Electrophysiol 2023;66:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Gallagher C, Nyfort-Hansen K, Rowett D, Wong CX, Middeldorp ME, Mahajan Ret al. Polypharmacy and health outcomes in atrial fibrillation: a systematic review and meta-analysis. Open Heart 2020;7:e001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Proietti M, Cesari M. Describing the relationship between atrial fibrillation and frailty: clinical implications and open research questions. Exp Gerontol 2021;152:111455. [DOI] [PubMed] [Google Scholar]
- 327. Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla Vecchia LAet al. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev 2022;79:101652. [DOI] [PubMed] [Google Scholar]
- 328. Diemberger I, Fumagalli S, Mazzone AM, Bakhai A, Reimitz PE, Pecen Let al. Perceived vs. objective frailty in patients with atrial fibrillation and impact on anticoagulant dosing: an ETNA-AF-Europe sub-analysis. Europace 2022;24:1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Dalgaard F, Xu H, Matsouaka RA, Russo AM, Curtis AB, Rasmussen PVet al. Management of atrial fibrillation in older patients by morbidity burden: insights from get with the guidelines-atrial fibrillation. J Am Heart Assoc 2020;9:e017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Rasmussen PV, Pallisgaard JL, Hansen ML, Gislason GH, Torp-Pedersen C, Ruwald Met al. Treatment of older patients with atrial fibrillation by morbidity burden. Eur Heart J Qual Care Clin Outcomes 2022;8:23–30. [DOI] [PubMed] [Google Scholar]
- 331. Mazzone A, Bo M, Lucenti A, Galimberti S, Bellelli G, Annoni G. The role of comprehensive geriatric assessment and functional status in evaluating the patterns of antithrombotic use among older people with atrial fibrillation. Arch Gerontol Geriatr 2016;65:248–54. [DOI] [PubMed] [Google Scholar]
- 332. Mongkhon P, Alwafi H, Fanning L, Lau WCY, Wei L, Kongkaew Cet al. Patterns and factors influencing oral anticoagulant prescription in people with atrial fibrillation and dementia: results from UK primary care. Br J Clin Pharmacol 2021;87:1056–68. [DOI] [PubMed] [Google Scholar]
- 333. Gugganig R, Aeschbacher S, Leong DP, Meyre P, Blum S, Coslovsky Met al. Frailty to predict unplanned hospitalization, stroke, bleeding, and death in atrial fibrillation. Eur Heart J Qual Care Clin Outcomes 2021;7:42–51. [DOI] [PubMed] [Google Scholar]
- 334. Campitelli MA, Bronskill SE, Huang A, Maclagan LC, Atzema CL, Hogan DBet al. Trends in anticoagulant use at nursing home admission and variation by frailty and chronic kidney disease among older adults with atrial fibrillation. Drugs Aging 2021;38:611–23. [DOI] [PubMed] [Google Scholar]
- 335. Wilkinson C, Clegg A, Todd O, Rockwood K, Yadegarfar ME, Gale CPet al. Atrial fibrillation and oral anticoagulation in older people with frailty: a nationwide primary care electronic health records cohort study. Age Ageing 2021;50:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Proietti M, Romiti GF, Vitolo M, Harrison SL, Lane DA, Fauchier Let al. Epidemiology and impact of frailty in patients with atrial fibrillation in Europe. Age Ageing 2022;51:afac192. [DOI] [PubMed] [Google Scholar]
- 337. Alexander KP, Brouwer MA, Mulder H, Vinereanu D, Lopes RD, Proietti Met al. Outcomes of apixaban versus warfarin in patients with atrial fibrillation and multi-morbidity: insights from the ARISTOTLE trial. Am Heart J 2019;208:123–31. [DOI] [PubMed] [Google Scholar]
- 338. Nicolau AM, Corbalan R, Nicolau JC, Ruff CT, Zierhut W, Kerschnitzki Met al. Efficacy and safety of edoxaban compared with warfarin according to the burden of diseases in patients with atrial fibrillation: insights from the ENGAGE AF-TIMI 48 trial. Eur Heart J Cardiovasc Pharmacother 2020;6:167–75. [DOI] [PubMed] [Google Scholar]
- 339. Deitelzweig S, Keshishian A, Kang A, Dhamane AD, Luo X, Klem Cet al. Use of non-vitamin K antagonist oral anticoagulants among patients with nonvalvular atrial fibrillation and multimorbidity. Adv Ther 2021;38:3166–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Dhamane AD, Ferri M, Keshishian A, Russ C, Atreja N, Gutierrez Cet al. Effectiveness and safety of direct oral anticoagulants among patients with non-valvular atrial fibrillation and multimorbidity. Adv Ther 2023;40:887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Lip GYH, Keshishian A, Kang A, Dhamane AD, Luo X, Klem Cet al. Effectiveness and safety of oral anticoagulants among non-valvular atrial fibrillation patients with polypharmacy. Eur Heart J Cardiovasc Pharmacother 2021;7:405–14. [DOI] [PubMed] [Google Scholar]
- 342. Zheng Y, Li S, Liu X, Lip GYH, Guo L, Zhu W. Effect of oral anticoagulants in atrial fibrillation patients with polypharmacy: a meta-analysis. Thromb Haemost (EPUB ahead of print: 2023 Jul 3). [DOI] [PubMed] [Google Scholar]
- 343. Grymonprez M, Petrovic M, De Backer TL, Steurbaut S, Lahousse L. The impact of polypharmacy on the effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Thromb Haemost (EPUB ahead of print: 2023 Jun 27). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Chen N, Alam AB, Lutsey PL, MacLehose RF, Claxton JS, Chen LYet al. Polypharmacy, adverse outcomes, and treatment effectiveness in patients ≥ 75 with atrial fibrillation. J Am Heart Assoc 2020;9:e015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Mentias A, Heller E, Vaughan Sarrazin M. Comparative effectiveness of rivaroxaban, apixaban, and warfarin in atrial fibrillation patients with polypharmacy. Stroke 2020;5:2076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Proietti M, Camera M, Gallieni M, Gianturco L, Gidaro A, Piemontese Cet al. Use and prescription of direct oral anticoagulants in older and frail patients with atrial fibrillation: a multidisciplinary consensus document. J Pers Med 2022;12:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Grymonprez M, Steurbaut S, De Backer TL, Petrovic M, Lahousse L. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. Front Pharmacol 2020;11:583311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Wilkinson C, Wu J, Clegg A, Nadarajah R, Rockwood K, Todd Oet al. Impact of oral anticoagulation on the association between frailty and clinical outcomes in people with atrial fibrillation: nationwide primary care records on treatment analysis. Europace 2022;24:1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Wilkinson C, Wu J, Searle SD, Todd O, Hall M, Kunadian Vet al. Clinical outcomes in patients with atrial fibrillation and frailty: insights from the ENGAGE AF-TIMI 48 trial. BMC Med 2020;18:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Grymonprez M, Petrovic M, De Backer TL, Steurbaut S, Lahousse L. Impact of frailty on the effectiveness and safety of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with atrial fibrillation: a nationwide cohort study. Eur Heart J Qual Care Clin Outcomes qcad019. (EPUB ahead of print: 2023 Mar 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Lip GYH, Keshishian AV, Kang AL, Dhamane AD, Luo X, Li Xet al. Oral anticoagulants for nonvalvular atrial fibrillation in frail elderly patients: insights from the ARISTOPHANES study. J Intern Med 2021;289:42–52. [DOI] [PubMed] [Google Scholar]
- 352. Kim DH, Pawar A, Gagne JJ, Bessette LG, Lee H, Glynn RJet al. Frailty and clinical outcomes of direct oral anticoagulants versus warfarin in older adults with atrial fibrillation: a cohort study. Ann Intern Med 2021;174:1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Freedman B, Boriani G, Glotzer TV, Healey JS, Kirchhof P, Potpara TS. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol 2017;14:701–14. [DOI] [PubMed] [Google Scholar]
- 354. Imberti JF, Bonini N, Tosetti A, Mei DA, Gerra L, Malavasi VLet al. Atrial high-rate episodes detected by cardiac implantable electronic devices: dynamic changes in episodes and predictors of incident atrial fibrillation. Biology (Basel) 2022;11:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Miyazawa K, Pastori D, Martin DT, Choucair WK, Halperin JL, Lip GYHet al. Characteristics of patients with atrial high rate episodes detected by implanted defibrillator and resynchronization devices. Europace 2022;24:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Proietti M, Romiti GF, Vitolo M, Borgi M, Rocco AD, Farcomeni Aet al. Epidemiology of subclinical atrial fibrillation in patients with cardiac implantable electronic devices: a systematic review and meta-regression. Eur J Intern Med 2022;103:84–94. [DOI] [PubMed] [Google Scholar]
- 357. Boriani G, Diemberger I, Ziacchi M, Valzania C, Gardini B, Cimaglia Pet al. AF Burden is important—fact or fiction? Int J Clin Pract 2014;68:444–52. [DOI] [PubMed] [Google Scholar]
- 358. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi Met al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (stroke preventiOn strategies based on atrial fibrillation information from implanted devices). Eur Heart J 2014;35:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Imberti JF, Mei DA, Vitolo M, Bonini N, Proietti M, Potpara Tet al. Comparing atrial fibrillation guidelines: focus on stroke prevention, bleeding risk assessment and oral anticoagulant recommendations. Eur J Intern Med 2022;101:1–7. [DOI] [PubMed] [Google Scholar]
- 360. Wolfes J, Ellermann C, Frommeyer G, Eckardt L. Evidence-based treatment of atrial fibrillation around the globe: comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev Cardiovasc Med 2022;23:56. [DOI] [PubMed] [Google Scholar]
- 361. Sgreccia D, Manicardi M, Malavasi VL, Vitolo M, Valenti AC, Proietti Met al. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: a systematic review and meta-analysis of 81,462 patients. J Clin Med 2021;10:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Boriani G, Vitolo M, Imberti JF, Potpara TS, Lip GYH. What do we do about atrial high rate episodes? Eur Heart J Suppl 2020;22:O42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Bertaglia E, Blank B, Blomström-Lundqvist C, Brandes A, Cabanelas N, Dan GAet al. Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Europace 2019;21:1459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Kitsiou A, Rogalewski A, Kalyani M, Deelawar S, Tribunyan S, Greeve Iet al. Atrial fibrillation in patients with embolic stroke of undetermined source during 3 years of prolonged monitoring with an implantable loop recorder. Thromb Haemost 2021;121:826–33. [DOI] [PubMed] [Google Scholar]
- 365. Vitolo M, Imberti JF, Maisano A, Albini A, Bonini N, Valenti ACet al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: a systematic review and meta-analysis. Eur J Intern Med 2021;92:100–6. [DOI] [PubMed] [Google Scholar]
- 366. Boriani G, Glotzer TV, Ziegler PD, De Melis M, Mangoni di S Stefano L, Sepsi Met al. Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018;15:376–83. [DOI] [PubMed] [Google Scholar]
- 367. Kalarus Z, Mairesse GH, Sokal A, Boriani G, Średniawa B, Casado-Arroyo Ret al. Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. Europace 2023;25:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Proietti M. Natural history of ‘silent’ atrial fibrillation from subclinical to asymptomatic: state of the art and need for research. Eur J Intern Med 2023;107:27–9. [DOI] [PubMed] [Google Scholar]
- 369. Savelieva I, Fumagalli S, Kenny RA, Anker S, Benetos A, Boriani Get al. EHRA Expert consensus document on the management of arrhythmias in frailty syndrome, endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace 2023;25:1249–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Boriani G, Pettorelli D. Atrial fibrillation burden and atrial fibrillation type: clinical significance and impact on the risk of stroke and decision making for long-term anticoagulation. Vascul Pharmacol 2016;83:26–35. [DOI] [PubMed] [Google Scholar]
- 371. Boriani G, Healey JS, Schnabel RB, Lopes RD, Calkins H, Camm JAet al. Oral anticoagulation for subclinical atrial tachyarrhythmias detected by implantable cardiac devices: an international survey of the AF-SCREEN group. Int J Cardiol 2019;296:65–70. [DOI] [PubMed] [Google Scholar]
- 372. Imberti JF, Tosetti A, Mei DA, Maisano A, Boriani G. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep 2021;23:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Ahmed FZ, Sammut-Powell C, Kwok CS, Tay T, Motwani M, Martin GPet al. Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2022;24:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Boriani G, Imberti JF, Bonini N, Carriere C, Mei DA, Zecchin Met al. Remote multiparametric monitoring and management of heart failure patients through cardiac implantable electronic devices. Eur J Intern Med S0953-6205(23)00122-X. (EPUB ahead of print: 2023 Apr 17) [DOI] [PubMed] [Google Scholar]
- 375. Wan D, Andrade J, Laksman Z. Thromboembolic risk stratification in atrial fibrillation-beyond clinical risk scores. Rev Cardiovasc Med 2021;22:353–63. [DOI] [PubMed] [Google Scholar]
- 376. Malavasi VL, Fantecchi E, Tordoni V, Melara L, Barbieri A, Vitolo Met al. Atrial fibrillation pattern and factors affecting the progression to permanent atrial fibrillation. Intern Emerg Med 2021;16:1131–40. [DOI] [PubMed] [Google Scholar]
- 377. Ding WY, Proietti M, Boriani G, Fauchier L, Blomström-Lundqvist C, Marin Fet al. Clinical utility and prognostic implications of the novel 4S-AF scheme to characterize and evaluate patients with atrial fibrillation: a report from ESC-EHRA EORP-AF long-term general registry. Europace 2022;24:721–8. [DOI] [PubMed] [Google Scholar]
- 378. Bonini N, Vitolo M, Imberti JF, Proietti M, Romiti GF, Boriani Get al. Mobile health technology in atrial fibrillation. Expert Rev Med Devices 2022;19:327–40. [DOI] [PubMed] [Google Scholar]
- 379. Corica B, Bonini N, Imberti JF, Romiti GF, Vitolo M, Attanasio Let al. Yield of diagnosis and risk of stroke with screening strategies for atrial fibrillation: a comprehensive review of current evidence. Eur Heart J Open 2023;3:oead031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Lowres N, Olivier J, Chao TF, Chen SA, Chen Y, Diederichsen Aet al. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med 2019;16:e1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Nemati S, Ghassemi MM, Ambai V, Isakadze N, Levantsevych O, Shah Aet al. Monitoring and detecting atrial fibrillation using wearable technology. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:3394–7. [DOI] [PubMed] [Google Scholar]
- 382. Yan BP, Lai WHS, Chan CKY, Chan SC, Chan LH, Lam KMet al. Contact-Free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018;7:e008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Brasier N, Raichle CJ, Dorr M, Becke A, Nohturfft V, Weber Set al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 2019;21:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Yet al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–75. [DOI] [PubMed] [Google Scholar]
- 385. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris Tet al. Apple heart study I. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Verbrugge FH, Proesmans T, Vijgen J, Mullens W, Rivero-Ayerza M, Van Herendael Het al. Atrial fibrillation screening with photo-plethysmography through a smartphone camera. Europace 2019;21:1167–75. [DOI] [PubMed] [Google Scholar]
- 387. Zhang H, Zhang J, Li HB, Chen YX, Yang B, Guo YTet al. Validation of single centre pre-mobile atrial fibrillation apps for continuous monitoring of atrial fibrillation in a real-world setting: pilot cohort study. J Med Internet Res 2019;21:e14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou Jet al. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm 2020;17:847–53. [DOI] [PubMed] [Google Scholar]
- 389. Lubitz SA, Faranesh AZ, Selvaggi C, Atlas SJ, McManus DD, Singer DEet al. Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation 2022;146:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Rizas KD, Freyer L, Sappler N, von Stulpnagel L, Spielbichler P, Krasniqi Aet al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 2022;28:1823–30. [DOI] [PubMed] [Google Scholar]
- 391. Gibson CM, Steinhubl S, Lakkireddy D, Turakhia MP, Passman R, Jones WSet al. Does early detection of atrial fibrillation reduce the risk of thromboembolic events? Rationale and design of the heartline study. Am Heart J 2023;259:30–41. [DOI] [PubMed] [Google Scholar]
- 392. Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann Jet al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN international collaboration. Circulation 2019;140:1834–50. [DOI] [PubMed] [Google Scholar]
- 393. Ungar A, Pescini F, Rafanelli M, De Angelis MV, Faustino M, Tomaselli Cet al. Detection of subclinical atrial fibrillation after cryptogenic stroke using implantable cardiac monitors. Eur J Intern Med 2021;92:86–93. [DOI] [PubMed] [Google Scholar]
- 394. Brandes A, Stavrakis S, Freedman B, Antoniou S, Boriani G, Camm AJet al. Consumer-led screening for atrial fibrillation: frontier review of the AF-SCREEN international collaboration. Circulation 2022;146:1461–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Koh KT, Law WC, Zaw WM, Foo DHP, Tan CT, Steven Aet al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicentre randomized controlled trial. Europace 2021;23:1016–23. [DOI] [PubMed] [Google Scholar]
- 396. Boriani G, Maisano A, Bonini N, Albini A, Imberti JF, Venturelli Aet al. Digital literacy as a potential barrier to implementation of cardiology tele-visits after COVID-19 pandemic: the INFO-COVID survey. J Geriatr Cardiol 2021;18:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Boriani G, Schnabel RB, Healey JS, Lopes RD, Verbiest-van Gurp N, Lobban Tet al. Consumer-led screening for atrial fibrillation using consumer-facing wearables, devices and apps: a survey of health care professionals by AF-SCREEN international collaboration. Eur J Intern Med 2020;82:97–104. [DOI] [PubMed] [Google Scholar]
- 398. Boriani G, Svennberg E, Guerra F, Linz D, Casado-Arroyo R, Malaczynska-Rajpold Ket al. Reimbursement practices for use of digital devices in atrial fibrillation and other arrhythmias: a European Heart Rhythm Association survey. Europace 2022;24:1834–43. [DOI] [PubMed] [Google Scholar]
- 399. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar Pet al. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Vitolo M, Ziveri V, Gozzi G, Busi C, Imberti JF, Bonini Net al. Digital health literacy after COVID-19 outbreak among frail and non-frail cardiology patients: the DIGI-COVID study. J Pers Med 2022;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk Ret al. Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. Europace euaa269. (EPUB ahead of print: 2020 Aug 27) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.