Abstract
Background
Lyme borreliosis (LB), caused by Borrelia burgdorferi (Bb), is the most common tick-borne infection in Germany. Antibodies against Bb are prevalent in the general population but information on temporal changes of prevalence and estimates of seroconversion (seroincidence) and seroreversion are lacking, especially for children and adolescents.
Aim
We aimed at assessing antibodies against Bb and factors associated with seropositivity in children and adolescents in Germany.
Methods
We estimated seroprevalence via two consecutive cross-sectional surveys (2003–2006 and 2014–2017). Based on a longitudinal survey component, we estimated annual seroconversion/seroreversion rates.
Results
Seroprevalence was 4.4% (95% confidence interval (CI): 3.9–4.9%) from 2003 to 2006 and 4.1% (95% CI: 3.2–5.1%) from 2014 to 2017. Seroprevalence increased with age, was higher in male children, the south-eastern regions of Germany and among those with a high socioeconomic status. The annual seroconversion rate was 0.3% and the annual seroreversion rate 3.9%. Males were more likely to seroconvert compared with females. Low antibody levels were the main predictor of seroreversion.
Conclusion
We did not detect a change in seroprevalence in children and adolescents in Germany over a period of 11 years. Potential long-term changes, for example due to climatic changes, need to be assessed in consecutive serosurveys. Seroconversion was more likely among children and adolescents than among adults, representing a target group for preventive measures. Seroreversion rates are over twice as high in children and adolescents compared with previous studies among adults. Thus, seroprevalence estimates and seroconversion rates in children are likely underestimated.
Keywords: Borrelia burgdorferi, antibodies, seroprevalence, Lyme disease, Lyme borreliosis, ticks, Germany
Key public health message.
What did you want to address in this study?
Tick-borne Lyme borreliosis is a widespread disease in Europe, caused by bacteria of Borrelia burgdorferi. We tested blood samples of children and adolescents from 2014 to 2017 in Germany for Borrelia burgdorferi-specific antibodies indicating a previous infection and compared our results to findings from 2003 to 2006. We wished to determine changes over time after a decade: new infections and loss of antibodies.
What have we learnt from this study?
We did not see a change in Bb antibodies 2003–2006 to 2014–2017 (4.4% and 4.1%). Infections with Borrelia burgdorferi were common throughout Germany, especially amongst males and in Southern Germany. In many, antibodies were no longer detectable when retested 11 years later.
What are the implications of your findings for public health?
As infections with Borrelia burgdorferi can lead to severe disease, our study has a significant public health relevance. This, as well as long-term trends, which may be affected by climatic changes, should be addressed in future studies. Furthermore, our study indicates that Borrelia burgdorferi infections continue to be common among children and adolescents and are likely underestimated, which underlines the need for prevention campaigns.
Introduction
Lyme borreliosis (LB) is a bacterial infection caused by spirochaetes belonging to the Borrelia burgdorferi sensu lato (Bb) genospecies complex. Lyme borreliosis is the most common tick-borne disease in Europe [1,2], and B. afzelii and B. garinii are the predominant species causing LB [3,4]. Borrelia are transmitted through bites of ticks of Ixodes species, in Germany mainly Ixodes ricinus.
Symptoms of LB may appear days to months, in rare cases even years, after the tick bite. The skin manifestation erythema migrans (EM) is by far the most common clinical form and occurs within 3 days up to several weeks after a bite [2,5,6]. More severe forms, such as acute neuroborreliosis (NB) or Lyme arthritis (LA), occur at a progressed stage [6-8]. Antibiotic treatment usually results in full recovery [3,6,8,9]. There is currently no approved vaccine for humans [1,6]. A previous infection with Bb does not provide reliable immunity and multiple courses of LB may occur [3,6]. Lyme borreliosis is mainly prevented by avoiding tick bites through individual protection measures, such as wearing long and light-coloured clothing, avoiding going through bushes or tick-infested areas, remaining on walkways when in nature or using repellents, as well as by correct early removal of ticks [1,10].
Yearly incidences of reported cases of LB per 100,000 inhabitants vary widely in Europe (from 0.5 in Ireland to 300 in Austria) [11] and also between the 214 German districts that report cases (0.5 to 138) [5]. In nine of the 16 German federal states, EM, NB and LA are mandatorily notifiable. Between 2013 and 2017, the LB incidence fluctuated between 26 and 41 cases per 100,000 inhabitants in Germany, without an apparent trend [5]. The incidence of reported LB cases follows a bimodal age distribution, peaking in children aged 5–9 years, especially in males, and in adults aged 60–69 years, especially in females [5]. The incidences of cranial nerve palsy and meningitis, two severe forms of LB, are higher in children than in adults and highest in the age group of 5–9 years compared with 20–29 years (incidence rate ratio (IRR) = 12.8 and IRR = 14.1) [5]. Furthermore, in 2019, physicians had diagnosed 128,177 LB cases in Germany using the International Classification of Diseases (ICD) code A69.2 [12], corresponding to an incidence of 179 per 100,000 inhabitants. The estimated incidence varied strongly between districts [13].
Seroprevalence estimates in population-based surveys provide more unbiased information about the exposure to Bb compared with surveillance data from notification systems, although prevalence of antibodies is not equivalent to clinical disease, as infections often are asymptomatic [4,14,15]. Consecutive serosurveys, preferably over a period of time, can help revealing trends and identifying risk groups. In Germany, a seroprevalence of 4% was measured in children in the period 2003 to 2006 [16] and 9% in adults in 1997 to 1999 and in 2008 to 2011 [17]. To assess the incidence of Bb infections in a population, however, repeated measurements of the same individuals are necessary to determine a seroconversion.
Estimates of seroprevalence and seroincidence are useful for prioritisation of public health interventions. They help to assess changes in the risk of acquiring Bb infections. Furthermore, seroprevalence estimates serve as a basis to account for pre-test probabilities of serological tests in the context of clinical diagnoses of LB in children and adolescents.
Here, we aimed at analysing and comparing the seroprevalence of Bb IgG antibodies among children and adolescents in Germany in two nationwide surveys conducted 11 years apart, identifying factors associated with seropositivity, seroconversion and seroreversion and estimating their annual rates based on longitudinal data of individuals.
Methods
Study procedures and description
The first cross-sectional survey (KiGGS Baseline) of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) of 0–17-year-olds residing in Germany was conducted from 2003 to 2006, the second survey (KiGGS Wave 2) from 2014 to 2017. In both studies, the enrolment was based on a two-step stratified, probability-clustered sampling approach. In the first step, a specified number of study locations was chosen, stratified by federal state and structural factors, with sampling probability proportional to 0–17-year-olds in the population. In the second step, a predefined number of participants per birth cohort, dependent on community size, was randomly selected within the study locations based on registry data. Comprehensive overviews of the study procedures, including sampling strategies, are available elsewhere [18].
A questionnaire study was included: the study participants or the parents of children 10 years or younger responded. There were questions on sociodemographic facts (sex, age, place of residence, population size of the place of residence, socioeconomic status (SES)), leisure time activities (media consumption, physical activity), animal contact (presence of pets in household) and migration background; we append a description of the variables and categorisation in the Supplement. Information on previous LB was not available. A physical examination and testing, including blood sampling, was done for children from the age of 1 year on in KiGGS Baseline and for a representative subset of children from the age of 3 years in KiGGS Wave 2.
To compare seroprevalence estimates of KiGGS Baseline [16] with KiGGS Wave 2, we used samples of 3–17-year-olds. Altogether, 17,640 children participated in KiGGS Baseline and 15,023 in the cross-sectional part of KiGGS Wave 2 (including children from whom blood samples were not available). Blood samples were available from 11,626 (65.9%) of the 17,640 KiGGS Baseline participants and from 2,891 (19.2%) of the 15,023 KiGGS Wave 2 participants.
Simultaneously to the conduct of the cross-sectional KiGGS Wave 2 survey, another blood sample was taken from former KiGGS Baseline participants, providing a longitudinal follow-up (KiGGS follow-up). We included 4,016 (37.0%) of the 10,853 KiGGS follow-up participants from whom blood samples were available to determine the Bb serostatus in both studies. In the Supplement, Figure S4 and S5 give an overview of survey and study participants.
Laboratory methods
Serum samples were tested for IgG at the National Reference Centre for Borrelia using same guidelines, assays and approach for all study groups (including the studies in adults [17]), which is also the standard for clinical diagnostics [6,8,19]. We used a two-step approach involving a screening with an enzyme-linked immunosorbent assay (ELISA) (Enzygnost Lyme link VlsE/IgG, Siemens Healthcare Diagnostics GmbH, Eschborn, Germany), followed by a confirmatory immunoblot (line blot) (Borrelia Europe plus TpN17 LINE, IgG, Virotech, Rüsselsheim, Germany) test in case of a positive or borderline ELISA result. The immunoblot test covered a range of specific Bb antigens and was considered positive if reactive to at least two bands. Potential cross-reactions were accounted for by including the antigen TpN17, testing a reaction specific to Treponema pallidum (causative agent of syphilis), the most relevant potential cross-reaction in Germany. More details on the test systems and test result categorisation are provided in the Supplement. Serum samples that tested either positive or borderline with the ELISA and positive with the immunoblot were considered seropositive. We refer to Supplementary Figure S1 for a scheme displaying this categorisation.
Statistical analyses
Seroprevalence and predictors of seropositivity
We used Pearson’s chi-squared test to test associations for categorical data and t-test for continuous data. We calculated two-sided p values and considered results statistically significant at a threshold of < 0.05. For all analyses, we used the statistical software R (version 4.2.1) [20]. Visualisations were realised using the R-package ggplot2. To improve representativeness, we adjusted for the clustered study design and applied study-specific weights [18,21], using the R-package survey. We assessed associations with seropositivity calculating odds ratios (OR) and 95% confidence intervals (CI), using univariable logistic regression. Variables for multivariable analyses were selected from available study variables. Relevant variables were identified by a priori selection based on biological and epidemiological plausibility, and on associations found in scientific literature. We refer to Supplementary Table S1 for a list of hypothesised causal relationships and to Supplementary Figure S2 for the corresponding directed acyclic graph (DAG), for which we used the webpage tool dagitty. To determine independent predictors of seropositivity, we estimated total effects of exposures of interest and based multivariable models on the DAG; detailed adjustment sets for seropositivity and seroconversion are appended in Supplementary Table S2. Total effects include both the direct effect of an exposure of interest and indirect effects through mediating factors, while adjusting for potential confounders. If no need for adjustment was indicated to test the effect of an individual variable on seropositivity based on the DAG, controlling for additional variables would possibly lead to over-adjustment, and the univariate analysis was considered sufficient. We estimated an age-standardised seroprevalence for KiGGS Wave 2, using the age distribution of participants in KiGGS Baseline, to account for potential effects of age on seroprevalence estimates due to changes in the age distribution of the German population between 2004 and 2015.
Seroconversion and seroreversion
We refer to seroconversion if participants tested seronegative in KiGGS Baseline and seropositive in KiGGS Wave 2. Seroreversion, on the other hand, was defined when a person tested seropositive in KiGGS Baseline and seronegative in KiGGS Wave 2. We refer to Supplementary Figure S5 B for a schematic overview. We assessed potential associations with seroconversion and seroreversion calculating ORs and 95% CIs, using univariable logistic regression models, respectively. For multivariable logistic regression, we included characteristics based on our a priori considerations, additionally including immune response at KiGGS Baseline in case of seroreversion. More details for both are appended to this manuscript in Supplementary Figures S2 and S3. Variables prone to change over time, such as pet ownership or leisure time activities, were only included on availability in KiGGS follow-up data.
Annual rates
The annual seroconversion (or seroincidence) rate between the two sampling points was calculated using paired samples, after stratifying by the number of years between sampling, using the following formula on each stratum: pconvyear = 1 − (1−pconvyearx)1/X, where pconvyear is the estimated probability of seroconverting per year and pconvyearx is the observed rate of seroconversion in the x years between the two time points of sampling. We assumed a constant rate of infection. The seroreversion rate was determined by dividing the total number of seroreversions by the average observation time of participants who tested seropositive in KiGGS Baseline and were resampled in KiGGS Wave 2.
Antigen reactivity
Reactivity to the individual antigens included in the immunoblot were read out; for details of the test systems, we refer to the Supplement. Immunogenicity of antigens varies by stage of infection [6,19]. For example, antibodies targeting OspC and VlsE seem to be associated with the early stages of LB, whereas DbpA, p83, p58, and p39 are associated with late stages of LB [3,6,9,19]. Reactions to a broader spectrum of antigens are indicative of an advanced stage of clinical LB at any point in life or even multiple courses of LB but may also be present after (multiple) asymptomatic infection(s) in some persons [6,19]. Thus, band patterns and the number of positive bands are associated with stages of infection [6,9,19]. Among seropositive KiGGS Baseline participants, we assessed if the individual and the cumulative antigen reactions were predictive of seroreversion in univariable logistic regression analysis. All individual antigen reactions were included in the multivariable regression model.
Results
Seroprevalence and seropositivity
Of the included 11,626 KiGGS Baseline participants, 511 were seropositive (4.4%, unweighted), and 131 among the 2,891 KiGGS Wave 2 participants (4.5%, unweighted). The overall weighted seroprevalence in children and adolescents aged 3–17 years in Germany was 4.4% (95% CI: 3.9–4.9%) in the period 2003 to 2006 and 4.1% (95% CI: 3.2–5.1%) in 2014 to 2017 (Table 1, Table 2). The age-standardised seroprevalence for KiGGS Wave 2, given the same population distribution as in the KiGGS Baseline, was 4.3%.
TABLE 1. Weighted prevalence of Borrelia burgdorferi-specific IgG antibodies by different characteristics and results of logistic regression analyses of potential determinants of seropositivity in a cross-sectional survey of children and adolescents (KiGGS Baseline), Germany, 2003–2006 (n = 11,626).
| Characteristics | Tested | Seropositive | Prevalence | Univariable analyses | Multivariable analysesa | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | OR | 95% CI | aOR | 95% CI | |||
| Sex | ||||||||
| Female | 5,658 | 212 | 3.51 | 2.93–4.08 | Reference | |||
| Male | 5,968 | 299 | 5.2 | 4.50–5.90 | 1.51 | 1.24–1.83 | No adjustment necessary | |
| Age group (years) | ||||||||
| 3–6 | 2,364 | 61 | 2.31 | 1.58–3.05 | Reference | |||
| 7–10 | 3,033 | 119 | 4.07 | 3.23–4.90 | 1.79 | 1.24–2.59 | No adjustment necessary | |
| 11–13 | 2,809 | 119 | 3.95 | 3.08–4.82 | 1.74 | 1.19–2.54 | ||
| 14–17 | 3,420 | 212 | 6.16 | 5.26–7.06 | 2.77 | 1.94–3.97 | ||
| Region of residenceb | ||||||||
| Baden-Württemberg | 1,347 | 56 | 4.09 | 2.72–5.45 | 1.13 | 0.70–1.81 | 1.11 | 0.69–1.81 |
| Bavaria | 1,474 | 105 | 6.97 | 5.39–8.54 | 1.98 | 1.32–2.97 | 1.94 | 1.28–2.93 |
| Central | 1,193 | 54 | 4.3 | 3.43–5.17 | 1.19 | 0.81–1.75 | 1.15 | 0.76–1.73 |
| North-west | 1,459 | 53 | 3.65 | 2.51–4.79 | Reference | |||
| North Rhine-Westphalia | 2,205 | 63 | 2.95 | 2.14–3.76 | 0.8 | 0.52–1.23 | 0.8 | 0.52–1.22 |
| East (north) | 2,219 | 94 | 4.44 | 3.41–5.48 | 1.23 | 0.82–1.84 | 1.2 | 0.80–1.81 |
| East (south) | 1,729 | 86 | 5.79 | 4.01–7.57 | 1.62 | 1.02–2.57 | 1.61 | 1.02–2.54 |
| Size of municipalityc | ||||||||
| Rural | 2,540 | 140 | 6.19 | 4.94–7.43 | 1.91 | 1.29–2.85 | 1.61 | 1.11–2.35 |
| Town | 5,480 | 262 | 4.8 | 4.06–5.55 | 1.46 | 1.01–2.12 | 1.35 | 0.96–1.91 |
| Medium-sized town | 2,598 | 78 | 2.69 | 2.01–3.37 | 0.8 | 0.52–1.22 | 0.84 | 0.57–1.23 |
| Metropolitan | 1,008 | 31 | 3.33 | 2.25–4.41 | Reference | |||
| Socioeconomic statusd (244 missing values) | ||||||||
| High | 2,755 | 155 | 5.46 | 4.45–6.47 | Reference | |||
| Medium | 6,856 | 294 | 4.39 | 3.80–4.99 | 0.8 | 0.64–0.99 | 0.85 | 0.68–1.07 |
| Low | 1,771 | 46 | 2.13 | 1.34–2.92 | 0.38 | 0.24–0.58 | 0.5 | 0.32–0.79 |
| Migration backgrounde (45 missing values) | ||||||||
| Yes | 1,751 | 25 | 1.38 | 0.82–1.94 | Reference | |||
| No | 9,830 | 483 | 5.01 | 4.47–5.55 | 3.76 | 2.53–5.59 | Not applicable | |
| Daily TV consumption (hours) (3,647 missing values) | ||||||||
| 0 | 507 | 33 | 5.88 | 3.71–8.04 | Reference | |||
| 0.5 | 2,701 | 113 | 3.95 | 3.00–4.89 | 0.66 | 0.41–1.04 | 0.63 | 0.39–1.03 |
| 1–2 | 4,122 | 140 | 3.32 | 2.67–3.96 | 0.55 | 0.36–0.84 | 0.5 | 0.31–0.80 |
| 3–4 | 557 | 10 | 1.49 | 0.40–2.57 | 0.24 | 0.10–0.56 | 0.26 | 0.11–0.61 |
| > 4 | 92 | 2 | 1.12 | 5.26–7.06 | 0.18 | 0.04–0.91 | 0.23 | 0.04–1.21 |
| TV consumption on weekends (h/day) (3,772 missing values) | ||||||||
| 0 | 245 | 19 | 7.55 | 3.94–11.16 | Reference | |||
| 0.5 | 1,016 | 39 | 3.49 | 2.22–4.77 | 0.44 | 0.23–0.85 | 0.41 | 0.22–0.79 |
| 1–2 | 4,193 | 156 | 3.5 | 2.82–4.18 | 0.44 | 0.26–0.77 | 0.35 | 0.19–0.62 |
| 3–4 | 2,001 | 67 | 3.35 | 2.38–4.33 | 0.42 | 0.23–0.80 | 0.32 | 0.16–0.62 |
| > 4 | 399 | 9 | 1.89 | 0.51–3.27 | 0.24 | 0.09–0.59 | 0.21 | 0.08–0.54 |
| Weekly media consumptionf (622 missing values) | ||||||||
| Low | 3,737 | 196 | 5.17 | 4.35–5.98 | Reference | |||
| Middle | 3,539 | 167 | 4.73 | 3.90–5.57 | 0.91 | 0.73–1.14 | 0.9 | 0.72–1.12 |
| High | 3,728 | 132 | 3.57 | 2.81–4.33 | 0.68 | 0.52–0.89 | 0.73 | 0.55–0.97 |
| Physical activity (only assessed in participants aged 11 years and older; 120 missing values) | ||||||||
| Never | 584 | 19 | 2.9 | 1.42–4.38 | Reference | |||
| Rarely | 320 | 16 | 5.39 | 2.64–8.13 | 1.91 | 0.87–4.19 | 1.48 | 0.64–3.38 |
| 1–2 times per week | 1,806 | 93 | 5.11 | 3.95–6.27 | 1.8 | 1.00–3.26 | 1.66 | 0.92–2.98 |
| 3–5 times per week | 1,933 | 120 | 6.19 | 5.02–7.37 | 2.21 | 1.29–3.79 | 2.1 | 1.24–3.55 |
| Almost every day | 1,466 | 78 | 5.34 | 4.02–6.67 | 1.89 | 1.09–3.28 | 1.83 | 1.07–3.13 |
| Current pet ownership (251 missing values) | ||||||||
| Yes | 5,655 | 286 | 5 | 4.33–5.66 | 1.34 | 1.09–1.63 | 1.21 | 0.94–1.57 |
| No | 5,720 | 215 | 3.78 | 3.16–4.41 | Reference | |||
| Cat as a pet (280 missing values) | ||||||||
| Yes | 2,220 | 140 | 6.48 | 5.33–7.62 | 1.72 | 1.37–2.15 | 1.37 | 1.00–1.86 |
| No | 9,126 | 359 | 3.88 | 3.35–4.40 | Reference | |||
| Dog as a pet (280 missing values) | ||||||||
| Yes | 1,865 | 89 | 4.9 | 3.85–5.96 | 1.16 | 0.91–1.47 | 0.89 | 0.62–1.29 |
| No | 9,481 | 410 | 4.27 | 3.74–4.79 | Reference | |||
| Small mammal as a pet (280 missing values) | ||||||||
| Yes | 1,700 | 86 | 4.87 | 3.75–5.98 | 1.14 | 0.87–1.49 | 1.22 | 0.86–1.73 |
| No | 9,646 | 413 | 4.29 | 3.75–4.84 | Reference | |||
aOR: adjusted odds ratio; CI: confidence interval; KiGGS: German Health Interview and Examination Survey for Children and Adolescents; OR: odds ratio.
a Multivariable regression models include adjustment variables according to the minimal sufficient adjustment sets listed in Supplementary Table S2.
b Central: Hesse, Rhineland-Palatinate and Saarland; North-west: Bremen, Hamburg, Lower Saxony and Schleswig-Holstein; East (north): Berlin, Brandenburg, Mecklenburg-Vorpommern and Saxony-Anhalt; East (south): Saxony and Thuringia.
c Size of municipality was classified as residential areas based on population sizes and categorized into rural (< 5,000), town (5,000– < 20,000), medium-sized town (20,000-– < 100,000) and metropolitan areas (≥ 100,000).
d Categorisation of socio-economic status was based on an index, which was calculated using information regarding education and occupational qualifications, occupational status and net equivalent income of the parents. More details are given in the Supplement.
e Participants were assigned a migration background if they had moved to Germany and at least one parent was born abroad, if both parents had moved to Germany or if neither parent had a German citizenship.
f Media consumption was categorised as high, middle or low based on an index. More details are given in the Supplement.
Entries in bold represent statistically significant findings.
TABLE 2. Weighted prevalence of Borrelia burgdorferi-specific IgG antibodies by different characteristics and results of logistic regression analyses of potential determinants of seropositivity in a cross-sectional survey of children and adolescents (KiGGS Wave 2), Germany, 2014–2017 (n = 2,891).
| Characteristics | Tested | Seropositive | Prevalence | Univariable analyses | Multivariable analysesa | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | OR | 95% CI | aOR | 95% CI | |||
| Sex | ||||||||
| Female | 1,472 | 49 | 2.78 | 1.87–3.70 | Reference | No adjustment necessary | ||
| Male | 1,419 | 82 | 5.44 | 3.82–7.06 | 2.01 | 1.28–3.15 | ||
| Age group (years) | ||||||||
| 3–6 | 567 | 11 | 1.35 | 0.18–2.51 | Reference | No adjustment necessary | ||
| 7–10 | 711 | 25 | 3.5 | 1.81–5.19 | 2.66 | 0.97–7.30 | ||
| 11–13 | 721 | 40 | 4.29 | 2.72–5.86 | 3.28 | 1.26–8.60 | ||
| 14–17 | 892 | 55 | 6.81 | 4.56–9.07 | 5.35 | 2.10–13.64 | ||
| Region of residenceb | ||||||||
| Baden-Württemberg | 322 | 14 | 4.75 | 2.44–7.07 | 2.38 | 0.95–5.97 | 2.21 | 0.88–5.55 |
| Bavaria | 360 | 18 | 5.26 | 2.70–7.82 | 2.65 | 1.05–6.66 | 2.33 | 0.90–6.01 |
| Central | 313 | 13 | 3.06 | 1.09–5.04 | 1.51 | 0.55–4.16 | 1.41 | 0.51–3.94 |
| North-west | 358 | 10 | 2.05 | 0.51–3.60 | Reference | |||
| North Rhine-Westphalia | 556 | 21 | 4.5 | 1.95–7.05 | 2.24 | 0.85–5.92 | 2.11 | 0.80–5.58 |
| East (north) | 535 | 23 | 4.15 | 0.98–7.33 | 2.06 | 0.68–6.24 | 1.86 | 0.60–5.74 |
| East (south) | 447 | 32 | 6.19 | 3.46–8.92 | 3.14 | 1.28–7.72 | 2.66 | 1.09–6.51 |
| Size of municipalityc | ||||||||
| Rural | 595 | 31 | 4.56 | 3.14–5.98 | 0.88 | 0.41–1.88 | 0.8 | 0.37–1.76 |
| Town | 1,377 | 70 | 4.74 | 3.21–6.27 | 0.92 | 0.43–1.97 | 0.87 | 0.41–1.82 |
| Medium-sized town | 617 | 15 | 2.13 | 0.73–3.53 | 0.4 | 0.15–1.05 | 0.35 | 0.13–0.95 |
| Metropolitan | 302 | 15 | 5.14 | 1.80–8.48 | Reference | |||
| Socioeconomic statusd (98 missing values) | ||||||||
| High | 667 | 30 | 3.89 | 2.29–5.48 | Reference | |||
| Medium | 1,712 | 85 | 5.25 | 3.81–6.69 | 1.37 | 0.81–2.32 | No adjustment possible | |
| Low | 414 | 11 | 1.39 | 0.30–2.48 | 0.35 | 0.15–0.84 | ||
| Daily TV consumption (hours) (1,667 missing values) | ||||||||
| 0 | 73 | 3 | 2.01 | 0–4.60 | Reference | |||
| < 1 | 658 | 25 | 3.49 | 1.71–5.26 | 1.76 | 0.45–6.92 | 1.29 | 0.32–5.27 |
| 1–2 | 334 | 2 | 0.67 | 0–1.78 | 0.33 | 0.05–2.29 | 0.23 | 0.03–1.55 |
| 2–3 | 117 | 3 | 1.89 | 0–4.52 | 0.94 | 0.14–6.43 | 0.72 | 0.11–4.60 |
| 3–4 | 34 | 0 | No adjustment necessary | |||||
| > 4 | 8 | 0 | ||||||
| Weekly media consumptione (232 missing values) | ||||||||
| Low | 974 | 47 | 3.62 | 2.35–4.89 | Reference | |||
| Middle | 607 | 27 | 5.84 | 3.18–8.51 | 1.65 | 0.95–2.86 | 1.06 | 0.60–1.89 |
| High | 1,078 | 50 | 3.93 | 2.60–5.25 | 1.09 | 0.67–1.77 | 0.66 | 0.39–1.12 |
aOR: adjusted odds ratio; CI: confidence interval; KiGGS: German Health Interview and Examination Survey for Children and Adolescents; OR: odds ratio.
a Multivariable regression models include adjustment variables according to the minimal sufficient adjustment sets listed in Supplementary Table S2.
b Central: Hesse, Rhineland-Palatinate and Saarland; North-west: Bremen, Hamburg, Lower Saxony and Schleswig-Holstein; East (north): Berlin, Brandenburg, Mecklenburg-Vorpommern and Saxony-Anhalt; East (south): Saxony and Thuringia.
c Size of municipality was classified as residential areas based on population sizes and categorized into rural (< 5,000), town (5,000– <20,000), medium-sized town (20,000–< 100,000) and metropolitan areas (≥ 100,000).
d Categorisation of socio-economic status was based on an index, which was calculated using information regarding education and occupational qualifications, occupational status and net equivalent income of the parents. More details are given in the Supplement.
e Media consumption was categorised as high, middle or low based on an index. More details are given in the Supplement.
Entries in bold represent statistically significant findings.
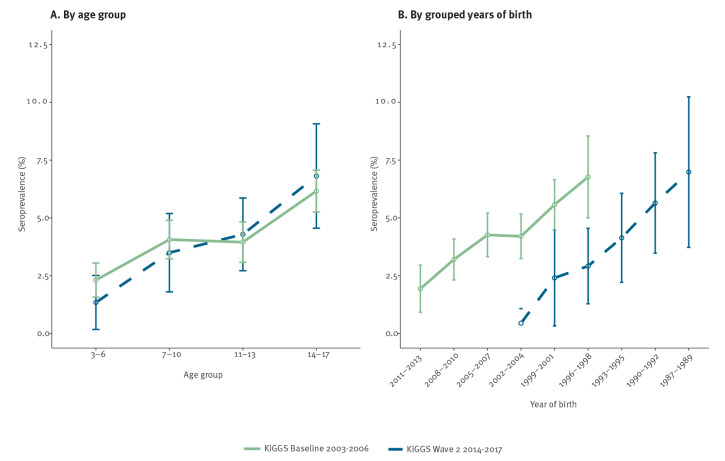
In both surveys, seroprevalence increased by age (Figure, Table 1 and 2), more in males than in females. More detailed data differentiating between males and females are provided in Supplementary Table S3. The chance of being seropositive was higher in all older age groups compared with the reference group of 3–6-year-olds and odds ratios were highest in the oldest age category of 14–17 years (Table 1 and 2). Overall, males were more likely to be seropositive than females, with ORs of 1.5 and 2 in the respective surveys (Table 1 and 2).
FIGURE.
Weighted prevalence of Borrelia burgdorferi-specific IgG antibodies in the cross-sectional surveys KiGGS Baseline (n = 11,626) and KiGGS Wave 2 (n = 2,891) of children and adolescents, Germany, 2003–2006 and 2014–2017
KiGGS: German Health Interview and Examination Survey for Children and Adolescents.
In both surveys, the highest seroprevalence was noted in Bavaria and the adjacent federal states Thuringia and Saxony. The prevalence was similar in participants living in medium-sized (20,000– < 100,000 inhabitants) towns (2.7% and 2.1%) but varied to some extent in the metropolitan areas (≥ 100,000 inhabitants) (3.3% vs 5.1%) between the two surveys, although the CIs overlapped. In KiGGS Baseline, children and adolescents living in rural areas (< 5,000 inhabitants) were more likely to be seropositive (OR = 1.6; 95% CI: 1.11–2.35) compared with those living in metropolitan areas. In both surveys, a low SES was associated with reduced odds of being seropositive compared with a high SES (OR = 0.50; 95% CI: 0.32–0.79 and 0.35; 95% CI: 0.15–0.84). More details on the SES are presented in the Supplement. In KiGGS Baseline, the OR of being seropositive was 3.8 (95% CI: 2.53–5.59) for children without migration background vs children with a migration background.
Children and adolescents with a high weekly media consumption were less likely to be seropositive compared with those with low media consumption in KiGGS Baseline (OR = 0.73; 95% CI: 0.55–0.97). Details on the categorisation of media consumption are given in the Supplement. KiGGS Baseline participants who stated being physically active 3–5 times per week had 2.1 times the odds of being seropositive compared with non-active participants. Pet owners in KiGGS Baseline had increased odds of being seropositive compared with children without pets (5.0% vs 3.8%; p < 0.001), particularly cat owners (6.5% vs 3.9%; p < 0.001) (Table 1). In multivariable analysis, the association between cat ownership and seropositivity was not significant. Owning a pet dog or a small mammal was not associated with seropositivity.
Seroconversion and seroreversion
In the KiGGS follow-up, paired serum samples were available for 4,016 participants, evenly from males (2,052; 51.1%) and females (1,964; 48.9%). Participants were retested after a median of 11 (interquartile range (IQR): 11–11, range: 11–14) years after the KiGGS Baseline study. Most of the 3,885 participants who tested seronegative in KiGGS Baseline were seronegative in KiGGS Wave 2 (3,753; 96.6%); 132 (3.4%) seroconverted. Of the 131 follow-up participants testing seropositive in KiGGS Baseline, 75 (57.3%) were still seropositive in KiGGS Wave 2, while 56 (42.7%) seroreverted. An overview can be accessed in Supplementary Figure S5 B. Being male and of younger age were predictive of seroconversion (Table 3). Median age of seroconverters was 6 years (IQR: 4–10) compared with 8 years (IQR: 4–12) in those who remained seronegative. Living in a rural area was associated with seroconversion compared with living in metropolitan areas, although the CI was wide (Table 3). Children aged 3–6 years in KiGGS Baseline had higher odds for seroreversion than those aged 14–17 years (OR: 4.2; 95% CI: 1.5–12.0). A lower antibody level in the ELISA test in KIGSS Baseline was associated with seroreversion (Table 4). Ten of 11 participants with antibody levels below 10 seroreverted, 39 of 70 with levels between 10 and 99 did so, and 6 of 49 with antibody levels of ≥ 100 seroreverted (Table 4). In the Supplementary Figure S6, a graphical overview is shown.
TABLE 3. Predictors of seroconversion of Borrelia burgdorferi-specific IgG antibodies among participants of a follow-up to a cross-sectional survey of children and adolescents (KiGGS), Germany, 2003–2006 and 2014–2017 (n = 3,885).
| Characteristics | Seronegative participants in KiGGS Baseline | Seroconversions | p valuea | Univariable analyses | Multivariable analysesb | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | aOR | 95% CI | ||
| Sex | |||||||||
| Female | 1,915 | 49.3 | 40 | 30.3 | < 0.01 | Reference | |||
| Male | 1,970 | 50.7 | 92 | 69.7 | 2.30 | 1.59–3.38 | No adjustment necessary | ||
| Age group (years) | |||||||||
| 1–2 | 455 | 11.7 | 17 | 12.9 | < 0.01 | 2.55 | 1.15–6.03 | No adjustment necessary | |
| 3–6 | 1,182 | 30.4 | 50 | 37.9 | 2.90 | 1.49–6.35 | |||
| 7–10 | 995 | 25.6 | 34 | 25.8 | 2.32 | 1.15–5.19 | |||
| 11–13 | 653 | 16.8 | 22 | 16.7 | 2.29 | 2.29–5.28 | |||
| 14–17 | 600 | 15.4 | 9 | 6.8 | Reference | ||||
| Region of residencec | |||||||||
| Baden-Württemberg | 469 | 12.1 | 17 | 12.9 | 0.79 | 1.19 | 0.59–2.44 | 1.18 | 0.58–2.42 |
| Bavaria | 509 | 13.1 | 21 | 15.9 | 1.36 | 0.70–2.72 | 1.35 | 0.69–2.70 | |
| Central | 393 | 10.1 | 11 | 8.3 | 0.91 | 0.40–2.00 | 0.91 | 0.40–1.99 | |
| North-west | 490 | 12.6 | 15 | 11.4 | Reference | ||||
| North Rhine-Westphalia | 792 | 20.4 | 23 | 17.4 | 0.95 | 0.49–1.87 | 0.95 | 0.50–1.89 | |
| East (north) | 680 | 17.5 | 22 | 16.7 | 1.06 | 0.55–2.10 | 1.07 | 0.55–2.12 | |
| East (south) | 552 | 14.2 | 23 | 17.4 | 1.38 | 0.72–2.72 | 1.36 | 0.71–2.69 | |
| Size of municipalityd | |||||||||
| Rural | 812 | 20.9 | 35 | 26.5 | 0.04 | 2.52 | 1.13–6.72 | 2.39 | 1.04–6.46 |
| Town | 1,854 | 47.7 | 70 | 53.0 | 2.20 | 1.03–5.71 | 2.13 | 0.98–5.58 | |
| Medium-sized town | 877 | 22.6 | 21 | 15.9 | 1.37 | 0.58–3.77 | 1.36 | 0.57–3.79 | |
| Metropolitan | 342 | 8.8 | 6 | 4.5 | Reference | ||||
| Socioeconomic statuse (33 missing values) | |||||||||
| High | 1,032 | 26.8 | 34 | 25.8 | 0.72 | Reference | |||
| Medium | 2,400 | 62.3 | 86 | 65.2 | 1.09 | 0.74–1.65 | 1.08 | 0.73–1.64 | |
| Low | 420 | 10.9 | 12 | 9.1 | 0.86 | 0.43–1.64 | 0.82 | 0.40–1.60 | |
| Migration statusf (18 missing values) | |||||||||
| Yes | 474 | 12.3 | 17 | 12.9 | 0.82 | Reference | |||
| No | 3,393 | 87.7 | 115 | 87.1 | 0.94 | 0.58–1.64 | Not applicable | ||
| Weekly media consumptiong (2,305 missing values) | |||||||||
| Low | 189 | 12.0 | 10 | 15.6 | 0.51 | Reference | |||
| Medium | 423 | 26.8 | 14 | 21.9 | 0.61 | 0.27–1.45 | 0.60 | 0.26–1.42 | |
| High | 968 | 61.3 | 40 | 62.5 | 0.77 | 0.39–1.66 | 0.80 | 0.40–1.75 | |
aOR: adjusted odds ratio; CI: confidence interval; KiGGS: German Health Interview and Examination Survey for Children and Adolescents; OR: odds ratio.
a To compare the proportion of seroconversions, we used the Pearson’s chi-squared test.
b Multivariable regression models include adjustment variables according to the minimal sufficient adjustment sets listed in Supplementary Table S2.
c Central: Hesse, Rhineland-Palatinate and Saarland; North-west: Bremen, Hamburg, Lower Saxony and Schleswig-Holstein; East (north): Berlin, Brandenburg, Mecklenburg-Vorpommern and Saxony-Anhalt; East (south): Saxony and Thuringia.
d Size of municipality was classified as residential areas based on population sizes and categorized into rural (<5,000), town (5,000-<20,000), medium-sized town (20,000-<100,000), and metropolitan areas (≥100,000).
e Categorisation of socio-economic status was based on an index, which was calculated using information regarding education and occupational qualifications, occupational status and net equivalent income of the parents. More details are given in the Supplement.
f Participants were assigned a migration background if they had moved to Germany and at least one parent was born abroad, if both parents had moved to Germany or if neither parent had a German citizenship.
g Media consumption was categorised as high, middle or low based on an index. More details are given in the Supplement.
Entries in bold represent statistically significant findings.
TABLE 4. Predictors of seroreversion of Borrelia burgdorferi-specific IgG antibodies among participants of a follow-up to a cross-sectional survey of children and adolescents (KiGGS), Germany, 2003–2006 and 2014–2017 (n = 131).
| Characteristics | Seropositive participants in KiGGS Baseline | Seroreversions | p valuea | Univariable analyses | Multivariable analysesb | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | aOR | 95% CI | ||
| Sex | |||||||||
| Female | 47 | 35.9 | 19 | 33.9 | 0.69 | Reference | |||
| Male | 84 | 64.1 | 37 | 66.1 | 1.16 | 0.56–2.41 | Not applicable | ||
| Age group (years) | |||||||||
| 1–2 | 2 | 1.5 | 1 | 1.8 | 0.10 | 2.55 | 0.10–68.29 | Not applicable | |
| 3–6 | 29 | 22.1 | 18 | 32.1 | 4.17 | 1.53–12.01 | |||
| 7–10 | 32 | 24.4 | 14 | 25.0 | 1.98 | 0.74–5.41 | |||
| 11–13 | 29 | 22.1 | 12 | 21.4 | 1.80 | 0.65–5.04 | |||
| 14–17 | 39 | 29.8 | 11 | 19.6 | Reference | ||||
| Place of residencec | |||||||||
| Baden-Württemberg | 17 | 13.0 | 5 | 8.9 | 0.27 | 0.54 | 0.12–2.23 | 0.53 | 0.12–2.21 |
| Bavaria | 27 | 20.6 | 10 | 17.9 | 0.76 | 0.21–2.71 | 0.82 | 0.23–2.95 | |
| Central | 9 | 6.9 | 3 | 5.4 | 0.64 | 0.10–3.43 | 0.65 | 0.10–3.57 | |
| North-west | 16 | 12.2 | 7 | 12.5 | Reference | ||||
| North Rhine-Westphalia | 21 | 16.0 | 11 | 19.6 | 1.41 | 0.38–5.37 | 1.40 | 0.38–5.34 | |
| East (north) | 22 | 16.8 | 14 | 25.0 | 2.25 | 0.61–8.72 | 2.16 | 0.58–8.51 | |
| East (south) | 19 | 14.5 | 6 | 10.7 | 0.59 | 0.14–2.36 | 0.61 | 0.15–2.42 | |
| Size of municipalityd | |||||||||
| Rural | 34 | 26.0 | 18 | 32.1 | 0.50 | 1.13 | 0.23–5.48 | 1.69 | 0.31–9.32 |
| Town | 70 | 53.4 | 27 | 48.2 | 0.63 | 0.14–2.86 | 0.85 | 0.17–4.14 | |
| Medium-sized town | 19 | 14.5 | 7 | 12.5 | 0.58 | 0.10–3.18 | 0.56 | 0.09–3.33 | |
| Metropolitan | 8 | 6.1 | 4 | 7.1 | Reference | ||||
| Socioeconomic statuse (2 missing values) | |||||||||
| High | 40 | 31.0 | 17 | 30.9 | 0.84 | Reference | |||
| Medium | 80 | 62.0 | 35 | 63.6 | 1.05 | 0.49–2.29 | 1.07 | 0.50–2.33 | |
| Low | 9 | 7.0 | 3 | 5.5 | 0.68 | 0.13–2.96 | 0.45 | 0.06–2.24 | |
| Antibody level (ELISA) | |||||||||
| < 10 | 12 | 9.2 | 11 | 19.6 | < 0.01 | Reference | |||
| 10–99 | 70 | 53.4 | 39 | 69.6 | 0.11 | 0.01–0.64 | 0.12 | 0.01–0.78 | |
| ≥ 100 | 49 | 37.4 | 6 | 10.7 | 0.01 | 0.00–0.08 | 0.01 | 0.00–0.08 | |
CI: confidence interval; KiGGS: German Health Interview and Examination Survey for Children and Adolescents; OR: odds ratio; aOR: adjusted odds ratio.
a To compare the proportion of seroreversions, we used the Pearson’s chi-squared test.
b Multivariable regression models include adjustment variables according to the minimal sufficient adjustment sets listed in Supplementary Table S2.
c Central: Hesse, Rhineland-Palatinate and Saarland; North-west: Bremen, Hamburg, Lower Saxony and Schleswig-Holstein; East (north): Berlin, Brandenburg, Mecklenburg-Vorpommern and Saxony-Anhalt; East (south): Saxony and Thuringia.
d Size of municipality was classified as residential areas based on population sizes and categorized into rural (<5,000), town (5,000-<20,000), medium-sized town (20,000-<100,000) and metropolitan areas (≥100,000).
e Categorisation of socio-economic status was based on an index, which was calculated using information regarding education and occupational qualifications, occupational status and net equivalent income of the parents. More details are given in the Supplement.
Entries in bold represent statistically significant findings.
Annual rates
Observation time for the 4,016 participants was 43,852 person-years. On average, participants contributed 10.9 years (standard deviation (SD): 0.2; range: 10.6–13.8). The average follow-up time was the same in participants with initially seronegative (10.9 years; SD: 0.3) and seropositive (11.0 years; SD: 0.2) test results. The annual seroconversion rate was 0.32% (95% CI: 0.26–0.38), the annual seroreversion rate was 3.91% (95% CI: 3.22–4.78). Eighteen of the 29 initially seropositive children in age group 3–6 years seroreverted whereas 11 of the 39 in age group 14–17 years did so. Although the numbers are small and the CIs are overlapping, we observed decreasing seroreversion rates with increasing age group (Table 5). Average observation times did not differ between age groups.
TABLE 5. Association between seroreversion and Borrelia burgdorferi antigen-specific IgG antibodies and annual rate of seroreversion by age group among participants of a follow-up to a cross-sectional survey of children and adolescents (KiGGS), Germany, 2003–2006 and 2014–2017 (n = 131).
| Characteristics | Seropositive participants in KiGGS Baseline | Seroreversions | p valuea | Univariable analyses | Multivariable analysesb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | aOR | 95% CI | |||
| Antigens (7 missing values) | ||||||||||
| OspC | 24 | 19.4 | 8 | 16.3 | 0.49 | 0.72 | 0.27–1.80 | 0.55 | 0.16–1.72 | |
| VlsE | 114 | 91.9 | 41 | 83.7 | 0.006 | 0.14 | 0.02–0.59 | 0.27 | 0.04–1.35 | |
| p39 | 49 | 39.5 | 11 | 22.4 | < 0.01 | 0.28 | 0.12–0.62 | 0.57 | 0.20–1.60 | |
| p58 | 76 | 61.3 | 19 | 38.8 | < 0.01 | 0.20 | 0.09–0.43 | 0.60 | 0.23–1.58 | |
| p83 | 39 | 31.5 | 5 | 10.2 | < 0.01 | 0.14 | 0.04–0.36 | 0.36 | 0.10–1.17 | |
| DbpA | 99 | 79.8 | 30 | 61.2 | < 0.01 | 0.14 | 0.05–0.36 | 0.25 | 0.07–0.77 | |
| Number of antigens (7 missing values) | ||||||||||
| 1 | 20 | 16.1 | 16 | 32.7 | < 0.01 | Reference | ||||
| 2 | 23 | 18.5 | 13 | 26.5 | 0.33 | 0.07–1.22 | Not applicable | |||
| 3 | 26 | 21.0 | 11 | 22.4 | 0.18 | 0.04–0.66 | ||||
| 4 | 26 | 21.0 | 6 | 12.2 | 0.08 | 0.02–0.29 | ||||
| 5 | 21 | 16.9 | 3 | 6.10 | 0.04 | 0.01–0.19 | ||||
| 6 | 8 | 6.5 | 0 | 0.0 | Not applicable | |||||
| Age group (years) | n | % | n | p value | Annual rate of seroreversion | 95% CI | ||||
| 1–2 | 2 | 1.5 | 1 | 0.10 | Not applicable | |||||
| 3–6 | 29 | 22.1 | 18 | 5.70 | 5.06–8.52 | |||||
| 7–10 | 32 | 24.4 | 14 | 4.01 | 2.57–5.74 | |||||
| 11–13 | 29 | 22.1 | 12 | 3.81 | 1.27–3.81 | |||||
| 14–17 | 39 | 29.8 | 11 | 2.56 | 0.69–2.78 | |||||
aOR: adjusted odds ratio; CI: confidence interval; KiGGS: German Health Interview and Examination Survey for Children and Adolescents; OR: odds ratio.
a To compare the proportion of seroreversions, we used the Pearson’s chi-squared test.
b The multivariable regression model includes all antigen reactions.
Entries in bold represent statistically significant findings.
Antigen reactivity
Information on antigen-specific antibodies was available for 124 of the 131 follow-up participants who tested seropositive in KiGGS Baseline. Among those, 49 were seroreverters. Considering the individual antigen reactions, the proportion of seroreverters was highest in participants with OspC-specific antibodies (8/24) and lowest among participants with p83-specific antibodies (5/39). In contrast to all other antigens, antibodies targeting the antigen OspC were not significantly associated with seroreversion in the univariable regression analysis (Table 5). In the multivariable regression, including all antigen reactions, only the reaction to DbpA was significantly associated with seroreversion. The number of reactive immunoblot bands in KiGGS Baseline was positively associated with remaining seropositive. Among the 20 participants with one positive band, 16 seroreverted, three of the 21 participants with five reactive bands and none among the eight participants with six reactive bands seroreverted (Table 5); additional figures showing the change in serostatus in relation to the number of bands and antibody levels are appended in Supplementary Figure S6 A.
Discussion
Comparing seroprevalence data of Bb in children and adolescents in Germany based on representative population data, we found no change over the period of a decade. Available for the first time for this age group, serological follow-up 11 years after 1–17-year-olds were first tested, revealed 56 seroreverters among 131 seropositive participants, which was more than we expected.
Similar seroprevalence estimates in the two surveys suggest no change in risk of exposure to Bb from 2003 to 2017. This is an interesting finding in light of the discussion about the potential influence of climate change on increases of tick abundance and tick-borne diseases [4,11,22-24]. The interface between climatic factors, ticks, Borrelia and hosts is complex, and determinants are poorly understood. The period of 11 years may be too short to detect effects of climate change on exposure to Bb. Thus, seroprevalence studies are needed to assess potential long-term effects.
Our estimated seroprevalence was similar to the one reported from Italy (based on ELISA only) [25], but higher than in Sweden [14] and Finland [26] and lower than in Belgium [27]. Differences in seroprevalence across Europe have been described [28], reflecting both differences in Borrelia infection rates as well as different study designs and laboratory methods. In Finland, seroprevalence was considerably higher in the late 1960s and early 1970s (20%) [29] than in 2011 (3.9%) [26]. However, due to different laboratory methods, a preponderance of older age groups with no adjustment in the older study and the fact that in the earlier time periods LB had not yet been a known disease with expected lack of early antibiotic treatment, which would have prevented antibody production, comparability of these studies is limited.
In Germany, Bb can be found throughout the country, but seroprevalence is heterogeneous between regions. It appears highest in Bavaria and Saxony/Thuringia. Living in a rural area was predictive of seropositivity in KiGGS Baseline, but inconclusive in KiGGS Wave 2. Although many studies have found rural settings to be associated with increased seroprevalence of LB [30,31], more recently, concern has been raised on increasing numbers of tick-borne infections in suburban and urban areas [32]. High infection rates of ticks in urban parks have been reported [33,34] and studies have found comparable seropositivity, LB incidence or risk of LB after a tick bite in urban and rural areas [35,36]. In Hannover, a German city of approximately half a million inhabitants, an increase in the number of Borrelia-infected ticks in urban parks was observed, but not in ticks found in forest areas between 2005 and 2015 [33]. However, findings from ticks from one area may not be easily extrapolated to another [33,37].
The increase of seroprevalence by age we found is widely observed [1,17,36]. The estimated annual seroincidence rate in our study was lower than reported in adults (0.32% vs 0.45%), whereas the estimated annual seroreversion rate was more than double compared with another study in adults (3.9% vs 1.5%) [17]. In addition, preschool age (3–6 years) and especially low antibody levels were predictors of seroreversion. This may indicate a less pronounced immune response either due to the evolving immune system in young children or in primary infections, assuming that most infections in the youngest were primary. Repeated exposure leads to higher seroreactivity [38]. Individuals with a previous Bb-infection were more likely to experience another infection, as certain risk characteristics remain [39]. Observed seroreversions, especially in the age group of 3–6 years, likely resulted in an underestimation of seroconversions during the follow-up period of 11 years.
We assessed the humoral immunity to Bb using serological diagnostic methods routinely used for LB. Seroprevalence studies reported a high proportion of seropositive individuals with no clinical symptoms of LB [4,14,15]. Our representative seroprevalence estimates serve as an important prerequisite to define pre-test probabilities in different age groups. Thus, they guide the interpretation of diagnostic antibody tests (e.g. in an individual with clinical symptoms), as the seroprevalence affects the negative and positive predictive value of diagnostic tests. Similar estimates have already been included in guidelines for clinical diagnoses [6,8] and may prevent misdiagnoses and unnecessary treatment.
Seroreversion rates were dependent on the immune response to Bb. Our antigen analyses suggest that participants with signs of a more pronounced immune response remained seropositive for a longer period and were less likely to serorevert. Reactivity to all antigens, except Ospc, was associated with remaining seropositive. Antibodies specific to DbpA were the only predictor of remaining seropositive, independently of the other antigens. This reaction is typically present at a later stage of infection [6,19]. The proportion of seroreverters was highest in participants with specific antibodies to the OspC antigen, a sign of early infection. These early infections are linked to fewer antigen reactions included in the immunoblot, whereas reactions to a broad spectrum of antigens are a sign of infection that occurred long ago [6,19]. Numbers addressing seroreversion, in particular, were small. Still, meaningful conclusions can be derived and these results provide the best evidence base for a population-based assessment of this phenomenon and for the evaluation of Bb seropositivity in the population.
Additional testing for Bb-specific IgM antibodies may have captured additional early infections, which could have led to higher seroincidence and seroprevalence estimates. Absence of seroconversion in a proportion of asymptomatic or mildly symptomatic (including EM) infected individuals, as well as waning antibody levels over time likely lead to a general underestimation of the actual exposure to Bb based on seroprevalence studies, which also applies to our study.
Although small-scale analyses considering diverse ecological or geographical characteristics were not possible with our data, our results still provide differentiation of seroprevalence on a regional scale in one country.
Conclusion
We found similar seroprevalence estimates in children and adolescents over time. Estimates differed by characteristics such as sex, age or region. We found a high rate of 42% of seroreversions in previously seropositive children. Our findings serve as an important source for further risk assessments, modelling studies and the empirical basis for public health interventions. For example, education should aim to increase awareness in children and their parents as a target group. At the same time, physical and outdoor activities should be promoted, while communicating adequate protection such as tick bite avoidance and correct tick removal. Consecutive serosurveys, preferably with a large proportion of follow-up participants, enable to track risk changes (i.e. due to climate change) over time.
Ethical statement
KiGGS Baseline was approved by the Ethics Committee of Charité - Universitätsmedizin Berlin (reference number 101/2000) and KiGGS2 was examined and approved by Hannover Medical School’s Ethics Committee (No. 2275-2014).
Funding statement
This project was funded by Robert Koch Institute and Bavarian Health and Food Safety Authority.
Data availability
Scientific Use Files are available for KiGGS Baseline and KiGGS Wave 2. Data not included in the scientific use files can be requested. More information can be found here: www.rki.de/fdz
Acknowledgements
We would like to thank Niklas Willrich for his statistical support.
Supplementary Data
Conflict of interest: VF: Continuing education (honoraria, travel expenses) Fenner laboratory Hamburg, REMMDI Regensburg, BML – LABORATORY Singen. Consultant activities (no honoraria) Pfizer, Global Lyme Alliance. Borrelia PCR and serology ring trials (no honoraria) INSTAND, QCMD. None of these organisations are involved in any aspect of the current manuscript.
Authors’ contributions: HW and VF conceived the study. VF managed laboratory testing and RK the survey data. SB and TW analysed the final data set. TW, SB, MMB, KK, MS, VF and HW contributed to the interpretation of results. SB drafted the first manuscript. All authors reviewed the manuscript and agreed on the final version of the manuscript.
References
- 1. Rizzoli A, Hauffe H, Carpi G, Vourc H G, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27):19906. 10.2807/ese.16.27.19906-en [DOI] [PubMed] [Google Scholar]
- 2. Stanek G, Strle F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol Rev. 2018;42(3):233-58. 10.1093/femsre/fux047 [DOI] [PubMed] [Google Scholar]
- 3. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461-73. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- 4. Radolf JD, Strle K, Lemieux JE, Strle F. Lyme disease in humans. Curr Issues Mol Biol. 2021;42:333-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enkelmann J, Böhmer M, Fingerle V, Siffczyk C, Werber D, Littmann M, et al. Incidence of notified Lyme borreliosis in Germany, 2013-2017. Sci Rep. 2018;8(1):14976. 10.1038/s41598-018-33136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hofmann H, Fingerle V, Hunfeld K-P, Huppertz H-I, Krause A, Rauer S, et al. Cutaneous Lyme borreliosis: guideline of the German dermatology society. Ger Med Sci. 2017;15:Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanek G, Fingerle V, Hunfeld KP, Jaulhac B, Kaiser R, Krause A, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17(1):69-79. 10.1111/j.1469-0691.2010.03175.x [DOI] [PubMed] [Google Scholar]
- 8. Rauer S, Kastenbauer S, Hofmann H, Fingerle V, Huppertz HI, Hunfeld KP, et al. Guidelines for diagnosis and treatment in neurology - Lyme neuroborreliosis. Ger Med Sci. 2020;18:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fingerle V, Sing A, Hofmann H. Lyme-Borreliose: Fallstricke bei Diagnose und Therapie. [Lyme borreliosis: pitfalls in diagnosis and therapy]. Dtsch Arztebl Int. 2015;112(23):15-7. [Google Scholar]
- 10. des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC, 3rd, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis. 2001;183(5):773-8. 10.1086/318818 [DOI] [PubMed] [Google Scholar]
- 11.Lindgren E, Jaenson TGT. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. Copenhagen: World Health Organization Regional Office for Europe; 2006. Available from: https://apps.who.int/iris/handle/10665/107800 [Google Scholar]
- 12.World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10 Version:2019). Geneva: WHO; 2019. Available from: https://icd.who.int/browse10/2019/en
- 13. Akmatov MK, Holstiege J, Dammertz L, Heuer J, Kohring C, Lotto-Batista M, et al. Epidemiology of Lyme borreliosis based on outpatient claims data of all people with statutory health insurance, Germany, 2019. Euro Surveill. 2022;27(32):2101193. 10.2807/1560-7917.ES.2022.27.32.2101193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skogman BH, Ekerfelt C, Ludvigsson J, Forsberg P. Seroprevalence of Borrelia IgG antibodies among young Swedish children in relation to reported tick bites, symptoms and previous treatment for Lyme borreliosis: a population-based survey. Arch Dis Child. 2010;95(12):1013-6. 10.1136/adc.2010.183624 [DOI] [PubMed] [Google Scholar]
- 15. Garro A, Bennett J, Balamuth F, Levas MN, Neville D, Branda JC, et al. Positive 2-tiered Lyme disease serology is uncommon in asymptomatic children living in endemic areas of the United States. Pediatr Infect Dis J. 2019;38(5):e105-7. 10.1097/INF.0000000000002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dehnert M, Fingerle V, Klier C, Talaska T, Schlaud M, Krause G, et al. Seropositivity of Lyme borreliosis and associated risk factors: a population-based study in children and adolescents in Germany (KiGGS). PLoS One. 2012;7(8):e41321. 10.1371/journal.pone.0041321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woudenberg T, Böhm S, Böhmer M, Katz K, Willrich N, Stark K, et al. Dynamics of Borrelia burgdorferi-specific antibodies: seroconversion and seroreversion between two population-based, cross-sectional surveys among adults in Germany. Microorganisms. 2020;8(12):1859. 10.3390/microorganisms8121859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann R, Lange M, Butschalowsky H, Houben R, Schmich P, Allen J, et al. KiGGS Wave 2 cross-sectional study - participant acquisition, response rates and representativeness. J Health Monit. 2018;3(1):78-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fingerle V, Eiffert H, Gessner A, Göbel UB, Hofmann H, Hunfeld KP, et al. MIQ12: Lyme-Borreliose. Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik. [MIQ12: Lyme borreliosis. Quality standards in microbiological-infectiological diagnostics]. Jena - München: Urban & Fischer; 2017. p. 1-68. German. [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org
- 21. Lange M, Hoffmann R, Mauz E, Houben R, Gößwald A, Rosario AS, et al. KiGGS Wave 2 longitudinal component - data collection design and developments in the numbers of participants in the KiGGS cohort. J Health Monit. 2018;3(1):92-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medlock JM, Leach SA. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect Dis. 2015;15(6):721-30. 10.1016/S1473-3099(15)70091-5 [DOI] [PubMed] [Google Scholar]
- 23. Kjær LJ, Soleng A, Edgar KS, Lindstedt HEH, Paulsen KM, Andreassen AK, et al. Predicting and mapping human risk of exposure to Ixodes ricinus nymphs using climatic and environmental data, Denmark, Norway and Sweden, 2016. Euro Surveill. 2019;24(9):1800101. 10.2807/1560-7917.ES.2019.24.9.1800101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Semenza JC, Suk JE. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett. 2018;365(2):fnx244. 10.1093/femsle/fnx244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castiglia P, Mura I, Masia MD, Maida I, Solinas G, Muresu E. Indagine sieroepidemiologica sulla presenza di anticorpi anti-Borrelia burgdorferi in giovani del Nord-Sardegna. [Prevalence of antibodies to Borrelia burgdorferi in Sardinian teen-agers]. Ann Ig. 2004;16(1-2):103-8. [PubMed] [Google Scholar]
- 26. van Beek J, Sajanti E, Helve O, Ollgren J, Virtanen MJ, Rissanen H, et al. Population-based Borrelia burgdorferi sensu lato seroprevalence and associated risk factors in Finland. Ticks Tick Borne Dis. 2018;9(2):275-80. 10.1016/j.ttbdis.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 27. Lernout T, Kabamba-Mukadi B, Saegeman V, Tré-Hardy M, de Laveleye M, Asikainen T, et al. The value of seroprevalence data as surveillance tool for Lyme borreliosis in the general population: the experience of Belgium. BMC Public Health. 2019;19(1):597. 10.1186/s12889-019-6914-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santino I, Sessa R, Del Piano M. Lyme borreliosis infection in Europe. Eur J Inflamm. 2006;4(2):69-75. 10.1177/1721727X0600400201 [DOI] [Google Scholar]
- 29. Cuellar J, Dub T, Sane J, Hytönen J. Seroprevalence of Lyme borreliosis in Finland 50 years ago. Clin Microbiol Infect. 2020;26(5):632-6. 10.1016/j.cmi.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 30. Wilking H, Fingerle V, Klier C, Thamm M, Stark K. Antibodies against Borrelia burgdorferi sensu lato among adults, Germany, 2008-2011. Emerg Infect Dis. 2015;21(1):107-10. 10.3201/eid2101.140009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Killilea ME, Swei A, Lane RS, Briggs CJ, Ostfeld RS. Spatial dynamics of lyme disease: a review. EcoHealth. 2008;5(2):167-95. 10.1007/s10393-008-0171-3 [DOI] [PubMed] [Google Scholar]
- 32. Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health. 2014;2:251. 10.3389/fpubh.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blazejak K, Raulf MK, Janecek E, Jordan D, Fingerle V, Strube C. Shifts in Borrelia burgdorferi (s.l.) geno-species infections in Ixodes ricinus over a 10-year surveillance period in the city of Hanover (Germany) and Borrelia miyamotoi-specific Reverse Line Blot detection. Parasit Vectors. 2018;11(1):304. 10.1186/s13071-018-2882-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A. Ticks and the city - are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors. 2017;10(1):573. 10.1186/s13071-017-2391-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huppertz HI, Böhme M, Standaert SM, Karch H, Plotkin SA. Incidence of Lyme borreliosis in the Würzburg region of Germany. Eur J Clin Microbiol Infect Dis. 1999;18(10):697-703. 10.1007/s100960050381 [DOI] [PubMed] [Google Scholar]
- 36. Cora M, Kaklıkkaya N, Topbaş M, Çan G, Yavuzyılmaz A, Tosun İ, et al. Determination of seroprevalence of Borrelia burgdorferi IgG in adult population living in Trabzon. Balkan Med J. 2017;34(1):47-52. 10.4274/balkanmedj.2015.0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okeyo M, Hepner S, Rollins RE, Hartberger C, Straubinger RK, Marosevic D, et al. Longitudinal study of prevalence and spatio-temporal distribution of Borrelia burgdorferi sensu lato in ticks from three defined habitats in Latvia, 1999-2010. Environ Microbiol. 2020;22(12):5033-47. 10.1111/1462-2920.15100 [DOI] [PubMed] [Google Scholar]
- 38. Zając V, Pinkas J, Wójcik-Fatla A, Dutkiewicz J, Owoc A, Bojar I. Prevalence of serological response to Borrelia burgdorferi in farmers from eastern and central Poland. Eur J Clin Microbiol Infect Dis. 2017;36(3):437-46. 10.1007/s10096-016-2813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, O’Keefe CF, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One. 2014;9(1):e84758. 10.1371/journal.pone.0084758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.