Abstract
The development of highly effective photosensitizers (PSs) for photodynamic therapy remains a great challenge at present. Most PSs rely on the heavy-atom effect or the spin–orbit charge-transfer intersystem crossing (SOCT-ISC) effect to promote ISC, which brings about additional cytotoxicity, and the latter is susceptible to the interference of solvent environment. Herein, an immanent universal property named photoinduced molecular vibrational torsion (PVT)-enhanced spin–orbit coupling (PVT-SOC) in PSs has been first revealed. PVT is verified to be a widespread intrinsic property of quinoid cyanine (QCy) dyes that occurs on an extremely short time scale (10–10 s) and can be captured by transient spectra. The PVT property can provide reinforced SOC as the occurrence of ISC predicted by the El Sayed rules (1ππ*–3nπ*), which ensures efficient photosensitization ability for QCy dyes. Hence, QTCy7-Ac exhibited the highest singlet oxygen yield (13-fold higher than that of TCy7) and lossless fluorescence quantum yield (ΦF) under near-infrared (NIR) irradiation. The preeminent photochemical properties accompanied by high biosecurity enable it to effectively perform photoablation in solid tumors. The revelation of this property supplies a new route for constructing high-performance PSs for achieving enhanced cancer phototherapy.
Short abstract
Photoinduced molecular vibrational torsion-enhanced spin−orbit coupling (PVT-SOC) in quinoid cyanine dyes has been revealed. This strategy enables cyanine photosensitizers to have enhanced intersystem crossing for cancer photodynamic therapy.
Introduction
Photodynamic therapy (PDT) has been recognized as an effective mild treatment that is minimally invasive and has high spatiotemporal selectivity for cancers.1−6 Among several essential factors of photodynamic therapy, the photosensitizer (PS) is the most important component, which is responsible for generating triplet excited states (Tn) through the intersystem crossing (ISC) process under photoexcitation to produce reactive oxygen species (ROS).7−14 Therefore, regulating the excited states of photosensitizers and developing new stimulative ISC strategies have been widely concerned.15−19
Cyanine dyes are a series of organic functional materials with excellent properties, including convenient synthesis, large molar extinction coefficients, and adjustable absorption and emission wavelengths from the visible to near-infrared (NIR, >650 nm) regions.20,21 In addition, the cationic structure of cyanine dyes allows them to be anchored to cell membranes or mitochondrial membranes, thereby enhancing the cellular uptake and further increasing the accumulation in tumor tissues.22 For quinoid cyanine (QCy) dyes, a special class of cyanine dyes, the reaction with the substrate could restore the cyanine-like structure of the molecule, which could enhance the intramolecular charge transfer (ICT) effect and recover the NIR fluorescence signal.23 Such a structure has only been used in the field of cancer diagnosis or fluorescence imaging so far, which has limited the further application of these intelligent molecules. Therefore, it is valuable to perform research to develop highly efficient photosensitizers with this cyanine framework.
Currently, the heavy-atom effect is a general approach to enhance ISC efficiency and 1O2 yield for cyanine photosensitizers by introducing halogen atoms with larger atomic numbers into their skeletons,24−26 which works because the spin–orbit coupling (SOC) constant is approximately proportional to Z4, where Z is the atomic number.16,27 Nevertheless, the fatal deficiency of this strategy is that it also enhances the ISC of T1 → S0, resulting in the drastically contracted triplet lifetimes,28,29 and besides, the connatural cytotoxicity and poor water solubility caused by heavy atoms also limit their further applications.30−32 In order to solve this problem, many strategies have been proposed,33−35 among which the spin–orbit charge-transfer ISC (SOCT-ISC) mechanism is a widely used method to enhance the SOC process through charge transfer (CT) and charge recombination (CR).36 In this process, the transfer of electrons between the two mutually orthogonal orbitals changes their spin angular momentum, therefore enhancing ISC, which was predicted by the El Sayed rules in the 1960s.37 Nonetheless, this mechanism is faced with the challenge of limited efficiency in the environments with different polarity due to the existence of a Marcus inverted region in electron transfer. With the increase of Gibbs energy change (ΔG) in the charge transfer process, molecules prefer to proceed via charge recombination to the ground state (CRS) rather than to the triplet state (CRT) after charge separation (CS), which leads to the decrease of ISC rate.38 Therefore, it is urgent to develop novel cyanine photosensitizer strategies to solve these problems.
Hence, according to our research, a photoinduced molecular vibrational torsion (PVT)-enhanced spin–orbit coupling (PVT-SOC)-induced intersystem crossing mechanism in QTCy7 dyes and its contribution to enhance ISC capability have been discovered, which we believe can be a novel approach to construct heavy-atom-free photosensitizers for PDT applications. The PVT-SOC property can provide a reinforced spin–orbit coupling through the formation of the vibrational first singlet excited state (S1V) as the occurrence of ISC at a conical intersection point between it and the second triplet excited state (T2) with partial 3nπ* characteristic predicted by the El Sayed rules (1ππ*–3nπ*) without the introduction of heavy atoms. The PVT effect can occur rapidly (kPVT ∼ 1010 s–1) in the excited state, and the intersystem crossing rate constant can be easily adjusted via introduction of different electron effect groups. By this means, the singlet oxygen yield (ΦΔ) of this series of photosensitizers can be promoted to above 20%. Therein, the introduction of a para-ester group extremely heightens this effect, resulting in the highest singlet oxygen yield (ΦΔ = 33.8%). In addition, QTCy7-Ac can quickly destroy the integrity of cell membranes under light irradiation with extremely low cytotoxicity, and the strong tumor targeting ability also improves the photoinhibition efficiency for solid tumors due to the inherent targeting capability of the QTCy7 scaffold. We believe that our discovery not only breaks through the restriction of QCy dyes used only in fluorescence detection but also provides a novel approach for constructing efficient photosensitizers.
Results and Discussion
Molecular Design and Synthesis
As a particular class of QCy dyes, QTCy7 compounds possess a long absorption and emission wavelength and adequate molar extinction coefficient in the NIR region, which benefits from the 1ππ* transition process of its excited state. By embedding a quinone structure in the conjugated chain of the molecule, the nonbonding orbital in the oxygen atom can participate in the process of excited-state electron transfer, resulting in the valid doping of the 3nπ* component in the low-lying triplet excited states, thus enhancing SOC from the 1ππ*–3nπ* mechanism.39−41 Simultaneously, the introduced rigid benzene ring structure also prevents cis–trans isomerization and rotation of the flexible chain, which partly reduce the thermal relaxation of the excited-state energy (Figure 1).
Figure 1.
Chemical structures of TCy7 and QTCy7-R.
For QTCy7-R, all of the compounds were synthesized according to the synthetic routes detailed in Scheme S1. In brief, hydroxyphenylaldehydes containing different substituents (−Me, −Ph, −Ac, −CHO) were prepared by Duff reaction of the corresponding phenols with urotropine, and then the benzothiazole salt was reacted with the above condensation agents via Knoevenagel condensations. All the reaction products were fully characterized by ESI-HRMS, 1H NMR, and 13C NMR (Figures S24–S43).
Determination of Spectra and Interpretation of Computational Mechanism
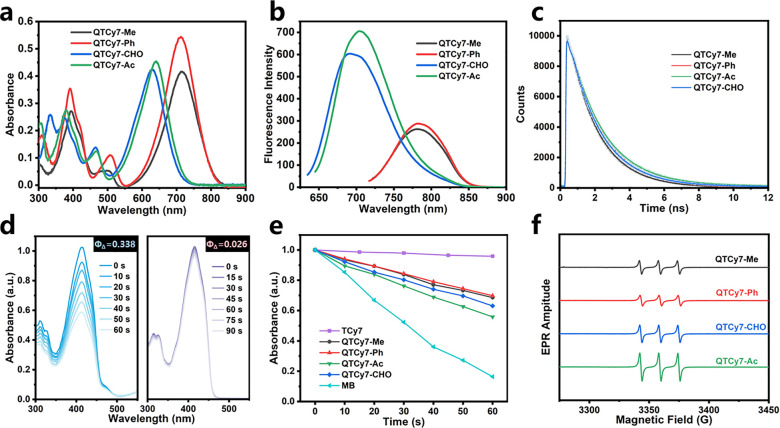
First, the UV–vis absorption and fluorescence emission spectra of the series of compounds were measured and analyzed. QTCy7-Me and QTCy7-Ph displayed intense UV–vis absorption peaks at about 710 nm with the molar extinction coefficient of approximately 4 × 104, while QTCy7-Ac and QTCy7-CHO exhibited a similar absorbance band at around 630 nm (Figure 2a), which indicated that the introduction of an electron-withdrawing group (EWG) could blue-shift the wavelength, and similarly, this blue-shift effect was also present in the emission spectra (Figure 2b). Instead, as shown in Table 1, conjugated EWG enhanced the fluorescence quantum yield (up to 28–31%), suggesting a strong NIR fluorescence imaging capability. Concurrently, the Stokes shifts of all four compounds were more than 60 nm (Figure 2a,b and Table 1), which could effectively improve the signal-to-noise ratio (SNR) of fluorescence compared to the ordinary TCy7. The fluorescence lifetimes of all four compounds were within 2 ns, indicating no delayed fluorescence generation (Figure 2c and Table 1). The UV–vis absorption spectra of the series compounds solvents of different polarity showed a small distinction, indicating a feeble solvation effect, which ensured the stability in different environments (Figure S1).
Figure 2.
Spectral tests of QTCy7-R. (a) UV–vis absorption spectra and (b) fluorescence emission spectra of QTCy7-R (5 μM) in DCM. (c) Time-correlated single-photon counting fluorescence intensity decay of QTCy7-R. (d) DPBF degradation induced by QTCy7-Ac (left) and TCy7 (right) under 660 nm irradiation (2 mW/cm2). (e) Normalized DPBF degradation (415 nm) caused by different compounds under 660 nm irradiation in DCM. (f) EPR signals of 1O2 induced by different compounds under 660 nm irradiation in DCM.
Table 1. Photophysical Properties of QTCy7-R.
| compd | λabs (nm)a | λem (nm)b | Δλ (nm)c | ΦF (%)d | τS (ns)e | ΦΔf |
|---|---|---|---|---|---|---|
| QTCy7-Me | 714 | 780 | 66 | 16.7 | 1.70 | 0.225 |
| QTCy7-Ph | 711 | 783 | 72 | 17.2 | 1.90 | 0.212 |
| QTCy7-CHO | 630 | 690 | 60 | 28.0 | 1.82 | 0.263 |
| QTCy7-Ac | 636 | 703 | 67 | 31.4 | 1.97 | 0.338 |
| TCy7 | 770 | 793 | 23 | 19.3 | 1.54 | 0.026 |
Maximum absorption wavelength of the compound in dichloromethane.
Maximum emission wavelength of the compound in dichloromethane.
Stokes shift of QTCy7-R.
Absolute fluorescence quantum yield.
Fluorescence lifetime of QTCy7-R in dichloromethane.
Singlet oxygen quantum yield in dichloromethane with methylene blue (MB) as the standard (ΦΔ = 0.57 in dichloromethane).
Afterward, the singlet oxygen yields of the series of molecules were preliminarily measured and analyzed by the 1,3-diphenylisobenzofuran (DPBF) decay curve method (Figures 2d,e and S2). Compared with the reference TCy7, all QTCy7-R showed spectacular singlet oxygen generation ability (Table 1), particularly for QTCy7-Ac, which possessed much higher ΦΔ than TCy7 (33.8% vs 2.6%). Meanwhile, in order to exclude the effect of the rigid structure, ΦΔ of a Cy7-like reference molecule (TCy7C) with a rigidified structure but absence of an oxygen atom was measured. The singlet oxygen yield of TCy7C was 2.2%, even lower than that of TCy7 under the same conditions (Figure S3). Furthermore, the enhanced characteristic singlet oxygen signal (with TEMP as the scavenger) in electron paramagnetic resonance (EPR) measurements also showed similar results (Figure 2f). On the basis of this experimental evidence, we hypothesized that introduction of the quinone structure could be proved to effectively change the excited-state properties of TCy7 and improve the ISC process. Notably, the oxygen atom of the quinone structure preferred the n−π* transition in some cases, which therefore boosted the spin-forbidden electronic transition of singlet to triplet excited states through the ISC process to generate triplet excitons. Hence, the SOC enhancement caused by the 1ππ*–3nπ* process may be the emphasis of the ISC process.
To probe the mechanism of the observed phenomenon and further validate our hypothesis, ultrafast excited-state dynamic behaviors were implemented by using femtosecond transient absorption spectroscopy (fs-TA) (Figures 3a and S4a–c). In order to obtain the complete process of excited states, a 350 nm laser was chosen as a pump to populate the high singlet excited states (Sn) from the ground state (S0). Taking QTCy7-Ac as an example, according to the steady-state absorption spectra, it displayed distinct negative absorption bands at its maximum absorbance, which were assigned to the ground-state bleaching (GSB). As shown in Figure 3a,b, with a period of 260 fs internal conversion (IC) (Sn–S1), QTCy7-Ac demonstrated a significant fast-rising positive absorption peak at ca. 505 nm, which was attributed to the excited-state absorption (ESA) of S1. With the growth of delay time, the ESA of S1 gradually decayed (τ = 7.65 ps); meanwhile, beyond our expectation, accompanied by a particularly noticeable peak at ca. 583 nm increasing promptly, this new emerging ESA peak reached its maximum within about 21.98 ps. Similar phenomena were found in the transient spectra of the other three compounds (Figures S4d–f). However, for the control TCy7, there was no obvious formation of another ESA peak in transient spectra (Figure S5). These peaks in QTCy7 were not simply ascribed to the generation of a triplet state, because its (hereinafter referred to as the X state) lifetime was similar to that of S1 (τX = 2.138 ns for this state and τS1 = 2.142 ns for S1). Furthermore, the rate of X state formation was calculated to be 4.54 × 1010 s–1 for QTCy7-Ac based on the rise time (kX = 1/τX,rise), which was close to the order of magnitude of the intramolecular distorted vibration behavior. Therefore, it was reasonable to assume that this process was controlled by the distorted vibration of the excited molecules. Moreover, such phenomenon in fs-TA of general cyanine photosensitizers with mediocre singlet oxygen yields was not observed in the earlier literature,35 indicating that the formation of the X state plays an important role in the ISC process (Figure 3c). Meanwhile, the triplet lifetime of this series of molecules was evaluated and inspected for further applications (Figures 3d and S4g–i). QTCy7-R exhibited almost reasonable triplet lifetimes, in which QTCy7-Me and QTCy7-Ph owned relatively long triplet lifetimes (about 12 μs) compared with QTCy7-Ac and QTCy7-CHO (about 2 μs). These lifetimes are basically enough for practical applications compared to the picosecond-level sensitization process between oxygen and molecules in excited states. In addition, due to the extremely weak triplet generation efficiency, the triplet lifetime of TCy7 was not obtained.
Figure 3.
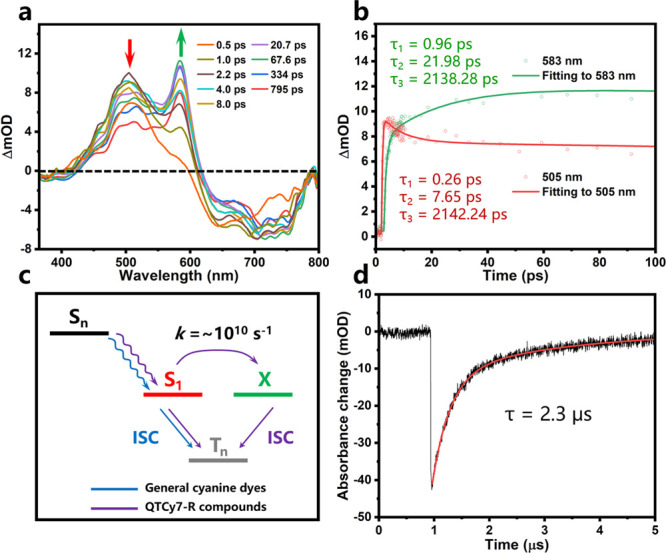
(a) Femtosecond transient absorption spectroscopy (fs-TA) analysis for QTCy7-Ac at different pump–probe delay times. Different color lines represent spectra at different times; λex = 350 nm. (b) Kinetic traces and fitting lines of QTCy7-Ac taken through the representative ESA wavelength. (c) Schematic diagram of intersystem crossing process of excited states in general cyanine dyes and QTCy7-R compounds. (d) Kinetic traces of the triplet state of QTCy7-Ac (5 μM) in deaerated dichloromethane at 635 nm. λex = 610 nm.
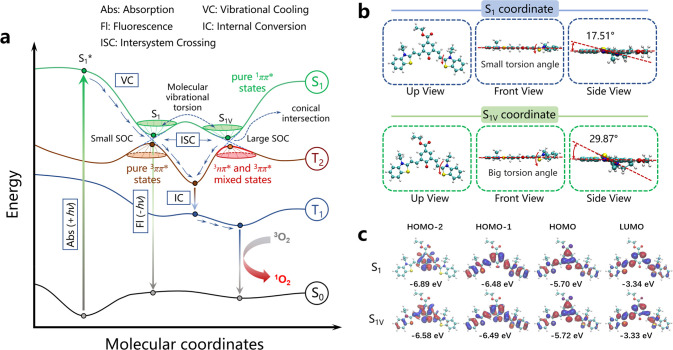
In general, the S1–T1 transition can occur in two ways: (1) S1 is directly spin–orbit-coupled to the higher vibrational energy level of T1; (2) S1 is spin–orbit-coupled to Tn, after which internal conversion from Tn to T1 proceeds. For method (1), the ISC rate depends on the energy gap between S1 and T1; however, for method (2), in addition to the energy gap between S1 and Tn, we also expect that S1 should have a necessary vibrational motion to allow the molecule to look for an effective spin–orbit coupling mechanism in different conformations. Therefore, ISC is possible in all of the conformations of S1 isoenergetic points. Thus, the first-principles time-dependent density functional theory (TD-DFT) research on both singlet and triplet excited states was executed. According to the TD-DFT calculations, as depicted in Figure 4a, unlike ordinary molecules, compounds QTCy7-R had two steady energy-similar S1 states (Franck–Condon minimum), S1 and S1V, at almost the same energy (ΔE ⩽ 0.01 eV; Table S1). However, as shown for QTCy7-Ac in Figure 4b, these states had completely different molecular configurations, embodying the fact that configuration in S1V had a larger out-of-plane torsion than S1, as its dihedral angle could reach about 30°. Simultaneously, the frontier molecular orbital (FMOs) at the S1 and S1V coordinates were quite different (Figure 4c), with the HOMO–1 in the S1V configuration exhibiting more n orbital characteristic on oxygen atom than the S1 configuration, while both S1 and S1V had obvious n orbital characteristic in HOMO–2 and π/π* orbital characteristic in HOMO/LUMO, which provided a prerequisite for orbital transitions between excited states. A similar situation was observed in the other three QTCy7-R compounds (Figures S6 and S7a,b). On the contrary, there was no n orbital characteristic in the FMO of TCy7 (Figure S7c,d).
Figure 4.
(a) Schematic diagram of the PVT-SOC mechanism in QTCy7-R. (b) Views from different locations (up, front, and side) of QTCy7-Ac and the torsion angles in the S1 and S1V coordinates. (c) Frontier molecular orbitals (FMOs) and the corresponding energies of QTCy7-Ac in the S1 and S1V coordinates.
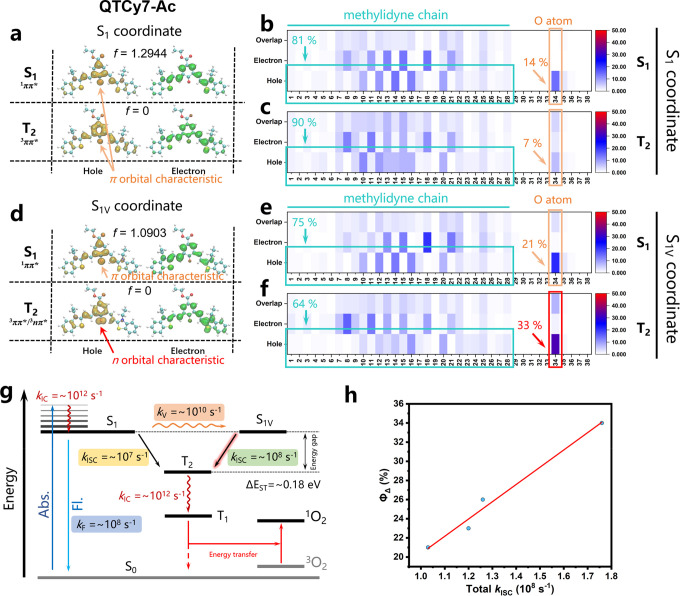
In order to describe the process of molecular excitation more clearly and intuitively, hole–electron analysis was utilized to investigate the characteristics of electron excitation, which was similar to natural transition orbital (NTO) analysis but more universal (all molecular orbital and hole–electron analyses were carried out using Multiwfn 3.842,43). The first singlet excited state of all four compounds (QTCy7-R) was a bright locally excited (LE) state with typical π–π* characteristic whether it was in the S1 or S1V configuration, as displayed for QTCy7-Ac in Figure 5a,d. As shown in the hole–electron distribution and heat map (Figure 5b,e; the atomic serial numbers are shown in Figure S8), the hole was concentrated on π orbitals of Q-carbonyl (ca. 14–21%) and the methylidyne chain (ca. 86–79%), while the electron was almost completely assembled at the methylidyne chain of QTCy7-Ac, indicating a large oscillator strength (f ≈ 1.2), which convincingly proved the formation of the LE singlet excited state with π–π* characteristic.
Figure 5.
Hole–electron distribution and excited-state process analysis. (a) Hole–electron distribution of QTCy7-Ac at S1 and T2 in the S1 coordinate. (b, c) Heat maps of the hole–electron distributions of QTCy7-Ac at (b) S1 and (c) T2 in the S1 coordinate. (d) Hole–electron distribution of QTCy7-Ac at S1 and T2 in the S1V coordinate. (e, f) Heat maps of the hole–electron distributions of QTCy7-Ac at (e) S1 and (f) in the S1V coordinate. (g) Schematic diagram of different processes in the excited state of QTCy7-Ac and the orders of magnitude of their rate constants (k). (h) Positive correlation between ΦΔ and total kISC.
Pursuant to the energy gap law, the ISC process tends to take place at singlet and triplet states with close energy levels. As discovered in TD-DFT calculations, the second triplet state (T2) possessed the smallest energy gap to the S1 or S1V state (ΔES1–T2 < 0.2 eV), which ensured efficient ISC premise. Furthermore, as depicted in hole–electron and heat map analysis (Figure 5a,c), the T2 state at the S1 configuration exhibited a significant π–π* characteristic, whose hole and electron were both restricted onto the π and π* orbitals of the methylidyne chain with tiny minority holes distributed around the n orbital of the oxygen atom. In this case, the SOC value between the S1(1ππ*) and T2(3ππ*) states would be relatively small, which was not conducive to the occurrence of ISC. Conversely, the T2 state at the S1V configuration preferred to display n−π* excitation characteristic, in which the hole at the oxygen atom displayed a distinct dumbbell-like shape hovering on either side of the atom (Figure 5d). The formation of the 3nπ* state was mainly ascribed to the excitation between the n orbital part (33%) of the oxygen atom in the hole distribution and the π* orbital part of the methylidyne chain in the electron distribution. Relatively, the T2 state at the S1 configuration did not have any obvious n orbital characteristics, and the hole concentrated on the oxygen atom (7%) was also distinctly less than that under the S1V configuration (Figure 5c,f). In other words, the T2 state at the S1V configuration tended to possess more charge transfer (CT) state characteristic than the S1 configuration, which promoted the SOC by constructing the transition of 1LE → 3CT. On the contrary, the 1LE → 3LE transition would reduce the SOC on account of the 1ππ–3ππ* transition in the S1 configuration. Similarly, this feature was also reflected in the three other compounds (QTCy7-R) with similar structures (Figures S9–S11). Nonetheless, no analogous properties were found in TCy7 due to the absence of the S1V coordinate (Figure S12).
In order to obtain an accurate evaluation of ISC theoretically, the ISC rate constants were calculated according to Marcus theory.44,45 According to the calculations, compared with the configuration at S1, the SOC values of S1V/T2 were significantly large due to the conservation of spin orbital angular momentum in the 1ππ*–3nπ* transition. Meanwhile, as described in Figure 5g and Table S2, the ISC rate constants (kISC) in QTCy7-R at the S1V configuration were an order of magnitude higher than those at the S1 configuration (∼108 s–1vs ∼107 s–1), which is large enough to compete with the fluorescence rate constant (kF ∼ 108 s–1). In sharp contrast, kISC in TCy7 of S1/T2 was extremely small (∼104 s–1), which indirectly leads to a relatively small ΦΔ. In development, the total ISC rate constants had an obvious positive correlation with ΦΔ, which proved that the enhancement of ISC was a necessary condition for the improvement of ΦΔ (Figure 5h).
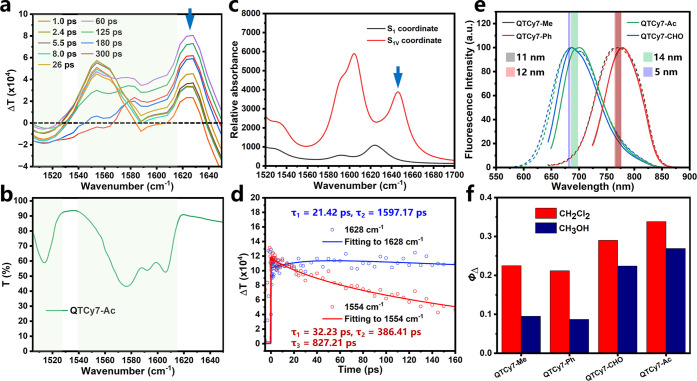
To further prove this conclusion, time-resolved infrared (TRIR) spectroscopy was conducted to study the vibrational modes of molecules at different coordinates in their excited states. Taking QTCy7-Ac as an example, the TRIR spectra possessed a superposed band containing both bleach vibrational bands and transient absorption bands at the wavenumbers from ca. 1505 cm–1 to ca. 1615 cm–1 (Figure 6a,b). With a longer time, a new prominent absorption band at ca. 1628 cm–1 appeared and gradually enhanced (Figure 6a, blue arrow), which indicated that a new vibrational pattern was generated in excited states. Therefore, the vibrational modes of QTCy7 in the S1 and S1V coordinates were calculated, and the results were plotted as simulated IR spectra. As depicted in Figure 6c, compared with the spectra at the S1 coordinate, a new absorption band at ca. 1644 cm–1 was observed only at the S1V coordinate, which possessed high similarity with the results measured in the TRIR experiment. Furthermore, the vibrational vector at this absorption band was analyzed (Figure S16), and the vibrational mode was ascribed to stretching and oscillating vibration of the methylidyne chain (It is worth noting that the obvious absorption band at ca. 1623 cm–1 in Figure 6b was ascribed to vibration of the benzene ring in benzothiazole group, which appeared in either the S1 coordinate or S1v coordinate.). Simultaneously, exponential fitting also indicates that the vibrational mode at ca. 1628 cm–1 tended to be at a maximum at about 22.65 ps (Figure 6d), which was on the same order of magnitude as the data obtained in the fs-TA experiments (21.98 ps). Analogously, similar phenomena had been found in TRIR experiments of QTCy7-Me and QTCy7-Ph (Figures S13, S14, and S16). Compared with QTCy7, the control TCy7 only showed bleach vibrational bands at the wavenumbers of ca. 1539 cm–1, but no similar transient absorption bands were observed (Figure S15). However, due to the poor photostability of QTCy7-CHO under the experimental conditions, effective data were not obtained. Hence, it is reasonable to suppose that the changes in TRIR spectra reflect the molecular coordinate change (S1–S1V) by PVT in excited states.
Figure 6.
TRIR experiments and the comparison of excited-state properties between the DCM and MeOH of QTCy7-R. (a) TRIR spectra for QTCy7-Ac at different pump–probe delay times. Different colored lines represent spectra at different times. (b) FTIR spectrum for QTCy7-Ac.(c) Simulated IR spectra for QTCy7-Ac at the S1 and S1V coordinates. (d) Kinetic traces and fitting lines of QTCy7-Ac taken through 1554 and 1628 cm–1. (e) Comparison of normalized emission spectra of QTCy7-R in dichloromethane and methanol. Solid lines represent spectra in dichloromethane, and dashed lines represent spectra in methanol. (f) Comparison of ΦΔ of QTCy7-R in methanol and dichloromethane.
Meanwhile, as a proof of principle, the fluorescence emission spectra and ΦΔ in DCM and MeOH of all four compounds were evaluated and analyzed to illustrate the forecast. The fluorescence emission spectra in DCM and MeOH exhibited similar wavelengths (Δλ < 15 nm), which implied that the energy of the 1ππ* state was scarcely influenced (Figure 6e). On the other hand, it was widely accepted that formation of a hydrogen bond between the carbonyl group and protic solvent would lead to instability of the 3nπ* state and therefore increase its energy,46,47 which further reduced the efficiency of the 1ππ*–3nπ* transition and ISC process. As we expected, the ΦΔ in MeOH demonstrated an apparent quenching compared with that in DCM (Figures 6f and S17), which supplied a strong piece of circumstantial evidence for the hypothesis that the formation of the 3nπ* state was important in the S1V → T2 transition process.
From what has been discussed above, the formation of X states from S1 in fs-TA can be attributed to a high probability of the S1 → S1V conversion process. As a matter of fact, the intramolecular vibrational torsion indeed occurs on approximately the picosecond time scale, which provides a credible precondition for the PVT-SOC mechanism to be proposed. In our theory, there are three necessary prerequisites for the PVT-SOC mechanism: (1) there must be an intramolecular vibrational torsion motion in the excited states which is faster than the emission of the fluorescence process; (2) the vibrational torsion motion must bring the molecule to an energy-close thermodynamic steady-state point (Franck–Condon minimum) at the potential energy surface; (3) the mixing of electronic states caused by the distortion vibration must inevitably lead to the increase of SOC at this Franck–Condon point/conical intersection point (schematic diagram of PVT-SOC in Figure 4a).
In Vitro Application
In order to further investigate the PDT ability of QTCy7-R on cancer cells, the UV–vis absorption and emission spectra and 1O2 production capacity of QTCy7-R were tested in phosphate-buffered solution (PBS) to better mimic the cellular environment. As shown in Figure S18, compared with UV–vis absorption spectra in organic solution, the spectra of QTCy7-R in PBS all have different degrees of blue shift. QTCy7-Me (701 nm) and QTCy7-Ac (610 nm) were relatively less affected, while QTCy7-Ph (613 nm) and QTCy7-CHO (552 nm) showed distinct blue shifts, which might be due to the aggregation of them in aqueous medium. Meanwhile, the fluorescence quenching of QTCy7-Ph and QTCy7-CHO also confirmed their aggregation in aqueous solution compared to the intense fluorescence emission of QTCy7-Ac. Furthermore, singlet oxygen sensor green (SOSG) was selected to investigate the 1O2 quantum yield in PBS. As depicted in Figure S18i, compared with the control TCy7, all QTCy7-R exhibited considerable 1O2 production capacity, especially QTCy7-Ac, whose 1O2 quantum yield was an order of magnitude bigger than those of the other QTCy7 and even 5.8 times as much as that of MB.
In view of QTCy7-R having strong singlet oxygen generation potency and reasonable wavelength, human hepatocellular carcinoma cells were selected for photoactivity experiments in vitro. On account of their low molecular weights and intrinsic positive cationic charge, QTCy7-R could be quickly taken up by cells within 90 min (Figure S19a,b). Therein, QTCy7-Me and QTCy7-Ph could get inside cells due to the evident fluorescence inside the cells. In contrast, it could be sufficiently indicated that QTCy7-Ac or QTCy7-CHO could effectively adhere to the cell membrane by the obvious red fluorescence signal on the cell edge. According to our supposition, the enhanced partial cationic charge caused by the introduction of the EWG in QTCy7-Ac or QTCy7-CHO made it easier for them to cling to the cell membrane with negative charge than to cross it.
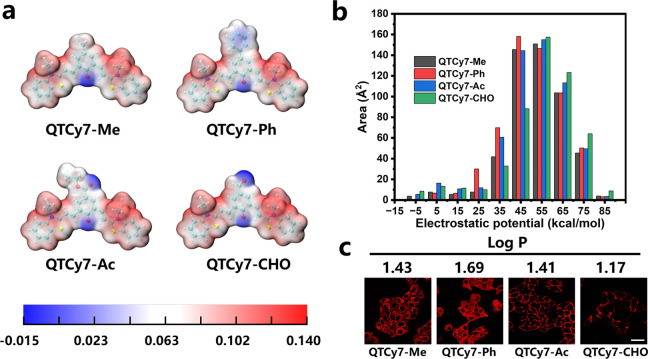
In order to guarantee the preciseness of the supposition, electrostatic potential (ESP) analysis of the molecular surface was conducted to quantify the surface charge distribution.48 With a unit positive charge, the surface potential of the molecule was mostly positive. As depicted in Figure 7a, most of the positive electrostatic potential regions on the surface of the molecules were located on the benzothiazole groups in all four compounds, while only a small amount of positive electrostatic potential and negative electrostatic potential are distributed on the benzoquinone group and the oxygen atom, respectively. Furthermore, in the high surface positive potential region (>55 kcal/mol), QTCy7-CHO possessed the largest effective surface area, which was ascribed to the strong electron withdrawing properties of the aldehyde group. On the contrary, QTCy7-Ph preferred a larger effective surface area at the low surface positive potential region (<55 kcal/mol). In addition, the areas of surface electrostatic potential in QTCy7-Me and QTCy7-Ac were maintained in a median (QTCy7-Ac was partly larger than QTCy7-Me) (Figure 7b). The oil–water partition coefficient (Log P) was also taken into account to study cell uptake. According to Log P (Figure 7c), the introduction of phenyl enhanced the lipophilicity of QTCy7-Ph, resulting a good membrane permeability, whereas the introduction of formyl enhanced the hydrophilicity of QTCy7-CHO, which decreased its cellular uptake capacity. For QTCy7-Me and QTCy7-Ac, although they had a similar Log P, QTCy7-Me was better than QTCy7-Ac in cell membrane permeability due to the influence of the surface potential distribution. Interestingly, the distribution of electrostatic potential on the surface and Log P of molecules was obviously correlated with the uptake of the four compounds: the stronger the positive electrostatic potential on the surface, the harder it is to cross the cell membrane, and the better the hydrophilicity, the harder the cellular uptake. This explains why only a small amount of QTCy7-CHO adhered on the membrane surface (Figure 7c).
Figure 7.
(a) Electrostatic potential analysis of QTCy7-R. (b) Area distribution of different ESP intervals in different compounds. (c) Log P and cellular uptake of QTCy7-R in HepG2 cells after being incubated for 90 min (scale bar: 20 μm).
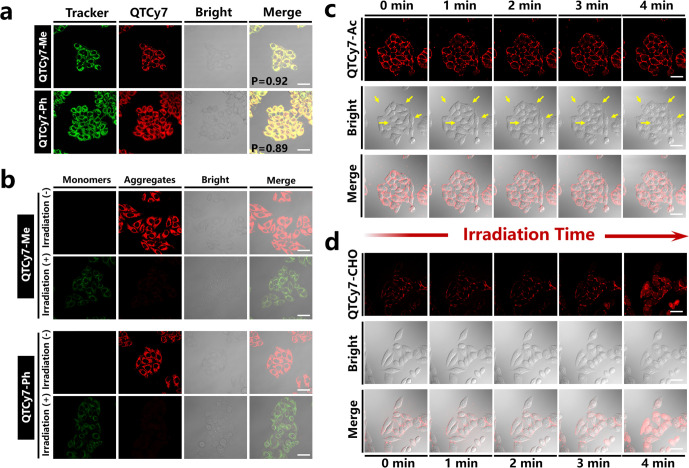
Interestingly, QTCy7-Me and QTCy7-Ph had highly efficient accumulation in cell mitochondria, which could be verified by the intracellular distribution measurement using commercial subcellular organelle localization trackers (Figure 8a). The red fluorescence signal produced by QTCy7-Me or QTCy7-Ph overlapped well with the green fluorescence representing MitoTracker Green in mitochondria (Pearson correlation coefficient of 0.92 and 0.89 for QTCy7-Me and QTCy7-Ph, respectively), which proved that there is a high affinity between QTCy7-Me or QTCy7-Ph and mitochondria. Next, in order to verify the disruption effect on cell structural integrity of the 1O2 generation by QTCy7-R under irradiation, JC-1 staining imaging was conducted to monitor the mitochondria membrane potential. As depicted in Figure 8b, under 660 nm irradiation (6 J/cm2), QTCy7-Me and QTCy7-Ph could induce severe mitochondrial depolarization accompanied by the significant weakening of red fluorescence signal (JC-1 aggregates) and enhancement of the green fluorescence signal (JC-1 monomers). On the other hand, cell membrane damage caused by the photooxidation of QTCy7-Ac and QTCy7-CHO could be confirmed by a visualized observation. With the extension of illumination time, the formation of distinct bubblelike structures could be observed on the surface of the cell membrane after being incubated with QTCy7-Ac (Figures 8c and S20a), which proved the destruction of the integrity of the cell membrane and extravasation of intracellular material caused by 1O2. In contrast, QTCy7-CHO did not cause severe cell membrane rupture because of a slight change in cell morphology (Figures 8d and S20b). Nevertheless, unlike QTCy7-Ac, the fluorescence intensity of QTCy7-CHO gradually enhanced from the membrane surface to the interior of the cell, indicating the change of cell membrane permeability, which thus inferred that QTCy7-CHO could also destroy the cell structure under irradiation. Simultaneously, more importantly, the fluorescence intensity in cells did not weaken during light irradiation, proving the good photostability of QTCy7-Ac/QTCy7-CHO as an efficient photosensitizer. Furthermore, the lactate dehydrogenase (LDH) content in cell culture medium was measured to characterize the extent of cell membrane damage.49 As shown in Figure S19c, the LDH content exhibited a remarkable increase after incubation with QTCy7-Ac compared to QTCy7-CHO under irradiation, and its release rate reached about 70%, indicating a fierce cell membrane destruction capacity.
Figure 8.
Confocal laser scanning microscopy (CLSM) imaging of cell uptake and damage. (a) Subcellular colocalization images of QTCy7-Me/QTCy7-Ph and MitoTracker Green (MTG) in HepG2 cells; P is the colocalization coefficient. (MTG: λex = 488 nm, λem = 500–550 nm; QTCy7-Me/QTCy7-Ph: λex = 640 nm, λem = 700–800 nm; scale bars: 20 μm). (b) Mitochondrial membrane potential detection assays implemented by JC-1 staining. (JC-1 monomer: λex = 488 nm, λem = 500–550 nm; JC-1 aggregate: λex = 488 nm, λem = 560–590 nm; scale bars: 20 μm). (c) QTCy7-Ac and (d) QTCy7-CHO caused cell membrane destruction images at 0–4 min (λex = 640 nm; λem = 650–750 nm; scale bars: 20 μm).
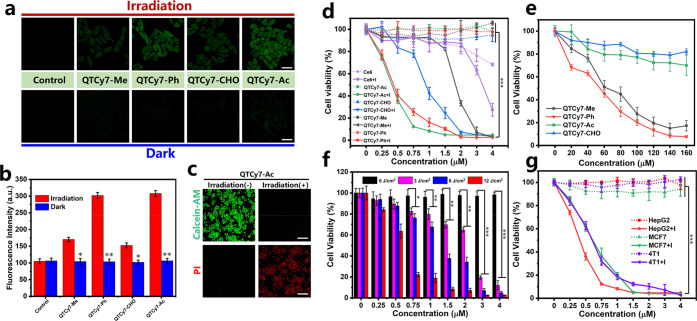
In order to explore the mechanism of cellular structure destruction caused by QTCy7-R, intracellular ROS generation was investigated by DCFH-DA reagent. As exhibited in Figure 9a, there was an obvious fluorescence enhancement upon treatment with QTCy7-R in contrast to the control group after light irradiation, indicating that these four molecules could produce ROS in cells. Among them, QTCy7-Ph and QTCy7-Ac showed a stronger fluorescence signal (Figure 9b). Ulteriorly, cell destruction caused by ROS generation was assessed by Calcein-AM/propidium iodide (PI)-mediated fluorescence imaging (Figures 9c and S21). Compared with the intense green fluorescence signal in living cells, after illumination with 660 nm irradiation (10 mW/cm2) for 5 min, the strong red fluorescence signal generated by PI entering the nuclei of dead cells indicated that all four molecules can effectively kill cells.
Figure 9.
Intracellular ROS production and cell destruction assays. (a) Images showing intracellular ROS production induced by QTCy7-R under irradiation and dark conditions (λex = 488 nm; λem = 500–550 nm; scale bars: 20 μm) and (b) corresponding fluorescence intensities. (c) Cell viability detection images measured by Calcein-AM/PI for QTCy7-Ac (Calcein-AM: λex = 488 nm, λem = 500–550 nm; PI: λex = 561 nm, λem = 580–630 nm; scale bars: 120 μm). (d) Quantitative detection of cell viability (MTT assay) for QTCy7-R and Ce6 with/without 660 nm irradiation (12 J/cm2). (e) Cell viability of HepG2 cells treated with QTCy7-R at different concentrations under dark condition. (f) Cell viability of HepG2 cells treated with QTCy7-Ac under different light doses. (g) Cell viability of different cancer cells (HepG2, MCF7, and 4T1) treated with QTCy7-Ac under 660 nm irradiation (12 J/cm2). Data are expressed as mean ± SD. **, P < 0.01; ***, p < 0.001; ****, P < 0.0001 as determined by Student’s t test.
To further quantitatively explore the in vitro anticancer potency of QTCy7-R, the methyl thiazolyltetrazolium (MTT) assay was employed to detect cell viability. As shown in Figure 9d, all four compounds exhibited negligible cytotoxicity toward HepG2 cells in dark condition, yet they could effectively inhibit cell proliferation in a concentration-dependent manner under the same irradiation condition. QTCy7-CHO and QTCy7-Me showed relatively general inhibition in cell growth, and their half-maximal inhibitory concentration (IC50) values were about 1.8 and 0.9 μM, respectively. In contrast, a powerful cell-killing capability was demonstrated for QTCy7-Ph and QTCy7-Ac with nearly the same IC50 of about 0.3 μM after exposure to NIR light. Notably, ROS production of these four molecules showed a positive correlation with the cell damage ability, illustrating that 1O2 generation was the direct cause of cell death. Moreover, the cell growth inhibition effect of all QTCy7-R was better than that of commercial chlorin e6 (Ce6) photosensitizer (IC50 = 3.5 μM), which demonstrated that this series of molecules possessed excellent cell killing capacity. Next, we evaluated the cytotoxicity under dark conditions of this series of compounds at high concentrations (Figure 9e), as due to the characteristics of mitochondrial accumulation, QTCy7-Me and QTCy7-Ph might lead to mitochondrial depolarization and apoptosis at high concentrations. However, its IC50 value could still reach about 60 μM, which was almost lower than those of all cyanine photosensitizers reported. On the other hand, 70–80% of cells could still survive even at an exceedingly high QTCy7-Ac or QTCy7-CHO concentration of around 160 μM, indicating that the intracellular microenvironment would not be disturbed owing to their adhesion properties on cell membranes. The photocytotoxicity index value (dark IC50/light IC50) of QTCy7-Ac was >500, manifesting that QTCy7-Ac is a PS with high efficacy but low cytotoxicity, which is extremely promising in clinical application.
Hence, the cell inhibition effect of QTCy7-Ac was further investigated under different light doses (0, 3, 6, and 12 J/cm2) (Figure 9f). The IC50 values were about 2.0, 1.2, and 0.3 μM in the light of 3, 6, and 12 J/cm2, respectively, which suggested a remarkable potency for QTCy7-Ac to generate 1O2 under weak light excitation. Moreover, to further prove the nonselective cell killing ability of QTCy7-Ac, two other cancer cells (murine mammary carcinoma cells (4T1) and human mammary carcinoma (MCF7)) were used to detect the growth inhibition potential. As shown in Figure 9g, the IC50 values for them were about 0.5 and 0.6 μM, indicating its broad-spectrum cancer cell destruction as a photosensitizer. These results clearly demonstrated that nascent QTCy7-Ac photosensitizer under the new ISC-enhanced strategy played a powerful role in the inhibition of cancer cells.
In Vivo Application
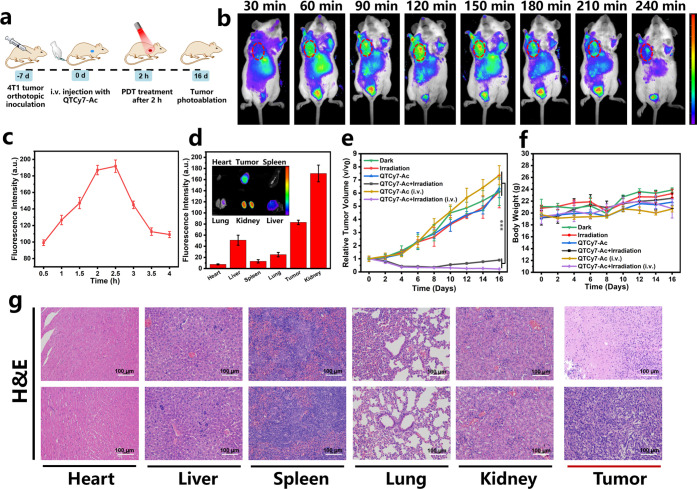
The prospective in vitro results prompted us to further explore in vivo application possibility of QTCy7-Ac, and a solid tumor model of BALB/c mice was established by inoculating 4T1 cells subcutaneously under the armpit (Figure 10a). All the BALB/c mice were purchased from Liaoning Changsheng Biotechnology Co., Ltd., and the animal experiments were approved by the Local Scientific Research Ethics Review Committee of the Animal Ethics Committee of Dalian University of Technology (ID no. // Ethics Approval No. 2021-043). When the tumor volume reached 100 mm3, fluorescence imaging was performed at different time points after intravenous injection of QTCy7-Ac. As we expected, due to the inherent targeting of Cy7-type molecules in cancer cells,50−52 the tumor region was quickly “lit up” around 60 min (Figure 10b), and the fluorescence signal gradually increased, reaching the maximum value around 2.5 h (Figure 10c). Subsequently, the fluorescence intensity in the tumor site gradually decreased significantly within 4 h, indicating the rapid metabolism and good safety of QTCy7-Ac in vivo. Meanwhile, in the process of fluorescence imaging, an interesting phenomenon was that the bladder region of the mice also showed strong fluorescence signal, which we speculated was related to the dye metabolism through the kidney into the urine. To test this hypothesis, the mice were executed and dissected 2.5 h after intravenous injection, and the fluorescence imaging of the main organs and tumor was performed. As shown in Figure 10d, as we suspected, unlike most cyanine dye molecules that were metabolized through the liver,24,25 the kidney showed a convincingly strong fluorescence signal, suggesting that the dye was metabolized primarily through the kidney. Compared with liver metabolism, drugs metabolized by the kidney have been proved to have better biocompatibility,53,54 which further indicated that QTCy7-Ac has certain medical application value. Simultaneously, the tumor also showed a non-negligible fluorescence signal stronger than the liver, which demonstrated excellent enrichment in the tumor site.
Figure 10.
In vivo solid tumor imaging and inhibition tests. (a) Schematic diagram of PDT for solid tumors mediated by QTCy7-Ac. (b) In vivo real-time fluorescence imaging of 4T1 subcutaneous solid tumor mice after iv injection of QTCy7-Ac. (c) Relative fluorescence intensities at different times (λex = 660 nm, λem = 720 ± 20 nm). (d) Fluorescence imaging and relative fluorescence intensities of main organs and tumor after iv injection of QTCy7-Ac for about 2.5 h (λex = 660 nm, λem = 720 ± 20 nm). (e) Relative tumor volumes with different treatments at different days. (f) Body weights of the mice in various groups at different days. (g) H&E staining assays of main organs and tumors in the QTCy7-Ac + irradiation (i.v.) group (top) and QTCy7-Ac (i.v.) group (bottom) after 16 days of treatment (scale bars: 100 μm). Data are expressed as mean ± SD. **, P < 0.01; ***, p < 0.001; ****, P < 0.0001 as determined by Student’s t test.
In view of the observation that the fluorescence intensity of QTCy7-Ac in the tumor site peaked around 2–2.5 h after intravenous injection, this period was chosen as the PDT time window. After a single PDT treatment (660 nm, 100 mW/cm2, 15 min) (Figure 10e), the groups containing QTCy7-Ac by intratumoral injection initially exhibited ideal tumor inhibition, but the tumor volume increased a little after 8 days, which was caused by the treatment blind spot ascribed to uneven diffusion of intratumoral injection drugs. Even so, this approach achieved partial tumor suppression; the tumor volume was 1-fold compared with the initial situation. However, the groups containing QTCy7-Ac by intravenous injection exposed to NIR light showed extraordinary tumor regression. After PDT treatment, the tumor kept shrinking in size (Figure S22a), which achieved a tumor suppression rate of nearly 95% (Figure S22b). Nevertheless, the tumors in PBS groups increased quickly with a 6-fold increment compared with the initial volume whether exposed to irradiation or not, which ruled out the influence of light on tumor growth. In addition, there was virtually no difference between the intratumoral and intravenous injection groups treated with QTCy7-Ac, with tumor volumes increasing by 6-fold and 7-fold, respectively, which excluded the cytotoxicity of the dye alone.
The efficacy and safety of each therapeutic group were also evaluated by performing a hematoxylin and eosin (H&E) staining assay of major organs and tumor tissue (Figures 10g and S23). Conspicuous morphological damage was observed in tumor tissues treated with QTCy7-Ac-mediated PDT. The distinct cell necrosis and inflammatory response in any major organ sections including heart, liver, spleen, lung, and kidney were not observed, and no abnormal body weight changes were obtained in mice during the treatment (Figure 10f), which further underscored that QTCy7-Ac possessed good biocompatibility and applicability in vivo.
Conclusion
In summary, a novel property called photoinduced molecular vibrational torsion (PVT)-enhanced spin–orbit coupling (PVT-SOC) to enhance intersystem crossing of QTCy7 photosensitizer was first revealed and applied for PDT successfully. By modifying QTCy7 with an ester group and utilizing intramolecular vibrational torsion motion, a series of NIR photosensitizers with high 1O2 yield (33.8%) and reasonable fluorescence quantum yield (31%) were successfully obtained. Combining computational theoretical calculation, transient absorption spectroscopy, and other experimental evidence, the enhanced ISC efficiency was clarified logically as the PVT-SOC mechanism: the photoinduced molecular vibrational torsion motion in the S1 state would bring the molecule to another energy-close Franck–Condon minimum (S1V) at the potential energy surface. Under this circumstance, the 1ππ*–3nπ* transition between S1V and T2 greatly increased the SOC, which subsequently strengthened kISC (∼108 s–1). QTCy7-Ac exhibited optimal photophysical properties and demonstrated excellent PDT potential owing to its low cytotoxicity and broad-spectrum cell membrane destruction at low light doses compared with commercial Ce6 photosensitizer (11-fold enhancement). The structural inherent targeting of tumors endowed QTCy7-Ac to possess a strong ability to accumulate in tumor sites and perform effective tumor photoablation (95%) under NIR irradiation. Meanwhile, great biocompatibility through renal metabolism also raised the possibility of clinical application. Our findings not only break the limitation of traditional QCy dyes used only for cell and molecular recognition but also provide an effective strategy for constructing efficient photosensitizers, which we believe can offer new thought in developing PSs for achieving enhanced cancer phototherapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Projects 22090011 and U1908202) and the Fundamental Research Funds for the Central Universities of China (DUT22LAB608).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.3c00611.
Detailed experimental conditions, methods, synthesis, characterization, biological assays, Figures S1–S43, and Tables S1 and S2 (PDF)
Author Contributions
All of the authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhao X.; Liu J.; Fan J.; Chao H.; Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. 10.1039/D0CS00173B. [DOI] [PubMed] [Google Scholar]
- Huang H.; Banerjee S.; Qiu K.; Zhang P.; Blacque O.; Malcomson T.; Paterson M. J.; Clarkson G. J.; Staniforth M.; Stavros V. G.; et al. Targeted photoredox catalysis in cancer cells. Nat. Chem. 2019, 11, 1041–1048. 10.1038/s41557-019-0328-4. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Kim D. Wireless metronomic photodynamic therapy. Nat. Biomed. Eng. 2019, 3, 5–6. 10.1038/s41551-018-0341-8. [DOI] [PubMed] [Google Scholar]
- Li X.; Lee S.; Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. 10.1039/C7CS00594F. [DOI] [PubMed] [Google Scholar]
- Celli J. P.; Spring B. Q.; Rizvi I.; Evans C. L.; Samkoe K. S.; Verma S.; Pogue B. W.; Hasan T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q.; Fan J.; Long S.; Zhao X.; Li H.; Du J.; Shao K.; Peng X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. 10.1016/j.chempr.2021.10.006. [DOI] [Google Scholar]
- Li M.; Xiong T.; Du J.; Tian R.; Xiao M.; Guo L.; Long S.; Fan J.; Sun W.; Shao K.; et al. Superoxide Radical Photogenerator with Amplification Effect: Surmounting the Achilles’ Heels of Photodynamic Oncotherapy. J. Am. Chem. Soc. 2019, 141, 2695–2702. 10.1021/jacs.8b13141. [DOI] [PubMed] [Google Scholar]
- Monro S.; Colón K. L.; Yin H.; Roque J.; Konda P.; Gujar S.; Thummel R. P.; Lilge L.; Cameron C. G.; McFarland S. A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.; Li M.; Zhang Z.; Yao Q.; Shao K.; Xu F.; Xu N.; Li H.; Fan J.; Sun W.; et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. 10.1016/j.biomaterials.2020.119755. [DOI] [PubMed] [Google Scholar]
- Shi C.; Zhou X.; Zhao Q.; Zhang Z.; Ma H.; Lu Y.; Huang Z.; Sun W.; Du J.; Fan J.; et al. CD44-Specific Targeting Nanoreactors with Glutathione Depletion for Magnifying Photodynamic Tumor Eradication. CCS Chem. 2022, 4, 2662–2673. 10.31635/ccschem.021.202101222. [DOI] [Google Scholar]
- Wang Z.; Sun Y.; Lin S.; Wang G.; Chang X.; Gou X.; Liu T.; Jin S.; He G.; Wei Y.; et al. Orthogonal carbazole-perylene bisimide pentad: a photoconversion-tunable photosensitizer with diversified excitation and excited-state relaxation pathways. Sci. China: Chem. 2021, 64, 2193–2202. 10.1007/s11426-021-1154-0. [DOI] [Google Scholar]
- Wei X.; Zhang C.; He S.; Huang J.; Huang J.; Liew S. S.; Zeng Z.; Pu K. A Dual-Locked Activatable Phototheranostic Probe for Biomarker-Regulated Photodynamic and Photothermal Cancer Therapy. Angew. Chem., Int. Ed. 2022, 61, e202202966. 10.1002/anie.202202966. [DOI] [PubMed] [Google Scholar]
- He S.; Liu J.; Zhang C.; Wang J.; Pu K. Semiconducting Polymer Nano-regulators with Cascading Activation for Photodynamic Cancer Immunotherapy. Angew. Chem., Int. Ed. 2022, 61, e202116669. 10.1002/anie.202116669. [DOI] [PubMed] [Google Scholar]
- Kim H.; Lee Y. R.; Jeong H.; Lee J.; Wu X.; Li H.; Yoon J. Photodynamic and photothermal therapies for bacterial infection treatment. Smart Mol. 2023, 1, e20220010. 10.1002/smo.20220010. [DOI] [Google Scholar]
- Sasikumar D.; John A. T.; Sunny J.; Hariharan M. Access to the triplet excited states of organic chromophores. Chem. Soc. Rev. 2020, 49, 6122–6140. 10.1039/D0CS00484G. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Wu W.; Sun J.; Guo S. Triplet photosensitizers: from molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. 10.1039/c3cs35531d. [DOI] [PubMed] [Google Scholar]
- Nguyen V.; Qi S.; Kim S.; Kwon N.; Kim G.; Yim Y.; Park S.; Yoon J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. 10.1021/jacs.9b09220. [DOI] [PubMed] [Google Scholar]
- Li M.; Gebremedhin K. H.; Ma D.; Pu Z.; Xiong T.; Xu Y.; Kim J. S.; Peng X. Conditionally Activatable Photoredox Catalysis in Living Systems. J. Am. Chem. Soc. 2022, 144, 163–173. 10.1021/jacs.1c07372. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Xu K.; Yang W.; Wang Z.; Zhong F. The triplet excited state of Bodipy: formation, modulation and application. Chem. Soc. Rev. 2015, 44, 8904–8939. 10.1039/C5CS00364D. [DOI] [PubMed] [Google Scholar]
- Sun W.; Guo S.; Hu C.; Fan J.; Peng X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. 10.1021/acs.chemrev.6b00001. [DOI] [PubMed] [Google Scholar]
- Schnermann M. J. Organic dyes for deep bioimaging. Nature 2017, 551, 176–177. 10.1038/nature24755. [DOI] [PubMed] [Google Scholar]
- Luo S.; Tan X.; Fang S.; Wang Y.; Liu T.; Wang X.; Yuan Y.; Sun H.; Qi Q.; Shi C. Mitochondria-Targeted Small-Molecule Fluorophores for Dual Modal Cancer Phototherapy. Adv. Funct. Mater. 2016, 26, 2826–2835. 10.1002/adfm.201600159. [DOI] [Google Scholar]
- Karton-Lifshin N.; Albertazzi L.; Bendikov M.; Baran P. S.; Shabat D. Donor-Two-Acceptor” Dye Design: A Distinct Gateway to NIR Fluorescence. J. Am. Chem. Soc. 2012, 134, 20412–20420. 10.1021/ja308124q. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Long S.; Li M.; Cao J.; Li Y.; Guo L.; Sun W.; Du J.; Fan J.; Peng X. Oxygen-Dependent Regulation of Excited-State Deactivation Process of Rational Photosensitizer for Smart Phototherapy. J. Am. Chem. Soc. 2020, 142, 1510–1517. 10.1021/jacs.9b11800. [DOI] [PubMed] [Google Scholar]
- Shi C.; Huang H.; Zhou X.; Zhang Z.; Ma H.; Yao Q.; Shao K.; Sun W.; Du J.; Fan J.; et al. Reversing Multidrug Resistance by Inducing Mitochondrial Dysfunction for Enhanced Chemo-Photodynamic Therapy in Tumor. ACS Appl. Mater. Interfaces 2021, 13, 45259–45268. 10.1021/acsami.1c12725. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Xu S.; Lai F.; Wang Y.; Zhang N.; Nazare M.; Hu H. Rapid Synthesis of γ-Halide/Pseudohalide-Substituted Cyanine Sensors with Programmed Generation of Singlet Oxygen. Org. Lett. 2019, 21, 2121–2125. 10.1021/acs.orglett.9b00404. [DOI] [PubMed] [Google Scholar]
- Palmer J. R.; Wells K. A.; Yarnell J. E.; Favale J. M.; Castellano F. N. Visible-Light-Driven Triplet Sensitization of Polycyclic Aromatic Hydrocarbons Using Thionated Perinones. J. Phys. Chem. Lett. 2020, 11, 5092–5099. 10.1021/acs.jpclett.0c01634. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Toffoletti A.; Hou Y.; Zhao J.; Barbon A.; Dick B. Insight into the drastically different triplet lifetimes of BODIPY obtained by optical/magnetic spectroscopy and theoretical computations. Chem. Sci. 2021, 12, 2829–2840. 10.1039/D0SC05494A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Dick B.; Zhao J. Twisted Bodipy Derivative as a Heavy-Atom-Free Triplet Photosensitizer Showing Strong Absorption of Yellow Light, Intersystem Crossing, and a High-Energy Long-Lived Triplet State. Org. Lett. 2020, 22, 5535–5539. 10.1021/acs.orglett.0c01903. [DOI] [PubMed] [Google Scholar]
- Kolemen S.; Işık M.; Kim G. M.; Kim D.; Geng H.; Buyuktemiz M.; Karatas T.; Zhang X.; Dede Y.; Yoon J.; et al. Intracellular Modulation of Excited-State Dynamics in a Chromophore Dyad: Differential Enhancement of Photocytotoxicity Targeting Cancer Cells. Angew. Chem., Int. Ed. 2015, 54, 5340–5344. 10.1002/anie.201411962. [DOI] [PubMed] [Google Scholar]
- Cakmak Y.; Kolemen S.; Duman S.; Dede Y.; Dolen Y.; Kilic B.; Kostereli Z.; Yildirim L. T.; Dogan A. L.; Guc D.; et al. Designing Excited States: Theory-Guided Access to Efficient Photosensitizers for Photodynamic Action. Angew. Chem., Int. Ed. 2011, 50, 11937–11941. 10.1002/anie.201105736. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Li H.; Shi C.; Xu F.; Zhang Z.; Yao Q.; Ma H.; Sun W.; Shao K.; Du J.; et al. An APN-activated NIR photosensitizer for cancer photodynamic therapy and fluorescence imaging. Biomaterials 2020, 253, 120089. 10.1016/j.biomaterials.2020.120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H.; Long S.; Cao J.; Xu F.; Zhou P.; Zeng G.; Zhou X.; Shi C.; Sun W.; Du J.; et al. New Cy5 photosensitizers for cancer phototherapy: a low singlet-triplet gap provides high quantum yield of singlet oxygen. Chem. Sci. 2021, 12, 13809–13816. 10.1039/D1SC04570A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Ge H.; Xu N.; Yang C.; Yao Q.; Long S.; Sun W.; Fan J.; Xu X.; Peng X. Radical induced quartet photosensitizers with high 1O2 production for in vivo cancer photodynamic therapy. Sci. China: Chem. 2021, 64, 488–498. 10.1007/s11426-020-9922-3. [DOI] [Google Scholar]
- Ma H.; Lu Y.; Huang Z.; Long S.; Cao J.; Zhang Z.; Zhou X.; Shi C.; Sun W.; Du J.; et al. ER-Targeting Cyanine Dye as an NIR Photoinducer to Efficiently Trigger Photoimmunogenic Cancer Cell Death. J. Am. Chem. Soc. 2022, 144, 3477–3486. 10.1021/jacs.1c11886. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yao Q.; Long S.; Chi W.; Yang Y.; Tan D.; Liu X.; Huang H.; Sun W.; Du J.; et al. An Approach to Developing Cyanines with Simultaneous Intersystem Crossing Enhancement and Excited-State Lifetime Elongation for Photodynamic Antitumor Metastasis. J. Am. Chem. Soc. 2021, 143, 12345–12354. 10.1021/jacs.1c06275. [DOI] [PubMed] [Google Scholar]
- El Sayed M. A. Spin-Orbit Coupling and the Radiationless Processes in Nitrogen Heterocyclics. J. Chem. Phys. 1963, 38, 2834–2838. 10.1063/1.1733610. [DOI] [Google Scholar]
- Buck J. T.; Boudreau A. M.; DeCarmine A.; Wilson R. W.; Hampsey J.; Mani T. Spin-Allowed Transitions Control the Formation of Triplet Excited States in Orthogonal Donor-Acceptor Dyads. Chem 2019, 5, 138–155. 10.1016/j.chempr.2018.10.001. [DOI] [Google Scholar]
- Penfold T. J.; Gindensperger E.; Daniel C.; Marian C. M. Spin-Vibronic Mechanism for Intersystem Crossing. Chem. Rev. 2018, 118, 6975–7025. 10.1021/acs.chemrev.7b00617. [DOI] [PubMed] [Google Scholar]
- Rajagopal S. K.; Nagaraj K.; Deb S.; Bhat V.; Sasikumar D.; Sebastian E.; Hariharan M. Extending the scope of the carbonyl facilitated triplet excited state towards visible light excitation. Phys. Chem. Chem. Phys. 2018, 20, 19120–19128. 10.1039/C8CP01023D. [DOI] [PubMed] [Google Scholar]
- Conrad-Burton F. S.; Liu T.; Geyer F.; Costantini R.; Schlaus A. P.; Spencer M. S.; Wang J.; Sánchez R. H.; Zhang B.; Xu Q.; et al. Controlling Singlet Fission by Molecular Contortion. J. Am. Chem. Soc. 2019, 141, 13143–13147. 10.1021/jacs.9b05357. [DOI] [PubMed] [Google Scholar]
- Lu T.; Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Lu T.; Chen Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. 10.1016/j.carbon.2020.05.023. [DOI] [Google Scholar]
- Marcus R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. V. Comparison and Properties of Electrochemical and Chemical Rate Constants. J. Phys. Chem. 1963, 67, 853–857. 10.1021/j100798a033. [DOI] [Google Scholar]
- Ou Q.; Subotnik J. E. Electronic Relaxation in Benzaldehyde Evaluated via TD-DFT and Localized Diabatization: Intersystem Crossings, Conical Intersections, and Phosphorescence. J. Phys. Chem. C 2013, 117, 19839–19849. 10.1021/jp405574q. [DOI] [Google Scholar]
- Su Y.; Li K.; Yu X. Theoretical Studies on the Fluorescence Enhancement of Benzaldehydes by Intermolecular Hydrogen Bonding. J. Phys. Chem. B 2019, 123, 884–890. 10.1021/acs.jpcb.8b10271. [DOI] [PubMed] [Google Scholar]
- Zhou P.; Tang Z.; Li P.; Liu J. Unraveling the Mechanism for Tuning the Fluorescence of Fluorescein Derivatives: The Role of the Conical Intersection and nπ* State. J. Phys. Chem. Lett. 2021, 12, 6478–6485. 10.1021/acs.jpclett.1c01774. [DOI] [PubMed] [Google Scholar]
- Manzetti S.; Lu T. The geometry and electronic structure of aristolochic acid: possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. 10.1002/poc.3111. [DOI] [Google Scholar]
- Wu M.; Liu X.; Chen H.; Duan Y.; Liu J.; Pan Y.; Liu B. Activation of Pyroptosis by Membrane-Anchoring AIE Photosensitizer Design: New Prospect for Photodynamic Cancer Cell Ablation. Angew. Chem., Int. Ed. 2021, 60, 9093–9098. 10.1002/anie.202016399. [DOI] [PubMed] [Google Scholar]
- Usama S. M.; Burgess K. Hows and Whys of Tumor-Seeking Dyes. Acc. Chem. Res. 2021, 54, 2121–2131. 10.1021/acs.accounts.0c00733. [DOI] [PubMed] [Google Scholar]
- Usama S. M.; Lin C.; Burgess K. On the Mechanisms of Uptake of Tumor-Seeking Cyanine Dyes. Bioconjugate Chem. 2018, 29, 3886–3895. 10.1021/acs.bioconjchem.8b00708. [DOI] [PubMed] [Google Scholar]
- Zhang E.; Luo S.; Tan X.; Shi C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. 10.1016/j.biomaterials.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Wang H.; Antaris A. L.; Li L.; Diao S.; Ma R.; Nguyen A.; Hong G.; Ma Z.; Wang J.; et al. Traumatic Brain Injury Imaging in the Second Near-Infrared Window with a Molecular Fluorophore. Adv. Mater. 2016, 28, 6872–6879. 10.1002/adma.201600706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antaris A. L.; Chen H.; Cheng K.; Sun Y.; Hong G.; Qu C.; Diao S.; Deng Z.; Hu X.; Zhang B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. 10.1038/nmat4476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.