Abstract
In pursuit of a more sustainable route to phosphorus–carbon (P–C) bond-containing chemicals, we herein report that phosphonates can be prepared by mechanochemical phosphorylation of acetylides using polyphosphates in a single step, redox-neutral process, bypassing white phosphorus (P4) and other high-energy, environmentally hazardous intermediates. Using sodium triphosphate (Na5P3O10) and acetylides, alkynyl phosphonates 1 can be isolated in yields of up to 32%, while reaction of sodium pyrophosphate (Na4P2O7) and sodium carbide (Na2C2) engendered, in an optimized yield of 63%, ethynyl phosphonate 2, an easily isolable compound that can be readily converted to useful organophosphorus chemicals. Highly condensed phosphates like Graham’s salt and bioproduced polyphosphate were also found to be compatible after reducing the chain length by grinding with orthophosphate. These results demonstrate the possibility of accessing organophosphorus chemicals directly from condensed phosphates and may offer an opportunity to move toward a “greener” phosphorus industry.
Short abstract
Solvent-free mechanochemical phosphorylation of acetylides using condensed phosphates, a more sustainable route to alkynyl phosphonates and organophosphorus chemicals.
Introduction
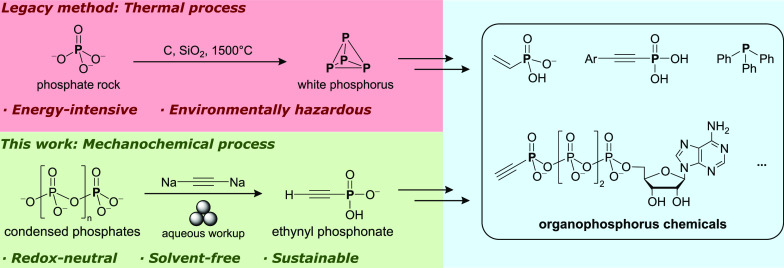
Phosphorus–carbon (P–C) bonds are widely found in organophosphorus compounds that have emerged as useful pharmaceuticals, flame retardants, agrochemicals, ligands, and materials.1−8 At present, P–C bond-containing chemicals are derived almost exclusively from white phosphorus (P4), the tetrahedral molecule originally discovered by German alchemist Hennig Brand in 16699 that has now become one of the most important feedstock materials in the modern phosphorus industry.1,8,10 Typically, mined phosphate rock goes through an energy intensive legacy process known as the “thermal process”, in which phosphate rock is treated with coke and sand at over 1500 °C to produce P4 (Figure 1A).10 The produced P4 is then oxidized to trichlorophosphine (PCl3) with chlorine gas (Cl2) and used in P–C bond forming reactions.1,11 Alternatively, P–C bonds can be accessed from hypophosphite (H2PO2–) and phosphane (phosphine gas, PH3) produced from P4 by cross-coupling or hydrophosphinylation12,13 and by hydrophosphination,1,6,14,15 respectively. Recent breakthroughs in P4 chemistry also allowed for direct functionalization of P4 into P–C bond-containing products.16
Figure 1.
(A) Overview of key P–C bond formation steps in P-chemical manufacture. (B) The envisioned mechanochemical phosphorylation of carbon nucleophiles. (C) Selected examples of useful phosphonate-based organophosphorus chemicals.
Although P4 remains at the center of P-chemical production, there are several drawbacks associated with its manufacture and utilization. The thermal process requires a massive amount of energy input for continuous electric arc furnace operation, limiting its facilities to regions with cheap sources of power.10 Furthermore, many substances involved in subsequent transformations, including Cl2, PCl3, PH3, and P4 itself, are environmentally hazardous and thus must be carefully regulated.17,18 In line with the principles of Green Chemistry19 and the United Nations’ Sustainable Development Goals,20−22 the issue of global phosphorus sustainability has received increasing attention over the past decade with the sustainable production of P-chemicals being one of its major targets.23−26 New strategies therefore need to be developed to address these energy and environmental issues.27
Another major portion of the mined phosphate rock, on the other hand, is treated with sulfuric acid to produce phosphoric acid and eventually fertilizers, in what is known as the “wet process”.7 Condensed phosphates (polyphosphates) can be prepared readily from phosphoric acid and its salts by dehydration.7 Apart from phosphate rock, phosphate removal protocols are being implemented worldwide to ameliorate eutrophication, and phosphates recovered from waste streams also constitute a new input stream of this nonrenewable resource.23−26,28 These phosphates can thus be considered as green starting materials.
Previously, we have shown that P–C bonds can be accessed from bis(trichlorosilyl)phosphide (Figure 1A, top), an intermediate directly prepared from phosphates, bypassing the hazardous P4.29−31 More recently, we demonstrated that phosphite can be produced from condensed phosphates and metal hydrides without traversing lower oxidation states than +3.32,33 This “hydride phosphorylation” breakthrough was made possible by mechanochemistry, an increasingly popular technique that is often recognized as green and sustainable.34−41 Moreover, polyphosphates recovered from microorganisms were also shown to be promising substrates.32
Building upon these principles, we sought to expand this redox-neutral mechanochemical phosphorylation to carbon nucleophiles and achieve direct P–C bond formation from polyphosphates (Figure 1B). The product phosphonate is found in many drugs, agrochemicals, and flame retardants (Figure 1C) and is currently manufactured starting from P4.5,8,42 Recent advances also enabled direct preparation of tertiary phosphines from organophosphonates.43
Results and Discussion
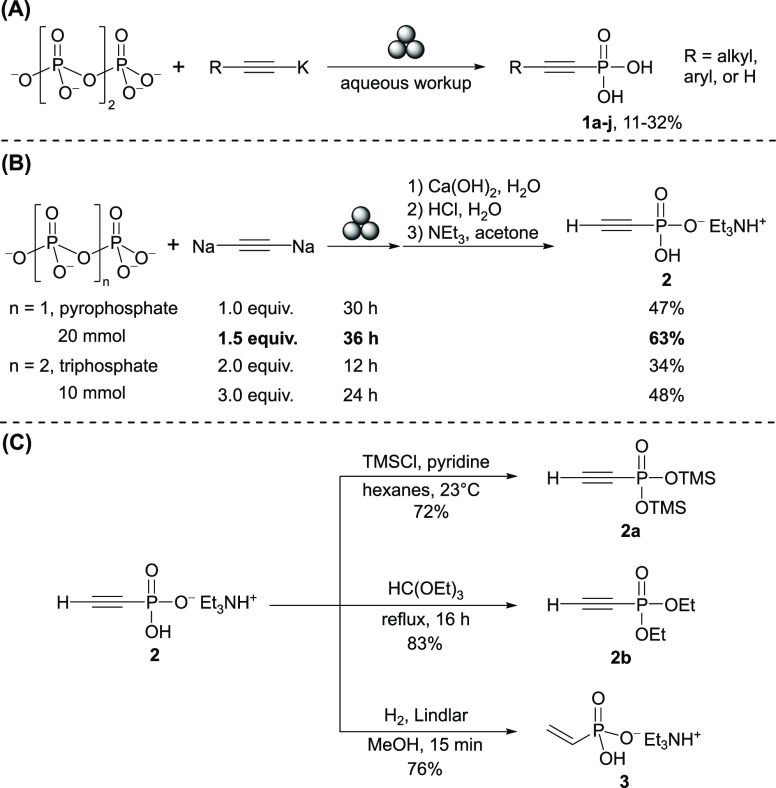
With the knowledge gained from the hydride phosphorylation reaction, we started out by exploring the mechanochemical reaction between sodium triphosphate (Na5P3O10) and common organometallic reagents (Supporting Information). Mechanochemical reactions were conducted in a Restch PM100 planetary ball mill using stainless steel jars and ball bearings at a rotational frequency of 450 rpm. We found that, among the tested organometallic reagents, phenylacetylide gave better results than the rest, with the best phosphonate yield of 33% achieved when using potassium phenylacetylide. Extending the substrates to common alkyl and aryl acetylides also afforded the corresponding alkynyl phosphonates 1a–j but in rather poor isolated yields possibly due to side reactions of acetylides under mechanochemical conditions (Figure 2A; see the Supporting Information for more details).
Figure 2.
(A) Synthesis of alkynyl phosphonates 1a–j. (B) Synthesis of ethynyl phosphonate 2, yield based on reducible phosphate (1 per Na4P2O7, 2 per Na5P3O10). (C) Synthesis of 2a, 2b, and 3 from 2.
In order to extend the synthetic applications of this “acetylide phosphorylation” reaction, we then targeted ethynyl phosphonate (HCCPO32–), a phosphonate with a terminal alkyne that allows for further functionalizations. Our first experiment involving treatment of Na5P3O10 with stoichiometric sodium acetylide (NaCCH) did not lead to the desired product, possibly due to the decomposition of NaCCH caused by deprotonation. To our delight, switching to sodium carbide (Na2C2) resulted in clean formation of HCCPO32– with an isolated yield of 34% after an aqueous workup, which could be improved to 48% when a higher carbide loading (Na2C2:reducible P = 1.5:1; for the definition of reducible P, see ref (32)) was used (Figure 2B). Sodium pyrophosphate (Na4P2O7) was found to give higher yields after elongated grinding times, with the best of 63% isolated yield achieved with the same carbide loading. Analytically pure ethynyl phosphonate can be isolated as the triethylammonium salt 2 simply by precipitation and recrystallization, while previous reports of its synthesis noted the formation of substantial side products and necessitated HPLC separation.44,45 The obtained 2 can be readily silylated or ethylated to afford the corresponding ester 2a or 2b, which can be used in further transformations (Figure 2C). More importantly, 2 can be partially hydrogenated to vinyl phosphonate 3 (Figure 2C), a useful monomer in the polymer industry for production of electrolyte membranes and other materials.46
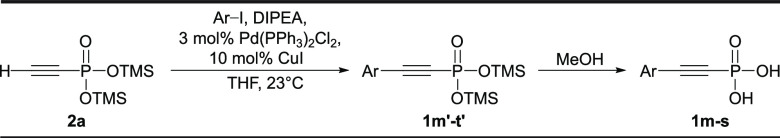
Having isolated pure 2 in decent yields, we envision that 2 (and its derivatives 2a and 2b) can now serve as a new, sustainable starting material for P–C bond containing chemicals. Treatment of 2a with aryl iodides under typical Sonogashira coupling conditions47,48 led to more alkynyl phosphonates (Table 1), including ones bearing functional groups, such as carboxylate ester, nitro, aldehyde and borate, that are typically not compatible with acetylides in ball milling. Use of diiodides and triiodides also afforded the corresponding bis- and tris-phosphonates, which may find applications as useful secondary building units in the construction of metal–organic framework (MOF) materials.49−53
Table 1. Synthesis of Alkynylphosphonic Acids from 2aa.
Reactions carried out on a 0.3 mmol scale. Yield over two steps except for 1t′.
In addition, phenyl rings can be constructed from 2b and cyclohexa-1,3-diene by Diels–Alder reaction with ethylene elimination (Figure 3A).54 The obtained phenyl phosphonate can be directly converted to triphenyl phosphine (PPh3) by treatment with the phenyl Grignard reagent and NaOTf followed by reduction.43 This is, to the best of our knowledge, the first example of PPh3 synthesis without the involvement of P4. There are many established procedures that convert 2b into other useful organophosphorus compounds as well.55−68 Moreover, ethynyl phosphonate offers opportunities for terminal phosphate modification of bioactive molecules. Nucleoside tetraphosphate 4 featuring a “clickable” moiety, for example, can be readily synthesized from the TBA salt 2′ using a diphosphorylation protocol recently developed by our group (Figure 3B).69 A number of such modified nucleotide analogues have found applications as probes to investigate biological processes and as tools for biotechnology and drug discovery.45,70−74 Similar strategies were also developed for labeling amino acids and peptides using ethynyl phosphonate.75−78
Figure 3.

Synthesis of triphenylphosphine from 2b (A) and synthesis of 4 from 2′ (B).
Lastly, we turned our attention to bioproduced condensed phosphates. Microorganisms are known to take up phosphate from their surroundings and store it as intracellular polyphosphate granules,79,80 forming the basis of the enhanced biological phosphorus removal (EBPR) process.81,82 A recent protocol of polyphosphate accumulation using Saccharomyces cerevisiae (baker’s yeast) allowed us to expand our mechanochemical phosphorylation to bioproduced polyphosphate (bio-polyP) of similar properties to what is recovered from waste streams by EBPR.32,83−86 As shown in Table S2, highly condensed phosphates like Graham’s salt afford the desired alkynyl phosphonates in poor yields. We therefore sought to break down these highly condensed phosphates into pyrophosphate, which has been shown to be a superior phosphorylation reagent. Treating Graham’s salt with stoichiometric sodium phosphate Na3PO4 under typical ball-milling conditions led to the clean formation of pyrophosphate, and subsequent reaction with Na2C2 afforded 2 in 42% yield (Figure 4, right). Similarly, a bio-polyP with an average chain length of 8.1 could be broken down to a phosphate mixture with an average chain length of 1.7. Ethynyl phosphonate 2 could be prepared from this mixture in 31% yield (Figure 4, left). These initial results demonstrate that polyphosphates recovered from microorganisms could be promising starting materials for sustainable production of P-chemicals, presenting an opportunity for a “closed-loop” phosphorus industry.25
Figure 4.
Synthesis of ethynyl phosphonate 2 from bio-polyP and Graham’s salt; yield is based on reducible phosphate.
Conclusions
We have achieved direct P–C bond formation from condensed phosphates via mechanochemical phosphorylation of common acetylides. This new method bypasses white phosphorus as a hazardous intermediate while replacing the carbon-intensive thermal process with a green, sustainable mechanochemical process. Pyrophosphate Na4P2O7 was found to be the optimal phosphorylation reagent for Na2C2 to afford ethynyl phosphonate 2, an easily isolable compound that is converted readily to useful organophosphorus chemicals. Bioproduced polyphosphate was also found to be a suitable phosphate source after breaking it down to pyrophosphate by grinding with orthophosphate. With mechanochemistry becoming more common in industrial chemistry,87 this study presents a new entry point into organophosphorus chemical production as an alternative to white phosphorus.
Acknowledgments
The authors gratefully acknowledge financial and logistical support received through the Université Mohammed VI Polytechnique-MIT Research Program, a partnership between UM6P and OCP Group in Morocco and the Massachusetts Institute of Technology dedicated to promoting sustainable development in Africa. We thank Dr. F. Zhai for independently reproducing the procedure for the synthesis of 2.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.3c00725.
General information, experimental procedures and results, characterization data, copies of 1H, 13C and 31P NMR spectra, and references (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Svara J.; Weferling N.; Hofmann T.. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd., 2006; Vol. 27; pp 19–49. [Google Scholar]
- Joly D.; Bouit P.-A.; Hissler M. Organophosphorus derivatives for electronic devices. J. Mater. Chem. C 2016, 4, 3686–3698. 10.1039/C6TC00590J. [DOI] [Google Scholar]
- Shameem M. A.; Orthaber A. Organophosphorus compounds in organic electronics. Chem.—Eur. J. 2016, 22, 10718–10735. 10.1002/chem.201600005. [DOI] [PubMed] [Google Scholar]
- Demkowicz S.; Rachon J.; Daśko M.; Kozak W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. 10.1039/C5RA25446A. [DOI] [Google Scholar]
- Velencoso M. M.; Battig A.; Markwart J. C.; Schartel B.; Wurm F. R. Molecular firefighting—how modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem., Int. Ed. 2018, 57, 10450–10467. 10.1002/anie.201711735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaroshenko V.Organophosphorus chemistry: from molecules to applications; John Wiley & Sons, 2019. [Google Scholar]
- Havelange S.; Van Lierde N.; Germeau A.; Martins E.; Theys T.; Sonveaux M.; Toussaint C.; Schrödter K.; Bettermann G.; Staffel T.; et al. Phosphoric Acid and Phosphates. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd., 2022; pp 1–55. [Google Scholar]
- Ung S. P.-M.; Li C.-J. From rocks to bioactive compounds: a journey through the global P(V) organophosphorus industry and its sustainability. RSC Sustain. 2023, 1, 11–37. 10.1039/D2SU00015F. [DOI] [Google Scholar]
- Weeks M. E. The discovery of the elements. XXI. Supplementary note on the discovery of phosphorus. J. Chem. Educ. 1933, 10, 302. 10.1021/ed010p302. [DOI] [Google Scholar]
- Diskowski H.; Hofmann T.. Phosphorus. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd., 2000; Vol. 26; pp 725–746. [Google Scholar]
- Bettermann G.; Krause W.; Riess G.; Hofmann T.. Phosphorus Compounds, Inorganic. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd., 2000; Vol. 27; pp 1–18. [Google Scholar]
- Montchamp J.-L. Phosphinate chemistry in the 21st century: a viable alternative to the use of phosphorus trichloride in organophosphorus synthesis. Acc. Chem. Res. 2014, 47, 77–87. 10.1021/ar400071v. [DOI] [PubMed] [Google Scholar]
- Abdou M. M. Synopsis of recent synthetic methods and biological applications of phosphinic acid derivatives. Tetrahedron 2020, 76, 131251. 10.1016/j.tet.2020.131251. [DOI] [Google Scholar]
- Koshti V.; Gaikwad S.; Chikkali S. H. Contemporary avenues in catalytic PH bond addition reaction: A case study of hydrophosphination. Coord. Chem. Rev. 2014, 265, 52–73. 10.1016/j.ccr.2014.01.006. [DOI] [Google Scholar]
- Novas B. T.; Waterman R. Metal-Catalyzed Hydrophosphination. ChemCatChem. 2022, 14, e202200988 10.1002/cctc.202200988. [DOI] [Google Scholar]
- Scott D. J. Recent Breakthroughs in P4 Chemistry: Towards Practical, Direct Transformations into P1 Compounds. Angew. Chem., Int. Ed. 2022, 61, e202205019 10.1002/anie.202205019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour R.Phosphorus. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd., 2019; pp 1–33. [Google Scholar]
- Gilmour R.Phosphorus Compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Ltd., 2019; pp 1–57. [Google Scholar]
- Anastas P.; Eghbali N. Green chemistry: principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]
- Axon S.; James D. The UN Sustainable Development Goals: How can sustainable chemistry contribute? A view from the chemical industry. Curr. Opin. Green Sustain. Chem. 2018, 13, 140–145. 10.1016/j.cogsc.2018.04.010. [DOI] [Google Scholar]
- Anastas P. T.; Zimmerman J. B. The United Nations sustainability goals: How can sustainable chemistry contribute?. Curr. Opin. Green Sustain. Chem. 2018, 13, 150–153. 10.1016/j.cogsc.2018.04.017. [DOI] [Google Scholar]
- Anastas P.; Nolasco M.; Kerton F.; Kirchhoff M.; Licence P.; Pradeep T.; Subramaniam B.; Moores A. The Power of the United Nations Sustainable Development Goals in Sustainable Chemistry and Engineering Research. ACS Sustainable Chem. Eng. 2021, 9, 8015–8017. 10.1021/acssuschemeng.1c03762. [DOI] [Google Scholar]
- Schipper W. Phosphorus: Too big to fail. Eur. J. Inorg. Chem. 2014, 2014, 1567–1571. 10.1002/ejic.201400115. [DOI] [Google Scholar]
- Withers P. J.; Elser J. J.; Hilton J.; Ohtake H.; Schipper W. J.; Van Dijk K. C. Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability. Green Chem. 2015, 17, 2087–2099. 10.1039/C4GC02445A. [DOI] [Google Scholar]
- Jupp A. R.; Beijer S.; Narain G. C.; Schipper W.; Slootweg J. C. Phosphorus recovery and recycling–closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. 10.1039/D0CS01150A. [DOI] [PubMed] [Google Scholar]
- Beijer S.; Slootweg J. C.. Sustainable Phosphorus. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd., 2021; pp 1–26. [Google Scholar]
- Geeson M. B.; Cummins C. C. Let’s make white phosphorus obsolete. ACS Cent. Sci. 2020, 6, 848–860. 10.1021/acscentsci.0c00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake H.; Tsuneda S.. Phosphorus recovery and recycling; Springer, 2019. [Google Scholar]
- Geeson M. B.; Cummins C. C. Phosphoric acid as a precursor to chemicals traditionally synthesized from white phosphorus. Science 2018, 359, 1383–1385. 10.1126/science.aar6620. [DOI] [PubMed] [Google Scholar]
- Geeson M. B.; Ríos P.; Transue W. J.; Cummins C. C. Orthophosphate and Sulfate Utilization for C–E (E = P, S) Bond Formation via Trichlorosilyl Phosphide and Sulfide Anions. J. Am. Chem. Soc. 2019, 141, 6375–6384. 10.1021/jacs.9b01475. [DOI] [PubMed] [Google Scholar]
- Geeson M. B.; Tanaka K.; Taakili R.; Benhida R.; Cummins C. C. Photochemical Alkene Hydrophosphination with Bis(trichlorosilyl)phosphine. J. Am. Chem. Soc. 2022, 144, 14452–14457. 10.1021/jacs.2c05248. [DOI] [PubMed] [Google Scholar]
- Zhai F.; Xin T.; Geeson M. B.; Cummins C. C. Sustainable production of reduced phosphorus compounds: Mechanochemical hydride phosphorylation using condensed phosphates as a route to phosphite. ACS Cent. Sci. 2022, 8, 332–339. 10.1021/acscentsci.1c01381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. Reducing the P-Cycle by Grinding. ACS Cent. Sci. 2022, 8, 303–305. 10.1021/acscentsci.2c00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friščić T.; Mottillo C.; Titi H. M. Mechanochemistry for Synthesis. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. 10.1002/anie.201906755. [DOI] [PubMed] [Google Scholar]
- Egorov I. N.; Santra S.; Kopchuk D. S.; Kovalev I. S.; Zyryanov G. V.; Majee A.; Ranu B. C.; Rusinov V. L.; Chupakhin O. N. Ball milling: an efficient and green approach for asymmetric organic syntheses. Green Chem. 2020, 22, 302–315. 10.1039/C9GC03414E. [DOI] [Google Scholar]
- Ferreira da Silva J. L.; Minas da Piedade M. F.; André V.; Domingos S.; Martins I. C.; Duarte M. T. The Lisbon Supramolecular Green Story: Mechanochemistry towards New Forms of Pharmaceuticals. Molecules 2020, 25, 2705. 10.3390/molecules25112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Venegas M.; Juaristi E. Mechanochemical and mechanoenzymatic synthesis of pharmacologically active compounds: A green perspective. ACS Sustainable Chem. Eng. 2020, 8, 8881–8893. 10.1021/acssuschemeng.0c01645. [DOI] [Google Scholar]
- Ardila-Fierro K. J.; Hernández J. G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. 10.1002/cssc.202100478. [DOI] [PubMed] [Google Scholar]
- Espro C.; Rodríguez-Padrón D. Re-thinking organic synthesis: Mechanochemistry as a greener approach. Curr. Opin. Green Sustain. Chem. 2021, 30, 100478. 10.1016/j.cogsc.2021.100478. [DOI] [Google Scholar]
- André V.; Duarte M. T.; Gomes C. S.; Sarraguça M. C. Mechanochemistry in Portugal—A step towards sustainable chemical synthesis. Molecules 2022, 27, 241. 10.3390/molecules27010241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill R. T.; Boulatov R. The many flavours of mechanochemistry and its plausible conceptual underpinnings. Nat. Rev. Chem. 2021, 5, 148–167. 10.1038/s41570-020-00249-y. [DOI] [PubMed] [Google Scholar]
- Horsman G. P.; Zechel D. L. Phosphonate biochemistry. Chem. Rev. 2017, 117, 5704–5783. 10.1021/acs.chemrev.6b00536. [DOI] [PubMed] [Google Scholar]
- Kendall A. J.; Salazar C. A.; Martino P. F.; Tyler D. R. Direct conversion of phosphonates to phosphine oxides: An improved synthetic route to phosphines including the first synthesis of methyl JohnPhos. Organometallics 2014, 33, 6171–6178. 10.1021/om500854u. [DOI] [Google Scholar]
- Hughes L. D.; Boxer S. G. DNA-based patterning of tethered membrane patches. Langmuir 2013, 29, 12220–12227. 10.1021/la402537p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat P.; Walczak S.; Wojtczak B. A.; Nowakowska M.; Jemielity J.; Kowalska J. Ethynyl, 2-propynyl, and 3-butynyl C-phosphonate analogues of nucleoside di-and triphosphates: Synthesis and reactivity in CuAAC. Org. Lett. 2015, 17, 3062–3065. 10.1021/acs.orglett.5b01346. [DOI] [PubMed] [Google Scholar]
- Macarie L.; Ilia G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. 10.1016/j.progpolymsci.2010.04.001. [DOI] [Google Scholar]
- Chinchilla R.; Nájera C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
- Chinchilla R.; Nájera C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]
- Gagnon K. J.; Perry H. P.; Clearfield A. Conventional and unconventional metal-organic frameworks based on phosphonate ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034–1054. 10.1021/cr2002257. [DOI] [PubMed] [Google Scholar]
- Taddei M.; Costantino F.; Vivani R. Robust Metal-Organic Frameworks Based on Tritopic Phosphonoaromatic Ligands. Eur. J. Inorg. Chem. 2016, 2016, 4300–4309. 10.1002/ejic.201600207. [DOI] [Google Scholar]
- Yücesan G.; Zorlu Y.; Stricker M.; Beckmann J. Metal-organic solids derived from arylphosphonic acids. Coord. Chem. Rev. 2018, 369, 105–122. 10.1016/j.ccr.2018.05.002. [DOI] [Google Scholar]
- Bao S.-S.; Shimizu G. K.; Zheng L.-M. Proton conductive metal phosphonate frameworks. Coord. Chem. Rev. 2019, 378, 577–594. 10.1016/j.ccr.2017.11.029. [DOI] [Google Scholar]
- Tholen P.; Zorlu Y.; Beckmann J.; Yücesan G. Probing Isoreticular Expansions in phosphonate MOFs and their applications. Eur. J. Inorg. Chem. 2020, 2020, 1542–1554. 10.1002/ejic.201901291. [DOI] [Google Scholar]
- Xu X.; Chen H.; Wang Y.; Gao Y.; Tang G.; Zhao Y. Catalyst-free synthesis of cycloalkenyl phosphonates. RSC Adv. 2014, 4, 14740–14743. 10.1039/C4RA00999A. [DOI] [Google Scholar]
- Acheson R. M.; Ansell P. J. The synthesis of diethyl p-tolylsulphonylethynylphosphonate and related acetylenes, and their reactions with nucleophiles, pyridinium-1-dicyanomethylides, and dienes. J. Chem. Soc., Perkin trans. 1987, 1, 1275–1281. 10.1039/p19870001275. [DOI] [Google Scholar]
- Huang X.; Zhang C.; Lu X. A Convenient Stereoselective Synthesis of 1,3-Dienylphosphonates and 1-En-3-ynylphosphonates and Their Phosphine Oxide Analogs. Synthesis 1995, 1995, 769–771. 10.1055/s-1995-4000. [DOI] [Google Scholar]
- Lazrek H.; Rochdi A.; Khaider H.; Barascut J.-L.; Imbach J.-L.; Balzarini J.; Witvrouw M.; Pannecouque C.; De Clercq E. Synthesis of (Z) and (E) α-alkenyl phosphonic acid derivatives of purines and pyrimidines. Tetrahedron 1998, 54, 3807–3816. 10.1016/S0040-4020(98)00107-0. [DOI] [Google Scholar]
- Gil J. M.; Oh D. Y. Carbocupration of Diethyl 1-Alkynyl Phosphonates: Stereo-and Regioselective Synthesis of 1,2,2-Trisubstituted Vinyl Phosphonates. J. Org. Chem. 1999, 64, 2950–2953. 10.1021/jo982123w. [DOI] [PubMed] [Google Scholar]
- Ageno T.; Okauchi T.; Minami T.; Ishida M. Generation of α-phosphonovinyl radicals and development of a new route to highly functionalized vinylphosphonates and vinylphosphonate-incorporated carbocyclic or heterocyclic compounds via a radical trapping sequence. Org. Biomol. Chem. 2005, 3, 924–931. 10.1039/B416394J. [DOI] [PubMed] [Google Scholar]
- Kettles T. J.; Cockburn N.; Tam W. Ruthenium-Catalyzed Homo Diels–Alder [2+2+2] Cycloadditions of Alkynyl Phosphonates with Bicyclo [2.2.1] hepta-2,5-diene. J. Org. Chem. 2011, 76, 6951–6957. 10.1021/jo2010928. [DOI] [PubMed] [Google Scholar]
- Thiery E.; You V.; Mora A.-S.; Abarbri M. Synthesis of 5-Substituted 1,2,3-Triazolyl-4-phosphonate through Cross-Coupling Reactions of 5-Iodo-1,2,3-triazolyl-4-phosphonate. Eur. J. Org. Chem. 2016, 2016, 529–534. 10.1002/ejoc.201501266. [DOI] [Google Scholar]
- Fopp C.; Romain E.; Isaac K.; Chemla F.; Ferreira F.; Jackowski O.; Oestreich M.; Perez-Luna A. Stereodivergent silylzincation of α-heteroatom-substituted alkynes. Org. Lett. 2016, 18, 2054–2057. 10.1021/acs.orglett.6b00680. [DOI] [PubMed] [Google Scholar]
- Fopp C.; Isaac K.; Romain E.; Chemla F.; Ferreira F.; Jackowski O.; Oestreich M.; Perez-Luna A. Stereodivergent synthesis of β-heteroatom-substituted vinylsilanes by sequential silylzincation–copper(I)-mediated electrophilic substitution. Synthesis 2017, 49, 724–735. 10.1055/s-0036-1588106. [DOI] [Google Scholar]
- Obijalska E.; Kowalski M. K.; Mlostoń G.; Heimgartner H. Application of diethyl ethynylphosphonate to the synthesis of 3-phosphonylated β-lactams via the Kinugasa reaction. Arkivoc 2017, 2017, 59–67. 10.3998/ark.5550190.p009.660. [DOI] [Google Scholar]
- Mlostoń G.; Pipiak P.; Heimgartner H. [3 + 2] cycloadditions of n-protected ‘(s)-diazoproline’ with selected acetylenes. Heterocycles 2017, 95, 223–231. 10.3987/COM-16-S(S)7. [DOI] [Google Scholar]
- de La Vega-Hernández K.; Romain E.; Coffinet A.; Bijouard K.; Gontard G.; Chemla F.; Ferreira F.; Jackowski O.; Perez-Luna A. Radical germylzincation of α-heteroatom-substituted alkynes. J. Am. Chem. Soc. 2018, 140, 17632–17642. 10.1021/jacs.8b09851. [DOI] [PubMed] [Google Scholar]
- Mitrofanov A. Y.; Bychkova V. A.; Nefedov S. E.; Beletskaya I. P. Selective Metal-Controlled Synthesis of Trifluoromethylated (Indolin-2-ylidene) methyl-and Quinolin-3-ylphosphonates. J. Org. Chem. 2020, 85, 14507–14515. 10.1021/acs.joc.0c00913. [DOI] [PubMed] [Google Scholar]
- Yu. Mitrofanov A.; Beletskaya I. P. A convenient one-pot two-step synthesis of pyrazolylphosphonates from ethynylphosphonate. Mendeleev Commun. 2021, 31, 536–537. 10.1016/j.mencom.2021.07.033. [DOI] [Google Scholar]
- Qian K.; Shepard S. M.; Xin T.; Park G.; Cummins C. C. Stabilized Molecular Diphosphorus Pentoxide, P2 O5 L2 (L= N-Donor Base), in the Synthesis of Condensed Phosphate–Organic Molecule Conjugates. J. Am. Chem. Soc. 2023, 145, 6045–6050. 10.1021/jacs.3c00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna H.; Caruthers M. H. Alkynyl phosphonate DNA: a versatile “click”able backbone for DNA-based biological applications. J. Am. Chem. Soc. 2012, 134, 11618–11631. 10.1021/ja3026714. [DOI] [PubMed] [Google Scholar]
- Walczak S.; Nowicka A.; Kubacka D.; Fac K.; Wanat P.; Mroczek S.; Kowalska J.; Jemielity J. A novel route for preparing 5′ cap mimics and capped RNAs: phosphate-modified cap analogues obtained via click chemistry. Chem. Sci. 2017, 8, 260–267. 10.1039/C6SC02437H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukáč M.; Hocková D.; Keough D. T.; Guddat L. W.; Janeba Z. Novel nucleotide analogues bearing (1H-1,2,3-triazol-4-yl) phosphonic acid moiety as inhibitors of Plasmodium and human 6-oxopurine phosphoribosyltransferases. Tetrahedron 2017, 73, 692–702. 10.1016/j.tet.2016.12.046. [DOI] [Google Scholar]
- Walczak S.; Sikorski P. J.; Kasprzyk R.; Kowalska J.; Jemielity J. Exploring the potential of phosphotriazole 5′ mRNA cap analogues as efficient translation initiators. Org. Biomol. Chem. 2018, 16, 6741–6748. 10.1039/C8OB01720D. [DOI] [PubMed] [Google Scholar]
- Wanat P.; Kasprzyk R.; Kopcial M.; Sikorski P. J.; Strzelecka D.; Jemielity J.; Kowalska J. ExciTides: NTP-derived probes for monitoring pyrophosphatase activity based on excimer-to-monomer transitions. Chem. Commun. 2018, 54, 9773–9776. 10.1039/C8CC04968H. [DOI] [PubMed] [Google Scholar]
- Kee J.-M.; Villani B.; Carpenter L. R.; Muir T. W. Development of stable phosphohistidine analogues. J. Am. Chem. Soc. 2010, 132, 14327–14329. 10.1021/ja104393t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T. E.; Nix M. G.; Webb M. E. Fmoc-chemistry of a stable phosphohistidine analogue. Chem. Commun. 2011, 47, 1297–1299. 10.1039/C0CC04238B. [DOI] [PubMed] [Google Scholar]
- McAllister T. E.; Webb M. E. Triazole phosphohistidine analogues compatible with the Fmoc-strategy. Org. Biomol. Chem. 2012, 10, 4043–4049. 10.1039/c2ob25517k. [DOI] [PubMed] [Google Scholar]
- Mukai S.; Flematti G. R.; Byrne L. T.; Besant P. G.; Attwood P. V.; Piggott M. J. Stable triazolylphosphonate analogues of phosphohistidine. Amino acids 2012, 43, 857–874. 10.1007/s00726-011-1145-2. [DOI] [PubMed] [Google Scholar]
- Mandala V. S.; Loh D. M.; Shepard S. M.; Geeson M. B.; Sergeyev I. V.; Nocera D. G.; Cummins C. C.; Hong M. Bacterial phosphate granules contain cyclic polyphosphates: evidence from 31P solid-state NMR. J. Am. Chem. Soc. 2020, 142, 18407–18421. 10.1021/jacs.0c06335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari A.; Wang Z.; He P.; Wang D.; Lee J.; Han I.; Li G.; Gu A. Z. Unrevealed roles of polyphosphate-accumulating microorganisms. Microb. Biotechnol. 2021, 14, 82–87. 10.1111/1751-7915.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi P.; Izadi P.; Eldyasti A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: a review. Rev. Environ. Sci. Biotechnol. 2020, 19, 561–593. 10.1007/s11157-020-09538-w. [DOI] [Google Scholar]
- Vučić V.; Müller S. New developments in biological phosphorus accessibility and recovery approaches from soil and waste streams. Eng. Life Sci. 2021, 21, 77–86. 10.1002/elsc.202000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ J. J.; Blank L. M. Saccharomyces cerevisiae containing 28% polyphosphate and production of a polyphosphate-rich yeast extract thereof. FEMS Yeast Res. 2019, 19, foz011 10.1093/femsyr/foz011. [DOI] [PubMed] [Google Scholar]
- Christ J. J.; Smith S. A.; Willbold S.; Morrissey J. H.; Blank L. M. Biotechnological synthesis of water-soluble food-grade polyphosphate with Saccharomyces cerevisiae. Biotechnol. Bioeng. 2020, 117, 2089–2099. 10.1002/bit.27337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Rahman S. M.; Li G.; Fowle W.; Nielsen P. H.; Gu A. Z. The composition and implications of polyphosphate-metal in enhanced biological phosphorus removal systems. Environ. Sci. Technol. 2019, 53, 1536–1544. 10.1021/acs.est.8b06827. [DOI] [PubMed] [Google Scholar]
- Wang D.; Li Y.; Cope H. A.; Li X.; He P.; Liu C.; Li G.; Rahman S. M.; Tooker N. B.; Bott C. B.; et al. Intracellular polyphosphate length characterization in polyphosphate accumulating microorganisms (PAOs): Implications in PAO phenotypic diversity and enhanced biological phosphorus removal performance. Water Res. 2021, 206, 117726. 10.1016/j.watres.2021.117726. [DOI] [PubMed] [Google Scholar]
- Gomollón-Bel F. Mechanochemists Want to Shake up Industrial Chemistry. ACS Cent. Sci. 2022, 8, 1474–1476. 10.1021/acscentsci.2c01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.