Abstract
In this study we have determined systemic and local antibody responses against different Helicobacter pylori antigens in H. pylori-infected and noninfected subjects. In addition, we studied whether differences in antibody responses between patients with duodenal ulcers and asymptomatic H. pylori carriers might explain the different outcomes of infection. Sera and in most instances gastric aspirates were collected from 19 duodenal ulcer patients, 15 asymptomatic H. pylori carriers, and 20 noninfected subjects and assayed for specific antibodies against different H. pylori antigens, i.e., whole membrane proteins (MP), lipopolysaccharides, flagellin, urease, the neuraminyllactose binding hemagglutinin HpaA, and a 26-kDa protein, by enzyme-linked immunosorbent assay. The H. pylori-infected subjects had significantly higher antibody titers against MP, flagellin, and urease in both sera and gastric aspirates compared with the noninfected subjects. Furthermore, the antibody titers against HpaA were significantly elevated in sera but not in gastric aspirates from the infected subjects. However, no differences in antibody titers against any of the tested antigens could be detected between the duodenal ulcer patients and the asymptomatic H. pylori carriers, either in sera or in gastric aspirates.
Infection with Helicobacter pylori can have several different outcomes including chronic active gastritis, peptic ulcer disease (PUD), and gastric cancer (3, 29, 31). However, most infected subjects never develop any symptoms but remain asymptomatic (AS) throughout life. It is still poorly understood which factors determine the result of infection, but variable expression of certain virulence factors may be one explanation. Although several putative virulence factors have been described for H. pylori, only some isoforms of the vacuolating cytotoxin (VacA) and the pathogenicity island including the gene for the cytotoxin-associated protein (CagA) have been shown to be more prevalent in strains causing PUD than in other strains (2, 36).
Different abilities of hosts to control the inflammation caused by H. pylori infection as well as the quality of the specific immune response may also be important for the outcome of infection. H. pylori infection is generally associated with a massive infiltration of the gastric mucosa with neutrophils and lymphocytes (38). The infection also gives rise to elevated levels of specific antibodies in serum, and significantly increased antibody levels have also been demonstrated in saliva, gastric juice, and feces (24, 38). Despite the usually strong antibody responses against H. pylori infection, the bacteria are rarely eliminated from the stomach and the infection is usually lifelong. However, animal studies have shown a correlation between mucosally derived immunoglobulin A (IgA) antibodies against urease and protection against colonization with H. felis in mice immunized with H. pylori urease (21).
In the present study, we have determined the levels of specific antibodies against several different H. pylori antigens in sera and gastric aspirates from H. pylori-infected and noninfected subjects. We have also compared the antibody levels in H. pylori-infected patients with duodenal ulcers (DU) and in AS H. pylori carriers to evaluate if there are any differences in antibody responses to infection between these groups that may explain the different outcomes of infection.
MATERIALS AND METHODS
Subjects and specimens.
Sera and gastric aspirates were obtained from 13 H. pylori-infected patients with DU, 12 AS H. pylori carriers, and 12 healthy, noninfected volunteers who were participating in other studies at the Department of Surgery, Sahlgrenska University Hospital, Göteborg, Sweden. The DU patients comprised four women and nine men (mean age, 49 years [range, 22 to 59]), the AS subjects were seven women and five men (mean age, 42 years [range, 23 to 66]), and the noninfected volunteers were five women and seven men (mean age, 32 years [range, 23 to 66]). In addition, we studied sera collected from six additional DU patients, three AS subjects, and eight noninfected volunteers of corresponding ages. The DU patients had chronic relapsing DU disease, as confirmed by endoscopy, but were at the time of the investigation in clinical remission and had not been taking antisecretory medication for at least 5 days before the study. The AS subjects were recruited from among blood donors who had been screened for H. pylori infection by serology (13). Neither the AS subjects nor the noninfected subjects had any history of gastrointestinal disease or any other relevant illness. Infection with H. pylori was confirmed by culturing of gastric biopsies, serology, or a urea-breath test (13). Subjects who were negative in both culture and serology were included as noninfected controls, while subjects with positive culture or urea-breath test were considered H. pylori infected.
Gastric aspirates were obtained by collecting gastric juice from the fasting subjects, by means of a nasogastric tube connected to a suction pump (Egnell, Trollhättan, Sweden). At least 20 ml of gastric juice was aspirated from each subject during 30 min. Immediately after collection, the aspirates were put on ice and neutralized to pH 6 to 8 with Sörensen’s buffer containing 65 mM Na2HPO4 and 2 mM KH2PO4. To prevent enzymatic degradation of immunoglobulins, the following substances were added to the aspirates: bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) to a final concentration of 1 mg/ml, phenylmethylsulfonyl fluoride (Sigma) to a final concentration of 0.01 mg/ml, and soybean trypsin inhibitor (Sigma) to a final concentration of 0.35 mg/ml. Gastric aspirates were stored at −70°C and analyzed within 2 to 6 months; repeated analyses up to 1 year later resulted in almost identical titers as observed 1 to 3 weeks after collection. Serum specimens were obtained by intravenous puncture and stored at −20°C.
Bacterial strains and growth conditions.
Three reference strains—CCUG 17874 (NCTC 11637), E32, and E50 (kindly provided by E. Falsen, Göteborg University, Göteborg, Sweden, and J.-P. Butzler, St. Pieter’s University Hospital, Brussels, Belgium)—and two strains from our own collection—Hel 73, isolated from a patient with chronic antral gastritis, and Hel 305, isolated from a DU patient—were used for purification of specific antigens. The latter two strains had been subcultured only twice before use. All strains were cultured on Columbia II agar base plates containing 10% horse blood (produced at the Clinical Bacteriological Laboratory, Sahlgrenska University Hospital) at 37°C for 3 days under microaerophilic conditions (10% CO2, 5% O2, 85% N2) and then inoculated on new blood agar plates for 2 days before harvesting.
Preparation of antigens. (i) MPs.
Whole membrane proteins (MPs) were prepared as described by Achtman et al. (1) from three different strains of H. pylori—CCUG 17874, E50, and Hel 305—since we have previously shown that immune responses against MPs from different strains may vary (35). Briefly, H. pylori bacteria were harvested in a buffer containing 10 mM Tris and 5 mM MgCl2, pH 8, and centrifuged at 17,400 × g for 10 min. The pellet was subsequently sonicated at 22 kHz for 30 s eight times in a Vibra-cell ultrasonic processor (Sonics & Materials Inc., Danbury, Conn.) on ice. After centrifugation at 1,390 × g for 10 min at 4°C the supernatant was centrifuged at 14,900 × g for 30 min at 4°C. The pelleted bacterial membranes were then suspended in Tris buffer. The protein concentration was calculated by determining the spectrophotometric A280-A310 value. The lipopolysaccharide (LPS) content of the MP preparations varied between 2 and 12% (wt/wt) as determined by an inhibition enzyme-linked immunosorbent assay (ELISA) using specific monoclonal antibodies (MAbs) against the homologous LPS (22).
(ii) LPS.
LPS was purified by the hot-phenol-water method of Westphal and Jann (37) from three H. pylori strains with different O antigens (35)—CCUG 17874, E50, and Hel 73. The preparations were further purified by treatment with DNase II (2,000 U/mg; Boehringer Mannheim Scandinavia AB, Bromma, Sweden), RNase (40 U/mg; Boehringer Mannheim), and protease (type XIV; Sigma) followed by ultracentrifugation as described elsewhere (17). The protein content in the LPS preparations was less than 1% as determined by Peterson’s modification of the method of Lowry et al. (30), using the Sigma Diagnostics protein assay kit. The low protein content was further confirmed by silver staining of sodium dodecyl sulfate (SDS)-polyacrylamide gels using Bio-Rad’s original silver staining kit (Bio-Rad Laboratories, Richmond, Calif.).
(iii) Flagellin.
H. pylori flagellin was purified as previously described (18) from strain E32 since this is the strain in our collection that expresses the highest levels of flagellin; we tested purified flagellin from only one strain since H. pylori flagellin is highly conserved (15). Briefly, bacteria were harvested in phosphate-buffered saline (PBS) (pH 7.2), homogenized with an Ultra-Turrax blender (IKA-Labortechnik, Staufen, Germany), and then centrifuged at 18,000 × g for 1 h at 4°C. The supernatant was subsequently centrifuged at 100,000 × g for 1 h, whereupon the resulting pellet was resuspended in 20 mM Tris-HCl, pH 7.8, containing 20 mM CaCl2 and 1 mg of trypsin (Boehringer Mannheim) per ml and incubated at 37°C for 80 min. The enzyme reaction was stopped by adding soybean trypsin inhibitor at a final concentration of 0.25 mg/ml. Further purification was achieved by CsCl equilibrium density gradient ultracentrifugation in 1.27-g/cm3 CsCl2 at 180,000 × g at room temperature for 20 h followed by dialysis of the flagellin-containing fraction against PBS at 4°C for 12 h. The purity of the flagellin preparations was assessed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with antisera against whole H. pylori bacteria. The specificity was confirmed by immunoblotting with specific MAbs against flagellin (22).
(iv) Urease.
Urease, which also is highly conserved in H. pylori (14), was purified from strain E32, which is a good producer of urease. A combination of the methods described by Dunn et al. (8) and Evans et al. (11) was used for purification of urease. Briefly, H. pylori bacteria were harvested in PBS and centrifuged at 17,000 × g for 10 min. The pellet was then suspended in 1% N-octylglucose and left for 20 min at room temperature. After centrifugation at 26,000 × g for 15 min the supernatant was dialyzed against PBS overnight at 4°C. Further purification was obtained by size exclusion chromatography on a Sepharose CL 6B column (Pharmacia LKB, Uppsala, Sweden). The urease-containing fractions were identified, pooled, and dialyzed against PBS. After filtration through a 0.45-μm-pore-size filter the suspension was subjected to anion-exchange chromatography by fast protein liquid chromatography on a Resource Q column (Pharmacia). The purity of the preparations was assessed by SDS-PAGE and immunoblotting with urease-specific MAbs, and urease activity was confirmed by using a commercial urease test.
(v) 26-kDa protein and HpaA.
The 26-kDa protein (28) and HpaA (10, 27) were purified from strain CCUG 17874, which expresses high amounts of the antigens in vitro (22); we used only one strain for purification since these proteins are conserved in H. pylori (9, 23). The 26-kDa protein and HpaA were purified from the supernatant formed after centrifugation of disintegrated H. pylori bacteria at 100,000 × g for 1 h. The supernatant was added to a 17% bisacrylamide separation gel, and the proteins were separated by SDS-PAGE at 200 V for 1 h according to the protocol of Laemmli (20). After electrophoresis, two bands of 26 and 30 kDa (HpaA) were cut out from the gel. The proteins were then electroeluted according to the manufacturer’s instructions (Bio-Rad). The eluted proteins were dialyzed against PBS and frozen at −70°C. The purity was determined by SDS-PAGE and transblotting with polyclonal antisera against whole H. pylori bacteria as well as with MAbs reacting with the respective antigen (4, 22).
Determination of total IgA concentrations and H. pylori-specific antibody titers.
Total IgA and total secretory IgA (SIgA) contents in gastric aspirates were determined by a modified microplate ELISA as previously described (33). Goat anti-human IgA (Jackson Immuno Research Laboratories, West Grove, Pa.) was used for coating, horseradish peroxidase (HRP)-labeled goat anti-human IgA (Jackson) was used for detection of total IgA, and HRP-labeled anti-secretory component antibodies (Nordic Immunological Laboratories, Tilburg, The Netherlands) were used for detection of SIgA. Gastric aspirates were added to the plates in duplicates in initial dilutions of 1:30 (total IgA) or 1:5 (total SIgA) and threefold diluted. Commercially available IgA standards (Sigma) were used as references. Specimens with a total IgA concentration of ≤20 μg/ml were excluded from the study since samples with lower amounts of total IgA did not give reproducible results on antibody determination.
Titers of specific antibodies in sera and gastric aspirates, against membrane preparations and different purified H. pylori antigens were determined by ELISA. Polystyrene microtiter plates (Nunc, Roskilde, Denmark) were coated with optimal concentrations, as determined by checkerboard titrations, of the different antigens dissolved in PBS at room temperature overnight. The following antigen concentrations were used: MP, 25 μg/ml; LPS, 10 μg/ml; flagellin, 5 μg/ml; urease, 2.5 μg/ml; 26-kDa protein, 1.5 μg/ml; HpaA, 1.5 μg/ml. After two washes with PBS, wells were blocked with 0.1% (wt/vol) bovine serum albumin-PBS at 37°C for 30 min. Subsequent incubations were performed at room temperature, and plates were washed three times with PBS containing 0.05% Tween (PBS-Tween) between incubations. Serum samples and gastric aspirates were added in duplicates in initial dilutions of 1:10 and 1:2, respectively, and threefold diluted. Wells to which only PBS-Tween was added were used for determination of background values. After incubation at room temperature for 90 min, HRP-labeled rabbit anti-human IgA or IgG antibodies (Jackson) were added and incubated for 60 min. Plates were read in a spectrophotometer (Labsystems Multiskan PLUS) 20 min after addition of H2O2 and ortho-phenylene-diamine dihydrochloride in 0.1 M sodium citrate buffer, pH 4.5. The end point titers were determined as the reciprocal interpolated dilution giving an absorbance of 0.4 (sera) or 0.2 (aspirates) above background at 450 nm (33). H. pylori-specific antibody titers in gastric aspirates were expressed as the specific titer divided by the total IgA concentration in the sample. The measurement error of the ELISA (16) was determined to vary between 20 and 30% in repeat analyses over time.
Statistical methods.
Differences in antibody titers between the study groups were evaluated by the Mann-Whitney hypothesis test, and P values <0.01 were considered to represent significant differences. Correlations were expressed as Spearman’s rank correlation coefficient, rS.
RESULTS
H. pylori-specific antibodies in serum.
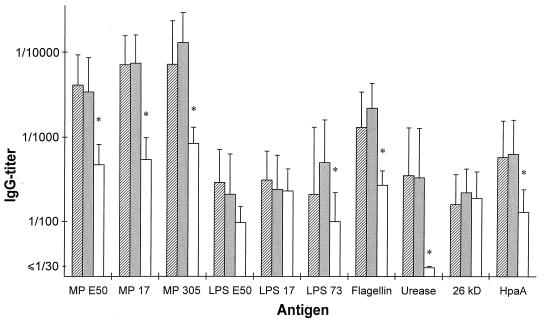
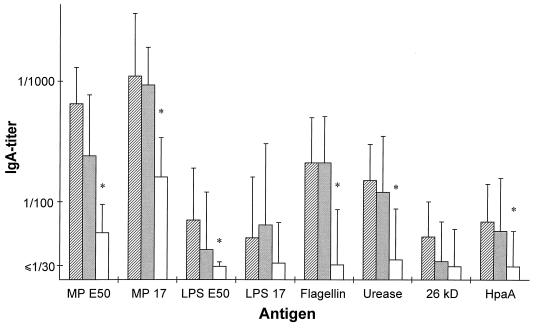
Serum samples from 19 H. pylori-infected patients with DU, 15 AS subjects, and 20 healthy, noninfected subjects were analyzed for specific antibodies in ELISA. Most of the H. pylori-infected subjects, i.e., both DU and AS subjects, had significant levels of H. pylori-specific antibodies in serum, while only a few of the noninfected subjects had antibodies that reacted with the H. pylori antigens (Table 1). The H. pylori-infected subjects had significantly higher IgG titers against MP prepared from H. pylori E50, CCUG 17874, or Hel 305 (P < 0.00006 for all strains) than the noninfected subjects (Fig. 1). Similarly, the serum IgG titers against LPS purified from strain Hel 73 (P = 0.0020), flagellin (P < 0.00006), urease (P < 0.00006), and HpaA (P < 0.00006) were significantly higher in the infected than in the noninfected group. However, IgG levels against LPS purified from strains E50 and CCUG 17874 and the 26-kDa protein did not differ significantly between the H. pylori-infected and the noninfected subjects (P, 0.018, 0.28, and 0.70, respectively). The subjects with high IgG titers against MP tended to have high titers also against flagellin, urease, and HpaA. When studying IgA responses, it was found that the H. pylori-infected subjects had significantly higher IgA titers against the different MPs, LPS from strain E50, flagellin, urease, and HpaA than the noninfected subjects, although the IgA titers were approximately 10 times lower than the IgG titers (Fig. 2). Comparison of serum antibody titers between the DU patients and the AS subjects did not reveal any significant differences between the two groups against any of the tested antigens (Fig. 1 and 2). Even though a higher proportion of the AS subjects had elevated IgG titers against LPS prepared from strain Hel 73 than the DU subjects (67% compared with 25%), this difference was not statistically significant.
TABLE 1.
Volunteers with significantly elevateda antibody titers in serum against different H. pylori antigens
| Antigen | % of subjectsb
|
|||||
|---|---|---|---|---|---|---|
| IgG
|
IgA
|
|||||
| DU (n = 19) | AS (n = 15) | HpÜn = 20) | DU (n = 19) | AS (n = 15) | HpÜn = 20) | |
| MP | ||||||
| E50 | 100 | 79 | 0 | 95 | 64 | 0 |
| CCUG 17874 | 100 | 100 | 0 | 68 | 67 | 5 |
| Hel 305 | 100 | 100 | 0 | NT | NT | NT |
| LPS | ||||||
| E50 | 70 | 60 | 0 | 90 | 70 | 0 |
| CCUG 17874 | 10 | 0 | 0 | 30 | 20 | 0 |
| Hel 73 | 25 | 67 | 0 | NT | NT | NT |
| Flagellin | 88 | 100 | 0 | 47 | 50 | 6 |
| Urease | 89 | 93 | 0 | 22 | 20 | 5 |
| 26 kDa | 0 | 0 | 0 | 22 | 0 | 5 |
| HpaA | 73 | 71 | 0 | 13 | 29 | 0 |
More than 2 standard deviations higher than the geometric mean titer of the noninfected subjects.
Hp−, noninfected subjects; NT, not tested.
FIG. 1.
Serum IgG titers (geometric means with standard deviation) against different H. pylori antigens in patients with DU (hatched bars; n = 19), AS H. pylori carriers (filled bars; n = 15), and noninfected subjects (open bars; n = 20). MP and LPS were purified from strains E50, CCUG 17874, Hel 73, and Hel 305 (E50, 17, 73, and 305, respectively). ∗, P < 0.01 (Mann-Whitney hypothesis test).
FIG. 2.
Serum IgA titers (geometric means with standard deviations) against different H. pylori antigens in patients with DU (hatched bars; n = 19), AS H. pylori carriers (filled bars; n = 15), and noninfected subjects (open bars; n = 20). MP and LPS were purified from strains E50 and CCUG 17874 (17 in the figure). ∗, P < 0.01 (Mann-Whitney hypothesis test).
H. pylori-specific antibodies in gastric aspirates.
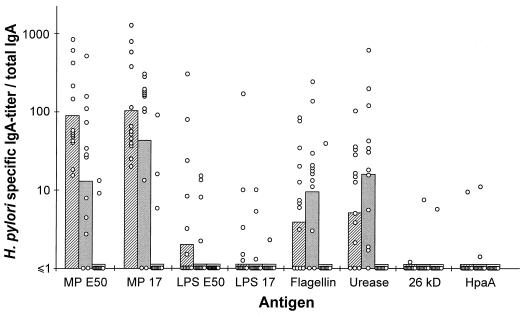
Gastric aspirates collected from the three groups of subjects were analyzed for total IgA as well as H. pylori-specific IgA antibodies in ELISA. Although the total IgA concentrations varied substantially in samples from different subjects, there were no significant differences in the mean values between the three groups—H. pylori-infected DU and AS volunteers and the noninfected subjects (data not shown). To adjust for the great variations in total IgA content in different samples, the H. pylori-specific antibody titers in the aspirates were expressed per microgram of total IgA per milliliter. The H. pylori-infected subjects had significantly higher proportions of IgA antibodies specific for the MPs (P < 0.00006), flagellin (P = 0.0014), and urease (P < 0.00006) than the noninfected subjects (Fig. 3). In contrast to the findings in serum, only very few of the infected subjects had gastric antibodies that reacted with HpaA and there were no significant differences between the H. pylori-infected and the noninfected subjects in IgA antibody levels against LPS from strain E50 (P = 0.011) or CCUG 17874 (P = 0.085) or against the 26-kDa protein (P = 0.29). No significant differences in gastric antibody levels against any of the antigens studied could be found between the DU and AS groups.
FIG. 3.
H. pylori-specific IgA antibodies in gastric aspirates from patients with DU (hatched bars), AS H. pylori carriers (filled bars), and noninfected subjects (open bars). The values are expressed as specific IgA titers divided by total IgA concentrations. The bars represent the geometric mean within each group, and each circle represents the value for one individual. MP and LPS were purified from strains E50 and CCUG 17874 (17 in the figure).
The correlation between serum IgA and gastric IgA antibody levels was low for all the antigens (rs < 0.5), as was the correlation between serum IgG and gastric IgA antibody levels (rs < 0.5). In no instance did an individual have antibodies against a particular antigen in the gastric aspirate and not in serum, while the opposite was frequently seen. To evaluate if the antibodies detected in the gastric aspirates were of mucosal or systemic origin, total SIgA concentrations were determined in all the gastric aspirates. These analyses showed that a majority of the samples contained SIgA and that there was a strong correlation between the SIgA and the total IgA levels (r = 0.830; P < 0.002). This suggests that at least a proportion of the specific antibodies detected in the gastric aspirates were mucosally derived.
DISCUSSION
Even though several previous studies have shown that H. pylori infection gives rise to specific antibody responses in the stomach, the antigenic specificities of these antibodies have not been analyzed in greater detail (32, 38). In the present study, we show strong antibody responses both in sera and in gastric aspirates against a number of different H. pylori antigens, e.g., MP, flagellin, and urease, in patients with DU and in AS carriers. We also demonstrate antibody responses against the neuraminyllactose binding hemagglutinin HpaA in serum, whereas specific immune responses against a prevalent 26 kDa protein could be detected neither in sera nor in gastric aspirates.
Despite the fact that the H. pylori-specific antibodies induced by natural infection are not capable of eliminating the bacteria, it is likely that a vaccine against H. pylori should induce strong secretory antibody responses locally in the stomach. In agreement with this belief, immunization of mice with H. pylori urease together with Escherichia coli heat-labile toxin has been shown to induce mucosal IgA antibodies which correlate with protection against infection with H. felis (21). In addition, it has been demonstrated that urease given to H. felis-infected mice cures the infection and eliminates the bacteria from the stomach (6). As we show in this study, natural infection with H. pylori induces strong systemic and mucosal antibody responses against urease in humans, and the urease has been considered a potent vaccine candidate for a long time. The effect of oral vaccination with urease on H. pylori infection was recently tested in a study in which AS H. pylori carriers were given recombinant urease (19). The urease was well tolerated by the subjects, but the vaccination did not have any effect on the infection. However, this might well be due to the fact that no mucosal adjuvant was included in the vaccine preparation.
In contrast to the strong antibody responses against urease, the antibody responses against the 26-kDa protein and HpaA were very poor, especially in the gastric aspirates. We have previously shown that H. pylori produces high amounts of the two proteins in vitro (22, 34), but the antigens might be poorly expressed in vivo or may be degraded by gastric juice since they induce strong antibody responses in serum when administered parenterally to experimental animals.
The H. pylori-infected subjects in this study had significantly higher antibody titers in sera against LPS prepared from H. pylori Hel 73 (IgG and IgA) and E50 (IgA) than the noninfected subjects, while no differences in antibody titers against LPS from strain CCUG 17874 were found between the two groups. It has previously been proposed that H. pylori strains could be classified into different serotypes on the basis of O antigens (26) and we have recently demonstrated considerably higher antibody responses against homologous LPS preparations than against LPS prepared from stock strains or heterologous clinical isolates (35). The reason for the low number of responders against LPS prepared from CCUG 17874 might well be due to the fact that LPS from strain CCUG 17874 lacks an O-specific side chain (35).
Although half of the human population is infected with H. pylori, only a relatively low proportion of the infected individuals develop DU while the majority remain AS throughout life. One aim of this study was to evaluate if there were any differences in antibody responses either in sera or in gastric aspirates between patients with DU and AS H. pylori carriers. However, the serum titers were strikingly similar in the two groups of subjects against all the antigens studied, and the only difference that could be detected was that a higher proportion of the AS subjects had elevated serum IgG titers against Hel 73 LPS than the DU patients. This might be explained by a higher proportion of strains having the same O antigen as strain Hel 73 in the AS group than among DU patients, but since we do not have access to the infecting strains from all the subjects, this could not be evaluated. In spite of large individual variations in antibody titers in the gastric aspirates, no differences in antibody responses could be detected between the AS and DU subjects. Thus, the different outcomes of infection could not be ascribed to differences in antibody responses against the antigens tested in this study, either in serum or in gastric aspirates. It would of course have been interesting to study immune responses against CagA and VacA since several papers have reported that H. pylori strains isolated from patients with PUD more often express VacA and CagA than strains isolated from individuals with other symptoms (2, 36) and also that it is more common for patients with PUD to have antibodies against CagA than it is for other H. pylori-infected individuals (5, 7). Unfortunately, we did not have access to these proteins and could not compare antibody levels against these antigens between the DU and AS subjects.
An obvious problem in assessing locally produced IgA antibodies in gastric aspirates is to evaluate whether the antibodies have been produced in the stomach or if they originate from the mouth or the intestine or even if they have transudated from serum. However, in another study we have shown that there is a high prevalence of H. pylori-specific antibody-secreting cells in the stomach in H. pylori-infected individuals and that these cells produce antibodies reacting with the same antigens as the antibodies detected in this study (25). This, together with the fact that we found SIgA in the aspirates from most of the H. pylori-infected subjects, suggests that at least a proportion of the H. pylori-specific antibodies in the gastric aspirates actually have been produced in the stomach. Another problem in using gastric aspirates to assess levels of antibody responses may be that the content of immunoglobulins varies significantly, not only between individuals but also within the same individual from one day to another and even in relation to the migrating motor complex, i.e., the IgA levels produced may vary at least threefold during the different phases of this complex (12). In order to compensate for these variations, we have expressed the H. pylori-specific antibodies in relation to the total IgA concentrations in each sample.
In conclusion, this study demonstrates that H. pylori infection induces strong antibody responses against MP, flagellin, and urease, both in serum and locally in gastric aspirates. However, no significant differences in antibody titers were found between the DU patients and the AS subjects, and thus the different outcomes of infection probably could not be explained by differences in specific antibody responses against the tested antigens.
ACKNOWLEDGMENTS
Financial support was obtained from The Bank of Sweden Tercentenary Foundation and the Swedish Medical Research Council.
All volunteers and the staff at the Laboratory of Gastroenterology, Sahlgrenska University Hospital, are gratefully acknowledged. We also thank Kerstin Andersson and Kristina Retteli for invaluable help.
REFERENCES
- 1.Achtman M, Schwuchow S, Helmuth R, Morelli G, Manning P A. Cell-cell interactions in conjugating Escherichia coli: Con− mutants and stabilization of mating aggregates. Mol Gen Genet. 1978;164:171–183. [Google Scholar]
- 2.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 4.Bölin I, Lönroth H, Svennerholm A M. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J Clin Microbiol. 1995;33:381–384. doi: 10.1128/jcm.33.2.381-384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ching C K, Wong B C, Kwok E, Ong L, Covacci A, Lam S K. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz I, Porta N, Glauser M, Saraga E, Vaney A C, Haas R, Kraehenbuhl J P, Blum A L, Michetti P. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology. 1995;109:115–121. doi: 10.1016/0016-5085(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompkins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunn B E, Campbell G P, Perez Perez G I, Blaser M J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 9.Evans D G, Evans D J, Jr, Lampert H C, Graham D Y. Restriction fragment length polymorphism in the adhesin gene hpaA of Helicobacter pylori. Am J Gastroenterol. 1995;90:1282–1288. [PubMed] [Google Scholar]
- 10.Evans D G, Karjalainen T K, Evans D J, Jr, Graham D Y, Lee C H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D J, Evans D G, Engstrand L, Graham D Y. Urease-associated heat shock protein of Helicobacter pylori. Infect Immun. 1992;60:2125–2127. doi: 10.1128/iai.60.5.2125-2127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fändriks L, Mattsson A, Dalenbäck J, Sjövall H, Olbe L, Svennerholm A-M. Gastric output of IgA in man: relation to migrating motor complexes and sham feeding. Scand J Gastroenterol. 1995;30:657–663. doi: 10.3109/00365529509096309. [DOI] [PubMed] [Google Scholar]
- 13.Hamlet A K, Erlandsson K I, Olbe L, Svennerholm A M, Backman V E, Pettersson A B. A simple, rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand J Gastroenterol. 1995;30:1058–1063. doi: 10.3109/00365529509101607. [DOI] [PubMed] [Google Scholar]
- 14.Höök-Nikanne J, Perez-Perez G I, Blaser M J. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin Diagn Lab Immunol. 1997;4:592–597. doi: 10.1128/cdli.4.5.592-597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtado A, Owen R J, Desai M. Flagellin gene profiling of Helicobacter pylori infecting symptomatic and asymptomatic individuals. Res Microbiol. 1994;145:585–594. doi: 10.1016/0923-2508(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 16.Jertborn M, Svennerholm A-M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonson G, Svennerholm A-M, Holmgren J. Vibrio cholerae expresses cell surface antigens during intestinal infection which are not expressed during in vitro culture. Infect Immun. 1989;7:1809–1815. doi: 10.1128/iai.57.6.1809-1815.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kostrzynska M, Betts J D, Austin J W, Trust T J. Identification, characterization, and spatial localization of two flagellin species in Helicobacter pylori flagella. J Bacteriol. 1991;173:937–946. doi: 10.1128/jb.173.3.937-946.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreiss C, Buclin T, Cosma M, Corthésy-Theulaz I, Michetti P. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet. 1996;347:1630–1631. doi: 10.1016/s0140-6736(96)91119-8. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–172. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Lindholm C, Osek J, Svennerholm A-M. Quantification of conserved antigens in Helicobacter pylori during different culture conditions. Infect Immun. 1997;65:5376–5380. doi: 10.1128/iai.65.12.5376-5380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundström, A., et al. Unpublished data.
- 24.Luzza F, Imeneo M, Maletta M, Monteleone G, Doldo P, Biancone L, Pallone F. Isotypic analysis of specific antibody response in serum, saliva, gastric and rectal homogenates of Helicobacter pylori-infected patients. FEMS Immunol Med Microbiol. 1995;10:285–288. doi: 10.1111/j.1574-695X.1995.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson, A., M. Quiding-Järbrink, H. Lönroth, A. Hamlet, I. Ahlstedt, and A.-M. Svennerholm. Antibody-secreting cells in the stomach of symptomatic and asymptomatic Helicobacter pylori infected subjects. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 26.Mills S D, Kurjanczyk L A, Penner J L. Antigenicity of Helicobacter pylori lipopolysaccharides. J Clin Microbiol. 1992;30:3175–3180. doi: 10.1128/jcm.30.12.3175-3180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Toole P W, Janzon L, Doig P, Huang J, Kostrzynska M, Trust T J. The putative neuraminyllactose-binding hemagglutinin HpaA of Helicobacter pylori CCUG 17874 is a lipoprotein. J Bacteriol. 1995;177:6049–6057. doi: 10.1128/jb.177.21.6049-6057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Toole P W, Logan S M, Kostrzynska M, Wadstrom T, Trust T J. Isolation and biochemical and molecular analyses of a species-specific protein antigen from the gastric pathogen Helicobacter pylori. J Bacteriol. 1991;173:505–513. doi: 10.1128/jb.173.2.505-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 30.Peterson G L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 31.Sipponen P, Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama, T., S. Furuyama, T. Awakawa, T. Kobayashi, T. Yabana, and A. Yachi. 1993. Local immune response in gastric mucosa to Helicobacter pylori infection with and without intestinal metaplasia. Eur. J. Gastroenterol. Hepatol. 5(Suppl. 1):119–122.
- 33.Svennerholm A-M, Jertborn M, Gothefors L, Karim A M M M, Sack D A, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B-subunit-whole-cell vaccine. J Infect Dis. 1984;149:884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- 34.Thoreson, A.-C., et al. Unpublished data.
- 35.Tinnert A, Mattsson A, Bölin I, Dalenbäck J, Hamlet A, Svennerholm A-M. Local and systemic immune responses in humans against Helicobacter pylori antigens from homologous and heterologous strains. Microb Pathog. 1997;23:285–296. doi: 10.1006/mpat.1997.0158. [DOI] [PubMed] [Google Scholar]
- 36.Weel J F, van der Hulst R W, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat G N, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173:1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- 37.Westphal O, Jann K. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–92. [Google Scholar]
- 38.Wyatt J I, Rathbone B J. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl. 1988;142:44–49. [PubMed] [Google Scholar]