Abstract
Introduction: Nutcracker syndrome (NS) is a rare condition in which the abdominal aorta and superior mesenteric artery compress the left renal vein (LRV). One treatment option is the placement of an endovascular stent into the LRV, which carries the risk of stent migration.
Case Report: A 30-year-old female with NS status-post LRV stenting 6 months prior presented to the emergency department with suprapubic pain. An incidental finding on abdominal computed tomography scan noted interval removal of LRV stent, which had not been surgically removed. A subsequent chest radiograph showed the stent lodged in the left pulmonary artery.
Discussion: To our knowledge, this is the first documented case of LRV stent migration to the pulmonary artery. This case demonstrates the importance of physician awareness of stent migration as a potential complication after stent placement, and careful review of all imaging findings, even if unrelated to the chief complaint.
INTRODUCTION
Nutcracker syndrome (NS) should be in the differential diagnosis of patients with abdominal pain/flank pain and hematuria. The name refers to the compression of the left renal vein (LRV) between the abdominal aorta and superior mesenteric artery resulting in renal vein hypertension [1]. A treatment option includes endovascular stenting (EVS) of the LRV [2]. A rare complication of EVS for NS is stent migration [1]. Complaints are variable and include pain and hematuria, while some are asymptomatic [2]. EVS migration has previously been shown to cause vessel damage, heart valve damage, dysrhythmias and pulmonary infarction [3]. This case demonstrates the importance of physician awareness of complications related to stent migration and careful review of all radiographic findings, even if incidental.
CASE REPORT
A 30-year-old female presented to the emergency department with a chief complaint of suprapubic pain. Her medical history was remarkable for NS status-post EVS with a 9 × 39 mm Abbott Omnilink Elite™ vascular balloon-expandable stent 6 months prior. The pain was ongoing for a few days, intermittent and sharp. The patient noted that the pain seemed different from when she was initially diagnosed with NS. Physical examination was unremarkable except for suprapubic tenderness to palpation. Blood work, vital signs and urinalysis were within normal limits, except for microscopic hematuria. A computed tomography abdomen and pelvis (CTAP) with intravenous contrast was performed and revealed a 2.2 cm right adnexal cystic structure, likely demonstrating a dominant ovarian follicle versus a hemorrhagic cyst. In addition, there was interval removal of the LRV stent and persistent LRV compression consistent with NS. A pelvic ultrasound was obtained and confirmed a hemorrhagic cyst. Upon discussing the findings with the patient, she denied subsequent LRV stent removal. The most recent imaging available was a CTAP on postoperative day 1 that confirmed proper positioning of the stent (Fig. 1). A chest radiograph was ordered and showed a metallic stent in the left hilum concerning for stent migration (Fig. 2). A CT angiogram (CTA) of the chest confirmed that the stent was in the left interlobar pulmonary artery and a heparin infusion was initiated (Fig. 3). The patient was transferred to a tertiary care center with interventional radiology and cardiothoracic capabilities. Interventional radiology attempted removal of the stent with balloon manipulation; however, the stent was embedded in the vessel wall. They then performed balloon angioplasty with 10-, 12- and 14-mm balloons. The patient was discharged on dual antiplatelet therapy with a recommendation of follow-up in 3 months for a CTA of the chest to confirm patency.
Figure 1.

CTAP without contrast demonstrating LRV stent in appropriate position postoperative day 1.
Figure 2.
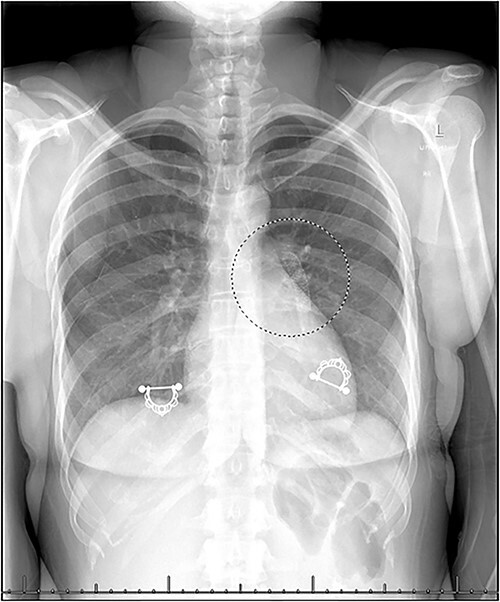
Chest radiograph demonstrating metallic stent in the left hilum.
Figure 3.

CTA Chest with IV contrast demonstrating stent in left interlobar pulmonary artery.
DISCUSSION
Intravenous stent usage continues to rise each year. Common indications include hemodialysis access, venous stenosis or extravascular venous occlusion. Stent migration is a rare but potentially serious complication of venous stenting. Sayed et al. reviewed EVS migration initially placed for various venous compression syndromes involving the superior vena cava, inferior vena cava, common iliac, hepatic, brachiocephalic, subclavian and LRVs. Of 54 documented migration events between 1994 and 2020, 41.6% presented asymptomatically [3]. Eleven of the 54 events migrated to the pulmonary artery, of which, none originated from the LRV. They concluded that a high degree of publication bias was present, with the true number of stent migrations likely much higher.
The incidence of LRV EVS migration for patients treated for NS has previously been estimated at 6.7% [1]. However, our review of the literature found only 11 previously documented cases of LRV stent migration [1, 2, 4–6]. Seven migrated partially or completely into the IVC, two migrated into the left side of the LRV, one presented into the right atrium and one into the right ventricle. Presenting complaints included flank pain, chest pain or hematuria, while others were asymptomatic. Migration of a LRV stent to the pulmonary artery has not been reported. In cases of stent migration to the pulmonary artery from other sites, presenting complaints included chest pain, dyspnea, cough or were asymptomatic [3]. It is important to note that stent migrations have been reported from 1-day postoperative to months or years later [1, 2]. It is possible that a stent may have been displaced days or months prior to the development of any complications. Thus, we recommend that the timing of stent placement to the discovery of migration should not be used to guide clinical decision-making. Factors contributing to migration may include stent sizing and intended indication. It has been noted that migration is more likely in stents shorter in length < 60 mm and narrower in diameter < 14 mm, and even more so in combination; in this case, the stent fits both criteria [3]. Additionally, per the manufacturer, The Omnilink Elite™ Vascular Balloon-Expandable Stent has the treatment of atherosclerotic iliac artery lesions as its only listed indication.
Several life-threatening complications of stent migrations have previously been reported. Migrations to the heart have damaged the tricuspid apparatus, leading to acute tricuspid insufficiency, damaged vessel walls or the myocardium or caused ventricular dysrhythmias [3, 7, 8]. Each of these outcomes necessitates urgent or emergent surgical management. Percutaneous retrieval may be the preferred option; however, open surgery may be needed in severe cases. Lastly, stent migration to the pulmonary vasculature can cause acute pulmonary infarctions [9]. If suspected, immediate heparinization is recommended until operative retrieval of the stent is possible. As in this case, removal may be deemed impossible or unnecessary. A ‘wait-and-see’ approach with or without preceding balloon angioplasty may be used in these patients. Discharge with appropriate antiplatelets or anticoagulation may be used until follow-up [10].
The migrated stent in this case was initially suspected due to an incidental finding on the CTAP. Incidental findings are a common occurrence in the emergency department. Prompt evaluation or communication for outpatient follow-up is essential for the well-being of the patient and the medicolegal liability of the physician. A low threshold for suspicion should be held for any incidental finding that could indicate a migrated stent, as early recognition can prompt further evaluation and escalation of care to prevent adverse outcomes.
ACKNOWLEDGEMENTS
None.
Contributor Information
Christopher Cooley, Department of Emergency Medicine, Corewell Health East – Farmington Hills, Framington Hills, MI, USA; College of Osteopathic Medicine, Michigan State University College of Osteopathic Medicine, East Lansing, MI, USA.
Robert A Alexander, College of Osteopathic Medicine, Michigan State University College of Osteopathic Medicine, East Lansing, MI, USA.
Pradeep Johns, Department of Emergency Medicine, Corewell Health East – Farmington Hills, Framington Hills, MI, USA.
Thomas Fanning, College of Arts and Sciences, Ohio State University, Columbus, OH, USA.
Jerry Z Oommen, Department of Emergency Medicine, Corewell Health East – Farmington Hills, Framington Hills, MI, USA; College of Osteopathic Medicine, Michigan State University College of Osteopathic Medicine, East Lansing, MI, USA.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No sources of funding.
ETHICAL APPROVAL
No ethical approval required.
CONSENT
No identifying information included in this report.
GUARANTOR
Christopher Cooley, DO.
REFERENCES
- 1. Wu Z, Zheng X, He Y, Fang X, Li D, Tian L et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord 2016;4:193–9. [DOI] [PubMed] [Google Scholar]
- 2. Rana MA, Oderich GS, Bjarnason H. Endovenous removal of dislodged left renal vein stent in a patient with nutcracker syndrome. Semin Vasc Surg 2013;26:43–7. [DOI] [PubMed] [Google Scholar]
- 3. Sayed MH, Salem M, Desai KR, O'Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord 2022;10:482–90. [DOI] [PubMed] [Google Scholar]
- 4. Hartung O, Grisoli D, Boufi M, Marani I, Hakam Z, Barthelemy P et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg 2005;42:275–80. [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Zhang H, Shi H, Tian L, Jin W, Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long-term followup. J Urol 2011;186:570–5. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Zhang Y, Li C, Zhang H. Results of endovascular treatment for patients with nutcracker syndrome. J Vasc Surg 2012;56:142–8. [DOI] [PubMed] [Google Scholar]
- 7. Khalid MO, Moskovits N, Frankel RA, Moskovits M, Saunders PC, Jacobowitz IJ et al. Venous stent migrating to the right heart causing severe regurgitation. J Investig Med High Impact Case Rep 2020;8:232470962097422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinberg E, Gentile C, Heller M, Kaban N, Bang E, Li T. Intracardiac venous stent migration: emergency department presentation of a catastrophic complication. J Emerg Med 2017;53:e11–3. [DOI] [PubMed] [Google Scholar]
- 9. Anand G, Lewanski CR, Cowman SA, Jackson JE. Superior vena cava stent migration into the pulmonary artery causing fatal pulmonary infarction. Cardiovasc Intervent Radiol 2011;34:198–S201. [DOI] [PubMed] [Google Scholar]
- 10. Kakisis JD, Vassilas K, Antonopoulos C, Sfyroeras G, Moulakakis K, Liapis CD. Wandering stent within the pulmonary circulation. Ann Vasc Surg 2014;28:1932.e9–12. [DOI] [PubMed] [Google Scholar]


