ABSTRACT
Background:
Prophylactic use of intra-aortic balloon pump (IABP) mainly depends on left ventricular (LV) systolic function. Global longitudinal strain (GLS) is a robust prognostic parameter for LV strain. It has proved to be more sensitive than LV ejection fraction (EF) as a measure of LV systolic function and is a strong predictor of outcome.
Aim:
To determine whether GLS can be used as a reliable marker and its cut-off value for IABP insertion in patients undergoing elective off-pump coronary artery bypass grafting (OPCABG).
Settings and Design:
A prospective observational clinical study which included 100 adult patients scheduled for elective OPCABG.
Materials and Methods:
Two-dimensional (2D) speckle tracking echocardiography (STE)-estimated GLS was computed and compared with LV EF measured by three dimensional (3D) echocardiography for the insertion of IABP. The intensive care unit (ICU) parameters were correlated with echocardiographic parameters to predict early post-operative outcome.
Results:
IABP insertion correlates better with GLS (post-revascularization > pre-revascularization) than with 3D LV EF. Receiver operating characteristic (ROC) curve analysis revealed the highest area under the curve (AUC, 0.972) with a cut-off value of > -9.8% for GLS compared to 3D LV EF (AUC, 0.938) with a cut-off value of ≤ 44%. ICU parameters show better correlation with E/e’> GLS > WMSI than 3D LV EF.
Conclusion:
GLS is a better predictor of IABP insertion compared to 3D LV EF in patients undergoing OPCABG.
Keywords: Ejection fraction, global longitudinal strain, intra-aortic balloon pump, low cardiac output state, off-pump coronary artery bypass grafting, trans-esophageal echocardiography
INTRODUCTION
Since its introduction in 1960s,[1] intra-aortic balloon pump (IABP) is the most commonly employed mechanical circulatory assist device and is often used to optimize cardiac function as a rescue therapy in case of failure of pharmacological therapy.[2,3] During off-pump coronary artery bypass grafting (OPCABG), use of IABP often reduces the requirement for urgent switching to on-pump coronary artery bypass grafting (CABG).[2,3] IABP has the advantage of optimizing the ratio between myocardial oxygen demand and supply, as compared to pharmacological treatment.[4,5] However, controversy still exists about the timing of IABP insertion, and a lot depends upon multiple variables, most important of which are left ventricle (LV) systolic function and clinical judgment with no well-defined criteria.[6,7,8]
The systole of the LV is a complex and coordinated effort performed by circumferential shortening, radial thickening, and longitudinal contraction.[9] Systolic function of the LV is assessed by various conventional echocardiographic techniques such as LV ejection fraction (EF), regional wall motion abnormalities (RWMA), wall motion score index (WMSI), tissue Doppler index (TDI-E/e’), or newer techniques based on speckle tracking echocardiographic (STE) assessment. Several studies have presented the advantages of global longitudinal strain (GLS) in comparison to EF for the assessment of LV systolic function.[10,11] The potential limitations of image quality, heart rate, and load dependence of LV EF are other reasons to support the increasing use of GLS. GLS also avoids geometric assumptions; it is less variable and sensitive to sub-clinical changes (low cardiac output state).[11,12]
Therefore, we planned to investigate the changes in myocardial function in order to figure out whether GLS can be used as a reliable marker compared to three-dimensional (3D) LVEF for IABP insertion in patients undergoing OPCABG.
The primary aim of our study was to determine whether pre-operative transesophageal echocardiography (TEE)-guided GLS can be used as a reliable marker compared to 3D LVEF and its cut-off value for IABP insertion in patients undergoing elective OPCABG. The secondary aim was to correlate echocardiographic parameters with intensive care unit (ICU) parameters to predict early post-operative outcome.
METHOD
After institutional ethics committee (IEC) approval (IECPG-424/26.08.2020) and obtaining informed written consent, this prospective observational study was conducted on 100 adult patients scheduled for elective OPCABG. Patients with acute coronary syndrome, pre-operative complication of myocardial infarction (ventricular septal rupture/LV aneurysm), significant pre-operative valvular heart disease, existing ventricular arrhythmias, post-cardiopulmonary resuscitation, and cardiomyopathies were excluded from the study. Patients were also excluded if they had prior mechanical ventilation and dialysis, had redo cardiac surgery, had emergency surgery, had pre-operative IABP, had any contra-indication to IABP insertion (e.g., severe aortic regurgitation (AR), mobile of grade 4 atheromas, peripheral vascular disease, etc.), required cardiopulmonary bypass (CPB) institution, or required surgical exploration.
After securing wide bore i.v. cannula and radial artery cannulation, anesthesia was induced and maintained as per the standard institutional protocol. Central venous cannulation was performed via the right internal jugular vein. Femoral artery was cannulated with a 16 G catheter. All patients were subjected to volume-controlled mechanical ventilation and ventilator settings optimized for an end-tidal carbon dioxide level target of 29–34 mm Hg to maintain PaCO2 35–45 mm Hg.
Transesophageal echocardiography
A Philips Healthcare IE 33 ultrasound machine and matrix array probe (Bothell, WA, USA) were used for the TEE examination. The TEE examination was performed and analyzed by an echocardiographer with 10 years of experience and who was blinded to the pre-operative echocardiography findings of the patients. The echocardiographic images were acquired before the surgical incision (pre-revascularization) and after completion of myocardial revascularization (post-revascularization) at stable hemodynamics (+/- 10% variation in mean arterial blood pressure and heart rate). One lead electrocardiogram was recorded simultaneously.
QLAB cardiovascular ultrasound quantification and cardiac motion quantification (CMQ) were used for the analysis of echocardiographic information, whereas 3DQ Advanced software was used for the analysis of 3D data (Philips Medical Systems, Andover, MA, USA).
For the strain analysis of LV, the myocardium was scanned using 2D - STE at a frame rate of >50 Hz, and cine loops of six consecutive beats from the mid esophageal 4 chamber (ME - 4C), 2 chamber (ME - 2C), and long-axis (ME - LAX) view were obtained. Before image acquisition, it was ensured that the entire LV along with the endocardium was visualized without any foreshortening throughout the cardiac cycle. For 2D strain measurements, an off-line analysis was performed. On the end-diastolic frame, the endocardium was traced, and the regions of interest were optimized by approaching the myocardium from endocardium to epicardium while avoiding the pericardium. To incorporate the different segments of the LV, the regions of interest were modified according to the myocardial wall thickness. The software automatically calculated the comprehensive GLS curve for all the myocardial segments in each of the three standard views (ME - 4C, ME - 2C, and ME - LAX). An inspection of the moving image was performed to confirm that the tracking was adequate [Figure 1a-d].
Figure 1.
The echocardiographic image panel. (a) longitudinal strain quantification ME- 4 chamber view; (b) longitudinal strain quantification ME- 2 chamber view; (c) longitudinal strain quantification ME – long axis view; (d) global longitudinal strain quantification (average of the 3 views; bull’s eye view); (e) 3D LV EF quantification
3DQ software was used for the calculation of ventricular volumes (end-systolic and end-diastolic) which also gave an estimate of LVEF by 3D. All measurements were averaged from at least three consecutive cardiac beats [Figure 1e].
Pulse wave Doppler was applied to determine the trans-mitral inflow velocity pattern, and the peak early diastolic (E) wave velocity was measured. Early mitral annular diastolic velocity (e’) was assessed using tissue Doppler imaging at the septal and lateral annuli to compute the TDI in the form of E/e’ ratio (average of septal and lateral values).
WMSI was calculated as the average of myocardial motion for each myocardial segment of the 17 segment model.
The coronary revascularization procedure was carried out by a single surgeon through the mid-sternotomy approach. Following surgery, the patients were shifted to the cardiothoracic surgical ICU. Adhering to the institutional protocol, standardized intensive care was implemented. The post-operative decision making was performed by cardiac anesthesiologists, who were kept blind to the objectives and nature of the study.
IABP insertion
The decision for timely insertion of IABP was taken based on hemodynamic instability [sustained hypotension (a systolic arterial pressure less than 90 mm Hg or 30 mm Hg below baseline), raised right atrial (RA) pressures, and signs of diminished tissue perfusion (cold periphery, clammy skin, confusion, oligouria, elevated lactate level) in the absence of hypovolemia] during or after OPCABG by the cardiac anesthesiologist who was blinded to intra-operative echocardiographic data. However, at the time of decision making, he used the conventional echocardiographic data except GLS and hemodynamic parameters for IABP insertion. IABP was inserted into the femoral artery via the femoral arterial access taken at induction. Using a percutaneous sheathless technique, the balloon of 8 Fr with 40 cc balloon (Arrow International, Everett, MA, USA) was introduced with its tip positioned just distal to the origin of the left subclavian artery.
Hemodynamic parameters and clinical outcomes
Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), pulse oximetry, vasoactive inotropic score (VIS), and any significant ST changes were recorded at the time of IABP insertion.
The secondary clinical variables observed were maximum VIS,[13] duration of inotropic support, mechanical ventilation, ICU stay, and any other significant complication.
Statistical analysis
The data were imported into MS Excel, and the analysis was completed with SPSS version 21.0. For continuous variables, the data were provided as mean and standard deviation, whereas for categorical variables, the data were presented as percentages. To compare two independent group means and paired sample means of the same group, the unpaired and paired t-tests were used. The connection between categorical variables was determined using the Chi-square or Fisher exact test. To determine the correlation between two continuous variables, the Pearson correlation coefficient was utilized. The proper cut-off values for each parameter were determined using receiver operating characteristic (ROC) curve analysis with the Youden index. Significance was defined as a P value of less than 0.05.
RESULTS
A total of 100 patients were screened and analyzed. The cohort of subjects with IABP insertion comprised 14 patients (intra-operative = 6, post-operative = 8), whereas 86 patients did not require IABP insertion. The two groups were comparable with regard to the baseline demographic characteristics such as age, sex, body mass index (BMI), European system for cardiac operative risk evaluation II (EuroSCORE II), hypertension, diabetes mellitus, and NYHA grading [Table 1].
Table 1.
Comparison of baseline demographic and echocardiographic characteristics between the patients with IABP insertion and without IABP insertion
| Variables | IABP | No IABP | P |
|---|---|---|---|
| Age (years) | 58.7±10.0 | 59.1±11.0 | 0.892 |
| Weight (kg) | 61.4±12.7 | 66.0±10.4 | 0.140 |
| Height (cm) | 160.1±7.6 | 163.0±7.5 | 0.180 |
| BMI (Kg/m2) | 24.0±4.7 | 24.8±3.4 | 0.403 |
| Male | 12/86 (14%) | 74/86 (86%) | 1.000* |
| Female | 2/14 (14.3%) | 12/14 (85.7%) | 1.000* |
| Diabetes | 7/59 (11.9%) | 52/59 (88.1%) | 0.460 |
| Hypertension | 9/59 (15.3%) | 50/59 (84.7%) | 0.665 |
| NYHA class II | 4/39 (10.3%) | 35/39 (89.7%) | |
| NYHA class III | 9/57 (15.8%) | 48/57 (84.2%) | 0.604 |
| NYHA class IV | 1/4 (25.0%) | 3/4 (75%) | |
| EuroSCORE II | 1.2±0.6 | 1.0±0.5 | 0.199 |
| Echocardiographic measurements | |||
| Pre-revascularization GLS (%) | -8.3±1.5 | -14.0±3.2 | <0.001 |
| Post-revascularization GLS (%) | -7.7±1.5 | -13.1±2.8 | <0.001 |
| Pre-revascularization 3D LV EF (%) | 36.0±8.4 | 52.0±8.9 | <0.001 |
| Post-revascularization 3D LV EF (%) | 36.3±6.9 | 51.3±7.0 | <0.001 |
| Pre-revascularization E/e’ | 13.3±2.5 | 10.9±2.5 | 0.001 |
| Post-revascularization E/e’ | 14.3±1.9 | 10.7±2.8 | <0.001 |
| Pre-revascularization WMSI | 2.4±0.6 | 1.8±0.6 | 0.001 |
| Post-revascularization WMSI | 2.4±0.5 | 1.7±0.5 | <0.001 |
(x±y=mean±standard deviation; P<0.05 is considered statistically significant), (Abbreviations: BMI, body mass index; NYHA, New York Health Association; GLS, global longitudinal strain; 3D LV EF, 3-dimensional left ventricular ejection fraction; WMSI, wall motion score index)
Table 1 also compares the echocardiographic variables among the two groups. The GLS, E/e’, and WMSI (both pre-revascularization and post-revascularization) were significantly greater in IABP group than in non-IABP group, whereas the LV EF (both pre-revascularization and post-revascularization) was significantly lower in the IABP group.
Table 2 demonstrates pre-revascularization and post-revascularization values of GLS, 3D LVEF, E/e’, and WMSI. There is a highly significant difference in pre-revascularization and post-revascularization values of GLS and WMSI. The postop GLS value is more than the pre-revascularization value, whereas the WMSI is less than the pre-revascularization value. There is no significant difference in other parameters [Table 2].
Table 2.
Comparison of pre-revascularization and post-revascularization values of echocardiographic parameters
| Variables | Time | Mean | Mean difference | Paired t test P |
|---|---|---|---|---|
| GLS (%) | Pre-revascularization | -13.239 | -0.9070 | <0.001 |
| Post-revascularization | -12.332 | |||
| 3D LV EF (%) | Pre-revascularization | 49.740 | 0.5600 | 0.248 |
| Post-revascularization | 49.180 | |||
| E/e’ | Pre-revascularization | 11.199 | 0.0339 | 0.825 |
| Post-revascularization | 11.165 | |||
| WMSI | Pre-revascularization | 1.860 | 0.0764 | 0.006 |
| Post-revascularization | 1.784 |
(GLS, global longitudinal strain; 3D LV EF, 3-dimensional left ventricular ejection fraction; WMSI, wall motion score index)
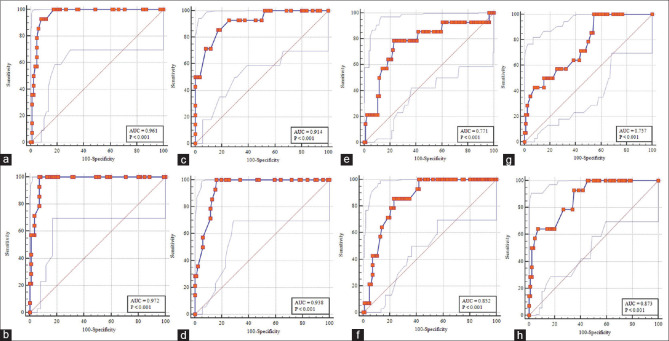
The optimal cut-off values for different echocardiographic parameters to predict IABP insertion are depicted in Table 3. Pre-revascularization GLS > -10.2%, post-revascularization GLS > -9.8%, post-revascularization LV EF ≤44%, and predicted IABP insertion with high sensitivity and specificity. Pre-revascularization WMSI >1.7 and post-revascularization WMSI >1.875 have high sensitivity for IABP insertion but with low specificity. However, on ROC curve analysis, the GLS was significantly more accurate as a predictor for IABP insertion compared to 3D LV EF [area under curve (AUC) higher for GLS than 3D LV EF] [Figure 2].
Table 3.
Appropriate cut-off for the different echocardiographic parameters to predict IABP insertion
| Parameters | Cut-off | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| EuroSCORE II | >0.93 | 0.609 | 71.4 | 58.1 | 21.7 | 92.6 | 60.0 |
| Pre-revascularization GLS (%) | >-10.2 | 0.961 | 92.9 | 91.9 | 65.0 | 98.8 | 92.0 |
| Post-revascularization GLS (%) | >-9.8 | 0.972 | 100.0 | 93.0 | 70.0 | 100.0 | 94.0 |
| Pre-revascularization 3D LV EF (%) | </=42 | 0.914 | 85.7 | 82.6 | 44.4 | 97.3 | 83.0 |
| Post-revascularization 3D LV EF (%) | </=44 | 0.938 | 100.0 | 83.7 | 50.0 | 100.0 | 86.0 |
| Pre-revascularization E/e’ | >12.6 | 0.771 | 78.6 | 76.7 | 35.5 | 95.6 | 77.0 |
| Post-revascularization E/e’ | >12.4 | 0.852 | 85.7 | 76.7 | 37.5 | 97.1 | 78.0 |
| Pre-revascularization WMSI | >1.7 | 0.757 | 100.0 | 45.4 | 23.0 | 100.0 | 53.0 |
| Post-revascularization WMSI | >1.875 | 0.873 | 92.9 | 65.1 | 30.2 | 98.3 | 69.0 |
(AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; GLS, global longitudinal strain; 3D LV EF, 3-dimensional left ventricular ejection fraction; WMSI, wall motion score index)
Figure 2.
ROC curve analysis showing the area under the curve (AUC) for the prediction of IABP insertion. (a) pre-revascularization GLS; (b) post-revascularization GLS; (c) pre-revascularization 3D LV EF; (d) post-revascularization 3D LV EF; (e) pre-revascularization E/e’; (f) post-revascularization E/e’; (g) pre-revascularization WMSI; (h) post-revascularization WMSI)
The demographic and hemodynamic parameters for patients at the time of IABP insertion are demonstrated in Table 4.
Table 4.
Indication for IABP insertion
| Age (Yr) | Sex (M/F) | BMI (Kg/m2) | Comorbidities (DM/HTN/Both) | NYHA | EURO SCORE II | IABP insertion time | Triggering event (ECG) | HR | SBP/DBP (MAP) | CVP | VIS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 | M | 19.67 | Both | III | 1.82 | POD0 | VT | 186 | 68/32 (48) | 18 | 28 |
| 61 | M | 24.61 | Both | III | 0.94 | POD1 | STE | 104 | 83/33 (55) | 17 | 35 |
| 65 | M | 29.75 | HTN | III | 1.05 | POD0 | QRS widening | 125 | 76/38 (56) | 11 | 38 |
| 60 | M | 33.25 | Both | III | 0.95 | OT | STE | 124 | 104/54 (69) | 22 | 28 |
| 51 | M | 30.33 | DM | III | 0.67 | POD0 | STE | 135 | 86/42 (60) | 20 | 59 |
| 58 | M | 20.07 | HTN | II | 1.07 | OT | STE | 132 | 74/35 (50) | 14 | 38 |
| 73 | M | 27.23 | Both | II | 1.36 | POD0 | VF | - | 70/22 (52) | 17 | 46 |
| 72 | M | 25.72 | DM | III | 1.49 | OT | VT | 196 | 73/38 (52) | 18 | 30 |
| 46 | F | 19.56 | Both | III | 2.72 | OT | STE + QRS widening | 116 | 111/68 (80) | 8 | 27 |
| 48 | M | 23.14 | - | II | 0.55 | POD3 | STE | 126 | 69/46 (57) | 16 | 35 |
| 47 | M | 21.07 | - | III | 2.01 | OT | STE | 56 | 74/42 (58) | 16 | 20 |
| 45 | F | 19.83 | DM | II | 0.76 | POD1 | STE | 142 | 82/36 (58) | 12 | 34 |
| 63 | M | 17.99 | HTN | IV | 1.29 | POD8 | VT | 178 | 70/32 (46) | 19 | 44 |
| 61 | M | 23.05 | - | III | 0.67 | OT | VF | - | 72/38 (53) | 14 | 30 |
(Yr, years; M, male; F, female; BMI, Body mass index; DM, Diabetes mellitus; HTN, Hypertension; HR, Heart rate; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; MAP, Mean arterial pressure; CVP, Central venous pressure; VIS, Vasoactive inotropic score)
The secondary outcomes, that is, ICU parameters, were computed for Pearson correlation [Table 5]. As per post-revascularization echocardiographic parameters, duration of ionotropic support correlates best with E/e’ > WMSI > GLS > 3D LV EF. Max VIS correlates best with E/e’ > GLS > WMSI > 3D LV EF. Duration of mechanical ventilation has a significant correlation only with E/e’ and WMSI. Duration of ICU stay correlates maximum with E/e’ > GLS > WMSI >3D LV EF.
Table 5.
Correlation of echocardiographic parameters for early post-operative clinical outcome
| Variables Pearson correlation (P) | Duration of ionotropic support (hrs) | Max VIS | Duration of mechanical ventilation (hrs) | Duration of ICU stay (days) |
|---|---|---|---|---|
| EuroSCORE II | 0.214 (0.035) | 0.131 (0.193) | 0.193 (0.055) | 0.209 (0.038) |
| Pre-revascularization GLS (%) | 0.433 (<0.001) | 0.393 (<0.001) | 0.118 (0.241) | 0.435 (<0.001) |
| Post-revascularization GLS (%) | 0.471 (<0.001) | 0.470 (<0.001) | 0.149 (0.139) | 0.485 (<0.001) |
| Pre-revascularization 3D LV EF (%) | -0.450 (<0.001) | -0.364 (<0.001) | -0.154 (0.126) | -0.435 (<0.001) |
| Post-revascularization 3D LV EF (%) | -0.468 (<0.001) | -0.417 (<0.001) | -0.222 (0.026) | -0.480 (<0.001) |
| Pre-revascularization E/e’ | 0.465 (<0.001) | 0.372 (<0.001) | 0.101 (<0.001) | 0.431 (<0.001) |
| Post-revascularization E/e’ | 0.586 (<0.001) | 0.471 (<0.001) | 0.239 (<0.001) | 0.566 (<0.001) |
| Pre-revascularization WMSI | 0.399 (<0.001) | 0.303 (0.002) | 0.154 (0.127) | 0.355 (<0.001) |
| Post-revascularization WMSI | 0.506 (<0.001) | 0.422 (<0.001) | 0.231 (0.021) | 0.483 (<0.001) |
P<0.05 is considered significant (Abbreviations: GLS, global longitudinal strain; 3D LV EF, 3-dimensional left ventricular ejection fraction; WMSI, wall motion score index; VIS, Vasoactive-Inotropic Score)
Complications such as arrhythmia (n = 11), acute kidney injury (AKI) (n = 5), respiratory failure (n = 4), and psychosis (n = 1) seen in patients underwent OPCABG. Mortality was reported in two patients (one patient developed sepsis, and another one could not be revived after sudden cardiac arrest).
DISCUSSION
The IABP is a valuable and established mechanical circulatory support used in the treatment of the acute decompensated phase of heart failure.[14] Along with the beneficial effect of significant afterload reduction and augmentation of diastolic pressures leading to the favorable myocardial blood supply, it also leads to improved perfusion of ischemic myocardial areas with redistribution of coronary blood flow.[5,15] The indication and optimal timing of IABP insertion still remain debatable. Some studies support prophylactic pre-operative IABP in OPCABG, whereas others use it to treat low cardiac output during or after surgery when required.[16,17,18,19] Prophylactic use of IABP mainly depends on LV systolic function. GLS is the best-evaluated parameter for LV strain. It has proved to be more sensitive than EF for the measurement of LV systolic function and a strong predictor of outcome in the recent literature.[20,21,22]
It has been demonstrated in the recent literature that GLS has been able to diagnose sub-clinical LV dysfunction even in patients with normal LV function or mild LV dysfunction as classified on the basis of LVEF.[22] Under these circumstances, the present study has been conducted to evaluate the comparison of GLS with LV EF as a predictor for IABP insertion in patients undergoing OPCABG. Moreover, there is no objective parameter for prophylactic IABP insertion till now.
In a review article, Elizabeth Potter et al.[21] reclassified the LV dysfuction based on GLS into very severe with GLS > -8%, severe with GLS > -12%, reduced with GLS -12% to -16%, borderline with GLS -16% to -18%, normal with GLS -18% to -20%, and supranormal with GLS < -20%. They concluded that the addition of GLS to LVEF enables comprehensive phenotyping and better risk assessment, and it can be a tool for present and future therapeutic advancement.
In 320 consecutive patients of triple vessel disease and EF >45%, Francesca D’Auria et al.[23] used STE to assess the improvement of myocardial activity following OPCABG. They came to the conclusion that there was a significant correlation between coronary lesions detected by angiogram and the corresponding impaired heart zones, as evidenced by a reduction in global and segmental longitudinal strain (P < 0.05), but no significant correlation was found between angiographic lesion and LV volume, LV dysfunction, or EF (P > 0.05).
Aude Mignot et al.[24] evaluated 147 patients with heart failure with LV EF ≤45% (74% men; mean age, 64 ± 14 years; mean LV EF, 29.9 ± 8.9%) for cardiac events in a multi-center study. The values of conventional echocardiographic parameters as well as segmental and GLS were assessed and compared to those of a control group. Using a cut-off value of -7%, they demonstrated that GLS had the greatest prognostic value (AUC, 0.83) and the strongest combination of specificity (83%) and sensitivity (73%) on ROC curve analysis.
In another study, Tony Stanton et al.[25] investigated the incremental value of EF, WMSI, and GLS to key clinical factors in their study prediction of all-cause mortality from GLS in comparison with EF and WMSI. They discovered that adding WMSI (HR, 1.28; P < 0.01) or EF (HR, 1.23; P = 0.03) increased the predictive power of clinical variables, and adding GLS (HR, 1.45; P < 0.001) increased the model power the most (χ2 = 34.9, P < 0.001). GLS was similarly beneficial in those with an EF of greater than 35% and those with and without aberrant wall motion. In terms of prognosis prediction, an LV EF ≤ 35% was shown to be similar to a GLS of > -12%.
The present study evaluated the incremental value of GLS and LVEF as a predictor of IABP insertion which will help in development of objective parameters over and above clinical markers of IABP insertion in patients undergoing OPCABG.
The present study predicted that IABP insertion better correlates with GLS (post-revascularization > pre-revascularization;) than with 3D LVEF. The analysis of ROC curve revealed the highest AUC (0.972) with a cut-off value of > -9.8% for post-revascularization GLS with maximum combined sensitivity and specificity, followed by LVEF (AUC, 0.938) with a cut-off value of ≤44%. The next parameter is WMSI (AUC, 0.873) with a cut-off value of > 1.875. The reason, the authors believe, was the ability of GLS to prognosticate LV systolic function in patients with LVEF > 40% above and beyond traditional indexes.[26] The author observed no significant difference in EuroSCORE II in both the groups and a minimum AUC (0.609) with a cut-off value of > 0.93. It is in accord with other studies in determining the uncertainness of Euro scores in predicting early post-operative outcome and the need to be supplemented with other echocardiographic variables.[27,28,29]
Vijayaraghavan G et al.[30] in their review article labeled GLS as mildly reduced if it is between − 15% and − 12.5%, moderately reduced if between − 8.1% and − 12.5%, and severely reduced when less than − 8.0%, which correlates well with our study.
In the present study, patients had a better early post-operative outcome with lower GLS, E/e’, and WMSI and with higher 3D LVEF. ICU parameters show better correlation with E/e’ > GLS > WMSI than 3D LVEF. Our findings are similar to the study by Anna Gozdzik et al.,[31] who observed that patients with E/e’ > 12 had a significantly prolonged intubation time and higher inotropic requirements. After ROC analysis, they demonstrated that LV dysfunction was a good predictor of early post-operative outcome and higher inotropic requirements.
The present study had several limitations. The TEE requires sedation or general anesthesia. It is possible that the anesthetic drugs could have changed the absolute value of echocardiographic measurements. However, a uniform and consistent protocol was followed, and nearly identical anesthetic drugs were given in all the patients, depending on baseline hemodynamic parameters, and the data were collected while the patients were in a stable hemodynamic condition. Consequently, it would not be justifiable to compare the parameters obtained by TEE to those obtained by transthoracic echocardiography (TTE). TEE, on the other hand, had greater control and picture quality than TTE, especially in situations requiring mechanical ventilation interruption and longer examination times in these forms of study. The other limitations are not having RV information and the fact that GLS requires pictures of high resolution that are HR-dependent. Therefore, pictures need to be taken in quick succession to prevent change in HR. The study was also limited to OPCABG, and the number of patients who required IABP insertion was quite low.
Furthermore, the study population is relatively small because of the continuing COVID-19 pandemic. The authors could have performed multi-variate analysis to find variables independently if they had a larger group of patients with a greater number of IABP insertions.
CONCLUSION
We demonstrated that echocardiographic parameters were good predictors for IABP insertion and early post-operative clinical outcome. The incremental predictive value of LV GLS was higher than the other conventional parameters (3D LVEF, WMSI, E/e’), conventional risk score (EuroSCORE II), and clinical baseline variables. These parameters have the potential to replace the classical indications of prophylactic IABP insertion. The present study highlights the importance of involvement of LV GLS measurement during routine echocardiographic assessment, particularly in high-risk patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kantrowitz A, Tjønneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL., Jr Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:113–8. [PubMed] [Google Scholar]
- 2.Kolh P, Windecker S, Alfonso F, Collet J, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;46:517–92. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 3.Ding W, Ji Q, Wei Q, Shi Y, Ma R, Wang C. Prophylactic application of an intra-aortic balloon pump in high-risk patients undergoing off-pump coronary artery bypass grafting. Cardiology. 2015;131:109–15. doi: 10.1159/000377720. [DOI] [PubMed] [Google Scholar]
- 4.Hanlon-Pena PM, Quaal SJ. Intra-aortic balloon pump timing: Review of evidence supporting current practice. Am J Crit Care. 2011;20:323–33. doi: 10.4037/ajcc2011542. [DOI] [PubMed] [Google Scholar]
- 5.De Silva K, Lumley M, Kailey B, Alastruey J, Guilcher A, Asrress KN, et al. Coronary and microvascular physiology during intra-aortic balloon counterpulsation. JACC Cardiovasc Interv. 2014;7:631–40. doi: 10.1016/j.jcin.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 6.de Waha S, Desch S, Eitel I, Fuernau G, Lurz P, Sandri M, et al. Intra-aortic balloon counterpulsation—basic principles and clinical evidence. Vascul Pharmacol. 2014;60:52–6. doi: 10.1016/j.vph.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Ranucci M, Ballotta A, Castelvecchio S, De Vincentiis C, Biondi A, Parisi A, et al. Perioperative heart failure in coronary surgery and timing of intra-aortic balloon pump insertion. Acta Anaesthesiol Scand. 2010;54:878–84. doi: 10.1111/j.1399-6576.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 8.Miceli A, Duggan SM, Capoun R, Romeo F, Caputo M, Angelini GD. A clinical score to predict the need for intraaortic balloon pump in patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2010;90:522–6. doi: 10.1016/j.athoracsur.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: The relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 10.Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. 2017;19:307–13. doi: 10.1002/ejhf.694. [DOI] [PubMed] [Google Scholar]
- 11.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–80. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 12.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–9. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–8. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 14.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42:3599–726. [Google Scholar]
- 15.Maccioli GA, Lucas WJ, Norfleet EA. The intra-aortic balloon pump: A review. J Cardiothorac Anesth. 1988;2:365–73. doi: 10.1016/0888-6296(88)90320-1. [DOI] [PubMed] [Google Scholar]
- 16.Christenson JT, Licker M, Kalangos A. The role of intra-aortic counterpulsation in high-risk OPCAB surgery: A prospective randomized study. J Card Surg. 2003;18:286–94. doi: 10.1046/j.1540-8191.2003.02030.x. [DOI] [PubMed] [Google Scholar]
- 17.Marra C, De Santo LS, Amarelli C, Della Corte A, Onorati F, Torella M, et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction: A prospective randomized study on the timing of perioperative intraaortic balloon pump support. Int J Artif Organs. 2002;25:141–6. doi: 10.1177/039139880202500209. [DOI] [PubMed] [Google Scholar]
- 18.Dyub AM, Whitlock RP, Abouzahr LL, Cinà CS. Preoperative intra-aortic balloon pump in patients undergoing coronary bypass surgery: A systematic review and meta-analysis. J Card Surg. 2008;23:79–86. doi: 10.1111/j.1540-8191.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 19.Pilarczyk K, Boening A, Jakob H, Langebartels G, Markewitz A, Haake N, et al. Preoperative intra-aortic counterpulsation in high-risk patients undergoing cardiac surgery: A meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2016;49:5–17. doi: 10.1093/ejcts/ezv258. [DOI] [PubMed] [Google Scholar]
- 20.Duncan AE, Alfirevic A, Sessler DI, Popovic ZB, Thomas JD. Perioperative assessment of myocardial deformation. Anesth Analg. 2014;118:525–44. doi: 10.1213/ANE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter E, Marwick TH. Assessment of left ventricular function by echocardiography: The case for routinely adding global longitudinal strain to ejection fraction. JACC Cardiovasc Imaging. 2018;11:260–74. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Luis SA, Chan J, Pellikka PA. Echocardiographic assessment of left ventricular systolic function: An overview of contemporary techniques, including speckle-tracking echocardiography. Mayo Clin Proc. 2019;94:125–38. doi: 10.1016/j.mayocp.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 23.D'Auria F, Consalvo V, Costagliola T, Leone R, Myat A, Itri F, et al. Speckle tracking to assess the improvement of myocardial activity after OPCABG. EC Cardiol. 2018;5:358–64. [Google Scholar]
- 24.Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, et al. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: A multicenter study. J Am Soc Echocardiogr. 2010;23:1019–24. doi: 10.1016/j.echo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 26.Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365–73. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 27.Jha AK, Malik V, Gharde P, Chauhan S, Kiran U, Hote MP. Echocardiographic predictors of immediate postoperative outcomes in patients with severe left ventricular systolic dysfunction undergoing on-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth. 2017;31:184–90. doi: 10.1053/j.jvca.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Merello L, Riesle E, Alburquerque J, Torres H, Aránguiz-Santander E, Pedemonte O, et al. Risk scores do not predict high mortality after coronary artery bypass surgery in the presence of diastolic dysfunction. Ann Thorac Surg. 2008;85:1247–55. doi: 10.1016/j.athoracsur.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 29.Diez C, Silber RE, Wächner M, Stiller M, Hofmann HS. EuroSCORE directed intraaortic balloon pump placement in high-risk patients undergoing cardiac surgery–retrospective analysis of 267 patients. Interact Cardiovasc Thorac Surg. 2008;7:389–95. doi: 10.1510/icvts.2007.165795. [DOI] [PubMed] [Google Scholar]
- 30.Vijayaraghavan G, Sivasankaran S. Global longitudinal strain: A practical step-by-step approach to longitudinal strain imaging. J Indian Acad Echocardiogr Cardiovasc Imaging. 2020;4:22–8. [Google Scholar]
- 31.Gozdzik A, Letachowicz K, Grajek BB, Plonek T, Obremska M, Jasinski M, et al. Application of strain and other echocardiographic parameters in the evaluation of early and long-term clinical outcomes after cardiac surgery revascularization. BMC Cardiovasc Disord. 2019;19:189. doi: 10.1186/s12872-019-1162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]