ABSTRACT
Background:
Congenital heart surgeries are associated with post-bypass renal and cardiac dysfunctions. The use of low-dose vasopressin has been found to be beneficial in adult cardiac surgeries.
Objective:
To assess the hemodynamic and renal effects of patients undergoing on-pump pediatric cardiac surgery under general anesthesia (GA) with low-dose vasopressin infusion.
Design:
Prospective randomized controlled study.
Setting:
Operation room and ICU, tertiary care teaching hospital.
Patients:
Fifty-five pediatric cardiac patients undergoing repair for congenital heart diseases (CHD).
Interventions:
Low-dose vasopressin infusion in the study group and placebo in the control group.
Measurements and Main Results:
Renal near-infrared spectroscopy (NIRS), serum NGAL, and inflammatory mediators—IL6 and IL8 along with other renal and hemodynamic parameters in the perioperative period were recorded. Diastolic blood pressure (DBP) and cardiac index were significantly higher in the vasopressin group. Inflammatory markers were significantly high in the immediate postoperative period in all patients which later stabilized in the next 48 h but showed similar trends in both groups. Low-dose vasopressin infusion did not improve either renal perfusion or function. The duration of mechanical ventilation and length of hospital stay, the incidence of AKI development, and transfusion requirements were marginally lower in the vasopressin group, although not significant.
Conclusion:
Low-dose vasopressin infusion improved hemodynamics and showed a decreased incidence of complications. However, it failed to show any benefit of renal function and overall outcome in pediatric cardiac surgery.
Keywords: Acute kidney injury, Congenital heart surgery, Interleukins, Renal NIRS, Serum NGAL, Vasopressin
INTRODUCTION
Repair of congenital heart diseases (CHD) using cardiopulmonary bypass (CPB) are prone to complications by persistent hypotension due to low systemic vascular resistance, in 5–22% of patients.[1] Different causes have been associated with this situation, like hypothermia, duration of CPB, total cardioplegic volume infused, reduced left ventricular function, hemodilution, systemic inflammatory response syndrome, or inappropriate low arginine-vasopressin secretion. Exposing the kidney to such brief periods of ischemia and reperfusion may induce a subsequent more prolonged renal insult, leading to acute kidney injury.[2] An intraoperative low dose of vasopressin may prevent postoperative vasodilatory shock. This low dose of vasopressin causes an increase in mean arterial pressure (MAP), as well as systemic vascular resistance mainly due to systemic vasoconstrictive action.[3,4] This would benefit renal perfusion in the perioperative period.
However, there are no studies indicating that low-dose vasopressin administration in pediatric cardiac surgery improves renal outcomes. Serum neutrophil gelatinase-associated lipocalin (NGAL) is a specific marker of early renal dysfunction and can be used in the pediatric population to assess renal damage.[5] Renal near-infrared spectroscopy (NIRS) can be used preoperatively for assessing renal oxygenation status in pediatric cardiac surgery.[6] Inflammatory markers like Interleukin 6 and 8 (IL6 and IL8) have been shown to be markers of renal injury in the pediatric population.[7] The primary aim of our study is to observe the effect of low-dose vasopressin infusion on renal perfusion and function (as measured by creatinine clearance, serum NGAL, IL6, IL8, and renal somatic NIRS) in pediatric cardiac surgery. The secondary objective is to see the effect of vasopressin administration on outcomes in pediatric cardiac surgery.
MATERIALS AND METHODS
Pediatric patients (<18 yrs) undergoing on-pump cardiac surgery at a tertiary care center, for congenital heart diseases, were included in the study. Ethical clearance by “Institutional Ethics Committee, Army Hospital (R and R), Delhi Cantt” IEC No 106/2020 dated September 01, 2020, for the study titled “Low-Dose Vasopressin And Renal Perfusion In Pediatric Cardiac Surgery” was obtained. All the procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki Declaration of 1975. Trial registration was done in the National Trial Registry (CTRI No: CTRI/202010/028316 dt October 09, 2020). Written informed consent was obtained from the mother of all minors participating in the trial. Patients with preoperative renal dysfunction and inotropes were excluded from the study. Patients were randomized into two groups—vasopressin and no-vasopressin groups using computer-generated random numbers.
All the patients were premedicated with intranasal ketamine 7 mg/kg and midazolam 0.5 mg/kg. General anesthesia (GA) was induced with ketamine (1–2 mg/kg), fentanyl (2 μg/kg), and rocuronium (1 mg/kg). After tracheal intubation, the lungs were ventilated with 100% O2 using a semi-closed circle system, with a tidal volume of 6–8 ml/kg, and the ventilatory rate was adjusted to maintain end-tidal CO2 (EtCO2) between 35 and 40 mmHg. Anesthesia was maintained by sevoflurane 1–2%. The epidural catheter was inserted at the T5-6 interspace after induction of anesthesia. An initial bolus of 1 ml/kg bupivacaine 0.25% with 50 mcg/kg morphine was injected and followed by a continuous infusion of 0.125% bupivacaine at a rate of 0.2 ml/kg/hr. In the vasopressin group, vasopressin @ 0.003 U/kg/min was started at the start of the surgery and continued postoperatively for 24–72 h. In the no-vasopressin group, 5% dextrose was used instead of vasopressin. The observer was blinded to the drug being administered as the label was hidden.
We measured renal oximetry with the INVOS monitor (Covidien/Somanetics, Dublin, Ireland) intraoperatively and 48 h postoperatively. The NIRS sensor was placed after intubation and before the incision, on the back left or right of the spine, at the level of T10-L2 for renal oximetry (rSO2). The rSO2 was recorded continuously during CPB and until the end of the surgical procedure. After admission to the ICU, the renal oximetry was restarted and recorded continuously for at least 48 h in each patient. Heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), MAP, oxygen saturation, and cerebral NIRS were monitored throughout the surgery. All surgical procedures were performed by experienced surgeons using standardized techniques. CPB and anesthesia management were performed according to our standard operating procedures. Perioperative goal-oriented hemodynamic support was established according to our institutional standards. Conventional and modified ultrafiltration both were used during CPB to achieve zero balance in all patients. Hematocrit was maintained at >30 before coming off CPB. Extubation protocol and postoperative sedation protocol were decided by the pediatric cardiac surgical team on a case-to-case basis. Continuous epidural infusion of 0.125% bupivacaine at the rate of 0.1 ml/kg/h was used for postoperative analgesia in the ICU, and fentanyl bolus of 1 mcg/kg was used as rescue analgesia. Age-appropriate balanced electrolyte solution was used for maintenance fluid at 2–4 ml/kg/h. The first line of ionotropes used was dopamine 5–10 mcg/kg/min and milrinone 0.3–0.7 mcg/kg/min along with low-dose vasopressin infusion (5% dextrose in the no-vasopressin group instead of vasopressin), and adrenalin 0.05–0.1 mcg/kg/min was added if post-bypass echocardiography revealed impaired ventricular function. The doses were titrated to maintain the perioperative hemodynamic goals according to institutional standards, that is, heart rate 80–100 beats/min, mean arterial pressure 65–85 mmHg, central venous pressure 8–12 mmHg at positive end-expiratory pressure 5 cm H2O, urine output >1 ml/kg/h, and mixed venous oxygen saturation >65. Diuretics (inj frusemide at 0.05-0.1 mg/kg/h) were used if urine output decreased to less than one ml/kg/h.
Apart from the demographic profile of the patient, collected operative data include aortic cross-clamping (AXC) time, cardiopulmonary bypass (CPB) time, and NIRS values. ICU measurements included serial 2D echocardiography measurement of cardiac index (CI), the incidence of low cardiac output syndrome (LCOS) (defined as maximum Vasopressor Ionotropic Score (VIS) of ≥15 for >30 mins), duration of mechanical ventilation (MV), length of hospital stay (LOS), AKI[8] requiring renal replacement therapy (RRT) given if any, transfusions, amount of chest tube drainage, and any other adverse events. Chest tube drainage for criteria classifying as bleeder included more than 10% blood volume within 6 h, or more than 8 ml/kg/in 2 h in first 12 h post-surgery, or more than 84 ml/kg in 24 h.
Laboratory investigations: Blood samples were collected preoperatively (T0), 4 h (T1), one (T2), and two (T3) days after surgery. Separated serum was assayed for enzyme-linked immunoassay (ELISA) estimation of serum neutrophil gelatinase-associated lipocalin (NGAL) and inflammatory markers (IL6 and IL8). NGAL samples were analyzed by a double sandwich ELISA technique using commercially available kits. The personnel measuring the biomarkers was blinded to clinical outcomes.
Statistical analysis
The distribution of the continuous data was tested with the Kolmogorov–Smirnov one-sample test. Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD). Dichotomous data were expressed as numbers and percentages. Obtained laboratory data were analyzed using the repeated measure ANOVA test with Bonferroni correction. A Chi-square test was used to compare categorical variables and a t-test for continuous variables. A median test was performed for patients in different STAT categories for MV duration and LOS. A P value <0.05 was considered statistically significant.
Sample size calculation: Wong et al. demonstrated post-cardiac surgery renal NIRS increase compared to pre-CPB value and reached a median value of 87.3 (IQR: 77.2, 89.5).[9] A sample size of 46 patients (23 in each arm) was sufficient to detect an increase of 10% in renal NIRS level due to increased renal perfusion with the use of vasopressin at a 5% significance level and 90% power. Statistical analysis was performed using SPSS software (IBM SPSS Statistics version 24, Chicago IL, USA).
OBSERVATIONS AND RESULTS
The data of all the patients admitted for congenital heart surgeries were collected from January 2021 to January 2022. A total of 88 patients underwent congenital heart surgery under CPB in the period [Figure 1]. The cohort has been grouped as per STAT scoring (The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery). Preoperative diagnoses and surgical procedures for study group patients are presented in Table 1. Maximum patients in STAT category I are acyanotic (93%) while in STAT categories III and IV are cyanotic (86% each).
Figure 1.
Consort diagram ECMO = Extra-corporeal membrane oxygenation, CPB = Cardiopulmonary bypass
Table 1.
Distribution of patients as per STAT mortality category
| STAT mortality category | Diagnosis and procedures | No | Vasopressin | No vasopressin | Chi-square value | P |
|---|---|---|---|---|---|---|
| 1 | ASD, VSD, PAPVC, partial/transitional AVCD, non-TAP TOF | 14 | 6 | 8 | 1.77 | 0.62 |
| 2 | DORV, TAP TOF, BDG, FONTAN, PA/TV/PV/AV plasty | 17 | 9 | 8 | ||
| 3 | TOF+pulmonary atresia, complete AVCD, TGA+IVS | 6 | 3 | 3 | ||
| 4 | EBSTEIN, TAPVC, DORV (IVTR), TGA+VSD, TOF+absent PV | 18 | 10 | 8 | ||
| Total | 55 | 28 | 27 |
AVCD=Atrioventricular canal defect, DORV=Double outlet right ventricle (IVTR-Intra-ventricular tunnel repair), VSD=Ventricular septal defect, ASD=Atrial septal defect, TOF=Tetralogy of Fallot (TAP-Trans-annular patch), TGA=Transposition of great vessels (IVS Intact ventricular septum), PAPVC/TAPVC=Partial/total anomalous pulmonary venous connection, TV=Tricuspid valve, AV=Aortic valve, PV=Pulmonary valve, PA=Pulmonary artery, BDG=Bidirectional Glenn
The preoperative and intraoperative characteristics of the patients are presented in Table 2. The median age of the patients was about 11 months (5–14.5) with a predominance of the male gender. Cyanotic patients coming for corrective surgeries were in majority and were younger infants. All the patients underwent post-procedure epicardial echo/TEE. Six patients required revision and underwent a second bypass. Preoperative laboratory investigations, hemodynamic, and renal parameters were comparable in both groups [Table 2].
Table 2.
Patient demographic characteristics, laboratory, and intraoperative variables
| Parameters | Mean±SD/n (%) | Vasopressin n=28 | No vasopressin n=27 | t/Chi-square# value | P |
|---|---|---|---|---|---|
| Age (months) | 12.3±10.2 | 12±9.7 | 12.5±11 | −0.17 | 0.9 |
| Sex (Male) | 28 (51%) | 15 (48.1%) | 13 (53.6%) | 0.2# | 0.8 |
| Weight (kg)* | 8.9±7.3 | 9.8±9.4 | 8.0±3.5 | 0.8 | 0.4 |
| BSA (sq m)* | 0.42±0.23 | 0.45±0.3 | 0.4±0.1 | 0.7 | 0.5 |
| STAT mortality category | 55 | 28 | 27 | 1.77 | 0.62 |
| ASD, VSD, PAPVC, partial/transitional AVCD, non-TAP TOF | 14 | 6 | 8 | ||
| DORV, TAP TOF, PA/TV/PV plasty | 17 | 9 | 8 | ||
| TOF+pulmonary atresia, complete AVCD, TGA+IVS | 6 | 3 | 3 | ||
| EBSTEIN, TAPVC, DORV (IVTR), TGA+VSD, TOF+absent PV | 18 | 10 | 8 | ||
| Cyanosis | 41 (74.5%) | 22 (78.6%) | 19 (70.4%) | 1.1# | 0.3 |
| Preop CCF | 23 (41.8%) | 12 (42.9%) | 11 (40.7%) | 0.4# | 0.6 |
| Preop severe PAH | 21 (38.2%) | 11 (39.3%) | 10 (37%) | 0.2# | 0.7 |
| CPB (min) | 118.8±61.3 | 131.9±61.7 | 103.2±58.5 | 1.6 | 0.1 |
| AXC (min) | 82.5±51.5 | 92.8±50.4 | 70.7±51.4 | 1.5 | 0.15 |
| Nadir temperature (degree Celsius) | 31.5±2.2 | 31.8±1.7 | 32.2±2.5 | −1.1 | 0.2 |
| Hemoglobin (mg/dl) | 12.8±2.7 | 13.6±2.7 | 14.2±2.6 | −0.3 | 0.7 |
| Total leucocyte count (/cu mm) | 9964.3±3586 | 9502.3±2752 | 10486.6±4348 | −0.9 | 0.4 |
| Total vasopressin dose (IU/kg/min* duration in h) | 65.5±37.3 | - | - | - | - |
| Duration of vasopressin therapy (h) | 26.2±15 | - | - | - | - |
| Cardiovascular Parameters | |||||
| HR (bpm) | 123.1+19.4 | 122+21.8 | 124.2+16.8 | 0.4 | 0.7 |
| SBP (mmHg) | 92.9+11.2 | 91.5+9.2 | 94.3+13 | 0.8 | 0.4 |
| DBP (mmHg) | 53+13.3 | 53.8+9.5 | 52+16.7 | 0.5 | 0.6 |
| MAP (mmHg) | 66.1+10.8 | 66.3+8.5 | 66+13.2 | 0.09 | 0.9 |
| Cardiac index (l/min/sq m) | 2.67+0.8 | 2.58+0.66 | 2.76+0.99 | 0.7 | 0.5 |
| Serum TROP I (mcg/L) | 0.31+0.2 | 0.33+0.3 | 0.29+0.3 | 0.07 | 0.8 |
| Renal Parameters | |||||
| Blood urea (mg/dl) | 11.4±4.8 | 10.7±5.1 | 12.3±4.4 | −1.2 | 0.2 |
| Serum creatinine (mg/dl) | 0.3±0.15 | 0.28±0.1 | 0.32±0.2 | 1.2 | 0.2 |
| Creatinine clearance (ml/min/1.73 sq m) | 144.6. ± 56.7 | 147.1±51.2 | 142.4±62.1 | −0.3 | 0.8 |
| Serum NGAL (ng/ml) | 2.2±1 | 2.3±1.1 | 2.2±0.8 | 0.3 | 0.7 |
| Renal NIRS (%) | 59.6±19.5 | 61.4±21.3 | 57.5±17.6 | 0.6 | 0.5 |
| Baseline Inflammatory Markers | |||||
| Interleukin-6 (pg/ml) | 3.38±2.14 | 3.28±2 | 3.5±2.3 | −0.4 | 0.7 |
| Interleukin-8 (pg/ml) | 105.4±109 | 98.5±104 | 113.2±116 | −0.46 | 0.6 |
BSA=Body surface area, STAT=The Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery, AVCD=Atrioventricular canal defect, DORV=Double outlet right ventricle (IVTR-Intra-ventricular tunnel repair), VSD=Ventricular septal defect, ASD=Atrial septal defect, TOF=Tetralogy of Fallot (TAP-Trans-annular patch), TGA=Transposition of great vessels (IVS Intact ventricular septum), PAPVC/TAPVC=Partial/total anomalous pulmonary venous connection, TV=Tricuspid valve, AV=Aortic valve, PV=Pulmonary valve, PA=Pulmonary artery, CCF=Congestive cardiac failure, PAH=Pulmonary arterial hypertension, CPB=Cardiopulmonary bypass, AXC=Aortic cross-clamp Time, NGAL=Neutrophil gelatinase-associated lipocalin, NIRS=Near-infrared spectroscopy. P <0.05* is considered significant
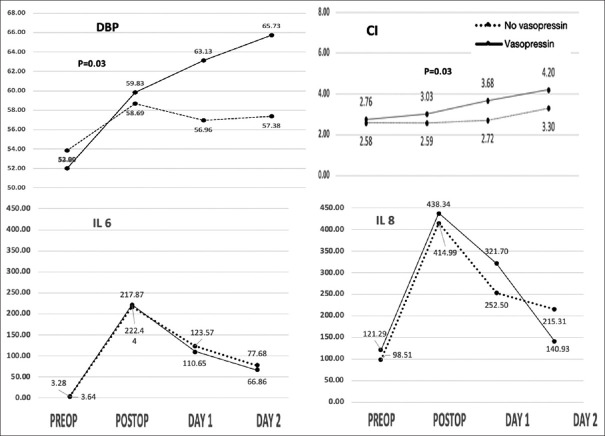
Trends of hemodynamic, cardiac, renal parameters, and various inflammatory markers were tested with an ANOVA test. DBP and CI were found to be significantly affected by the use of vasopressin [Figure 2]. Diastolic blood pressure was lower, while cardiac index improved in the vasopressin group as compared to the no-vasopressin group in the perioperative period. Renal (BUN, serum creatinine, creatinine clearance, serum NGAL, renal NIRS), rest of the hemodynamics, and cardiac parameters (HR, SBP, MAP, and serum cardiac troponin I (TROP-I)) were similar in both the groups in the perioperative period, and the use of vasopressin showed no significant effects on these parameters among the two groups (See supplementary file). Although renal NIRS remained high in the vasopressin group and so was creatinine clearance on day 1, it was not significant [Figure 3]. However, AKI incidence was more in the no-vasopressin group, although the sample size is small to comment on it. The patients who developed AKI did receive higher mean packed red blood cell transfusions; however, the incidence was too small to conclude any association (126.2 Vs 72.6 ml/kg). Inflammatory markers IL6 and IL8 were significantly high in the immediate postoperative period which later stabilized in the next 48 h in all patients. Inflammatory markers were comparable in both the groups during the postoperative period [Figure 2].
Figure 2.
Line chart showing trends of cardiac parameters and inflammatory markers. DBP = Diastolic blood pressure, CI = Cardiac index, IL = Interleukins
Figure 3.

Line chart showing trends of renal parameters Cr Cl = Creatinine clearance, NIRS = Near-infrared spectroscopy, AKI = Acute kidney injury
Although the mean MV duration was 22 h, we found vasopressin group patients being ventilated less although it was not statistically significant (P = 0.5). The mean LOS which included ICU stay was 11.5 days which was comparable in both groups (P = 0.9) [Table 3]. Two patients died in the no-vasopressin group. Both of them belonged to STAT category 4 and developed LCOS with high TROP-I and lactate levels in the immediate postoperative period. They had prolonged mean MV duration (124.5 h), and one of them developed culture-proven sepsis and AKI requiring RRT before succumbing to death. In terms of complication rates, both groups were comparable [Table 3]. Patients who developed AKI requiring RRT did have higher serum NGAL levels and low renal NIRS values at various time points [Figure 3], but the incidence is too low to compare any difference between the two groups. Also, patients who developed AKI had longer mean MV duration (90.5 h) and LOS (17.3 days). Blood and blood products transfusion requirement was more in the vasopressin group but none reached statistical significance. Chest drains were comparable in both groups (P > 0.05). Platelet count also showed no significant decrease with the use of vasopressin when compared between the two groups (See supplementary file). None of the patients were reexplored.
Table 3.
Outcome parameters
| Parameters | n=55 Mean±SD | Vasopressin n=28 | No vasopressin n=27 | t/z value | P |
|---|---|---|---|---|---|
| MV Duration (h) | 22.2±29 | 19.1+31.6 | 25±26.8 | 0.7 | 0.5 |
| Hospital Stay (days) | 11.5±9.5 | 11.3±9.3 | 11.5±10.6 | 0.1 | 0.9 |
| Complications | n | ||||
| Mortality | 2 | 0 | 2 | 0.8 | 0.5 |
| Acute Kidney injury requiring RRT | 4 | 1 | 3 | 0.9 | 0.4 |
| Sepsis | 4 | 1 | 3 | 0.7 | 0.5 |
| Pneumonia | 3 | 1 | 2 | 0.5 | 0.6 |
| Arrythmia/block | 4 | 2 | 2 | 0.07 | 0.8 |
| Low cardiac output State | 8 | 2 | 6 | 1.3 | 0.2 |
| Neurological (focal seizures) | 1 | 0 | 1 | 0.9 | 0.3 |
| Reintubation | 2 | 1 | 1 | 0.09 | 0.9 |
| Surgical site infection | 1 | 1 | 0 | -0.9 | 0.35 |
| Transfusions (ml/kg) | |||||
| PRBC | 72.7±44.1 | 70.8±38 | 73.96±49.1 | 1 | 0.3 |
| FFP | 9.2±12.3 | 8.1±10.6 | 10±13.2 | 1.1 | 0.3 |
| PC | 6.5±5.1 | 5.8±5.1 | 6.9±4.7 | 1.3 | 0.2 |
| CRYO | 12.5±16.8 | 12.1±16.2 | 13.2±17.8 | 0.9 | 0.3 |
| Chest Drains (ml/kg) | |||||
| 4 h | 7.9±6.2 | 7.4±5.9 | 8.5±6.5 | 0.6 | 0.5 |
| 12 h | 17.4±7.5 | 18.3±8.2 | 16.4±6.7 | 0.8 | 0.4 |
| Day 1 | 7.9±4.4 | 8.4±12.6 | 7.5±9.3 | 0.3 | 0.8 |
| Day 2 | 2±4.4 | 2.9±5.4 | 1±2.7 | 1.5 | 0.1 |
MV=Mechanical ventilation, PRBC=Packed red blood cells, FFP=Fresh frozen plasma, PC=Platelet concentrate, CRYO=Cryoprecipitate. Results of the one-way ANOVA test of different parameters show the overall effect of vasopressin use on trends in the perioperative period
DISCUSSION
During the repair of a congenital heart defect, the child may be exposed to transient hypotension and renal hypoxia. Trials have shown that vasopressin improves the glomerular filtration rate in adult cardiac surgery but does not improve renal perfusion.[10] Indeed, several studies in the past have shown that the perioperative administration of vasopressin restores the vascular tone in patients following cardiopulmonary bypass, especially in cases that are refractory to norepinephrine.[11] The use of low-dose vasopressin has been shown to reduce the requirement for catecholamines and improve MAP and renal perfusion.[12]
Near-infrared spectroscopy (NIRS) is a noninvasive tool that utilizes oxyhemoglobin and deoxyhemoglobin to provide a continuous estimate of regional tissue oxygen saturation (rSO2).[13] Reduction in NIRS over the organ in which it is placed may be considered a surrogate marker for hypoperfusion and tissue ischemia. In our study, we have used a decline in the overall trend rather than the absolute value for renal NIRS to consider further clinical evaluation in cyanotic patients. Studies have demonstrated a significant correlation between intraoperative renal NIRS score and the postoperative occurrence of AKI in infants after cardiac surgery.[14,15] Cerebral NIRS was also used, and it collaborated with the somatic NIRS in our study. However, guidelines for what is considered to be a clinically relevant decline in cerebral or renal NIRS have not yet been established, especially in children with congenital heart disease.[16,17] Although renal NIRS remained high in the vasopressin group and so was creatinine clearance on day 1 in our study, it was not significant.
Also, serum NGAL has emerged as one of the most promising biomarkers for the early diagnosis of acute kidney injury. In children, a significant correlation has been found between acute renal injury and serum NGAL concentration 2 h after cardiopulmonary bypass[3]. Koch et al. in their study found that NGAL was weakly correlated with the severity of the pRIFLE score.[18] In our study, patients who developed AKI requiring RRT did have higher serum NGAL levels, but it failed to reach any statistical significance.
Inflammatory cytokines are promising biomarkers of AKI progression because systemic and intrarenal inflammation plays an integral role in the development and progression of AKI.[19,20,21] Furthermore, systemic inflammation is heightened after cardiac surgery, which raises blood cytokine levels and puts patients at increased risk for AKI.[22] Several studies have previously reported that plasma IL8 is strongly associated with the development of AKI and other adverse outcomes after pediatric cardiac surgery.[23,24,25] We did not find any difference in the inflammatory markers between the two groups. The incidence of AKI is very less in our study to comment on its correlation with these markers.
The increased urine output represents a remarkable result of infused vasopressin due to the increased mean arterial pressure of the patient and therefore the improvement of the glomerular filtration rate.[26] Several studies confirm that vasopressin receptors in the renal vasculature are in the efferent arterioles, in contrast to the catecholamine receptors, which are in the afferent arterioles. Therefore, although the vasoconstrictive action of catecholamines leads to a decrease in the filtration fraction, the action of vasopressin leads to an increase in the filtration fraction and, hence, to an increase in urine output.[3,26] Morales D, et al. proposed the long-term administration (up to 12 h) of vasopressin in patients with post-cardiotomy vasodilatory shock associated with renal insufficiency (instead of 2 to 3 h in patients with normal renal function), to maintain an improved filtration rate and urine output.[27] In our study, although the urine output was more in the vasopressin group, it failed to reach statistical significance (P = 0.14). A trial with more pediatric patients is required to further prove that low-dose vasopressin infusion leads to increase urine output in pediatric cardiac surgery patients.
The positive findings of the present study were that low-dose vasopressin infusion resulted in a significant increase in CI and DBP. The rest of the hemodynamics parameters (HR, SBP, MAP, and TROP-I) were not affected. DBP and CI were found to be significantly affected by the use of vasopressin in our study. Bragadottir G et al.[10] in their study found that systemic vascular resistance, central venous pressure, and pulmonary capillary wedge pressure increased in the vasopressin group, which is in line with our study which found that DBP increased significantly in the vasopressin group. However, their finding which showed that HR, CO, and Mean pulmpnary artery pressure (MPAP) decreased in the vasopressin group is against the finding of our study which showed a significant increase in CI in the vasopressin group. This difference could be due to multiple reasons, Bragadottir G et al. used low to moderate dose vasopressin for a short period, whereas we used low-dose vasopressin infusion for 24–48 h; the target population in their study was adult patients for CABG/valve surgery, whereas we did a study on pediatric patients with CHD.
Acute kidney injury (AKI) is a frequent complication after cardiac surgery with cardiopulmonary bypass (CPB) in infants. In a recent multicenter study including 311 pediatric patients after on-pump cardiac surgery, 42% developed AKI postoperatively. Among them, patients younger than 2 years showed an increased risk for AKI.[2] We found the incidence of AKI requiring RRT to be 8.16% in our study. Also, the incidence of AKI requiring RRT was higher in the no-vasopressin group. Three out of four patients who developed AKI requiring RRT were in the no-vasopressin group, though statistically not significant. Patients who developed AKI had a higher requirement of ionotropes and vasopressors after low cardiac output syndrome. They had poor hemodynamic and cardiac function, lower NIRS values, and high serum NGAL levels. However, vasopressin infusion failed to show any benefit on renal outcome in this cohort. AKI after CPB surgery is associated with longer time on mechanical ventilation and intensive care unit (ICU) stay[28,29,30,31] and possibly increased mortality.[28,29] In our study, patients with AKI had a significantly longer duration of mechanical ventilation and longer LOS. Although the mean mechanical ventilation duration was 29.4 h, we found patients in the no-vasopressin group being ventilated more (P = 0.2). The mean length of hospital stay which included ICU stay was 12 days which was also more in the vasopressin group, although not significant (P = 0.7).
In terms of complications, the vasoconstrictor action of vasopressin infusion can lead to adverse effects like coronary ischemia, increased myocardial afterload, tachyarrhythmias, splanchnic hypoperfusion, skin gangrene, and poor wound healing.[32] The adverse effects of vasopressin are dose-related with limited or no side effects at doses up to 0.04 units/min. Moreover, complications are evident with the concomitant use of norepinephrine.[26,33,34] In our study, the overall rate of complications was comparable in both groups and none were statistically significant. However, it was noteworthy that three out of four patients who developed AKI requiring RRT and six out of eight patients who developed LCOS belonged to the no-vasopressin group. This only shows an inclination toward improved hemodynamics with the use of low-dose vasopressin but fails to reach any statistical significance. In our study, the overall mortality benefit was better in the vasopressin group, while it was 8.69% in the no-vasopressin group although the number was too less to reach any significant conclusion. Both the patients who died belonged to the placebo group and did not correlate with the preoperative existence of congestive cardiac failure (CCF) or pulmonary arterial hypertension (PAH). Both of them belonged to STAT category 4. Both the patients had LCOS with high TROP-I and lactate levels in the immediate postoperative period. They had prolonged mean MV duration (124.5 h) and one of them developed culture-proven sepsis and AKI requiring RRT before succumbing to death.
The limitations of our study are that it is a single-center study with a small sample size. Also, we used surrogate markers instead of direct methods for the assessment of renal perfusion. However, this was beneficial for the patients as the procedures were noninvasive, reducing the chances of infections and other related complications. We also did not measure serum lactate levels in our study to study the perfusion effects of the vasopressin in relation to the vasoconstrictive effect on the one hand and the increase in arterial pressures on the other hand. However, we did measure the end organ perfusion with various renal and cardiac parameters.
In summary, low-dose vasopressin infusion did improve hemodynamics. The rate of complications was reduced by the administration of vasopressin infusion, although statistically not significant. Also, renal NIRS values were marginally higher in the patients who were administered vasopressin infusion. However, low-dose vasopressin infusion failed to show any significant benefit to renal function and overall outcome in pediatric cardiac surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY FILE
Results of the one-way ANOVA test of different parameters show the overall effect of vasopressin use on trends in the perioperative period.
| Parameters | F (df1, df2) | Mean square | P |
|---|---|---|---|
| Platelet count (/cumm) (See Graph Below) | 1.94 (2.02, 107) | 224000 | 0.15 |
| Heart rate (Bpm) | 0.32 (2.5,119) | 98 | 0.78 |
| Systolic blood pressure (mmHg) | 1.17 (2.7,121.2) | 142.6 | 0.32 |
| Diastolic blood pressure (mmHg) | 3.5 (2.5, 114.5) | 360.2 | 0.03* |
| Mean arterial pressure (mmHg) | 0.8 (2.3, 107.2) | 125.3 | 0.47 |
| Central venous pressure (mmHg) | 2.9 (2, 94.1) | 16.7 | 0.06 |
| Cardiac index (L/min/m2) | 3.8 (1.8, 85.8) | 2.85 | 0.03* |
| Serum cardiac trop-I (mcg/L) | 0.93 (1.8, 84.5) | 7.4 | 0.39 |
| Vasopressor-ionotropic score | 2.2 (2.8, 123.8) | 20.6 | 0.08 |
| Serum BUN (mg/dl) | 3 (2, 95.3) | 71.5 | 0.051 |
| Serum creatinine (mg/dl) | 1.1 (1, 47.5) | 9.6 | 0.29 |
| Creatinine clearance (ml/min/1.73 sq m) | 1.4 (2.6, 121.6) | 4204.1 | 0.24 |
| Serum NGAL (ng/ml) | 0.3 (2.3, 108.5) | 6.1 | 0.77 |
| Renal NIRS (%) | 0.4 (3.2, 53.9) | 36.6 | 0.78 |
| Serum IL6 (pg/ml) | 1.17 (2.4, 105.4) | 20433 | 0.32 |
| Serum IL8 (pg/ml) | 1.1 (2.6, 124.2) | 42651 | 0.34 |
Figure.

Platelet count showing no significant decrease with the use of vasopressin when compared between the two groups, p=0.15
REFERENCES
- 1.Kristof AS, Magder S. Low systemic vascular resistance state in patients undergoing cardiopulmonary bypass. Crit Care Med. 1999;27:1121–7. doi: 10.1097/00003246-199906000-00033. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit Care Med. 2011;39:1493–9. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulos G, Sintou E, Siminelakis S, Koletsis E, Baikoussis NG, Apostolakis E. Perioperative infusion of low- dose of vasopressin for prevention and management of vasodilatory vasoplegic syndrome in patients undergoing coronary artery bypass grafting-A double-blind randomized study. J Cardiothorac Surg. 2010;5:17. doi: 10.1186/1749-8090-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales DLS, Garrido MJ, Madigan JD, Helman DN, Faber J, Williams MR, et al. A double-blind randomized trial: Prophylactic vasopressin reduces hypotension after cardiopulmonary bypass. Ann Thorac Surg. 2003;75:926–30. doi: 10.1016/s0003-4975(02)04408-9. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaleski KL, Kussman BD. Near-infrared spectroscopy in pediatric congenital heart disease. J Cardiothorac Vasc Anesth. 2020;34:489–500. doi: 10.1053/j.jvca.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury working group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 9.Wong JJ-M, Chen CK, Moorakonda RB, Wijeweera O, Tan TYS, Nakao M, et al. Changes in near-infrared spectroscopy after congenital cyanotic heart surgery. Front Pediatr. 2018;6:97. doi: 10.3389/fped.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragadottir G, Redfors B, Nygren A, Sellgren J, Ricksten SE. Low-dose vasopressin increases glomerular filtration rate, but impairs renal oxygenation in post-cardiac surgery patients. Acta Anesthesiol Scand. 2009;53:1052–9. doi: 10.1111/j.1399-6576.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 11.Masetti P, Murphy SF, Kouchoukos NT. Vasopressin therapy for vasoplegic syndrome following cardiopulmonary bypass. J Card Surg. 2002;17:485–9. doi: 10.1046/j.1540-8191.2002.01002.x. [DOI] [PubMed] [Google Scholar]
- 12.Choong K, Kissoon N. Vasopressin in pediatric shock and cardiac arrest. Pediatr Crit Care Med. 2008;9:372–9. doi: 10.1097/PCC.0b013e318172d7c8. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–87. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 14.Ruf B, Bonelli V, Balling G, Hörer J, Nagdyman N, Braun SL, et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: A case control study. Crit Care. 2015;19:27. doi: 10.1186/s13054-015-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183–8. doi: 10.1007/s00246-010-9839-x. [DOI] [PubMed] [Google Scholar]
- 16.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–7. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, 3rd, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 18.Koch AM, Dittrich S, Cesnjevar R, Rüffer A, Breuer C, Glöckler M. Plasma neutrophil gelatinase-associated lipocalin measured in consecutive patients after congenital heart surgery using point of care technology. Interact Cardiovasc Thorac Surg. 2011;13:133–6. doi: 10.1510/icvts.2011.269647. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al. TRIBE-AKI Consortium: Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30:1519–27. doi: 10.1007/s00467-015-3088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabb H, Griffin MD, McKay DB, Swaminathan S, Pickkers P, Rosner MH, et al. Acute dialysis quality initiative consensus XIII work group: Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27:371–9. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: Mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013;24:529–36. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 22.Brix-Christensen V, Petersen TK, Ravn HB, Hjortdal VE, Andersen NT, Tønnesen E. Cardiopulmonary bypass elicits a pro- and anti-inflammatory cytokine response and impaired neutrophil chemotaxis in neonatal pigs. Acta Anaesthesiol Scand. 2001;45:407–13. doi: 10.1034/j.1399-6576.2001.045004407.x. [DOI] [PubMed] [Google Scholar]
- 23.Powell TC, Powell SL, Allen BK, Griffin RL, Warnock DG, Wang HE. Association of inflammatory and endothelial cell activation biomarkers with acute kidney injury after sepsis. Springerplus. 2014;3:207. doi: 10.1186/2193-1801-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kambhampati G, Ejaz NI, Asmar A, Aiyer RK, Arif AA, Pourafshar N, et al. Fluid balance and conventional and novel biomarkers of acute kidney injury in cardiovascular surgery. J Cardiovasc Surg (Torino) 2013;54:639–46. [PubMed] [Google Scholar]
- 25.Oncel MY, Canpolat FE, Arayici S, Dizdar EA, Uras N, Oguz SS. Urinary markers of acute kidney injury in newborns with perinatal asphyxia. Ren Fail. 2016;38:882–8. doi: 10.3109/0886022X.2016.1165070. [DOI] [PubMed] [Google Scholar]
- 26.Holmes CL, Walley KR, Chittock DR, Lehman T, Russel JA. The effects of vasopressin on hemodynamics and renal function in severe septic shock: A case series. Intensive Care Med. 2001;27:1416–21. doi: 10.1007/s001340101014. [DOI] [PubMed] [Google Scholar]
- 27.Morales DL, Gregg D, Helman DN, Williams MR, Naka Y, Landry DW, et al. Arginine vasopressin in the treatment of fifty patients with postcardiotomy vasodilatory shock. Ann Thorac Surg. 2000;69:102–6. doi: 10.1016/s0003-4975(99)01197-2. [DOI] [PubMed] [Google Scholar]
- 28.Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–92. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen K. Acute kidney injury in children undergoing surgery for congenital heart disease. Eur J Pediatr Surg. 2012;22:426–33. doi: 10.1055/s-0032-1322540. [DOI] [PubMed] [Google Scholar]
- 30.Blinder JJ, Goldstein SL, Lee VV, Baycroft A, Fraser CD, Nelson D, et al. Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–74. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Aydin SI, Seiden HS, Blaufox AD, Parnell VA, Choudhury T, Punnoose A, et al. Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg. 2012;94:1589–95. doi: 10.1016/j.athoracsur.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Núñez A, Fernández-Sanmartín M, Martinón-Torres F, González-Alonso N, Martinón-Sánchez JM. Terlipressin for catecholamine-resistant septic shock in children. Intensive Care Med. 2004;30:477–80. doi: 10.1007/s00134-003-2114-3. [DOI] [PubMed] [Google Scholar]
- 33.Cartotto R, McGibney K, Smith T, Abadir A. Vasopressin for the septic burn patient. Burns. 2007;33:441–51. doi: 10.1016/j.burns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Dünser MW, Mayr AJ, Tür A, Pajk W, Barbara F, Knotzer H, et al. Ischemic skin lesions as a complication of continuous vasopressin infusion in catecholamine resistant vasodilatory shock: Incidence and risk factors. Crit Care Med. 2003;31:1394–8. doi: 10.1097/01.CCM.0000059722.94182.79. [DOI] [PubMed] [Google Scholar]