Abstract
A lytic bacteriophage, which was previously isolated from sewage and which attaches to the K1 capsular antigen, has been used to prevent septicemia and a meningitis-like infection in chickens caused by a K1+ bacteremic strain of Escherichia coli. Protection was obtained even when administration of the phage was delayed until signs of disease appeared. The phage was able to multiply in the blood. In newly borne colostrum-deprived calves given the E. coli orally, intramuscular inoculation of phage delayed appearance of the bacterium in the blood and lengthened life span. With some provisos there is considerable potential for this approach to bacterial-disease therapy.
After the discovery of bacteriophages by Twort in 1915 and, independently, by d’Herelle in 1917 (34) a great deal of faith was initially placed in their use for bacterial-disease therapy. However, a poor understanding of mechanisms of bacterial pathogenesis and of the nature of phage-host interactions, including lysogeny and phage DNA restriction, together with the absence of animal models of disease led to a succession of badly designed and executed experiments and field trials. Phages were claimed to be highly effective, for example, against Vibrio cholerae infection, and favorable results have been reported in field trials against this organism (14). However, much of this work was uncontrolled; for example, anti-V. cholerae phage was assessed by pouring undisclosed quantities of phage down the drinking wells of villages where cholera was expected and determining the incidence of infection before and after this treatment (14). In some areas overenthusiasm for phage for cholera control led to the replacement of hygienic measures by phage prophylaxis with disastrous results. Consequently, the World Health Organization came to the conclusion that with the success of tetracycline therapy there did not seem to be any reason to continue investigations into the use of phage (16).
Most of the controlled experimental work that was carried out early on indicated that phages had little influence on the course of infection in the case of Salmonella typhimurium or Salmonella enteritidis in mice (35, 36), staphylococcal infections in a number of animal models (7), or other miscellaneous experimental infections including Yersinia pestis in rats, anthrax in mice, streptococcal infection in rabbits, and salmonellosis in fowl (for a review of this early work, see reference 34). A few studies showed some beneficial effects. Ward (37) showed some protection against Salmonella typhi infection in mice, as did Asheshov et al. (1); however, in the latter case, phage was given simultaneously with the pathogen and in a large dose. Simultaneous administration of bacterium and phage has also continued experimentally to the present day (5, 13, 32). However, Dubos and colleagues were able to prevent death in mice following intracerebral inoculation with Shigella dysenteriae by intraperitoneal inoculation with an undefined phage preparation (6). This suggested that phages might be able to cross the blood-brain barrier. Lowbury and Hood demonstrated a reduction in the proportion of phage-sensitive strains of Staphylococcus aureus on the wounds of burn patients after phage treatment (12). Similarly, and more recently, phages have been usefully shown, under experimental conditions, to be able to control growth of Pseudomonas aeruginosa on pig skin, which is sometimes used for burn treatment (33).
After having been forgotten for many years the idea of phage therapy and prophylaxis was reassessed more recently by Smith and coworkers after the realization that colicin V could be used to treat Escherichia coli septicemia caused by a colicin V-sensitive strain (23). With a more profound understanding of bacterial virulence factors, which had been lacking earlier in the century, it became possible to use infections with strains of bacteremic and enterotoxigenic E. coli in target animal species which had been used earlier to determine the role of different E. coli virulence determinants in these infections (20, 25). Using lytic phages which attached to the lipopolysaccharide of the enterotoxigenic serotypes pathogenic for calves, Smith and colleagues were able to prevent morbidity and mortality and were even able to use the phages successfully for therapy once the first signs of diarrhea had appeared in the animals (28, 30, 31). An experimental model of septicemia was also set up in mice by using a K1+ E. coli, and anti-K1 phages were shown to be equally useful (27).
However, large numbers of uncontrolled clinical studies continue to be published on the use of phage therapy, for example, in the treatment of suppurative bacterial infections, in which there is information on neither the criteria used for selecting the phages nor the phages themselves (18, 19).
In this study we have used E. coli septicemia in chickens and calves and a meningitis-like infection in chickens to demonstrate the value of bacteriophage administration in a controlled study in target animals and the potential for the treatment of at least some other diseases of animals and humans. Septicemia and other sequelae caused by a limited number of E. coli serotypes, some of them possessing the capsular K1 antigen, is not an uncommon occurrence following infection of poultry with a variety of respiratory viruses (8, 21). Similar serotypes are able to produce acute septicemia in newly born colostrum-deprived calves (15).
MATERIALS AND METHODS
Bacterial strains and bacteriophage.
E. coli H247 (O18:K1:H7) is a ColV-possessing strain which is able to produce experimental bacteremia in chickens, mice, and other laboratory animals (29). This strain was used as a spontaneous mutant resistant to nalidixic acid. Resistance to this antibiotic has been found not to affect virulence and intestinal colonization ability in E. coli (22, 24). Other strains of E. coli which were O1:K1 or O2:K1 were isolated from diseased chickens and have been described previously (21). The bacterial strains were stored in Luria-Bertani (LB) broth containing 30% glycerol at −70°C and on revival were checked for colicin V production (22), smoothness (26), and K1 production (17).
Bacteriophage R was originally isolated from human sewage by standard methods (2, 27) using strain H247 as host. It is specific for K1-possessing strains as indicated by its lysis of H247 but not of a K1-negative derivative of this strain (27). The phage was stored at 4°C in a suspension in LB broth.
Propagation methods.
Broth cultures were made in 10-ml volumes of LB broth (Difco) in a 20-ml bottle incubated at 37°C for 24 h in a shaking incubator (150 rpm). This produced viable counts of between 3 × 109 and 6 × 109 CFU/ml. Phage R was cultured by an adaptation of this method. A 30-ml volume of LB broth in a 100-ml conical flask was inoculated with aliquots of broth culture of H247 to contain approximately 107 CFU/ml and a phage R preparation to contain 106 PFU/ml. A similar volume of broth was inoculated with H247 only. The cultures were incubated at 37°C with shaking until the culture containing the phage had cleared. This occurred after about 2 h. At this time 3 × 107 CFU of H247 was again added, and the culture was reincubated until clearing occurred. This was done twice more until no further clearing occurred. At this point the flask was placed at 4°C overnight for additional lysis to occur. The culture was then centrifuged at 7,000 rpm for 30 min at 4°C, and the supernatant was filtered (0.45-μm pore size).
Bacterium and phage enumeration.
To enumerate E. coli, decimal dilutions of broth cultures and tissue homogenates were plated onto MacConkey agar (CM7; Oxoid, Basingstoke, United Kingdom). For calf experiments, MacConkey agar with and without sodium nalidixate (20 μg/ml) and novobiocin (1 μg/ml) was used. Phage R was counted by plating decimal dilutions of cultures or tissue and fecal samples onto a lawn of H247 Nalr prepared on a plate of LB agar containing sodium nalidixate (20 μg/ml) by using a standard method (27).
Experimental animals.
Specific-pathogen-free Rhode Island Red chickens were used from the Institute flock. These are of moderate sensitivity to E. coli infection, have been used previously (21), and were used at 3 weeks of age. They were housed and reared on a vegetable-protein-based diet under conditions described previously. Newly hatched commercial broiler chicks were obtained from a hatchery and were reared in cardboard containers with brooder lamps as described previously (10).
Aberdeen Angus calves were obtained by hysterotomy and were thus colostrum deprived. They were housed on plastic mats in fiberglass tanks both of which had been autoclaved to prevent environmental infection. Twice a day, calves were given 1 to 1.5 liter of a mixture of equal volumes of sterilized condensed milk and sterile water, both kept at 37°C. Feeding was by mouth with a disinfected bottle except for the first feed, when the calves were infected and fed by stomach tube (see below).
Inoculation procedures and experimental protocol.
Chickens were inoculated intramuscularly (gastrocnemius, left leg) with 50 μl of cultures of H247 diluted in LB broth. Intracranial inoculation was made between the plates of the skull, at a median point immediately anterior to the cerebellum. Volumes of 20 μl of culture dilutions were inoculated. Chickens were inoculated intramuscularly with 50-μl dilutions of phage preparations (gastrocnemius muscle, right leg).
Within 2 h of delivery, calves were inoculated orally with 3 × 1010 CFU of H247 Nalr in 10 ml of undiluted broth culture by using a stomach tube, followed immediately by the first feed. Dilutions of phage were inoculated intramuscularly into the thigh in two 2-ml volumes. Immediately afterwards the inoculation site was swabbed liberally with a solution of the virucidal disinfectant Virkon (Antec, Sudbury, United Kingdom) to try to prevent the calf from ingesting phage. Preliminary experiments showed that Virkon was very effective against phage R (data not presented).
In some experiments at intervals after inoculation, groups of three chickens from different groups were killed for examination. Samples were taken of cardiac blood, spleen, liver, and brain in order. These tissue samples were weighed, diluted in phosphate-buffered saline, and homogenized with a Griffith’s tube prior to further dilution for counting. Calves were sampled frequently by taking feces from the rectum and blood from the jugular vein.
Statistical analyses were carried out with the χ2 test.
RESULTS
In vitro characteristics of bacteriophage R.
Phage R was able to multiply very rapidly on a culture of E. coli H247, reaching counts of 109 PFU/ml within 2 h. A similar rate of multiplication was seen at both 37°C and 44°C. It was thus likely that the phage would be able to multiply rapidly at the temperature of the chicken (41.5°C).
Suspensions of phage R were spotted directly onto lawns of 19 other strains of E. coli, isolated from diseased chickens and identified as possessing the K1 antigen. Areas of lysis were seen in six of them. In the areas of lysis observed with H247 and with the other susceptible strains, colonies were seen to appear as secondary growth. In all cases these were found to be K1 negative.
Effect of bacteriophage administration on mortality in chickens.
After intramuscular inoculation of 3-week-old chickens with 106 CFU of the E. coli strain alone, birds became ill after 12 h or so. They became increasingly lethargic, standing with drooping wings, and eventually collapsed. Birds in this condition were killed for humane reasons 24 to 30 h after inoculation rather than allowing them to die. Chickens inoculated intracranially with 103 CFU of E. coli showed similar signs, but these generally appeared after 8 h, and humane killing occurred between 18 and 22 h after inoculation.
The (log10) geometric mean viable counts of E. coli per gram of spleen, blood, and brain in birds which were killed when severely ill were 7.2, 5.5, and 3.9, respectively, for those inoculated intramuscularly, and 6.1, 5.9, and 8.9, respectively, for those inoculated intracranially.
In the absence of phage the E. coli produced almost 100% mortality in both 3-week-old and newly hatched chickens and by both routes of inoculation (Table 1). When both E. coli and phage were given by the intramuscular route (in different muscles) and equal numbers of both (106 CFU and PFU) were administered, no morbidity or mortality was observed at all. The administration of 104 PFU of phage also produced significant protection, indicating that the phage had multiplied in vivo. Administration of 102 PFU produced some protection, which was not, however, statistically significant.
TABLE 1.
Mortality in chickens infected with E. coli K1+ with and without simultaneous administration of phage
| Chickens | Intramuscular inoculation (106 CFU)
|
Intracranial inoculation (103 CFU)
|
||
|---|---|---|---|---|
| Phage dose (PFU)a | Mortalityb | Phage dose (PFU)c | Mortalityb | |
| 3 weeks old | 106 | 0/5 | 108 | 0/5* |
| 104 | 0/5 | —d | 3/5 | |
| 102 | 2/5* | |||
| — | 5/5 | |||
| Newly hatched | 106 | 0/10 | 108 | 2/10*** |
| — | 10/10 | 106 | 6/10** | |
| — | 10/10 | |||
E. coli was administered into one leg, and phage was administered into the other leg.
Number of chickens dead out of the number infected. Statistically significant differences from the corresponding values for chickens not given phage are indicated as follows: *, P ≅ 0.03; **, P = 0.02; ***, P < 0.01.
Phage was given intramuscularly.
—, no phage was administered.
In the 3-week-old birds good protection against morbidity and mortality following intracranial inoculation with E. coli was obtained when 108 PFU of phage was given but not with smaller doses. However, in the younger birds statistically significant protection was also obtained with 106 PFU of phage. The reason for this difference is unclear.
Kinetics of phage R multiplication in chickens inoculated intracranially with E. coli.
Studying the kinetics of infection and protection when the E. coli was given by the intracranial route showed that in the absence of phage the E. coli multiplied in the brain almost as fast as in a static broth culture (Table 2). The organisms quickly reached the blood and were taken up by the spleen, where counts higher than those in the blood suggested that multiplication was taking place primarily in this organ. However, it is likely that death occurred because of the very high concentration of bacteria in the brain and the associated toxic effects that would result from this. When phage was given to these birds intramuscularly, small numbers rapidly reached the brain. The phage multiplied rapidly on the bacteria and prevented massive multiplication, resulting in a decline in bacterial numbers. Considerable numbers of phage were still present in the spleen at the termination of the experiment.
TABLE 2.
Kinetics of infection in 3-week-old chickens simultaneously administered E. coli K1+ intracranially (103 CFU) and bacteriophage intramuscularly (108 PFU)
| Time (h) postinfection |
E. coli count (log10 CFU/g) in:
|
Phage count (log10 PFU/g) in:
|
||||
|---|---|---|---|---|---|---|
| Spleen | Blood | Brain | Spleen | Blood | Brain | |
| E. coli only | ||||||
| 0 | <2a | <2 | <2 | <2 | <2 | <2 |
| 3 | 4.1 | 2.0 | 4.2 | <2 | <2 | <2 |
| 6 | 4.7 | 3.7 | 6.4 | <2 | <2 | <2 |
| 12 | 5.4 | 5.2 | 8.2 | <2 | <2 | <2 |
| 24 | —b | — | — | — | — | — |
| E. coli and phage | ||||||
| 0 | <2 | <2 | 2.6 | <2 | 3.1 | <2 |
| 3 | 2.8 | 1.6 | 4.2 | 7.2 | 6.5 | 3.8 |
| 6 | 3.9 | 2.1 | 5.5 | 6.6 | 6.4 | 7.3 |
| 12 | 3.0 | <2 | 3.3 | 6.7 | 6.6 | 7.6 |
| 24 | <2 | <2 | 4.3 | 7.1 | 4.3 | 7.2 |
| 48 | <2 | <2 | 1.7 | 6.9 | 1.6 | 4.2 |
| 120 | <2 | <2 | 1.2 | 5.9 | <2 | <2 |
<2 = <100.
—, all birds dead.
Administration of bacteriophage before and after inoculation of E. coli.
Because the slow rate of decline in the numbers of phage R in the spleen in the previous experiment suggested that they may persist for several days in therapeutically useful numbers, 106 PFU of phage was inoculated intramuscularly several days before the E. coli was administered also by this route. In this experiment the dose of E. coli was accidentally low (105 CFU), but this dose nevertheless killed four of nine chickens, and phage given simultaneously as positive control resulted in no dead or diseased animals out of seven. The numbers of dead or diseased chickens out of seven in each group given phage R 1, 2, and 5 days prior to the E. coli were one, one, and three, indicating a degree of protection for at least 2 days before challenge.
In addition, the E. coli was inoculated into two groups of 10 chickens by the intracranial route, and when signs had begun to appear in both groups after 8 h the birds in one group were treated with phage by the intramuscular route. The numbers dead or killed in the control and treated groups were 10 and 3, respectively. All birds which died had done so by 18 to 22 h after inoculation of the E. coli. All the remaining birds in the group treated with phage remained completely healthy.
Effect of bacteriophage administration on the course of E. coli infection in colostrum-deprived calves.
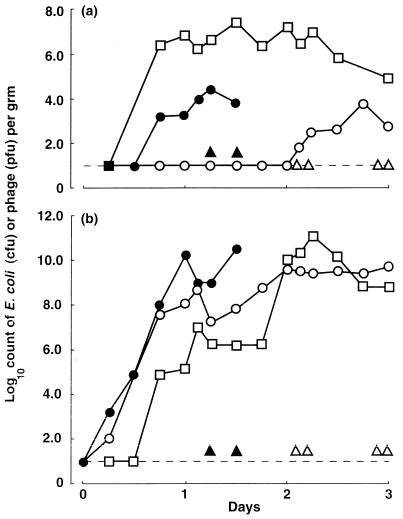
Two colostrum-deprived calves were inoculated orally, by stomach tube, with 1010 CFU of the Nalr mutant of H247. The kinetics of infection monitored by blood and fecal counts are shown in Fig. 1. The fecal counts rose rapidly, and detectable numbers appeared in the blood by 18 h after inoculation. These animals then quickly became lethargic and comatose, necessitating humane killing of both within 36 h of inoculation. It seemed unlikely that the high inoculum resulted in direct endotoxemia, since high doses of avirulent K1- or ColV-negative derivatives of H247 did not result in illness or death (22, 26).
FIG. 1.
E. coli and bacteriophage R in blood (a) and feces (b) of colostrum-deprived calves. •, E. coli in control (untreated) animals; ○, E. coli in animals to which phage was administered intramuscularly 8 h after E. coli infection; □, bacteriophage counts in phage-treated animals. Triangles indicate time at which control (▴) or treated (▵) calves were killed. Dashed lines indicate the limit of sensitivity of the bacterial counting method.
Four additional calves were housed, fed, and inoculated in the same way but were given 1010 PFU of phage R intramuscularly 8 h after the E. coli. One was ill and a second was healthy when they were killed more than 2 days after infection, but the remaining two animals were healthy when killed 3 days after the initial infection. The experiment was not prolonged further to avoid the possibility of infection with other pathogens which might have confused the clinical picture. The counts of E. coli in the intestine rose rapidly as in the control animals, and counts of phage also rose. In the blood phage was detected soon after the intramuscular inoculation, but E. coli also appeared in numbers similar to those in the control calves, although 1 day later. Colonies were picked from these plates and tested for the presence of K1 and for susceptibility to phage R. By 1 day after initial inoculation of the E. coli, phage-resistant K1+ mutants of the Nalr strain appeared in the gut of the animal that became ill and these became the majority strain in the blood 2 days after infection. It seemed possible that the reduction in the phage titer in the blood of these calves was related to the absence of phage-susceptible bacteria in the tissues.
DISCUSSION
We have demonstrated that a bacteriophage which is highly active and rapidly lytic in vitro and which attaches to the K1 capsule of bacteremic strains of E. coli was very effective in preventing and treating septicemia and cerebritis or meningitis in chickens and also, in a limited experiment, in delaying onset of signs of and disease in E. coli bacteremia in colostrum-deprived calves. These experiments are an extension of the initial demonstration of the success of this approach, under experimental conditions, in mice (27). Dubos and colleagues (6) were also able to demonstrate a therapeutic effect using an undefined (not purified) phage preparation administered intraperitoneally after intracranial inoculation of Shigella in mice.
As in mice (27) phage R was obviously able to multiply in the tissues. It seems unclear whether the main site of multiplication was the spleen or the blood. It seems unlikely to be the former, since we have found (4a) that several phages, highly active against Salmonella gallinarum in vitro, had absolutely no effect on the course of infection in vivo. At the time of the termination of the experiment there were very few phage particles and E. coli in the brain whereas considerable numbers of phage were still present in the spleen.
It seemed likely that, in the chickens inoculated intracranially with E. coli, phage had crossed the blood-brain barrier. The phage counts in the brain could not have been solely a result of blood contamination, since the kinetics of the appearance of phage in blood and brain were different. Whether this was a result of the inflammation induced by the E. coli infection or whether it might occur anyway is difficult to determine and would be very difficult to detect experimentally. It was also thought to have occurred in the experiments of Dubos et al. (6). Appearance in the brain following intraperitoneal inoculation of Bacillus phage has been reported (10), but those phages in the brain could have resulted from blood contamination.
Phage R persisted long enough in the tissues to be effective when administered to the chickens a day or two before challenge with the E. coli. This was also reported previously with mice (27) and suggests that there are possibilities for prophylaxis. More interestingly, it was possible to delay administration of the phage until signs of disease were evident in the chickens and yet retain considerable protection, suggesting that acute infections might also be amenable to phage treatment.
Despite the success of this experimental investigation, phage treatment could only become a practical measure for treating or preventing avian E. coli infections under certain conditions. The fact that a relatively small proportion of strains possessing the K1 antigen were lysed by phage R indicates that an active anti-K1 phage used for this purpose would have to be adapted to strains causing particular problems in the field. Parenteral inoculation of phage could be used for mass treatment, but oral administration would be more convenient, and although there have been reports on the translocation of phages administered orally across the gut (9), it would seem unlikely that the phages could accumulate in the tissues and blood to a sufficiently high concentration to be practically useful.
Too few calves were used for statistical analysis because of the high costs associated with hysterotomy, and caution should therefore be used in interpreting the results. However, it seemed likely that the use of phage in calves was able to control infection but not prevent it. Large numbers of bacteriophage appeared in the alimentary tract after inoculation, but despite attempts at eliminating oral ingestion of phage by disinfecting the skin this cannot be ruled out. One of the treated animals became ill, and phage-resistant K1+ mutants were found in the blood. These were interesting, but their nature was not pursued further. However, there seemed to be insufficient numbers of E. coli in the blood to have caused the problem, and it seemed likely that illness in these animals resulted from absorption of toxins from the intestine. Thus, although most phage-resistant mutants that arise would be K1 negative, the large reservoir of organisms in the intestine in all probability allowed other mutants to arise that might not do so from a smaller population. Under commercial conditions such calves would be fed normal milk which, containing antibodies against miscellaneous lipopolysaccharide antigens, would assist in reducing multiplication of a single clone in the gut. The problem of the appearance of virulent, phage-resistant mutants may be, therefore, an artifact of the conditions of the experiment.
The results indicate that bacteriophages have the potential for the prevention and/or treatment of certain bacterial infections of animals and, by extension, of humans. It is tempting to advocate research investigations into many bacterial infections for which animal models of infection are available and for which phages may be isolated. However, a number of problems associated with the use of phages may restrict the numbers of types of infections for which such an approach may be appropriate. These have been discussed recently (3, 4). In summary, phages are most likely to be able to multiply in vivo where a stage of the pathogenesis of the disease mimics conditions under which phages multiply optimally and are able to spread between susceptible bacterial cells in vitro. These in vitro conditions include liquid broth, for which septicemias and meningites (e.g., those caused by E. coli and Haemophilus influenzae) may be a suitable analogy, and the surface of agar plates, whose analogy in vivo is intestinal colonization by enterotoxigenic pathogens, such as V. cholerae, or burn colonization by organisms such as P. aeruginosa. However, lysis can also be produced in vivo without phage multiplication by administration of overwhelming numbers of phage (5). This might be applicable to clearing staphylococci from the anterior nares. Problems with DNA restriction may limit this to pathogens that are largely clonal, although this problem could be circumvented. The use of phages which attach to surface virulence determinants, such as the K1 antigen here, may avoid the problem of resistance development. Phages are unlikely to be effective for intracellular pathogens or where spread between susceptible bacteria is hindered by other material, as in areas of inflammation and in the large bowel. There is some evidence for this (5, 34).
Despite continued skepticism over this area of research, carefully controlled experimental work shows that it can be very effective. Given the increasing problems of bacterial disease and bacterial antibiotic resistance worldwide, it would seem timely to begin to look afresh at this approach and begin a period of renewed and rational assessment.
ACKNOWLEDGMENTS
We thank K. Page and A. Gray for technical assistance and P. M. Biggs for farsighted encouragement.
We thank the British Egg Marketing Board, CNPq, and FAPESP for financial support.
REFERENCES
- 1.Asheshov I N, Wilson J, Topley W W C. The effect of an anti-Vi bacteriophage on typhoid infection in mice. Lancet. 1937;i:319–320. [Google Scholar]
- 2.Barrow P A. Bacteriophages mediating somatic antigenic conversion in Salmonella cholerae-suis; their isolation from sewage and other Salmonella serotypes possessing the somatic 6 antigen. J Gen Microbiol. 1986;132:835–837. doi: 10.1099/00221287-132-3-835. [DOI] [PubMed] [Google Scholar]
- 3.Barrow P A. Novel approaches to control of bacterial infections in animals. Acta Vet Hung. 1997;45:317–329. [PubMed] [Google Scholar]
- 4.Barrow P A, Soothill J S. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 4a.Berchieri, A., Jr., and P. Barrow. Unpublished data.
- 5.Berchieri A, Jr, Lovell M A, Barrow P A. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res Microbiol. 1991;142:541–549. doi: 10.1016/0923-2508(91)90187-f. [DOI] [PubMed] [Google Scholar]
- 6.Dubos R J, Straus J H, Pierce C. The multiplication of bacteriophage in vivo and its protective effect against an experimental infection with Shigella dysenteriae. J Exp Med. 1943;20:161–168. doi: 10.1084/jem.78.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elek S D. Staphylococcus pyogenes and its relation to disease. E. & S. London, United Kingdom: Livingstone; 1959. [Google Scholar]
- 8.Gross W B. Colibacillosis. In: Calnek B W, Barnes H J, Beard C W, Reid W M, Yoder H W, editors. Diseases of poultry. Ames: Iowa State University Press; 1991. pp. 138–144. [Google Scholar]
- 9.Hildebrand G J, Wolochow H. Translocation of bacteriophage across the intestinal wall of the rat. Proc Soc Exp Biol Med. 1962;109:183–185. doi: 10.3181/00379727-109-27146. [DOI] [PubMed] [Google Scholar]
- 10.Iba A M, Berchieri A, Jr, Barrow P A. Interference between Salmonella serotypes in intestinal colonization of chickens: correlation with in vitro behaviour. FEMS Microbiol Lett. 1995;131:153–159. doi: 10.1111/j.1574-6968.1995.tb07770.x. [DOI] [PubMed] [Google Scholar]
- 11.Keller R, Engley F B., Jr Fate of bacteriophage particles introduced into mice by various routes. Proc Soc Exp Biol Med. 1958;98:577–580. doi: 10.3181/00379727-98-24112. [DOI] [PubMed] [Google Scholar]
- 12.Lowbury E J L, Hood A M. The acquired resistance of Staphylococcus aureus to bacteriophage. J Gen Microbiol. 1953;9:524–535. doi: 10.1099/00221287-9-3-524. [DOI] [PubMed] [Google Scholar]
- 13.Merril C R, Biswas B, Carlton R, Jensen N C, Creed G J, Zullo S, Adhya S. Long-lasting bacteriophage as antibacterial agents. Proc Natl Acad Sci USA. 1996;93:3188–3192. doi: 10.1073/pnas.93.8.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morison J. Bacteriophage in the treatment and prevention of cholera. H. K. London, United Kingdom: Lewis; 1932. [Google Scholar]
- 15.Morris J A, Sojka W J. Escherichia coli as a pathogen in animals. In: Sussman M, editor. The virulence of Escherichia coli. London, United Kingdom: Academic Press; 1985. pp. 47–77. [Google Scholar]
- 16.Pollitzer R. Cholera. Geneva, Switzerland: World Health Organization; 1959. [Google Scholar]
- 17.Sarff L D, McCracken G H, Jr, Sciffer M S, Glode M P, Robbins J B, Ørskov I, Ørskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975;i:1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- 18.Slopek S, Durlakowa I, Weber-Dabrowska B, Kucharewicz-Krukowska A, Dabrowski M, Bisikiewicz R. Results of bacteriophage treatment of suppurative bacterial infections. I. General evaluation of results. Arch Immunol Ther Exp. 1983;31:267–291. [PubMed] [Google Scholar]
- 19.Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative infections in the years 1981–1986. Arch Immunol Ther Exp. 1987;35:569–583. [PubMed] [Google Scholar]
- 20.Smith H W. Transmissible pathogenic characteristics of invasive strains of Escherichia coli. J Am Vet Med Assoc. 1978;173:601–607. [PubMed] [Google Scholar]
- 21.Smith H W, Cook J K A, Parsell Z E. The experimental infection of chickens with mixtures of infectious bronchitis virus and Escherichia coli. J Gen Virol. 1985;66:777–786. doi: 10.1099/0022-1317-66-4-777. [DOI] [PubMed] [Google Scholar]
- 22.Smith H W, Huggins M B. Further observations on the association of the colicine V plasmid of Escherichia coli with pathogenicity and with survival in the alimentary tract. J Gen Microbiol. 1976;92:335–350. doi: 10.1099/00221287-92-2-335. [DOI] [PubMed] [Google Scholar]
- 23.Smith H W, Huggins M B. Treatment of experimental Escherichia coli infection in mice with colicine V. J Med Microbiol. 1977;10:479–482. doi: 10.1099/00222615-10-4-479. [DOI] [PubMed] [Google Scholar]
- 24.Smith H W, Huggins M B. The effect of plasmid-determined and other characteristics on the survival of Escherichia coli in the alimentary tract of two human beings. J Gen Microbiol. 1978;109:375–379. doi: 10.1099/00221287-109-2-375. [DOI] [PubMed] [Google Scholar]
- 25.Smith H W, Huggins M B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tract of piglets, calves and lambs. J Med Microbiol. 1978;11:471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- 26.Smith H W, Huggins M B. The association of the O18, K1 and H7 antigens and the ColV plasmid of a strain of Escherichia coli with its virulence and immunogenicity. J Gen Microbiol. 1980;121:384–400. doi: 10.1099/00221287-121-2-387. [DOI] [PubMed] [Google Scholar]
- 27.Smith H W, Huggins M B. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol. 1982;128:307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 28.Smith H W, Huggins M B. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol. 1983;129:2659–2675. doi: 10.1099/00221287-129-8-2659. [DOI] [PubMed] [Google Scholar]
- 29.Smith H W, Huggins M B. Acquisition of genes from an O18:K1:H7 ColV+ strain of Escherichia coli renders intra-cranially-inoculated E. coli K12 highly virulent for chickens, ducks and guinea-pigs but not mice. J Hyg. 1985;95:363–374. doi: 10.1017/s0022172400062781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith H W, Huggins M B, Shaw K M. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol. 1987;133:1111–1126. doi: 10.1099/00221287-133-5-1111. [DOI] [PubMed] [Google Scholar]
- 31.Smith H W, Huggins M B, Shaw K M. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol. 1987;133:1127–1135. doi: 10.1099/00221287-133-5-1127. [DOI] [PubMed] [Google Scholar]
- 32.Soothill J S. Treatment of experimental infections of mice with bacteriophages. J Med Microbiol. 1992;37:258–261. doi: 10.1099/00222615-37-4-258. [DOI] [PubMed] [Google Scholar]
- 33.Soothill J S, Lawrence J C, Ayliffe G A J. The efficacy of phages in the prevention of the destruction of pig skin in vitro by Pseudomonas aeruginosa. Med Sci Res. 1988;16:1287–1288. [Google Scholar]
- 34.Topley W W C, Wilson G S. Principles of bacteriology and immunity. 2nd ed. London, United Kingdom: Edward Arnold; 1936. [Google Scholar]
- 35.Topley W W C, Wilson J. Further observations on the role of the Twort-d’Herelle phenomenon in the epidemic spread of murine typhoid. J Hyg. 1925;24:295–300. doi: 10.1017/s0022172400008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topley W W C, Wilson J, Lewis E R. The role of the Twort-d’Herelle phenomenon in epidemics of mouse typhoid. J Hyg. 1925;24:17–36. doi: 10.1017/s0022172400031697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward W E. Protective action of Vi bacteriophage in Erbethella typhi infections in mice. J Infect Dis. 1943;72:172–176. [Google Scholar]