Abstract
We have developed a simple chromatographic procedure for the partial purification of substance P (SP) from acidified plasma and serum samples. We have evaluated a sensitive antigen competition enzyme immunoassay (EIA) for the quantitation of SP. The chromatographic procedure has recovery efficiencies ranging from 94.8 to 125%. The immunoreactivity of unknown amounts of purified SP subjected to the preparative procedure yielded a coefficient of variance of 9.4%. The EIA yielded reproducible standard curves having an interassay (n = 8) correlation coefficient of 0.984. The evaluation of normal adult control serum yielded a mean value of 51 pg/ml (range, 35 to 61 pg/ml). The evaluation of 3.33× concentrates of serum-derived partially purified SP provided uncorrected SP values of 117 to 201 pg/ml, which fell within the midpoint of the three-decalog standard curve. These studies indicate that both the preparative and quantitative procedures are required for the detection of SP in plasma or serum samples collected from patients with several clinical disorders.
Substance P (SP), a bioactive undecapeptide, was discovered in 1930 (7) and is found at varying concentrations in the central nervous system (11). Observations by Lembeck that there is a much higher concentration of SP in the dorsal than in the ventral roots of the spinal cord led to the speculation that SP was associated with sensory neuron transmission (18). Chang and Leeman (5) reported the chemical structure of SP, which they determined to be an undecapeptide (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-amide), leading to its synthesis and availability for experimentation. Recently, immunoassay techniques have made it possible to study the distribution of SP in different tissues (6). SP is widely distributed in the central and peripheral nervous systems and has been implicated in immune (14) and hematopoietic (20, 21) modulation. We have recently demonstrated that human immune cells such as monocytes and macrophages express SP at both mRNA and protein levels (10). SP may have a major role in the pathogenesis of several inflammatory diseases such as rheumatoid arthritis, bullous pemphigus, asthma, inflammatory bowel disease (Crohn’s disease), and ulcerative colitis as well as in the transmission of pain (4, 16).
An area of intense investigation within the field of psychoneuroimmunology centers on the elucidation of the roles played by certain neuropeptides in the modulation of immunological responses in various disease states. SP, one of three well-characterized neuropeptides of the tachykinin peptide family, has been extensively studied in terms of its role in physiological processes such as vasodilation, smooth-muscle contraction, and nerve conduction (3, 12, 27). More recently, SP has been shown to play an important role in the induction of cytokines such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha, which are central to the initiation of inflammatory responses leading to modulation of both specific and nonspecific immune responses (13, 24). With the observation of SP receptors on both T and B lymphocytes (26), several studies have strengthened the hypothesis that SP plays a pivotal role in the trafficking of lymphocytes (15) leading to enhanced humoral (25) as well as cellular humoral responses (16, 17). Elevated plasma SP and immunoglobulin A levels were noted in human immunodeficiency virus type 1 (HIV-1)-infected infants compared to their uninfected cohorts (2, 23). Macrophages play a central role in both inflammatory responses and antigen presentation. The finding of SP augmentation of lipopolysaccharide (LPS)-induced cytokine production (IL-1, IL-6, and tumor necrosis factor alpha) by macrophages lends additional evidence for the role of SP in the modulation of host immune function (4).
The measurement of SP levels in various body fluids may serve as an important surrogate marker in monitoring host immune responses in a variety of disease states. Therefore, development of techniques to accurately measure SP is of obvious importance. Historically, attempts to measure neuropeptides in various body fluids, particularly serum or plasma, have been difficult due to the nonspecific binding of neuropeptides to other plasma or serum proteins (1, 22). Thus, various liquid organic extraction methods have been described for the partial purification of neuropeptides including SP with varying levels of success (9, 22). Further, until recently, labor-intensive radioimmunoassays (RIA) were the only methods available for the quantitation of SP after partial purification (9). The RIA technique, however, is less than satisfactory in terms of safety, ease of handling, and sensitivity.
In an effort to improve upon existing procedures for both the preparation and quantitation of SP in serum or plasma, the present paper describes the development of a simple chromatographic procedure for the partial purification of SP and describes the evaluation of a sensitive enzyme immunoassay (EIA) for the determination of SP level in serum and plasma samples from patients with several clinical disorders.
MATERIALS AND METHODS
Patient populations.
The 305 samples were from adults undergoing a diagnostic procedure three times, twice in the hospital and once at home (n = 23 [69 samples]), children hospitalized with sickle-cell disease (n = 51), women with human papillomavirus (HPV) infection (n = 11), patients infected with HIV (n = 43), patients with chronic inflammatory demyelinating polyneuropathy (n = 3), patients with multiple sclerosis (n = 23), healthy adult volunteers (n = 19), umbilical cord blood from HIV-positive mothers (n = 4), cultured monocytes from umbilical cord blood treated with cytokines (n = 77), and a cultured monocyte cell line from umbilical cord blood (n = 5). The choice of these diverse groups was based on sample availability as well as the desire to test the procedures on a wide range of patients.
Specimen collection.
Blood was collected in serum separation tubes and centrifuged at 1,500 × g for 20 min. The blood was processed in the laboratory approximately 2 h after the specimen was drawn. The serum was collected, aprotinin (5 U/ml), a protease inhibitor (Sigma Chemical Co., St. Louis, Mo.), was added to each specimen, and the specimen was stored at −70°C until analysis was performed. The mean storage time prior to analysis was 8 days. The samples were dried under nitrogen gas and reconstituted with deionized water to 1/3 of the initial volume and analyzed with the EIA as described below.
Extraction of SP from plasma and sera.
The method used for the extraction of SP from acidified plasma or serum samples is a modification of a previously described procedure in which plasma samples were extracted with acetone followed by ether. The extracts were then air dried and reconstituted with assay buffer (9). Rissler (22) reviews the problems associated with liquid-liquid extraction of peptides such as SP from body fluids and advocates solid-phase extraction of samples on small disposable cartridges such as the ones used in this study.
Briefly, 1.0-ml C18 reverse-phase columns (Bond Elute; Varton, Harbor City, Calif.) were activated by first rinsing with 5 ml of high-performance liquid chromatography grade methanol followed by a final rinse with 5 ml of distilled water. Plasma samples (0.5 ml) were diluted 1:4 with 4% (vol/vol) acetic acid. Initially, acidified plasma samples were spiked with known amounts of radiolabeled SP to evaluate SP recovery from the C18 reverse-phase columns. Further, known amounts of purified SP were processed through the column to evaluate the influence of preparation on immunoreactivity of the eluted peptide. The acidified samples were then added to the activated reverse-phase columns and allowed to pass through the columns by gravity. The columns were then washed five times with 2 ml of 4% acetic acid to remove all unbound material. Three buffer systems were evaluated for efficiency of elution of SP bound to C18 reverse-phase columns. The buffer systems tried were (i) 90% (vol/vol) ethanol, 10% (vol/vol) water, 0.4% (vol/vol) acetic acid; (ii) 80% (vol/vol) acetonitrile, 1% (vol/vol) trifluoroacetic acid (prepared in distilled water); and (iii) 60% (vol/vol) acetonitrile, 1% (vol/vol) trifluoroacetic acid (prepared in distilled water). The evaluation of the elution buffers was accomplished by binding known amounts of 125I-radiolabeled SP (125 pg/ml) added to acidified plasma samples. A gamma counter was used to compare the eluates to an appropriately diluted 125I-radiolabeled SP standard which was not processed through the column. The buffer which reproducibly yielded more than 90% recovery was made up of 60% (vol/vol) acetonitrile prepared in 1% (vol/vol) trifluoroacetic acid (prepared in distilled water). Specimens to be evaluated were processed through the column in the manner described above. The bound SP was then eluted from the columns by the addition of 1 ml of the chosen buffer system. The eluted samples were then dried at 45°C under a constant flow of N2 gas, followed by reconstitution in 0.15 ml of distilled water. Total resolubilization was achieved by incubation of the sample for 30 min at 45°C. SP was then quantified by an antigen competition EIA described below.
SP EIA.
The quantification of SP in plasma and serum samples, prepared as described above, was accomplished by an antigen competition EIA (Caymen Chemical Co., Ann Arbor, Mich.). The assay employs SP conjugated to acetylcholinesterase with acetylthiocholine as the substrate which is hydrolyzed to thiocholine and in turn reacts with 5,5′-dithio-bis-2-nitrobenzoic acid, producing the product 5-thio-2-nitrobenzoic acid, which has a maximum absorbance at 412 nm. Acetylcholinesterase exhibits several advantages as the tracer tag over other commonly used enzymes such as horseradish peroxidase in that it does not autoinactivate during turnover, allowing for multiple development of the assay. Additionally, acetylcholinesterase is highly stable under assay conditions with a wide pH range (pH 5 to 10) and is not inhibited by common buffer salts and preservatives. The assay was calibrated by the use of an SP standard over a range of 7 to 1,000 pg/ml. The reported limit of detection of this assay is 17.2 pg/ml. Briefly, 0.05 ml of an appropriately diluted unknown sample or a known SP standard was added to each well of the microtiter plate precoated with monoclonal antibody specific for rabbit immunoglobulin G, followed by adding 0.05 ml of acetylcholinesterase-conjugated SP to each well. Finally, 0.05 ml of rabbit anti-SP was added to all wells except nonspecific binding wells, which received 0.05 ml of buffer. The plates were incubated at 4°C for 18 h, followed by five washes with wash buffer. Enzyme substrate (0.2 ml) was added to each well, followed by 2-h incubation at room temperature in the dark with rotation. The absorbance (412 nm) of each well was then measured with a Labrepco Elx 800 microplate reader (Bio-Tek Instrument, Inc., Horsham, Pa.). A computer program was developed which converts net optical density (OD) values to B/Bo values (net OD of sample/net OD of maximum binding), which were plotted versus the concentration of SP standards. From this standard curve, sample B/Bo values were then used to determine unknown-sample SP concentrations in picograms per milliliter. An accuracy control (125 pg of SP per ml) was included with all evaluations to monitor assay variability. Any assay in which the value for the accuracy control was determined to be outside the 95% confidence limit was repeated.
RESULTS
Recovery of SP from C18 reverse-phase columns.
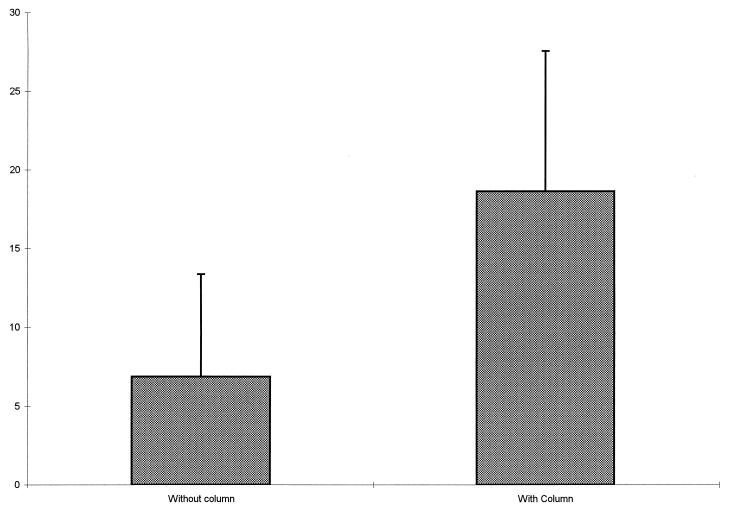
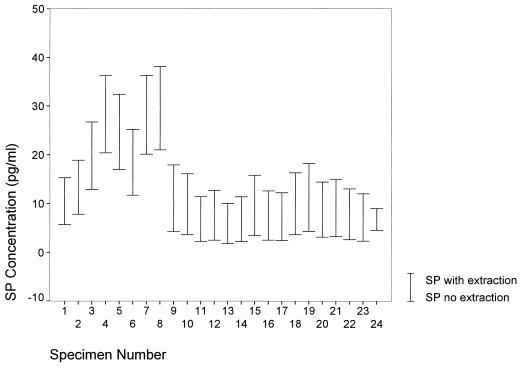
In order to determine the efficiency and efficacy of using the chromatographic extraction procedure on clinical specimens, 24 specimens were processed in duplicate both with and without the C18 reverse-phase columns. The paired specimens were plated and EIA readings were obtained at the same times. Values were obtained for these duplicate specimens from B/Bo plots and were compared statistically. The mean value for SP obtained for the duplicate specimens without the column extraction was 6.87 with a standard deviation (SD) of 6.49. By comparison, the mean value of SP obtained from the duplicates with the column was 18.63 with an SD of 8.91 (Fig. 1). The paired coefficient of correlation between values obtained with and without the column was 0.98 (P < 0.001). The changes in the SP concentrations for the individual specimens based on the extraction procedure are graphically presented in Fig. 2. In addition to eliminating the interference with EIA measurement due to the nonspecific binding of SP to plasma or serum proteins, the purification procedure provides an additional advantage. Since the mean values for the specimens subjected to the chromatographic procedure were consistently three times the values for specimens not treated, and the intertreatment coefficient of correlation was very high, the purification procedure represents a worthwhile step in measuring SP levels since it also serves to place the values closer to the center of the EIA measurement scale.
FIG. 1.
Comparison of mean SP values obtained with and without the extraction procedure (error bars represent 1 SD).
FIG. 2.
Comparison of SP concentrations in individual specimens treated with and without the extraction procedure.
Determination of immunoreactivity of eluted SP.
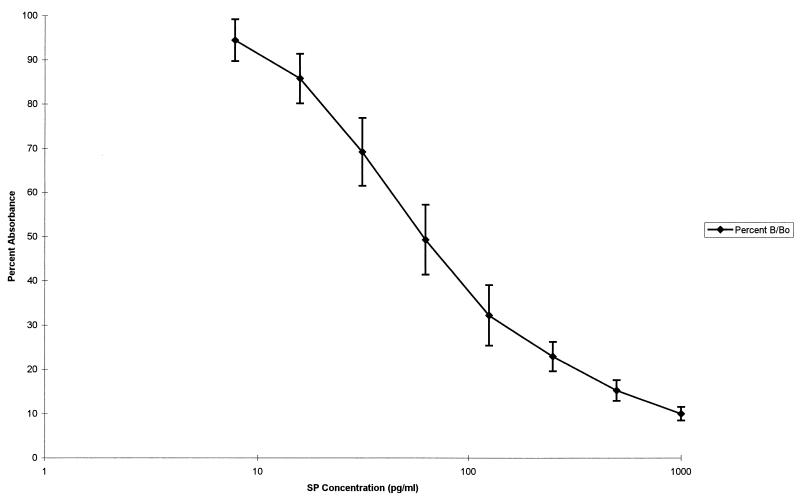
In order to evaluate the influence of sample preparation on the immunoreactivity of SP, known amounts of synthetic SP (0.5 ml of 125 pg/ml) provided with the EIA kits were subjected to the same preparative procedure as that performed on unknown plasma and serum samples and then quantified by EIA. The results of eight individual experiments yielded a mean value of 124.5 pg/ml with an SD of 21.4 pg/ml and a coefficient of variance of 9.4%. The 95% confidence limits (mean ± 2 SD) were 81.7 to 167.4 pg/ml. We therefore used those limits for acceptance or rejection of individual evaluations of unknown samples. Figure 3 illustrates the mean B/Bo values ± SD for each SP standard used to produce reference standard curves for eight individual EIA experiments and represents the typical calibration curve for this EIA of SP. A linear displacement of acetylcholinesterase-linked SP by synthetic SP standard concentrations was obtained, when plotted as a semilogarithmic function from 7.8 to 1,000 pg of SP per ml. The coefficient of variation for each curve was greater than 0.98, while the coefficient of variation for the composite curve was 0.97. These data demonstrate an acceptable level of interassay precision for the EIA formatted assay, particularly when the coefficient of variance (9.4%) obtained with the multiple evaluations of the accuracy control mentioned above is taken into consideration.
FIG. 3.
SP standard curve of mean absorbance for the eight calibration concentrations used (±1 SD).
Evaluation of SP level in normal control specimens.
The limit of detection of SP in plasma and serum samples by the EIA evaluated in the present study was stated by the manufacturer to be 17.5 pg/ml, which places the limit of detection near the level of normal ranges reported elsewhere for control subjects (21.94 ± 18 pg/ml) (9). We, therefore, elected to reconstitute eluted and dried samples in a volume (0.15 ml) which represented a 3.33× concentrate of the original sample volume (0.5 ml), which was applied to the C18 reverse-phase columns. This was found to yield SP values near the middle of the three-decalog standard curve or about 100 pg/ml. The evaluation of 19 control sera yielded an uncorrected mean value of 126 pg of SP per ml (range, 17 to 382 pg/ml), with corrected values having a mean of 38 pg of SP per ml (range, 5 to 115 pg/ml), which is well within the quantitation range of the assay. The correction used was for the 3.33× concentration effect of the purification procedure. These conditions allow for the evaluation of pathological samples which might have both higher and lower levels of SP compared to a normal cohort.
Evaluation of SP levels among patient populations.
Among all 306 specimens analyzed, the mean value for SP was 32 pg/ml, with corrected values ranging from a low of 2 pg/ml to a high of 242 pg/ml (SD, 39 pg/ml). The mean values for each group of subjects are presented in Table 1. Statistical analysis of the mean SP values using analysis of variance revealed significant differences among the groups (F(9,312) = 3.7; P < 0.01). The post hoc Tukey-B test revealed that the SP values obtained from the cord blood from mothers infected with HIV-1 were significantly higher than those in the diagnostic-procedure group, the children hospitalized with sickle-cell disease, the women with HPV, patients infected with HIV-1, and patients with multiple sclerosis. The concentrations of SP released by monocyte cell cultures in serum-free medium were similar to those obtained from the plasma or serum samples presented in Table 1 (18 ± 31.5 pg/ml for monocytes treated with cytokines [n = 77] and 35 ± 52.1 pg/ml for untreated monocytes [n = 5]; the value for lymphocytes reported in reference 10 is 32 pg/ml [n = 3]).
TABLE 1.
Comparison of corrected SP concentrations among groups
| Group (n) | Mean SP concn (pg/ml)a | SD |
|---|---|---|
| Healthy adult volunteers (19) | 38 | 35.7 |
| Diagnostic-procedureb patients (23) | 35∗ | 27.2 |
| Children with sickle-cell disease (51) | 45∗ | 51.1 |
| Women with HPV (11) | 14∗ | 8.4 |
| Patients with multiple sclerosis (23) | 33∗ | 23.8 |
| Patients with CIDPc (3) | 40 | 19.2 |
| Patients with HIV (43) | 35∗ | 45.2 |
| Umbilical cord blood from HIV+ mothers (4) | 98∗ | 68.5 |
Adjusted for the extraction concentration effect. ∗, P ≤ 0.05 (post hoc Tukey-B analysis of variance).
Performed at three different time points.
CIDP, chronic inflammatory demyelinating polyneuropathy.
DISCUSSION
This study illustrates the usefulness of the procedures in obtaining valid measures of SP from a variety of clinical settings. The mean values we obtained compare favorably with those obtained by means of RIA from plasma samples from healthy blood bank donors (20 to 151 pg/ml) reported by Powell et al. (19). Similar results were also reported by Fernandez-Rodriguez et al. (9), who used an RIA technique to measure SP and found mean values ranging from 65 to 128 pg/ml in a comparison between normal control volunteers and patients with cirrhosis. In the experiments designed to study the relationship between SP and anxiety we found that they are significantly correlated and that EIA is sufficiently sensitive to detect changes within individual subjects over time (8). Interestingly, umbilical cord plasma from HIV-positive mothers had significantly higher SP levels than the other groups studied. Although we had only four samples of cord blood, these data support the results obtained by Azzari and coworkers (2, 23), who found that the SP levels of HIV-seropositive children born to HIV-seropositive mothers were significantly higher than the SP levels of HIV-seronegative children born to HIV-seropositive mothers. Elevated SP levels in the HIV-seropositive children might be associated with the imbalance between T-helper lymphocyte subsets and the cytokines they secrete.
The variability of SP concentration within certain groups such as children with sickle-cell disease (SD = 51.1) and umbilical cord blood from HIV-positive mothers (SD = 68.5) might be due to the heterogeneous factors within the groups. The children with sickle-cell disease may have had different levels of pain or have been at different stages of the disease, resulting in the high variability exhibited by the group. Similarly, the umbilical cord blood from HIV-positive mothers might or might not have been infected with HIV, possibly causing the high variability demonstrated. Thus, the variability of SP values seen within the diagnostic groups might be a further demonstration of the usefulness of the techniques described in this paper.
We have demonstrated that the described procedures for purifying and quantifying SP are reliable and useful. The SP measures obtained from diverse sources were comparable to the published results obtained by other researchers using different techniques. The use of the chromatographic procedure to partially purify SP in plasma and serum samples can be omitted when cell culture medium is serum free.
ACKNOWLEDGMENTS
This study was supported in part by NIH grant R01 MH 49981 and a grant from the Xi Chapter of Sigma Theta Tau, Inc. (International Honor Society of Nursing).
Some tissue and fluid specimens were obtained from the National Neurological Research Specimen Bank, VAMC, Los Angeles, Calif., which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society, Hereditary Disease Foundation, Comprehensive Epilepsy Program, Tourette’s Syndrome Association, Dystonia Medical Research Foundation, and Veterans Health Services and Research Administration, Department of Veterans Affairs.
REFERENCES
- 1.Arimura A, Lundqvist G, Rothman J, Chang R, Fernandez-Durango R, Elde R, Coy D, Meyers C, Schally A. Radioimmunoassay of somatostatin. Metabolism. 1978;27:1139–1144. doi: 10.1016/0026-0495(78)90032-x. [DOI] [PubMed] [Google Scholar]
- 2.Azzari C, Rossi M, Resti M, Caldini A, Legra L, Galli L, Fico E, Vierucci A. Imbalance in the levels of substance P and somatostatin in HIV positive children. Ped Med Chir. 1992;14:577–581. [PubMed] [Google Scholar]
- 3.Bartho L, Holzer P. Search for a physiological role of substance P in gastrointestinal motility. Neuroscience. 1985;16:1–32. doi: 10.1016/0306-4522(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 4.Chancellor-Freeland C, Zhu G, Kage R, Beller D, Leeman S, Black P. Substance P and stress induced changes in macrophages. Ann N Y Acad Sci. 1995;771:472–484. doi: 10.1111/j.1749-6632.1995.tb44703.x. [DOI] [PubMed] [Google Scholar]
- 5.Chang M, Leeman S. Amino-acid sequence of substance P. Nature. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 6.Euler U. Historical notes. In: Euler U, Pernow B, editors. Substance P. New York, N.Y: Raven Press; 1977. pp. 1–3. [Google Scholar]
- 7.Euler U, Gaddum J. An unidentified depressor substance in certain tissue extracts. J Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fehder W, Sachs J, Uvaydova M, Douglas S. Substance P as an immune modulator of anxiety. Neuroimmunomodulation. 1997;4:42–48. doi: 10.1159/000097314. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Rodriguez C, Prieto J, Quiroga J, Zozoya J, Andrade A, Nunez M, Sangro B, Penas J. Plasma levels of substance P in liver cirrhosis: relationship to the activation of vasopressor systems and urinary sodium excretion. Hepatology. 1995;21:35–40. doi: 10.1002/hep.1840210108. [DOI] [PubMed] [Google Scholar]
- 10.Ho W, Lai J, Zhu X, Uvaydova M, Douglas S. Human monocytes and macrophages express substance P and neurokinin 1 receptor. J Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- 11.Hokfelt T, Johansson O, Kellerth J, Ljungdahl A, Nilsson G, Nygards A, Pernow B. Immunohistochemical distribution of substance P. In: Euler U, Pernow B, editors. Substance P. New York, N.Y: Raven Press; 1977. pp. 117–145. [Google Scholar]
- 12.Lembeck F, Gamse R, Juan H. Substance P and sensory nerve endings. In: Euler U, Pernow B, editors. Substance P. New York, N.Y: Raven Press; 1977. pp. 169–181. [Google Scholar]
- 13.Lotz M, Vaughan J, Carson D. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 14.McGillis J, Mitsuhashi M, Payan D. Immunomodulation by tachykinin neuropeptides. Ann N Y Acad Sci. 1990;594:85–94. doi: 10.1111/j.1749-6632.1990.tb40470.x. [DOI] [PubMed] [Google Scholar]
- 15.Moore T. Modification of lymphocyte traffic by vasoactive neurotransmitter substances. Immunology. 1984;52:511. [PMC free article] [PubMed] [Google Scholar]
- 16.Payan D. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- 17.Payan D, Brewster D, Goetzl E. Specific stimulation of human T lymphocytes by substance P. J Immunol. 1983;131:1613–1615. [PubMed] [Google Scholar]
- 18.Pernow B. Opening address. Regul Pept. 1993;46:4–6. [Google Scholar]
- 19.Powell D, Skrabanek P, Cannon D. Substance P: radioimmunoassay studies. In: Euler U, Pernow B, editors. Substance P. New York, N.Y: Raven Press; 1977. pp. 35–40. [Google Scholar]
- 20.Rameshwar P, Doina G, Gascon P. In vitro stimulatory effect of substance P on hematopoiesis. Blood. 1993;81:391–398. [PubMed] [Google Scholar]
- 21.Rameshwar P, Gascon P. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood. 1995;86:482–490. [PubMed] [Google Scholar]
- 22.Rissler K. Sample preparation, high-performance liquid chromatographic separation and determination of substance P-related peptides. J Chromatogr B. 1995;665:233–270. doi: 10.1016/0378-4347(94)00533-b. [DOI] [PubMed] [Google Scholar]
- 23.Rossi M, Resti M, Azzari C, Calabri G, DeMartino M, Galli L, Carbonella R, Vierucci A. High levels of IgA in HIV-1-perinatally-infected children: antigen specificity and possible role of increased substance P plasma levels. Pediatr Allergy Immunol. 1994;5:240–243. doi: 10.1111/j.1399-3038.1994.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 24.Sacerdote P, Carrabba M, Galante A, Pisati R, Manfredi B, Panerai A. Plasma and synovial fluid interleukin-1, interleukin-6 and substance P concentrations in rheumatoid arthritis patients: effect of the nonsteroidal anti-inflammatory drugs indomethacin, diclofenac and naproxen. Inflammatory Res. 1995;44:486–490. doi: 10.1007/BF01837915. [DOI] [PubMed] [Google Scholar]
- 25.Stanisz A, Befus D, Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferation by lymphocytes from Peyer’s patches, mesenteric lymph nodes, and spleen. J Immunol. 1986;136:152–156. [PubMed] [Google Scholar]
- 26.Stanisz A, Scicchitano R, Dazin P, Bienenstock J, Payan D. Distribution of substance P receptors on murine spleen and Peyer’s patch T and B cells. J Immunol. 1987;139:749–754. [PubMed] [Google Scholar]
- 27.Withrington P. The actions of two sensory neuropeptides, substance P and calcitonin gene-related peptide, on the canine hepatic arterial and portal vascular beds. Br J Pharmacol. 1992;107:296–302. doi: 10.1111/j.1476-5381.1992.tb12741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]