Abstract
The humoral immune response to Chlamydia outer membrane protein 2 (Omp2) was studied. Omp2 is a highly genus-conserved structural protein of all Chlamydia species, containing a variable N-terminal fragment. To analyze where the immunogenic parts were localized, seven highly purified truncated fusion proteins constituting different regions of the protein were produced (Chlamydia pneumoniae-Omp2aa23-aa93, Chlamydia psittaci-Omp2aa23-aa94, and Chlamydia trachomatis-Omp2aa23-aa84, aa87-aa547, aa23-aa182, aa167-aa434, aa420-aa547). By an enzyme-linked immunosorbent assay with serologically defined patient sera, Omp2 was found to be a major immunogen of both C. pneumoniae and C. trachomatis infections (P ≪ 0.0001). The humoral immune responses were not confined to any particular region of the Omp2 protein, and no species-specific anti-Omp2 immunoglobulins were detected.
There are four recognized species of the Chlamydia genus. Three of these species are etiological agents of a variety of human infections. Chlamydia trachomatis trachoma inclusion conjunctivitis biovar elicits local mucosal epithelial infections of the eye or the urogenital tract. Infections can progress to chronic stages such as trachoma and pelvic inflammatory disease, respectively. Pelvic inflammatory disease leads to tubal obstruction or ectopic pregnancy. The lymphogranuloma venereum biovar of C. trachomatis infects both macrophages and epithelial cells and is spread systemically through the lymphatic tissue, causing the invasive disease known as lymphogranuloma venereum. Chlamydia pneumoniae causes pneumonia, bronchitis, and pharyngitis (9). Additionally, C. pneumoniae is associated with a wide range of chronic disease states, such as asthma, chronic bronchitis, acute myocardial infarction, and coronary artery disease (10, 18, 24). Chlamydia psittaci is a rare but opportunistic pathogen of humans, causing a severe infection of the respiratory tract, known as psittacosis (27).
Disease stages developed upon infection with Chlamydia are mediated by the immune response, since the organism possesses little intrinsic toxicity (26). Animal models have demonstrated the importance of the immune response in determining the outcome of infections caused by C. trachomatis (30). Being an obligate intracellular bacterium, Chlamydia propagates through a unique biphasic life cycle, which involves the proliferation of the organism at an intracellular site inaccessible to circulating antibodies. Chlamydia-derived peptides do not appear to readily enter the class I or class II antigen presentation pathway (19). This facilitates widespread, persistent, or subclinical infections by Chlamydia. It is estimated that 90% of individuals infected by C. pneumoniae show little or no clinical symptoms, and it has been proposed that chronic chlamydial disease evolves upon an ineffective triggering of the immune system (8, 30). At present, only a few immunodominant Chlamydia-specific proteins have been identified. The major outer membrane protein (MOMP) is considered the primary target of T-cell response in C. trachomatis, and serovar-specific, neutralizing antibodies can be obtained upon immunization of mice with this antigen (28, 29). Being a membrane-spanning porin, parts of the protein are located at the surface of C. trachomatis. Consequently, the humoral immune response is directed towards the variable, surface-exposed domains of this protein. In C. pneumoniae, MOMP does not appear to be surface exposed and consequently it is less immunogenic in infections caused by this pathogen (2, 3). Outer membrane protein 2 (Omp2) is a target of the immune system in both C. trachomatis and C. pneumoniae infections (7, 12, 21, 31). Animal models have identified helper T-cell epitopes in Omp2 (1). The humoral immune response in rabbits towards recombinant Omp2 has identified both genus- and species-specific epitopes (34). The protein is a constituent of the chlamydial outer membrane complex, which can be purified upon disruption of Chlamydia elementary bodies. The topology has not been clarified, but the protein can be extracted from the chlamydial outer membrane complex fraction upon addition of a reducing agent, and no surface exposure of the protein has been detected (5, 34). This indicates that Omp2 resides in the periplasmic lumen, constituting the structural integrity possibly as a chlamydial equivalent of peptidoglycan (13). The present study was devoted to characterization of the humoral immune response to overlapping parts of Omp2 during human Chlamydia infections. Seven truncated Omp2 fusion proteins were generated and affinity purified. A large selection of sera from patients with antibodies to C. trachomatis, C. pneumoniae, or mixed serology were used for truncated Omp2 proteins in an enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Chlamydial strains.
C. pneumoniae VR-1310 was obtained from the American Type Culture Collection, whereas C. trachomatis L2/434/Bu and C. psittaci cal10 were received from The State Serum Institute in Copenhagen, Denmark.
Serum samples.
Sera of group I (positive for both C. pneumoniae and C. trachomatis by microimmunofluorescence [MIF]) were selected from women who were culture positive for C. trachomatis. Group II consisted of sera from normal healthy women who had been screened for antichlamydial antibodies (measured by MIF) and found positive for C. trachomatis only. Sera from patients examined for C. pneumoniae infection or ornithosis where only anti-C. pneumoniae antibodies had been found constituted group III. This group also included 11 sera from apparently healthy donors (asymptomatic patients). Sera without antichlamydial antibodies (measured by MIF) at routine screening constituted the fourth group.
MIF.
Prototype strains were grown in yolk sacs of embryonated hens’ eggs. Semipurified yolk sac material was treated with 0.1% formalin and then used as antigen. C. pneumoniae IOL-207 and C. psittaci 6BC were used. A pool of C. trachomatis serovars D through K was also included. One small dot of each antigen in a group of three was placed on microscope slides. Twelve such antigen clusters in two rows were included on each slide. Different dilutions of patient serum were placed on each antigen cluster. Using a fluorescein-labelled anti-human immunoglobulin G (IgG) conjugate, it was possible to measure antibodies to the different species of Chlamydia. End-point titrations of reactive sera were performed and geometric mean titers were calculated for positive sera. The protocol has previously been described (22).
Sequence analysis.
DNA and protein sequences were analyzed by using programs in the Wisconsin Genetic Computer Group sequence analysis software package (4). Chlamydia Omp2 sequences were obtained through the EMBL-GenBank-DDBJ sequence database and the SwissProt database.
Cloning of gene fragments.
In order to generate gene fragments, oligonucleotides specific for selected regions of the omp2 genes were produced and used as primers for PCR (DNA-Technology, Aarhus, Denmark) (Table 1). To facilitate subsequent cloning of amplified DNA, EcoRI and BamHI restriction endonuclease sites were introduced in primers. PCR with Chlamydia template DNA was done as described by the manufacturer (Boehringer Mannheim GmbH, Mannheim, Germany). The PCR protocol used included 30 cycles (30 s at 94°C, 30 s at 47°C, and 60 s at 72°C). Products were directly ligated into pCR II vector, and recombinant DNA was transformed into INVαF′ Escherichia coli as described (Invitrogen, San Diego, Calif.). Isolation of recombinant plasmid was done by alkaline lysis (25). Positive clones were obtained and control sequenced as described by Hattori and Sakaki (14), by using [α-32P]dATP (Amersham International, Little Chalfont, Buckinghamshire, United Kingdom) and T7 DNA polymerase (Pharmacia, Uppsala, Sweden). To express recombinant proteins, omp2 fragments were moved into the expression vector pEV40 (15, 25). This was performed by using vector and/or primer-defined restriction endonuclease sites (EcoRI and BamHI). Recombinant pEV40 vectors were electrotransformed into E. coli pl248. Plasmid preparation and restriction enzyme digestion were used to detect clones containing omp2 fragments.
TABLE 1.
Oligonucleotides used to generate recombinant fusion proteins of Omp2
| Oligonucleotidesa | pEV40-encoded fusion protein | Sizeb (kDA) |
|---|---|---|
| Sense: 5′-GAATTCAGCGGGGGTATAGAGGC-3′ | C. pneumoniae-Omp2aa23-aa93 | 10.0 |
| Antisense: 5′-GGATCCTACTGAGCCTCTACAGG-3′ | ||
| Sense: 5′-GAATTCAGCGGGAAGATAGAGG-3′ | C. psittaci-Omp2aa23-aa94 | 10.9 |
| Antisense: 5′-GGATCCTATGTAGCGTCTACTGG-3′ | ||
| Sense: 5′-AGCGGGGTGTTAGAGACC-3′ | C. trachomatis-Omp2aa23-aa84 | 9.8 |
| Antisense: 5′-GGATCCTTATTTAGGTCCTGTAGCTTTAG-3′ | ||
| Sense: 5′-CAGGATTCTTGCTTTGGC-3′ | C. trachomatis-Omp2aa87-aa547 | 53.7 |
| Antisense: 5′-CGGATCCTTAATAGATGTGTGTATTCTCTG-3′ | ||
| Sense: 5′-AGCGGGGTGTTAGAGACC-3′ | C. trachomatis-Omp2aa23-aa182 | 20.6 |
| Antisense: 5′-CGGGATCCTTAAGGTTTTACCCATACAG-3′ | ||
| Sense: 5′-CGCTTAGGACAAGGCG-3′ | C. trachomatis-Omp2aa167-aa437 | 32.5 |
| Antisense: 5′-CGGGATCCTTAATGAGTAGCAGCAACTCC-3′ | ||
| Sense: 5′-TGCGCAGAAGCGACAA-3′ | C. trachomatis-Omp2aa420-aa547 | 17.1 |
| Antisense: 5′-CGGATCCTTAATAGATGTGTGTATTCTCTG-3′ |
Underlined sequences are of omp2 origin.
Theoretical size of fusion protein.
Production and purification of fusion proteins.
Generation of fusion proteins from recombinant pEV40-E. coli pl248 is controlled by a heat-labile promoter, and by induction at 42°C, fragments of Omp2 were produced. The pEV40 plasmid carries a nucleotide fragment encoding a hexamer histidine tag at the N-terminal end of the fusion protein, and by affinity chromatography under denaturing conditions using Ni2+-nitrilotriacetic acid resin fusion proteins were purified (Qiagen Inc., Valencia, Calif.). The purifications were done essentially as described by the manufacturer (Qiagen Inc.). Briefly, the recombinant culture was induced (3 h, 42°C), harvested by centrifugation (6,000 × g), and resuspended in a guanidine-hydrochloride buffer (6 M guanidine-HCl, 0.1 M NaH2PO4, 10 mM Tris, 14 mM β-mercaptoethanol [pH 8]). Upon centrifugation, the supernatant was loaded onto a column of Ni2+-nitrilotriacetic acid resin and washed, and by applying a linear pH gradient of urea buffer, the fusion protein was purified and eluted from the column (8 M urea, 0.1 M NaH2PO4, 10 mM Tris, 14 mM β-mercaptoethanol [pH 8 to pH 4.5]). The eluate was neutralized by a one-tenth volume of Tris buffer (1 M, pH 8). Samples of eluates were diluted in sodium dodecyl sulfate (SDS) buffer (125 mM Tris [pH 6.8], glycerol [10%, wt/vol], SDS [2.3%, wt/vol], β-mercaptoethanol [5%, wt/vol]), boiled, and analyzed by SDS-polyacrylamide gel electrophoresis and Coomassie blue staining.
ELISA.
The antigenicities of purified fusion proteins were measured by ELISA by using the selected panel of sera. Prior to the determination of antigenicity, the optimal coating concentration of each protein was determined by using a monoclonal antibody directed against the 6-mer histidine fragments of the antigen. MaxiSorb microtiter plates (Nunc, Roskilde, Denmark) were then coated with 50 μl of recombinant protein per well (2 to 10 μg/ml), suspended in 50 mM carbonate buffer (pH 9.6), and incubated for 2 h. The plates were washed twice in phosphate-buffered saline (PBS) (pH 7.4) containing 0.05% Tween 20 (PBS–T) (Sigma, St. Louis, Mo.), and excess binding capacity was blocked by adding 200 μl of 20% fetal calf serum in PBS–T. In order to absorb any cross-reacting antibodies directed against fetal calf serum the patient sera were diluted in the blocking agent (1/200 and 1/1,000). Antigen-coated microtiter plates and preabsorbed sera were stored overnight (4°C). Plates were then washed four times in PBS–T, and diluted patient sera were added (50 μl/well). Following a 2-h incubation period, plates were washed again, four times in PBS–T. Antigen-directed Ig was detected by incubation with 50 μl of peroxidase-labelled goat anti-human Ig, diluted 1/3,000 in PBS–T (Bio-Rad, Richmond, Calif.). After 1 h of incubation, microtiter plates were washed four times in PBS–T followed by four times in PBS. Fifty microliters of tetramethylbenzidine (Sigma) was added as described by the manufacturer. The color development was terminated after 20 min by adding an equal amount of 1 M HCl. The optical density at 450 nm (OD450) was measured on a Bio-Kinetics Reader by using the KC3 software program (Bio-Tek Instruments, Winooski, Vt.). All procedures were carried out at room temperature (20°C).
Accession numbers.
EMBL-GenBank-DDBJ DNA sequences are m23001, m61116, and x53511 and SwissProt protein sequences are P23700, P23701, P26758, P18151, P23603, P21264, and P18586.
RESULTS
Sequence analysis of Omp2.
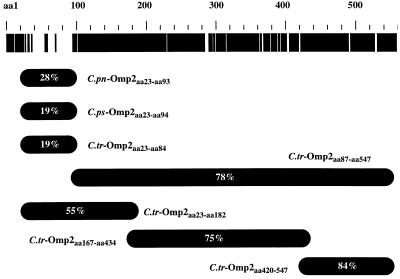
The nucleotide sequence of omp2 has been determined for nine strains (serovars) covering all Chlamydia species (C. trachomatis B, C, E, L1, L2, and L3, C. psittaci eae and 6BC, and C. pneumoniae IOL-207). The nucleotide sequences encode proteins of 547 to 557 amino acids with no homology to proteins of other bacteria. A multiple alignment of Omp2 reveals remarkable conservation of the protein. A graphic representation of the overall Omp2 genus homology among Chlamydia species is seen at the top of Fig. 1. The intraspecies homology is greater than 98%, whereas the homology among chlamydia species is more than 71%, with 85% homology between serovars of C. psittaci and C. pneumoniae. Due to the high intraspecies homology, only C. pneumoniae VR-1310, C. psittaci Cal10, and C. trachomatis L2 were included in this study. The omp2 sequence of C. pneumoniae (VR-1310) is identical to C. pneumoniae (IOL-207), and that of C. psittaci (Cal10) is identical to C. psittaci (6BC). Despite the overall sequence conservation, the N-terminal region (∼70 amino acids) of Omp2 is highly variable among species (33). The genus homology of this region ranges between 19 and 28% (Fig. 1).
FIG. 1.
Homology in the Omp2 protein of Chlamydia pneumoniae (IOL-207), C. psittaci (6BC), and C. trachomatis (L2). Homology among all species is indicated in black in the top band, whereas heterology is indicated in white. Genus homology (%) and the situation of each fusion protein produced are illustrated below. pn, pneumoniae; ps, psittaci; tr, trachomatis.
Generation of truncated Omp2 fusion proteins.
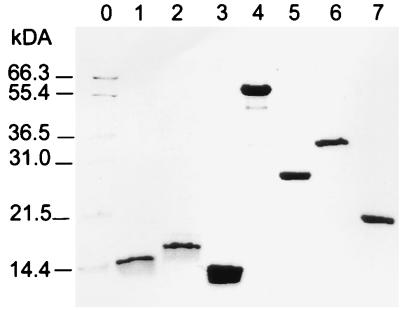
In order to identify immunodominant regions of Omp2 recognized by a selected panel of human sera, we produced truncated Omp2 fusion proteins. Figure 1 shows the Omp2 fragments that were expressed and purified as histidine-tagged proteins using the pEV40 expression vector. The variable region was produced for all three Chlamydia species (C. pneumoniae-Omp2aa23-aa93, C. psittaci-Omp2aa23-aa94 and C. trachomatis-Omp2aa23-aa84). Four other constructs were also generated in order to cover the remaining part of the protein (C. trachomatis-Omp2aa87-aa547, C. trachomatis-Omp2aa23-aa182, C. trachomatis-Omp2aa167-aa434, C. trachomatis-Omp2aa420-aa547). Oligonucleotides were used as primers to amplify the selected regions of the omp2 genes (Table 1). The calculated sizes of the resulting fusion proteins are included. By nickel-affinity chromatography, all seven proteins were purified under denaturing conditions to more than 95% purity. Protein concentrations of 1 to 8 mg/ml were obtained by using this procedure. Figure 2 shows the purified proteins on a Coomassie blue-stained SDS-polyacrylamide gel. The lower-molecular-weight fragments observed in some lanes are presumably proteolytic degradation products. The size of fusion proteins encoding the variable region of Omp2 was seen to deviate from the theoretically predicted size. This is possibly due to the high percentage of charged residues in this region (37 to 43% of amino acid residues). Each purified fusion protein was confirmed immunologically by ELISA with rabbit polyclonal sera directed against purified elementary bodies of C. trachomatis (L2).
FIG. 2.
SDS-polyacrylamide gel electrophoresis of purified Omp2 fusion proteins. Lanes: 1, C. pneumoniae-Omp2aa23-aa93; 2, C. psittaci-Omp2aa23-aa94; 3, C. trachomatis-Omp2aa23-aa84; 4, C. trachomatis-Omp2aa87-aa547; 5, C. trachomatis-Omp2aa23-aa182; 6, C. trachomatis-Omp2aa167-aa434; 7, C. trachomatis-Omp2aa420-aa547. Molecular size markers are indicated to the left (lane 0).
ELISA and MIF.
In order to evaluate the humoral immune response against Omp2, a panel of 78 patients’ sera were tested in duplicate by ELISA. Sera were sorted into four groups, according to serum reactivity measured by MIF (Table 2). When the smaller variable fragments of Omp2 were used as antigen in ELISA, a slightly higher coating concentration was required (∼10 μg/ml) compared to the larger fragments (∼2 μg/ml). Optimal antigen coated microtiter plates were incubated with sera, and bound antibodies were detected by using horseradish peroxidase-conjugated goat anti-human Ig and visualized by using tetramethylbenzidine as the chromogen. Optical densities hereby reported are averaged values of duplicates, with deviations of <10%.
TABLE 2.
Serologic reactivity of sera group I to IV
| Group | No. | MIF
|
C. trachomatisOmp2aa87-aa547
|
||
|---|---|---|---|---|---|
| C. trachomatis | C. pneumoniae | ||||
| + | − | ||||
| I | 19 | POS | POS | 19 (100%) | 0 (0%) |
| II | 20 | POS | NEG | 18 (90%) | 2 (10%) |
| III | 30 | NEG | POS | 24 (80%) | 6 (20%) |
| IV | 9 | NEG | NEG | 0 (0%) | 9 (100%) |
POS, positive; NEG, negative.
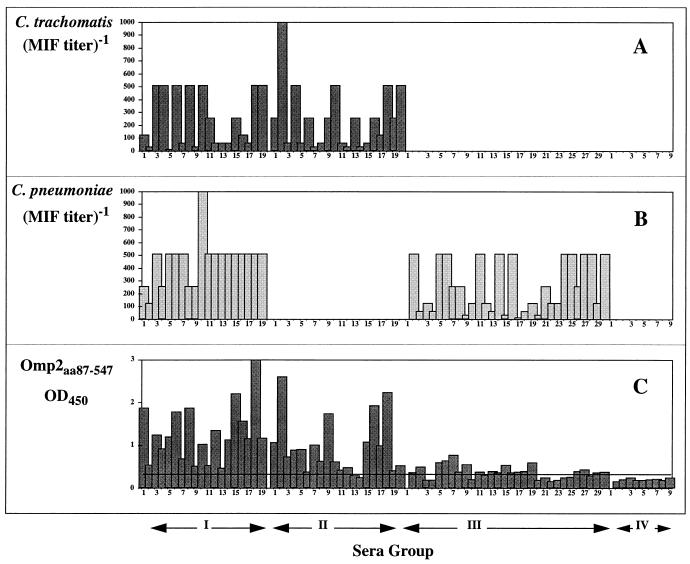
Figure 3 illustrates the serum reactivity towards the large conserved fragment of Omp2, namely C. trachomatis-Omp2aa87-aa547. Furthermore, the figure presents MIF titers obtained using each serum group (lowest positive titer was 16). The Omp2 reactivity of serum group IV (MIF-negative samples) is markedly lower. The mean value obtained in this group is an OD450 of ∼0.20. A positive reacting serum sample was arbitrarily defined by an OD450 greater than 0.25 (125% of the negative mean OD). Using this criterion, 61 of 69 (88%) samples, positively defined by MIF (groups I to III), showed a positive reaction with C. trachomatis-Omp2aa87-aa547. In group IV (negative by MIF serology) no samples were positive by using the above criteria. The statistical significance of these results was confirmed by the χ2 test (P ≪ 0.0001). It is evident that group I (C. trachomatis MIF positive [POS]–C. pneumoniae MIF POS) shows the highest mean titer against C. trachomatis-Omp2aa87-aa547 (OD450, ∼1.28). All sera (19 of 19, 100%) of this group reacted positively with the Omp2 fragment. In group II (C. trachomatis MIF POS–C. pneumoniae MIF NEG) and group III (C. trachomatis MIF NEG–C. pneumoniae MIF POS), serum reactivity was somewhat lower, with a sensitivity of 90 and 80%, respectively. Also the mean OD measured against C. trachomatis-Omp2aa87-aa547 was lower (OD450, ∼0.96 and OD450, ∼0.38). There was no quantitative correlation between MIF titers and antibodies measured against C. trachomatis-Omp2aa87-aa547 (Fig. 1).
FIG. 3.
Serological data representing all four serum groups. Titers obtained using C. trachomatis antigen in microimmunofluorescence are illustrated by bars in panel A and titers of C. pneumoniae-MIF are indicated by bars in panel B. ELISA readings, using C. trachomatis-Omp2aa87-aa547 as the coating antigen, are represented in panel C. Sera were diluted 1/1,000 for the ELISA measurements. Solid lines indicate 125% of the mean OD450 value obtained by group IV (MIF-negative sera).
The serum reactivity towards the remaining species-specific variable region of Omp2 was also tested (C. pneumoniae-Omp2aa23-aa93, C. psittaci-Omp2aa23-aa94, and C. trachomatis-Omp2aa23-aa84). No significant differences in serologic reactivity were observed between serum groups using these antigens (serum dilutions of 1/1,000 and 1/100) (data not shown). The cutoff point for a positive result was arbitrarily defined to be 125% of the negative mean OD450 (group 4). The serum reactivity was poor, with an OD450 of <1.0. Cross-reaction and/or nonspecific reactivity was generally observed using these antigens. It was therefore concluded that this region did not possess immunodominant epitopes, reflected by the lack of specific reactivity in the majority of positive sera defined as (groups I to III).
Finally, we analyzed the immune response against Omp2 in serologically defined C. trachomatis sera (group I and II). We used three fusion proteins constituting total C. trachomatis Omp2 in large overlapping fragments (C. trachomatis-Omp2aa23-aa182, C. trachomatis-Omp2aa167-aa434, C. trachomatis-Omp2aa420-aa547). Results obtained with 20 high-titer sera (OD450 of C. trachomatis-Omp2aa87-aa547 >400% of negative mean OD450) are presented in Fig. 4. The results show that epitopes recognized by human Ig are spread throughout the sequence of Omp2. The N-terminal fragment (C. trachomatis-Omp2aa23-aa182) shows a generally lower antigenicity, which is in accordance with the lack of reactivity when C. trachomatis-Omp2aa23-aa84 is used as the coating antigen.
FIG. 4.
Antibodies towards C. trachomatis-Omp2aa23-aa182 (black bars), C.trachomatis-Omp2aa167-aa434 (grey bars), and C.trachomatis-Omp2aa420-aa547 (white bars) as measured by ELISA with selected patient samples. Serum dilution, 1/1,000.
DISCUSSION
Serology has provided important information regarding the wide range of diseases associated with C. trachomatis, especially infant pneumonia, lymphogranuloma venereum, and chronic genital infections. MOMP, Omp2, GroEL, and lipopolysaccharide (LPS) are the major immunogens in infections caused by C. trachomatis. This paper evaluates the humoral immune response to overlapping parts of Omp2. By using seven truncated fusion proteins of Omp2, the immune response was characterized in 78 sera covering four serologically defined groups. Sera from different patient groups were selected according to MIF results. Thus, sera with antibody titers against both C. pneumoniae and C. trachomatis constituted group I and those with antibodies to either species or without antichlamydial antibodies made up three separate groups (groups II to IV).
The results confirm that Omp2 is a major target in the humoral immune response in infections caused by C. trachomatis. In sera defined by C. trachomatis MIF (serum group I and II), 37 of 39 (95%) samples showed significant antibody titers against C. trachomatis-Omp2aa87-aa547. On the contrary, none of the MIF-negative sera (sera group IV) showed antibodies to this antigen. Previous studies have indicated that the MIF test and the qualitative detection of antibodies to Omp2 were equally sensitive and specific when correlated with culture isolation in patients with C. trachomatis infections (21). The results presented in this paper confirm these results and suggest that antibodies against Omp2 are also a major feature of C. pneumoniae infections. Since MOMP is not a major target of the humoral immune response in infections caused by C. pneumoniae, this is the first major antigen to be identified in C. pneumoniae. In group III (C. pneumoniae MIF-positive sera), 24 of 30 (80%) showed antibodies to C. trachomatis-Omp2aa87-aa547. However, this group of sera did show lower amounts of antibodies to Omp2, but whether this is due to the fact that C. trachomatis derived Omp2 was used in these assays remains unsolved. The results are supported by those of Freidank et al. (7), who found a weaker cross-reactivity towards C. trachomatis 60- to 62-kDa antigen (Omp2, presumably) in sera that were C. pneumoniae-positive by MIF. In a paper by Watson et al. (34) major cross-reacting epitopes recognized by experimentally immunized rabbits were located in the C-terminal region of Omp2 (amino acid 495 to 510). In the present study, we did not detect a greater antigenic cross-reactivity in this region when using C. pneumoniae MIF-positive sera (data not shown). The high homology is constituted by long stretches of nearly identical sequences, which does not indicate a species-specific reaction of this fragment (C. trachomatis-Omp2aa87-aa547) (Fig. 1).
The existence of an interspecies variable amino-terminal fragment in Omp2 could indicate a role of this part of Omp2 as a potential antigen. In order to characterize a species-specific humoral immune response in Chlamydia-positive sera, the variable parts of Omp2 from the three Chlamydia species were used as antigens in ELISA. Our results indicate that this region has a limited immunogenicity and thus is of little differential serodiagnostic use, with no quantitative or qualitative correlation between titers obtained in ELISA and MIF serology. If this region is recognized by the humoral immune response, this may reflect that this part constitutes conformational epitopes not present when denatured, truncated fusion proteins are used. The lack of immunodominant linear epitopes in this variable region of Omp2 is supported by the study of Watson et al. (34). They found no dominant epitopes in this region recognized by antibodies from experimentally immunized rabbits. Antibodies to Omp2 have not been shown to be neutralizing. Therefore, Omp2 alone is not suited as a vaccine candidate, but since it has been shown that Omp2 does contain helper T-cell epitopes, it may be used as an additional vaccine component as suggested by Allen and Stephens (1).
Species-specific serum reactivity towards Chlamydia has previously been studied by Jones et al. (17). They found that peptides constituting the variable segment 1 (VS1) in MOMP were immunodominant in trachoma patients (in agreement with MIF results). MOMP-VS1 has also been shown to be immunodominant in rabbits immunized with C. trachomatis elementary bodies (16). Whereas MOMP-VS1 is a surface-exposed protein fragment in C. trachomatis, the variable part of Omp2 does not seem to be surface exposed in any Chlamydia species (34).
The use of whole-cell Chlamydia organisms and particularly the MIF has long been applied in the species-specific serological detection of Chlamydia infections (32). However, Chlamydia genus-specific LPS induces problems when interpreting results obtained by MIF. C. trachomatis MOMP is immunoreactive in MIF in a species- and serovar-specific manner, but this reaction is hardly distinguishable from the serum reaction towards LPS. With the discovery of the highly prevalent C. pneumoniae, the need for an exact species-specific serological assay has become evident. Contrary to C. trachomatis, the surface antigens responsible for a positive reaction in C. pneumoniae MIF have not been identified. A large retrospective study showed that antibodies to C. pneumoniae account for up to half of all Chlamydia IgG-positive patients attending genitourinary clinics (20).
Sequence-defined serology has successfully been applied in the detection of other bacterial infections, including Borrelia burgdorferi and Mycobacterium leprae (6, 23). The fact that human antibodies bind to conserved regions of Omp2 reduces the specificity of a serological assay with this antigen. Predominantly species-specific antibody and T-cell activity in C. pneumoniae infections has been shown to be directed against noncharacterized proteins of 54 and 94 to 98 kDa (2, 7, 11). The identification of these immunodominant species-specific antigens of C. pneumoniae may enable us to define the importance of this new Chlamydia species in human infections.
ACKNOWLEDGMENTS
This work was supported by EU Grant ERBCHRXCT920040 from the Human Capital and Mobility Program, the Danish Health Research Council (20-3503-1), the Danish Veterinary and Agricultural Research Council (12-1620-1), the Danish Pasteur Association, the University of Aarhus Research Foundation, “Fonden til Lægevidenskabens Fremme,” and “Nationalforeningen til Bekæmpelse af Lungesygdomme.”
We are grateful to Karin Skovgaard Soerensen and Inger Andersen for excellent technical assistance.
REFERENCES
- 1.Allen J E, Stephens R S. An intermolecular mechanism of T cell help for the production of antibodies to bacterial pathogen, Chlamydia trachomatis. Eur J Immunol. 1993;23:1169–1172. doi: 10.1002/eji.1830230529. [DOI] [PubMed] [Google Scholar]
- 2.Campbell L A, Kuo C-C, Wang S-P, Grayston J T. Serological response to Chlamydia pneumoniae infection. J Clin Microbiol. 1990;28:1261–1264. doi: 10.1128/jcm.28.6.1261-1264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiansen G, Østergaard L, Birkelund S. Analysis of the Chlamydia pneumoniae surface. In: Orfila J, et al., editors. Chlamydial infections: proceedings of the Eighth International Symposium on Human Chlamydial Infections. Bologna, Italy: Esculapio; 1994. pp. 173–176. [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett K D, Hatch T P. Architecture of the cell envelope of Chlamydia psittaci 6BC. J Bacteriol. 1995;177:877–882. doi: 10.1128/jb.177.4.877-882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filley E, Thole J E R, Rook G A W, Nagai S, Waters M, Drijfhout J W, Rinke de Wit T F, De Vries R R P, Abou-Zeid C. Identification of an antigenic domain on Mycobacterium leprae protein antigen 85B, which is specifically recognized by antibodies from patients with leprosy. J Infect Dis. 1994;169:162–169. doi: 10.1093/infdis/169.1.162. [DOI] [PubMed] [Google Scholar]
- 7.Freidank H M, Clad A, Herr A S, Wiedmann-Al-Ahmad M, Jung B. Identification of Chlamydia pneumoniae-specific protein antigens in immunoblots. Eur J Clin Microbiol Infect Dis. 1993;12:947–951. doi: 10.1007/BF01992171. [DOI] [PubMed] [Google Scholar]
- 8.Grayston J T, Wang S P, Yeh L J, Kuo C C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 9.Grayston J T, Kuo C C, Wang S P, Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory infections. N Engl J Med. 1986;315:161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- 10.Hahn D L, Dodge W, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult onset asthma. JAMA. 1991;266:225–230. [PubMed] [Google Scholar]
- 11.Halme S, Saikku P, Surcel H M. Characterization of Chlamydia pneumoniae antigens using human T cell clones. Scand J Immunol. 1997;45:378–384. doi: 10.1046/j.1365-3083.1997.d01-413.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanuka N, Glasner M, Sarov I. Detection of IgG and IgA antibodies to Chlamydia trachomatis in sera of patients with chlamydial infection: use of immunoblotting and peroxidase assay. Sex Transm Dis. 1988;15:93–99. doi: 10.1097/00007435-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hatch, T. P. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J. Bacteriol. 178:1–5. [DOI] [PMC free article] [PubMed]
- 14.Hattori M, Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986;152:232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- 15.Hochuli E, Bannwarth W, Döbeli H, Gentz R, Stüber D. Genetic approach to facilitate purification of recombinant protein with a novel metal chelate adsorbent. Bio/Technology. 1988;6:1321–1325. [Google Scholar]
- 16.Jones, H. M., M. Volpicelli, and R. S. Stephens. Immunoassay of polyclonal antibody responses to Chlamydia trachomatis variable segment one and variable segment two major outer membrane protein peptides, p. 109–112. In W. R. Bowie et al. (ed.), Chlamydial infections: proceedings of the Seventh International Symposium on Human Chlamydial Infections. Cambridge University Press, New York, N.Y.
- 17.Jones H M, Schachter J, Stephens R S. Evaluation of the humoral immune response in trachoma to Chlamydia trachomatis major outer membrane proteins by sequence-defined immunoassay. J Infect Dis. 1992;166:915–919. doi: 10.1093/infdis/166.4.915. [DOI] [PubMed] [Google Scholar]
- 18.Kuo C C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maarack P, Kappler J. Subversion of the immune system by pathogens. Cell. 1994;76:323–332. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 20.Moss T R, Darougar S, Woodland R M, Nathan M, Dines R J, Cathrine V. Antibodies to Chlamydia species in patients attending a genitourinary clinic and the impact of antibodies to C. pneumoniae and C. psittaci on the sensitivity and specificity of C. trachomatis serology tests. Sex Transm Dis. 1993;20:61–65. doi: 10.1097/00007435-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Newhall W J, Batteiger B, Jones R B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982;38:1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson K, Treharne J. Diagnosis of infection caused by chlamydia pneumoniae (strain TWAR) in patients with ‘ornithosis’ in southern Sweden 1981–1987. Scand J Infect Dis. 1989;21:675–679. doi: 10.3109/00365548909021697. [DOI] [PubMed] [Google Scholar]
- 23.Robinson J M, Pilot-Matias T J, Pratt S D, Patel C B, Bevirt T S, Hunt J C. Analysis of the humoral response to the flagellin protein of Borrelia burgdorferi: cloning of regions capable of differentiating Lyme disease from syphilis. J Clin Microbiol. 1993;31:629–635. doi: 10.1128/jcm.31.3.629-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Serological evidence of an association of novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;ii:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schachter J, Caldwell H D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- 27.Schachter J. The intracellular life of Chlamydia. Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- 28.Stag A J, Elsley W A J, Pickett M A, Ward M E, Knight S C. Primary human T-cell responses to the major outer membrane protein of Chlamydia trachomatis. Immunology. 1993;79:1–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Su H, Caldwell H D. In vitro neutralization of Chlamydia trachomatis by monovalent Fab antibody specific to the major outer membrane protein. Infect Immun. 1991;59:2843–2845. doi: 10.1128/iai.59.8.2843-2845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor H R, Johnson S L, Schachter J, Caldwell H D, Prendergast R A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987;138:3023–3027. [PubMed] [Google Scholar]
- 31.Wagar E A, Schachter J, Bavoil P, Stephens R S. Differential human serologic response to two 60.000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990;162:922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- 32.Wang S P, Grayston J T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970;70:367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- 33.Watson M W, Lambden P R, Clark I N. Genetic diversity and identification of human infection by amplification of the chlamydial 60-kilodalton cysteine-rich outer membrane protein gene. J Clin Microbiol. 1991;29:1188–1193. doi: 10.1128/jcm.29.6.1188-1193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson M W, Lambden P R, Everson J S, Clarke I N. Immunoreactivity of the 60 kDa cysteine-rich proteins of Chlamydia trachomatis, Chlamydia psittaci and Chlamydia pneumoniae expressed in Escherichia coli. Microbiology. 1994;140:2003–2011. doi: 10.1099/13500872-140-8-2003. [DOI] [PubMed] [Google Scholar]