Abstract
Low concentrations of mannose-binding protein (MBP; also known as mannose-binding lectin) are associated with common opsonic defect in immunodeficient children. We compared the concentrations of MBP in the sera of 47 adults with non-human immunodeficiency virus-related recurrent infections (group I) and 50 healthy adult controls. Mean serum MBP concentrations in the patient group did not differ significantly from those in the control group (P < 0.4). Nevertheless, the proportion of individuals with less than 5 ng of serum MBP per ml was significantly larger in the patient group (21%, P = 0.01) than in the control group (4%). Group II consisted of 73 pediatric and 56 adult patients with recurrent infections. Pediatric patients had significantly lower mean concentrations of serum MBP than their controls (P < 0.005), and there was no significant difference between the concentrations in sera of adult patients and adult controls (P < 0.4). Again, the proportion of individuals with less than 5 ng of serum MBP per ml was significantly larger in both pediatric (22%, P = 0.045) and adult (38%, P = 0.000016) patients than in their respective controls (4%). Our results demonstrate that, as in children, low concentrations of serum MBP can be associated with recurrent infections in adults.
Human mannose-binding protein (MBP) is a calcium-dependent lectin secreted by the liver as an acute-phase protein which plays an important role in innate immunity (16). By binding to mannose or N-acetylglucosamine on the surfaces of various pathogens, MBP mediates opsonization and subsequent phagocytosis (4). MBP can also associate with serine proteases (MASP-1 and MASP-2) to activate the third complement activation pathway independent of antibody or C1q (7, 14).
The low concentrations of MBP in human serum associated with a common defect in opsonization (12) are characterized by three different point mutations in one of the coding regions of the MBP gene with resultant amino acid changes in the collagen-like region of the molecule (5, 6, 10). Individuals homozygous or heterozygous for one or a combination of any of the three mutations are at risk for developing recurrent infections, not only early in life before development of their own antibody responses (12, 17) but also as older children and adults (2, 11).
Recent discoveries of an association between deficiencies in MBP and several diseases, such as systemic lupus erythematosus (1), recurrent spontaneous abortion (3), hepatitis B (15), and tuberculosis (9), implicate MBP variance as one of the genetic susceptibility factors in various infections (16). The present study was undertaken to ascertain whether there is an association between deficiencies in MBP and suspected immunodeficiency in adult patients.
MATERIALS AND METHODS
Patients.
Patients’ specimens were remnants of serum samples sent to Specialty Laboratories for routine clinical testing and were from two different groups. Group I consisted of 47 adult patients (33 females, ages 29 to 86, and 14 males, ages 24 to 72) with non-human immunodeficiency virus (HIV)-related recurrent infections and a history of repeated hospitalization. Group II consisted of 73 pediatric patients (30 females and 43 males, ages <19 years) and 56 adult patients (35 females and 21 males, ages >19 years) with recurrent infections whose sera had been sent for a humoral immune response survey following pneumococcal vaccination.
Control subjects.
Group III (the control group) included 23 healthy pediatric subjects and 50 healthy adult subjects. Sera from the healthy pediatric subjects (ages <19 years) were remnant samples from a previous study on Bartonella-HIV encephalopathy conducted in collaboration with Benjamin Estrada, New Orleans, La. Sera from the adult subjects were obtained from volunteers who were employed at Specialty Laboratories. All the adult serum donors were remunerated.
Purification of human MBP.
Human MBP was purified from pooled human AB serum (American Qualex, San Clemente, Calif.) as described earlier (13). Briefly, 500 ml of pooled human AB serum was dialyzed extensively against loading buffer containing 20 mM Tris (pH 7.4), 0.15 M NaCl, 1.0 mM CaCl2, and 0.05% NaN3. Following dialysis, the serum was loaded on a mannan-agarose column (Sigma Chemical Co., St. Louis, Mo.) which was equilibrated with loading buffer. The loaded column was washed extensively with loading buffer, and the bound protein was eluted with a solution of 20 mM Tris (pH 7.4), 0.15 M NaCl, 0.01 M EDTA, and 0.05% NaN3. The fractions with higher absorbances (280 nm) were pooled, and the serum amyloid P component was removed by CaCl2 precipitation. Following concentration by ultrafiltration (filter from Amicon), the presence of MBPs was confirmed by Western blotting with mouse monoclonal antibodies to human MBP (Harlan Bioproducts for Science, Inc., Indianapolis, Ind.). Protein concentrations were determined with the Bio-Rad protein assay reagent with bovine serum albumin as the standard.
Generation of rabbit antibodies to human MBP.
One milligram of purified human MBP was mixed with 1 ml of Titermax (Sigma Chemical Co.) and injected subcutaneously into three rabbits (Universal Animal Care, Bloomington, Calif.). Three weeks following immunization, the rabbits were bled for sera. The sera were tested for MBP-specific antibodies by Western blotting. The immunoglobulin G fraction of the MBP-specific antibodies was purified from the serum with a high titer by using a protein A-Sepharose column (13).
Assay for MBP.
Serum MBP concentrations were determined by a sandwich enzyme-linked immunosorbent assay (sandwich ELISA) as described earlier (13). Briefly, Immulon 2 ELISA plates (Dynatech Laboratories, Inc., Chantilly, Va.) were coated overnight at 4°C with 100 μl of purified rabbit anti-human MBP immunoglobulin G per well at a concentration of 1 μg/ml in carbonate buffer (50 mM Na2CO3, pH 9.6). The following morning, the plates were washed three times with washing solution (phosphate-buffered saline with 0.05% Tween 20 [PBS-T]) and blocked with 1% bovine serum albumin in PBS-T for 1 h at room temperature (RT). One-hundred-microliter aliquots of serum samples or standards per well were added to the antibody-coated plates and incubated overnight at 4°C. Different concentrations of MBP were used as standards in twofold dilutions. Following incubation, the plates were washed as before and 100 μl of biotinylated anti-MBP mouse monoclonal antibody (0.5 μg/ml) was added per well. The plates were incubated at RT for 2 h. At the end of the incubation, the plates were washed, 100 μl of 1:2,000-diluted alkaline phosphatase-conjugated streptavidin (Boehringer Mannheim Corporation, Indianapolis, Ind.) was added to each well, and the plates were incubated at RT for 1 h. Following incubation, the plates were washed and 150 μl of the substrate, p-nitrophenyl phosphate (1 mg/ml) (Sigma Chemical Co.), was added to all the wells. The plates were kept in the dark for 30 min and then read in an ELISA reader. Data were analyzed by using a four-parameter standard curve (Softmax software; Molecular Devices, Sunnyvale, Calif.).
Reference range.
The cutoff value of 5 ng of serum MBP per ml was chosen on the basis of the earlier report that individuals with homozygosity of abnormal MBP alleles had <10 ng of serum MBP per ml (10) and that homozygosity for MBP mutant alleles predisposes individuals to recurrent infections (2).
Statistical analysis.
Student’s t test and Fisher’s exact test were employed to evaluate differences between control and patient groups.
RESULTS
MBP concentrations in adult patients with recurrent infections.
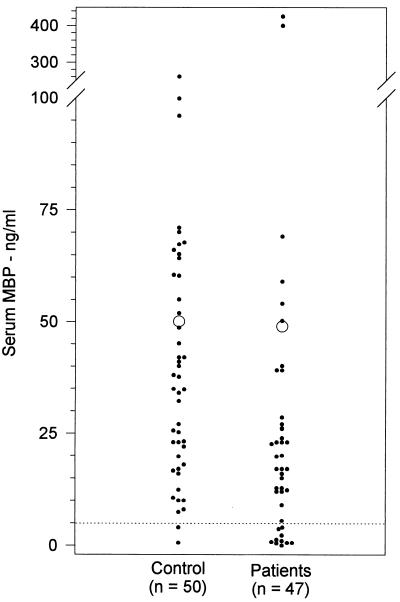
The serum samples from 47 adults with non-HIV-related recurrent infections (group I) were screened for MBP concentrations by ELISA and compared with those from 50 healthy adults. The mean concentration of MBP in sera of the patient group (48.9 ± 12 ng/ml) did not differ significantly from that in sera of the control individuals (50.0 ± 7 ng/ml, P < 0.4) (Fig. 1). Patients’ serum samples had a median MBP concentration of 20 ng/ml (with a range of 0 to 425 ng/ml), while the controls’ serum samples had a median MBP concentration of 38 ng/ml (with a range of 0 to 360 ng/ml). However, the number of individuals with less than 5 ng of MBP per ml in their serum was significantly larger (10 of 47 [21%], P = 0.01) than the number in the control group (2 of 50 [4%]).
FIG. 1.
Serum MBP concentrations in adult patients with recurrent infections. MBP concentrations were determined by the enzyme immunoassay method as described in Materials and Methods. Open circles represent the mean values, and the dotted line indicates the cutoff value.
MBP concentrations in pediatric and adult patients with recurrent infections.
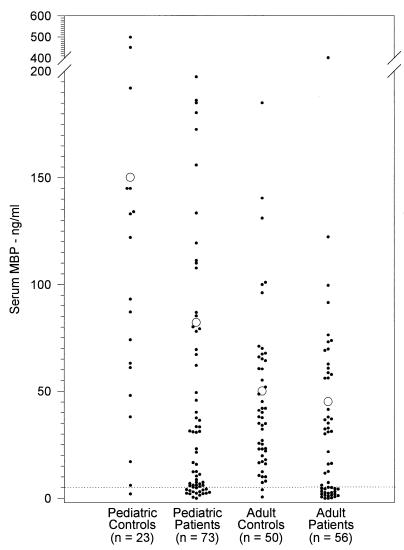
We also measured serum MBP concentrations in 73 pediatric and 56 adult patients with recurrent infections (group II). The sera from pediatric patients had a significantly lower mean concentration of MBP (78.9 ± 13 ng/ml; median, 31 ng/ml, with a range of 0 to 199 ng/ml) than sera from controls (150.2 ± 30 ng/ml, P < 0.005; median, 122 ng/ml, with a range of 0 to 499 ng/ml) (Fig. 2). However, the mean concentration of MBP in the sera of pediatric patients was significantly higher than that in the sera of adult patients (45.2 ± 10 ng/ml, P < 0.025). In contrast, there was no significant difference between the mean serum MBP concentrations in adult patients and those in controls (50.0 ± 7 ng/ml, P < 0.4).
FIG. 2.
Serum MBP concentrations in pediatric and adult patients with recurrent infections. Serum samples from pediatric and adult patients with suspected immunodeficiency were screened for MBP as described in Materials and Methods. Open circles represent the mean values, and the dotted line indicates the cutoff value.
Sixteen of 73 (22%, P = 0.045) pediatric immunodeficiency patients had less than 5 ng of serum MBP per ml, compared to 1 of 23 (4%) healthy children. Similarly, 21 of 56 adult immunodeficiency patients (38%, P = 0.000016) and 4% of healthy adult controls had less than 5 ng of MBP per ml of their serum.
DISCUSSION
The mean concentration of MBP in the sera of adults with non-HIV-related recurrent infections did not differ from that in the sera of healthy control individuals. In a childhood syndrome, recurrent infections, failure to thrive, and chronic diarrhea can be associated with low levels or the absence of MBP (12). Because low serum MBP concentrations can result in a common opsonic defect, MBP is thought to play a critical role during infancy, especially before the development of the antibody repertoire (12, 17). Identification of the homozygous phenotype caused by two mutations of the MBP gene (at codons 54 and 52) in an adult immunodeficiency patient suggests that MBP confers a lifelong risk of infection on both children and adults (11).
This paper is the first comprehensive report that the average concentrations of MBP in serum are similar in adult patients and healthy adult subjects. However, an absence or lower concentrations of serum MBP (less than 5 ng/ml) were present in 21% of the adults with recurrent infections. This significant increase in frequency might reflect a higher incidence of the homozygous mutations in MBP genes in adult patients (9). The proportion of patients with lower concentrations of serum MBP (<5 ng/ml) is similar to that in earlier reports regarding patients with suspected immunodeficiency (2). Further analysis of these patients by genotypic investigation for various mutations will be required to confirm these results.
Group II included both pediatric and adult patients with suspected immunodeficiency. The children had significantly higher concentrations of serum MBP than adult patients, which is in agreement with earlier reports (13). Interestingly, in contrast to adult patients, pediatric patients had a significantly lower mean concentration of serum MBP than did controls. This may be due to the possible increase in heterozygous mutations in the patients, as reported earlier (9). The serum MBP concentrations in adult patients were not significantly lower than those in control subjects, as in group I. On the other hand, the number of patients in group II with less than 5 ng of MBP per ml of serum is significantly higher than in group III. Again, this may be due to the increase in homozygous mutations in the groups of patients, as previously reported (9).
Alternatively, the increase in the proportion of patients with less than 5 ng of MBP per ml of serum may be due to the clearance of serum MBP during an infection in which it is involved. However, studies on the levels of circulating MBP during HIV infection showed a higher level of MBP in HIV-seropositive patients than in members of the control group (8). Therefore, it is possible that the absence of MBP in a significant number of our patients may not be due to the clearance of MBP during infection. Thus, our results, along with the recent discovery of MBP mutations in patients with infectious diseases, such as tuberculosis (9) and hepatitis B (15), are consistent with a lifelong increased risk of infections in some patients with low levels or an absence of serum MBP.
ACKNOWLEDGMENTS
We thank Ashu Kumar, Foaad Hanna, and Narsis Bhasharkhah for excellent technical assistance.
REFERENCES
- 1.Davies E J, Snowden N, Hillarby M C, Carthy D, Grennan D M, Thomson W, Ollier W E R. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 1995;38:110–114. doi: 10.1002/art.1780380117. [DOI] [PubMed] [Google Scholar]
- 2.Garred P, Madsen H O, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunedeficiency. Lancet. 1995;346:941–943. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick D C, Beven B H, Liston W A. Association between mannan binding protein deficiency and recurrent miscarriage. Hum Reprod. 1995;10:2501–2505. doi: 10.1093/oxfordjournals.humrep.a136330. [DOI] [PubMed] [Google Scholar]
- 4.Kuhlman M, Joiner K, Ezekowitz R A. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–1745. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipscombe R J, Sumiya M, Hill A V S, Lau Y L, Levinsky R J, Summerfield J A, Turner M W. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–715. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 6.Madson H O, Garred P, Kurtzhals A L, Lamm L U, Ryder L P, Thiel S, Svejgaard A. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 7.Matsushita M, Fujita T. Activation of the classical complement pathway by mannose-binding protein in association with a novel C1s-like serine protease. J Exp Med. 1992;176:1497–1502. doi: 10.1084/jem.176.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senaldi G, Davies E T, Mahalingam M, Lu J, Pozniak A, Peakman M, Reid K B, Vergani D. Circulating levels of mannose binding protein in human immunodeficiency virus infection. J Infect. 1995;31:145–148. doi: 10.1016/s0163-4453(95)92185-0. [DOI] [PubMed] [Google Scholar]
- 9.Sumiya M, Summerfield J A. Mannose-binding protein, genetic variants and the risk of infection. Q J Med. 1996;89:723–726. doi: 10.1093/qjmed/89.10.723. [DOI] [PubMed] [Google Scholar]
- 10.Sumiya M, Super M, Tabona P, Levinsky R J, Arai T, Turner M W, Summerfield J A. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–1570. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 11.Summerfield J A, Ryder S, Sumiya M, Thursz M, Gorchein A, Monteil M A, Turner M W. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–889. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 12.Super M, Thiel S, Lu J, Levinsky R J, Turner M W. Association of low levels of mannan-binding protein with a common defect in opsonisation. Lancet. 1989;2:1236–1239. doi: 10.1016/s0140-6736(89)91849-7. [DOI] [PubMed] [Google Scholar]
- 13.Terai I, Kobayashi K, Fujita T, Hagiwara K. Human serum mannose binding protein (MBP): development of an enzyme-linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem Med Metab Biol. 1993;50:111–119. doi: 10.1006/bmmb.1993.1052. [DOI] [PubMed] [Google Scholar]
- 14.Thiel S, Vorup-Jensen T, Stover C M, Schwaeble W, Laursen S B, Poulsen K, Willis A C, Eggleton P, Hansen S, Holmskov U, Reid K B M, Jensenius J C. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–510. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 15.Thomas H C, Foster G R, Sumiya M, McIntosh D, Jack D L, Turner M W, Summerfield J A. Mutation of gene for mannose-binding protein associated with chronic hepatitis B viral infection. Lancet. 1996;348:1417–1419. doi: 10.1016/s0140-6736(96)05409-8. [DOI] [PubMed] [Google Scholar]
- 16.Turner M W. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–540. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 17.Turner, M. W., M. Super, S. Singh, and R. J. Levinsky. 1991. Molecular basis of a common opsonic defect. Clin. Exp. Allergy 21(Suppl. 1):182–188. [DOI] [PubMed]