Abstract
Simple Summary
Understanding the molecular mechanisms involved in feather color formation is important because coloration is one of the most recognizable characteristics in chickens. In this study, after transcrip-tome sequencing of the wing and neck feather follicle tissues of chickens with different plumage colors, we retrieved differentially expressed genes (DEGs) with the same trends in both the wing and neck and then identified DEGs that may be associated with melanin deposition through GO and KEGG annotation and PPI analysis. Finally, we verified that two genes in chicken melanocytes, SLC45A2 and GPNMB, promote melanocyte melanin deposition in chickens.
Abstract
As an essential genetic and economic trait, chicken feather color has long been an important research topic. To further understand the mechanism of melanin deposition associated with coloration in chicken feathers, we selected feather follicle tissues from the neck and wings of chickens with differently colored feathers (yellow, sub-Columbian, and silver) for transcriptome analysis. We focused on genes that were expressed in both the wings and neck and were expressed with the same trends in breeds with two different plumage colors, specifically, SLC45A2, GPNMB, MLPH, TYR, KIT, WNT11, and FZD1. GO and KEGG enrichment analyses showed the DEGs were enriched in melanin-related pathways, such as tyrosine metabolic pathway and melanogenesis, and PPI analysis highlighted the genes SLC45A2 and GPNMB as associated with melanin deposition. Verification experiments in chicken melanocytes demonstrated that these two genes promote melanocyte melanin deposition. These data enrich our knowledge of the mechanisms that regulate chicken feather color.
Keywords: transcriptome, melanin, melanocytes, SLC45A2, GPNMB
1. Introduction
There are a variety of color combinations and patterns in chicken plumage, making it one of the most colorful terrestrial vertebrates; therefore, these color variations have attracted interest from researchers. In addition to attracting individuals of the opposite sex, feather color helps keep chickens away from predators [1]. In addition, the color of the plumage affects the first impression consumers have when buying chickens, and so plumage color is considered an important economic trait in the industry.
The variation in plumage colors is attributed to the distribution and deposition of melanin, including eumelanin (gray or black) and pheomelanin (yellow or red), produced within melanosomes in melanocytes, and the presence of structural colors resulting from the reflection, refraction, and scattering of light within the feathers [2,3]. Studies have shown that the biosynthesis process of melanin is a series of biochemical reactions initiated by tyrosinase catalyzing tyrosine hydroxylation in the body [4]. After tyrosinase synthesis, it enters the melanosome from the Golgi apparatus, and the active transport of the membrane sends tyrosine into melanocytes and begins to synthesize melanin [5].
The deposition of melanin in chicken feathers is more affected by genetic factors than by environmental factors [6]. With the development of modern technology and the efforts of scientists, more and more causative mutations that control chicken feather color genes have been located, such as the dominant white (I), recessive white (c), spots (mo), barring (B), dark brown (DB), extended black (E), Columbia, and others [7,8,9,10,11,12]. However, the deposition of melanin pigmentation in chicken feathers is not limited to gene polymorphisms, but also influenced by the expression of various genes related to pigment formation in feather follicles during the growth period [13,14,15,16]. The transcriptome sequencing procedure is an efficient method for identifying genes that are DEGs. In recent years, several mRNAs have been shown to affect the deposition of melanin in chickens through transcriptome sequencing analysis. For example, by transcriptome sequencing of the breast muscle tissue of Xichuan black-bone chickens of the tyrosine-added group and the normal feeding group, dopachrome tautomerase (DCT) and endothelin receptor B subtype 2 (EDNRB2) were screened for effects on the deposition of eumelanin [17]. Using transcriptome sequencing to study genes related to eyelid color in chickens, it was found that premelanosome protein (PMEL), DCT, and tyrosinase (TYR) were expressed much more strongly in black eyelids than in light yellow eyelids (p < 0.05), and these coding proteins positively regulate melanin deposition [18]. RNA sequencing technology was used to study the expression profiles of mRNA in black and white back skin tissues of Muchuan black-bone chicken, and a total of eight melanin deposition-related genes: KIT proto-oncogene receptor tyrosine kinase (KIT), tyrosinase-related protein 1 (TYRP1), DCT, solute carrier family 45 member 2 (SLC45A2), OCA2 melanosomal transmembrane protein (OCA2), EDNRB2, transient receptor potential cation channel subfamily M member 1 (TRPM1), and RAB38 were identified [19]. There have also been some studies that have been conducted by transcriptome analysis of the feather follicle tissue of chickens to screen for the deposition of melanin pigmentation in chicken feathers. For example, transcriptome sequencing of feather follicles of yellow and white feathers was performed to identify the differentially expressed genes TYRP1, DCT, PMEL, melan-A (MLANA), and hematopoietic prostaglandin D synthase (HPGDS) associated with pheomelanin deposition [20]. High-throughput sequencing technology was used to compare the differences in the transcriptome of black and white chicken feather bulbs, revealing the differential genes homeobox B9 (HOXB9), homeobox C8 (HOXC8), homeobox A9 (HOXA9), ChaC glutathione specific gamma-glutamylcyclotransferase 1 (CHAC1), and glutathione peroxidase 3 (GPX3) involved in the melanin production of black-bone chicken feathers [21]. Sub-Columbian chicken dorsal neck black and white striped feather follicles and abdominal neck white feather follicles were selected for transcriptome sequencing, and it was found that mediator complex subunit 23 (MED23) and G protein subunit alpha q (GNAQ) played a crucial role in melanin deposition [22]. However, the formation of feather color in many chicken breeds is the result of a combination of eumelanin and pheomelanin, so it is necessary to study the change in total melanin. At the same time, few transcriptome-screened genes are functionally verified in chicken melanocytes.
The “Yufen I” hybrid lines of Chinese commercial laying hens, developed by Henan Agricultural University, consist of three lines. Among these, the H line exhibits predominantly white plumage with black bars on the hackles, primaries, secondaries, and tail. This coloration closely resembles the Columbian plumage pattern, except that it features barring instead of black vertical striping [23]. Hence, we call this plumage coloration “sub-Columbian” (Figure 1A). In this experiment, we collected the follicle tissue of the wing and neck of chickens with three different plumage colors: yellow feathers (Y group) (Figure 1B), sub-Columbian feathers (H group), and silver feathers (S group) (Figure 1C). Then, we identified SLC45A2 and glycoprotein nmb (GPNMB) from the differentially expressed genes revealed by transcriptome sequencing. By constructing an overexpression vector, we confirmed that these two genes promote the deposition of melanin in chicken melanocytes. Overall, our study provided a theoretical basis for the molecular mechanism underlying the formation of chicken feather color and revealed the roles of SLC45A2 and GPNMB in regulation of melanin deposition during feather color growth.
Figure 1.
The feather colors of different colored hens, as well as their neck and wing feathers. (A) line chicken, sub-Columbian feathers: almost white with black barring only in the hackles, primary and secondary feathers, and tail; (B) Gushi chicken, yellow feathers: yellow with black spots all over the body feathers; (C) Hy-Line chicken, silver feathers: pure white feathers all over the body.
2. Materials and Methods
2.1. Ethics Statement
In accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Henan Agricultural University, China (Permit Number: 11-0085; date: 13 June 2011), all sample collection and treatment procedures were carried out with strict adherence to ethical guidelines to ensure animal welfare and minimize suffering.
2.2. Laboratory Animal Samples and Collection
Hens with different plumage colors were selected as experimental subjects (H line chicken: sub-Columbian feathers; Gushi chicken: yellow feathers; Hy-Line chicken: silver feathers). Chickens were housed and raised in our laboratory using standard methods. The tissues were categorized into six groups: HN (sub-Columbian plumage nuchal follicle tissue), HW (sub-Columbian plumage wing follicle tissue), YN (yellow plumage nuchal follicle tissue), YW (yellow plumage wing follicle tissue), SN (white plumage nuchal follicle tissue), and SW (white plumage wing follicle tissue). Each group consisted of three biological replicates, and each replicate contained three follicle tissue samples.
Nine 5-week-old hens were selected for each group, and nuchal follicle tissue and wing follicle tissue were collected immediately. First, the surface of the feather was cleaned with 75% alcohol cotton, the feather root was clamped with tweezers, and the feather follicles were quickly removed; then, another small forceps was used to separate the feather follicle tissue at the root of the feather. The feather was cleaned with PBS, quickly placed in a cryopreservation tube, and finally frozen in a liquid nitrogen tank.
2.3. RNA Extraction, Library Construction, and High-Throughput Sequencing
RNA was extracted from the follicles was performed using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). The RNA quantity and quality were assessed through 1% agarose gel electrophoresis. Eighteen RNA sequence libraries were constructed—HW1, HW2, HW3, HN1, HN2, HN3, YW1, YW2, YW3, YN1, YN2, YN3, SW1, SW2, SW3, SN1, SN2, and SN3. The sequencing libraries for the NEBNext® UltraTM Directional RNA Library Prep KIT (New England Biolabs, Ipswich, MA, USA) were generated on the Illumina platform. Initially, the raw data in fastq format were processed using an in-house Perl script. During this step, the raw data were filtered to obtain clean reads by excluding those containing adapters, poly-N sequences, and low-quality reads. The clean reads were mapped to the Gallus_gallus-6.0 genome (http://ftp.ensembl.org/pub/release-96/fasta/gallus_gallus/dna/) URL (accessed on 11 March 2019) using HISAT2 [24], and the expression levels of each gene were calculated using fragments per kilobase of transcript per million mapped fragments (FPKM) [25]. The formula is shown as follows: FPKM= [25]. Differential expression analysis of the 6 groups was performed using DESeq2 [4]. To control the false discovery rate (FDR), only genes with p adjusted < 0.05 were considered differentially expressed.
To screen for alternative splicing events of DEGs associated with melanin deposition, equal amounts of RNA were collected from the nuchal and wing of sub-Colombian and yellow feathers into four mixing pools: the HN group, HW group, YN group, and YW group. The four libraries were then analyzed for full-length transcriptomes using the PacBio Iso-seq platform.
2.4. Quantitative Real-Time PCR (qRT–PCR)
For qPCR analysis, nine DEGs were chosen, including collagen type I alpha 1 chain (COL1A1), aquaporin 1 (AQP1), cysteine rich angiogenic inducer 61 (CYR61), regucalcin (RGN), keratin 23 (KRT23), SLC45A2, GPNMB, InaF motif containing 2 (INAFM2), and NFKB inhibitor epsilon (NFKBIE). Three biological replicates and three technical replicates were performed for each group. The gene expression levels were measured using quantitative real-time PCR (qRT-PCR) with SYBR Premix Ex TaqTM II (TaKaRa) on a LightCycler 96 Instrument (Roche Applied Science, Indianapolis, IN, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal reference gene. Primers were designed using Oligo 6.0 software. The primer sequences can be found in Supplementary Materials Table S1.
2.5. GO and KEGG Enrichment Analysis
Gene Ontology (GO) enrichment analysis of the DEGs was conducted with the GOseq R package (https://www.rdocumentation.org/packages/goseq/versions/1.24.0/topics/goseq, accessed on 23 June 2023). This analysis employed Wallenius noncentral hypergeometric distribution and adjusted for length bias in the DEGs [26]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database resource that provides insights into the advanced functions and utilities of biological systems according to the pathways to which they belong (http://www.genome.jp/kegg/). To evaluate the statistical enrichment of DEGs in KEGG pathways, we utilized the KOBAS software. Pathways with q values < 0.05 for either GO or KEGG were regarded as significantly enriched.
2.6. Protein–Protein Interaction Analysis
To examine the interactive connections between DEGs, we obtained protein–protein interaction (PPI) data for the DEGs from the STRING database (http://string-db.org, Organism: Chicken). Subsequently, the DEG PPI network was constructed and visualized using Cytoscape version 3.6.1 (http://www.cytoscape.org/).
2.7. Plasmid Construction
To construct an overexpression plasmid for the SLC45A2 and GPNMB genes, the coding DNA sequences (CDSs) of the SLC45A2 and GPNMB genes were amplified from H-line chicken feather follicle cDNA by PCR using gene-specific homologous recombinant clone primers (Supplementary Materials Table S1).
Subsequently, the coding DNA sequence (CDS) was inserted into the pcDNA3.1-EGFP vector (Invitrogen, Carlsbad, CA, USA) through homologous recombination, resulting in the creation of pcDNA3.1-SLC45A2-EGFP. The accuracy of pcDNA3.1-SLC45A2-EGFP construction was confirmed by both agarose gel electrophoresis and sequencing. The procedure for constructing pcDNA3.1-GPNMB-EGFP followed the same method as that for pcDNA3.1-SLC45A2-EGFP.
2.8. Cell Culture, Identification, and Transfection
Melanocytes were isolated from the peritoneum of 20-day-old Xichuan black-bone chickens. The peritoneum tissue was washed multiple times with phosphate-buffered saline (PBS) containing penicillin and streptomycin. Next, the tissue was cut into pieces and digested with dispase II (Roche, China) and trypsin-EDTA (Gibco, Waltham, MA, USA) at 37 °C for 1 h with gentle shaking every 15 min. To stop the digestion, Medium 254 (Gibco, Waltham, MA, USA) supplemented with 1% Human Melanocyte Growth Supplement (HMGS; Gibco; Waltham, MA, USA) was added at a 1:3 ratio. The resulting cell suspension was then filtered through screens of varying mesh sizes (100, 200, and 500). Next, the filtered suspension was centrifuged at 1000 rpm for 5 min. The obtained cell pellet was resuspended to obtain melanocytes. These melanocytes were cultured at 37 °C and 5% CO2 [27].
Immunofluorescence analysis was used to confirm that isolated primary cells were melanocytes. Primary cells were fixed with 4% paraformaldehyde for 10 min and then permeabilized with 0.1% Triton X-100 for 1 h, followed by 3 washes with PBS. Then, 3% bovine serum albumin (BSA) was added for 1 h to block nonspecific binding, and melanocyte inducing transcription factor-melanocyte inducing transcription factor (MITF; 1:200, ab122982) (Abcam, Cambridge, UK) was added and incubated at 4 °C for 16 h, followed by 3 washes with PBS. The secondary antibodies used for staining were goat anti-mouse IgG conjugated to Alexa® Fluor 488 (ab150078; Abcam) and goat anti-rabbit IgG conjugated to Alexa Fluor 555 (ab150117; Abcam). The samples were then incubated with the secondary antibody for 1 h in the dark and washed 3 times with PBS. The cells were incubated with DAPI (Abcam) for 10 min at room temperature in the dark and quickly washed 3 times with PBS to stain the nuclei. Finally, the cells were observed under an inverted fluorescence microscope.
Transient transfections were carried out using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and the cells were collected after 48 h of incubation.
2.9. Western Blotting (WB)
The proteins were extracted from the cells and analyzed by western blotting. The protein expression of MITF and GAPDH was examined through western blot analysis. Primary antibodies, including anti-MITF (MITF; 1:1000, bs-1990R) (Bioss, Beijing, China) and anti-GAPDH (GAPDH; 1:10,000; AF7021) (Affinity, West Bridgeford, UK), were used in this study.
2.10. Tyrosinase and Melanin Content Measurements
After transfection for 48 h, the melanocytes were washed twice with ice-cold PBS, digested with trypsin and centrifuged for 10 min to obtain the pellet. Then, intracellular tyrosinase was measured by enzyme-linked immunosorbent assay (ELISA) using a Tyrosinase ELISA Assay KIT (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. Melanin content was quantitated using the Amplite™ Fluorimetric Melanin Assay Kit (ATT Bioquest, CA, USA).
2.11. Statistical Analyses
Statistical analysis was conducted using SPSS Statistics 27 to assess differences among groups. One-way ANOVA was performed, and significance levels were indicated by * (p < 0.05) and ** (p < 0.01). The data are presented as the mean ± SEM (standard error of the mean).
3. Results
3.1. Transcriptome Sequencing and Quality Control
Eighteen libraries were sequenced on an Illumina HiSeq platform. As shown in Supplementary Materials Table S2, clean reads, mapped reads, uniquely mapped reads, and Q20, Q30, and GC contents for each library were identified. A total of 476.78 Mb of clean data was obtained, with an average of 26.49 Mb of clean data for each sample. Of these, the uniquely mapped reads to the chicken reference genome (Gallus_gallus-6.0) ranged from 77.06% to 84.86%. The sequencing data obtained from the experiment exhibited high quality, as indicated by Q20 (>95%) and Q30 (>90%) values and the similar GC content. To ensure accessibility and transparency, the raw sequence data have been deposited in the NCBI Sequence Read Archive under accession number PRJNA859098.
3.2. Differential mRNA Expression in Feather Follicles
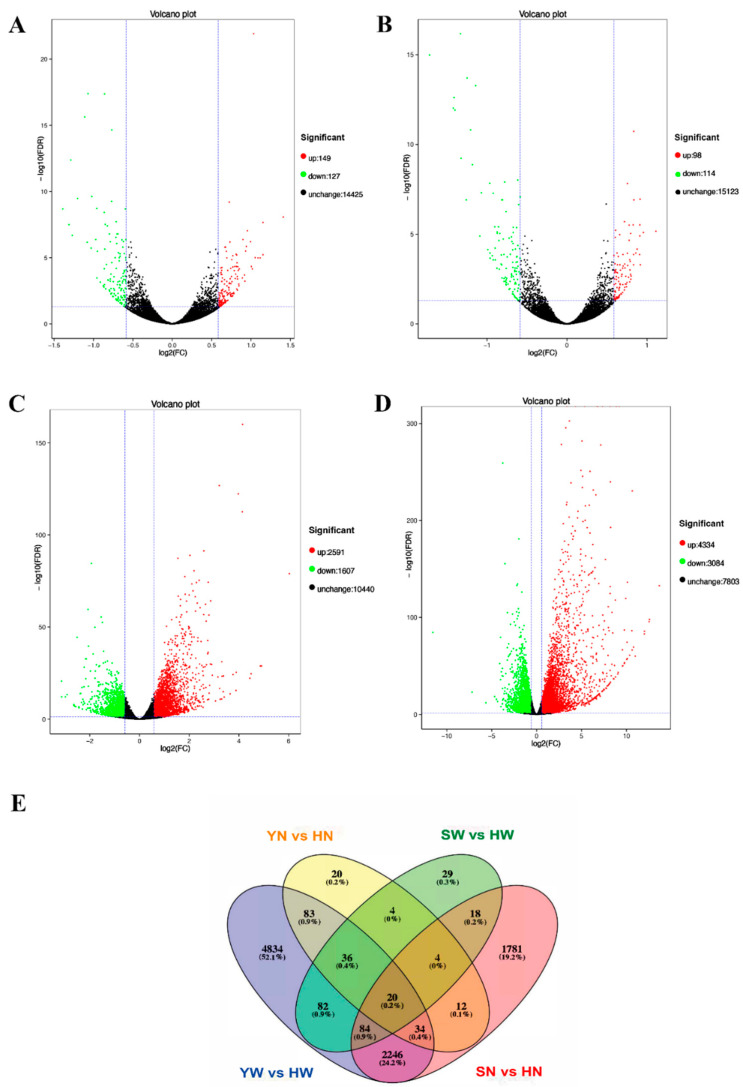
DESeq2 was used to analyze the DEGs between the groups. Fold change ≥ 1.5 and FDR < 0.05 were used as screening criteria for DEG detection. A total of 12104 genes were detected in this study; 276 DEGs were obtained from the YW group and the HW group, of which 149 genes were upregulated and 127 genes were downregulated; and 212 DEGs were obtained from the YN group and the HN group, of which 98 genes were upregulated and 114 genes were downregulated (Figure 2A,B). Compared to the SW group, the HW group had 4198 DEGs identified, with 2591 genes upregulated and 1607 genes downregulated. A total of 7418 DEGs were obtained in the SN group and the HN group, including 4334 upregulated and 3084 downregulated genes (Figure 2C,D). To identify the DEGs responsible for the formation of plumage color, we targeted DEGs that had the same trend in the YW vs. HW groups and in the YN vs. HN groups or that had the same trend in the SW vs. HW groups and in the SN vs. HN groups (p < 0.05) (Figure 2E). If a DEG had the same trend in the YN vs. HN group and the YW vs. HW group, we denoted it as the Y vs. H group and similarly for the S vs. H group.
Figure 2.
Analyses of DEGs in four comparisons. (A–D) YW vs. HW, YN vs. HN, SW vs. HW, and SN vs. HN groups, respectively. Red and green points indicate the genes with significantly increased or decreased expression, respectively (FDR < 0.05). The x-axis shows the log2-fold change in expression, and the y-axis shows the log10-fold likelihood of a gene being differentially expressed. (E) Venn diagram showing the overlap of the DEGs in four comparisons.
To some extent, alternative splicing allows the same gene to produce multiple isoforms. Four AS forms were detected, namely, the alternative 3′ splice site (A3SS), alternative 5′ splice site (A5SS), retained intron (RI), and skipped exon (SE) [28]. We identified AS forms of genes expressed in feather follicle tissues of the HN vs. YN group and HW vs. YW group, which showed similar proportions for the two groups. Unfortunately, we did not find different alternative splicing events in the DEGs of the HN vs. YN groups or in the HW vs. YW groups.
3.3. Data Validation
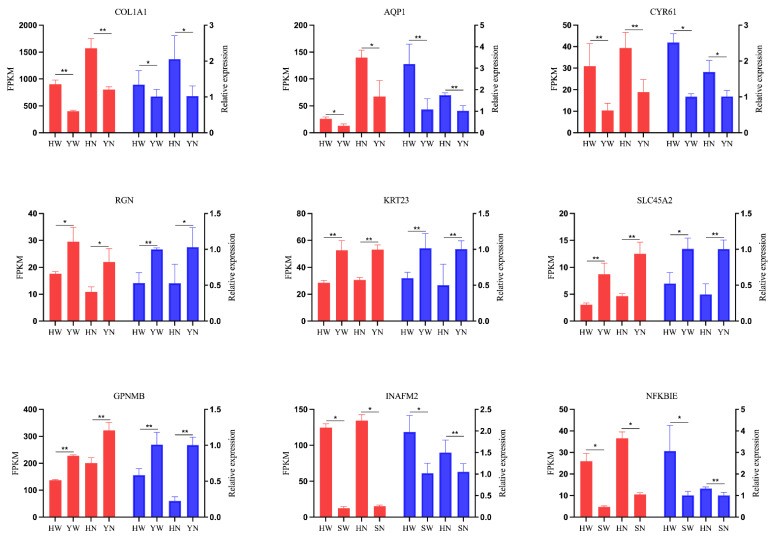
We randomly selected nine DEGs for validation using qRT-PCR and found that the expression levels of all genes were consistent with the results of RNA-seq, indicating that the detection and expression of our RNA-seq are accurate (Figure 3).
Figure 3.
RT–qPCR validation of RNA-seq results. The left Y-axis displays the FPKM derived from the RNA-seq, while the data from RT–qPCR are shown on the Y-axis on the right. The data are represented as the means ± SEDs; red represents the FPKM value of RNA-seq, blue represents the RT–qPCR with GAPDH as the internal reference gene; * indicates p < 0.05; ** indicates p < 0.01.
3.4. GO Analysis and KEGG Pathway Enrichment Analysis
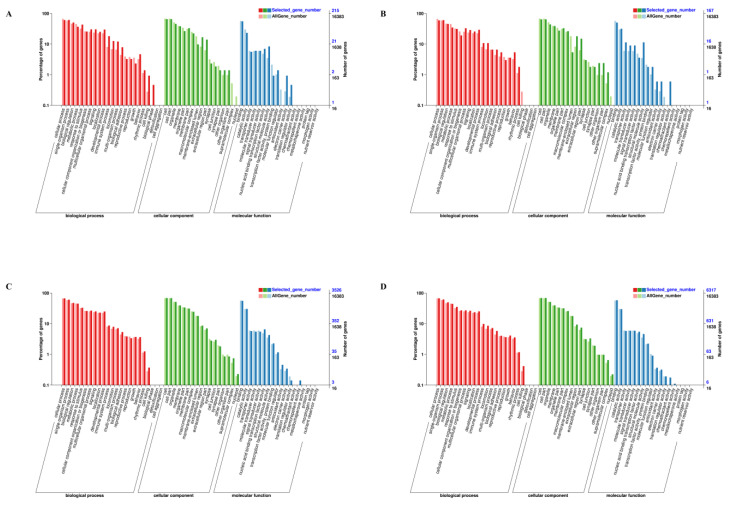
Gene enrichment analysis was conducted to investigate the distribution of GO items and reveal differential enrichment in the biological process, cellular component, and molecular function categories. The GO classification of the DEGs can be observed in Figure 4 and Supplementary Materials Table S3. Within the biological process category, the predominant subcategories identified in all four groups included cellular process, single-organism process, biological regulation, and metabolic process.
Figure 4.
GO classification of DEGs. (A) YW vs. HW groups. (B) YN vs. HN groups. (C) SW vs. HW groups. (D) SN vs. HN groups. The x-axis indicates the secondary classification in the GO database; the y-axis on the left side indicates the ratio of the number of genes annotated with this GO classification to all genes. The y-axis on the right side indicates the number of genes annotated with this GO entry. The denominator of ‘Selected_gene_number’ is the number of DEGs, and the denominator of ‘AllGene_number’ is the total number of genes.
The cellular process was the most affected of the subcategories, including upregulated genes AQP1 in the Y vs. H groups, ras homolog family member C (RHOC), frizzled class receptor 1 (FZD1), frizzled class receptor 6 (FOXO6), dihydroorotate dehydrogenase (quinone) (DHODH) in the S vs. H groups, downregulated genes TYR, KIT, transient receptor potential cation channel subfamily M member 2 (TRPM2), GPNMB in the Y vs. H groups, Wnt family member 11 (WNT11) in the S vs. H groups, etc. Among the cellular components, the cell, cell part, and organelle categories were dominant. The DEGs involved in these GO terms included the upregulated genes DHODH, FZD1, myocardin (MYOCD), LIM domain kinase 1 (LIMK1), and semaphorin 6B (SEMA6B) in the S vs. H groups and the downregulated genes SLC45A2, TYR, MLPH, major facilitator superfamily domain containing 12 (MFSD12), and TRPM2 in the Y vs. H groups. For molecular function, the most affected subcategories were bound together (upregulated genes included aquaporin 1 (AQP1) and CYR61 in the Y vs. H groups, RHOC, SRY-box 9 (SOX9), FZD1, and forkhead box O6 (FOXO6) in the S vs. H groups, and downregulated genes included TYR, MLPH, and GPNMB in the Y vs. H groups, and WNT11 in the S vs. H groups), catalytic activity (upregulated genes included LIM domain kinase 1 (LIMK1), RHOC, alkaline ceramidase 1 (ACER1), protein phosphatase, Mg2+/Mn2+ dependent 1J (PPM1J), and aprataxin (APTX) in the S vs. H groups, and downregulated genes included TYR, KIT, and TRPM2 in the Y vs. H groups), and transporter activity (upregulated genes included gap junction protein beta 1 (GJB1), tRNA-yW synthesizing protein 5 (TYW5), cyclin, and CBS domain divalent metal cation transport mediator 1 (CNNM1), and solute carrier family 11 member 1 (SLC11A1) in the S vs. H groups, and downregulated genes included SLC45A2 in the Y vs. H groups and glutamate ionotropic receptor kainate type subunit 1 (GRIK1) in the S vs. H groups. These genes play an important role in the deposition of melanin.
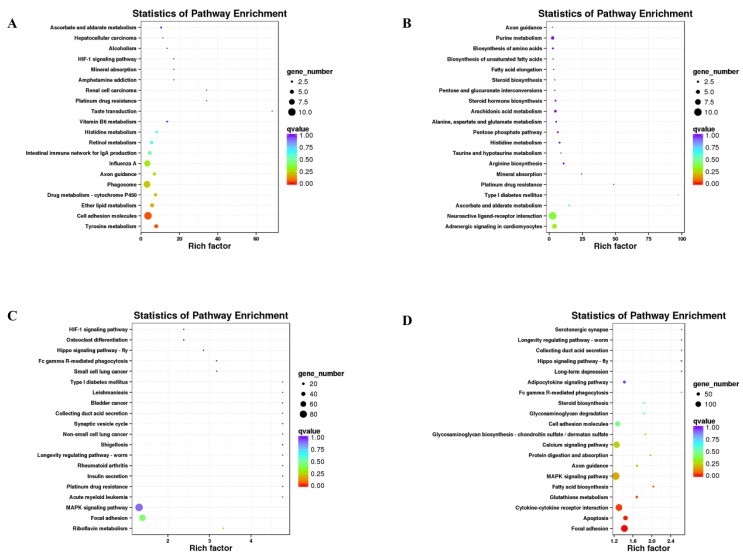
The results of KEGG pathway enrichment analysis for differentially expressed genes are shown (Figure 5, Supplementary Materials Table S4), listing the top 20 pathways with the lowest Q values. Among the pathways that are significantly associated with melanin are tyrosine metabolism, the MAPK signaling pathway, adrenergic signaling in cardiomyocytes, and the calcium signaling pathway. Among these pathways, the tyrosine metabolism pathway was the most significantly enriched pathway in the Y vs. H groups (p < 0.05). By analyzing the top 20 pathways with the lowest Q values in the four comparison groups, we found the distinctly expressed gene TYR in the tyrosine metabolic pathway.
Figure 5.
Scatterplot of the top 20 pathways in KEGG enrichment. (A) YW vs. HW groups. (B) YN vs. HN groups. (C) SW vs. HW groups. (D) SN vs. HN groups. The x-axis represents the rich factor, which is the ratio of the DEGs annotated with the pathway term to the total number of genes annotated with the pathway term. The greater the rich factor is, the greater the degree of enrichment. The y-axis shows each KEGG pathway name. Each round point represents a specific KEGG pathway. The circle size indicates the number of DEGs associated with each significantly enriched pathway. The circle color indicates the significance level (q-value). A q-value < 0.05 was considered to indicate significant enrichment. Light purple indicates the least significant, and orange represents the most significant.
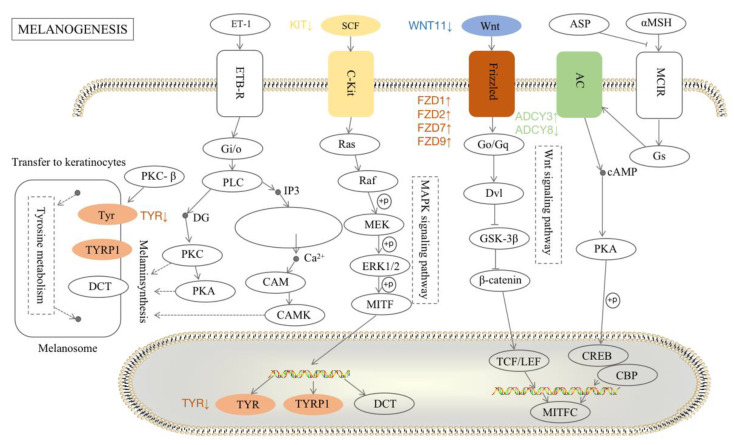
In addition, the KEGG classification table shows that there were other melanin-related pathways, such as melanogenesis, Wnt signaling, Notch signaling, calcium signaling, adherens junction, and mTOR signaling in the four comparison groups (Supplementary Materials Table S5). Interestingly, we found that the melanogenesis pathway was common in all four groups, so we focused on that pathway (Figure 6). We found that nine DEGs were enriched in the melanogenesis pathway—TYR, KIT, WNT11, FZD1, frizzled class receptor 2 (FZD2), frizzled class receptor 7 (FZD7), frizzled class receptor 9 (FZD9), adenylate cyclase 3 (ADCY3), and adenylate cyclase 3 (ADCY8), which had the same trend in the YW vs. HW groups and the YN vs. HN groups, or had the same trend in the SW vs. HW groups and SN vs. HN group (Supplementary Materials Table S6).
Figure 6.
The networks of DEGs in the melanogenesis pathway. Proteins and DEGs in the same families are represented by the same color.
3.5. PPI Analysis of DEGs
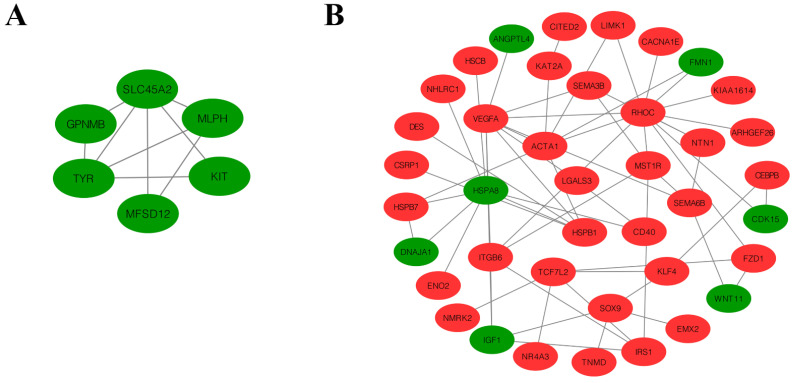
In the Y vs. H groups, the DEG PPI network was composed of nine proteins and nine pairs of PPIs. Moreover, TYR interacted with SLC45A2, KIT, GPNMB, and MFSD12. Melanophilin (MLPH) interacted with SLC45A2, TYR, and MFSD12 (Figure 7A). In addition, TYR was mainly enriched in tyrosine metabolism and melanogenesis. Furthermore, the PPI network in the S vs. H groups consisted of 161 proteins and 107 pairs of PPIs (Supplementary Materials Table S7). The key core node FZD1 exceeded seven interactions, including WNT11, transcription factor 7-like 2 (TCF7L2), and RHOC. Among them, both heat shock protein family A (Hsp70) member 8 (HSPA8) and heat shock protein family B (small) member 1 (HSPB1) were enriched in the MAPK signaling pathway, while WNT11 and TCF7L2 were enriched in the melanogenesis pathway and the Wnt signaling pathway (Figure 7B).
Figure 7.
PPI network of DEGs. (A,B) represent Y vs. H and S vs. H, respectively. Red nodes represent upregulated DEGs, and green nodes represent downregulated DEGs.
3.6. Effects of SLC45A2 and GPNMB Overexpression on Melanin Deposition
Notably, we found that the expression of SLC45A2 and GPNMB in the H line was significantly lower than that in the Gushi line using transcriptome sequencing and qRT–PCR. Moreover, in the transcriptome sequencing, the eumelanin-related genes MLPH and TYR were significantly reduced in Group H compared to Group Y (p < 0.05). Given that SLC45A2 has the same expression trend as MLPH and TYR, high expression of SLC45A2 and GPNMB is also speculated to affect the deposition of melanin.
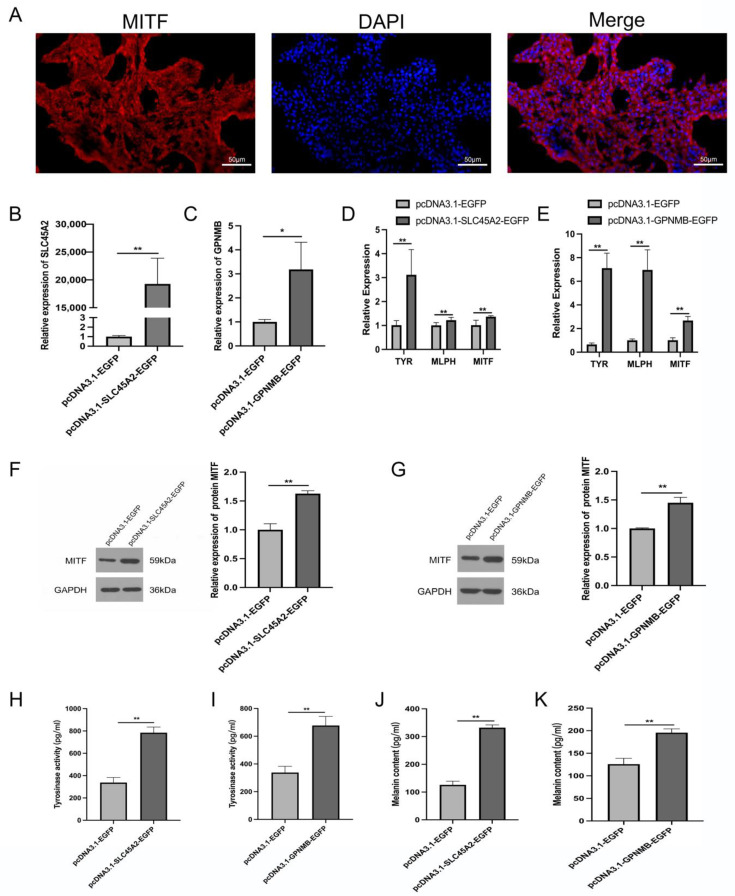
To explore the biological role of SLC45A2 and GPNMB in eumelanin in chickens, we overexpressed the two genes separately in chicken primary melanocytes. Primary melanocytes were identified by immunofluorescence staining for MITF (Figure 8A). The mRNA expression levels of the SLC45A2 and GPNMB genes in melanocytes were increased by approximately 18000-fold and 3-fold after transfection with pcDNA3.1-SLC45A2-EGFP and pcDNA3.1-GPNMB-EGFP, respectively, compared to the control group that was transfected with the pcDNA3.1-EGFP plasmid (Figure 8 B,C).
Figure 8.
SLC45A2 and GPNMB promote the deposition of melanin in chicken melanocytes. (A) Marker gene identification in chicken primary melanocytes (scale 50 μm). (B,C) The mRNA expression of SLC45A2 and GPNMB after 48 h of transfection of the SLC45A2 overexpression vector pcDNA3.1-SLC45A2-EGFP and the GPNMB overexpression vector pcDNA3.1-GPNMB-EGFP in melanocytes. (D,E) Effects of SLC45A2 and GPNMB overexpression on the expression of genes related to pigmentation melanocytes. (F,G) Effects of SLC45A2 and GPNMB overexpression on MITF protein levels. (H,I) Effect of SLC45A2 and GPNMB overexpression on the tyrosinase content of melanocytes. (J,K) Effect of SLC45A2 and GPNMB overexpression on the melanin content of melanocytes. * p < 0.05; ** p < 0.01.
After overexpression of SLC45A2 or GPNMB, the mRNA expression levels of the eumelanin marker genes TYR, MLPH, and MITF were significantly increased (p < 0.01; Figure 8D,E). Consistently, western blotting also confirmed that the protein levels of MITF were upregulated following melanocytes’ transfection with pcDNA3.1-SLC45A2-EGFP and pcDNA3.1-GPNMB-EGFP, respectively, compared to the control group (Figure 8F,G). Full WB images can be found in Supplementary Materials Figure S1. Furthermore, the tyrosinase and melanin contents in cells transfected with the recombinant plasmids pcDNA3.1-SLC45A2-EGFP or pcDNA3.1-GPNMB-EGFP were significantly higher than those in the control group (p < 0.01) (Figure 8H–K).
4. Discussion
In chicken feathers, melanin distribution and deposition are responsible for plumage color variations, and feather follicles are the only place where plumage melanin is produced [29]. During feather follicle melanogenesis, mature melanocytes produce melanosomes; newly synthesized melanin then migrates to the feather keratinocytes, giving the feathers their particular color [30]. Pigmentation is a complex feature, and many studies have shown that melanin pigmentation is strongly genetically controlled [31]. In recent years, several key genes involved in melanin deposition have been identified. These include MLPH, TYR, KIT, PMEL, MITF, agouti signaling (ASIP), melanocortin 1 receptor (MC1R), and endothelin 3 (EDN3) [32]. However, there has been little research on the association of pigmentation on chicken feathers. In particular, because most of the feather color is caused by eumelanin and pheomelanin, and most of the marker genes related to melanin deposition, for example TYR, affect both eumelanin and pheomelanin, it is particularly important to further study the colorful plume color due to total melanin deposition [33,34]. In our transcriptome data, there were significant differences in the expression of MLPH, TYR, and KIT (p < 0.05). MLPH is an important structural protein that assists in the transport of melanosomes, ensuring that melanosome bodies accumulate at the dendritic borders of melanocytes and release them into surrounding tissues for deposition, regulating the color of the animal’s skin and coat. MLPH gene mutations result in chickens with the Japanese quail feather lavender diluted phenotype [35,36]. The expression of the MLPH gene in the goose has tissue specificity, with the highest expression in black eyes and the lowest expression in back skin, abdominal skin, and fins, but it was not detected in the heart, liver, and other organs [37]. This expression pattern is consistent with the melanin deposition pattern of geese, showing that the MLPH gene is related to melanin deposition [37]. TYR is a key gene in the biosynthesis of melanin in animals, and the tyrosinase it encodes has the catalytic activity of tyrosine hydroxylase and dopa oxidase and is a rate-limiting enzyme in the process of melanin biosynthesis. The lack of tyrosinase catalytic function hinders melanin synthesis, resulting in corresponding albinism traits in the animal’s coat and skin [38]. At present, many research results show that functional mutations in the TYR gene and differences in gene expression levels in animals such as cattle, sheep, dogs, cats, martens, rabbits, rats, and poultry will lead to albino traits in their coats [14,39,40]. The insertion of an intact avian retroviral sequence in intron 4 in the chicken TYR gene causes deletion of transcript exon 5, eventually leading to chicken recessive white feather mutation [8]. The mast stem cell factor receptor encoded by the KIT gene is a member of the tyrosine kinase receptor family, which acts on blood cells and melanin, affecting the growth and development of blood cells and melanocytes [41]. When KIT is expressed in a melanocyte precursor, its ligand SCF activates receptors on the cell membrane through the MAPK signaling pathway, ultimately initiating melanin synthesis [42]. Together with the previous results, we speculate that the expression of MLPH, TYR, and KIT plays an important role in the deposition of melanin in chicken feathers.
To understand the potential functions of the identified mRNAs, the DEGs were subjected to functional enrichment analyses. KEGG pathway analysis showed that the differentially expressed genes TYR, KIT, WNT11, FZD1, FZD2, FZD7, FZD9, ADCY3, and ADCY8 were significantly enriched in tyrosine metabolic pathway or melanogenesis. This result suggested that the DEGs identified in the feather follicles play an important role in melanin biosynthesis and pigmentation. In previous studies, we collected dorsal feather follicles (sub-Columbian feathers) and ventral feather follicles (white feathers) of the nuchal area of the H line for RNA-seq and found that the expression of WNT11 and SLC45A2 significantly differed in these two sites, while WNT11 was enriched in the melanogenesis pathway [22]. WNT11 also plays a crucial role in the Wnt signaling pathway. Several studies have demonstrated that the Wnt pathway is involved in melanin synthesis [43,44]. In addition, we found that the expression of WNT11 in the feather follicles of the H line was significantly increased after feeding 1.0% tyrosine daily [22]. However, there are very few reports on the synthesis of WNT11 in melanin. In this study, the expression level of WNT11 in sub-Columbian feathers was significantly higher than that in white feathers (p < 0.05). Using KEGG enrichment analysis, we found that WNT11 was enriched in the melanogenesis pathway and Wnt signaling pathway. In the PPI network, WNT11 interacted with FZD1, which is also involved in the melanogenesis pathway. We speculate that FZD1 and WNT11 are responsible for the formation of dark spots (melanin) at specific sites in sub-Columbian feathers, but the exact genetic mechanism needs to be further investigated.
In our transcriptome data, we focused on DEGs (p < 0.05) between different plumage colors and selected DEGs with the same trend in both wing and neck. Moreover, through PPI analysis, genes that are consistent with the expression trend of MLPH, TYR, and KIT, such as SLC45A2 and GPNMB, are likely to exhibit similar functions to melanin marker genes.
SLC45A2 is often found to be overexpressed in black tissues compared with white tissues [45]. Moreover, after transfection of SLC45A2 into mouse melanocytes, MITF, TYR, TYRP1, DCT, and tyrosine contents were significantly increased (p < 0.05) [46]. This is similar to the result of significantly elevated MITF and TYR after we overexpressed SLC45A2 on chicken primary melanocytes (p < 0.05). According to the results of previous experiments in our laboratory, the expression of SLC45A2 on the dorsal side of the nuchal (sub-Columbian plumage) area of the H line was significantly higher than that on the ventral side of the nuchal (white plumage) area, and the addition of different concentrations of tyrosine to melanocytes significantly affected the expression of SLC45A2 [22]. In our experimental results, after overexpressing SLC45A2, the tyrosinase content and melanin content in cells were significantly increased, so we hypothesized that the high expression of SLC45A2 could promote the deposition of melanin. Notably, SLC45A2 is the known pathogenic gene of an autosomal recessive hypopigmentary disorder, oculocutaneous albinism type 4 (OCA4) [45]. Moreover, the missense mutation of SLC45A2 results in a dominant silver locus that dilutes pheomelanin [47,48]. Similarly, no effect on eumelanin was observed in SLC45A2 mutant horses, with some effect on pheomelanin dilution [49]. Cysteine is a key amino acid in the synthesis of pheomelanin, so we speculate that this mutation prevents cysteine transport to melanosomes. In addition, SLC45A2 transports sugar in yeast cells in an acid-dependent manner [50]. The anti-melanogenic effects of sugar and sugar derivatives are probably due to three different mechanisms: by increasing melanosomal pH, by interfering with melanosome maturation, or by inhibiting TYR maturation by blocking N-glycosylation [51]. It has been previously demonstrated that the proton/glucose exporter SLC45A2 mediates melanin synthesis and melanoma metastasis primarily by modulating melanosomal glucose metabolism. In human melanocytes and melanoma cell lines, the SLC45A2 gene is highly enriched, and MATP, the gene’s protein, is found in melanosomes [52]. By knocking down MATP using siRNAs, melanin content and tyrosinase activity were reduced. It helps maintain an appropriate melanosome pH, so that copper ions can be incorporated into the tyrosinase active site correctly [52]. Therefore, we speculate that in the melanocytes of chicken feather follicles, SLC45A2 may regulate the pH of melanosomes by regulating the glucose metabolism of melanosomes, leading to the deposition of melanin.
At the cyclin-dependent kinase inhibitor 2A (CDKN2A) tumor suppressor locus, there are two non-coding and two missense mutations associated with sex-linked barring [53]. Schwochow Thalmann found that one or both of the non-coding changes cause an upregulation of CDKN2A expression in feather follicles during feather growth [10]. Melanocyte progenitor cells may stop dividing prematurely when CDKN2A expression is high, distinguishing into pigment-producing cells instead [10]. In the KASP typing results published earlier in our laboratory, the silver feather only had the mutations of SLC45A2 and no mutation of cyclin-dependent kinase inhibitor 2A (CDKN2A), while sub-Columbian plumage have both SLC45A2 mutations and CDKN2A mutations. We speculate that CDKN2A is also a key gene influencing melanin deposition in chicken feathers. But CDKN2A did not appear in the differentially expressed genes in our transcriptome data. However, we verified that the gene was significantly different in the sub-Columbian plumage vs. yellow plumage by q-PCR (p < 0.05) (Supplementary Materials Figure S2). The results were consistent with the previous studies.
Melanin is synthesized in the melanosomes of melanocytes, and its formation goes through four stages [54]. GPNMB is a type I transmembrane glycoprotein, which is present in all stages of melanosome formation (I–IV), and especially enriched in mature (stage III and IV) melanosomes [55,56]. The expression level of GPNMB in melanocytes was found to be inversely correlated with the metastatic capacity of human melanomas and was shown to be associated with the development of the retinal pigment epithelium and the iris [57,58]. In mice with pigmentary glaucoma, a premature stop codon mutation in the GPNMB gene results in iris pigment dispersal [59]. Combined with the results of this experiment, in chicken melanocytes, overexpression of GPNMB can increase intracellular tyrosinase content and melanin content, we speculated that the high expression of GPNMB could promote the deposition of melanin. During development, GPNMB is expressed in a pattern similar to that of MITF, DCT, and PMEL [60]. GPNMB and PMEL are highly homologous proteins that play an important role in melanosome biogenesis [61,62]. In addition, GPNMB adheres to PAM212 keratinized cells in an RGD-dependent manner and is possibly involved in melanocyte development, melanin synthesis, and melanoma production [63]. MITF has been regarded as an essential transcription factor for the regulation of more than 25 pigmentation genes, such as TYR, TYRP1, DCT, PMEL17, and so on [64]. In addition, MITF is involved in the Wnt/β-catenin signaling pathway, MC1R/α-MSH signaling pathway, and SCF/C-KIT signaling pathway [65,66,67]. The first two pathways can increase MITF expression, and these pathways affect melanin production by regulating the transcription of TYR, TYRP1, and DCT through MITF [68]. Based on this experiment, after overexpression of GPNMB in chicken melanocytes, the expression of TYR and MITF increased. From this, we speculate that GPNMB affects the deposition of melanin by upregulating the transcription of TYR, TYRP1, and DCT by upregulating MITF.
It is worth noting that by screening for differentially expressed genes with the same expression trend as marker genes such as TYR in the two groups of this experiment, combined with PPI correlation analysis, we finally speculated that SLC45A2 and GPNMB genes may affect the deposition of total melanin on chicken feather follicles. However, since the experimental cells were obtained from the peritoneal tissue of black chickens, the cells can only secrete eumelanin, so we can only prove that SLC45A2 and GPNMB have an effect on the deposition of eumelanin, and whether they have an effect on pheomelanin needs further experimental verification.
5. Conclusions
In this study, transcriptome sequencing and subsequent GO, KEGG, and PPI analyses were used to screen for genes with the same trend as melanin deposition marker genes and DEGs (SLC45A2, GPNMB, MLPH, TYR, KIT, WNT11, and FZD1) in the neck and wing feather follicles of chickens. Moreover, we found that SLC45A2 and GPNMB can promote the deposition of melanin in chicken feathers. Overall, the candidate genes detected in these experiments can help provide novel insights into the mechanisms regulating feather color development in chickens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani13162608/s1, Table S1. List of overexpression vector construction and qPCR primers; Table S2. Statistics of quality control and reads mapping of sequencing date; Table S3. GO classification of the differentially expressed genes; Table S4. The top 20 pathways in KEGG enrichment in the four comparisons; Table S5. KEGG classification of the differentially expressed genes; Table S6. DEGs in the Melanogenesis pathway; Table S7. PPI network of DEGs; Figure S1: Full Western Blots representing images used in Figure 8F,G; Figure S2: Differences in the relative expression of CDKN2A in the two comparison groups.
Author Contributions
Conceptualization, X.K. and Z.L.; Data Curation, Y.L. and D.L.; Methodology, R.L and Y.W.; Software, Y.T. and X.L.; Validation, R.L., Y.L. and D.L.; Visualization, Y.T. and X.L.; Writing—Original Draft, R.L and Y.W.; Writing—Review & Editing, X.K. and Z.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Guide for the Care and Use of Laboratory Animals: Eighth Edition’ by National Research Council (ISBN-10 0309154006). The study proposal was approved by the Institutional Animal Care and Use Committee (IACUC) of Henan Agricultural University (approval number: 11-0085). All procedures were carried out in accordance with relevant guidelines and regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data used in this study are publicly available and can be obtained upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the China Agriculture Research System of MOF and MARA (CARS-40), the Zhongyuan Youth Talent Support Program (ZYYCYU202012156), and the Key Research Project of the Shennong Laboratory (SN01-2022-05).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Roulin A., Ducrest A.L. Genetics of colouration in birds. Semin. Cell Dev. Biol. 2013;24:594–608. doi: 10.1016/j.semcdb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Singaravelan N., Pavlicek T., Beharav A., Wakamatsu K., Ito S., Nevo E. Spiny mice modulate eumelanin to pheomelanin ratio to achieve cryptic coloration in “evolution canyon,” Israel. PLoS ONE. 2010;5:e8708. doi: 10.1371/journal.pone.0008708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cone R.D., Lu D., Koppula S., Vage D.I., Klungland H., Boston B., Chen W., Orth D.N., Pouton C., Kesterson R.A. The melanocortin receptors: Agonists, antagonists, and the hormonal control of pigmentation. Recent. Prog. Horm. Res. 1996;51:287–317, discussion 318. [PubMed] [Google Scholar]
- 4.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando H., Ichihashi M., Hearing V.J. Role of the ubiquitin proteasome system in regulating skin pigmentation. Int. J. Mol. Sci. 2009;10:4428–4434. doi: 10.3390/ijms10104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galván I., Solano F. Bird Integumentary Melanins: Biosynthesis, Forms, Function and Evolution. Int. J. Mol. Sci. 2016;17:520. doi: 10.3390/ijms17040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerje S., Sharma P., Gunnarsson U., Kim H., Bagchi S., Fredriksson R., Schütz K., Jensen P., von Heijne G., Okimoto R., et al. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics. 2004;168:1507–1518. doi: 10.1534/genetics.104.027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C.M., Coville J.L., Coquerelle G., Gourichon D., Oulmouden A., Tixier-Boichard M. Complete association between a retroviral insertion in the tyrosinase gene and the recessive white mutation in chickens. BMC Genom. 2006;7:19. doi: 10.1186/1471-2164-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita K., Akiyama T., Mizutani M., Shinomiya A., Ishikawa A., Younis H.H., Tsudzuki M., Namikawa T., Matsuda Y. Endothelin receptor B2 (EDNRB2) is responsible for the tyrosinase-independent recessive white (mo(w)) and mottled (mo) plumage phenotypes in the chicken. PLoS ONE. 2014;9:e86361. doi: 10.1371/journal.pone.0086361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwochow Thalmann D., Ring H., Sundström E., Cao X., Larsson M., Kerje S., Höglund A., Fogelholm J., Wright D., Jemth P., et al. The evolution of Sex-linked barring alleles in chickens involves both regulatory and coding changes in CDKN2A. PLoS Genet. 2017;13:e1006665. doi: 10.1371/journal.pgen.1006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunnarsson U., Kerje S., Bed’hom B., Sahlqvist A.S., Ekwall O., Tixier-Boichard M., Kämpe O., Andersson L. The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell Melanoma Res. 2011;24:268–274. doi: 10.1111/j.1755-148X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwochow D., Bornelöv S., Jiang T., Li J., Gourichon D., Bed’Hom B., Dorshorst B.J., Chuong C.M., Tixier-Boichard M., Andersson L. The feather pattern autosomal barring in chicken is strongly associated with segregation at the MC1R locus. Pigment Cell Melanoma Res. 2021;34:1015–1028. doi: 10.1111/pcmr.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon J.D., Peles D., Wakamatsu K., Ito S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009;22:563–579. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- 14.Cieslak M., Reissmann M., Hofreiter M., Ludwig A. Colours of domestication. Biol. Rev. Camb. Philos. Soc. 2011;86:885–899. doi: 10.1111/j.1469-185X.2011.00177.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi Y., Hearing V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014;4:a017046. doi: 10.1101/cshperspect.a017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S., Wang G., Liao J., Tang M. Transcriptome profile analysis identifies candidate genes for the melanin pigmentation of breast muscle in Muchuan black-boned chicken. Poult. Sci. 2018;97:3446–3455. doi: 10.3382/ps/pey238. [DOI] [PubMed] [Google Scholar]
- 17.Li D., Wang X., Fu Y., Zhang C., Cao Y., Wang J., Zhang Y., Li Y., Chen Y., Li Z., et al. Transcriptome Analysis of the Breast Muscle of Xichuan Black-Bone Chickens under Tyrosine Supplementation Revealed the Mechanism of Tyrosine-Induced Melanin Deposition. Front. Genet. 2019;10:457. doi: 10.3389/fgene.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan H., Zhang X., Zhang Q., Wang Y., Wang S., Li Y., Zhang Y., Jing J., Qiu J., Wang Z., et al. Comparative transcriptome profiles of Lindian chicken eyelids identify melanin genes controlling eyelid pigmentation. Br. Poult. Sci. 2019;60:15–22. doi: 10.1080/00071668.2018.1544414. [DOI] [PubMed] [Google Scholar]
- 19.Yu S., Wang G., Liao J., Shen X., Chen J. Integrated analysis of long non-coding RNAs and mRNA expression profiles identified potential interactions regulating melanogenesis in chicken skin. Br. Poult. Sci. 2023;64:19–25. doi: 10.1080/00071668.2022.2113506. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X., Zhang B., Zhang Y., Zhong H., Nie R., Li J., Zhang H., Wu C. Transcriptome analysis of feather follicles reveals candidate genes and pathways associated with pheomelanin pigmentation in chickens. Sci. Rep. 2020;10:12088. doi: 10.1038/s41598-020-68931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu S., Wang G., Liao J., Tang M., Sun W. Transcriptome Profile Analysis of Mechanisms of Black and White Plumage Determination in Black-Bone Chicken. Cell Physiol. Biochem. 2018;46:2373–2384. doi: 10.1159/000489644. [DOI] [PubMed] [Google Scholar]
- 22.Wang X., Li D., Song S., Zhang Y., Li Y., Wang X., Liu D., Zhang C., Cao Y., Fu Y., et al. Combined transcriptomics and proteomics forecast analysis for potential genes regulating the Columbian plumage color in chickens. PLoS ONE. 2019;14:e0210850. doi: 10.1371/journal.pone.0210850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R., Wang X., Wang Y., Liu D., Zhang Y., Liu Y., Niu X., Han R., Li H., Jiang R., et al. Research Note: Combined analysis of BSA-seq based mapping and RNA-seq reveals candidate genes associated with sub-Columbian plumage in H line chickens. Poult. Sci. 2023;102:102665. doi: 10.1016/j.psj.2023.102665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortazavi A., Williams B.A., McCue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 26.Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P., Cao Y., Fu Y., Zhu H., Xu S., Zhang Y., Li W., Sun G., Jiang R., Han R., et al. Revealing the Regulatory Mechanism of lncRNA-LMEP on Melanin Deposition Based on High-Throughput Sequencing in Xichuan Chicken Skin. Genes. 2022;13:2143. doi: 10.3390/genes13112143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Liu J., Huang B.O., Xu Y.M., Li J., Huang L.F., Lin J., Zhang J., Min Q.H., Yang W.M., et al. Mechanism of alternative splicing and its regulation. Biomed. Rep. 2015;3:152–158. doi: 10.3892/br.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter L.L., Hou L., Loftus S.K., Pavan W.J. Spotlight on spotted mice: A review of white spotting mouse mutants and associated human pigmentation disorders. Pigment. Cell Res. 2004;17:215–224. doi: 10.1111/j.1600-0749.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 30.Raposo G., Marks M.S. Melanosomes—Dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddodi N., Jayanthy A., Setaluri V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012;88:1075–1082. doi: 10.1111/j.1751-1097.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J., Liu F., Cao J., Liu X. Skin transcriptome profiles associated with skin color in chickens. PLoS ONE. 2015;10:e0127301. doi: 10.1371/journal.pone.0127301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterson E.K., Fielder T.J., MacGregor G.R., Ito S., Wakamatsu K., Gillen D.L., Eby V., Boissy R.E., Ganesan A.K. Tyrosinase Depletion Prevents the Maturation of Melanosomes in the Mouse Hair Follicle. PLoS ONE. 2015;10:e0143702. doi: 10.1371/journal.pone.0143702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L., Guo C., Yan L., Li H., Sun J., Huo X., Xie X., Hu J. Syntenin regulates melanogenesis via the p38 MAPK pathway. Mol. Med. Rep. 2020;22:733–738. doi: 10.3892/mmr.2020.11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaez M., Follett S.A., Bed’hom B., Gourichon D., Tixier-Boichard M., Burke T. A single point-mutation within the melanophilin gene causes the lavender plumage colour dilution phenotype in the chicken. BMC Genet. 2008;9:7. doi: 10.1186/1471-2156-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bed’hom B., Vaez M., Coville J.L., Gourichon D., Chastel O., Follett S., Burke T., Minvielle F. The lavender plumage colour in Japanese quail is associated with a complex mutation in the region of MLPH that is related to differences in growth, feed consumption and body temperature. BMC Genom. 2012;13:442. doi: 10.1186/1471-2164-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Liu Y., Wang C., Yang Y., Wang H., Liu A., He D. Molecular cloning, tissue expression and SNP identification of MLPH gene in geese. China Poult. 2016;38:4–9. [Google Scholar]
- 38.Boonanuntanasarn S., Yoshizaki G., Iwai K., Takeuchi T. Molecular Cloning, Gene Expression in Albino Mutants and Gene Knockdown Studies of Tyrosinase mRNA in Rainbow Trout. Pigment Cell Res. 2004;17:413–421. doi: 10.1111/j.1600-0749.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 39.Hu S., Zhai P., Chen Y., Zhao B., Yang N., Wang M., Xiao Y., Bao G., Wu X. Morphological Characterization and Gene Expression Patterns for Melanin Pigmentation in Rex Rabbit. Biochem. Genet. 2019;57:734–744. doi: 10.1007/s10528-019-09929-x. [DOI] [PubMed] [Google Scholar]
- 40.Watson A., Wayman J., Kelley R., Feugier A., Biourge V. Increased dietary intake of tyrosine upregulates melanin deposition in the hair of adult black-coated dogs. Anim. Nutr. 2018;4:422–428. doi: 10.1016/j.aninu.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizutani Y., Hayashi N., Imokawa K.G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch. Dermatol. Res. 2010;302:283–294. doi: 10.1007/s00403-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 42.Jie L., Yi L., Ke H.Y.J., Qian H.D., Fang L.A. Research Progress of the Genes KIT and MLPH for Melanin Pigmentation in Animals. Newsl. Sericultural Sci. 2015;35:16–21. [Google Scholar]
- 43.Bellei B., Flori E., Izzo E., Maresca V., Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell. Signal. 2008;20:1750–1761. doi: 10.1016/j.cellsig.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Bellei B., Pitisci A., Catricalà C., Larue L., Picardo M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011;24:309–325. doi: 10.1111/j.1755-148X.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 45.Le L., Escobar I.E., Ho T., Lefkovith A.J., Latteri E., Haltaufderhyde K.D., Dennis M.K., Plowright L., Sviderskaya E.V., Bennett D.C., et al. SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation. Mol. Biol. Cell. 2020;31:2687–2702. doi: 10.1091/mbc.E20-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haidong W., Bingling Z., Tianzhi C., Xiuju Y.U., Yujing Y., Ying L., Lucheng C., Xiao-Yan H.E., Linli X. Slc45a2 Regulates Melanogenesis in Mouse Melanocytes. Chin. J. Biochem. Mol. Biol. 2017;33:406–413. [Google Scholar]
- 47.Gunnarsson U., Hellström A.R., Tixier-Boichard M., Minvielle F., Bed’hom B., Ito S., Jensen P., Rattink A., Vereijken A., Andersson L. Mutations in SLC45A2 cause plumage color variation in chicken and Japanese quail. Genetics. 2007;175:867–877. doi: 10.1534/genetics.106.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brumbaugh J.A. Ultrastructural differences between forming eumelanin and pheomelanin as revealed by the pink-eye mutation in the fowl. Dev. Biol. 1968;18:375–390. doi: 10.1016/0012-1606(68)90047-X. [DOI] [PubMed] [Google Scholar]
- 49.Mariat D., Taourit S., Guérin G. A mutation in the MATP gene causes the cream coat colour in the horse. Genet. Sel. Evol. 2003;35:119–133. doi: 10.1186/1297-9686-35-1-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartölke R., Heinisch J.J., Wieczorek H., Vitavska O. Proton-associated sucrose transport of mammalian solute carrier family 45: An analysis in Saccharomyces cerevisiae. Biochem. J. 2014;464:193–201. doi: 10.1042/BJ20140572. [DOI] [PubMed] [Google Scholar]
- 51.Bin B.H., Kim S.T., Bhin J., Lee T.R., Cho E.G. The Development of Sugar-Based Anti-Melanogenic Agents. Int. J. Mol. Sci. 2016;17:583. doi: 10.3390/ijms17040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bin B.H., Bhin J., Yang S.H., Shin M., Nam Y.J., Choi D.H., Shin D.W., Lee A.Y., Hwang D., Cho E.G., et al. Membrane-Associated Transporter Protein (MATP) Regulates Melanosomal pH and Influences Tyrosinase Activity. PLoS ONE. 2015;10:e0129273. doi: 10.1371/journal.pone.0129273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellström A.R., Sundström E., Gunnarsson U., Bed’Hom B., Tixier-Boichard M., Honaker C.F., Sahlqvist A.S., Jensen P., Kämpe O., Siegel P.B., et al. Sex-linked barring in chickens is controlled by the CDKN2A/B tumour suppressor locus. Pigment Cell Melanoma Res. 2010;23:521–530. doi: 10.1111/j.1755-148X.2010.00700.x. [DOI] [PubMed] [Google Scholar]
- 54.Diehl C. Melanogenesis—An update. Ukr. J. Dermatol. Venerol. Cosmetol. 2021;1:58–66. doi: 10.30978/UJDVK2021-1-58. [DOI] [Google Scholar]
- 55.Chi A., Valencia J.C., Hu Z.Z., Watabe H., Yamaguchi H., Mangini N.J., Huang H., Canfield V.A., Cheng K.C., Yang F., et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J. Proteome Res. 2006;5:3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 56.Hoashi T., Sato S., Yamaguchi Y., Passeron T., Tamaki K., Hearing V.J. Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein. FASEB J. 2010;24:1616–1629. doi: 10.1096/fj.09-151019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weterman M.A., Ajubi N., van Dinter I.M., Degen W.G., van Muijen G.N., Ruitter D.J., Bloemers H.P. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- 58.Bächner D., Schröder D., Gross G. mRNA expression of the murine glycoprotein (transmembrane) nmb (Gpnmb) gene is linked to the developing retinal pigment epithelium and iris. Brain Res. Gene Expr. Patterns. 2002;1:159–165. doi: 10.1016/S1567-133X(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 59.Anderson M.G., Smith R.S., Hawes N.L., Zabaleta A., Chang B., Wiggs J.L., John S.W. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- 60.Loftus S.K., Antonellis A., Matera I., Renaud G., Baxter L.L., Reid D., Wolfsberg T.G., Chen Y., Wang C., Prasad M.K., et al. Gpnmb is a melanoblast-expressed, MITF-dependent gene. Pigment Cell Melanoma Res. 2009;22:99–110. doi: 10.1111/j.1755-148X.2008.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theos A.C., Watt B., Harper D.C., Janczura K.J., Theos S.C., Herman K.E., Marks M.S. The PKD domain distinguishes the trafficking and amyloidogenic properties of the pigment cell protein PMEL and its homologue GPNMB. Pigment Cell Melanoma Res. 2013;26:470–486. doi: 10.1111/pcmr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomihari M., Hwang S.H., Chung J.S., Cruz P.D., Jr., Ariizumi K. Gpnmb is a melanosome-associated glycoprotein that contributes to melanocyte/keratinocyte adhesion in a RGD-dependent fashion. Exp. Dermatol. 2009;18:586–595. doi: 10.1111/j.1600-0625.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schallreuter K.U., Kothari S., Chavan B., Spencer J.D. Regulation of melanogenesis--controversies and new concepts. Exp. Dermatol. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 64.Vachtenheim J., Borovanský J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010;19:617–627. doi: 10.1111/j.1600-0625.2009.01053.x. [DOI] [PubMed] [Google Scholar]
- 65.Schepsky A., Bruser K., Gunnarsson G.J., Goodall J., Hallsson J.H., Goding C.R., Steingrimsson E., Hecht A. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol. Cell Biol. 2006;26:8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheli Y., Luciani F., Khaled M., Beuret L., Bille K., Gounon P., Ortonne J.P., Bertolotto C., Ballotti R. αMSH and Cyclic AMP elevating agents control melanosome pH through a protein kinase A-independent mechanism. J. Biol. Chem. 2009;284:18699–18706. doi: 10.1074/jbc.M109.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu W., Gong L., Haddad M.M., Bischof O., Campisi J., Yeh E.T., Medrano E.E. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res. 2000;255:135–143. doi: 10.1006/excr.2000.4803. [DOI] [PubMed] [Google Scholar]
- 68.Bentley N.J., Eisen T., Goding C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996-8006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data used in this study are publicly available and can be obtained upon reasonable request to the corresponding author.