Abstract
Skin conditions are a significant cause of fatal and nonfatal disease burdens globally, ranging from mild irritations to debilitating diseases. Oxidative stress, which is an imbalance between reactive oxygen species and the cells’ ability to repair damage, is implicated in various skin diseases. Antioxidants have been studied for their potential benefits in dermatologic health, but the evidence is limited and conflicting. Herein, we conducted a systematic review of controlled trials, meta-analyses, and Cochrane review articles to evaluate the current evidence on the utility of antioxidant supplementation for adjunct prevention and treatment of skin disease and to provide a comprehensive assessment of their role in promoting dermatologic health. The Cochrane Library, PubMed, EMBASE, and Epistemonikos databases were queried. Eligibility criteria included (1) primary focus on nanoparticle utility for skin cancer; (2) includes measurable outcomes data with robust comparators; (3) includes a number of human subjects or cell-line types, where applicable; (4) English language; and (5) archived as full-text journal articles. A total of 55 articles met the eligibility criteria for the present review. Qualitative analysis revealed that topical and oral antioxidant supplementation has demonstrated preliminary efficacy in reducing sunburns, depigmentation, and photoaging. Dietary exogenous antioxidants (namely vitamins A, C, and E) have shown chemopreventive effects against skin cancer. Antioxidant supplementation has also shown efficacy in treating non-cancer dermatoses, including rosacea, psoriasis, atopic dermatitis, and acne vulgaris. While further studies are needed to validate these findings on a larger scale, antioxidant supplementation holds promise for improving skin health and preventing skin diseases.
Keywords: antioxidant supplementation, vitamins, reactive oxygen species, skin cancer, dermatology
1. Introduction
According to the Global Burden of Disease project, skin conditions are the fourth-leading source of nonfatal disease burden globally [1]. These conditions can range from mild irritations to chronic, debilitating diseases that significantly impact a person’s quality of life. Importantly, skin manifestations can give way to new diagnoses of otherwise occult systemic diseases, aiding in the timely initiation of important care and optimizing patient outcomes [2,3].
The management of dermatologic diseases requires a detailed evaluation of each patient and highly customized medical management plans with comorbidities and environmental factors in mind. The effects of oxidative stress on the skin and the potential utility of antioxidants in dermatology are being investigated in the more recent literature, highlighting a link between antioxidant imbalance and cutaneous diseases. In the skin, oxidative stress has been implicated in the pathogenesis of numerous skin diseases and disorders, including psoriasis, vitiligo, atopic dermatitis, pemphigus vulgaris, lichen planus, alopecia areata, melanoma, allergic contact dermatitis, acne vulgaris, solar elastosis, and pigmentary disorders. While the role of antioxidants is well-understood in diseases such as obesity, Alzheimer’s, and atherosclerosis, their use in numerous dermatologic diseases has not been well-established [4,5]
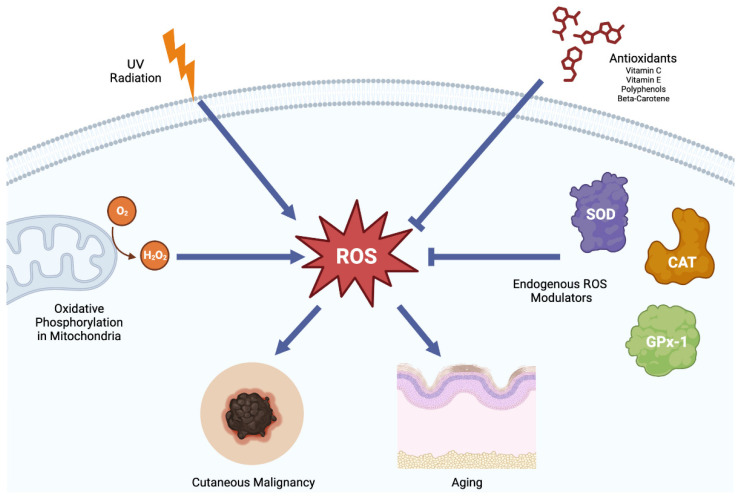
Oxidative stress is a state of imbalance between the production of reactive oxygen species (ROS) and the ability of cells to detoxify or repair their damage. ROS are a natural byproduct of cellular metabolism and play a critical role in cellular signaling and homeostasis [6]. However, excessive ROS production can lead to oxidative stress, which can cause damage to cellular components, including lipids, proteins, and DNA, and ultimately lead to cellular dysfunction and death [7]. Antioxidants are compounds that can neutralize ROS and prevent oxidative damage (Figure 1).
Figure 1.
Summary of the balance between reactive oxygen species and the endogenous antioxidant system. ROS play a critical role in the development of skin cancer and dermatoses, such as skin aging and hyperpigmentation. ROS in the skin can build up with long-term exposure to UV radiation. They are also a natural by-product of a cell’s native metabolism. The cell has devoted many mechanisms to ensure that ROS levels are kept at a physiological state. However, when the system becomes overwhelmed, ROS can accumulate and lead the to the development of skin cancer and dermatoses. Antioxidants have shown to be an effective treatment in a wide range of skin issues. SOD: Superoxide dismutase. GPx-1 glutathione peroxidase-1. CAT: catalase. Figure created with BioRender.com.
Various antioxidants have been studied for their potential benefits to dermatologic health, including vitamins C and E, beta-carotene, astaxanthin, and selenium. Vitamin C is a water-soluble antioxidant that can scavenge free radicals and regenerate other antioxidants, such as vitamin E. Vitamin E is a fat-soluble antioxidant that can protect the skin’s lipid membranes from oxidative damage. Beta-carotene is a precursor of vitamin A, which is vital for skin health and immune function. Astaxanthin is a carotenoid with potent antioxidant and anti-inflammatory properties which has been shown to improve skin hydration, elasticity, and texture. Selenium is a trace element that plays a role in antioxidant defense and immune function and has been suggested to have a protective effect against skin cancer [8]. Although the potential benefits of antioxidant supplementation in dermatologic health are promising, the evidence is still limited and conflicting. Optimal dosages and formulations of antioxidants for dermatologic use are not commonly recognized. Moreover, excessive intake of certain antioxidants may have adverse effects and interfere with other physiological processes [9]. Thus, it is essential to carefully evaluate the efficacy and safety of antioxidant supplementation in specific dermatologic conditions and tailor the supplementation to individual needs, taking risk factors into consideration.
It is also important to consider the efficacy of orally taken antioxidants, which involves considering several factors, including their ability to reach the skin in sufficient amounts and whether they may accumulate in other tissues instead. Studies indicate that orally administered antioxidants can reach detectable levels in the skin. For instance, Keen and Hassan demonstrated increased vitamin E levels in the skin following oral administration of vitamin E [10]. Similarly, Pullar et al. showed increased vitamin C levels in the skin after oral supplementation with vitamin C [11]. Certain antioxidants, such as vitamin C and vitamin E, have been found to accumulate in the skin layers requiring antioxidation. However, the distribution and accumulation of orally taken antioxidants in the skin may vary depending on the antioxidant and individual factors. Additionally, some antioxidants may be distributed and accumulate in other tissues, as shown in studies by Darvin et al. with beta-carotene, which increased levels in the skin, plasma, and adipose tissue [12].
In this systematic review, we aim to evaluate the current evidence regarding the utility of antioxidant supplementation for the prevention and adjunct treatment of various skin diseases and disorders. We will review the pathophysiology of oxidative stress in dermatologic conditions and summarize the findings from preclinical and clinical studies on the effects of antioxidant supplementation on skin health and function. We will also discuss the potential mechanisms of action of different antioxidants, the safety and tolerability of supplementation, and the implications for clinical practice and future research. Overall, this review aims to provide a comprehensive and evidence-based evaluation of the role of antioxidant supplementation in promoting dermatologic health.
2. Methods
2.1. Study Design
A systematic review of controlled trials, randomized controlled trials (RCTs), meta-analyses, case reports, and review articles was conducted in accordance with the latest (2020) Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRIMSA) guidelines [13]. This review protocol was registered in the international prospective register of systematic review (PROSPERO) (CRD42023416336).
2.2. Search Strategy
The Cochrane Library, PubMed, EMBASE, and Epistemonikos databases were broadly queried on 10 March 2023 to retrieve all relevant articles since the database’s inception. The main keyword search terms were “antioxidant” AND “skin disease” OR “skin cancer”. Queries were restricted to the Title and Abstract fields (“[tiab]”). Search records were maintained with Covidence (www.covidence.org, accessed on 10 March 2023), a web-based collaborative platform designed to streamline and automate many aspects of the systematic review process [14].
2.3. Eligibility Criteria
All initial search results were subjected to the following inclusion criteria: (1) has a primary focus on antioxidant supplementation for dermatologic conditions and/or diseases, (2) includes measurable outcomes data with robust comparators (i.e., changes compared with baseline or comparison to age-matched, healthy human patients or non-exposed controls), (3) includes a number of human subjects or cell-line types, where applicable. Criteria for exclusion were (1) non-English articles, (2) Abstract-only text, and (3) not-yet-published clinical trials.
2.4. Data Extraction
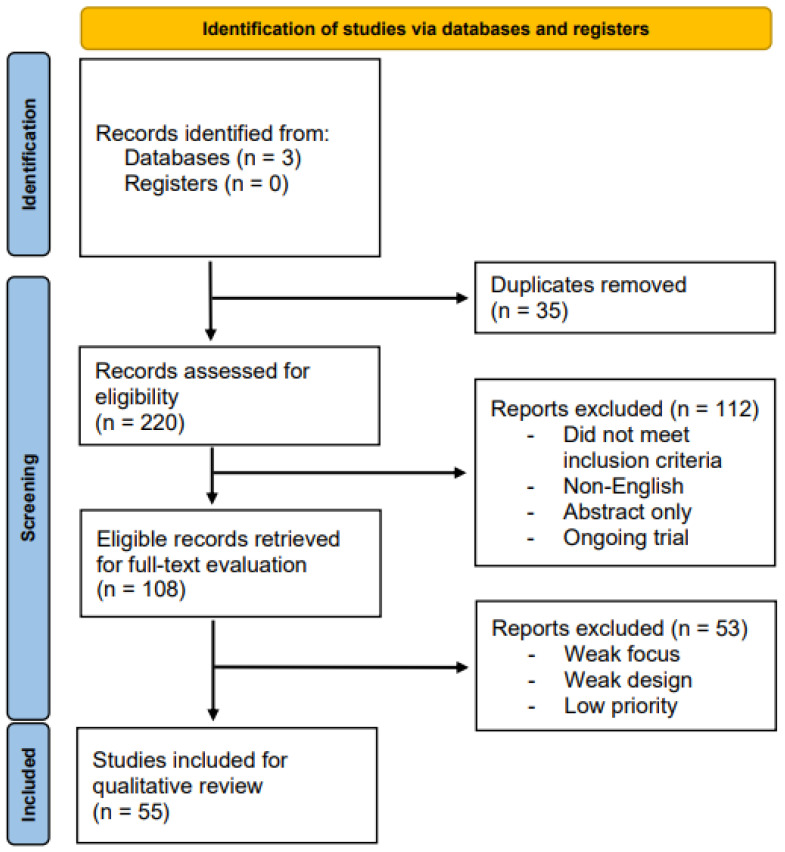
The initial sensitivity search returned 255 total records. Following the elimination of duplicates, 220 articles underwent independent Abstract and Title screening for eligibility assessment by three of the authors (BG, DG, and GK). From these records, 108 articles were deemed eligible for full-text review. After a thorough examination of the relevant studies, 55 studies were deemed suitable for qualitative synthesis. Any discrepancies in the selection process were resolved through discussion or by seeking the input of an impartial third party (JTT). Figure 2 depicts the PRISMA selection process flowchart used for this systematic review.
Figure 2.
PRISMA flow diagram.
3. Results
3.1. Primer on Antioxidant Supplementation
Exposure of the skin to ultraviolet radiation (UVR) generates reactive oxygen species (ROS) that can deplete endogenous antioxidants and cause acute and chronic changes to the skin, such as sunburns, depigmentation, photoaging, and cutaneous malignancy [15,16].
Several studies have shown the clinical efficacy of topical and oral antioxidant supplementation on mitigating UV-mediated damage by neutralizing free radicals and protecting the skin. Vitamins C and E are naturally strong antioxidants and can be combined with other antioxidant supplementation to promote cutaneous radical scavenging activity after UV radiation, as shown in many studies [16,17]. One such study used electron paramagnetic resonance in subjects who consumed a vitamin C supplement or a chokeberry peel extract (Aronia) [17]. Oral supplementation with vitamin C and Aronia significantly increased the radical scavenging capacity of the skin by 22% and 23%, respectively [17]. Another study demonstrated that a stable and permeable vitamin C derivative, tetra-isopalmitoyl ascorbic acid (VC-IP), could effectively suppress UVB-induced skin pigmentation, reduce melanocyte proliferation, and reduce oxidative stress [18]. Additionally, a study analyzed the effect of long-term oral intake of vitamin D and E analogues for 3 months and found a significant reduction in sunburn reaction and significantly fewer thymine dimers following UVB exposure [16]. Further, the effects of a topical antioxidant mixture consisting of vitamin C, ferulic acid, and phloretin, a natural phenol, showed the increases in sunburn cell formation and thymine dimer formation to be attenuated in subjects treated with the topical antioxidant cream compared with the vehicle control [19]. Pretreatment of the skin with the cream also blocked the suppression of CD1a-expressing Langerhans cells after UV irradiation [19]. The unique topical antioxidant mixture of vitamin C and ferulic acid was further analyzed but with vitamin E instead of phloretin [20]. Subjects who received a topical antioxidant solution containing 15% vitamin C, 1% vitamin E, and 0.5% ferulic acid (CEFer) experienced a significantly decreased erythema and total number of sunburn cells. Immunohistochemical analyses also revealed a significant decrease in thymine dimer formation [20].
Tocopherols and tocotrienols, two major forms of vitamin E, were also studied [21]. The prophylactic efficacy of a topical agent containing tocopherols 10% and tocotrienols 0.3% was evaluated [21]. Erythema was significantly lower in areas treated with the topical vitamin E formulation compared with those treated with the simple vehicle or vitamin A [21]. Thus, high concentrations of tocotrienols and tocopherols in a topical formulation show a promising strategy to reduce photoinduced skin damage. Vitamin E is also a popular active ingredient found in sunscreens in the form of alpha-tocopherol acetate [22]. A study evaluated if topical application of alpha-tocopherol acetate is absorbed in human skin and metabolizes to alpha-tocopherol, the free form of vitamin E, since it is known that the free unesterified form of alpha-tocopherol significantly reduces experimental UVB carcinogenesis [22]. The study found no evidence of the systemic availability or biotransformation of topically applied alpha-tocopherol acetate to the free form of alpha-tocopherol in plasma or skin, which may be concerning since many commercial sunscreens and lotions contain this synthetic form of vitamin E, which does not protect against UV radiation [22].
Additional antioxidant supplements have been investigated for their photoprotective capacities. Polyphenols, such as the natural botanical compound resveratrate, have shown powerful antioxidant capacities [23]. Resveratrate-treated skin decreased the appearance of erythema and resulted in a significant reduction in the formation of sunburn cells after UVR exposure [23]. Further, topical 12.5% melatonin cream also demonstrated the ability to protect against erythema induced by UVR from natural sunlight by acting as a radical scavenger [24]. Additionally, reduced glutathione (GSH) has important properties in the protection of UVB-induced damage to DNA [25]. A study evaluated the efficacy of the application of a cream containing GSH conjugated to a long-chain polyunsaturated fatty acid called S-linolenoyl-glutathione (Lin-GSH) and found that Lin-GSH was able to significantly inhibit erythema [25]. A portion of this observed protective effect exerted by the Lin-GSH cream was attributed to molecular absorption of the UV radiation by multiple double bonds of the hydrocarbon chain of unsaturated fatty acids [25]. Additionally, topical administration of a dried melon juice concentrate, particularly rich in superoxide dismutase (SOD), showed promising results in reducing UV-induced cytotoxicity [15]. The topical melon juice concentrate demonstrated a significant increase in minimal erythema dose (MED), defined as the amount of UV radiation that will produce noticeable redness on an individual’s skin 24 h after sun exposure [15]. Topical melon concentrate application significantly decreased the formation of sunburn cells, or keratinocytes, undergoing apoptosis after irradiation in human skin explants compared with the placebo cream [15]. The level of glutathione peroxidase-1 (GPx-1), catalase (CAT), and SOD were significantly increased in irradiated skin explants after topical application of the active cream when compared with untreated explants and placebo-irradiated skin [15]. Further, the combination of antioxidants with DNA-repair enzyme complexes has shown improvement in the genomic and proteomic integrity of skin cells exposed to UVR [26].
Three studies analyzed the efficacy of oral supplementation with beta-carotene, a known antioxidant, compared with sunscreen for protection against skin cancer [27,28,29]. Daily beta-carotene supplementation did not reduce the incidence of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) after a 4.5-year follow-up compared to that with daily sunscreen with a sun protection factor of 15 [27]. A separate long-term placebo-controlled trial found that 12 years of beta-carotene supplementation did not affect the incidence of BCC and SCC [28]. Further, a separate study conducted over four years randomly assigned subjects to daily sunscreen use or beta-carotene supplement, and the incidence of solar keratoses (SKs) was measured since SK is a strong determinant of skin cancer. It was found that sunscreen was able to slow the rate of SK acquisition, but beta-carotene supplementation did not influence the occurrence of SKs [29].
Landmark articles describing the general utility of antioxidant supplementation for the treatment of dermatologic disease, to date, are summarized in Table 1.
Table 1.
Summary of studies retrieved to develop a primer on the utility of antioxidant supplementation for dermatologic diseases (n = 15).
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Egoumenides, 2018 [15] | Randomized controlled trial |
|
| Placzek, 2005 [16] | Controlled trial |
|
| Meinke, 2012 [17] | Randomized controlled trial |
|
| Ochiai, 2006 [18] | Controlled trial |
|
| Oresajo, 2008 [19] | Randomized controlled trial |
|
| Murray, 2008 [20] | Controlled trial |
|
| Pedrelli, 2012 [21] | Controlled trial |
|
| Alberts, 1996 [22] | Controlled trial |
|
| Wu, 2013 [23] | Randomized controlled trial |
|
| Scheuer, 2017 [24] | Randomized controlled trial |
|
| Grandi, 2019 [25] | Randomized controlled trial |
|
| Emanuele, 2014 [26] | Controlled trial |
|
| Green, 2000 [27] | Randomized controlled trial |
|
| Frieling, 2000 [28] | Randomized controlled trial |
|
| Darlington, 2003 [29] | Randomized controlled trial |
|
3.2. Non-Cancer Utility
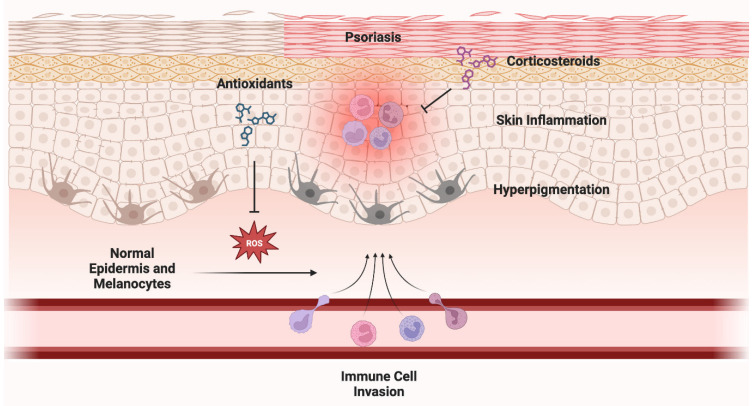
Numerous studies have demonstrated the efficacy of antioxidant supplementation against various dermatoses [30,31,32]. For rosacea, oral diammonium glycyrrhizinate combined with oral clarithromycin, or isotretinoin, was more effective and rapid than clarithromycin or isotretinoin alone in treating rosacea primarily characterized by papules and pustules [30]. Turmeric has demonstrated antioxidant and anti-inflammatory effects and increases skin moisture. It has proven effective in reducing thickness, erythema, pruritus, burning, and pain in psoriasis lesions and improving radiodermatitis lesions [31]. Moreover, a trial led by Dall’oglio and colleagues reported preliminary evidence that prescription-strength (15%) azelaic acid with 1% dihydroavenanthramide D yielded a significant decrease in inflammatory lesions and erythema associated with inflammatory rosacea, which could serve as an adjunct to the current therapeutic interventions for managing inflammatory rosacea [32]. See Figure 3 for exemplification of the role of ROS in the development of non-cancer skin conditions. Landmark articles describing the utility of antioxidant supplementation for the treatment of non-cancer dermatologic conditions are summarized in Table 2.
Figure 3.
Role of ROS in the development of non-cancer skin conditions. ROS can lead to 2 major outcomes: (1) skin inflammation and (2) hyperpigmentation through activation of melanocytes. ROS have been implicated in the development of erythema, psoriasis, rosacea, and acne vulgaris. Studies have shown that antioxidants (sometimes combined with corticosteroids) can drastically improve these conditions. Figure created with BioRender.com.
Table 2.
Summary of studies retrieved to remark on the utility of antioxidant supplementation for non-cancer skin conditions (n = 17).
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Xie, 2022 [30] | Randomized controlled trial |
|
| Barbalho, 2021 [31] | Systematic review |
|
| Dall’Oglio, 2021 [32] | Open-label trial |
|
| Al-Oudah, 2022 [33] | Clinical trial |
|
| Xu, 2022 [34] | Controlled trial |
|
| Bahraini, 2018 [35] | Randomized controlled trial |
|
| Mason, 2013 [36] | Systematic review |
|
| Al-Katib, 2018 [37] | Randomized controlled trial |
|
| Ahmad, 2023 [38] | Randomized controlled trial |
|
| Iraji, 2022 [39] | Randomized controlled trial |
|
| Javanbakht, 2010 [40] | Randomized controlled trial |
|
| Maralit Bruan, 2019 [41] | Clinical trial |
|
| Panahi, 2012 [42] | Randomized controlled trial |
|
| Woolery-Lloyd, 2010 [43] | Randomized controlled trial |
|
| Shubber, 2020 [44] | Randomized controlled trial |
|
| Klock, 2005 [45] | Clinical study |
|
| Kus, 2005 [46] | Clinical trial |
|
Antioxidant supplementation has also evidenced efficacy for psoriasis. Studies suggest oral CoQ10 adjuvant therapy may be an effective supplement as it improves the correlation between the Psoriasis Area and Severity Index (PASI) and the Dermatology Life Quality Index (DLQI) after 12 weeks of treatment [33]. A second indicated antioxidant is astilbin, a component of Rhizoma smilacis glabrae that may function against autoimmune diseases [34]. Topical administration of low-dose astilbin has reduced psoriasis-like lesions and psoriasis-specific cytokine expression, thus attenuating psoriasis [34]. Oral supplementation with turmeric tonic was also shown to significantly reduce erythema, scaling, and induration of lesions and improve the overall quality of life in patients [35]. Compared with the control group, turmeric tonic resulted in significant improvements in the management of scalp psoriasis [35]. A review on vitamin D effectiveness in patients with chronic plaque psoriasis found that when vitamin D analogues were used on the body, they were significantly more effective than the placebo [36]. For scalp psoriasis, calcipotriol monotherapy alone was considerably less effective than corticosteroids; additionally, potent corticosteroids were less likely than calcipotriol to cause local adverse reactions such as burning or irritation [36]. However, for both body and scalp psoriasis, the combined treatment of topical corticosteroids with vitamin D analogues performed significantly better than vitamin D analogues or corticosteroids alone, with minimal side effects [36]. Unlike vitamin D, oral vitamin C has demonstrated insignificant effects against psoriasis [37]. Although oral vitamin C supplementation directly correlated with GSH levels, a weak, negative, and negligible correlation was found between vitamin C and PASI scores [37].
Additional supplements have been investigated for various dermatoses. The herbal supplements Majoon Ushba (MU) and Marhaam Raal (MR), which have antioxidant, antimicrobial, antifungal, and anti-inflammatory properties, were utilized topically against tinea corporis [38]. MU and MR demonstrated equal effectiveness against tinea corporis compared to traditional/conventional treatments as measured by erythema, scaling, margins, size of the lesion, and itching [38]. The herbal cream containing silymarin and the antioxidant fumaria officinalis may help improve the severity and symptoms of atopic dermatitis in patients, comparable to the control mometasone cream [39]. Additionally, vitamins D(3) and E (600 IU synthetic all-rac-ɑ-tocopherol) may be beneficial against atopic dermatitis [40]. Javanbakht et al. showed significant reductions in SCORing Atopic Dermatitis (SCORAD) in groups receiving vitamin D, E, or D and E [40]. Further, the antioxidant 4% gumamela leaf extract ointment may be effective in venous leg ulcer (VLU) closures [41]. Findings showed that the gumamela leaf extract ointment used with compression stockings closed VLUs in less than 12 weeks in 83% of patients (N = 12) [41]. Oral curcumin supplementation was also investigated against chronic pruritis induced by sulfur mustard (SM) exposure [42]. Curcumin supplementation was associated with significant reductions in pruritus severity [42]. Polyphenon E, a green tea catechin, has also been indicated as a treatment against HPV warts. Polyphenon E 15% and 10% ointment demonstrated significant effectiveness in the complete clearance of baseline and new external anogenital warts (EGWs), as well as possibly lowering recurrence rates [42]. Polyphenon, however, was not compared with the principal treatment, imiquimod [42].
Moreover, several studies examined the effects of antioxidant supplementation against acne vulgaris. Multiple vitamin C analogues were investigated since vitamin C is too unstable [43,44,45]. A study has indicated Sodium L-ascorbyl-2-phosphate (APS) as effective as a monotherapy for the treatment of acne, as it showed improvement in 61% of subjects as measured by the investigator (n = 50) [43]. Further, vitamin C capsules taken with doxycycline are significantly more efficient than doxycycline alone therapeutically and in reducing serum levels of IL-8, IL-1β, IFN-γ, TNF-α, and TLR-2 [44]. Sodium ascorbyl phosphate (SAP) at 1% demonstrated a strong antimicrobial effect as well as effectiveness in the treatment and prevention of acne vulgaris with minimal side effects [45]. Lastly, topical vitamin E proved ineffective in reducing the side effects of isotretinoin for the treatment of acne vulgaris [46].
3.3. Anti-Cancer Utility
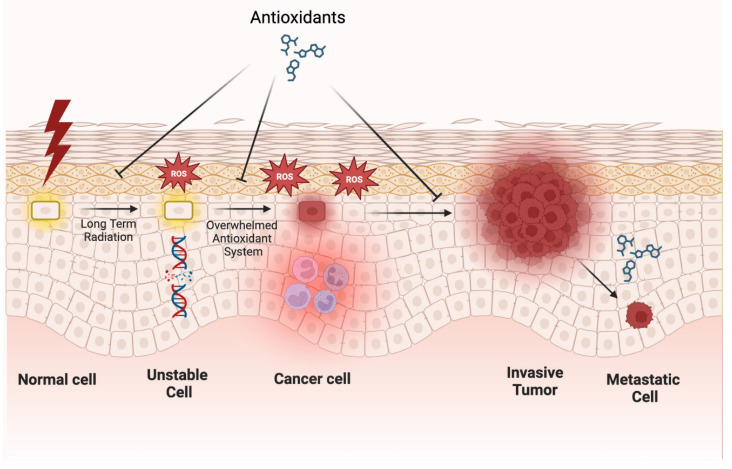
Skin cancer can arise when skin cells are exposed to highly unstable reactive oxygen species (ROS), which, in excess, are associated with the proliferation of cancer cells. ROS can be generated after the skin is exposed to ultraviolet radiation (UVR), leading to oxidative stress if antioxidant levels are too low to compensate for increased DNA damage caused by ROS. While the antioxidant system in the skin is normally well-equipped to manage oxidative stress, chronic or excessive exposure to UVR or other oxidizing agents can overwhelm the system and result in melanoma [47]. See Figure 4 for a representation of the role of ROS in the development of skin cancer.
Figure 4.
Role of ROS in the development of skin cancer. Long-term UV radiation can lead to ROS, which can lead to DNA damage. The combination of ROS and DNA damage can overwhelm a cell’s inherent antioxidant system, eventually leading to a cancer cell. Antioxidants have been shown to greatly inhibit the development of skin cancer. Interestingly, oxidative stress can inhibit the development of metastatic cells, and antioxidants can promote the development of these cells. Figure created with BioRender.com.
The antioxidant system acts as a defense against melanoma in that it will either work to prevent the generation of ROS and eliminate any free radicals, which are similarly highly reactive, or it will function through a repair circuit to remove damaged biomolecules, preventing their accumulation and the subsequent effect on cell function [47]. A key component of the antioxidant defense system is manganese-superoxide dismutase (MnSOD). Poswig et al. found that the adaptive antioxidant response of manganese-superoxide dismutase can be induced following repeated exposure to UVR, with increasing induction of the MnSOD antioxidant mRNA quantity and degree of activity observed after UVR in fibroblasts [48]. While the MnSOD antioxidant seems to be linked positively to UVR, not all individuals’ MnSOD increases with UVR. Furthermore, Poswig et al.’s association between MnSOD levels and UVR does not necessarily imply all antioxidants respond identically to UVR exposure. Using mouse skin, Fuchs et al. showed that acute UVR exposure led to a decrease in glutathione reductase and catalase activity in mouse skin, but to insignificant changes in superoxide dismutase and glutathione peroxidase, all of which are antioxidants [49].
In addition to endogenous antioxidants, dietary exogenous antioxidants such as vitamins A, C, and E may offer a source of protection against melanoma, for which UVR exposure is a major risk factor. Elmore (2005) reported evidence that magnesium ascorbyl phosphate (which contains the exogenous antioxidant ascorbic acid) administered immediately after exposure in hairless mice significantly delayed skin-tumor formation and hyperplasia induced by chronic exposure to UVR and that ascorbic acid applied to mice and pig skin prior to UVR reduced skin-cancer-related damage [50]. Evidence of magnesium ascorbyl phosphate’s toxicity was obtained at ingested levels significantly higher than what one would encounter in typical cosmetic products with magnesium ascorbyl phosphate or ascorbic acid.
Another exogenous antioxidant, silymarin, has been shown to have chemopreventive effects against chemical carcinogenesis as well as photocarcinogenesis in various animal tumor models [51]. Topical treatment of silymarin inhibited several tumor promoters and showed evidence of preventing UVR-induced skin carcinogenesis [51]. Important to note is the difference in the antioxidant’s administration route within these studies. While silymarin was applied topically, magnesium ascorbyl phosphate was injected peritoneally in animal models. The means of delivery may have some effect on the antioxidant response. Another limitation to these studies is that most were conducted on animal or human in vitro models. Human in vivo studies are preferred for studying the utility of antioxidant supplementation in humans. In human subjects, dietary beta-carotene has been found to manage UVR-induced DNA damage in the skin in vivo [52]. Other dietary antioxidants such as curcumin and nobiletin, found naturally in turmeric and citrus fruits, respectively, were shown to have an anti-tumor initiating effect when used to treat tumors experimentally initiated by the reactive oxidant peroxynitrite [53]. In animal models, topical treatment with or oral consumption of green tea polyphenols (GTP) inhibited chemical carcinogen- or UVR-induced skin carcinogenesis [54]. High concentrations of selenium applied topically through serums were associated with a lower risk of basal or squamous cell carcinoma [55].
While endogenous and dietary antioxidants have been shown to protect against cancer, some more recent contradictory clinical findings have found a carcinogenic effect of antioxidants. Le Gal et al. found oral administration of the antioxidant N-acetylcysteine (NAC) increased lymph node metastases in an endogenous mouse model of malignant melanoma [56]. Additionally, NAC and the soluble vitamin E analogue Trolox markedly increased the migration and invasive properties of human malignant melanoma cells, but notably did not affect their proliferation [56]. Another study found that a combination of dietary antioxidants [120 mg vitamin C (sodium ascorbate), 30 mg vitamin E (dl-α-tocopherol), 6 mg β-carotene, 100 μg selenium (selenium-enriched yeast), and 20 mg zinc (zinc gluconate)] increased the risk of melanoma in female subjects, but interestingly not in male subjects, possibly attributed to metabolism differences between men and women [57]. Further linking dietary antioxidants and the risk of cancer are findings that the supplementation of vitamins C and A accelerates malignant melanoma metastasis in mice [58]. Piskounova et al. provide additional evidence that antioxidants can promote cancer metastasis. In mouse models of melanoma, the authors found that levels of oxidative stress were higher in circulating cancer cells than in primary-tumor cancer cells [59]. They found oxidative stress interfered with the formation of metastatic tumors, contrary to the current line of thinking that oxidative stress is solely carcinogenic. Treating these mice with antioxidants decreased oxidative stress in the circulating cancer cells and increased their ability to metastasize. In another study investigating the potential delayed effect of oral antioxidant supplementation on skin cancer incidence, after a 5-year post-intervention follow-up, it was found that the elevated melanoma risk associated with the original antioxidant treatment group receded when antioxidant supplementation was stopped, suggesting a potential causative role of antioxidant supplementation in the appearance of melanomas [60]. Many of these studies find a link between antioxidants and melanoma risk, but more research is needed to understand the different roles antioxidants have in various contexts, as well as to broaden the investigation of the effect of antioxidants to UVR-induced skin cancers other than melanoma.
Landmark articles describing the utility of antioxidant supplementation for the treatment of skin cancer are summarized in Table 3.
Table 3.
Summary of studies retrieved to remark on the anti-skin-cancer utility of antioxidant supplementation (n = 14).
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Godic, 2014 [47] | Review |
|
| Poswig, 1999 [48] | Randomized controlled trial |
|
| Fuchs, 1989 [49] | Randomized controlled trial |
|
| Elmore, 2005 [50] | Review |
|
| Katiyar, 2005 [51] | Randomized controlled trial |
|
| Cho, 2010 [52] | Randomized controlled trial |
|
| Nishino, 2004 [53] | Randomized controlled trial |
|
| Katiyar, 2003 [54] | Randomized controlled trial |
|
| van der Pols, 2009 [55] | Prospective cohort study |
|
| Le Gal, 2015 [56] | Randomized controlled trial |
|
| Hercberg, 2007 [57] | Randomized controlled trial |
|
| Kashif, 2023 [58] | Randomized controlled trial |
|
| Piskounova, 2015 [59] | Randomized controlled trial |
|
| Ezzedine, 2010 [60] | Randomized controlled trial |
|
3.4. Emerging Research
There is a magnitude of new advances in antioxidant supplementation. Burke and colleagues found that concentrated ascorbic acid in DMSO is a well-tolerated, inexpensive, and easy-to-use topical preparation that was superior to 5% topical imiquimod at 8 weeks and non-inferior at 12 weeks for low-risk nodular and superficial BCC [61,62,63]. The presence or absence of residual tumor was determined using a 2 mm punch biopsy after 8 weeks of treatment. Non-resolved lesions that received topical imiquimod treatment were treated an additional 4 weeks. Ascorbic acid was also associated with fewer adverse effects than imiquimod, although there was no significant difference in recurrence during a 30-month observation period [61]. This new topical treatment could potentially reduce costs and improve outcomes for BCC, particularly for lesions on the face where surgical scarring and cosmesis are significant concerns. While the study did not explore the mechanism of action of ascorbic acid on nodular BCC, which can extend several millimeters in depth, Burke and Bailie hypothesized that indirect inflammation-related effects may be involved [61].
Larger controlled clinical trials are needed to confirm these preliminary findings [62,63,64], with desired aims to identify cotreatment options and ideal dosing regimens informed by a better understanding of the mechanisms of action [64,65,66]. Furthermore, Farajzadeh et al. recently evaluated the effectiveness of carboxytherapy combined with narrowband-ultraviolet B (NB-UVB) in treating vitiligo, with results indicating that combination therapy resulted in significantly higher rates of repigmentation compared with NB-UVB alone, and there were no significant differences based on the demographic or clinical features of the patients [67].
Several double-blind clinical trials showcase higher clinical efficacy through probiotic supplementation [68,69]. The objective of one study was to assess the efficacy of a food supplement containing specific strains of probiotics in ameliorating atopic dermatitis (AD) symptoms and skin conditions in adult participants [69]. The research involved a randomized controlled trial with 80 adults exhibiting mild-to-severe AD symptoms who received either a placebo or the probiotic mixture for a period of 56 days. The group who received the probiotics experienced an improvement in skin smoothness, moisturization, and self-perception, as well as a reduction in inflammatory markers linked to AD. This positive trend continued for a month after the cessation of the supplement. The study concluded that the administration of the chosen probiotic strains led to a prompt and sustained improvement in AD-related symptoms and skin conditions. A second study by Moludi et al. aimed to evaluate the effects of probiotics on the quality of life, oxidative stress, inflammatory markers, and clinical outcome of psoriasis patients [68]. Fifty patients were randomized into two groups, one receiving a probiotic drink with Lactobacillus strains for eight weeks and the other receiving a placebo. The probiotic group showed significant improvements in depression scores, quality of life, psoriasis area and severity index, psoriasis symptom scale, total antioxidant capacity levels, and decreased levels of inflammatory markers. The study suggests that probiotics may improve the quality of life and inflammatory biomarkers in psoriatic patients. Further studies are needed to establish probiotics as a routinely prescribed therapy for inflammatory dermatoses. The range of probiotic research seeking to improve dermatological diseases can benefit from future research in a few key areas. The development of specific probiotic formulations may lead to higher success. The creation of such formulations is dependent on an understanding of the probiotic’s mechanism of action as well as the role of the gut microbiome in mediating probiotic effects. By better characterizing how probiotics work and what influences their effect, further research can personalize and specialize probiotic therapy, further leading to improved clinical outcomes.
Landmark articles describing emerging findings in antioxidant supplementation are summarized in Table 4.
Table 4.
Summary of studies retrieved to remark on emerging evidence for antioxidant supplementation for dermatologic conditions (n = 9).
| Author, Year | Study Design | Key Findings |
|---|---|---|
| Burke, 2022 [61] | Randomized controlled trial |
|
| Huang, 2020 [62] | Meta-analysis |
|
| Bánvölgyi, 2020 [63] | Controlled trial |
|
| Neville, 2007 [64] | Controlled trial |
|
| Shumack, 2002 [65] | Randomized controlled trial |
|
| Holló, 2016 [66] | Controlled trial |
|
| Farajzadeh, 2022 [67] | Randomized controlled trial |
|
| Moludi, 2021 [68] | Randomized controlled trial |
|
| Michelotti, 2021 [69] | Randomized controlled trial |
|
4. Discussion
The role of antioxidants in the prevention and treatment of numerous dermatoses has been examined by recent papers, highlighting a link between antioxidant imbalance and dermatological diseases. The skin serves an essential role as the body’s first line of defense against pathogens, toxins, and ultraviolet (UV) radiation [8]. Its frequent exposure to environmental stressors, including UV radiation, pollution, and lifestyle habits such as smoking, can lead to oxidative stress and damage to the skin that increases the risk of cutaneous diseases [70]. Oxidative stress occurs when an imbalance exists between the production of ROS and antioxidant defense mechanisms, where ROS levels exceed the body’s antioxidant defenses, causing cellular damage [71]. This damage can lead to the onset and/or progression of dermatological diseases and disorders, such as photoaging, pigmentation disorders, atopic dermatitis, psoriasis, and skin cancer [72]. Recent studies depict the promising utility of antioxidant supplementation in both the prevention and treatment of skin diseases and disorders through the promotion of maintaining the physiological balance of the skin barrier by reducing oxidative stress and inflammatory processes [73].
Several antioxidant agents, such as vitamin C, vitamin E, polyphenols, and carotenoids, have demonstrated efficacy in neutralizing ROS to prevent oxidative damage associated with inducing or irritating various dermatoses [8,74]. Previous research has identified a role for oxidative stress in pigmentation disorders, such as melasma and post-inflammatory hyperpigmentation, where ROS activate melanocytes, leading to increased melanin production and subsequent hyperpigmentation [75]. This led to investigations of numerous antioxidants for melasma therapy that suggested the use of antioxidants as a monotherapy or in combination with other melasma therapies [76]. Inflammatory skin diseases, such as atopic dermatitis and psoriasis, have found that oxidative stress is involved in the pathogenesis of these diseases [77,78].
Multiple studies have investigated the use of antioxidant supplements in the prevention of skin cancer. Antioxidants neutralize free radicals and enhance DNA enzyme repair systems [9]. The DNA-repair capacity of human skin cells is, therefore, related to the carcinogenesis initiation probability. Accumulating evidence depicts how dietary changes and supplementation with specific micronutrients may help prevent oxidative stress and the formation of free radicals that promote the skin-damage process [47]. A randomized controlled trial of 386 women found that oral supplementation with selenium, vitamin E, and beta-carotene reduced the incidence of basal cell carcinoma [79]. Since the human body cannot synthesize exogenous antioxidants like vitamins C and E, they must be obtained through the diet [80]. Although endogenous and dietary antioxidants have demonstrated some efficacy in protecting against cancer, some recent clinical studies reported contradictory findings, with antioxidants having a carcinogenic effect. These studies note how chronic antioxidant consumption could foster harmful side effects that increase the risk of developing malignancies [81]. Moreover, emerging research in antioxidant supplementation characterizes promising new therapeutics in the form of topical treatments, combination therapy, and probiotic supplementation that offer antioxidant properties aimed at improving outcomes of dermatological diseases [61,68,69].
A primary limitation of this present review is the lack of available clinical data conducted on a larger scale to strengthen conclusions drawn about the role of antioxidant supplementations. It is important to note that many of these studies revealed an in vitro role of antioxidants and investigated biomarkers in different tissues employing different methods. The results of in vitro study may not necessarily correspond to clinical outcomes [8]. Additionally, although some of the studies, especially the ones on topical antioxidants, are well-designed, they may contain biases as they are promoted by cosmetic enterprises, which can influence the research agenda in favor of corporate interest. Additional long-term studies are warranted to validate our findings and shed light on the potential clinical and therapeutic implications. With these limitations in mind, some considerations on the role of antioxidant supplementation in various dermatoses can be postulated from the literature data available. Antioxidant supplementation is a promising approach for improving skin health and preventing the development of skin diseases and disorders.
5. Conclusions
Here, we provided a systematic appraisal and critique of the available body of relevant literature on antioxidant supplementation for dermatologic health. This review collates varying levels of evidence on the effects of antioxidants in the prevention and treatment of dermatological diseases and disorders, revealing notable associations between the imbalance of antioxidant systems and various dermatoses. Existing data are accumulating on the efficacy of antioxidant supplementation in promoting skin health. Strengths of this review include the adherence to current PRISMA guidelines and the inclusion of multiple literature types. Potential limitations include the lack of a formal qualitative analysis and data synthesis. Further long-term studies on a larger scale are needed to corroborate the large wealth of literature data available on the role of antioxidants in dermatology to determine the implications for clinical applications, and we hope that future research will expand on these salient aspects.
Author Contributions
Conceptualization, J.T.T. and M.J.D.; methodology, J.T.T., M.J.D. and P.A.; validation, S.B. and M.F.; formal analysis, J.T.T., M.J.D., D.R., G.K., S.A., S.P. and P.A.; writing—original draft preparation, J.T.T., M.J.D., D.R., G.K., S.A., S.P. and P.A.; writing—review and editing, J.T.T., M.J.D., D.R., G.K., S.A., S.P. and P.A.; visualization, K.T.; supervision, M.F. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Seth D., Cheldize K., Brown D., Freeman E.F. Global Burden of Skin Disease: Inequities and Innovations. Curr. Dermatol. Rep. 2017;6:204–210. doi: 10.1007/s13671-017-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelman D., Fuller L.C., Solomon A.W., McCarthy J.S., Hay R.J., Lammie P.J., Steer A.C. Opportunities for Integrated Control of Neglected Tropical Diseases That Affect the Skin. Trends Parasitol. 2016;32:843–854. doi: 10.1016/j.pt.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Tschachler E., Bergstresser P.R., Stingl G. HIV-related skin diseases. Lancet Lond. Engl. 1996;348:659–663. doi: 10.1016/S0140-6736(96)01032-X. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; Oxford, UK: 2007. pp. 79–186. Antioxidant Defences: Endogenous and Diet Derived. [Google Scholar]
- 5.Gomes E.C., Silva A.N., de Oliveira M.R. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid. Med. Cell. Longev. 2012;2012:756132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valko M., Leibfritz D., Moncol J., Cronin M.T.D., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Addor F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017;92:356–362. doi: 10.1590/abd1806-4841.20175697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keen M.A., Hassan I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016;7:311–315. doi: 10.4103/2229-5178.185494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullar J.M., Carr A.C., Vissers M.C.M. The Roles of Vitamin C in Skin Health. Nutrients. 2017;9:866. doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darvin M.E., Lademann J., von Hagen J., Lohan S.B., Kolmar H., Meinke M.C., Jung S. Carotenoids in Human SkinIn Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants. 2022;11:1451. doi: 10.3390/antiox11081451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veritas Health Innovation; Melbourne, Australia: [(accessed on 10 March 2023)]. Covidence Systematic Review Software. Available online: www.covidence.org. [Google Scholar]
- 15.Egoumenides L., Gauthier A., Barial S., Saby M., Orechenkoff C., Simoneau G., Carillon J. A Specific Melon Concentrate Exhibits Photoprotective Effects from Antioxidant Activity in Healthy Adults. Nutrients. 2018;10:437. doi: 10.3390/nu10040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Placzek M., Gaube S., Kerkmann U., Gilbertz K.-P., Herzinger T., Haen E., Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J. Investig. Dermatol. 2005;124:304–307. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- 17.Meinke M.C., Lauer A.C., Haag S.F., Darvin M.E., Groth N., Lademann J. Cutaneous radical scavenging effects of orally administered antioxidants measured by electron paramagnetic resonance spectroscopy. E-SPEN J. 2012;7:e160–e166. doi: 10.1016/j.clnme.2012.06.001. [DOI] [Google Scholar]
- 18.Ochiai Y., Kaburagi S., Obayashi K., Ujiie N., Hashimoto S., Okano Y., Masaki H., Ichihashi M., Sakurai H. A new lipophilic pro-vitamin, C.; tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J. Dermatol. Sci. 2006;44:37–44. doi: 10.1016/j.jdermsci.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Oresajo C., Stephens T., Hino P.D., Law R.M., Yatskayer M., Foltis P., Pillai S., Pinnell S.R. Protective effects of a topical antioxidant mixture containing vitamin, C.; ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008;7:290–297. doi: 10.1111/j.1473-2165.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 20.Murray J.C., Burch J.A., Streilein R.D., Iannacchione M.A., Hall R.P., Pinnell S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008;59:418–425. doi: 10.1016/j.jaad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Pedrelli V.F., Lauriola M.M., Pigatto P.D. Clinical evaluation of photoprotective effect by a topical antioxidants combination (tocopherols and tocotrienols) J. Eur. Acad. Dermatol. Venereol. JEADV. 2012;26:1449–1453. doi: 10.1111/j.1468-3083.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- 22.Alberts D.S., Goldman R., Xu M.J., Dorr R.T., Quinn J., Welch K., Guillen-Rodriguez J., Aickin M., Peng Y., Loescher L., et al. Disposition and metabolism of topically administered alpha-tocopherol acetate: A common ingredient of commercially available sunscreens and cosmetics. Nutr. Cancer. 1996;26:193–201. doi: 10.1080/01635589609514475. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Jia L.L., Zheng Y.N., Xu X.-G., Luo Y.-J., Wang B., Chen J., Gao X.-H., Chen H.-D., Matsui M., et al. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J. Eur. Acad. Dermatol. Venereol. JEADV. 2013;27:345–350. doi: 10.1111/j.1468-3083.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- 24.Scheuer C. Melatonin for prevention of erythema and oxidative stress in response to ultraviolet radiation. Dan. Med. J. 2017;64:B5358. [PubMed] [Google Scholar]
- 25.Grandi V., Milanesi N., Sessa M., Gola M., Cappugi P., Pimpinelli N. Efficacy and safety of S-acyl glutathione 2% cream vs. placebo against UVB-induced erythema: A randomized, double-blinded clinical trial. G. Ital. Dermatol. E Venereol. Organo Uff. Soc. Ital. Dermatol. E Sifilogr. 2019;154:632–637. doi: 10.23736/S0392-0488.17.05603-6. [DOI] [PubMed] [Google Scholar]
- 26.Emanuele E., Spencer J.M., Braun M. An experimental double-blind irradiation study of a novel topical product (TPF 50) compared to other topical products with DNA repair enzymes, antioxidants, and growth factors with sunscreens: Implications for preventing skin aging and cancer. J. Drugs Dermatol. JDD. 2014;13:309–314. [PubMed] [Google Scholar]
- 27.Green A., Williams G., Neale R. Does daily use of sunscreen or beta-carotene supplements prevent skin cancer in healthy adults? West. J. Med. 2000;173:332. doi: 10.1136/ewjm.173.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frieling U.M., Schaumberg D.A., Kupper T.S., Muntwyler J., Hennekens C.H. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch. Dermatol. 2000;136:179–184. doi: 10.1001/archderm.136.2.179. [DOI] [PubMed] [Google Scholar]
- 29.Darlington S., Williams G., Neale R., Frost C., Green A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch. Dermatol. 2003;139:451–455. doi: 10.1001/archderm.139.4.451. [DOI] [PubMed] [Google Scholar]
- 30.Xie Y., Huang J., Liu J., Zhang Q. Efficacy of diammonium glycyrrhizinate in the treatment of rosacea with papules and pustules: A randomized, double-blind, placebo-controlled study. Dermatol. Ther. 2022;35:e15905. doi: 10.1111/dth.15905. [DOI] [PubMed] [Google Scholar]
- 31.Barbalho S.M., de Sousa Gonzaga H.F., de Souza G.A., de Alvares Goulart R., de Sousa Gonzaga M.L., de Alvarez Rezende B. Dermatological effects of Curcuma species: A systematic review. Clin. Exp. Dermatol. 2021;46:825–833. doi: 10.1111/ced.14584. [DOI] [PubMed] [Google Scholar]
- 32.Dall’Oglio F., Tedeschi A., Lacarrubba F., Fabbrocini G., Skroza N., Chiodini P., Micali G. A novel azelaic acid formulation for the topical treatment of inflammatory rosacea: A multicentre, prospective clinical trial. J. Cosmet. Dermatol. 2021;20:28–31. doi: 10.1111/jocd.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Oudah G.A., Sahib A.S., Al-Hattab M.K., Al-Ameedee A.A. Effect of CoQ10 Administration to Psoriatic Iraqi Patients on Biological Therapy Upon Severity Index (PASI) and Quality of Life Index (DLQI) Before and After Therapy. J. Popul. Ther. Clin. Pharmacol. J. Ther. Popul. Pharmacol. Clin. 2022;29:e52–e60. doi: 10.47750/jptcp.2022.931. [DOI] [PubMed] [Google Scholar]
- 34.Xu Q., Liu Z., Cao Z., Shi Y., Yang N., Cao G., Zhang C., Sun R., Zhang C. Topical astilbin ameliorates imiquimod-induced psoriasis-like skin lesions in SKH-1 mice via suppression dendritic cell-Th17 inflammation axis. J. Cell. Mol. Med. 2022;26:1281–1292. doi: 10.1111/jcmm.17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahraini P., Rajabi M., Mansouri P., Sarafian G., Chalangari R., Azizian Z. Turmeric tonic as a treatment in scalp psoriasis: A randomized placebo-control clinical trial. J. Cosmet. Dermatol. 2018;17:461–466. doi: 10.1111/jocd.12513. [DOI] [PubMed] [Google Scholar]
- 36.Mason A.R., Mason J., Cork M., Dooley G., Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst. Rev. 2013;3:CD005028. doi: 10.1002/14651858.CD005028.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Katib S.R., Al-Wakeel H.A., Al-Rawaf R.F. Role of vitamin c as antioxidant in psoriasis patients treated with NB-UVB phototherapy. Indian J. Public Health Res. Dev. 2018;9:375. doi: 10.5958/0976-5506.2018.01372.4. [DOI] [Google Scholar]
- 38.Ahmad M., Mobeen A. Efficacy and safety of Majoon Ushba oral and Marham Raal topical in tinea corporis—A randomized open-labeled active-controlled clinical trial. Explore. 2023 doi: 10.1016/j.explore.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Iraji F., Sharif Makhmalzadeh B., Abedini M., Aghaei A., Siahpoush A. Effect of herbal cream containing Fumaria officinalis and silymarin for treatment of eczema: A randomized double-blind controlled clinical trial. Avicenna J. Phytomed. 2022;12:155–162. doi: 10.22038/AJP.2022.19492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javanbakht M.H., Keshavarz S.A., Djalali M., Siassi F., Eshraghian M.R., Firooz A., Seirafi H., Ehsani A.H., Chamari M., Mirshafiey A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J. Dermatol. Treat. 2011;22:144–150. doi: 10.3109/09546630903578566. [DOI] [PubMed] [Google Scholar]
- 41.Maralit Bruan M.J., Tianco E.A. Efficacy and Safety of 4% Hibiscus rosa-sinensis Leaf Extract Ointment as an Adjunct Treatment to Compression Stockings on the Closure of Venous Leg Ulcers: A Pilot Study. Wounds Compend. Clin. Res. Pract. 2019;31:236–241. [PubMed] [Google Scholar]
- 42.Panahi Y., Sahebkar A., Amiri M., Davoudi S.M., Beiraghdar F., Hoseininejad S.L., Kolivand M. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2012;108:1272–1279. doi: 10.1017/S0007114511006544. [DOI] [PubMed] [Google Scholar]
- 43.Woolery-Lloyd H., Baumann L., Ikeno H. Sodium L-ascorbyl-2-phosphate 5% lotion for the treatment of acne vulgaris: A randomized, double-blind, controlled trial. J. Cosmet. Dermatol. 2010;9:22–27. doi: 10.1111/j.1473-2165.2010.00480.x. [DOI] [PubMed] [Google Scholar]
- 44.Al Mukhtar E.J., Shubber Z.I.J., Al-Shibly I.K. Clinical and Immunological Response to Doxycycline Versus Doxycycline Plus Vitamin C in Patients with Acne Vulgaris. Int. J. Pharm. Qual. Assur. 2020;11:69–75. doi: 10.25258/ijpqa.11.1.10. [DOI] [Google Scholar]
- 45.Klock J., Ikeno H., Ohmori K., Nishikawa T., Vollhardt J., Schehlmann V. Sodium ascorbyl phosphate shows in vitro and in vivo efficacy in the prevention and treatment of acne vulgaris. Int. J. Cosmet. Sci. 2005;27:171–176. doi: 10.1111/j.1467-2494.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 46.Kus S., Gün D., Demirçay Z., Sur H. Vitamin E does not reduce the side-effects of isotretinoin in the treatment of acne vulgaris. Int. J. Dermatol. 2005;44:248–251. doi: 10.1111/j.1365-4632.2004.02072.x. [DOI] [PubMed] [Google Scholar]
- 47.Godic A., Poljšak B., Adamic M., Dahmane R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014;2014:860479. doi: 10.1155/2014/860479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poswig A., Wenk J., Brenneisen P., Wlaschek M., Hommel C., Quel G., Faisst K., Dissemond J., Krieg T., Scharffetter-Kochanek K., et al. Adaptive antioxidant response of manganese-superoxide dismutase following repetitive UVA irradiation. J. Investig. Dermatol. 1999;112:13–18. doi: 10.1046/j.1523-1747.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 49.Fuchs J., Huflejt M.E., Rothfuss L.M., Wilson D.S., Carcamo G., Packer L. Acute effects of near ultraviolet and visible light on the cutaneous antioxidant defense system. Photochem. Photobiol. 1989;50:739–744. doi: 10.1111/j.1751-1097.1989.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 50.Elmore A.R. Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics. Int. J. Toxicol. 2005;24:51–111. doi: 10.1080/10915810590953851. [DOI] [PubMed] [Google Scholar]
- 51.Katiyar S.K. Silymarin and skin cancer prevention: Anti-inflammatory, antioxidant and immunomodulatory effects (Review) Int. J. Oncol. 2005;26:169–176. doi: 10.3892/ijo.26.1.169. [DOI] [PubMed] [Google Scholar]
- 52.Cho S., Lee D.H., Won C.H., Kim S.M., Lee S., Lee M.-J., Chung J.H. Differential effects of low-dose and high-dose beta-carotene supplementation on the signs of photoaging and type I procollagen gene expression in human skin in vivo. Dermatology. 2010;221:160–171. doi: 10.1159/000305548. [DOI] [PubMed] [Google Scholar]
- 53.Nishino H., Tokuda H., Satomi Y., Masuda M., Osaka Y., Yogosawa S., Wada S., Mou X.Y., Takayasu J., Murakoshi M., et al. Cancer prevention by antioxidants. BioFactors. 2004;22:57–61. doi: 10.1002/biof.5520220110. [DOI] [PubMed] [Google Scholar]
- 54.Katiyar S.K. Skin photoprotection by green tea: Antioxidant and immunomodulatory effects. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003;3:234–242. doi: 10.2174/1568008033340171. [DOI] [PubMed] [Google Scholar]
- 55.van der Pols J.C., Heinen M.M., Hughes M.C., Ibiebele T.I., Marks G.C., Green A.C. Serum antioxidants and skin cancer risk: An 8-year community-based follow-up study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009;18:1167–1173. doi: 10.1158/1055-9965.EPI-08-1211. [DOI] [PubMed] [Google Scholar]
- 56.Le Gal K., Ibrahim M.X., Wiel C., Sayin V.I., Akula M.K., Karlsson C., Dalin M.G., Akyürek L.M., Lindahl P., Nilsson J., et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015;7:308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 57.Hercberg S., Ezzedine K., Guinot C., Preziosi P., Galan P., Bertrais S., Estaquio C., Briançon S., Favier A., Latreille J., et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J. Nutr. 2007;137:2098–2105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 58.Kashif M., Yao H., Schmidt S., Chen X., Truong M., Tüksammel E., Liu Y., Bergo M.O. ROS-lowering doses of vitamins C and A accelerate malignant melanoma metastasis. Redox Biol. 2023;60:102619. doi: 10.1016/j.redox.2023.102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ezzedine K., Latreille J., Kesse-Guyot E., Galan P., Hercberg S., Guinot C., Malvy D. Incidence of skin cancers during 5-year follow-up after stopping antioxidant vitamins and mineral supplementation. Eur. J. Cancer Oxf. Engl. 1990. 2010;46:3316–3322. doi: 10.1016/j.ejca.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Burke B., Bailie J.E. Randomized trial of topical ascorbic acid in DMSO versus imiquimod for the treatment of basal cell carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2022;148:112710. doi: 10.1016/j.biopha.2022.112710. [DOI] [PubMed] [Google Scholar]
- 62.Huang C.M., Kirchhof M.G. Topical Imiquimod as a Treatment Option for Nodular Basal Cell Carcinoma: A Systematic Review. J. Cutan. Med. Surg. 2020;24:495–503. doi: 10.1177/1203475420931770. [DOI] [PubMed] [Google Scholar]
- 63.Bánvölgyi A., Lőrincz K., Kiss N., Avci P., Fésűs L., Szipőcs R., Krenács T., Gyöngyösi N., Wikonkál N., Kárpáti S., et al. Efficiency of long-term high-dose intravenous ascorbic acid therapy in locally advanced basal cell carcinoma—A pilot study. Postepy Dermatol. Alergol. 2020;37:548–558. doi: 10.5114/ada.2019.83027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neville J.A., Williford P.M., Jorizzo J.L. Pilot study using topical imiquimod 5% cream in the treatment of nodular basal cell carcinoma after initial treatment with curettage. J. Drugs Dermatol. JDD. 2007;6:910–914. [PubMed] [Google Scholar]
- 65.Shumack S., Robinson J., Kossard S., Golitz L., Greenway H., Schroeter A., Andres K., Amies M., Owens M. Efficacy of topical 5% imiquimod cream for the treatment of nodular basal cell carcinoma: Comparison of dosing regimens. Arch. Dermatol. 2002;138:1165–1171. doi: 10.1001/archderm.138.9.1165. [DOI] [PubMed] [Google Scholar]
- 66.Holló P., Jókai H., Hársing J., Soós G., Kárpáti S., Németh K. Topically applied ascorbic acid solution for the treatment of basal cell carcinoma (BCC) J. Am. Acad. Dermatol. 2016;75:212–213. doi: 10.1016/j.jaad.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Farajzadeh S., Yazdanpanah F., Khalili M., Mohammadi S., Iranmanesh B., Aflatoonian M. Combination of carboxytherapy with narrowband-ultraviolet B in the treatment of recalcitrant areas of vitiligo: A randomized clinical trial. Dermatol. Ther. 2022;35:e15229. doi: 10.1111/dth.15229. [DOI] [PubMed] [Google Scholar]
- 68.Moludi J., Khedmatgozar H., Saiedi S., Razmi H., Alizadeh M., Ebrahimi B. Probiotic supplementation improves clinical outcomes and quality of life indicators in patients with plaque psoriasis: A randomized double-blind clinical trial. Clin. Nutr. ESPEN. 2021;46:33–39. doi: 10.1016/j.clnesp.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Michelotti A., Cestone E., De Ponti I., Giardina S., Pisati M., Spartà E., Tursi F. Efficacy of a probiotic supplement in patients with atopic dermatitis: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Dermatol. EJD. 2021;31:225–232. doi: 10.1684/ejd.2021.4019. [DOI] [PubMed] [Google Scholar]
- 70.Parrado C., Mercado-Saenz S., Perez-Davo A., Gilaberte Y., Gonzalez S., Juarranz A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019;10:759. doi: 10.3389/fphar.2019.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kammeyer A., Luiten R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Azevedo Martins T.E., Sales De Oliveira Pinto C.A., Costa De Oliveira A., Velasco M.V.R., Guitiérrez A.R.G., Rafael M.F.C., Tarazona J.P.H., Retuerto-Figueroa M.G. Contribution of Topical Antioxidants to Maintain Healthy Skin—A Review. Sci. Pharm. 2020;88:27. doi: 10.3390/scipharm88020027. [DOI] [Google Scholar]
- 74.Calniquer G., Khanin M., Ovadia H., Linnewiel-Hermoni K., Stepensky D., Trachtenberg A., Sedlov T., Braverman O., Levy J., Sharoni Y. Combined Effects of Carotenoids and Polyphenols in Balancing the Response of Skin Cells to UV Irradiation. Molecules. 2021;26:1931. doi: 10.3390/molecules26071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seçkin H.Y., Kalkan G., Baş Y., Akbaş A., Önder Y., Özyurt H., Şahin M. Oxidative stress status in patients with melasma. Cutan. Ocul. Toxicol. 2014;33:212–217. doi: 10.3109/15569527.2013.834496. [DOI] [PubMed] [Google Scholar]
- 76.Babbush K.M., Babbush R.A., Khachemoune A. The Therapeutic Use of Antioxidants for Melasma. J. Drugs Dermatol. JDD. 2020;19:788–792. doi: 10.36849/JDD.2020.5079. [DOI] [PubMed] [Google Scholar]
- 77.Cannavò S.P., Riso G., Casciaro M., Di Salvo E., Gangemi S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019;53:829–840. doi: 10.1080/10715762.2019.1648800. [DOI] [PubMed] [Google Scholar]
- 78.Bertino L., Guarneri F., Cannavò S.P., Casciaro M., Pioggia G., Gangemi S. Oxidative Stress and Atopic Dermatitis. Antioxidants. 2020;9:196. doi: 10.3390/antiox9030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen A.C., Martin A.J., Choy B., Fernández-Peñas P., Dalziell R.A., McKenzie C.A., Scolyer R.A., Dhillon H.M., Vardy J.L., Kricker A., et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015;373:1618–1626. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 80.Moussa Z., Judeh Z.M.A., Ahmed S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In: Das K., Das S., Shivanagouda Biradar M., Bobbarala V., Subba Tata S., editors. Free Radical Medicine and Biology. IntechOpen; London, UK: 2020. [DOI] [Google Scholar]
- 81.Seifirad S., Ghaffari A., Amoli M.M. The antioxidants dilemma: Are they potentially immunosuppressants and carcinogens? Front. Physiol. 2014;5:245. doi: 10.3389/fphys.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]