Abstract
Simple Summary
Histological and/or cytological evaluation of the mediastinal lymph nodes is essential for the successful treatment of lung cancer. This study analyzes the role of endobronchial ultrasound (EBUS) in the preoperative staging of non-small cell lung cancer. We carried out a prospective study between December 2019 and December 2022 on 217 lung cancer patients eligible for surgical resection. The lymph nodes biopsied, the number of samples, and the likelihood ratio for positive and for negative outcomes were the variables considered. All patients were discharged from hospital on day one. A downstaging and upstaging were noted in 16 patients (8 and 8, respectively, 7.4%). The sensitivity, specificity, positive and negative predictive value, and diagnostic accuracy were 90%, 90%, 82%, 94%, and 90%, respectively. The likelihood ratio for positive and negative results confirmed cancer when present, excluding it when absent. EBUS is the only minimally invasive and easy procedure for mediastinal staging. The direct visualization of the vessels, especially if posterior to the lymph node, allows for method-checking at every step and makes it safe and effective. Therefore, the endoscopist and the histologist/cytologist must have carried out an adequate learning curve in order not to negatively affect the method.
Abstract
Background: The treatment of lung cancer depends on histological and/or cytological evaluation of the mediastinal lymph nodes. Endobronchial ultrasound/transbronchial needle aspiration-biopsy (EBUS/TBNA-TBNB) is the only minimally invasive technique for a diagnostic exploration of the mediastinum. The aim of this study is to analyze the reliability of EBUS in the preoperative staging of non-small cell lung cancer (NSCLC). Methods: A prospective study was conducted from December 2019 to December 2022 on 217 NSCLC patients, who underwent preoperative mediastinal staging using EBUS/TBNA-TBNB according to the ACCP and ESTS guidelines. The following variables were analyzed in order to define the performance of the endoscopic technique—comparing the final staging of lung cancer after pulmonary resection with the operative histological findings: clinical characteristics, lymph nodes examined, number of samples, and likelihood ratio for positive and negative outcomes. Results: No morbidity or mortality was noted. All patients were discharged from hospital on day one. In 201 patients (92.6%), the preoperative staging using EBUS and the definitive staging deriving from the evaluation of the operative specimen after lung resection were the same; the same number of patients were detected in downstaging and upstaging (8 and 8, 7.4%). The sensitivity, specificity, positive and negative predictive value, and diagnostic accuracy were 90%, 90%, 82%, 94%, and 90%, respectively. The likelihood ratio for positive and negative results was 9 and 0.9, respectively, confirming cancer when present and excluding it when absent. Conclusions: EBUS is the only low-invasive and easy procedure for mediastinal staging. The possibility to check the method in each of its phases—through direct visualization of the vessels regardless of their location in relation to the lymph nodes—makes it safe both for the endoscopist and for the patient. Certainly, the cytologist/histologist and/or operator must have adequate expertise in order not to negatively affect the outcome of the method, although three procedures appear to reduce the impact of the individual professional involved on performance.
Keywords: NSCLC, mediastinal staging, ultrasonography of the mediastinum
1. Introduction
Lung cancer treatment is closely related to the stage of the tumor at diagnosis [1,2]. The involvement of the mediastinum radically modifies the therapeutic approach; therefore, it is crucial to carry out a correct preoperative evaluation of the lymph node stations [3]. On the one hand, invasive diagnostic methods of the mediastinum involve high costs and disadvantages, on the other hand, they ensure a wide collection of tissue samples and a histological diagnosis in most cases [4,5,6]; however, today there are alternative methods that have the same diagnostic yield as conventional approaches but with significantly less invasiveness. Endobronchial ultrasound-transbronchial needle aspiration/biopsy (EBUS-TBNA/TBNB) represents a turning point since it allows us to obtain results comparable to more invasive techniques in terms of diagnostic accuracy, sensitivity, and specificity, with minimal discomfort of the patient [7,8,9]. Despite the comforting data from the literature [10,11,12], some authors advocate and re-propose surgical methods as essential in the staging of lung cancer, considering EBUS to be too dependent on the surgeon/endoscopist and on the cytologist and/or pathologist [13,14,15]. The purpose of this study is to highlight the real role of EBUS in the preoperative evaluation of the mediastinum in non-small cell lung cancer (NSCLC) ahead of surgery.
2. Method
Two hundred and seventeen non-small cell lung cancer (NSCLC) patients, eligible for surgery, underwent preoperative mediastinal staging using EBUS after total-body computed tomography (CT) scan—with and without contrast enhancement—and/or total body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT). The study was conducted between December 2019 and December 2022, according to the ACCP [16] and ESTS [17] guidelines; the Internal Review Board of the University of L’Aquila approved the prospective research (protocol number: 70302). Furthermore, the lymph nodes examined were chosen on the basis: (1) CT, if the diameter of the lymph nodes was ≥1 cm in the respective minor axis, independently of whether there was uptake on PET/CT; (2) PET/CT, if there was uptake (SUVmax > 2.5) in lymph nodes with a diameter < 1 cm in the minor axis on CT. The clinical characteristics of patients are reported in Table 1 and Table 2. The choice between anesthesia (propofol) and moderate sedation, the patient’s decubitus, and the type of needles used depended on each center’s experience and practice. On average, three procedures (range: 2–5) for 50–60 needle passes were performed for each lymph node station sampled.
Table 1.
Patient demographics and clinical characteristics.
| Age | ||||
|---|---|---|---|---|
| Mean ± DS | Min–Max | Median | ||
| 71.7143 ± 8.35 | 36–87 | 73 | ||
| Sex | ||||
| Male (%) | Female (%) | |||
| 135 (62) | 82 (38) | |||
| BMI | ||||
| <18.5 | 31 (14.3) | |||
| 18.5–24.9 | 134 (61.7) | |||
| >25.0 | 52 (23.9) | |||
| Oncological History | ||||
| YES (%) | NO (%) | |||
| 57 (26) | 160 (74) | |||
| Nodal Station Sampling | ||||
| n (%) | N1 (%) | N2 (%) | ||
| 2L | 0 (0) | |||
| 2R | 8 (1.5) | |||
| 4L | 51 (10) | |||
| 4R | 96 (20) | |||
| 5 | 10 (2) | |||
| 6 | 5 (1) | |||
| 7 | 195 (40) | |||
| 10L | 30 (6) | |||
| 10R | 54 (11) | |||
| 11L | 14 (3) | |||
| 11R | 28 (5.5) | |||
| Total number of lymph nodes sampled | 491 | 126 (26) | 365 (74) | |
| Primary Tumor Location | ||||
| n (%) | p value | |||
| Central | 45 (21) | |||
| Periphery | 172 (79) | p < 0.00001 | ||
Table 2.
Procedures and histological diagnosis and morbidity indices. Br: fiber-optic bronchoscopy; CT-N: CT-guided needle biopsy; EB: EBUS-TBNA-TBNB; Int: intra-operative; Eastern Cooperative Oncology Group (ECOG); Charlson Comorbidity Index (CCI).
| Histological Diagnostic Methods | |||||
|---|---|---|---|---|---|
| n (%) | p value | ||||
| Bronchoscopy | 31 (14) | Br vs. CT-N | p = 0.0001 | ||
| CT-Needle Biopsy | 93 (43) | Br vs. EB | p = 0.1613 | ||
| EBUS | 41 (19) | Br vs. Int | p = 0.0082 | ||
| Intra-operative | 52 (24) | CT-N vs. EB | p = 0.0001 | ||
| CT-N vs. Int | p = 0.0001 | ||||
| EB vs. Int | p = 0.2056 | ||||
| Tumor Histology | |||||
| n (%) | |||||
| Squamous | 57 (25) | ||||
| Adenocarcinoma | 143 (66) | ||||
| Undiffer. Carc. | 3 (2) | ||||
| Carcinoid (atypical) | 3 (2) | ||||
| Other | 11 (5) | ||||
| Average Rank | Sum of Ranks | Mean | Std.Dev | p value | |
| ECOG 0–5 | 1.099.078 | 238.5 | 0.493088 | 0.653469 | |
| CHARLSON | 1.900.922 | 412.5 | 3.470.046 | 2.325.438 | p < 0.00001 |
2.1. Primary Endpoints
Analysis of specificity, sensitivity, diagnostic accuracy, positive predictive value, negative predictive value of EBUS.
Evaluation if there is a “Threshold Value” of procedures that positively affects the diagnostic yield of the EBUS.
2.2. Secondary Endpoint
Analysis of diagnostic performance of cytology versus histology based on the different types of needles used.
2.3. Statistical Analysis
The analysis was performed using SPSS 10.0. Data were entered into a database using SPSS Data Entry II (SPSS, Inc., Chicago, IL, USA). Spearman’s Rank-Order Correlation was used for both dichotomous and continuous variables. The Multiple Regression test allowed us to verify the correlation between early and final staging. All p values < 0.05 were considered to indicate significance with a 95% confidence interval.
3. Results
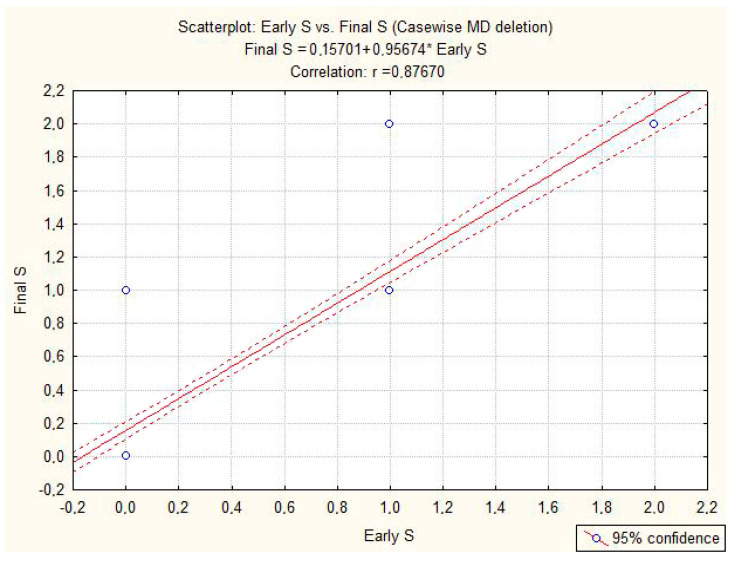
No complications during or after the procedure were noted. The mean duration of an EBUS was 27 min (range: 22–32 min) with a length of stay of 1 day. The average number of lymph nodes sampled was 2 hilar and 5 mediastinal nodes. The pathological tumor stage is described in Table 3. The outcomes relating to the primary endpoints are described in Table 4. Upstaging and downstaging were the same (8 vs. 8 patients) and statistically insignificant (p = 0.61). The negative predictive value (NPV)—which indicates the probability that a negative lymph node (EBUS test) is really negative at definitive histology—is 94% with a diagnostic accuracy of 90%. The positive likelihood ratio—which indicates the ratio between the probability that a lymph node is positive for both EBUS and definitive histology and the probability that a lymph node negative for EBUS (false negative) is positive for definitive histology—showed a good confidence level (LR+: 9). The inverse ratio is to be considered in the negative likelihood ratio, which also showed a good level of confidence (LR−: 0.9). Three procedures represented the cut-off for improving the diagnostic yield of EBUS. Concerning the secondary endpoint, we did not highlight any statistically significant difference (p = 0.78) in the performance of the procedure using the cytology needles or the biopsy needles. Spearman’s Rank-Order Correlation showed a statistically significant correlation between the different paired variables examined (Table 5), validating the analysis performed and the results obtained. Table 6 identifies the regression coefficient between the early stage and final stage (R = 0.877) and displays that 88% of the data did not deviate from the mean (Figure 1; 95% confidence interval; p = 0.00001). This indicates that the preoperative evaluation of the mediastinum in EBUS is equivalent to that obtained after surgical resection, testifying to the high degree of reliability of the endoscopic method.
Table 3.
Lung cancer stage according to TNM classification, eighth edition.
| Pathological Tumor Stage | ||||
|---|---|---|---|---|
| n (%) | p Value * | |||
| Early Stage | Final Stage | |||
| pT1a | 18 (8) | 24 (11) | 0.2891 | |
| pT1b | 36 (17) | 37 (17) | ns | |
| pT1c | 39 (18) | 33 (15) | 0.2 | |
| pT2a | 40 (18) | 45 (21) | 0.215 | |
| pT2b | 27 (12) | 21 (10) | 0.25 | |
| pT3 | 38 (18) | 41 (19) | 0.21 | |
| pT4 | 19 (9) | 16 (6) | 0.11 | |
| Total | 217 | 217 | ||
| p value * | ||||
| Pathological Nodal Stage | ||||
| pN0 | 133 (61) | 140 (65) | 0.194 | |
| pN1 | 39 (18) | 40 (18) | ns | |
| pN2 | 45 (21) | 37 (17) | 0.144 | |
| 217 | 217 | |||
* p values were calculated using Pearson’s Chi-square test.
Table 4.
Analysis of primary endpoints. The highly positive linear correlation (r = 0.98) indicates that 3 procedures are necessary for the best performance of EBUS. * = Number of Procedure.
| Primary Endpoints | |||
|---|---|---|---|
| Early Stage | |||
| Nodal downstaging | Nodal Upstaging | ||
| N0 | 7 (3%) | 0 | |
| N1 | 1 (1%) | 0 | |
| N2 | 0 | 8 (4%) | |
| Accuracy of test | |||
| Early Stage–Final Stage | |||
| Sensitivity | 90% | ||
| Specificity | 90% | ||
| PPV | 82% | ||
| NPV | 94% | ||
| Accuracy | 90% | ||
| LR+ | 9 | ||
| LR− | 1 | ||
| Number of Procedure * | |||
| Early Stage | n (%) | ||
| 2 * | N0 | 35 (16) | |
| 2 * | N1–N2 | 15 (7) | |
| 3 * | N0 | 71 (33) | R = 0.98786 |
| 3 * | N1–N2 | 41 (19) | |
| 4 * | N0 | 27 (12) | |
| 4 * | N1–N2 | 28 (13) | |
| 217 | |||
Table 5.
Spearman’s correlations show the strength of the relationship between the variables considered, with a highly significant p.
| Pair of Variables | Valid N |
Spearman’s R |
T (N-2) | p-Level |
|---|---|---|---|---|
| ECOG:0.5 and CCL | 217 | 0.175368 | 2.61187 | 0.009640 |
| ECOG:0.5 and Early S. | 217 | −0.200989 | −3.00846 | 0.002939 |
| ECOG:0.5 and Final S. | 217 | −0.215217 | −3.23143 | 0.001425 |
| CCL and Early S. | 217 | 0.316724 | 4.89615 | 0.000002 |
| CCL and Final S. | 217 | 0.428564 | 6.95508 | 0.000000 |
| Early S. and Final S. | 217 | 0.820610 | 21.05470 | 0.000000 |
Marked correlations are significant at p < 0.05.
Table 6.
Correlation analysis between early stage and final stage. The results are shown graphically in Figure 1.
| Dependent Variable |
Multiple R | Multiple R2 |
Adjusted R2 |
SS Model |
df Model |
MS Model |
SS Residual |
df Residual |
MS Residual |
F | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Final Stage | 0.876699 | 0.768602 | 0.767525 | 87.66309 | 1 | 87.66309 | 26.39221 | 215 | 0.122754 | 714.1335 | 0.000 |
Figure 1.
The two dashed curves display the high agreement (R = 0.87670) around the mean.
4. Discussion
This prospective multicenter study displayed that systematic endosonography in NSCLC patients with a resectable lung tumor is a highly reliable method in the preoperative staging of the mediastinum, being characterized by an overall performance of 90% and a good confidence interval for the likelihood ratio. Our results are in agreement with the experience of Bousema et al. [18], who, after a negative EBUS, carried out surgical resection immediately in 171 patients and, subsequently, a confirmatory mediastinoscopy in 155. Mediastinoscopy determined eight minor (4.6%) and three major (1.7%) complications and an unforeseen reduction in N2 by only 1.03%. The authors concluded that confirmatory mediastinoscopy can be omitted in patients with resectable NSCLC, also reducing delays in treatment. Based on these considerations, the use of video-assisted mediastinoscopic lymphadenectomy (VAMLA) or transcervical extended mediastinal lymphadenectomy (TEMLA) [19,20] does not appear justified due to the continuous search for minimally invasive diagnostic and surgical approaches; hence, the need for a re-evaluation of the indications is imperative in order to tailor the procedures on each patient. This concept is stressed by Mullins et al. [21] regarding early-stage inoperable NSCLC patients, who underwent stereotactic body radiotherapy (SBRT) after CT-guided needle biopsy (Group 1: 79 patients) and navigational bronchoscopy with EBUS for hilar and mediastinal staging (Group 2: 79 patients). The authors, having not found statistically significant differences in the recurrence and survival outcomes between the two groups, concluded that the choice of nodal assessment must be carefully evaluated, especially in patients with borderline clinical conditions. One question seems extremely timely: if the use of EBUS—the only minimally invasive method—must also be weighed on the patient, how can mediastinoscopy be promoted? Rami-Porta et al. [22], in an update on lung cancer staging through a review of the literature, showed a sensitivity for EBUS ranging between 17% and 41% in N0 patients at PET/CT and between 38% and 53% in N1 resectable patients at PET/CT. On the contrary, videomediastinoscopy, presenting a sensitivity between 78% and 97% and a negative predictive value between 83% and 99%, could be considered the preferred method for preoperative mediastinal staging. The authors confirmed this belief in an editorial [23], commenting on the predictive model proposed by Verdial et al. [24] concerning lung cancer staging. This model, through a false-positive rate of 44% and a true-positive rate of 97%, could potentially reduce the use of invasive procedures. In the literature, there is some variability about NPV ranging from 87.7% to 93.4% and sensitivity ranging from 35% to 60% [25,26]; our study revealed a sensitivity of 90% and a negative predictive value of 94%, although the use of cytology needles and biopsy needles did not show any statistically significant improvements in the accuracy of EBUS (p = 0.78). This secondary endpoint can be explained, as cytology needles often allow the removal of tissue fragments to be used for histological evaluation. In our experience, three procedures represented the cut-off for improving the diagnostic yield of EBUS, reducing the influence of the operator and the pathologist related to their individual skills, as demonstrated by the linear regression analysis performed on the number of procedures applied for each lymph node station. Our outcomes seem to contradict the findings made by Czarnecka-Kujawa et al. [27] about the difficulty of sampling micrometastases or the inaccessibility of some lymph nodes in the same station, which negatively affects the diagnostic performance of EBUS. In fact, the use of different needles according to the morphological characteristics (i.e., rubbery, necrotic, or hard consistency) of the lymph nodes detected using CT or PET as well as the possibility of visualizing the procedure in each of its phases using ultrasound allows us to adapt the technical option to the anatomy, optimizing the risk/benefit ratio according to the patient. The preference for anesthesia or sedation and the discretion of the type of needle used by each individual center—although this did not affect the homogeneity of the data collected—could represent a bias in this study. Also not to be overlooked is the incidence of SARS-CoV-2 infection, which on the one hand reduced the number of patients who could be enrolled, on the other could have affected mediastinal lymphadenopathy by influencing the diagnostic yield of the EBUS.
5. Conclusions
EBUS is the only minimally invasive technique with a high performance index for preoperative staging of lung cancer [28,29,30]. Its greatest advantage is the visualization of the lymph node station in real-time from the front and back, which helps to understand the relationships of the vessels and have a mastery over the depth of the biopsy. Concerning this, mediastinoscopy, guaranteeing only a frontal view, can be burdened by a non-negligible rate of hemorrhage as it fails to evaluate the distance or the infiltration of a vessel posteriorly. Furthermore, in our experience, we never received negative feedback regarding the analysis of biomarkers (EGFR, ALK, ROS, PD-L1, etc.) in biopsies performed through the EBUS; it is obvious that this depends on the quality and suitability of the material sent to the anatomopathologist but it is a problem common to every diagnostic procedure that cannot be exclusively attributed to EBUS.
A prospective international study would be desirable to establish which approach is indicated for the staging of NSCLC patients with the same clinical conditions and comorbidities.
Author Contributions
(I) Conception and design: D.D.; (II) Provision of study materials or patients: all authors; (III) Collection and assembly of data: all authors; (IV) Data analysis and interpretation: D.D. and G.D.L.; (V) Manuscript writing: all authors; (VI) Final approval of manuscript: all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wakeam E., Byrne J.P., Darling G.E., Varghese T.K. Surgical Treatment for Early Small Cell Lung Cancer: Variability in Practice and Impact on Survival. Ann. Thorac. Surg. 2017;104:1872–1880. doi: 10.1016/j.athoracsur.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Howington J.A., Blum M.G., Chang A.C., Balekian A.A., Murthy S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 3.Krantz S.B., Lutfi W., Kuchta K., Wang C.-H., Kim K.W., Howington J.A. Improved Lymph Node Staging in Early-Stage Lung Cancer in the National Cancer Database. Ann. Thorac. Surg. 2017;104:1805–1814. doi: 10.1016/j.athoracsur.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Brunelli A. European Society of Thoracic Surgeons preoperative mediastinal staging guidelines: From face validity to external validity. J. Thorac. Cardiovasc. Surg. 2018;155:796–797. doi: 10.1016/j.jtcvs.2017.09.115. [DOI] [PubMed] [Google Scholar]
- 5.Liu H., Zhou J., Feng Q.L., Wan G., Xie Y.-J., Gu H.-T. Minimally invasive endoscopic staging for mediastinal lymphadenopathy in lung cancer: A systematic review protocol. BMJ Open. 2014;4:e005707. doi: 10.1136/bmjopen-2014-005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho J.H., Kim J., Kim K., Choi Y.S., Kim H.K., Shim Y.M. A comparative analysis of video-assisted mediastinoscopy and conventional mediastinoscopy. Ann. Thorac. Surg. 2011;92:1007–1011. doi: 10.1016/j.athoracsur.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Um S.W., Kim H.K., Jung S.H., Han J., Lee K.J., Park H.Y., Choi Y.S., Shim Y.M., Ahn M.-J., Park K., et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J. Thorac. Oncol. 2015;10:331–337. doi: 10.1097/JTO.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 8.Ge X., Guan W., Han F., Guo X., Jin Z. Comparison of Endobronchial Ultrasound-Guided Fine Needle Aspiration and Video-Assisted Mediastinoscopy for Mediastinal Staging of Lung Cancer. Lung. 2015;193:757–766. doi: 10.1007/s00408-015-9761-3. [DOI] [PubMed] [Google Scholar]
- 9.Divisi D., Zaccagna G., Barone M., Gabriele F., Crisci R. Endobronchial ultrasound-transbronchial needle aspiration (EBUS/TBNA): A diagnostic challenge for mediastinal lesions. Ann. Transl. Med. 2018;6:92. doi: 10.21037/atm.2017.12.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Bussy S., Labarca G., Canals S., Caviedes I., Folch E., Majid A. Diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal staging in lung cancer. J. Bras. Pneumol. 2015;41:219–224. doi: 10.1590/S1806-37132015000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueiredo V.R., Cardoso P.F., Jacomelli M., Demarzo S.E., Palomino A.L.M., Rodrigues A.J., Terra R.M., Pego-Fernandes P.M., Carvalho C.R.R. Endobronchial ultrasound-guided transbronchial needle aspiration for lung cancer staging: Early experience in Brazil. J. Bras. Pneumol. 2015;41:23–30. doi: 10.1590/S1806-37132015000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckardt J., Licht P.B. Endobronchial ultrasound-guided transbronchial needle aspiration is a sensitive method to evaluate patients who should not undergo pulmonary metastasectomy. Interact. Cardiovasc. Thorac. Surg. 2015;20:482–485; discussion 485. doi: 10.1093/icvts/ivu443. [DOI] [PubMed] [Google Scholar]
- 13.Dooms C., Tournoy K.G., Schuurbiers O., Decaluwe H., De Ryck F., Verhagen A., Beelen R., van der Heijden E., De Leyn P. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: A prospective multicenter study. Chest. 2015;147:209–215. doi: 10.1378/chest.14-0534. [DOI] [PubMed] [Google Scholar]
- 14.Lin J., Fernandez F. Indications for invasive mediastinal staging for non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2018;156:2319–2324. doi: 10.1016/j.jtcvs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Call S., Rami-Porta R. Cervical mediastinoscopy and video-assisted mediastinoscopic lymphadenectomy for the staging of non-small cell lung cancer. Mediastinum. 2019;3:31. doi: 10.21037/med.2019.07.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silvestri G.A., Gonzalez A.V., Jantz M.A., Margolis M.L., Gould M.K., Tanoue L.T., Harris L.J., Detterbeck F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 17.De Leyn P., Dooms C., Kuzdzal J., Lardinois D., Passlick B., Rami-Porta R., Turna A., Schil P.V., Venuta F., Waller D., et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2014;45:787–798. doi: 10.1093/ejcts/ezu028. [DOI] [PubMed] [Google Scholar]
- 18.Bousema J.E., Dijkgraaf M.G.W., van der Heijden E.H.F.M., Verhagen A.F., Annema J.T., Broek F.J.v.D., Papen-Botterhuis N.E., Soud M.Y.-E., van Boven W.J., Daniels J.M., et al. Endosonography with or without confirmatory mediastinoscopy for resectable lung cancer: A randomized clinical trial. J. Clin. Oncol. 2023;41:JCO2201728. doi: 10.1200/JCO.22.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zieliński M. Video-assisted mediastinoscopic lymphadenectomy and transcervical extended mediastinal lymphadenectomy. Thorac. Surg. Clin. 2012;22:219–225. doi: 10.1016/j.thorsurg.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Hartert M., Tripsky J., Huertgen M. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) for staging & treatment of non-small cell lung cancer (NSCLC) Mediastinum. 2020;4:3. doi: 10.21037/med.2019.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullins B.T., Moore D.T., Rivera M.P., Marks L.B., Akulian J., Pearlstein K.A., Wang K., Burks A.C., Weiner A.A. The impact of pathologic staging of the hilar/mediastinal nodes on outcomes in patients with early-stage NSCLC receiving stereotactic body radiotherapy. J. Thorac. Dis. 2021;13:1045–1054. doi: 10.21037/jtd-20-2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rami-Porta R., Call S., Dooms C., Obiols C., Sánchez M., Travis W.D., Vollmer I. Lung cancer staging: A concise update. Eur. Respir. J. 2018;51:1800190. doi: 10.1183/13993003.00190-2018. [DOI] [PubMed] [Google Scholar]
- 23.Obiols C., Call S., Rami-Porta R. The importance of the false-negative rate to validate a staging protocol for non-small cell lung cancer. Transl. Lung Cancer Res. 2019;8((Suppl. S4)):S400–S402. doi: 10.21037/tlcr.2019.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdial F., Madtes D., Hwang B., Mulligan M.S., Odem-Davis K., Waworuntu R., Wood D.E., Farjah F. A prediction model for nodal disease among patients with non-small cell lung cancer. Ann. Thorac. Surg. 2019;107:1600–1606. doi: 10.1016/j.athoracsur.2018.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shingyoji M., Nakajima T., Yoshino M., Yoshida Y., Ashinuma H., Itakura M., Tatsumi K., Iizasa T. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann. Thorac. Surg. 2014;98:1762–1767. doi: 10.1016/j.athoracsur.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 26.Ong P., Grosu H., Eapen G.A., Rodriguez M., Lazarus D., Ost D., Jimenez C.A., Morice R., Bandi V., Tamara L., et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann. Am. Thorac. Soc. 2015;12:415–419. doi: 10.1513/AnnalsATS.201409-429OC. [DOI] [PubMed] [Google Scholar]
- 27.Czarnecka-Kujawa K., Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J. Thorac. Dis. 2017;9((Suppl. S2)):S83–S97. doi: 10.21037/jtd.2017.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guarize J., Sedda G., Bonizzoni G., Donghi S.M., Casiraghi M., Petrella F., Spaggiari L. The role of endobronchial ultrasound transbronchial needle aspiration in patients candidate to pneumonectomy. Shanghai Chest. 2020;4:39. doi: 10.21037/shc.2020.03.07. [DOI] [Google Scholar]
- 29.Dong X., Qiu X., Liu Q., Jia J. Endobronchial ultrasoundguided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: A meta-analysis. Ann. Thorac. Surg. 2013;96:1502–1507. doi: 10.1016/j.athoracsur.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Asano F., Aoe M., Ohsaki Y., Okada Y., Sasada S., Sato S., Suzuki E., Semba H., Fukuoka K., Fujino S., et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: A nationwide survey by the Japan Society for Respiratory Endoscopy. Respir. Res. 2013;14:50. doi: 10.1186/1465-9921-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared up on request.