Abstract
Cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), are used to assist in bone marrow recovery during cancer chemotherapy. Interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) play important roles in inflammatory processes, including exacerbation of periodontal diseases, one of the most common complications in patients who undergo this therapy. A human monocyte cell line (THP-1) was utilized to investigate IL-1β and TNF-α production following GM-CSF supplementation with lipopolysaccharide (LPS) from two oral microorganisms, Porphyromonas gingivalis and Fusobacterium nucleatum. LPS of P. gingivalis or F. nucleatum was prepared by a phenol-water extraction method and characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and determination of total protein and endotoxin contents. Resting THP-1 cells were treated with LPS of P. gingivalis or F. nucleatum and/or GM-CSF (50 IU/ml) by using different concentrations for various time periods. Production of IL-1β and TNF-α in THP-1 cells was measured by solid-phase enzyme-linked immunosorbent assay. Reverse transcription (RT)-PCR was used to evaluate the gene expression of resting and treated THP-1 cells. IL-1β was not detected in untreated THP-1 cells. IL-1β production was, however, stimulated sharply at 4 h. GM-CSF amplified IL-1β production in THP-1 cells treated with LPS from both oral anaerobes. No IL-1β-specific mRNA transcript was detected in untreated THP-1 cells. However, IL-1β mRNA was detected by RT-PCR 2 h after stimulation of THP-1 cells with LPS from both organisms. GM-CSF did not shorten the IL-1β transcriptional activation time. GM-CSF plus F. nucleatum or P. gingivalis LPS activated THP-1 cells to produce a 1.6-fold increase in TNF-α production at 4 h over LPS stimulation alone. These investigations with the in vitro THP-1 model indicate that there may be an increase in the cellular immune response to oral endotoxin following GM-CSF therapy, as evidenced by production of the tissue-reactive cytokines IL-1β and TNF-α.
Inflammation of the supporting tissues of the teeth produces one of the most common groups of human diseases, periodontal diseases (26). The mechanisms associated with these common oral inflammatory diseases are poorly understood. Interaction of bacterial products and antigens of periodontal pathogens with host inflammatory cells results in the release of cytokines. Periodontitis may involve both the direct cytotoxic and proteolytic effects of oral microorganisms and the indirect pathologic consequences of the host immune response to these microorganisms (33, 43).
Periodontitis is a relatively common infectious disease, leading to tooth loss in adults worldwide. Porphyromonas gingivalis is considered to be one of the important pathogens in the etiology of rapidly progressive periodontitis and adult periodontitis (38, 39). Fusobacterium nucleatum is routinely isolated in high numbers from subgingival plaque in patients with periodontitis (17, 38–40). The role of the lipopolysaccharide (LPS) of these two oral microorganisms in cytokine-mediated inflammatory and destructive lesions of the gingiva and periodontium merits investigation.
Interleukin-1β (IL-1β) is an important mediator of various immunological and inflammatory reactions produced primarily by monocytes (3). As a prototype of the proinflammatory cytokines, IL-1β induces the expression of a variety of genes and the synthesis of several proteins, in turn inducing acute and chronic inflammatory changes (3). Higher levels of IL-1β have been demonstrated in periodontitis tissue (23). IL-1β may play a pivotal role in the pathogenesis and onset of chronic inflammatory periodontal disease (30). IL-1β is one of the factors known to stimulate bone resorption and secretion of proteinase and may be involved in the attachment loss and bone resorption which are characteristic features of periodontitis (25, 36, 46).
Tumor necrosis factor (TNF), or cachectin, is a cytokine originally thought to play a role in host surveillance against neoplasms (7). Endotoxin-stimulated macrophages are the most important source of TNF. TNF alpha (TNF-α) was initially identified as a factor produced by leukocytes and was thought to be responsible for infection-induced cachexia. It has been recognized subsequently that TNF has a broader range of effects on host immune responsiveness, such as enhancing polymorphonuclear neutrophil-endothelial interactions and facilitating phagocytosis and bacterial killing. Recently, a role for TNF in the generation of free radicals and the pathophysiological changes during sepsis and septic shock has been proposed (7).
The biological properties of TNF have remarkable similarities to those of IL-1. Similar to IL-1, TNF induces fever by its ability to stimulate hypothalamic prostaglandin E2 synthesis directly (16). Levels of circulating TNF increase rapidly in humans injected with endotoxin (9). IL-1 acts synergistically with TNF to protect rats exposed to lethal hyperoxia or radiation. IL-1 cytotoxic effects on the insulin-producing beta cells of the islets of Langerhans are dramatically augmented by TNF. IL-1 can synergize with TNF to induce lethality in animal models, and in endotoxin-induced shock, the lethality is the result of the synergistic action of IL-1 and TNF rather than overproduction of TNF alone (15).
Human granulocyte-macrophage colony-stimulating factor (GM-CSF) is a glycoprotein functionally involved in the proliferation and differentiation of normal hematopoietic cells (35). This factor stimulates the growth and differentiation of granulocytes, monocytes, erythrocytes, and megakaryocytes (1) from progenitor cells, and it also activates mature granulocytes and macrophages (21, 35). In the last few years, recombinant human GM-CSF has been used in the treatment of chemotherapy-induced bone marrow suppression in patients undergoing transplantation for the treatment of cancer (8, 12). During chemotherapy and bone marrow recovery, with the administration of GM-CSF, these patients often suffer from periodontal infections involving complications. The relationship of GM-CSF-treated monocytes and their responses to LPS from two putative periodontal pathogens, P. gingivalis and F. nucleatum, were investigated by using a human monocytic leukemia cell line, THP-1 (49).
In the last few years, many studies have been done to investigate the role of LPS of aerobic bacteria on monocyte or THP-1 cell activation. There is little, if any, knowledge regarding IL-1β or TNF-α production by monocytes or THP-1 cells in response to LPS of putative periodontal pathogens. The complex interplay between the activation of monocyte-type cells and the release of these tissue-active cytokines in the complex oral environment was the focus of this study. The effect of GM-CSF on monocyte differentiation and activation in the presence of oral LPS has, in fact, never been investigated. It is hypothesized that GM-CSF-stimulated THP-1 cells are immunologically and functionally hyperactivated in the presence of oral LPS. Therefore, the purpose of this study was to elucidate the IL-1β and TNF-α expression of THP-1 cells after treatment with GM-CSF and in response to LPS of P. gingivalis and F. nucleatum to exploit this cell culture model, leading to more precise design of in vivo oral investigations.
MATERIALS AND METHODS
Preparation and characterization of LPS of P. gingivalis and F. nucleatum.
LPS of P. gingivalis and F. nucleatum was prepared by the method of Westphal and Jahn (50). Briefly, P. gingivalis (ATCC 33277) and F. nucleatum (ATCC 25586) were grown in Trypticase soy broth containing 1.5% yeast extract, 5-μg/ml hemin, and 1-μg/ml menadione and incubated anaerobically at 37°C for 72 h in a Coy anaerobic chamber (Coy Laboratory Products Inc., Ann Arbor, Mich.) containing 85% N2, 10% H2, and 5% CO2. The bacterial cells were then harvested, washed three times in 10 mM phosphate-buffered saline (pH 7.4), and suspended at a concentration of approximately 10 mg (dry weight)/ml in cold, distilled water. This thick suspension was poured into 10 volumes of cold acetone (−20°C), and the sedimented cells were dried under a vacuum. The acetone-dried bacterial cells were ground in a mortar and pestle and suspended in water at a concentration of approximately 6% (wt/vol) in a water bath at 65 to 68°C. An equal volume of a 90% (wt/vol) aqueous solution of phenol (Sigma, St. Louis, Mo.), at the same temperature, was then added, and the mixture was stirred. After 30 min of incubation at 65 to 68°C, the mixture was centrifuged (4°C) at 10,000 × g for 10 min. The upper aqueous layer was carefully removed, and the LPS was finally precipitated by pouring the solution into 10 volumes of cold acetone (−20°C). The precipitate after centrifugation was collected, resuspended in a small amount of distilled water, and freeze-dried. The crude LPS was further purified by being dissolved three times in water to give a 3% (wt/vol) solution and ultracentrifuged (100,000 × g) for 6 h.
The protein concentration of the LPS of P. gingivalis and F. nucleatum was determined by the method of Bradford (6). The LPS of P. gingivalis and F. nucleatum was also characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a Bio-Rad vertical electrophoresis apparatus (Bio-Rad, Hercules, Calif.). The stacking gel was 4% acrylamide, and the resolving gel was 10% acrylamide in Tris buffer. Running buffer was prepared as described by Laemmli (31). The endotoxin concentration (in endotoxin units [EU] per milliliter) of the LPS extracts of P. gingivalis and F. nucleatum was measured by the Limulus amebocyte lysate test using E-Toxate multiple test vials (Sigma).
Treatment of THP-1 cells with GM-CSF and/or LPS.
THP-1 cells (ATCC TIB 202) were grown in suspension in 75-cm2 plastic tissue culture flasks (Corning Glass Works, Corning, N.Y.) in RPMI 1640 (GIBCO, Gaithersburg, Md.) complete medium (CM) with addition of the antibiotics penicillin G sodium (100 U/ml) and streptomycin sulfate (100 μg/ml) and supplementation with 10% (vol/vol) heat-inactivated fetal bovine serum, l-glutamine (2 mM), HEPES buffer (10 mM), and minimum essential medium containing sodium pyruvate (1 mM). Cells were incubated at 37°C in a humidified atmosphere consisting of 5% CO2. All of the media and ingredients used in the cell culture system were tested with the E-Toxate test (Sigma) and found to be negative for endotoxin activity. After 3 to 4 days of growth, THP-1 cells were harvested, THP-1 cells at 106/ml were distributed among the wells of a 24-well microtiter plate, and the cells were then considered to be ready for the various treatment experiments. Concentrations of GM-CSF (Collaborative Biomedical Products, Bedford, Mass.) of 500, 50, 5, and 0.5 IU/ml in RPMI 1640 CM were added to duplicate wells of 24-well microtiter plates containing THP-1 cells at 106/ml in RPMI 1640 CM. Different concentrations (100, 10, 1, and 0.1 μg/ml) of P. gingivalis or F. nucleatum LPS were then added. Comparable concentrations (10−7, 10−8, 10−9, and 10−10 mol/ml) of phorbol-12-myristate-13 acetate (PMA) (positive control) in RPMI 1640 CM were also added to wells, and one row of THP-1 cells was left untreated as a negative control to which only 100 μl of RPMI 1640 CM was added. Plates were then incubated with 5% CO2 for 2, 4, 8, and 12 h and 1, 2, 4, and 7 days at 37°C. Supernatant fluids were collected, centrifuged, and stored at −80°C for later cytokine assays.
IL-1β and TNF-α cytokine assay.
Supernatant fluids of untreated THP-1 cells and those treated at different times and with different doses and substances were stored at −80°C until used for measurement of IL-1β and TNF-α with commercial enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, Minn.). The basic principle of the ELISA was the quantitative solid-phase sandwich enzyme immunoassay technique in which a monoclonal antibody specific for IL-1β or TNF-α was used to coat the microtiter plate provided in the kit. Duplicate readings for each standard, control, and sample were taken and averaged. The average absorbance for each duplicate set of standards, controls, and samples was calculated by using a standard curve. Results are expressed as picograms of IL-1β or TNF-α per milliliter of supernatant fluid.
Isolation of RNA.
RNA was isolated with TRIzol Reagent (GIBCO) by using a single-step isolation method originally developed by Chomczynski and Sacchi (10). RNase-free plastic and water were used throughout. THP-1 cells grown for 3 days in a 75-cm2 flask were harvested, and 3 × 106 THP-1 cells per ml were distributed among the wells of a 24-well microtiter plate. The cells were then treated with 100 μl of the LPS (1-μg/ml final concentration) of F. nucleatum and P. gingivalis and PMA (10−8 mol/ml final concentration) with or without GM-CSF (50 IU/ml) for 5, 15, or 30 min or 1 or 2 h. After each treatment period, the cells were harvested and lysed by resuspending the cell pellet with 1 ml of TRIzol Reagent (GIBCO) and repetitive pipetting. A 100-μl volume of chloroform was then added, and the samples were centrifuged at 4,500 × g for 30 min at 4°C.
Following centrifugation, the colorless upper aqueous phase containing RNA was transferred to a fresh tube. The RNA was precipitated from the aqueous phase by mixing with 0.5 ml of isopropyl alcohol. Samples were incubated at room temperature for 10 min and centrifuged at 4,500 × g for 20 min at 4°C. The RNA pellet was washed once with 75% ethanol. The concentration and purity of the RNA thus isolated were determined by measuring the optical density at 260 and 280 nm in a spectrophotometer and by agarose gel electrophoresis.
Determination of the optimal number of THP-1 cells and the purity of the RNA isolated.
The number of THP-1 cells required to produce an optimal quantity of isolated total RNA was determined by using 1 × 106, 5 × 106, and 1 × 107 THP-1 cells in preliminary experiments. A concentration of 3 × 106 THP-1 cells was chosen for the subsequent RNA isolation procedures after evaluation of the amounts of RNA isolated from the different numbers of cells. In all reverse transcription (RT)-PCRs, the isolated total RNA was run in a 1.2% agarose gel with ethidium bromide to see if pure RNA was present prior to quantitation of the optical density at 260 and 280 nm in a spectrophotometer.
RT-PCR detection of cytokine mRNA.
RT-PCR was performed to determine whether IL-1β, which was not detectable by the ELISA, could be detected by RT-PCR. For TNF-α, RT-PCR was performed to detect the presence of mRNA in untreated and treated THP-1 cells. RT-PCR was also utilized to detect the start time of IL-1β transcription after stimulation of THP-1 cells with LPS of P. gingivalis or F. nucleatum. IL-1β sense (upstream): 5′-ATGAAGTGCTCCTTCCAGGACCTG-3′) and antisense (downstream: 5′-CCTGGAGTGGAGAGCTTCAGTT-3′) and TNF-α sense (upstream: 5′-GGACGTGGAGCTGGCCGAGG-3′) and antisense (downstream: 5′-TGGGAGTAGATGAGGTACAGGCCC-3′) primers were selected from published sequences (11). The primers were prepared by Oligo (Wilsonville, Oreg.). An RT reaction reagent cocktail was prepared by using a thermostable rTth RNA reverse transcriptase PCR Kit (Perkin Elmer, Norwalk, Conn.). All of the components in the right proportions were added together in the master mixture for the number of reactions needed. A 15-microliter aliquot of the RT master mixture was added to each of the RT-PCR tubes with the appropriate RNA and then incubated at the appropriate temperature for the RT reaction. The reaction was stopped by placing the tubes on ice until needed for the PCR.
The PCR master mixture (80 μl) was dispensed into each RT reaction tube and placed in the PCR machine for amplification of the cDNA. After the template was denatured at 94°C for 30 s, DNA was amplified for 35 cycles in a Perkin Elmer 9600 thermal cycler. For IL-1β, cycles consisted of denaturing at 94°C for 10 s and annealing-extending at 66°C for 15 s. For TNF-α, cycles consisted of 10 s at 94°C, 10 s at 55°C, and 10 s at 72°C. After cycling, the reaction mixtures were maintained at 72°C for an additional 10 min and then chilled to 4°C.
For PCR product detection, an agarose gel was made with 1.2% Tris-borate-EDTA buffer and 0.5 μg of ethidium bromide with a 100-bp DNA molecular size ladder (GIBCO). The PCR products were separated in a Horizon 58 GIBCO Bethesda Research Laboratories gel electrophoresis apparatus attached to a Bio-Rad 300Xi computer-controlled electrophoresis power supply.
Statistical analysis.
Data collected were first examined for normality by using the Kolmogorov-Smirnov test. For a normally distributed data set, the parametric statistical tests were chosen for analyses. The paired Student t test was performed to compare the effects of LPS and/or GM-CSF treatments on the production of IL-1β and TNF-α at each time point. Experiments were always run in duplicate and repeated at least twice. Differences between results were considered statistically significant at P < 0.05.
RESULTS
Characterization of the LPS of P. gingivalis and F. nucleatum.
The endotoxin content of F. nucleatum LPS was determined to be 3 × 106 EU/mg, and that of P. gingivalis LPS was 6 × 106 EU/mg. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the isolated LPS displayed low- and intermediate-molecular-weight (10,000 to 60,000) bands. P. gingivalis LPS showed a prominent band of lipid smudges which was not seen in the F. nucleatum LPS preparation (data not shown).
Results of IL-1β estimation.
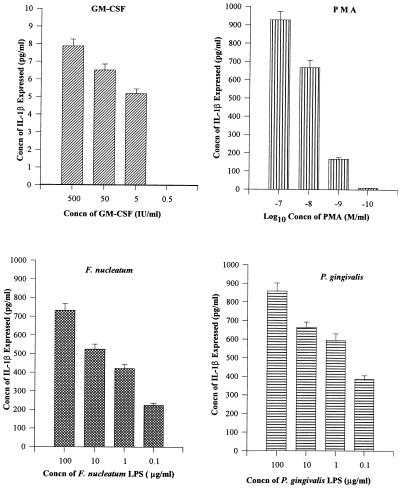
The effects of P. gingivalis and F. nucleatum LPS (100, 10, 1, and 0.1 μg/ml), PMA (10−7, 10−8, 10−9, and 10−10 mol/ml), and GM-CSF (500, 50, 5, and 0.5 IU/ml) on IL-1β production by THP-1 cells were evaluated. Dose-response experiments with THP-1 cells after 24 h of incubation with GM-CSF (500, 50, 5, and 0.5 IU/ml) demonstrated that treatment with GM-CSF at 50 IU/ml produced IL-1β at 6.5 pg/ml, while 8 pg/ml was produced by 500 IU/ml and 5.2 pg/ml was produced by 5 IU/ml (Fig. 1). A GM-CSF concentration of 50 IU/ml was selected for further experimentation (Fig. 1). Similarly, a PMA dose of 10−8 mol/ml was chosen. F. nucleatum LPS at 10 μg/ml produced IL-1β at 525 pg/ml, 1 μg/ml produced 421 pg/ml, and 0.1 μg/ml produced 223 pg/ml (Fig. 1). One microgram of F. nucleatum LPS per milliliter was selected as a suitable concentration for IL-1β stimulation. P. gingivalis LPS at 10 μg/ml produced IL-1β at 665 pg/ml, 1 μg/ml produced 595 pg/ml, and 0.1 μg/ml produced 386 pg/ml (Fig. 1). A P. gingivalis LPS dose of 1 μg/ml was also selected for further experimentation.
FIG. 1.
Different dose-response experiments for IL-1β assay. THP-1 cells (106/ml) were treated with different doses of GM-CSF for 24 h, PMA for 12 h, F. nucleatum LPS for 8 h, and P. gingivalis LPS for 8 h, and supernatant fluids were tested for IL-1β. A GM-CSF concentration (concn) of 50 IU/ml was chosen for future experimentation. A PMA concentration of 10−8 mol/ml was chosen as optimal. One microgram of F. nucleatum or P. gingivalis LPS was used for IL-1β stimulation.
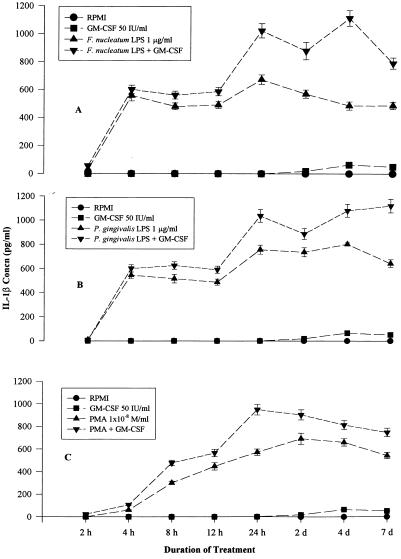
Untreated THP-1 cells did not produce IL-1β (Fig. 2). There was a sharp rise in IL-1β production to 600 pg/ml after 4 h of treatment with the LPS (1 μg/ml) of F. nucleatum (Fig. 2). Significantly greater (P < 0.05) IL-1β production resulted when GM-CSF was used along with F. nucleatum LPS than when F. nucleatum LPS alone was used. GM-CSF had a synergistic effect when combined with treatment with the LPS of F. nucleatum from 1 to 7 days (Fig. 2). LPS (1 μg/ml) of P. gingivalis produced a similar trend in IL-1β production (Fig. 2). There was also a significant (P < 0.05) increase in IL-1β production due to supplementation with GM-CSF (50 IU/ml) (Fig. 2).
FIG. 2.
(A) IL-1β production after F. nucleatum LPS and GM-CSF treatment. Significantly (P < 0.05) greater IL-1β production was observed at 8 h of treatment and afterwards when F. nucleatum LPS (1 μg/ml) was supplemented with GM-CSF (50 IU/ml) than when F. nucleatum LPS or GM-CSF alone was used. The data points represent average values of two replicative samples. (B) IL-1β production after P. gingivalis LPS and GM-CSF treatment. Significantly (P < 0.05) greater IL-1β production was observed at 8 h of treatment and afterwards when P. gingivalis LPS (1 μg/ml) was supplemented with GM-CSF (50 IU/ml) than when P. gingivalis LPS or GM-CSF alone was used. The data points represent average values of two replicative samples. (C) IL-1β production after treatment with PMA (10−8 mol/ml) and GM-CSF. Significantly (P < 0.05) greater IL-1β production was observed at 8 h after treatment when PMA was supplemented with GM-CSF (50 IU/ml) than when PMA or GM-CSF alone was used. The data points represent average values of two replicative samples.
PMA (10−8 mol/ml) treatment caused a gradual increase in IL-1β production to 600 pg/ml after 24 h (Fig. 2). When GM-CSF (50 IU/ml) was added with PMA, IL-1β production peaked at 950 pg/ml at 24 h and then declined to 700 pg/ml at 7 days. There was significantly (P < 0.05) greater IL-1β production when PMA treatment was supplemented with GM-CSF than when PMA alone was used at the 12-h, 24-h, and 2-day time points (Fig. 2).
TNF-α estimation results.
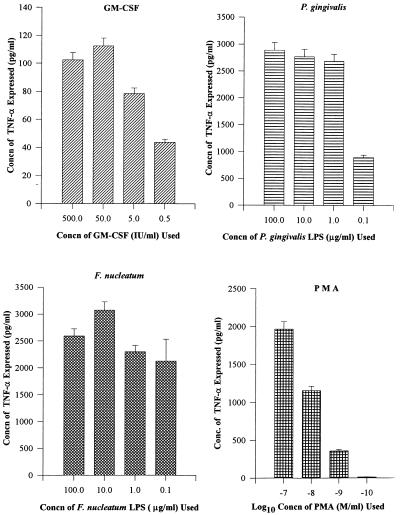
The dose-response effect of TNF-α was initially evaluated by using the LPS of P. gingivalis or F. nucleatum (100, 10, 1, and 0.1 μg/ml), PMA (10−7, 10−8, 10−9 and 10−10 mol/ml), and GM-CSF (500, 50, 5, and 0.5 IU/ml) to select a minimum concentration at which measurable TNF-α was produced. The maximum concentration (100 μg/ml) of P. gingivalis LPS produced 2,887 pg of TNF-α per ml, while 1 μg/ml produced 2,700 pg/ml. Hence, P. gingivalis LPS at 1 μg/ml was selected for later TNF-α experimentation. Similarly, 1 to 100 μg of F. nucleatum per ml produced >3,000 pg of TNF-α per ml (Fig. 3) after 8 h of stimulation. Therefore, 1 μg of F. nucleatum LPS per ml was also selected for later TNF-α experiments. A GM-CSF concentration of 50 IU/ml produced a maximum response of 112 pg of TNF-α per ml and was selected as suitable for experimentation (Fig. 3). A PMA concentration of 10−7 mol/ml produced 1,965 pg of TNF-α per ml, 10−8 mol/ml produced 1,155 pg/ml, and 10−9 mol/ml produced 358 pg/ml at 8 h of stimulation (Fig. 3). A PMA concentration of 10−8 mol/ml was selected for future experimentation.
FIG. 3.
Different dose-response experiments for TNF-α assay. THP-1 cells (106/ml) were treated with different doses of GM-CSF for 24 h, PMA for 12 h, and F. nucleatum or P. gingivalis LPS for 8 h, and supernatant fluids were tested for TNF-α. A 50-IU/ml GM-CSF concentration (concn) was chosen for future experimentation. A 10−8 mol/ml PMA concentration was chosen as optimal. One microgram of F. nucleatum or P. gingivalis LPS was used for TNF-α stimulation.
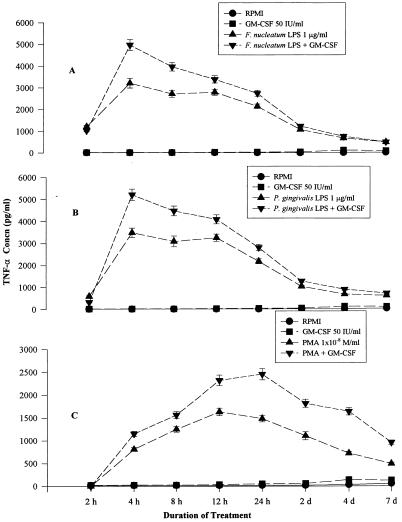
A very low level of TNF-α was detected in 2-h culture supernatant fluids of untreated THP-1 cells (Fig. 4). This indicated that THP-1 cells produced TNF-α constitutively, a finding which was supported later by our RT-PCR results. The TNF-α level rose sharply after 4 h of treatment with F. nucleatum LPS with or without GM-CSF treatment and then gradually declined to the baseline level at 7 days (Fig. 4). The TNF-α level after treatment with P. gingivalis LPS (1 μg/ml) and GM-CSF (50 IU/ml) demonstrated a sharp rise to 3,500 pg/ml at 4 h and then decreased gradually and came back to the baseline at 7 days (Fig. 4). TNF-α production was significantly (P < 0.05) higher when the LPS of F. nucleatum or P. gingivalis was used with GM-CSF than when the LPS of either organism was used alone.
FIG. 4.
(A) TNF-α production after treatment with F. nucleatum LPS and/or GM-CSF. There was a sharp rise in TNF-α production at 4 h of F. nucleatum LPS (1 μg/ml) stimulation, and the level gradually returned to the baseline by 48 h. Significantly (P < 0.05) greater TNF-α production was observed from 4 to 48 h when F. nucleatum LPS (1 μg/ml) was supplemented with GM-CSF (50 IU/ml) than when F. nucleatum LPS or GM-CSF alone was used. The data points represent average values of two replicative samples. (B) TNF-α production after treatment with P. gingivalis LPS and/or GM-CSF. TNF-α production rose sharply at 4 h after stimulation with P. gingivalis LPS (1 μg/ml) and then gradually returned to the baseline level after 48 h. Significantly (P < 0.05) greater TNF-α production was observed from 4 to 48 h when P. gingivalis LPS (1 μg/ml) was supplemented with GM-CSF (50 IU/ml) than when P. gingivalis LPS or GM-CSF alone was used. The data points represent average values of two replicative samples. (C) TNF-α production after treatment with PMA (10−8 mol/ml) and/or GM-CSF. TNF-α production gradually increased up to 24 h of stimulation with PMA (10−8 mol/ml) and then gradually declined. The data points represent average values of two replicative samples. Concn, concentration.
After treatment of THP-1 cells with PMA and GM-CSF, TNF-α production gradually increased, reached a peak at 24 h, and then gradually decreased to the baseline level at 7 days (Fig. 4). TNF-α production was significantly (P < 0.05) higher when GM-CSF was used with PMA than when PMA was used alone.
RT-PCR detection of IL-1β mRNA.
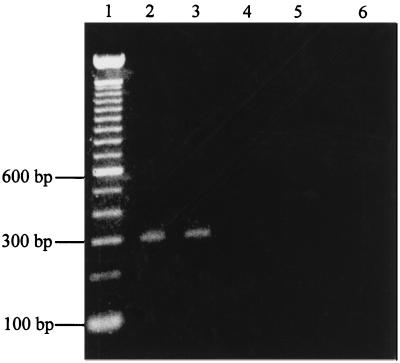
The RT-PCR products of IL-1β from THP-1 cells (Fig. 5) revealed the 300-bp IL-1β cDNA in THP-1 cells after 2 h of treatment with either P. gingivalis or F. nucleatum LPS. Untreated, GM-CSF (50 IU/ml)-treated, P. gingivalis or F. nucleatum LPS (1 μg/ml)-treated, and PMA (10−8 mol/ml)-treated THP-1 cells were studied over different periods of time for IL-1β-specific cDNA. P. gingivalis or F. nucleatum LPS stimulated IL-1β mRNA transcription after 2 h of stimulation (Table 1). Untreated THP-1 cells produced no mRNA within 24 h, indicating that IL-1β was not produced constitutively by THP-1 cells.
FIG. 5.
Agarose (1.2%) gel stained with ethidium bromide containing the 300-bp IL-1β RT-PCR product from treated and untreated THP-1 cells. Lanes: 1, 100-bp DNA ladder; 2, THP-1 cells treated for 2 h with F. nucleatum LPS (1 μg/ml); 3, THP-1 cells treated for 2 h with P. gingivalis LPS (1 μg/ml); 4, negative control; 5, untreated THP-1 cells; 6, THP-1 cells treated with GM-CSF for 2 h.
TABLE 1.
RT-PCR results for IL-1β gene expression in THP-1 cells after treatment with different substances for different durations of timea
| Duration of treatment | RPMI | GMb | Fnc | Fn + GM | Pgd | Pg + GM | PMAe | PMA + GM |
|---|---|---|---|---|---|---|---|---|
| 5 min | − | − | − | − | − | − | − | − |
| 15 min | − | − | − | − | − | − | − | − |
| 30 min | − | − | − | − | − | − | − | − |
| 1 h | − | − | − | − | − | − | − | − |
| 2 h | − | − | + | + | + | + | − | − |
| 4 h | − | − | NT | NT | NT | NT | + | + |
| 12 h | − | − | NT | NT | NT | NT | + | + |
+, IL-1β mRNA transcription in THP-1 cells detected by RT-PCR. −, IL-1β mRNA transcription in THP-1 cells not detected by RT-PCR. NT, not tested.
GM-CSF at 50 IU/ml.
F. nucleatum LPS at 1 μg/ml.
P. gingivalis LPS at 1 μg/ml.
PMA at 10−8 mol/ml.
RT-PCR detection of TNF-α mRNA.
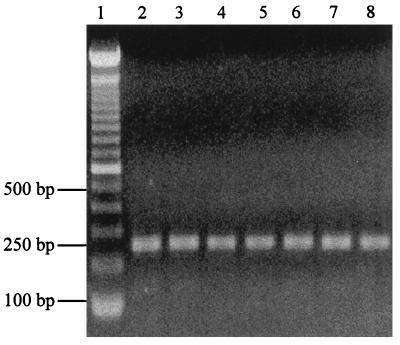
An agarose gel (1.2%) containing the TNF-α RT-PCR product (Fig. 6) showed a 250-bp TNF-α cDNA in untreated and treated THP-1 cells, indicating constitutive production of TNF-α mRNA in THP-1 cells. The negative control (no band) was in the other half of the gel (not shown). All THP-1 cells, including untreated, GM-CSF (50 U/ml)-treated, P. gingivalis and F. nucleatum LPS (1 μg/ml)-treated, and PMA (10−8 mol/ml)-treated cells, produced a 250-bp TNF-α-specific cDNA.
FIG. 6.
Agarose (1.2%) gel stained with ethidium bromide displaying the 250-bp TNF-α RT-PCR product from treated and untreated THP-1 cells. Lanes: 1, 100-bp DNA ladder; 2, untreated THP-1 cells; 3, GM-CSF (50 IU/ml)-treated THP-1 cells; 4, F. nucleatum LPS (1 μg/ml)-treated (2 h) THP-1 cells; 5, P. gingivalis LPS (1 μg/ml)-treated (2 h) THP-1 cells; 6, F. nucleatum-plus-GM-CSF-treated (2 h) THP-1 cells; 7, P. gingivalis LPS-plus-GM-CSF-treated (2 h) THP-1 cells; 8, PMA-treated (2 h) THP-1 cells.
DISCUSSION
F. nucleatum and P. gingivalis LPSs were selected for THP-1 cell stimulation in an attempt to develop a model system which would allow the study of monocyte-macrophage activation in oral diseases. There are few publications related to macrophage interactions with LPS from these periodontal pathogens, as most investigators have used Escherichia coli LPS in their studies (5, 32, 41). The composition of P. gingivalis LPS is unique in that it contains phosphorylated 2-keto-3-deoxyoctonate, which is not in the LPS of E. coli (20). F. nucleatum LPS differs from the classical E. coli LPS in that it contains a significant amount of heptose and small quantities of 2-keto-3-deoxyoctonate (27).
The cytokine IL-1 has a central role in many biologic processes, including inflammation (44). We observed a sharp rise in IL-1β production after 4 h of treatment with F. nucleatum or P. gingivalis LPS. The production reached a peak at 24 h and lasted for 4 days after treatment. An amplified effect was produced when GM-CSF was used with F. nucleatum or P. gingivalis LPS compared to treatment with LPS alone. These data agree with those of Hart et al. (28), who reported that the combination of GM-CSF and LPS (E. coli) induced synergistic IL-1 release by THP-1 cells and human monocytes. In combination with E. coli LPS, GM-CSF was reported to be a weak inducer of monocyte IL-1β activity (28).
No evidence of IL-1β gene expression in circulating peripheral blood mononuclear cells of healthy subjects has been obtained by Northern hybridization, in situ hybridization, or PCR (47). Many reports of “spontaneous” IL-1 production in various disease states, such as AIDS, or in the laboratory by infection of mononuclear cells with the human immunodeficiency virus are likely artifactual because of endotoxin contamination (37).
In our investigations, the 300-bp IL-1 β cDNA was found in THP-1 cells after 2 h of treatment with P. gingivalis or F. nucleatum LPS but was absent in untreated cells and cells treated for 2 h with GM-CSF. Hence, IL-1β mRNA was not produced constitutively by the THP-1 cells. GM-CSF alone did not induce IL-1β mRNA production by 2 h, but F. nucleatum or P. gingivalis LPS induced IL-1 β mRNA production at 2 h with or without GM-CSF supplementation. Other investigators (24) could not detect IL-1β mRNA in both unstimulated control monocytes and macrophages, whereas a marked accumulation of this transcript was observed in both cell types after LPS treatment (24).
In agreement with the results of our study, treatment of human monocytes with E. coli O11:B4 LPS (100 ng/ml) induced IL-1β mRNA transcription by 4 h and mRNA transcripts were still detectable 48 h after LPS treatment (42). As in our study, GM-CSF treatment did not shorten the mRNA expression in the study of Newman et al. (42). No specific IL-1β mRNA transcripts for the cytokine IL-1β from control noninfected macrophages were observed (42).
In contrast to our findings, Lee and Benvenniste (32) reported IL-1β mRNA in untreated THP-1 cells and within 2 h after stimulation with E. coli LPS (1 μg/ml). Their results showing IL-1β mRNA in untreated THP-1 cells may be explained by the fact that there might have been inadvertent stimulation, at some stage, of the THP-1 cells used for IL-1β mRNA detection. Variation of the annealing temperature may also have been a factor in the positive result observed by Lee and Benvenniste (32).
Gatanaga et al. (22) found TNF-α cytolytic activity in the supernatant of THP-1 cells stimulated by PMA. TNF-α began to appear at 4 h, reached a peak at 8 h, and then declined. For THP-1 cells stimulated with LPS, the TNF-α activity peaked at 4 h and then declined. Their Northern blotting showed mRNA for the 55-kDa receptor which increased during a 1- to 12-h period (22).
TNF-α appears to be more rapidly down-regulated than IL-1β during LPS stimulation of THP-1 cells (34). This could be related to the fact that TNF-α is also produced earlier than IL-1β in LPS-stimulated cells (34). Down-regulation of TNF-α possibly occurs at the mRNA level (34).
Delahooke et al. (14) found TNF-α peaks at both 4 and 8 h in THP-1 cells treated with LPS of Bacteroides species or E. coli. The pattern of TNF-α production was similar to the pattern we obtained with LPS stimulation. Undifferentiated THP-1 cells did not release significant amounts of TNF-α into the medium, but differentiation with PMA led to a release of TNF-α of about 800 to 1,400 pg/106 cells, which was detectable after 24 h and kept constant up to 72 h.
The network of cytokines is complex. Since TNF-α can also induce the synthesis of IL-1β by monocytes-macrophages, it is possible that the observed TNF-α effect was dependent on the synthesis of IL-1β (4). The kinetics of cytokine production by LPS-stimulated monocytes differed for IL-1β and TNF-α (34). In our study, TNF-α reached a plateau by 8 h after stimulation and then gradually declined. Also, all of our untreated, GM-CSF-treated, and P. gingivalis or F. nucleatum LPS-treated THP-1 cells showed a 250-bp TNF-α cDNA. This finding suggested that TNF-α is produced constitutively in THP-1 cells, as determined by RT-PCR. This is in conformity with the results of others (13, 19, 32), who also found low levels of TNF-α mRNA in untreated THP-1 cells.
The findings of Asakura et al. (2) and Essner et al. (18) are consistent with our finding that TNF-α, but not IL-1β, may be produced constitutively in human monocytes. In the THP-1 cell system, this appears to be the case. The findings of Terao et al. (48) are also consistent with our in vitro model. In sarcoidosis patients treated with GM-CSF, an enhanced inflammatory response, as evidenced by increased production of TNF-α and IL-1β, might be relevant to the pathogenesis of the disease. Periodontal disease and the cellular response to the LPS of oral microorganisms might likewise be affected by GM-CSF treatment.
Hays and Zoon (29) referred to a “priming effect” of GM-CSF on human monocytes. In our in vitro model with LPS of oral microorganisms, the goal was to identify any synergism between LPS and GM-CSF. The clinical importance in our model is that preexisting periodontal disease may predispose cancer or other patients to periodontal complications following GM-CSF therapy due to previous stimulation with the LPS of oral organisms. In fact, Perkins et al. found that patients receiving continuous infusion of GM-CSF demonstrated enhanced production of TNF-α and IL-1β in monocytes (45). Those researchers concluded that this effect may enhance the patient’s resistance to new infection, but based on our model, in an inflammatory disease like periodontal disease, exacerbation of disease activity might be observed.
The sequence of events in periodontal diseases is still in need of in-depth study. The studies described herein have evaluated the effect of the growth factor GM-CSF and the LPSs of two putative periodontal pathogens on macrophage lineage cells. The results of this study imply that macrophages have an active role in acute and chronic periodontal exacerbations in the presence of the GM-CSF growth factor and LPS. Periodontal diseases are clearly multifactorial, perhaps beginning with the activation of the immune system at the cellular level by the LPS of a potential pathogen such as F. nucleatum or P. gingivalis. Simultaneously, genes are up-regulated to express tissue-active inflammatory cytokines such as IL-1β, IL-6, and TNF-α. The events become cyclic, leading to periodontal attachment and tissue damage. The effects of these agents clearly are directly related to the oral disease activity observed clinically in immunologically healthy and immunocompromised patients. Activation and differentiation of THP-1 cells by oral LPS in the presence of GM-CSF may suggest a role for human macrophages in acute and chronic periodontal diseases.
ACKNOWLEDGMENT
This work was supported by National Institute of Dental Research grant DE 11373 from the National Institutes of Health.
REFERENCES
- 1.Aglietta M, Monzeglio C, Sanario F, Apra F, Morelli S, Stacchini A, Piacibello W, Bussolino F, Bagnara G P, Zauli G, Stern A C, Gavosto F. In vivo effect of human granulocyte-macrophage colony-stimulating factor on megakaryocytopoiesis. Blood. 1991;77:1191–1194. [PubMed] [Google Scholar]
- 2.Asakura E, Hanamura T, Umemura A, Yada K, Yamauchi T, Tanabe T. Effects of macrophage colony-stimulating factor (M-CSF) on lipopolysaccharide (LPS)-induced mediator production from monocytes in vitro. Immunobiology. 1996;195:300–313. doi: 10.1016/S0171-2985(96)80047-7. [DOI] [PubMed] [Google Scholar]
- 3.Barthelson R A, Potter T, Valone F H. Synergistic increases in IL-1 synthesis by the human monocytic cell line THP-1 treated with PAF and endotoxin. Cell Immunol. 1990;125:142–150. doi: 10.1016/0008-8749(90)90069-4. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E M, Young L S, Gupta S. 1,25 Dihydroxyvitamin D3-dependent inhibition of growth or killing of Mycobacterium avium complex in human macrophages is mediated by TNF and GM-CSF. Cell Immunol. 1990;127:432–441. doi: 10.1016/0008-8749(90)90144-g. [DOI] [PubMed] [Google Scholar]
- 5.Boutten A, Dehoux M, Deschenes M, Rouzeau J-D, Bories P N, Durand G. Alpha 1-acid glycoprotein potentiates lipopolysaccharide-induced secretion of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha by human monocytes and alveolar and peritoneal macrophages. Eur J Immunol. 1992;22:2687–2695. doi: 10.1002/eji.1830221032. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brouckaert P, Libert C, Everaerdt B, Takahashi N, Cauwels A, Fiers W. Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiology. 1993;187:317–329. doi: 10.1016/S0171-2985(11)80347-5. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood S, Doyle J J, Hitzler J K, Saunders E F, Freedman M H. Administration of recombinant human granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation in children with acute myelogenous leukemia: a note of caution. Bone Marrow Transplant. 1996;18:87–91. [PubMed] [Google Scholar]
- 9.Cannon J G, Tompkins R G, Gelfand J A, Michie H R, Stanford G G, Van der Meer J W M, Endres S, Lonnemann G, Corsetti J, Chernow B, Wilmore D W, Wolf S W, Dinarello C A. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombat P, Delain M, Desbois I, Domenech J, Binet C, Tabah I, Lamagnere J P, Linassier C. Granulocyte-macrophage colony-stimulating factor accelerates hematopoietic recovery after autologous bone marrow or peripheral blood progenitor cell transplantation and high dose chemotherapy for lymphoma. Bone Marrow Transplant. 1996;18:293–299. [PubMed] [Google Scholar]
- 13.Datta R, Imamura K, Goldman S J, Dianoux A C, Kufe D W, Sherman M L. Functional expression of the macrophage colony-stimulating factor receptor in human THP-1 monocytic leukemia cells. Blood. 1992;79:904–912. [PubMed] [Google Scholar]
- 14.Delahooke D M, Barclay G R, Poxton I R. Tumor necrosis factor induction by an aqueous phenol-extracted lipopolysaccharide complex from Bacteroides species. Infect Immun. 1995;63:840–846. doi: 10.1128/iai.63.3.840-846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello C A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 16.Dinarello C A, Cannon J G, Wolf S M, Bernheim H A, Beutler B, Cerami A, Figari I S, Palladino M A J, O’Connor J V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin-1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzink J L, Socransky S S, Haffajee A D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal disease. J Clin Periodontol. 1988;15:316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 18.Essner R, Rhoades K, McBride W H, Morton D L, Economou J S. Differential effects of granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor on tumor necrosis factor and interleukin-1 production in human monocytes. Clin Lab Immunol. 1990;32:161–166. [PubMed] [Google Scholar]
- 19.Frankenberger M, Pforte A, Sternsdorf T, Passlick B, Baeuerle P A, Ziegler-Heitbrock H W L. Constitutive nuclear NF-κB in cells of the monocyte lineage. Biochem J. 1994;304:87–94. doi: 10.1042/bj3040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara T, Ogawa T, Sobue S, Hamada S. Chemical, immunobiological and antigenic characterizations of lipopolysaccharides from Bacteroides gingivalis strains. J Gen Microbiol. 1990;136:319–326. doi: 10.1099/00221287-136-2-319. [DOI] [PubMed] [Google Scholar]
- 21.Gasson J C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 22.Gatanaga T, Hwang C, Gatanaga M, Cappuccini F, Yamamoto R S, Granger G A. The regulation of TNF receptor mRNA synthesis, membrane expression, and release by PMA- and LPS-stimulated human monocytic THP-1 cells in vitro. Cell Immunol. 1991;138:1–10. doi: 10.1016/0008-8749(91)90127-w. [DOI] [PubMed] [Google Scholar]
- 23.Gemmell E, Seymour G J. Interleukin 1, interleukin 6 and transforming growth factor-beta production by human gingival mononuclear cells following stimulation with Porphyromonas gingivalis and Fusobacterium nucleatum. J Periodontal Res. 1993;28:122–129. doi: 10.1111/j.1600-0765.1993.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 24.Gessani S, Testa U, Varano B, Di Marzio P, Borghi P, Conti L, Barberi T, Tritarelli E, Martucci R, Seripa D, Peschle C, Belardelli F. Enhanced production of LPS-induced cytokines during differentiation of human monocytes to macrophages. J Immunol. 1993;151:3758–3766. [PubMed] [Google Scholar]
- 25.Gowen M, Wood D D, Ihrie E J, McGuire M K B, Russel R G G. An interleukin-1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan J S, editor. Orodental diseases. 8th ed. Norwalk, Conn: Appleton and Lange; 1994. [Google Scholar]
- 27.Hamada S, Koga T, Nishihara T, Fujiwara T, Okahashi N. Characterization and immunobiologic activities of lipopolysaccharides from periodontal bacteria. Adv Dent Res. 1988;2:284–291. doi: 10.1177/08959374880020021301. [DOI] [PubMed] [Google Scholar]
- 28.Hart P H, Whitty G A, Piccoli D S, Hamilton J A. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. J Immunol. 1988;141:1516–1521. [PubMed] [Google Scholar]
- 29.Hayes M P, Zoon K C. Priming of human monocytes for enhanced lipopolysaccharide responses: expression of alpha interferon, interferon regulatory factors, and tumor necrosis factor. Infect Immun. 1993;61:3222–3227. doi: 10.1128/iai.61.8.3222-3227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F. Increased interleukin-1 beta (IL-1 beta) concentration in gingival tissue from periodontal patients. J Periodontol. 1989;24:362–367. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y-J, Benvenniste E N. Stat 1-alpha expression is involved in IFN-gamma induction of the class II transactivator and class II MHC genes. J Immunol. 1996;157:1559–1568. [PubMed] [Google Scholar]
- 33.Listgarten U A. Nature of periodontal disease: pathogenic mechanisms. J Periodontal Res. 1987;22:172–178. doi: 10.1111/j.1600-0765.1987.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 34.Lozanski G, Ballou S P, Kushner I. Effect of flurbiprofen on cytokine production by human monocytes and U-937 and THP-1 cell lines. J Rheumatol. 1992;19:921–926. [PubMed] [Google Scholar]
- 35.Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986;67:257–267. [PubMed] [Google Scholar]
- 36.Mochan E, Armor L, Sporer R. Interleukin-1 stimulation of plasminogen activator production in cultured gingival fibroblasts. J Periodontal Res. 1988;23:28–32. doi: 10.1111/j.1600-0765.1988.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 37.Molina J-M, Scadden D T, Amirault C, Woon A, Vannier E, Dinarello C A, Groopman J E. Human immunodeficiency virus does not induce interleukin-1, interleukin-6, or tumor necrosis factor in mononuclear cells. J Virol. 1990;64:2901–2906. doi: 10.1128/jvi.64.6.2901-2906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore W E C, Holdeman L V, Cato E P, Smibert R M, Burmeister J A, Ranney R R. Bacteriology of moderate (chronic) periodontitis in mature adult humans. Infect Immun. 1983;42:510–515. doi: 10.1128/iai.42.2.510-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore W E C, Holdeman L V, Smibert R M, Hash D E, Burmeister J A, Ranney R R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982;38:1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore W E C, Moore L V H, Ranney R R, Smibert R M, Burmeister J A, Schenkein H A. The microflora of periodontal sites showing active destructive progression. J Clin Periodontol. 1991;18:729–739. doi: 10.1111/j.1600-051x.1991.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 41.Nathan C F. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman G W, Kelley T G, Gan H, Kandil O, Newman M J, Pinkston P, Rose R M, Remold H G. Concurrent infection of human macrophages with HIV-1 and Mycobacterium avium results in decreased cell viability, increased M. avium multiplication and altered cytokine production. J Immunol. 1993;151:2261–2272. [PubMed] [Google Scholar]
- 43.Nisengard R J. The role of immunology in periodontal disease. J Periodontol. 1988;48:505–516. doi: 10.1902/jop.1977.48.9.505. [DOI] [PubMed] [Google Scholar]
- 44.Nylander-Lundqvist E, Back O, Egelrud T. IL-1-beta activation in human epidermis. J Immunol. 1996;157:1699–1704. [PubMed] [Google Scholar]
- 45.Perkins R C, Vadhan-Raj S, Scheule R K, Hamilton R, Holian A. Effects of continuous high dose rhGM-CSF infusion on human monocyte activity. Am J Hematol. 1993;43:279–285. doi: 10.1002/ajh.2830430410. [DOI] [PubMed] [Google Scholar]
- 46.Sato K, Fujii Y, Kasono K, Sazi M, Tsushima T, Shizume T. Stimulation of prostaglandin E2 and bone resorption by recombinant human interleukin-1 alpha in fetal mouse bone. Biochem Biophys Res Commun. 1986;138:618–624. doi: 10.1016/s0006-291x(86)80541-1. [DOI] [PubMed] [Google Scholar]
- 47.Schindler R, Lonnemann G, Shaldon S, Kock K M, Dinarello C A. Transcription, not synthesis, of interleukin-1 and tumor necrosis factor by complement. Kidney Int. 1990;37:85–93. doi: 10.1038/ki.1990.12. [DOI] [PubMed] [Google Scholar]
- 48.Terao I, Hashimoto S, Horie T. Effect of GM-CSF on TNF-alpha and IL-1-beta production by alveolar macrophages and peripheral blood monocytes from patients with sarcoidosis. Int Arch Allerg Immunol. 1993;102:242–248. doi: 10.1159/000236532. [DOI] [PubMed] [Google Scholar]
- 49.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 50.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]