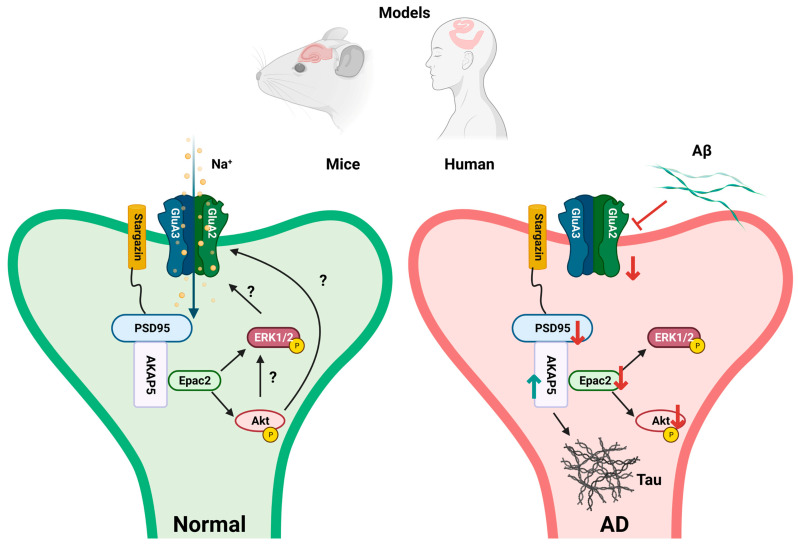
Figure 1.
Schematic illustration of postsynaptic Epac2 and GluA3 signaling complexes in the hippocampus. Shown are healthy controls (Normal) and alterations under disease conditions (AD). In postsynaptic areas, Epac2 and AMPARs are anchored by AKAP5 and stargazin/PSD95 [25,31]. Complex formation of AKAP5 and PSD95 [31] potentially facilitates complex formation with Epac2. Epac2 increases phospho-Akt and/or phospho-ERK and, thereby, contributes to synaptic plasticity and memory [30,32,33,34]. In postmortem samples of AD patients, the protein expression of Epac2 and phospho-Akt are downregulated, and GluA3 and PSD95 tend to be downregulated—these processes bear the potential to impair synaptic plasticity. On the other hand, AKAP5, reported to mediate Tau phosphorylation by PKA, is upregulated [35,36,37]. In the experimental AD model J20 mice, Epac2, GluA3, and phospho-Akt were downregulated. Epac2: exchange factor directly activated by cAMP 2; AMPARs: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors; PSD95: postsynaptic density 95; AKAP5: A kinase anchoring protein 5 (AKAP79/150); Akt: Ak strain transforming; ERK1/2: extracellular signal-regulated kinase 1/2. Model created with BioRender.com.