Abstract
Dipeptidyl peptidase IV (DPP IV), also identified as the glycoprotein CD26, is a transmembrane 110- to 120-kDa serine aminopeptidase involved in immune responses by influencing T-cell costimulation and by cleaving cytokines. Additionally, CD26 is a nonintegrin receptor that contains a binding site for extracellular matrix and other molecules. In order to further define the expression and functional activity of this membrane exopeptidase in human T cells, we developed a nondisruptive, four-color cytofluorogenic assay that utilizes three separate antibodies to cell-surface molecules (e.g., CD4/CD8/CD26 and CD19/CD56/CD26) along with a rhodamine 110-conjugated dipeptide substrate that allows the measurement of DPP IV activity in phenotypically defined cells. We found normal human thymi to have notable differences in time-dependent DPP IV activity among the thymocyte subsets defined by their CD4/CD8 phenotype, with CD4−/CD8− thymocytes containing less DPP IV activity than cells expressing CD4 and/or CD8 (i.e., maturing). CD26 positivity was moderately intense in thymocytes and tended to identify cells with higher DPP IV activity. The four-color technique was also used to examine mature peripheral blood lymphocytes, along with an assortment of leukemias and transformed T-cell lines. These experiments revealed that while DPP IV was consistently evident in normal T cells, neoplastic T cells could vary in their expression patterns. Furthermore, the presence (or intensity) of surface CD26 in some abnormal T cells and certain normal peripheral blood mononuclear cells was separable from the level of DPP IV measured intracellularly. Our results established that multicolor cytofluorographic analysis can be a practical means to measure DPP IV activity in various human cell populations. Furthermore, we found that DPP IV activity could vary in T cells according to their differentiation status and that under certain circumstances surface CD26 expression can be disassociated from the level of measured enzyme (i.e., DPP IV) activity.
The membrane-associated and cytoplasmic glycoprotein CD26 is a 110- to 120-kDa molecule encoded on chromosome 2 (1) that possesses inherent enzymatic activity known as dipeptidyl peptidase IV (DPP IV) (11, 27). DPP IV is a serine aminopeptidase that bears the capacity of cleaving polypeptides at locations containing amino-terminal dipeptides that have either l-alanine or l-proline in position 2 (30). In this regard, CD26 serves as a prototypical member of a multifunctional and heterogeneous group of molecules which possess enzymatic activity and which concurrently participate in a variety of cellular functions. For example, CD26 is a nonintegrin receptor which has the capacity to bind to collagen (26), CD45 (28), and adenosine deaminase (17), raising the likelihood that CD26 is involved in cellular trafficking throughout the interstitial compartments (e.g., collagen) and that this molecule participates in cellular transduction pathways (e.g., CD45 and ADA).
CD26 has been detected in a variety of tissues (1) and cell types, including cells involved in the immune system such as lymphocytes of T (27), B (8), or NK (7) lineage. Moreover, different T-cell subsets vary in their level of CD26 expression (29), with an upregulation of this molecule as T cells undergo cell activation (10). Commensurate with its phenotypic expression on immune cells, CD26 appears to be involved in a spectrum of immunological functions. For instance, CD26 has been found to provide an adjunct “second signal” during T-cell activation, thereby behaving as a costimulatory molecule which regulates interleukin 2 (IL-2) production and IL-2 receptor expression (5, 9). CD26/DPP IV has also been shown to be a modifier of cytokine activity (24) and antibody production (20). Finally, we (23) and others (4) have reported that CD26 in the thymus may play a role in T-lymphocyte ontogeny. It is not surprising, therefore, that interference with the molecule’s activity by using specific tripeptides or antibody has been reported to have an immunosuppressive effect (18).
We recently developed a flow cytometry-based assay that utilizes a unique fluorogenic dipeptide (glycine-proline) that is specific for DPP IV and which upon cleavage emits a fluorescence signal (via rhodamine 110) in the fluorescein isothiocyanate (FITC) wavelength range. We felt that it would be useful, particularly with thymocytes, to be able to determine intracellular DPP IV activity in cells that were distinctly characterized by simultaneous surface expression of three molecules, especially CD4, CD8, and CD26. To address this issue, we developed a four-color flow cytometry assay for the measurement of DPP IV that also utilized concurrent staining for several surface molecules (including the aforementioned markers) and employed this technique to examine human thymocytes as well as other normal and atypical human T-cell populations.
MATERIALS AND METHODS
Reagents.
Rhodamine 110-conjugated glycine-proline (Gly-Pro) and rhodamine 110-conjugated alanine-alanine (Ala-Ala) dipeptides (3, 19) were synthesized (Cell Probe) and generously provided by Coulter Corporation (Miami, Fla.). These two dipeptide compounds serve as substrates for DPP IV and parallel each other in terms of detection of enzyme activity. All of the results presented were obtained with Gly-Pro but were often substantiated with Ala-Ala, and our data demonstrating the specificity of these substrates have been previously reported (23). Four-color cytofluorographic analysis was performed in conjunction with antibodies directly conjugated to spectrally nonoverlapping fluorochromes; the antibody-fluorochrome combinations were CD4 (T4)-ECD, CD26-phycoerythrin (PE), and PE-Cy5 (PE5)-conjugated CD8 (T8) (the first four-color combination) and CD56-PE5, CD19-ECD, and CD26-PE (Immunotech, Marseilles, France).
Cell preparation.
Human thymus tissue was aseptically obtained during surgical procedures involving the mediastinal region. All of the donors (n = 8) were pediatric aged patients and did not have any systemic illness or thymic disorder, thereby ensuring that the thymic tissue being utilized was normal in composition and function. The thymic tissue was pushed through a wire mesh screen (50-μm mesh; Tekco, Inc., Lancaster, N.Y.) to disaggregate it, and thereafter, the cells were washed twice with serum-free RPMI 1640 (Media Facility, University of Miami) supplemented with 500 U of penicillin-streptomycin (Media Facility)/ml, counted, and used as described below. In several experiments, thymocytes were incubated in vitro in the presence of RPMI 1640 medium supplemented with 10% complement-inactivated AB human serum (North American Biologicals, Inc., Miami, Fla.) plus penicillin-streptomycin, 0.02 M HEPES buffer, and 10 ng of recombinant human IL-2 (R&D, Minneapolis, Minn.)/ml for 18 or 72 h at 37°C, 5% CO2; the cells were then harvested, counted, and used in the assay described below. Peripheral blood from normal volunteers and from patients with lymphoproliferative disorders was obtained aseptically by venipuncture and collected into EDTA-containing sterile Vacutainer tubes. The blood was then used unseparated in the flow cytometry assays outlined below. The cytoenzymatic studies were approved by the University of Miami Institutional Review Board.
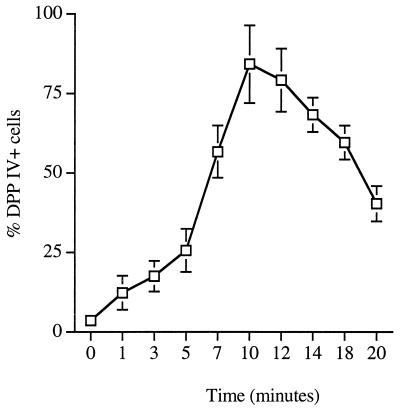
Cytoenzymatic analysis of DPP IV activity.
Thymocytes that were freshly isolated, as well as other cell populations (e.g., peripheral blood), were evaluated for DPP IV activity by using flow cytometry based upon a modification of a method we have previously reported upon (23). Essentially, thymocyte and other cell populations were resuspended in 50 μl of supplemented RPMI 1640 medium and incubated with 5 μl of a 3 × 10−4 M solution of rhodamine 110-conjugated dipeptides (Gly-Pro or Ala-Ala) for 1 and 10 min at 37°C; these time periods were established in previous studies and confirmed in the present study (Fig. 1) to be useful incubation times to show early and optimal DPP IV activities, respectively, with these substrates. No significant differences between the substrates insofar as activity levels could be detected. At the end of the incubation period, enzymatic activity was stopped by placing the tubes in an ice bath. The control for the enzyme reaction was the addition of substrate with a maintenance of the tube at 4°C in order to determine background fluorescence. In the majority of experiments, four-color staining was utilized to evaluate concomitant antigen expression of other markers along with the DPP IV activity: the first four-color combination with DPP IV was CD4-ECD, CD26-PE, and PE-Cy5-conjugated CD8, and the second four-color combination was CD56-PE-Cy5, CD19-ECD, and CD26-PE. Cells were designated positive based upon a comparison of values (fluorescence intensity) with those for cells stained with a mixture of irrelevant isotype controls conjugated to the same fluorochromes. The antibodies were added to the cells after the dipeptide incubation and washing steps, and the combinations were incubated at 4°C for 30 min and then washed twice. For peripheral blood samples, after a brief incubation, erythrocytes were lysed with Optilyse C (Coulter) and washed once with phosphate-buffered saline. The cells were then washed twice with phosphate-buffered saline containing 0.1% sodium azide at 4°C (5,000 rpm, 5 min) and then fixed with 2% paraformaldehyde. Samples were analyzed in less than 2 h after being stained on an XL flow cytometer (Coulter). The instrument was calibrated before experiments for electronic stability and fluorescence compensation with the Cyto-Comp Reagent kit (Coulter), which consists of four two-color fluorescent reagents composed of two monoclonal antibodies each. The antibodies were labeled with specific combinations of the fluorochromes FITC, PE, ECD, and PE5. The cells used for compensation were from the Cyto-Comp Cell kit (Coulter). A backgated scattergram of propidium iodide-stained cells (in a separate tube from the antibody-labeled cells) was sometimes used to determine which region of the gate contained dead cells (propidium iodide-positive cells), which were excluded from subsequent analysis. The values shown are for the populations that have the scatter characteristics of small lymphocytes. Fluorescence emission from a 488-nm 15-mW argon ion air-cooled laser was detected at 525-nm bandpaths (FL-1 for FITC and rhodamine), 575-nm bandpaths (FL-2 for PE), 620-nm bandpaths (FL-3 for ECD), and 675-nm bandpaths (FL-4 for PE5). Listmode data on 10,000 gated cells were collected with 1,024 channel resolution and later analyzed by using System II, version 1.0, software (Coulter). For the dipeptides, fluorescence intensity was calculated as the arithmetic mean channel fluorescence of log-amplified data measured on 10,000 cells. Backgating of listmode files was utilized.
FIG. 1.
Kinetic study of total DPP IV activity in normal peripheral blood, as determined by single-color flow cytometry analysis of lymphocytes, gated with typical low-forward and side-scatter characteristics. Values are means of separate determinations of lymphocytes from five subjects. Error bars indicate standard deviations.
Statistical analysis.
Student’s t test was utilized to determine whether there were statistical differences between various groups.
RESULTS
Cytoenzymatic analysis of DPP IV activity in normal human lymphocyte populations.
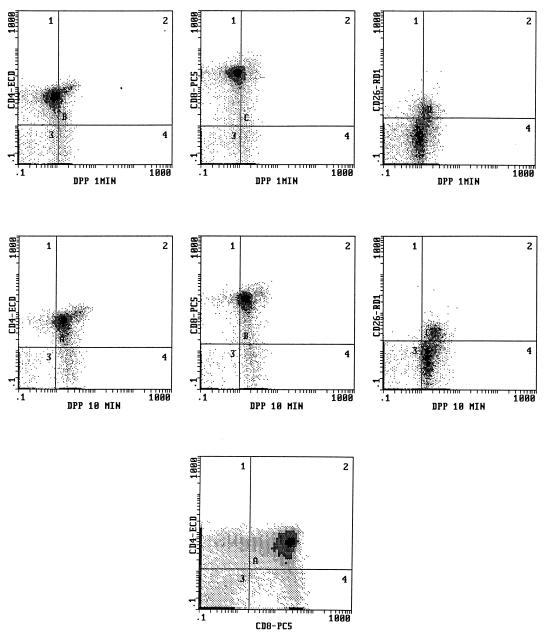
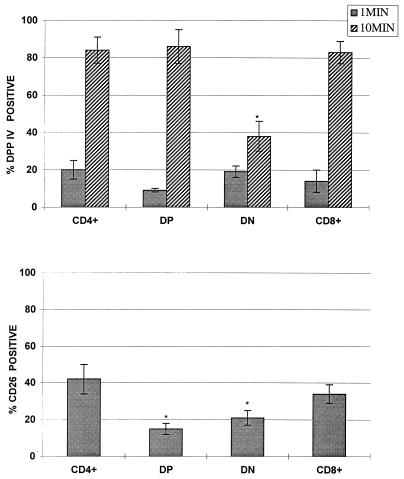
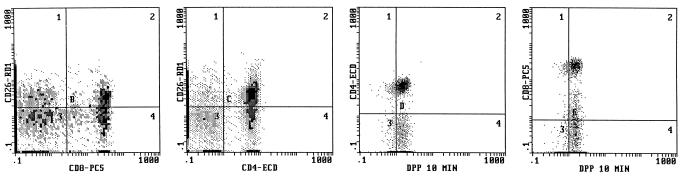
In this set of experiments we utilized our four-color cytofluorographic assay to measure intracellular DPP IV activity in normal human thymocytes and in peripheral blood; the cells were also characterized simultaneously by their expression patterns for CD4, CD8, or CD26 in the first combination with the DPP IV substrate and by their phenotypic profile for CD56, CD19, and CD26 (i.e., the second four-color combination with DPP IV). Figure 2 shows representative flow cytometry multiparameter histograms of freshly isolated human thymocytes that demonstrate a gradual increase in the amount of DPP IV activity over the time course studied, with the highest level evident at 10 min. As expected, the largest population of cells present were the thymocytes positive for both CD4 and CD8, followed by lesser percentages of the single-positive (SP) and dual-negative (DN) populations. Overall, surface CD26 expression was at a moderate level in the human thymi evaluated. Although two-parameter histograms are shown in Fig. 2, the use of this technique allowed backgating analysis in order to determine the amount of DPP IV activity in the various subpopulations in the thymus as defined by their expression of CD4 and CD8, as well as CD26. Figure 3 depicts the mean values of DPP IV activity at 1 and 10 min in all of the thymi (n = 8) evaluated. We found that the immature thymocyte populations, based upon dual negativity for CD4 and CD8, had the least amount of DPP IV activity (Fig. 3) at 10 min. Thymocytes that were either dual positive (DP) for CD4 and CD8 or SP for either CD4 or CD8 had higher DPP IV activities at 10 min, and the levels of activity among these three groups were comparable. Our data also suggested that subsets differed in their kinetics of enzyme utilization such that the SP and DN thymocytes had higher activities at 1 min of incubation compared to that of the DP cells. Backgating was also performed based on the CD26+ cells, and as shown in Fig. 3, there were differences in the amounts of CD26 expressed on the thymocyte subsets. Our results revealed that SP CD4+ cells had the largest percentage of CD26+ cells, followed by SP CD8+ cells. Interestingly, DP cells had the least amount of CD26+ cells, being slightly surpassed by DN cells (Fig. 3). This latter finding implied that there could be a discordance between the amount of surface CD26 expressed and the level of DPP IV activity measured within the cell.
FIG. 2.
Four-color flow cytometry analysis of human thymus cells for the simultaneous determination of DPP IV activity and expression of other surface molecules, as described in Materials and Methods. Shown are two-parameter histograms with DPP IV activity in CD4+, CD8+, and CD26+ cells at 1 (top) and at 10 (middle) min of incubation. The predominant pattern of CD4 and CD8 coexpression is demonstrated in the bottom histogram.
FIG. 3.
DPP IV activity in human thymocyte subsets distinguished by their CD4 and CD8 expression patterns. Values are means of eight separate determinations of DPP IV activity (top) and CD26 expression (bottom) in unstimulated, freshly isolated human thymus lymphocytic cells. ∗, P ≤ 0.05 compared to other subsets within either the 1- or the 10-min group. Error bars indicate standard deviations.
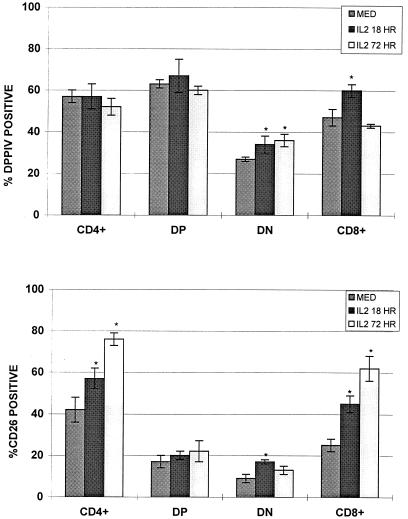
Culturing of thymocytes was also performed in an effort to increase CD26 expression and to measure whether there was any association between the level of expression and the amount of DPP IV activity. As shown in Fig. 4, CD26 expression increased in thymocytes incubated with IL-2, most dramatically in the mature SP CD8 and SP CD4 cells. There were alterations in the amount of DPP IV following incubation with IL-2, with the greatest changes evident in the SP CD8 and the DN cells (Fig. 4). SP CD4 and DP cells did not show any significant changes in the amount of DPP IV, and generally, the DPP IV pattern was not directly associable with the surface CD26 changes that were seen.
FIG. 4.
DPP IV activity in human thymocyte subsets distinguished by their CD4 and CD8 expression patterns. Values are means of eight separate determinations of DPP IV activity (top) and CD26 expression (bottom) in unstimulated, freshly isolated human thymus cells and thymus cells cultured with IL-2 as indicated (see Materials and Methods). ∗, P ≤ 0.05 compared with the control group (medium [med] alone). Error bars indicate standard deviations.
Normal human peripheral blood lymphocytes were also evaluated for DPP IV activity in conjunction with the surface phenotype by utilizing the four-color flow cytometry technique. Figure 5 shows a representative set of histograms that demonstrate CD26 expression and a significant amount of DPP IV activity in CD4+ and CD8+ cells after 10 min of incubation. An evaluation of multiple peripheral blood samples (n = 10) showed this overall pattern of DPP IV activity (Table 1) and similar levels and percentages of CD26 staining on these cells. CD4+, CD8+, and CD56+ cells had similar patterns and levels of DPP IV activity with a gradual increase in activity until almost all of the cells were positive at 10 min (Table 1), while B cells had lower DPP IV activity at 10 min compared to that of other lymphocyte subpopulations. Interestingly, the amount of CD26 expression on the surface of these cells was not predictive of the associated enzyme activity such that CD56+ NK cells, while having high levels of DPP IV activity, had minimal levels of surface CD26 expression.
FIG. 5.
Four-color flow cytometry analysis of human peripheral blood mononuclear cells for the simultaneous determination of DPP IV activity and expression of other surface molecules, as described in Materials and Methods. Shown are two-parameter histograms with DPP IV activity (10-min incubation) and CD26 expression in CD4+ and CD8+ cells.
TABLE 1.
DPP IV levels and CD26 expression in normal peripheral blood mononuclear cellsa
| Backgated populationb | % Cells positive for enzyme or protein at indicated time of incubation
|
|||||
|---|---|---|---|---|---|---|
| DPP IV
|
CD26
|
|||||
| 0 min | 1 min | 10 min | 0 min | 1 min | 10 min | |
| CD4 | 1.46 ± 0.24 | 9.06 ± 1.76 | 91.63 ± 2.46 | 65.23 ± 1.67 | 69.43 ± 2.22 | 68.43 ± 2.04 |
| CD8 | 2.91 ± 0.46 | 8.83 ± 1.20 | 90.04 ± 3.31 | 20.87 ± 0.76 | 19.49 ± 0.78 | 19.30 ± 0.74 |
| CD19 | 2.14 ± 0.85 | 2.72 ± 1.06 | 51.07 ± 4.00 | 0.32 ± 0.12 | 0.52 ± 0.12 | 0.66 ± 0.22 |
| CD56 | 5.11 ± 1.13 | 15.26 ± 4.94 | 93.46 ± 1.21 | 2.00 ± 0.21 | 2.14 ± 0.28 | 2.08 ± 0.32 |
| CD26 | 2.80 ± 0.66 | 5.95 ± 2.02 | 93.07 ± 0.93 | |||
DPP IV measurements were made by using a four-color flow cytometry assay as described in Materials and Methods. Values are means ± standard deviations (n = 10).
Backgating analysis of the listmode files was performed on the indicated populations. See Materials and Methods for description.
Cytoenzymatic analysis of DPP IV activity in abnormal T-cell populations.
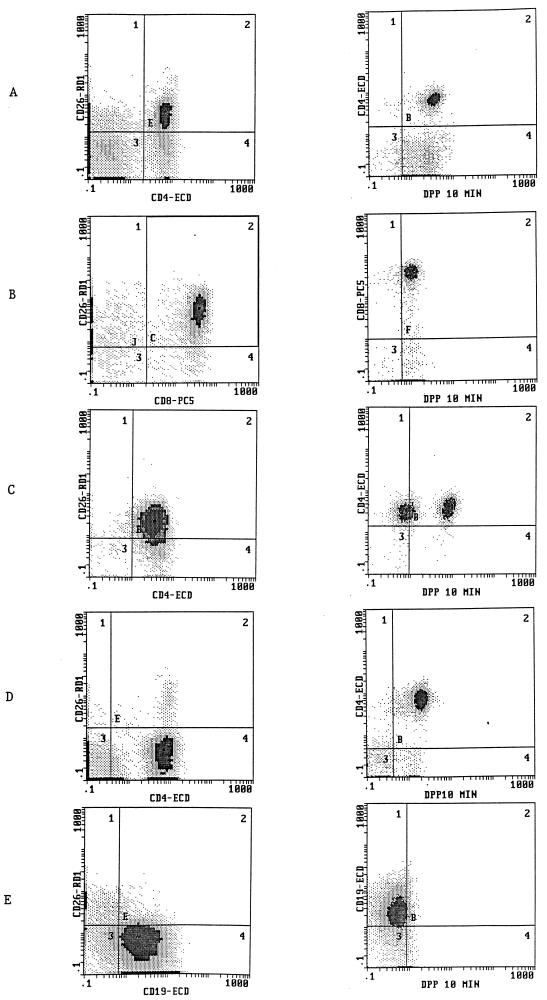
In addition to normal T-cell populations, we also used our cytoenzymatic technique to evaluate surface antigen (e.g., CD26) expression and DPP IV activity in several hematopoietic cell malignancies. Figure 6 demonstrates the phenotypic profiles of several T-cell tumors and a B-cell tumor. The T-cell tumors displayed heterogeneous patterns of CD26 expression and DPP IV activity, with the other positive surface markers on these cells correlating with the phenotype separately obtained by flow cytometric analysis of leukemias and/or lymphomas (data not shown). For example, several of the acute T-cell leukemias expressed CD26 (three examples are shown in Fig. 6) with no correlation seen with the CD4 or CD8 phenotype. One tumor (Fig. 6C) showed a clear bimodal pattern of DPP IV activity despite a unimodal pattern of CD26 expression, while another T-cell tumor (Fig. 6D) had no surface CD26 expression but did have significant DPP IV activity. As demonstrated in Fig. 6, the B-cell tumor was devoid of any significant CD26 expression and contained minimal DPP IV activity.
FIG. 6.
Four-color flow cytometry analysis of human leukemias for the simultaneous determination of DPP IV activity and expression of other surface molecules, as described in Materials and Methods. Gating was performed on populations almost exclusively comprised of tumor cells. Shown are two-parameter histograms with DPP IV activity (after 10 min of incubation) (right) or CD26 expression (left) in either CD4+, CD8+, or CD19+ cells. (A to D) Four separate T-cell acute lymphoblastic leukemias; (E) B-cell acute lymphoblastic leukemia.
DISCUSSION
DPP IV activity associated with CD26 is representative of a broadly distributed group of membrane-associated and intracellular proteolytic enzymes that are displayed in numerous cell types. The functions ascribed to DPP IV and to many of these other enzymes are diverse, including cleavage of inactive forms of proteins to active ones (e.g., cytokines) (14, 24), regulation of cellular activation pathways (2), acting as ligands for a variety of dynamic cell membrane activities (e.g., exocytosis and fusion) (22), and cellular differentiation (12). CD26/DPP IV has several roles in the immune system, including being an integral costimulatory molecule in antigen-specific activation (5, 9), a modifier of cytokine activity (15), and a cofactor in the generation of antibodies (21), and appears to play a role in T-lymphocyte ontogeny (4, 23). Moreover, DPP IV can serve as a binding site for connective tissue elements, thereby facilitating how immune cells traffic and remain in the extracellular matrix of organs (e.g., interstitium). The preferential substrate affinity DPP IV has for proline or alanine in the penultimate position of a polypeptide (30) is the likely means that this enzyme utilizes to activate or deactivate other molecules.
A principal component of the interrelationship of CD26/DPP IV with the immune system is the differential distribution of this molecule among T cells with mature (16) and memory (CD45RO+) (29) T lymphocytes containing higher levels of CD26 expression. CD26 is moderately displayed in the thymus, and Bauvois was among the first to suggest that DPP IV participates in the differentiation and selection processes of T cells that occur in this organ (4). We expanded on these latter studies by using a temperature- and time-dependent flow cytometry-based assay that utilizes a unique fluorogenic dipeptide that is specific for DPP IV; with this assay we can measure DPP IV activity in thymocyte cell populations simultaneously with the measurement of surface cellular molecules by employing antibodies conjugated to fluorochromes that are nonoverlapping in their emission spectra. For example, we have found that DPP IV activity patterns among murine thymus subpopulations (determined by CD4 and CD8 expression patterns) differ between adult and neonatal animals (23).
In the present study, we have modified the flow cytometry procedure to a four-color based method that allows DPP IV measurement within human thymocytes and other T-cell populations concurrently with three surface molecules. Our findings revealed that DPP IV was differentially expressed among human thymocytes, which were cytofluorographically identified on the basis of their CD4 and CD8 phenotype. For example, we found that similar to murine thymocytes, CD4−/CD8− cells contained the least amount of DPP IV activity compared to that of all the other thymus subpopulations. As the human thymocytes acquired a more mature phenotype, there was an increased expression of DPP IV among the DP and SP thymocyte populations; there was no significant difference between the latter populations concerning the degree of activity. Thymocytes cultured in vitro in the presence of IL-2 contained a higher level of enzyme activity among the SP CD8+ cells compared to that of freshly isolated thymocytes, and there were also increases in CD26 expression with the activation of the T cells. However, no statistically significant association could be made between the level of surface CD26 expression and the amount of internal DPP IV activity. These latter results imply that thymocytes are capable of having their DPP IV activity moderated in vivo via activation processes.
Peripheral blood lymphocytes and T-cell tumors were also examined with our four-color technique. Peripheral blood T cells tended to have high levels of CD26 and DPP IV while B cells had reduced levels of CD26 and DPP IV, as well as slower enzyme kinetics compared to that of T cells. Certain cell types were again found where the level of CD26 expression on the surface could not be correlated with the absence or presence of DPP IV activity. For example, we found that NK cells had low CD26 expression on the surface but had a moderate level of intracellular enzyme activity. One T-cell tumor which was negative for CD26 had significant DPP IV activity. We have also found that certain alloreactive T cells and human immunodeficiency virus-positive cells contain high levels of CD26 but no significant amounts of DPP IV (unpublished results). Relatedly, other investigators have demonstrated a lack of correlation between CD26 surface expression and DPP IV activity (9). Since we and others have shown that these Gly-Pro substrates are specific for DPP IV (6, 23), one potential explanation for these latter findings is that intracellular CD26 levels are a more accurate correlate with the enzyme activity measured by this method. Another possibility is that alternate forms of CD26 (known to exist [13]) are present with DPP IV activity but that these isoforms are not measurable with the antibody reagents available due to different antigenic epitopes. In summary, we have described a reliable, accurate, and nondisruptive flow cytometry-based method which has revealed differing levels of DPP IV in T lymphocytes and other cell types, which were characterized on the basis of their expression of a variety of surface antigens. Our data demonstrate that intracellular DPP IV activity is inducible and is influenced by other factors besides the density of membrane-associated CD26. An understanding of the regulatory pathways of DPP IV activity will help determine how the CD26 molecule influences normal T-cell function and ontogeny as well as pathological processes.
ACKNOWLEDGMENTS
We thank R. Morera for his assistance in tissue procurement and R. Lam for excellent clerical support.
REFERENCES
- 1.Abbott C A, Baker E, Sutherland G R, McCaughan G W. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. doi: 10.1007/BF01246674. [DOI] [PubMed] [Google Scholar]
- 2.Alhanaty E, Shaltiel S. Limited proteolysis of the catalytic subunit of cAMP-dependent protein kinase: a membranal regulatory device 656. Biochem Biophys Res Commun. 1979;89:323–332. doi: 10.1016/0006-291x(79)90633-8. [DOI] [PubMed] [Google Scholar]
- 3.Assfalg-Machleidt I, Rothe G, Klingel S, Banati R, Mangel W F, Valt G, Machleidt W. Membrane permeable fluorogenic rhodamine substrates for selective determination of cathepsin L. Biol Chem Hoppe-Seyler. 1992;373:433–440. doi: 10.1515/bchm3.1992.373.2.433. [DOI] [PubMed] [Google Scholar]
- 4.Bauvois B. Murine thymocytes possess specific cell surface-associated exoaminopeptidase activities: preferential expression by immature CD4-CD8-subpopulation. Eur J Immunol. 1990;20:459–468. doi: 10.1002/eji.1830200302. [DOI] [PubMed] [Google Scholar]
- 5.Bednarczyk J, Carroll S M, Marin C, McIntyre B. Triggering of the proteinase dipeptidyl peptidase IV (CD26) amplifies human T lymphocyte proliferation. J Cell Biochem. 1991;46:206–218. doi: 10.1002/jcb.240460304. [DOI] [PubMed] [Google Scholar]
- 6.Brandt W, Lehmann T, Thondor I, Born I, Schutkowski M, Rahfeld J U, Neubert K, Barth A. A model of the active site of dipeptidyl peptidase IV predicted by comparative molecular field analysis and molecular modelling simulations. Int J Peptide Prot Res. 1995;46:494–507. doi: 10.1111/j.1399-3011.1995.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 7.Buhling F, Dunz D, Reinhold D, Ulmer A J, Ernst M, Flad H D, Ansorgf S. Expression and functional role of dipeptidyl peptidase IV on human natural killer cells. Nat Immun. 1994;13:270–279. [PubMed] [Google Scholar]
- 8.Buhling F, Junker U, Reinhold D, Neubert K, Jager L, Ansorge S. Functional role of CD26 on human B lymphocytes. Immunol Lett. 1995;45:47–51. doi: 10.1016/0165-2478(94)00230-o. [DOI] [PubMed] [Google Scholar]
- 9.Dang N H, Torimoto Y, Deusch K, Schlossman S F, Morimoto C. Comitogenic effect of solid phase immobilized anti-IF7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol. 1990;144:4092–4100. [PubMed] [Google Scholar]
- 10.Dang N H, Torimoto Y, Shimamura K, Tanaka T, Daley J F, Schlossman S F, Morimoto C. IF7 (CD26): a marker of thymic maturation involved in the differential regulation of the CD3 and CD2 pathways of human thymocyte activation. J Immunol. 1991;147:2825–2832. [PubMed] [Google Scholar]
- 11.De Meester I, Vanhoof G, Hendriks D, Demuth H U, Yaron A, Scarpe S. Characterization of dipeptidyl peptidase IV (CD26) from human lymphocytes. Clin Chim Acta. 1992;210:23–34. doi: 10.1016/0009-8981(92)90042-o. [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano J F, Beck G, Lane B, Zucker S. Role of tumor cell membrane-bound serine proteases in tumor-induced target cytolysis. Cancer Res. 1982;42:207–218. [PubMed] [Google Scholar]
- 13.Duke-Cohan J S, Morimoto C, Rocker J A, Schlossman S F. A novel form of dipeptidyl peptidase IV found in human serum isolation, characterization, and comparison with T lymphocyte membrane dipeptidyl peptidase IV (CD26) J Biol Chem. 1995;270:14107–14114. doi: 10.1074/jbc.270.23.14107. [DOI] [PubMed] [Google Scholar]
- 14.Frank M M, Fries L F. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 15.Fukiwara H, Fukuoka M, Yasuda K, Ueda M, Imai K, Goto Y, Suginami H, Kanzaki H, Maeda M, Mori T. Cytokines stimulate dipeptidyl peptidase-IV expression on human luteinizing granulosa cells. J Clin Endocrinol Metab. 1994;79:1007–1011. doi: 10.1210/jcem.79.4.7962267. [DOI] [PubMed] [Google Scholar]
- 16.Kameoka J, Sato T, Torimoto Y Y, Sugita K, Soiffer R J, Schlossman S F, Ritz J, Morimoto C. Differential CD26-mediated activation of the CD3 and CD2 pathways after CD6-depleted allogeneic bone marrow transplantation. Blood. 1995;85:1132–1137. [PubMed] [Google Scholar]
- 17.Kameoka J, Tanaka T, Nojima Y, Schlossman S F, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, Flentke G R, Bachovchin W W, Stollar B D. Involvement of dipeptidyl peptidase IV in an in vivo immune response. Clin Exp Immunol. 1992;89:192–197. doi: 10.1111/j.1365-2249.1992.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leytus S P, Patterson W L, Mangel W F. New class of sensitive and selective fluorogenic substrates for serine proteinases. Biochem J. 1983;215:253–260. doi: 10.1042/bj2150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morimoto C, Schlossman S F. CD26: a key costimulatory molecule on CD4 memory T cells. Immunologist. 1994;2:4–7. [Google Scholar]
- 21.Morimoto C, Torimoto Y, Levison G, Rudd C E, Schrieber M, Dang N H, Letvin N L, Schlossman S F. 1F7, a novel cell surface molecule involved in helper function of CD4 cells. J Immunol. 1989;143:3430–3439. [PubMed] [Google Scholar]
- 22.Mundy D I, Strittmatter W J. Requirement for metalloendoprotease in exocytosis. Evidence in mast cells and adrenal chromaffin cells. Cell. 1985;40:645–656. doi: 10.1016/0092-8674(85)90213-2. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz P, Nassiri M, Steele B, Viciana A V. Cytofluorographic evidence that thymocyte dipeptidyl peptidase IV (CD26) activity is altered with stage of ontogeny and apoptotic status. Cytometry. 1996;23:322–329. doi: 10.1002/(SICI)1097-0320(19960401)23:4<322::AID-CYTO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Scholz W, Mentlein R, Heymann E, Feller A C, Ulmer A J, Flad H D. Interleukin 2 production by human T lymphocytes identified by antibodies to dipeptidyl peptidase IV. Cell Immunol. 1985;93:199–211. doi: 10.1016/0008-8749(85)90400-9. [DOI] [PubMed] [Google Scholar]
- 25.Schon E, Jahn S, Kiessig H U, Demuth K, Neubert A, Barth R, Baehr V, Ansorge S. The role of dipeptidyl peptidase IV in human T lymphocyte activation inhibitors and antibodies against dipeptidyl peptidase IV suppress lymphocyte proliferation and immunoglobulin synthesis in vitro. Eur J Immunol. 1987;17:1821–1826. doi: 10.1002/eji.1830171222. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu Y, Shaw S. Lymphocyte interactions with extracellular matrix. FASEB J. 1991;5:2292–2299. doi: 10.1096/fasebj.5.9.1860621. [DOI] [PubMed] [Google Scholar]
- 27.Shipp M A, Look A L. Hematopoietic differentiation antigens that are membrane associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 28.Torimoto Y, Dang N H, Vivier E, Tanaka T, Schlossman S F, Morimoto C. Coassociation of CD26 (dipeptidyl peptidase IV) with CD45 on the surface of human T lymphocytes. J Immunol. 1991;147:2514–2517. [PubMed] [Google Scholar]
- 29.Vanham G, Kesterns L, De Meester L, Vingerhoets J, Penne G, Vanhoof G, Scharpe S, Heyligen H, Bosmanns E, Ceuppens J L, Gigase P. Decreased expression of the memory marker CD26 on both CD4(+) and CD8(+) T lymphocytes of HIV infected subjects. J Acquired Immune Defic Syndr. 1993;6:749–757. [PubMed] [Google Scholar]
- 30.Walter R, Simmons W H, Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980;30:111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]