Abstract
Early diagnosis and appropriate treatments are crucial to reducing mortality risk in septic patients. Low SOFA scores and current biomarkers may not adequately discern patients that could develop severe organ dysfunction or have an elevated mortality risk. The aim of this prospective observational study was to evaluate the predictive value of the biomarkers mid-regional pro-adrenomedullin (MR-proADM), procalcitonin (PCT), C-reactive protein (CRP), and lactate for 28-day mortality in patients with sepsis, and patients with a SOFA score ≤6. 284 were included, with a 28-day all-cause mortality of 8.45% (n = 24). Non-survivors were older (p = 0.003), required mechanical ventilation (p = 0.04), were ventilated for longer (p = 0.02), and had higher APACHE II (p = 0.015) and SOFA (p = 0.027) scores. Lactate showed the highest predictive ability for all-cause 28-day mortality, with an area under the receiver-operating characteristic curve (AUROC) of 0.67 (0.55–0.79). The AUROC for all-cause 28-day mortality in patients with community-acquired infection was 0.69 (0.57–0.84) for SOFA and 0.70 (0.58–0.82) for MR-proADM. A 2.1 nmol/L cut-off point for this biomarker in this subgroup of patients discerned, with 100% sensibility, survivors from non-survivors at 28 days. In patients with community-acquired sepsis and initial SOFA score ≤ 6, MR-proADM could help identify patients at risk of 28-day mortality.
Keywords: sepsis biomarkers, mortality, MR-proADM, SOFA score, lactate, molecular diagnosis
1. Introduction
Sepsis remains a critical public health issue [1] and is widely recognized as a leading cause of global mortality [2,3,4]. Therefore, early diagnosis and effective, appropriate treatments are crucial to reducing mortality risk [5].
Various definitions and scoring systems have been developed to aid in screening and quick diagnosis. The Sequential Organ Failure Assessment (SOFA) score, a well-established and widely used tool, objectively stratifies the risk of multi-organ failure by considering variables from six organ systems [6]. In 2016, the Third International Consensus Conference (Sepsis-3) for sepsis definition considered an increase of two or more in the SOFA score as diagnostic criteria, replacing the previously used SIRS criteria [2,7,8].
SOFA score has been shown to be associated with mortality [2,7,9], and in those with initial low SOFA values less than six, mortality is lower than 10% [10,11]. However, even with a reported low mortality risk in patients with a SOFA ≤ 6, a subgroup of sepsis patients with a low SOFA score still experiences high mortality rates. It is crucial to identify this specific subgroup of patients with early sepsis and high mortality to initiate timely and appropriate treatment. Although score systems such as the qSOFA have been designed to screen for sepsis, they may lack the sensitivity required, which has led to the Surviving Sepsis Campaign 2021 guidelines recommending against using the qSOFA as a single-screening tool for sepsis [12]. Blood biomarkers may provide the extra information required to identify this subgroup of patients at high risk or those who are developing organ failure despite a low SOFA score. Hence, there is a need to identify biomarkers that may assist in mortality risk-stratification in patients with an initial low SOFA score. The combined use of mid-regional pro-adrenomedullin (MR-proADM), procalcitonin (PCT), and other commonly used biomarkers, such as C-reactive protein (CRP), Interleukin-6 (IL-6), and lactate, have been suggested in previous studies [5,7,13,14,15,16]. Nonetheless, MR-proADM has shown recent usefulness with better prognostic accuracy than CRP and APACHE [5], and it is also correlated with a 30-day mortality [7,17]. MR-proADM may also indicate risk for multiple organ failure in sepsis patients despite a low or moderate SOFA score [18], with its concentrations corresponding to endothelial permeability [19]. Moreover, its combination with PCT also improves the diagnosis of sepsis [20]. PCT and CRP have also been well-documented for their use in sepsis and in discriminating between infectious and non-infectious etiology. However, neither has shown strong evidence for mortality prediction [5].
Therefore, we hypothesized that the biomarkers mid-regional pro-adrenomedullin (MR-proADM), procalcitonin (PCT), C-reactive protein (CRP), and lactate can help identify 28-day mortality in patients with sepsis and SOFA score ≤ 6.
2. Materials and Methods
2.1. Study Design
The present study involved a prospective observational analysis. This is a single-center observational study of patients who met the criteria for the activation of the in-hospital sepsis code (ISC) [5,21], between April 2016 and July 2018. The study endpoint was 28-day all-cause mortality following enrollment.
2.2. Setting and Participants
Patients for whom the attending physician triggered the in-hospital sepsis code (ISC) at Hospital Universitari Vall d’Hebron in the emergency department (ED), hospital wards, and intensive care unit (ICU) with a diagnosis of sepsis or septic shock and initial SOFA score ≤ 6 were enrolled consecutively from April 2016 to July 2018.
The inclusion criteria were as follows: adult patients ≥18 years of age presenting with either a suspected or documented infection and met at least one of the two sets of variables of the Vall d’Hebron University Hospital in-hospital sepsis code (ISC) [5,21]. The ISC variables include (1) an acute alteration in the level of consciousness not explained by other clinical conditions, (2) hyperthermia (axillary temperature > 38.3 °C), hypothermia (axillary temperature < 36.0 °C) and/or tachycardia (>110 beats per minute), tachypnea (>30 breaths per minute), or low oxygen saturation (SpO2 < 90%), as well as arterial hypotension (systolic arterial pressure < 90 mmHg, mean arterial pressure < 65 mmHg, or a decrease of >40 mmHg of baseline systolic arterial pressure).
Patients younger than 18 years, patients who were pregnant, or patients for who no blood sample could be obtained were excluded. Patients were admitted to the ICU or a relevant ward according to clinical criteria.
2.3. Variables and Data Sources
Following the activation of the sepsis code, relevant data, including patient comorbidities, demographics, site of admission (ED vs. ward), and the classification of sepsis as nosocomial or community-acquired, were prospectively collected in the database. Triage data, laboratory results, microbiology tests, and final clinical diagnosis were also recorded.
The SOFA and APACHE II scores were calculated retrospectively upon enrollment.
Sepsis code activation also triggered the collection of blood samples, which were stored in the Sepsis Bank of Vall d’Hebron University Hospital Biobank until analysis. The samples used in this project were provided by the Sepsis Bank of Vall d’Hebron University Hospital Biobank and complied with appropriate ethics approval. Routine biomarker measurements tests included PCT (chemiluminescent immunoassay (CLIA)), CRP (immune turbidimetric test), and L-lactate (enzymatic color test). MR-proADM was analyzed retrospectively. MR-proADM was tested by blood sampling from the central catheter, and samples were stored at −80 °C. Samples were batch tested using TRACE technology (Time-Resolved Amplified Cryptate Emission, KRYPTOR® platform, Thermo Fisher, Hennigsdorf, Germany). MR-proADM results were unavailable to the corresponding physician throughout patient enrollment and hospitalization.
2.4. Statistical Methods
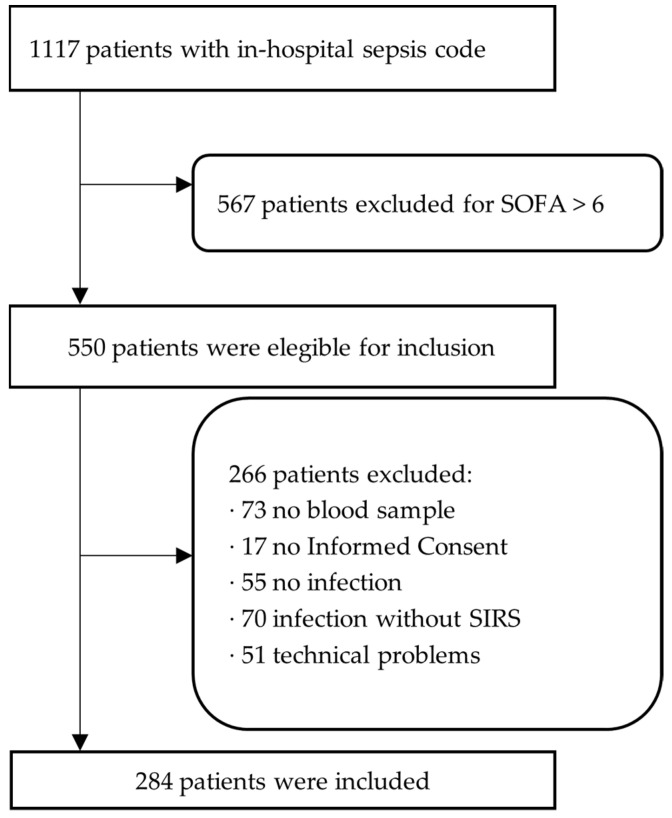
To test our hypothesis, we estimated, using the Cochran formula, that an optimal sample of patients would be 300. During the study period, the in-hospital sepsis code was activated in 1117 patients. A total of 567 patients were excluded for presenting a SOFA score > 6. Of the remaining 550 patients, 284 were included in our final sample (Figure 1).
Figure 1.
Flow chart of patients included in the present study.
Descriptive data for continuous variables were displayed using mean and standard deviation; for discrete variables, median and the first to third quartile intervals were used, and categorical variables are represented in frequency and percentages. Variables were compared between surviving and non-surviving patients at 28 days following sepsis code activation. Differences were assessed using the chi-square test for categorical variables, Student’s t-test, and the Mann-Whitney U test for all other continuous and discrete variables.
The area under the receiver operating characteristic (AUROC) curves were used to evaluate each biomarker’s predictive ability and to identify the biomarker or clinical score with the greatest predictive value for the study endpoint. An AUROC of 0.5 was considered non-predictive, and 1.0 was considered a perfect predictive ability. An AUROC of 0.70 to 0.80 was considered acceptable [19].
The predictive performance of each indicator for mortality was assessed using univariate and multivariate logistic regression models. In addition, each indicator was also assessed for the following subgroups: 28-day mortality vs. 90-day mortality, nosocomial vs. community infection, medical vs. surgical patient, and ward v. ICU admission. Results are presented as odds ratio (OR) for mortality prediction with the corresponding 95% confidence intervals.
Kaplan–Meier survival analyses were performed, and Log-rank test results were included when analyzing MR-proADM usefulness to detect differences in survivability according to both AUROC results in this study and cut-off points previously reported in the pertaining literature. Finally, since we hypothesized that patients with a SOFA score ≤ 6 would have a particular biomarker profile, we conducted Pearson’s correlations to measure associations between MR-proADM, Lactate, PCT, and other clinical variables of interest. All reported p-values were two-sided and set with significance levels at <0.05. Statistical analysis was performed with both SPSS version 18.0 (IBM) and Stata version 12.0 (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Participant Characteristics
A total of 284 patients were identified that met the inclusion criteria, with a 28-day all-cause mortality of 8.4% (N = 24). Of the 284 patients included, 184 (64.8%) patients had a diagnosis of sepsis, and 100 (35.2%) had septic shock. Patients had a mean age of 63 years, female patients accounted for 37.7%, and most cases were identified in the emergency department (48.6%). Positive blood cultures were obtained in 38.2% (n = 108) with predominantly Gram-negative bacteria growth (63.1%, n = 70). ISC was activated in 56 (19.7%) patients already admitted into the ICU. Of the remaining 228 patients, 62 (27.2%) were admitted into the ICU from the emergency department or another hospital ward. The median of the length of stay in the ICU was 4 days (2–8), and the median of the length of stay in the hospital was 13 (6–30) (Table 1).
Table 1.
Clinical patient characteristics upon activation of the sepsis code with respect to the total infected patient population and subsequent 28-day mortality.
| Patient Population (N = 284) |
Survivors (n = 260) |
Non-Survivors (n = 24) |
p-Value | |
|---|---|---|---|---|
| Age (years) (mean, S.D.) | 63 (16) | 62 (16) | 70 (9) | 0.001 |
| Female gender (N, %) | 107 (37.7) | 100 (38.5) | 7 (29.2) | 0.36 |
| Definition of sepsis | ||||
| Severe sepsis (N, %) | 184 (64.8) | 170 (65.4) | 14 (58.3) | 0.489 |
| Septic shock (N, %) | 100 (35.2) | 90 (34.6) | 10 (41.7) | 0.489 |
| Location of sepsis code activation | ||||
| Emergency department (N, %) | 138 (48.6) | 127 (48.8) | 11 (45.8) | 0.793 |
| Ward (N, %) | 90 (31.7) | 83 (31.9) | 7 (29.2) | 0.781 |
| ICU (N, %) | 56 (19.7) | 50 (19.2) | 6 (25) | 0.497 |
| Surgical admissions (N, %) | 104 (36.6) | 95 (36.5) | 9 (37.5) | 0.925 |
| Medical admissions (N, %) | 180 (63.4) | 165 (63.5) | 15 (62.5) | 0.925 |
| ICU length of stay (days) (median, IQR) | 4 [2–9] | 3 [2–8] | 11 [5–15] | 0.125 |
| Hospital length of stay (days) (median, IQR) | 13 [6–30] | 14 [7–32] | 7 [3–17] | 0.016 |
| Life-supporting and intensive care therapies | ||||
| Vasopressors (N, %) | 100 (35.2) | 90 (34.6) | 10 (41.7) | 0.489 |
| Renal replacement therapy (N, %) | 11 (8.5) | 9 (7.5) | 2 (20) | 0.172 |
| Mechanical ventilation (N, %) | 51 (18) | 43 (16.5) | 8 (33.3) | 0.04 |
| Mechanical ventilation duration (days) (median, IQR) | 5 [3–12] | 4 [2–8] | 12 [7–16] | 0.02 |
| High-flow nasal cannula use (N, %) | 41 (31.5) | 35 (29.2) | 6 (60) | 0.044 |
| Pre-existing comorbidities | ||||
| Cardiopathy (N, %) | 71 (25) | 65 (25) | 6 (25) | 1.00 |
| Chronic kidney disease (N, %) | 50 (17.6) | 47 (18.1) | 3 (12.5) | 0.492 |
| COPD (N, %) | 49 (17.3) | 45 (17.3) | 4 (16.7) | 0.937 |
| Immunosuppression (N, %) | 129 (45.4) | 112 (43.1) | 17 (70.8) | 0.009 |
| Liver cirrhosis (N, %) | 12 (4.2) | 9 (3.5) | 3 (12.5) | 0.035 |
| Microbiology | ||||
| Positive blood culture (N, %) | 108 (38.2) | 99 (38.2) | 9 (37.5) | 0.622 |
| Gram-positive (N, %) | 39 (35.1) | 34 (33.3) | 5 (55.6) | 0.291 |
| Gram-negative (N, %) | 70 (63.1) | 66 (64.7) | 4 (44.4) | 0.343 |
| Fungal (N, %) | 2 (1.8) | 2 (2) | 0 (0) | 0.666 |
| Origin of infection | ||||
| Abdominal (N, %) | 76 (26.8) | 72 (27.7) | 4 (16.7) | 0.243 |
| Bacteria-primary (N, %) | 11 (3.9) | 10 (3.8) | 1 (4.2) | 0.938 |
| Catheter-related (N, %) | 11 (3.9) | 10 (3.8) | 1 (4.2) | 0.938 |
| Central nervous system (N, %) | 1 (0.4) | 1 (0.4) | 0 (0) | 0.761 |
| Respiratory (N, %) | 68 (23.9) | 57 (21.9) | 11 (45.8) | 0.009 |
| Soft-tissue (N, %) | 18 (6.3) | 16 (6.2) | 2 (8.3) | 0.675 |
| Urinary (N, %) | 84 (29.6) | 79 (30.4) | 5 (20.8) | 0.327 |
| Unknown (N, %) | 8 (2.8) | 8 (3.1) | 0 (0) | 0.383 |
| Other (N, %) | 7 (2.5) | 7 (2.7) | 0 (0) | 0.416 |
| Source control | ||||
| Debridement of infectious foci (N, %) | 15 (5.4) | 14 (5.5) | 1 (4.3) | 0.799 |
| Drainage (N, %) | 40 (14.4) | 38 (14.9) | 2 (8.7) | 0.397 |
| Surgery (N, %) | 35 (12.6) | 33 (12.9) | 2 (8.7) | 0.534 |
| Biomarker and severity scores | ||||
| Leucocytes (x10E9/L) (mean, SD) | 13.82 (8.88) | 13.79 (8.88) | 14.09 (9.46) | 0.438 |
| Platelets (x10E9/L) (mean, SD) | 235 (148) | 236 (146) | 228 (166) | 0.600 |
| MR-proADM (nmol/L) (median, IQR) | 2.48 [1.46–4.38] | 2.43 [1.45–4.2] | 2.85 [1.8–4.98] | 0.236 |
| PCT (ng/mL) (median, IQR) | 3.09 [0.7–16.1] | 3.10 [0.7–17.5] | 3.51 [0.7–8.8] | 0.665 |
| Lactate (mmol/L) (mean, SD) | 2.75 (2.09) | 2.70 (2.10) | 3.48 (1.84) | 0.147 |
| CRP (mg/dL) (mean, SD) | 56.43 (288.39) | 46.18 (258.14) | 156.57 (494) | 0.312 |
| SOFA (points) (median, IQR) | 4 [3–6] | 4 [3–6] | 5 [4–6] | 0.027 |
| APACHE II (points) (mean, SD) | 19.71 (8.29) | 19.10 (8.11) | 26.50 (7.61) | 0.015 |
3.2. Biomarker Predictive Ability in Patients with Sepsis and a SOFA Score ≤ 6
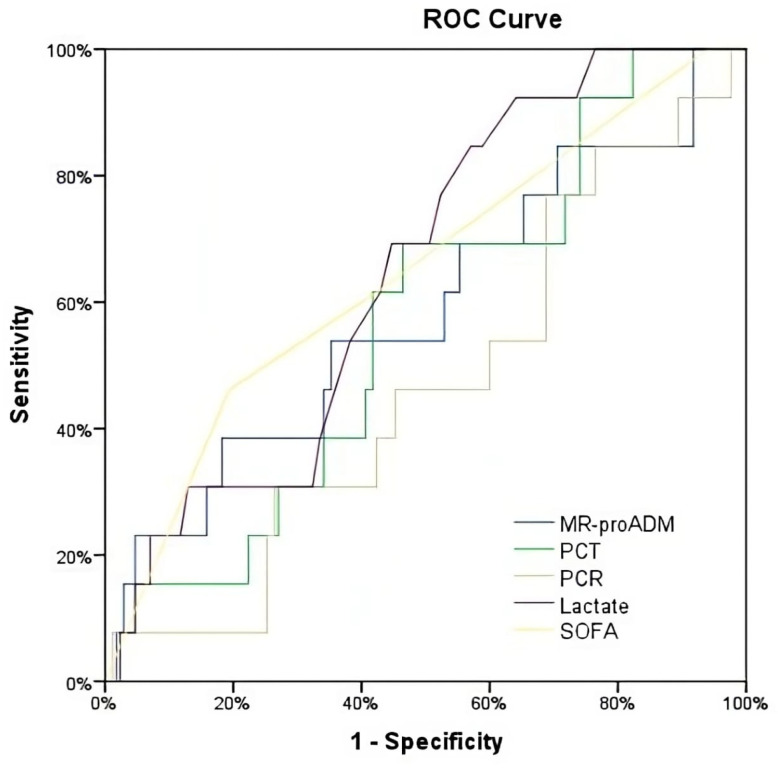
Lactate showed the highest predictive ability for all-cause 28-day mortality with an AUROC of 0.67 (0.55–0.79). In contrast, the AUROC for MR-proADM was low, with an AUROC of 0.57 (0.45–0.69). Parallelly, the AUROC for CRP was 0.49 (0.36–0.62), and for PCT, it was 0.47 (0.35–0.59) (Table 2, Figure 2 and Figure 3).
Table 2.
Area under the ROC for the prediction of mortality at 28 days and 90 days.
| Analyses | |||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| Biomarker or Clinical Score | Patients (N) | AUROC | OR IQR (95% CI) | OR IQR (95% CI) | |
| M28d | MR-proADM | 284 | 0.57 [0.45–0.69] | 1.08 [0.94–1.24] | 1.13 [0.82–1.56] |
| PCT | 274 | 0.47 [0.35–0.59] | 0.99 [0.98–1.01] | 0.99 [0.97–1.02] | |
| CRP | 237 | 0.49 [0.36–0.62] | 1.00 [0.99–1.01] | 1.01 [0.98–1.04] | |
| Lactate | 229 | 0.67 [0.55–0.79] | 1.13 [0.95–1.34] | 0.85 [0.49–1.49] | |
| SOFA | 284 | 0.63 [0.52–0.73] | 1.43 [1.03–1.97] | 1.63 [0.69–3.84] | |
| M90d | MR-proADM | 284 | 0.59 [0.49–0.68] | 1.08 [0.96–1.21] | 0.93 [0.72–1.20] |
| PCT | 274 | 0.44 [0.34–0.54] | 1.00 [0.99–1.00] | 0.99 [0.97–1.01] | |
| CRP | 237 | 0.54 [0.43–0.65] | 1.00 [0.99–1.01] | 1.00 [0.98–1.03] | |
| Lactate | 229 | 0.66 [0.55–0.77] | 1.25 [1.07–1.46] | 1.50 [0.90–2.50] | |
| SOFA | 284 | 0.62 [0.54–0.71] | 1.40 [1.08–1.81] | 1.31 [0.70–2.43] | |
Abbreviations: AUROC, area under the ROC; CI, confidence interval; OR, odds ratio; IQR, interquartile range; M28d, 28-day mortality; M90d, 90-day mortality. Multivariate analyses show the OR for predictive ability of every biomarker when combined between themselves and the inclusion of other variables: age, SOFA, APACHE II, and nosocomial infection.
Figure 2.
ROC for the prediction of mortality at 28 days.
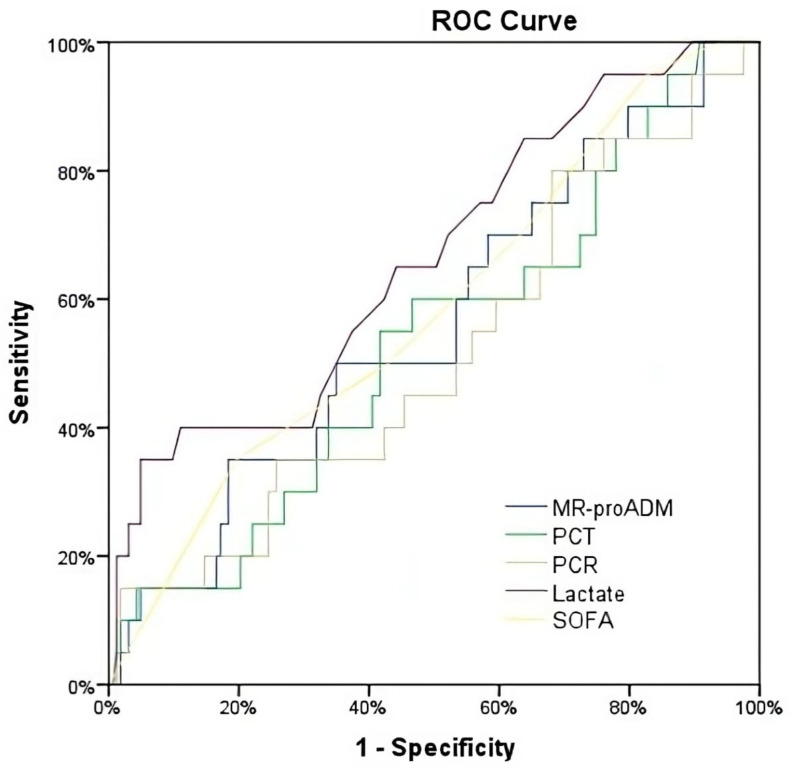
Figure 3.
ROC for the prediction of mortality at 90 days.
Both the univariate and multivariate logistic regression analyses of mortality at 28 days by each and all biomarkers were not statistically significant; however, the SOFA score had a significant OR (95% CI) of 1.43 for the same endpoint (Table 2).
When considering 90-day all-cause mortality, the AUROC for all biomarkers showed similar AUROCs to those established for 28-day mortality. In the same manner, univariate and multivariate ORs of all biomarkers and SOFA scores were not statistically significant (Table 2).
The predictive ability of each biomarker for 28-day mortality was assessed individually for either community-acquired or hospital-acquired infections. In patients with community-acquired infection, the best predictive AUROCs corresponded to MR-proADM (0.70 [0.58–0.82]) and SOFA score (0.69 [0.54–0.84]). In contrast, for hospital-acquired infection, only lactate showed a similarly high AUROC (0.70 [0.57–0.87]). Nonetheless, univariate and multivariate ORs of all biomarkers and SOFA score were not statistically significant (Table 3).
Table 3.
AUROCs and ORs for 28-day mortality in hospital-acquired vs. community-acquired infections.
| Analyses | |||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| Biomarker or Clinical Score | Patients (N) | AUROC | OR IQR (95% CI) | OR IQR (95% CI) | |
| Community | MR-proADM | 176 | 0.70 [0.58–0.82] | 1.11 [0.91–1.35] | 2.11 [0.7–6.39] |
| PCT | 168 | 0.41 [0.30–0.52] | 0.92 [0.82–1.04] | 0.93 [0.79–1.11] | |
| CRP | 147 | 0.55 [0.32–0.78] | 1.01 [1.00–1.03] | 1.01 [0.83–1.25] | |
| Lactate | 142 | 0.65 [0.48–0.82] | 1.09 [0.75–1.56] | 1.31 [0.85–20.20] | |
| SOFA | 176 | 0.69 [0.54–0.84] | 1.80 [0.98–3.30] | - | |
| Hospital | MR-proADM | 108 | 0.48 [0.30–0.66] | 1.07 [0.88–1.30] | 1.00 [0.69–1.46] |
| PCT | 106 | 0.53 [0.35–0.71] | 1.00 [0.99–1.00] | 1.01 [0.96–1.06] | |
| CRP | 87 | 0.49 [0.32–0.66] | 0.99 [0.98–1.01] | 1.00 [0.95–1.04] | |
| Lactate | 93 | 0.70 [0.54–0.86] | 1.14 [0.93–1.38] | 0.86 [0.44–1.65] | |
| SOFA | 108 | 0.57 [0.43–0.71] | 1.23 [0.85–1.77] | 1.18 [0.54–2.59] | |
Abbreviations: AUROC, area under the ROC; CI, confidence interval; OR, odds ratio; IQR, interquartile range; M28d, 28-day-mortality; M90d, 90-day mortality. Multivariate analyses show the OR for the predictive ability of every biomarker when combined between themselves and the inclusion of SOFA and APACHE II scores.
3.3. Inter-Biomarker Associations and 28-Day Survival Analysis in Patients with Sepsis and a SOFA Score ≤ 6
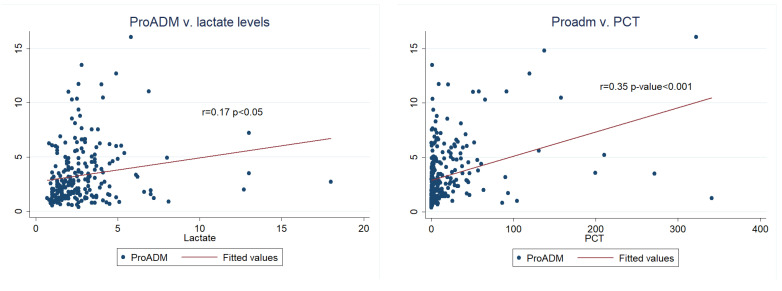
We found positive and significant correlations between MR-proADM, lactate, and PCT (Figure 4). Interestingly, from this secondary analysis, we also found a positive correlation between MR-proADM and age (r = 0.12, p < 0.05). Additionally, renal SOFA score was calculated, and we found a positive significant correlation with MR-proADM (r = 0.43, p < 0.0001).
Figure 4.
Correlation between MR-proADM and lactate and MR-proADM and PCT.
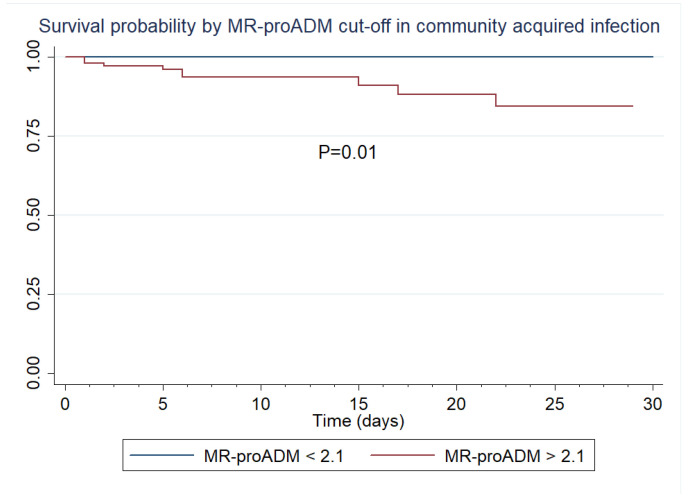
Additionally, according to our best predictive AUROC, we established that an MR-proADM cut-off point of 2.1 nmol/L classified, with 100% sensitivity, all non-survivors in patients with community-acquired infection. We generated a survival analysis for 28 days for this subgroup of patients using the 2.1 nmol/L MR-proADM cut-off. A Log-rank test of the Kaplan–Meier survival curves found significant statistical differences between survivors and non-survivors (Figure 5).
Figure 5.
Kaplan–Meier survival curves by MR-proADM in community-acquired infection.
4. Discussion
The present study analyzed the predictive ability of various biomarkers for 28-day all-cause mortality in sepsis patients with a SOFA score ≤ 6. The overall mortality rate observed in this study was 8.45%, aligning with the mortality rates reported in the existing literature on this particular subject [11].
MR-proADM showed an acceptable AUROC for 28-day mortality prediction in sepsis patients with community-acquired infection. In contrast, lactate showed the highest predictive ability in sepsis patients with hospital-acquired infection. Other biomarkers showed low predictive ability in sepsis patients with a SOFA ≤ 6.
Scoring systems and clinical and laboratory variables as tools to aid in early diagnosis and to determine severity are well-established, and many biomarkers have been extensively studied and implemented in ICUs and emergency departments [5,23,24,25,26,27,28]. The SOFA score is widely applied and provides a screening score that indicates the risk of a poor outcome [29]. However, this score is not without inherent limitations and may not always be easy to apply [30,31,32]. Moreover, despite the low mortality risk in sepsis patients presenting with a SOFA < 6 [10], a subgroup of patients still appears to have a high mortality rate. Identifying this subgroup of patients may allow for the early initiation of appropriate treatment strategies, avoiding over- or under-treatment and reducing mortality [33]. Interestingly in the present study, even in patients with a SOFA ≤ 6, SOFA remained the best individual predictor of survival. Thus, even in the lower SOFA range, organic dysfunction still discriminated severity better than the inflammatory response or hypoperfusion measured by biomarkers. Nonetheless, recent studies have indicated the potential of novel biomarkers such as MR-proADM and PCT [5,34].
MR-proADM has been proposed as a potentially useful early marker in critically ill patients, as its concentrations correspond to microcirculatory and endothelial damage in the early stages of organ dysfunction [35,36,37,38]. Hence, it is considered an indicator of early microcirculatory damage in patients with a low SOFA score. Previous studies support this consideration, showing that MR-proADM may be elevated in those sepsis patients likely to develop multiple organ failure even with a low or moderate SOFA score [17]. Our results are in line with these affirmations, as inflammatory biomarkers are correlated with each other even with low SOFA scores.
Other authors have reported that MR-proADM indicates the development of organ failure 24 h in advance [39]. Furthermore, even with a SOFA score that does not fulfill the criteria for sepsis, an elevated MR-proADM identifies septic patients, allowing for early treatment initiation [7]. Parallelly, in this study, elevated MR-proADM levels correlated with age increase, a finding that has not been thoroughly investigated and that may lead to age-dependent cut-off points, increasing the diagnostic accuracy of this inflammatory biomarker. MR-proADM has also shown good predictability in identifying patients that would require ICU admission and had good accuracy in identifying 28-day and 90-day mortality [5].
The current study results are in line with previous studies and provide further supportive evidence for the consideration of its use as part of a panel of early biomarkers in sepsis patients. These results support those found by Elke et al. [17] and Andaluz-Ojeda et al. [40], with MR-proADM showing a reasonable AUROC for 28-day mortality in patients with a SOFA ≤ 6. Although the study by Andaluz-Ojeda et al. reported a higher AUROC for 28-day mortality for MR-proADM, their study only included ICU patients. In contrast, the present study included all hospital patients that activated the sepsis code regardless of their ward.
Moreover, in our study, a MR-proADM cut-off point of 2.1 nmol/L had 100% sensibility to discern non-survivors at 28 days for patients with community-acquired infection, making MR-proADM a potentially useful screening biomarker for severity in out-of-hospital sepsis.
Our results align with those of Spoto et al. [7,20], who regarded MR-proADM as crucial for early sepsis diagnosis in those with a negative SOFA score. Hence, these studies, along with the results of this study, point to the potential of MR-proADM to guide clinical decisions and stratify risk, including in those with less-severe disease or early sepsis.
Biomarkers may vary based on whether the infection is nosocomial or has its origin in the community. In particular, hospital-acquired sepsis patients were originally admitted for another cause that can affect these biomarkers—for example, major surgery, or other major illness—and may confound the results. In contrast, in community-acquired sepsis patients, any changes in biomarker values are most likely to represent only the effect of sepsis. Along these lines, the present study showed that MR-proADM might be more useful to predict mortality in community-acquired infections, while lactate may be better to forecast mortality for hospital-acquired infections.
Limitations
The results of this study should be interpreted considering some limitations. First, the relatively low number of enrolled patients in a single center may have resulted in many subgroups being underpowered. Further, larger studies are needed to build on this study and determine the usefulness of the biomarkers studied. Second, the present study also reflected real clinical practice, and we cannot ensure that during the study period the in-hospital sepsis code was activated in all patients with sepsis. Nevertheless, our results are consistent with those previously discussed in the literature [16,40], despite the patient population enrolled in this study being preselected based on fulfilling a particular hospital sepsis criterion. Hence, this study provides additional supportive data for these biomarkers and their use at different hospital settings and varying levels of illness.
5. Conclusions
The findings of the present study suggest that, in patients with sepsis acquired in the community and an initial SOFA score ≤ 6, MR-proADM could help identify patients at risk of 28-day mortality. In those with hospital-acquired infection, lactate provided better predictive ability for 28-day mortality. Therefore, the initial measurement of these biomarkers could aid in implementing early treatment strategies based on risk stratification after activation of a hospital sepsis code in patients with a SOFA ≤ 6 and sepsis or septic shock.
Acknowledgments
The authors are grateful to the Vall d’Hebron University Hospital staff for their continued vigilance in identifying patients presenting with infections and for enrolling patients in this study. The authors want to thank all patients with sepsis for donating their blood, making them able to create the sepsis biobank. The authors wish to thank David Suárez for providing statistical advice and also Ingrid de Ruiter, MBChB, Ph.D., for editorial support in the preparation of the draft of this manuscript.
Author Contributions
J.B., J.C.R.-R. and R.F. conceived and designed the study. J.B. was the primary author and editor of the manuscript and the lead clinical investigator. All authors contributed to the enrollment of patients and sample collection. All authors critically reviewed and approved the final manuscript. R.F. and J.C.R.-R. take responsibility for the paper as a whole. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Clinical Research Ethics Committee of Vall d’Hebron University Hospital (PR(AG)333/2016, PR(AG)336/2016, PR(AG)11/2016) without the need for informed consent and in agreement with the Declaration of Helsinki and its later amendments.
Informed Consent Statement
Informed consent was signed by the patient or representatives for the collection and use of blood samples in the sepsis bank for research purposes (as per PR(AG)336/2016).
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors have provided information on potential conflicts of interest directly or indirectly related to the work submitted in the journal’s disclosure forms. RF received fees for participating in an advisory board of Thermo Fisher.
Funding Statement
This research was funded by a restricted grant from Thermo Fisher (Hennigsdorf, Germany), consisting of free-of-charge kits. However, the funding organization had no role in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yébenes J.C., SOCMIC (Catalonian Critical Care Society) Sepsis Working Group. Ruiz-Rodriguez J.C., Ferrer R., Clèries M., Bosch A., Lorencio C., Rodriguez A., Nuvials X., Martin-Loeches I., et al. Epidemiology of sepsis in Catalonia: Analysis of incidence and outcomes in a European setting. Ann. Intensiv. Care. 2017;7:19. doi: 10.1186/s13613-017-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent J.L., Marshall J.C., Ñamendys-Silva S.A., François B., Martin-Loeches I., Lipman J., Reinhart K., Antonelli M., Pickkers P., Njimi H., et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir. Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K., International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 5.Baldirà J., Ruiz-Rodríguez J.C., Wilson D.C., Ruiz-Sanmartin A., Cortes A., Chiscano L., Ferrer-Costa R., Comas I., Larrosa N., Fàbrega A., et al. Biomarkers and clinical scores to aid the identification of disease severity and intensive care requirement following activation of an in-hospital sepsis code. Ann. Intensiv. Care. 2020;10:7. doi: 10.1186/s13613-020-0625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones A.E., Trzeciak S., Kline J.A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 2009;37:1649–1654. doi: 10.1097/CCM.0b013e31819def97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spoto S., Nobile E., Carnà E.P.R., Fogolari M., Caputo D., De Florio L., Valeriani E., Benvenuto D., Costantino S., Ciccozzi M., et al. Best diagnostic accuracy of sepsis combining SIRS criteria or qSOFA score with Procalcitonin and Mid-Regional pro-Adrenomedullin outside ICU. Sci. Rep. 2020;10:16605. doi: 10.1038/s41598-020-73676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez M.L., Ruiz-Rodríguez J.C., Ferrer R. Improving knowledge about sepsis 3 definition in critically ill patients: New insights. J. Emerg. Crit. Care Med. 2018;2:39. doi: 10.21037/jeccm.2018.04.05. [DOI] [Google Scholar]
- 9.Sprung C.L., Schein R.M.H., Balk R.A. The new sepsis consensus definitions: The good, the bad and the ugly. Intensiv. Care Med. 2016;42:2024–2026. doi: 10.1007/s00134-016-4604-0. [DOI] [PubMed] [Google Scholar]
- 10.Vincent J.L., de Mendonca A., Cantraine F., Moreno R., Takala J., Suter P.M., Sprung C.L., Colardyn F., Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira F.L., Bota D.P., Bross A., Mélot C., Vincent J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 12.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021;49:e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 13.Angeletti S., Spoto S., Fogolari M., Cortigiani M., Fioravanti M., De Florio L., Curcio B., Cavalieri D., Costantino S., Dicuonzo G. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. Apmis. 2015;123:740–748. doi: 10.1111/apm.12406. [DOI] [PubMed] [Google Scholar]
- 14.Angeletti S., Dicuonzo G., Fioravanti M., De Cesaris M., Fogolari M., Presti A.L., Ciccozzi M., De Florio L. Procalcitonin, MR-Proadrenomedullin, and Cytokines Measurement in Sepsis Diagnosis: Advantages from Test Combination. Dis. Markers. 2015;2015:951532. doi: 10.1155/2015/951532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angeletti S., Ciccozzi M., Fogolari M., Spoto S., Presti A.L., Costantino S., Dicuonzo G. Procalcitonin and MR-proAdrenomedullin combined score in the diagnosis and prognosis of systemic and localized bacterial infections. J. Infect. 2016;72:395–398. doi: 10.1016/j.jinf.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela-Sánchez F., Valenzuela-Méndez B., Rodríguez-Gutiérrez J.F., Estella-García Á., González-García M.Á. New role of biomarkers: Mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016;4:329. doi: 10.21037/atm.2016.08.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H., Hur M., Struck J., Bergmann A., Di Somma S. Circulating Biologically Active Adrenomedullin Predicts Organ Failure and Mortality in Sepsis. Ann. Lab. Med. 2019;39:454–463. doi: 10.3343/alm.2019.39.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elke G., Bloos F., Wilson D.C., Meybohm P., SepNet Critical Care Trials Group Identification of developing multiple organ failure in sepsis patients with low or moderate SOFA scores. Crit. Care. 2018;22:147. doi: 10.1186/s13054-018-2084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harbarth S., Bergmann A., Müller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: An observational study. Crit. Care. 2005;9:R816–R824. doi: 10.1186/cc3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spoto S., Fogolari M., De Florio L., Minieri M., Vicino G., Legramante J., Lia M.S., Terrinoni A., Caputo D., Costantino S., et al. Procalcitonin and MR-proAdrenomedullin combination in the etiological diagnosis and prognosis of sepsis and septic shock. Microb. Pathog. 2019;137:103763. doi: 10.1016/j.micpath.2019.103763. [DOI] [PubMed] [Google Scholar]
- 21.Ferrer R., Ruiz-Rodriguez J.C., Larrosa N., Llaneras J., Molas E., González-López J.J. Sepsis code implementation at Vall d’Hebron University Hospital: Rapid diagnostics key to success. ICU Manag. Pract. 2017;17:214–215. [Google Scholar]
- 22.Li F., He H. Assessing the Accuracy of Diagnostic Tests. Shanghai Arch Psychiatry. 2018;30:207–212. doi: 10.11919/j.issn.1002-0829.218052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuetz P., Albrich W., Christ-Crain M., Chastre J., Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Rev. Anti-Infect. Ther. 2010;8:575–587. doi: 10.1586/eri.10.25. [DOI] [PubMed] [Google Scholar]
- 24.Schuetz P., Aujesky D., Müller C., Müller B. Biomarker-guided personalised emergency medicine for all—Hope for another hype? Swiss Med. Wkly. 2015;145:w14079. doi: 10.4414/smw.2015.14079. [DOI] [PubMed] [Google Scholar]
- 25.Schuetz P., Christ-Crain M., Thomann R., Falconnier C., Wolbers M., Widmer I., Neidert S., Fricker T., Blum C., Schild U., et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 26.Schuetz P., Batschwaroff M., Dusemund F., Albrich W., Bürgi U., Maurer M., Brutsche M., Huber A.R., Müller B. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: A post-study survey. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:269–277. doi: 10.1007/s10096-009-0851-0. [DOI] [PubMed] [Google Scholar]
- 27.Schuetz P., Chiappa V., Briel M., Greenwald J.L. Procalcitonin algorithms for antibiotic therapy decisions: A systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch. Intern. Med. 2011;171:1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 28.Schuetz P., Wirz Y., Sager R., Christ-Crain M., Stolz D., Tamm M., Bouadma L., E Luyt C., Wolff M., Chastre J., et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2017;10:CD007498. doi: 10.1002/14651858.CD007498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer A.J., Ng J., Thode H.C., Jr., Spiegel R., Weingart S. Quick SOFA Scores Predict Mortality in Adult Emergency Department Patients with and without Suspected Infection. Ann. Emerg. Med. 2017;69:475–479. doi: 10.1016/j.annemergmed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Simpson S.Q. New Sepsis Criteria: A Change We Should Not Make. Chest. 2016;149:1117–1118. doi: 10.1016/j.chest.2016.02.653. [DOI] [PubMed] [Google Scholar]
- 31.Simpson S.Q. SIRS in the Time of Sepsis-3. Chest. 2018;153:34–38. doi: 10.1016/j.chest.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Simpson S.Q. Diagnosing sepsis: A step forward, and possibly a step back. Ann. Transl. Med. 2017;5:55. doi: 10.21037/atm.2017.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Does Y., Limper M., Jie K.E., Schuit S.C.E., Jansen H., Pernot N., van Rosmalen J., Poley M.J., Ramakers C., Patka P., et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: A multicentre non-inferiority randomized clinical trial (HiTEMP study) Clin. Microbiol. Infect. 2018;24:1282–1289. doi: 10.1016/j.cmi.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Saeed K., del Castillo J.G., Backous C., Drevet S., Ferrer R., Gavazzi G., Gluck E., Jensen J.-U., Kanizsai P., Ruiz-Rodríguez J.C., et al. Hot topics on procalcitonin use in clinical practice, can it help antibiotic stewardship? Int. J. Antimicrob. Agents. 2019;54:686–696. doi: 10.1016/j.ijantimicag.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Temmesfeld-Wollbrück B., Hocke A.C., Suttorp N., Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb. Haemost. 2007;98:944–951. doi: 10.1160/th07-02-0128. [DOI] [PubMed] [Google Scholar]
- 36.Pittard A.J., Hawkins W.J., Webster N.R. The role of the microcirculation in the multi-organ dysfunction syndrome. Clin. Intensiv. Care. 1994;5:186–190. [PubMed] [Google Scholar]
- 37.Xie Z., Chen W.S., Yin Y., Chan E.C., Terai K., Long L.M., Myers T.G., Dudek A.Z., Druey K.M. Adrenomedullin surges are linked to acute episodes of the systemic capillary leak syndrome (Clarkson disease) J. Leukoc. Biol. 2018;103:749–759. doi: 10.1002/JLB.5A0817-324R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vigué B., Leblanc P.E., Moati F., Pussard E., Foufa H., Rodrigues A., Figueiredo S., Harrois A., Mazoit J.-X., Rafi H., et al. Mid-regional pro-adrenomedullin (MR-proADM), a marker of positive fluid balance in critically ill patients: Results of the ENVOL study. Crit. Care. 2016;20:363. doi: 10.1186/s13054-016-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viaggi B., Poole D., Tujjar O., Marchiani S., Ognibene A., Finazzi S. Mid regional pro-adrenomedullin for the prediction of organ failure in infection. Results from a single centre study. PLoS ONE. 2018;13:e0201491. doi: 10.1371/journal.pone.0201491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andaluz-Ojeda D., Nguyen H.B., Meunier-Beillard N., Cicuéndez R., Quenot J.-P., Calvo D., Dargent A., Zarca E., Andrés C., Nogales L., et al. Superior accuracy of mid-regional proadrenomedullin for mortality prediction in sepsis with varying levels of illness severity. Ann. Intensiv. Care. 2017;7:15. doi: 10.1186/s13613-017-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.