Abstract
The somatic cell count (SCC; leukocytes and epithelial cells) in milk is used as an indicator of udder health status. A SCC above the regulatory standard is generally considered as an indication of mastitis. Therefore, milk with a SCC equal to or greater than the regulatory limit cannot be sold to the public because it is unsuitable for human consumption. This study was performed to determine whether SCC levels above the regulatory limit observed in goats during late lactation are a physiologic or a pathological response of the goat mammary gland. Differential counts of cells in nonmastitic goat milk samples during late lactation revealed that approximately 80% of the cells were polymorphonuclear leukocytes (PMNs). In addition, microchemotaxis assay results indicated that normal nonmastitic late-lactation-stage goat milk is significantly higher (P < 0.001) in PMN chemotactic activity than early-lactation-stage goat milk, with a mean chemotactic activity of 14.9 and 42.7/mg of protein for early and late lactation stages, respectively. Physicochemical analyses also suggest that the PMN infiltration observed in normal late-lactation-stage goat milk is due to a PMN chemotactic factor(s) that is different from the PMN chemotactic factor(s) present in mastitic milk. Interestingly, the PMN chemotactic factor in late-lactation-stage goat milk is highly acid resistant (pH 2), suggesting that the factor is able to survive the highly acidic gastric environment and may therefore be important in the augmentation of the immune systems of sucklings. These results indicate that the chemotactic factor(s) present in the milk of normal late-lactation-stage goats is nonpathological and may play a physiologic regulatory role in mammary gland involution. Hence, the regulatory standard for goat milk needs to be redefined in order to reflect this.
Chemotactic cytokines have been shown to modulate leukocyte infiltration in a variety of diseases (4, 12, 46). In particular, chemokines, a group of chemotactic cytokines whose hallmark is the conservation of four cysteine residues, have been implicated in numerous inflammatory conditions. Interleukin-8 (IL-8), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1 alpha (MIP-1α) have been shown to be involved in diseases such as rheumatoid arthritis, septic shock, gastric cancer, asthma, cystic fibrosis, inflammatory bowel disease, alcoholic hepatitis, glomerulonephritis, and atherosclerosis (2, 7, 24, 25, 30, 41). More recently, the chemokine coreceptor CCR5 has been shown to be important for human immunodeficiency virus entry into cells (43). Increasing evidence also suggests that chemotactic cytokines are present in milk (11, 13, 15, 38, 42). Skansen-Saphir and coworkers (38) reported that lipopolysaccharide-stimulated milk mononuclear cells (MNCs) induced extensive production of the chemotactic cytokines IL-8 and tumor necrosis factor alpha in addition to other cytokines. As evidenced by these studies, chemotactic cytokine production is almost always associated with pathological conditions (35). Few studies have focused on evidence suggesting chemotactic cytokine involvement in normal physiologic processes.
During the course of mastitis, the release of chemotactic cytokines results in the infiltration of somatic cells into the mammary gland. “Somatic cells” is a term which refers to the leukocytes, specifically lymphocytes, macrophages, and polymorphonuclear leukocytes (PMNs), in addition to the small percentage of epithelial cells that is present in milk (31). This local population of somatic cells serves as one of the most important defense mechanisms of the mammary gland against infection. Numerous studies have shown that the somatic cell count (SCC) of mammary secretions is directly correlated to infection status (8, 22, 36). As such, the SCC is used by the dairy industry as a reliable indicator of mastitis and milk quality. The major factor influencing the SCC is an infection of the mammary gland (17, 31). The SCC of milk from an uninfected bovine udder is usually less than 200,000/ml. During inflammation however, the SCC of mammary secretions increases to millions per milliliter (31). According to official regulatory standards, analysis of bulk-tank milk samples from a given herd needs to be performed once a month. Goat milk samples having SCCs of 1 million cells/ml or higher on three consecutive tests are rejected and are prohibited from being sold. Cow milk samples are rejected at a SCC equal to or greater than 750,000 cells/ml.
Interestingly, numerous investigators have reported that SCC values above the regulatory limit are commonly observed in nonmastitic goat milk during late lactation. Dulin and coworkers (9) reported that total SCCs and the percentage of PMNs in goat milk increased as lactation progressed for both infected and uninfected glands, and as a result, the percentages of lymphocytes and macrophages decreased. These findings have been confirmed by other researchers (20). A study of uninfected goat milk revealed that milk samples from only 34.5% of producers were under the caprine regulatory limit of 1 million cells/ml (8). PMNs were prevalent in these samples, comprising 87% of the leukocyte populations in late-stage milk, despite the absence of infection. In another study, 71% of the samples exceeded the regulatory limit during the last 3 months of lactation (45). This phenomenon was observed despite the absence of signs and symptoms of mastitis in any of the does with SCCs of over 1 million/ml. Bacteriological tests of the samples showed only a trace (<5 CFU/ml) of mastitis-causing pathogens, if they were present at all. More importantly, examination of late-lactation-stage mammary gland tissue revealed that there was no histological or pathological evidence of tissue injury in the does with SCCs of >0.9, 1.5, and 3.3 million/ml (44). These data indicated that high SCCs were prevalent and that increased PMNs in goat milk during late lactation contributed to the high SCCs.
Few studies have attempted to explain the basis for the increase in SCCs and the predominance of PMNs during late lactation in goats (8, 9, 44, 45). The physiologic cause(s) for this phenomenon needs to be established. The aim of our study was to determine the causes of the increase in SCCs and the prevalence of PMNs in late-lactation-stage goat milk. Specifically, the focus was on the detection of lactation stage-dependent leukocyte chemotactic activity in mammary secretions of the goat. We provide evidence that a physiologic chemotactic factor(s) in the mammary gland is responsible for the increase in SCC and PMN infiltration in the mammary secretions of goats in the absence of mastitis at the late lactation stage.
MATERIALS AND METHODS
Milk samples.
Twelve 2-year-old Anglo-Nubian dairy goats were used for these studies (10 from farm 1 and 2 from farm 2). All animals received hay and water ad libitum and grain (Blue Seal Foods, Inc., Londonderry, N.H.) twice a day. All the goats tested negative for caprine arthritis and encephalitis virus and were clinically free of mastitis throughout the sampling period. Samples with bacterial growths on blood agar plates were designated as subclinical mastitic milk samples. Samples with five or more colonies of the same type in conjunction with a cell count of ≥1 million cells/ml were defined as (clinical) mastitic samples (National Mastitis Council standard). All mastitic samples (clinical and subclinical) were excluded from data analysis unless otherwise stated. The goats were milked twice daily at the same time each day, and milk production was recorded daily. Mammary gland secretions from udder halves of each animal were collected at weekly intervals beginning at parturition and continuing until the end of the lactation cycle (i.e., involution, beginning of the dry period, or weaning). Aliquots of the weekly samples were then processed by the Diagnostic Testing Services at the University of Connecticut for bacteriological analysis and direct microscopic SCC. For the assay of chemotactic activity, secretions were first centrifuged at 700 × g for 25 min. Following removal of the cell pellet and fat layer, the milk was respun at 1,000 × g for 1 h, the fat layer was removed, and the whey was then stored at −20°C. Prior to being tested, the samples were thawed and centrifuged again at 1,500 × g for 10 min to remove any residual fat and cell debris and then sterilized with a 0.45-μm-pore-size Millipore membrane (Micron Separations Inc., Westborough, Mass.).
Milk cell enumeration.
The cellular portion of the milk was separated from other milk components by centrifugation as described above. The cell pellet was then washed twice in phosphate (0.01 M)-buffered saline (PBS) (pH 7.2) containing 10 mM glucose by centrifugation at 500 × g for 10 min. Following the washing, the cell viability was determined by the trypan blue exclusion method. Slides for differential cell counting were then prepared by cytocentrifugation and staining with Leukostat (Fisher Scientific, Pittsburgh, Pa.). Two hundred cells per slide were identified and counted as MNCs or PMNs.
Determination of protein concentration.
The total milk protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). The assay is based on the reaction of copper with bicinchoninic acid. Dilutions of stock bovine serum albumin (BSA) were used as protein standards, and the standard protocol (incubation at 37°C for 30 min) was used. Absorbances were determined at 562 nm on a SpectraMax 250 spectrophotometer (Molecular Devices, Sunnyvale, Calif.).
Separation of MNCs by F-D.
Varying densities of Ficoll-diatrizoate (F-D) were prepared by a technique modified from Boyum (3). Briefly, 65 ml of a 9.5% solution of Ficoll (type 400; Sigma Chemical Co., St. Louis, Mo.) was combined with 40 ml of a 34% solution of sodium diatrizoate (Sigma Chemical Co.) and adjusted to pH 7.2. The density was then determined with a hydrometer (Fisher Scientific). Densities were adjusted to 1.090, 1.100, and 1.105 by the addition of sodium diatrizoate solution. The solutions were then filter sterilized through a 0.45-μm-pore-size Millipore membrane. For separation of MNCs, 3.0-ml aliquots of EDTA-treated caprine blood were first centrifuged at 400 × g for 20 min. The buffy coats were then isolated, diluted 1:4 with PBS, and layered over 3 ml of F-D gradient with specific densities of 1.090, 1.100, and 1.105. Following centrifugation at 400 × g for 35 min, the MNCs at the interface were collected and the numbers and percentages of recovered lymphocytes and monocytes (approximately 98% lymphocytes and 2% monocytes) were compared.
PMN isolation.
For the assay of PMN chemotactic activity, 15 ml of EDTA-treated caprine blood was centrifuged at 400 × g for 20 min. Following the removal of the plasma, buffy coat, and one-fourth of the erythrocyte pellet, PMNs were obtained by lysing the remaining erythrocyte pellet with 10 ml of 0.2% sodium chloride for 30 s, followed by 10 ml of 1.6% sodium chloride to restore isotonicity. The PMNs obtained were then washed twice and resuspended in PBS with glucose. The cell viability, as determined by the trypan blue exclusion method, was consistently found to be over 95%. The cell preparation was approximately ≥97% PMNs. Heparin was not used as an anticoagulant because it has been shown to bind neutrophils and induce apoptosis (26).
Microchemotaxis assay of chemotactic activity in goat milk.
MNC and PMN populations isolated from caprine blood as described above were resuspended in Hanks’ balanced salt solution containing BSA (0.05%) (Sigma Chemical Co.) at a concentration of 2 × 106 cells/ml. Chemotactic activities of goat milk from different lactation stages were then assayed by using a 48-well chemotaxis chamber (Neuroprobe, Cabin John, Md.) and a polyvinylpyrrolidone-free micropore filter (pore size, 5 μm; Poretics, Livermore, Calif.). Briefly, triplicate milk samples (30 μl) were added to the lower wells of the chamber, and responder cells (PMNs and MNCs) were then added to the upper wells of the chamber (105 cells/well). Mastitic goat milk and medium (Hanks’ balanced salt solution with 0.05% BSA) served as positive and negative controls, respectively. Following incubation at 37°C for 30 min (for PMNs) and 1 h and 30 min (for MNCs), the filter separating the upper and lower chambers was removed.
After elimination of nonmigratory cells on the filter side in contact with the upper well by using repeated washes with PBS and a wiper blade, the filter was stained with Leukostat (Fisher Scientific). PMNs and MNCs that migrated completely through the filter were counted with an Olympus (Woodbury, N.Y.) AH-2 microscope and a model Q4-Cue-4 image analyzer (Galai, Galai, Israel). The number of migrated cells was determined by finding the binary threshold of the image acquired at a total magnification of ×200. The results were expressed as the mean ± standard deviation (SD) of triplicate chemotactic differentials or triplicate chemotactic differentials per milligram of protein, which are defined as follows: chemotactic differential = (number of cells migrated per field) − (number of cells migrating randomly) and chemotactic differential per milligram of protein = (chemotactic differential × 103 × protein concentration−1)/30, where 103 is the dilution factor, 30 is the volume (in microliters) of goat whey loaded in the microchemotaxis chamber, and protein concentration is measured in milligrams per milliliter. For example, if the chemotactic differential is 80 and the protein concentration is 75 mg/ml, then chemotactic differential per milligram of protein = [80 × 103 × (1/75)]/30 = 35.56.
In order to determine whether goat milk induces chemotaxis or chemokinesis of PMNs and MNCs, standard checkerboard analyses were also performed. Increasing dilutions of milk were placed above and below the filters so that various concentration gradients were established across the filters. After incubation, the filters were analyzed as described previously.
Physicochemical characterization of PMN chemoattractants in goat milk. (i) Molecular mass.
For the assessment of possible differences between the molecular masses of chemoattractants in normal and mastitic milk, samples were dispensed into Microsep centrifugal concentrators (Filtron, Northborough, Mass.) with cutoff filters of 3, 10, and 30 kDa and processed according to the manufacturer’s instructions. The chemotactic activities of various milk filtrate fractions were then assayed by the microchemotaxis assay as described previously. To examine the possibility that casein protein present in the milk was responsible for any chemotactic activity detected in the milk fractions, goat casein was also tested at the concentration present in normal milk (22 g/liter).
(ii) Heat stability.
The heat stability of normal and mastitic milk fractions was assessed by heating the aliquots at 60°C for 30 min. Chemotactic activities of the milk samples were then tested as described above.
(iii) pH stability.
pH stability was assessed by treatment of 1-ml aliquots of normal milk fractions with 1 N HCl or 1 N NaOH. Extreme pH conditions (pH 2 and 11) were maintained for 30 min. Subsequently, the pH was brought back to neutral with HCl and NaOH and the milk fractions were concentrated to their original volumes by ultrafiltration and then tested for chemotactic activity.
Statistical analysis.
Values for PMN and MNC chemotactic activity per milligram of protein were analyzed by the mixed-model analysis of variance (ANOVA) with the statistical software package SAS (SAS Institute, Cary, N.C.) (both the GLM and MIXED procedures were utilized). Sources of variations included the fixed effects of farm, udder half, and stage of lactation and the random effects of individual goats. Expected mean squares with the random statement were incorporated to establish the validity of the F tests in the ANOVA, as some data were missing.
All mastitic samples were excluded from the analysis. Adjusted means and standard errors are presented as appropriate estimates of the means of the population farm, udder half, and stage of lactation and are computed from the LSMEAN procedure in the MIXED procedure in SAS. Examination of the residuals indicated that the log transformation provided an appropriate transformation of the data, as the residuals were consistent with a normal distribution (P > 0.05 with the Shapiro-Wilk statistic).
RESULTS
SCC and milk volume.
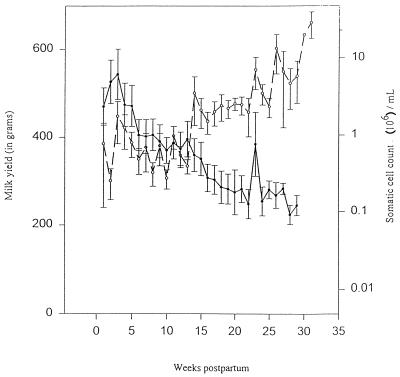
In order to determine whether a concentration effect was responsible for the increase in SCCs observed by previous investigators, both milk volume and SCCs were analyzed throughout the lactation cycle. As shown in Fig. 1, an inverse relationship between milk volume and SCC was observed. As the milk yield decreased toward the late lactation stage, there was a concomitant increase in the SCC, indicating that a concentration effect contributed, at least partially, to the increase in the SCC during late lactation.
FIG. 1.
Concentration-dependent SCC increase in goat milk during late lactation. The milk yield (•) and SCC (○) in goat milk were analyzed throughout the lactation cycle. Milk yield values represent milk production of an udder half for each animal per milking. SCCs were performed by the direct microscopic SCC method. The data represent the mean ± the standard error of the mean for 12 animals (n = 24 halves).
Differential counts of cells in goat milk.
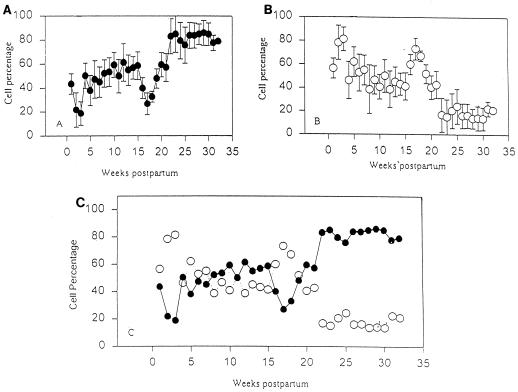
To verify reports by previous investigators on the increase in PMNs during late lactation, we examined the leukocyte populations in milk throughout the lactation cycle. Leukocyte populations in mammary secretions during the lactation cycle are shown in Fig. 2. During weeks 2 and 3 postpartum (transitional milk), MNCs were the predominant cell type in milk (∼80%). In contrast, normal (mature) milk from the early lactation stage (weeks 4 to 18) was characterized by slightly higher percentages of PMNs (∼50%) than of MNCs (∼45%), although the differences were not significant (P > 0.05). A switch in leukocyte profiles occurred during weeks 16 to 18. During this brief interval, MNCs were the predominant cell population (∼70%). A significant increase in the proportion of PMNs (P < 0.05 compared to that in early lactation) with a concomitant decrease in MNCs occurred during late lactation (≥19 weeks postpartum). During this stage, approximately 80% of the cells were PMNs (Fig. 2A and B).
FIG. 2.
Time course analysis of the cell populations in goat milk during the lactation cycle. Leukostat-stained cytocentrifuge smears of milk samples from each udder half were analyzed as either PMNs or MNCs. Individual profiles of PMN (•) (A) and MNC (○) (B) populations and a composite (C) are shown. The data represent the mean ± SD for 12 animals (n = 24 halves).
Separation of MNCs by F-D.
Typical recovery rates for peripheral blood MNCs obtained from F-D gradients with densities of 1.090, 1.100, and 1.105 were 15, 80, and 90%, respectively.
Leukocyte chemotactic activity in goat milk at different stages of the lactation cycle. (i) Chemotaxis assay.
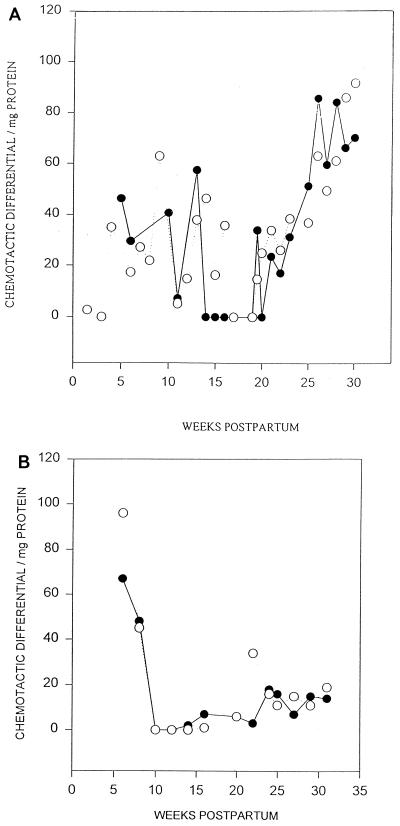
In order to determine whether leukocyte chemotactic factors contributed to the observed influx of leukocytes into milk, the PMN and MNC chemotactic differential per milligram of protein of whey from different stages of the lactation cycle was assayed. As shown in Fig. 3A, which contains the data from the typical response of a majority of the herd, there was a significant increase in PMN chemotactic activity during the late lactation stage compared to that of early lactation. In contrast, as shown in Fig. 3B, which also contains the data for the typical response of a majority of the herd, the MNC chemotactic activity was greater in milk from early lactation.
FIG. 3.
(A) Lactation stage-dependent PMN chemotactic activity in goat mammary secretions. Goat milk was tested for its ability to elicit PMN chemotaxis, as assayed by the microchemotaxis technique. PMNs (105/well) were placed in the upper wells, and whey was placed in the lower wells. After a 30-min incubation, the PMNs that had migrated to the underside of the micropore filter were quantitated. The data show the chemotactic differential per milligram of whey protein of one animal and reflect the typical response of a majority of the herd (n = 2 halves). Both left (•) and right (○) udder halves were analyzed. (B) Lactation stage-dependent MNC chemotactic activity in goat mammary secretions. Goat milk was tested for its ability to elicit MNC chemotaxis, as assayed by the microchemotaxis technique. MNCs (105/well) were placed in the upper wells, and whey was placed in the lower wells. After a 1.5-h incubation, the MNCs that had migrated to the underside of the micropore filter were quantitated. The data show the chemotactic differential per milligram of whey protein of one animal and reflect the typical response of a majority of the herd (n = 2 halves). Both left (•) and right (○) udder halves were analyzed.
(ii) Statistical analysis.
Using both the mean and maximum chemotactic activities per milligram of protein, we performed a repeated-measures ANOVA to investigate udder half, stage of lactation, and farm differences, with the farm being the “between goat” factor and udder half and stage of lactation being the “within goat” factors. There were no significant udder half effects in the analysis of PMN chemotactic activity. There was a significant farm-by-stage-of-lactation interaction (P < 0.001) with the mean PMN chemotactic activity per milligram of protein, with the late-stage mean being higher in farm 1 than in farm 2. There were no differences between the farms in maximum PMN chemotactic activity per milligram of protein. In subsequent analyses, goats from the two farms were used as a combined sample in order to estimate more efficiently the statistical differences for udder half and stage of lactation. Significant differences were found only for stage of lactation, with the late stage being higher (P < 0.001) for both the mean PMN chemotactic activity per milligram of protein and the maximum PMN chemotactic activity per milligram of protein. The means and the adjusted geometric means (the antilog of the adjusted mean) for PMN chemotactic activity per milligram of protein and maximum PMN chemotactic activity per milligram of protein are shown in Table 1.
TABLE 1.
PMN and MNC chemotactic activities in goat milk from early and late stages of the lactation cycle
| Cell type | Stage | Chemotactic activity per milligram of protein
|
Maximum chemotactic activity per milligram of protein
|
||
|---|---|---|---|---|---|
| Mean | Geometric mean | Mean | Geometric mean | ||
| PMNa | Early | 14.9 | 4.8 | 50.6 | 39.1 |
| Late | 42.7 | 13.1 | 78.9 | 71.4 | |
| MNCb | Early | 37.6 | 18.9 | 70.2 | 68.8 |
| Late | 21.9 | 16.9 | 36.3 | 33.5 | |
Goat whey was tested throughout the lactation cycle for PMN chemotactic activity by the microchemotaxis technique. PMNs and whey were placed above and below the micropore filter, respectively. Following a 30-min incubation, the PMNs that had migrated to the underside of the filter were quantitated. Values for PMN chemotactic activity per milligram of whey protein were analyzed by the mixed-model ANOVA. Significant differences were found between the lactation stages, with the late-stage values being higher (P < 0.001) for both the mean PMN chemotactic activity per milligram of protein and the maximum PMN chemotactic activity per milligram of protein.
Analysis was the same as that for PMN activity, except that incubation was for 1.5 h. Significant differences were found between the lactation stages for the maximum MNC chemotactic activity per milligram of protein, with the early-stage values being higher (P < 0.002) than those of the late stage. There were no differences between the stages for the mean MNC chemotactic activity per milligram of protein.
There were no significant udder half effects or differences between the farms for the mean and maximum MNC chemotactic activities per milligram of protein. Significant differences were found for stage of lactation with the maximum MNC chemotactic activity per milligram of protein, with that of the early stage being higher (P < 0.002) than that of the late stage. There were no differences between stages of lactation for the mean MNC chemotactic activity per milligram of protein. The means and the adjusted geometric means (antilog of the adjusted mean) for MNC chemotactic activity per milligram of protein and maximum MNC chemotactic activity per milligram of protein are shown in Table 1.
Induction of leukocyte chemokinesis and chemotaxis by milk.
In order to determine whether the observed leukocyte migration was due to chemokinesis and/or chemotaxis, a checkerboard analysis was done. Table 2 shows the results of the PMN and MNC checkerboard analyses. Chemokinesis is defined as increased random migration in the absence of a gradient. In contrast, chemotaxis is defined as directed migration along a positive concentration gradient. Migration of PMNs and MNCs increased when the concentration of milk was increased equally above and below the filter (see the diagonals of Table 2), suggesting that chemokinesis occurred. However, significant increases in PMN and MNC migration were also seen when the milk concentration was increased below the filter (see the verticals of Table 2). Therefore, goat milk induced both the chemotaxis and chemokinesis of PMNs and MNCs.
TABLE 2.
Checkerboard analyses of PMN and MNC migration toward goat milka
| % Milk below filter | No. of cells migrated (±SD)b
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PMN
|
MNC
|
|||||||
| 0c | 33 | 66 | 100 | 0 | 33 | 66 | 100 | |
| 0 | 26 ± 6 | 8.3 ± 3 | 7 ± 3 | 12 ± 4 | 35 ± 9 | 24 ± 6 | 30 ± 3 | 49 ± 6 |
| 33 | 60 ± 21 | 65 ± 7 | 63 ± 20 | 42 ± 14 | ||||
| 66 | 64 ± 10 | 58 ± 25 | 109 ± 2 | 43 ± 4 | ||||
| 100 | 74 ± 10 | 44 ± 3 | 50 ± 5 | 54 ± 5 | ||||
Increasing concentrations of goat whey were placed above and below the micropore filter so that various concentration gradients were established across the filter. After a 30-min or 1.5-h incubation, respectively, the PMNs and MNCs that had migrated to the underside of the filter were quantitated. The data suggest that goat whey is both chemokinetic and chemotactic for PMNs and MNCs.
Results are expressed as the mean number of cells (±SD) migrated per field for triplicate samples.
% Milk above the filter.
Physicochemical characterization of PMN chemoattractants in normal and mastitic milk.
In order to determine whether the PMN chemotactic factors present in normal and mastitic goat milk were different, various whey fractions were assayed for chemotactic activity. Table 3 is a summary of the physicochemical characteristics of the chemoattractant(s) present in mastitic milk and normal late-stage milk. The molecular masses of most of the chemotactic factors present in mastitic milk were <10 and ≥3 kDa. In contrast, the molecular mass of the chemotactic factor(s) present in normal late-stage milk was <30 and ≥10 kDa. Heat stability tests showed that the <10-kDa mastitic milk fraction retained its chemotactic activity following heating at 60°C for 30 min (P < 0.05 compared to the untreated fraction). In contrast, the chemotactic activity of the <30- and >10-kDa fraction of normal late-stage milk was destroyed by heat treatment. Furthermore, pH stability assays of normal late-stage milk fractions suggested that the <30- and >10-kDa fraction was stable at pH 2 whereas it lost 74% of its chemotactic activity at pH 11. Casein was also tested in the microchemotaxis assay for its chemotactic ability. Results showed that casein was not able to induce any PMN migration.
TABLE 3.
Physicochemical characteristics of PMN chemoattractants in mastitic and normal late-lactation-stage milka
| Milk type | Estimated molecular mass (kDa)b | Heat stabilityc | pH stability
|
|
|---|---|---|---|---|
| pH 2 | pH 11 | |||
| Mastitic | ≥3–<10 | Stable | NDd | ND |
| Normal late stage | ≥10–<30 | Unstable | Stable | 74% activity loss |
Results are representative of two separate experiments.
Molecular mass estimations were made with centrifugal concentrators with cutoff filters of 3, 10, and 30 kDa.
At 60°C for 30 min. Stable, full chemotactic activity remained; unstable, complete absence of chemotactic activity.
ND, not determined.
DISCUSSION
The local population of leukocytes (somatic cells) in the udder is necessary for an animal to mount an effective immune response against intramammary pathogens. The SCC has been shown to be directly correlated with infection status and is used in the dairy industry as a reliable indicator of mastitis (8, 17, 22, 36). Interestingly, numerous investigators have reported that SCC increases and the predominance of PMNs is consistently observed in normal late-lactation-stage goat milk (19, 29, 32, 33). This study provides a physiologic explanation for this phenomenon. Our work demonstrates that there are physiologic chemoattractants which are responsible for the increase in SCC and PMN infiltration that is observed in normal late-lactation-stage goat milk. Furthermore, we provide evidence which suggests that there are differences between the chemotactic cytokine profiles of mastitic and normal late-lactation-stage milk.
Differential counts of leukocytes in normal milk reveal that MNCs are the major cell population found in colostrum. This is consistent with the concept that milk provides passive protection for the neonate (6, 16) and has immunostimulatory capabilities (18, 40). During the late lactation stage, PMNs are the predominant cell type, despite the absence of infection. This confirms prior reports that an increase in PMNs is the cause of the increase in SCCs during the late stage of lactation in goats (8, 9, 32, 37, 44). It is of interest to note that morphologic characteristics of the phagocytic cells in goat milk and those of their blood counterparts were different. Milk phagocytic cells contained numerous vacuoles which distorted the shape of the cells, and extracellular particles were present in the milk despite extensive washing. This contributed to the complexity of differentiating the leukocyte populations in goat milk, which was also experienced by previous investigators (6, 14).
Examination of SCCs throughout the lactation cycle reveals that the SCC is inversely related to the milk yield. This supports the prevalent belief that a concentration effect is the main reason for the increase in SCCs found during the late stage of lactation in goats in the absence of infection (8, 44). However, chemotaxis assays of goat milk show that there is a significant increase in PMN chemotactic activity during the late stage of lactation even when the milk protein concentration is taken into consideration. This suggests that a decrease in milk volume is not the only cause of the observed rise in SCCs. An increase in PMN chemotactic activity during this period is also responsible for the dramatic increase in the SCC that is observed in late-lactation-stage goat milk. Changes in PMN/MNC ratios at different lactation stages are additional evidence that a concentration effect is not the only cause of the SCC increase during late lactation. These results challenge the predominant concept that infection status is the only reason for an increase in the SCC in milk (8, 9, 17, 37). Based on our findings, we propose that a normal physiologic program is responsible for the increase in SCCs (due to PMNs) that is observed during the late lactation stage in goats. In preparation for the dry period (i.e., weaning or involution), PMN infiltration occurs in order to participate in the involution process (apoptosis?) and to provide protection for the mammary gland during the period when it is most susceptible to intramammary infection. Susceptibility of the mammary gland to infection is highest during the first 2 weeks of the dry period (16, 31). In dairy cows, Jensen and Eberhart (21) have previously reported that PMNs are the predominant milk leukocyte population during the first week of the dry period. Macrophages and lymphocytes then become the predominant cell populations throughout the rest of the dry period. Hence, the importance of the PMN population during the first week of involution should be noted. PMNs in milk are able to phagocytize and destroy bacteria and to remove tissue debris (34). Thus, the activity of PMNs is the most important defense and cleaning mechanism of the mammary gland (34). This concept reinforces our belief that the observed increase in the PMN population in goats at the late stage of lactation also serves a physiologic function for the remodeling of the gland during the involution period.
Evaluation of the MNC chemotactic activity in goat milk showed that milk MNC chemotactic activity was highest during the first few weeks of early lactation (weeks 4 to 6). A transient increase in MNC chemotactic activity also occurred during late lactation (week 20 or later). In addition, PMN chemotactic activity increased significantly during late lactation (week 20 or later). It is of interest to speculate that the differential chemotactic activities detected at different stages of the lactation cycle (a MNC activity during the early stage and a PMN activity during the late stage) may be due to two entirely different chemotactic factors, i.e., a programmed signaling mechanism in the mammary gland induces the release of the MNC and PMN chemotactic factors during early and late lactation, respectively. To further support our hypothesis, conditioned media from a caprine mammary epithelial cell (CMEC) line developed in our laboratory (28) was tested for PMN chemotactic activity. Significant PMN chemotactic activity was shown to be present in the conditioned media of CMECs. Several investigators have previously shown that epithelial cells have the capacity to release chemotactic factors (1, 10, 24). It is tempting to speculate that a preprogrammed mechanism in the mammary gland signals the epithelial cells or the MNCs (upon receiving signals from the mammary gland epithelial cells) to secrete a PMN chemotactic factor during late lactation, which then results in the influx of PMNs into the milk. During late lactation and the first weeks of involution, PMNs may serve as physiologic regulators for the early phase of the involution process. Subsequently, MNCs are mobilized to participate in the completion of the involution process. This is in agreement with the results of prior investigators, who have observed that PMNs have the capacity to release soluble mediators which are chemotactic for MNCs upon exposure to a variety of stimuli (5). This process results in the predominance of MNCs (macrophages and lymphocytes) in the mammary gland during the involution stage. At that time, monocytes are activated and are particularly aggressive at phagocytizing milk residues and cell debris in the apoptotic or involuting mammary gland (34).
The predominance of PMNs during the late lactation stage is interpreted by many to be the result of an inflammation in the mammary gland. However, our study revealed that goat mammary glands produced chemotactic factors for PMNs and MNCs in the absence of any signs of mastitis or bacteria in the milk. Histopathological studies of late-lactation-stage goat mammary glands by other investigators showed no signs of tissue injury (44) or changes in milk composition (19). An increase in the SCC (due to PMNs) is the only similarity between normal late-lactation-stage milk and mastitic milk in the goat. However, the increase of PMNs in the milk should not be perceived solely as evidence of infection of the mammary gland. Smith and Goldman (39), who have observed high PMN counts in colostrum (despite the absence of infection) from nonnursing mothers, proposed that these maternal cells are potentially beneficial, and possibly serve to facilitate the development of immunocompetence in the neonate. Hence, the reported mobilization of the PMN population (responsible for most of the increase in SCCs) during the late lactation stage may not be indicative of a pathological condition but simply a normal physiologic regulatory mechanism in the mammary gland. In support of our hypothesis, physicochemical analyses of the chemotactic factors in normal late-lactation-stage and mastitic milk were performed. The results suggest that different cytokines are present in normal late-lactation-stage and mastitic milk. Molecular mass (<10 and ≥3 kDa) and heat stability (chemotactic post-heat-treatment) tests suggest that most of the PMN chemotactic activity in mastitic milk may be due to the chemokine IL-8. Numerous reports have implicated IL-8 in various inflammatory conditions (2, 23, 24, 41). In contrast, the physicochemical characteristics of the chemoattractant(s) present in normal late-lactation-stage milk are different. Its molecular mass is <30 and ≥10 kDa, and the chemotactic activity is heat labile. Based on these data, it is possible that the PMN chemotactic activity that was detected in normal late-lactation-stage milk is a hitherto uncharacterized factor or a factor related or similar to those detected in human milk (27). Further analysis is needed to definitively identify the chemotactic factor(s) in normal late-lactation-stage milk. The pH stability test of the PMN chemotactic factor present in normal late-lactation-stage milk indicates that it is highly acid resistant (pH 2). This result suggests that the chemotactic factor is able to survive the highly acidic environment of the gastrointestinal tract of sucklings. Thus, passive acquisition of this chemotactic factor may be of importance to the development of sucklings.
In summary, we have provided evidence to suggest the presence of normal lactation stage-dependent chemotactic factors in mammary secretions of the goat, thereby implicating chemotactic cytokines as physiologic regulators in the mammary gland. This may be the reason for the increase in the SCC and the predominance of the PMN population that are seen in the late lactation stage, despite the absence of infection. The infiltration of PMNs into the mammary gland during this period seems to be a normal physiologic homeostatic regulatory mechanism and is not a result of a pathological process. Hence, the SCC regulatory standards for goat milk need to be redefined in order to reflect this physiologic phenomenon. Isolation, further purification, and physicochemical characterization of the PMN chemotactic factor in normal late-lactation-stage milk is needed to determine whether it is a novel chemotactic cytokine.
ACKNOWLEDGMENTS
This work was supported by the American Dairy Goat Association Research Foundation.
We are very grateful to Ann Engel, Lynn Miller, Kathy Orovitz, the Roillards, and Lorraine Wheeler for assisting us in milk sample collection.
REFERENCES
- 1.Bare-Martin M T, Vidrich A, Lynch D H, Targan S R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-α and ligation of Fas antigen. J Immunol. 1995;155:4147–4154. [Google Scholar]
- 2.Bittleman D B, Erger R A, Casale T B. Cytokines induce selective granulocyte chemotactic responses. Inflamm Res. 1996;45:89–95. doi: 10.1007/BF02265121. [DOI] [PubMed] [Google Scholar]
- 3.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 4.Callard R, Gearing A. The cytokine factsbook. San Diego, Calif: Academic Press, Inc.; 1994. pp. 2–200. [Google Scholar]
- 5.Cassatella M A. Cytokines produced by polymorphonuclear neutrophils: molecular and biological aspects. New York, N.Y: Chapman and Hall; 1996. pp. 41–43. [Google Scholar]
- 6.Crago S S, Prince S J, Pretlow T G, McGhee J R, Metecky J. Human colostral cells. I. Separation and characterization. Clin Exp Immunol. 1979;38:585–597. [PMC free article] [PubMed] [Google Scholar]
- 7.Driscol K E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 8.Droke E A, Paape M J, Di Carlo A L. Prevalence of high somatic cell counts in bulk tank goat milk. J Dairy Sci. 1993;76:1035–1039. doi: 10.3168/jds.S0022-0302(93)77431-7. [DOI] [PubMed] [Google Scholar]
- 9.Dulin A M, Paape M J, Schultze W D, Winland B T. Effect of parity, stage of lactation, and intramammary infection on concentration of somatic cells and cytoplasmic particles in goat milk. J Dairy Sci. 1983;66:2426–2433. doi: 10.3168/jds.S0022-0302(83)82101-8. [DOI] [PubMed] [Google Scholar]
- 10.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eglinton E A, Roberton D M, Cummins A G. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol Cell Biol. 1994;72:306–313. doi: 10.1038/icb.1994.46. [DOI] [PubMed] [Google Scholar]
- 12.Fava R, Olsen N, Postelwaite A E, Broadley K, Davidson J M, Nanney L B, Lucas C, Townes A. Transforming growth factor-β1 (TGF-β1) induced neutrophil recruitment of synovial tissues: implications for TGF-β-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173:1121–1132. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garofalo R, Cheda S, Mei F, Palkowetz K H, Rudloff H E, Schmalstieg F C, Rassin D K, Goldman A S. Interleukin-10 in human milk. Pediatr Res. 1995;37:444–449. doi: 10.1203/00006450-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Garssen G J, Borgstein A M, Olderbroek J K. Total and differential somatic cells in milk of bST-treated cows. Report B-357. Zeist, The Netherlands: Research Institute for Animal Production; 1990. [Google Scholar]
- 15.Goldman A S, Rudloff H E, Schmalstieg F C. Are cytokines in human milk? Adv Exp Med Biol. 1991;310:93–97. doi: 10.1007/978-1-4615-3838-7_10. [DOI] [PubMed] [Google Scholar]
- 16.Guidry A J. Mastitis and the immune system of the mammary gland. In: Larson B, editor. Lactation. Ames: Iowa State University Press; 1985. p. 29. [Google Scholar]
- 17.Harmon R J. Physiology of mastitis and factors affecting somatic cell counts. J Dairy Sci. 1994;77:2103–2112. doi: 10.3168/jds.S0022-0302(94)77153-8. [DOI] [PubMed] [Google Scholar]
- 18.Head J R. Immunobiology of lactation. Semin Perinatol. 1977;1:195–210. [PubMed] [Google Scholar]
- 19.Hinckley L S. Somatic cell count in relation to caprine mastitis. Vet Med Small Anim Clin Zeist The Netherlands. 1983;1983:1267–1269. [Google Scholar]
- 20.Hinckley L S. Cell count and ADGA research. Am Dairy Goat Assoc News Events Zeist The Netherlands. 1996;2:14. [Google Scholar]
- 21.Jensen D L, Eberhart R J. Total and differential cell counts in secretions of the nonlactating bovine mammary gland. Am J Vet Res. 1981;42:743–747. [PubMed] [Google Scholar]
- 22.Kalogridou-Vassiliadou D, Manolkidis K, Tsigoida A. Somatic cell counts in relation to infection status of the goat udder. J Dairy Res. 1992;59:21–28. doi: 10.1017/s002202990003020x. [DOI] [PubMed] [Google Scholar]
- 23.Kay A B. Inflammatory cells in bronchial asthma. J Asthma. 1989;26:335–344. doi: 10.3109/02770908909073275. [DOI] [PubMed] [Google Scholar]
- 24.Lindley I J D, Westwick J, Kunkel S, editors. Advances in experimental medicine and biology. 351. The chemokines: biology of the inflammatory peptide supergene family II. New York, N.Y: Plenum Press; 1993. p. 210. [Google Scholar]
- 25.Lukas N W, Junkel S L, Strieter R M, Warmington K, Chensue S W. Role of macrophage inflammatory protein (MIP-1α) in granulomatous inflammation. In: Lindley I J D, Westwick J, Kunkel S, editors. Advances in experimental medicine and biology. 351. The chemokines: biology of the inflammatory peptide supergene family II. New York, N.Y: Plenum Press; 1993. p. 215. [Google Scholar]
- 26.Manaster J, Chezar J, Shurtz-Swirski R, Shapiro G, Tendler Y, Kristal B, Shasha S M, Sela S. Heparin induces apoptosis in human peripheral blood neutrophils. Br J Haematol. 1995;94:48–52. doi: 10.1046/j.1365-2141.1996.6202063.x. [DOI] [PubMed] [Google Scholar]
- 27.Noda K, Umeda M, Ono T. Transforming growth factor activity in human colostrum. Gann. 1984;75:109–112. [PubMed] [Google Scholar]
- 28.Pantschenko A G, Yang T J, Woodcock-Mitchell J, Bushmich S L. New England Animal Biotechnology Symposium. Storrs: University of Connecticut; 1997. Establishment and characterization of a caprine mammary epithelial cell line; p. 37. [Google Scholar]
- 29.Park Y W, Humphrey R D. Bacterial cell counts in goat milk and their correlations with somatic cell counts, percent fat, and protein. J Dairy Sci. 1986;69:32–37. doi: 10.3168/jds.S0022-0302(86)80366-6. [DOI] [PubMed] [Google Scholar]
- 30.Petrek M, Du Bois R M, Sirova M, Wigl E. Chemotactic cytokines and their role in physiological and immunopathological reactions. Folia Biol (Prague) 1995;41:263–283. [PubMed] [Google Scholar]
- 31.Philpot W N, Nickerson S C. Mastitis: counterattack. Naperville, Ill: Babson Bros. Co.; 1991. pp. 1–133. [Google Scholar]
- 32.Politis I, White J H, O’Hare K, Zavizion B, Gilmore J, Caler W. Distribution of plasminogen activator forms in fractions of goat milk. J Dairy Sci. 1994;77:2900–2906. doi: 10.3168/jds.S0022-0302(94)77230-1. [DOI] [PubMed] [Google Scholar]
- 33.Rota A M, Gonzalo C, Rodriguez P L, Rojas A I, Martin L, Tovar J J. Effects of stage of lactation and parity on somatic cell counts in milk of Verata goats and algebraic models of their lactation curves. Small Ruminant Res. 1993;12:211–219. [Google Scholar]
- 34.Sandholm M, Honkanen-Buzalski T, Kaartinen L, Pyorla S. The bovine udder and mastitis. Jväskylä, Finland: Gummerus Kirjapaino, Oy; 1995. [Google Scholar]
- 35.Schall T J. The chemokines. In: Thomson A, editor. The cytokine handbook. 2nd ed. San Diego, Calif: Academic Press Inc.; 1994. pp. 419–460. [Google Scholar]
- 36.Schukken Y, Weersink A, Leslie K, Martin S W. Dynamics and regulation of bulk milk somatic cell counts. Can J Vet Res. 1993;57:131–135. [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldrake R F, Hoare R J, Woodhouse V. Relationship of somatic cell counts and cell volume analysis of goat’s milk to intramammary infection with coagulase-negative staphylococci. J Dairy Res. 1981;48:393–403. doi: 10.1017/s0022029900021877. [DOI] [PubMed] [Google Scholar]
- 38.Skansen-Saphir U A, Lindfors A, Andersson U. Cytokine production in mononuclear cells of human milk studied at the single-cell level. Pediatr Res. 1993;34:213–216. doi: 10.1203/00006450-199308000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Smith C W, Goldman A S. The cells of human colostrum. I. In vitro studies of morphology and functions. Pediatr Res. 1968;2:103–109. doi: 10.1203/00006450-196803000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Soder O. Isolation of interleukin-1 from human milk. Int Arch Allergy Appl Immunol. 1987;83:19–23. doi: 10.1159/000234325. [DOI] [PubMed] [Google Scholar]
- 41.Warner J O. Immunology of cystic fibrosis. Br Med Bull. 1992;48:893–911. doi: 10.1093/oxfordjournals.bmb.a072584. [DOI] [PubMed] [Google Scholar]
- 42.Wirt D P, Adkins L T, Palkowetz K H, Schmalstieg F C, Goldman A S. Activated and memory T lymphocytes in human milk. Cytometry. 1992;13:282–290. doi: 10.1002/cyto.990130310. [DOI] [PubMed] [Google Scholar]
- 43.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature. 1996;384:179. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 44.Zeng S S, Escobar E N. Somatic cells and milk of small ruminants: proceedings of international symposium. Bella, Italy. Brussels, Belgium: International Dairy Federation; 1994. Factors affecting somatic cell count of goat milk throughout lactation: parity and milk production; pp. 16–19. [Google Scholar]
- 45.Zeng S S, Escobar E N. National Mastitis Council 1995 annual meeting proceedings. Arlington, Va: National Mastitis Council, Inc.; 1995. Factors affecting somatic cell count of goat milk: breed and farm; pp. 168–169. [Google Scholar]
- 46.Ziff M. Role of endothelium in chronic inflammation. Springer Semin Immunopathol. 1989;11:199–214. doi: 10.1007/BF00197189. [DOI] [PubMed] [Google Scholar]