Abstract
Simple Summary
Pancreatic carcinomas are among the most aggressive cancers and have a poor prognosis. The influence of postoperative analgesia on the prognosis of these patients has recently drawn considerable attention. We therefore conducted a retrospective study to evaluate the effect of postoperative analgesia on cancer-specific survival among patients after radical surgery for pancreatic cancer. We also investigated the effect of opioid and cannabinoid receptor gene expressions on overall survival. Our results showed that cancer-specific survival was increased by postoperative analgesia with morphine, cannabinoid receptor 2, and opioid growth factor receptor cancer tissue gene expressions but was reduced by delta opioid receptor gene expressions. The determination of opioid and cannabinoid receptor gene expression levels in pancreatic cancer cells and possibly also other cancer cells could thus provide important guidance on the selection of postoperative analgesia regimes and the prognosis of overall survival.
Abstract
Pancreatic cancer (PDAC) has a poor prognosis despite surgical removal and adjuvant therapy. Additionally, the effects of postoperative analgesia with morphine and piritramide on survival among PDAC patients are unknown, as are their interactions with opioid/cannabinoid receptor gene expressions in PDAC tissue. Cancer-specific survival data for 71 PDAC patients who underwent radical surgery followed by postoperative analgesia with morphine (n = 48) or piritramide (n = 23) were therefore analyzed in conjunction with opioid/cannabinoid receptor gene expressions in the patients’ tumors. Receptor gene expressions were determined using the quantitative real-time polymerase chain reaction. Patients receiving morphine had significantly longer cancer-specific survival (CSS) than those receiving piritramide postoperative analgesia (median 22.4 vs. 15 months; p = 0.038). This finding was supported by multivariate modelling (p < 0.001). The morphine and piritramide groups had similar morphine equipotent doses, receptor expression, and baseline characteristics. The opioid/cannabinoid receptor gene expression was analyzed in a group of 130 pancreatic cancer patients. Of the studied receptors, high cannabinoid receptor 2 (CB2) and opioid growth factor receptor (OGFR) gene expressions have a positive influence on the length of overall survival (OS; p = 0.029, resp. p = 0.01). Conversely, high delta opioid receptor gene expression shortened OS (p = 0.043). Multivariate modelling indicated that high CB2 and OGFR expression improved OS (HR = 0.538, p = 0.011, resp. HR = 0.435, p = 0.001), while high OPRD receptor expression shortened OS (HR = 2.264, p = 0.002). Morphine analgesia, CB2, and OGFR cancer tissue gene expression thus improved CSS resp. OS after radical PDAC surgery, whereas delta opioid receptor expression shortened OS.
Keywords: pancreatic cancer, pancreatic surgery, morphine, piritramide, postoperative analgesia, cancer recurrence, opioid receptors, cannabinoid receptors, patient survival
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) has a low 5-year survival of 5–7% [1]. Moreover, its median OS after surgery is just 18 months, and its incidence is rising [2,3]. Survival remains poor despite the use of multidisciplinary treatment strategies that combine surgical removal, adjuvant and neo-adjuvant chemotherapy, and radiotherapy [4,5,6].
For over a decade, many mostly retrospective studies have yielded conflicting results concerning the influence of postoperative analgesia on recurrence and survival for various cancers [7,8]. Mechanisms supporting the use of regional analgesia rather than potent opioids have been proposed [9], but several clinical studies have found no survival benefits for such approaches.
At the cellular level, our group and others have shown that the persistence of circulating tumor cells (CTCs) after radical cancer surgery is a negative prognostic factor [10,11,12]. We also showed that piritramide opioid analgesia reduces the presence of CTCs in colon cancer patients after surgery, potentially affecting survival [13,14], and that the expression of the cannabinoid-2 receptor (CB2) in cancer tissues improves survival in small-cell lung cancer [15]. Similar effects were also observed in hepatocellular cancer [16].
We therefore believe that the choice of postoperative analgesia may significantly affect survival in cancer patients. However, we also believe that these effects should be evaluated in relation to opioid and cannabinoid receptor expression in specific cancer tissues because the effects of individual opioids in cancer patients are likely to depend on the tumor’s unique receptor profile and biology.
Few studies on the influence of cannabinoid and opioid receptors on survival in PDAC have been reported. Cannabinoids mitigate cancer progression mainly by promoting apoptosis and autophagia via accumulation of the sphingolipid ceramide, which targets the stress-regulated protein p8 [17]. They also inhibit angiogenesis and invasiveness and have immunomodulatory properties [18,19,20,21]. Michalski et al. found that cannabinoid-1 receptor (CB1) and CB2 were expressed more strongly in PDAC cells than in normal pancreatic cells, and a survival analysis indicated that a low expression of CB1 in cancer cells was associated with better outcomes, while low levels of cannabinoid-metabolizing enzymes in cancer cells shortened survival [22].
With regards to opioid receptors, Zhang et al. found that high μ opioid receptor (OPRM) expression combined with a high perioperative dose of sufentanil was associated with significantly shorter OS and disease-free survival (DFS) in PDAC stages I-III, but high OPRM expression alone did not affect OS/DFS [23]. Zagon et al. showed that a native opioid peptide, opioid growth factor, suppressed the replication of PDAC cells in vitro in a dose-dependent manner via its receptor, OGFR [24]. Additionally, Haque et al. found that OPRM overexpression in murine and human PDAC cell lines increased proliferation and cancer stemness in vitro, whereas knocking down OPRM had the opposite effects. Moreover, direct morphine stimulation of OPRM and macrophages caused dose-dependent increases in proliferation, invasion, and levels of stemness markers in cancer cells, and morphine induced chemoresistance to chemotherapeutics used in PDAC treatment [25]. However, clinical studies on the role of perioperative morphine analgesia in PDAC progression following radical surgery are lacking.
The extent of radical PDAC surgery can make it challenging to provide adequate postoperative analgesia. In cases where perioperative epidural analgesia is not feasible, high doses of potent opioids are typically administered. This may be a problem if opioid receptor activity influences cancer development because there is evidence that patients are particularly vulnerable to cancer development during the perioperative period: Shakhar et al. showed that major surgery can suppress the anticancer immune response [26]. For decades, morphine has been the gold-standard agent for opioid analgesia worldwide. Piritramide, a potent opioid with a unique chemical structure, has only been used in a few European countries (i.e., Germany, Czech Republic, Netherlands, Austria) [27,28,29,30] and is thus is a less well-known option for postoperative analgesia. It is a 4-amino piperidine derivative (2,2,-diphenyl-4-[1-(4-carbamoyl-4-piperidino)-piperidine]-butyro-nitrile) [31] and has a relative potency of 0.75 compared to morphine [32]. It is typically administered parenterally to treat moderate to severe acute pain [33]. Given the effects of cannabinoid/opioid systems on cancer progression and the influence of morphine and piritramide on CTC levels following major surgery, we hypothesized that survival after radical PDAC surgery would differ between morphine and piritramide analgesia groups and may also depend on opioid and/or cannabinoid receptor expression in cancer tissues.
2. Materials and Methods
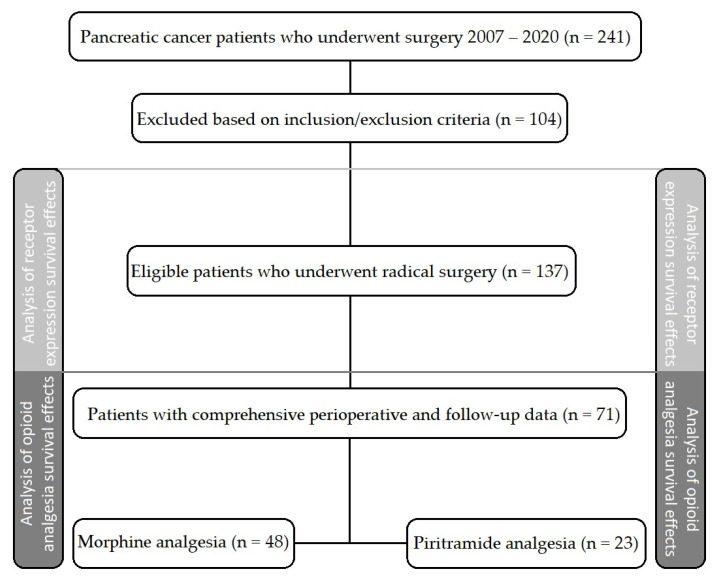
Data and samples representing 241 patients who underwent pancreatic cancer surgery at the University Hospital Olomouc and University Hospital Brno were biobanked between 2007 and 2020 and evaluated (Figure 1). Based on the inclusion and exclusion criteria (Table 1), the opioid receptor expression was analyzed in 137 tumor tissue samples. The comprehensive perioperative and follow-up data were mined in seventy-one patients, including the cause of death. Total doses of morphine and piritramide administered for postoperative analgesia were obtained, and piritramide doses were converted to morphine equivalents according to the equation 1 mg piritramide = 0.75 mg morphine [34]. Patients received either morphine or piritramide. No patients received both morphine and piritramide (Table 2). The quantitative real-time polymerase chain reaction was used to analyze the expression of the following opioid and cannabinoid receptors in tumor tissue samples: OGFR, OPRM, OPRD, kappa opioid receptor (OPRK), lambda opioid receptor (OPRL), CB1, and CB2.
Figure 1.
Study flow diagram.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age > 18 years | Tumor duplicity |
| Pancreatic adenocarcinoma (except ampullary carcinoma) | Death or reoperation within 30 days of surgery |
| Stage I, II, III (TNM classification) | |
| R0/R1 resection radicality | |
| Morphine or piritramide postoperative analgesia |
Table 2.
Summary of the patients’ basic characteristics. Abbreviations: OS = overall survival, CSS = cancer-specific survival, NA = not available, CI = confidence interval.
| Patient Characteristics | All Patients (n = 137) n (%) |
Morphine (n = 48) n (%) |
Piritramide (n = 23) n (%) |
|---|---|---|---|
| Sex | |||
| Male | 66 (48.5) | 24 (50) | 16 (69.6) |
| Female | 70 (51.5) | 24 (50) | 7 (30.4) |
| Tumor stage | |||
| I | 14 (10.2) | 7 (14.6) | 7 (30.4) |
| II | 109 (79.6) | 40 (83.3) | 16 (69.6) |
| III | 14 (10.2) | 1 (2.1) | 0 (0) |
| Tumor grade | |||
| 1 | 6 (4.4) | 2 (4.2) | 4 (17.4) |
| 2 | 78 (56.9) | 26 (54.2) | 11 (47.8) |
| 3 | 53 (38.7) | 20 (41.7) | 8 (34.8) |
| Age (years) | |||
| Median (q1–q3) | 63 (58.5–69) | 63 (58.5–69) | 65 (58.5–69) |
| Resection | |||
| R0 | 80 (58.4) | 48 (100) | 20 (87) |
| R1 | 57 (41.6) | 0 (0) | 3 (13) |
| Dosage (milligrams) | |||
| Median (q1–q3) | NA | 90 (70–120) | 101.2 (61.88–135) |
| OS (months) Median (95% CI) |
20.2 (16.4; 23.4) | 22.4 (16.6; NA) | 15.0 (13.4; 20.9) |
| CSS (months) Median (95% CI) |
NA | 22.4 (16.6; NA) | 15.0 (13.4; 20.9) |
2.1. Analysis of Opioid and/or Cannabinoid Receptor Expression in Tumor Tissues
Total RNA extraction from 20 to 40 mg tumor tissue samples fixed in RNAlater (ThermoScientific, Wilmington, DE, USA) was performed using the TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) and chloroform (Sigma-Aldrich s.r.o, St. Louis, MO, USA). The resulting RNA was then resuspended in diethylpyrocarbonate (DEPC)-treated water (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The purity and concentration of the RNA were assessed using a Nanodrop ND 1000 instrument (ThermoScientific, Wilmington, DE, USA).
Reverse transcription was performed using 3 µg of total RNA with random primers (Promega, Madison, WI, USA), RNAsin ribonuclease inhibitor (Promega, Madison, WI, USA), and RevertAid H Minus M-MuLV Reverse Transcriptase (Fermentas, Vilnius, Lithuania) in a 30 µL reaction volume according to the manufacturer’s instructions. The cDNA products were then stored at −20 °C until qPCR analysis.
Quantitative RT-PCR reactions were performed in LightCycler 384 Multiwell plates (Roche, Basel, Switzerland). In each reaction, 50 ng of cDNA was mixed with LightCycler 480 DNA Probes Master (Roche, Basel, Switzerland) and the appropriate TaqMan Gene Expression Assay (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA; CB1: Hs01038532_m1; CB2: Hs00275635_m1; OPRK: Hs00175127_m1; OPRD: Hs00538331_m1; OPRM: Hs01053957_m1; OPRL: 00173471_m1; OGFR: Hs01071266_m1; ACTB: Hs99999903_m1) [15]. The volumes of the reagent mixture and the sample were 9 µL and 1 µL, respectively, and each sample was applied to the plate in four replicates. Plates were amplified by performing 50 cycles with a LightCycler 480 instrument using the temperatures and amplification times specified in the protocol supplied with the TaqMan Gene Expression Assays. ACTB (encoding actin β) was amplified as a reference gene. Fluorescence signals and cycle threshold values (CT) were evaluated using LightCycler 480 Software, ver. 1.1. ΔCT values were calculated by normalization against ACTB.
2.2. Statistical Analysis
Statistical analysis was performed using R, ver. 3.5.2 (Core Team, 2018). The significance threshold was p < 0.05. Specific cut-off values for opioid and cannabinoid receptor expression were determined using the maxstat() function (maxstat R package, v. 0.7–25), which estimates cut-points based on the maximally selected log-rank statistic (using overall and cancer-specific survival as an outcome variable). The expression of individual receptors was then classified as low (>cut-off) or high (≤cut-off) based on the cut-off values. Pearson’s chi-squared test, Fisher’s exact test, the Wilcoxon exact test, and the t-test were used to compare patient groups receiving different analgesic treatments and having different levels of receptor expression. Univariate survival analysis was performed using the log-rank test and Cox proportional hazard models. In multivariate Cox regression models, age and sex were used as adjusting variables and disease stage was used as a stratification variable.
3. Results
3.1. Opioid and Cannabinoid Receptor Gene Expressions’ Effects on Overall Survival
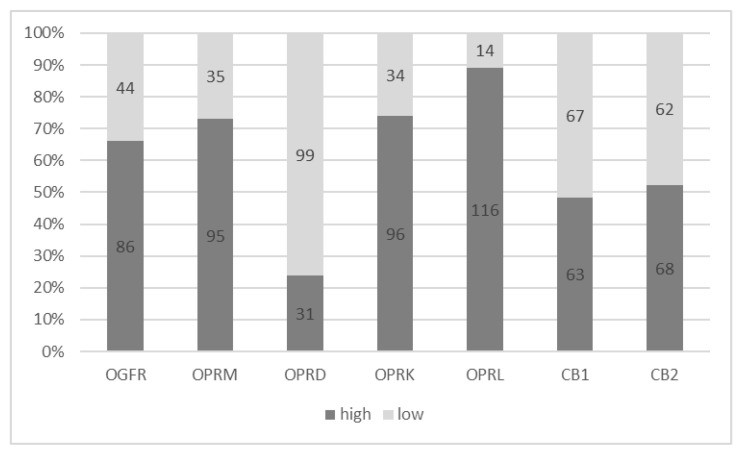
In total, 7 of the 137 patients initially included in the study had inconclusive receptor gene expression data, leaving 130 eligible for inclusion in the receptor gene expression analysis (Figure 1). Receptor gene expressions were categorized using estimated cut-off values (see Methods). All receptors were highly expressed in pancreatic tumor tissues except the OPRD (Figure 2).
Figure 2.
Opioid and cannabinoid receptor gene expressions. Abbreviations: OGFR = opioid growth factor receptor, OPRM = mu opioid receptor, OPRD = delta opioid receptor, OPRK = kappa opioid receptor, OPRL = lambda opioid receptor, CB1 = cannabinoid receptor 1, CB2 = cannabinoid receptor 2.
The age, sex, and grading have no significant influence on opioid and cannabinoid receptor gene expressions in tumor tissues. The CB1 and CB2 receptor gene expressions in tumor tissues were significantly associated with disease stage (p = 0.013, resp. p = 0.002). The higher the stage, the lower the cannabinoid receptor gene expressions.
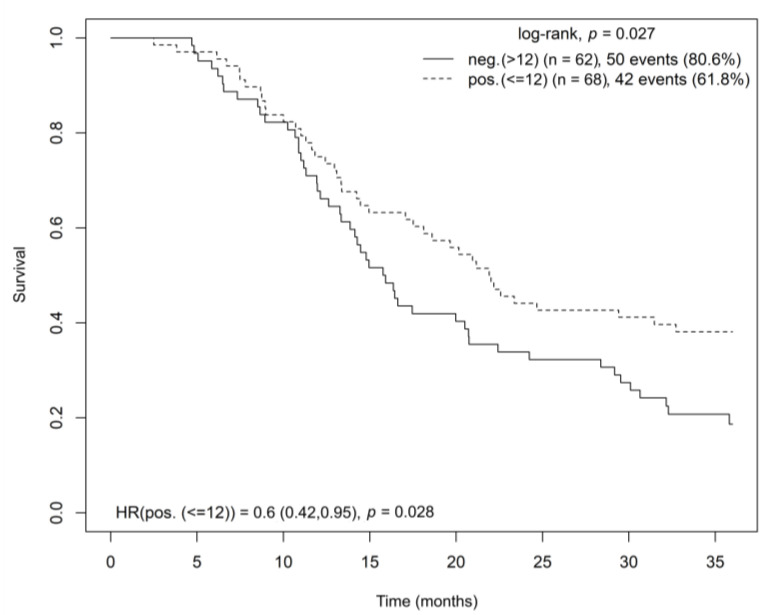
In patients with high CB2 receptor gene expression in tumor tissue, a significantly longer OS was found (log-rank test, p = 0.027; HR = 0.6, p = 0.028) (Figure 3). The multivariate Cox model analysis stratified by disease stage and adjusted for age and sex confirmed the findings (HR = 0.650; CI = (0.420–1.006); p = 0.053).
Figure 3.
Kaplan–Meier curve showing overall survival for radically resected patients with pancreatic adenocarcinoma based on CB2 gene expression in tumor tissue. Abbreviations: HR = hazard ratio, CB2 = cannabinoid receptor 2.
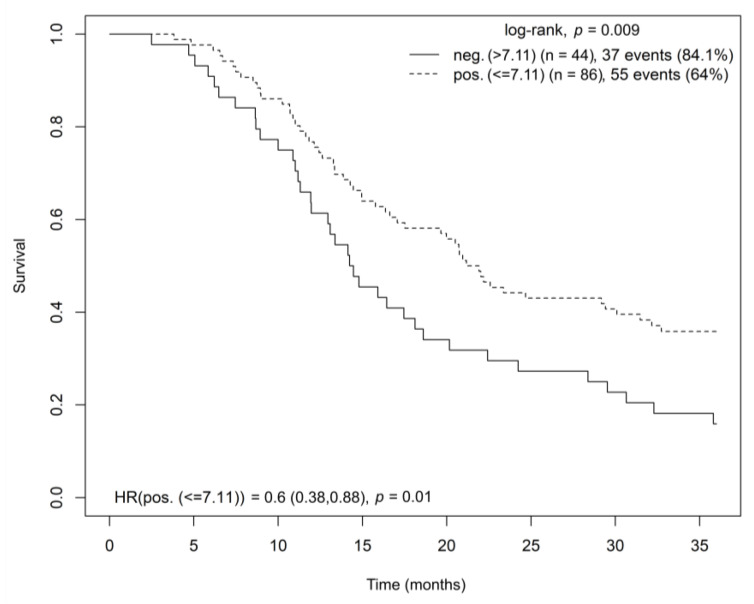
Patients with high OGFR receptor gene expression in tumor tissue had significantly longer OS (log-rank test, p = 0.009; HR = 0.631, p = 0.01) (Figure 4). This finding was confirmed by a multivariate Cox model analysis stratified by disease stage and adjusted for age and sex (HR = 0.588; CI = (0.372–0.927); p = 0.022).
Figure 4.
Kaplan–Meier curve showing overall survival for radically resected patients with pancreatic adenocarcinoma based on OGFR gene expression in tumor tissue. Abbreviations: HR = hazard ratio, OGFR = opioid growth factor receptor.
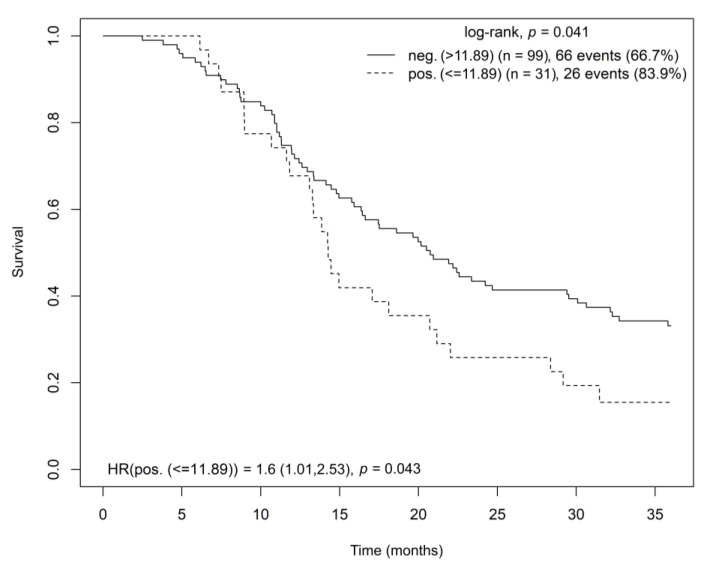
On the contrary, the high OPRD receptor gene expression negatively affected overall survival (log-rank test, p = 0.041; HR = 1.6, p = 0.043) (Figure 5). The multivariate Cox model analysis stratified by disease stage and adjusted for age and sex confirmed the findings (HR = 1.655; CI = (1.012–2.707); p = 0.045).
Figure 5.
Kaplan–Meier curve showing overall survival for radically resected patients with pancreatic adenocarcinoma based on OPRD gene expression in tumor tissue. Abbreviations: HR = hazard ratio, OPRD = opioid receptor delta.
An additional multivariate Cox model analysis with stepwise selection was performed that included the expression levels of all opioid and cannabinoid receptors stratified by disease stage and adjusted for age and sex. As shown in Table 3, this revealed that patients with high CB2 and OGFR receptor expressions had a significantly longer OS (HR = 0.538, p = 0.011, resp. HR = 0.435, p = 0.001), while those with high OPRD receptor expression had a significantly shorter OS (HR = 2.264, p = 0.002).
Table 3.
Multivariate Cox model analyzing the effects of all studied opioid and cannabinoid receptors on overall survival among patients with pancreatic adenocarcinoma. Abbreviations: HR = hazard ratio, CI = confidence interval, OGFR = opioid growth factor receptor, OPRM = mu opioid receptor, OPRD = delta opioid receptor, OPRK = kappa opioid receptor, OPRL = lambda opioid receptor, CB2 = cannabinoid receptor 2.
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.011 | 0.987–1.036 | 0.363 |
| Sex | 1.057 | 0.677–1.65 | 0.806 |
| OGFR | 0.435 | 0.264–0.717 | 0.001 |
| OPRM | 2.076 | 1.199–3.594 | 0.009 |
| OPRD | 2.264 | 1.334–3.843 | 0.002 |
| OPRK | 0.480 | 0.286–0.805 | 0.005 |
| OPRL | 3.017 | 1.344–6.775 | 0.007 |
| CB2 | 0.538 | 0.333–0.869 | 0.011 |
3.2. Postoperative Analgesia Effects on Cancer-Specific Survival
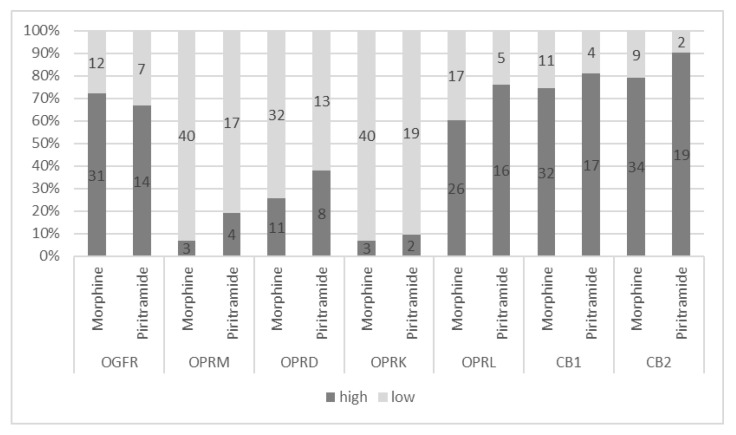
Of the 71 analyzed patients (31 female and 40 male, median age 63 years), 48 (67.6%) received morphine analgesia and 23 (32.4%) received piritramide analgesia in the postoperative period. The median morphine dose was 90 (70–120) mg and that for piritramide was 135 (82.5–180) mg, corresponding to a morphine equivalent dose of 101.2 (61.9–135) mg. The two groups thus had similar morphine equivalent dosage regimes and baseline characteristics (Table 2). The opioid and cannabinoid receptor expressions in tumor tissues were similar in both groups (Figure 6).
Figure 6.
Opioid and cannabinoid receptor expressions in different opioid treatment groups. Abbreviations: OGFR = opioid growth factor receptor, OPRM = mu opioid receptor, OPRD = delta opioid receptor, OPRK = kappa opioid receptor, OPRL = lambda opioid receptor, CB1 = cannabinoid receptor 1, CB2 = cannabinoid receptor 2.
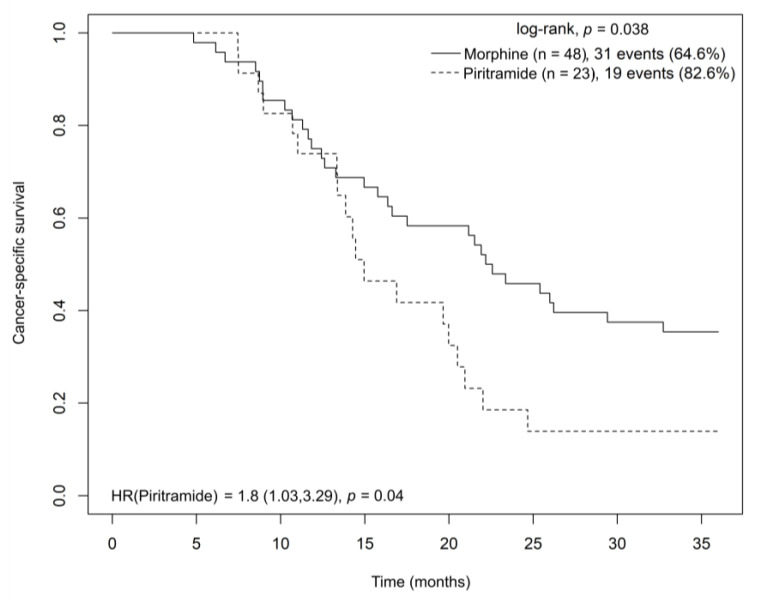
Patients receiving morphine analgesia had a significantly longer cancer-specific survival (CSS) than those receiving piritramide analgesia (22.4 vs. 15 months) according to a log-rank test (HR = 1.8, p = 0.04) (Figure 7). In a multivariate Cox model analysis stratified by disease stage and adjusted for age and sex, piritramide had a negative effect on CSS (HR = 2.904; CI = 1.485–5.679; p = 0.002) when compared to morphine.
Figure 7.
Kaplan–Meier curve showing cancer-specific survival for radically resected pancreatic adenocarcinoma patients treated with morphine or piritramide during the perioperative period. Abbreviations: HR = hazard ratio.
An additional multivariate Cox model analysis with stepwise selection was performed that included the expression levels of all opioid and cannabinoid receptors as well as the applied analgesic treatment, stratified by disease stage and adjusted for age and sex. As shown in Table 4, this revealed that patients with high CB2 receptor expression had significantly longer CSS (HR = 0.186, p < 0.001), while those with high OPRD receptor expression had significantly shorter CSS (HR = 4.886, p < 0.001).
Table 4.
Multivariate Cox model analyzing the effects of all studied opioid and cannabinoid receptors on cancer-specific survival among patients with pancreatic adenocarcinoma. Abbreviations: HR = hazard ratio, CI = confidence interval, OPRM = mu opioid receptor, OPRD = delta opioid receptor, CB2 = cannabinoid receptor 2.
| HR | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.023 | 0.978–1.07 | 0.318 |
| Sex | 1.234 | 0.632–2.409 | 0.538 |
| Piritramide | 3.060 | 1.478–6.337 | 0.003 |
| OPRM | 0.203 | 0.055–0.750 | 0.017 |
| OPRD | 4.886 | 2.228–10.717 | <0.001 |
| CB2 | 0.185 | 0.079–0.435 | <0.001 |
4. Discussion
We found that morphine analgesia improves cancer-specific survival (CSS) after radical PDAC surgery when compared to piritramide analgesia. Additionally, overall survival (OS) is increased by high CB2 and OGFR tumor tissue gene expression and reduced by high delta OPRD tumor tissue gene expression. Finally, all receptors were highly expressed in pancreatic tumor tissues except the OPRD. To our knowledge, this is the first study describing the survival benefits of morphine analgesia compared to piritramide analgesia after radical PDAC surgery and also the first study describing the effects of CB2 and OGFR gene expression on survival in PDAC patients.
Morphine analgesia has often been regarded as a negative factor that is associated with cancer recurrence. Several mechanisms describing its effects on cancer cells and anticancer immunity have been proposed to justify this position. Both in vivo and in vitro studies have shown that morphine enhances cancer cell proliferation, tumor progression, and cancer recurrence [35]. However, the evidence concerning its effects on cancer cell invasion [36,37] and angiogenesis promotion [38,39,40] is inconclusive and there are little data on its effects in PDAC. Zagon et al. found that OGF and OGFR suppressed the growth of PDAC in culture and in nude mice. Modulation of the OGF-OGFR pathway may potentially have a therapeutic effect in patients with pancreatic cancer [24,41,42,43]. Our results are consistent with those of Zagon et al. in that OGFR cancer tissue gene expressions improve overall survival in patients with pancreatic cancer. However, in our study, morphine analgesia proved beneficial for OS. There are several possible explanations for this discrepancy. First and foremost, our findings are based on data from a clinical setting involving major surgery. As such, they reflect the influence of several factors that are absent from controlled in vitro environments. In this context, it is notable that two recent prospective clinical trials examining lung and colon cancer surgery detected no survival benefits for regional analgesia when compared to morphine [8,44]. The effects of morphine analgesia in clinical cancer surgery thus remain unclear. Another important point is that our study compared morphine to piritramide and was not designed to determine whether morphine provides any survival benefit per se. However, since the median survival following PDAC surgery is only 18 months [2,3], but the OS of the morphine group in our study was 22.4 months, negative effects of morphine analgesia seem unlikely.
The effects of piritramide analgesia on cancer have received less attention than those of morphine. However, our group recently found that piritramide analgesia reduced CTC levels following colon cancer surgery [13,45] and could thus positively influence cancer recurrence/survival after major surgery. The fact that it had unfavorable effects on PDAC in the current study strongly suggests that individual cancer types differ in their molecular biology and responses to opioid and/or cannabinoid stimulation. Because our results indicated that high CB2 and OGFR tumor tissue gene expression improved OS, we hypothesize that piritramide’s negative effects may be linked to interactions with specific receptors in cancer and immune system cells. Unfortunately, studies on these interactions and comparisons between piritramide and morphine are lacking.
Our data on cannabinoid receptors revealed high CB2 gene expression in PDAC tissue, in accordance with the results of Michalsky et al. [22]. However, contrary to their findings, we observed that CB1 gene expression remained low, and OS was increased by high CB2 expression but unaffected by CB1 expression. These differences may be explained by the following observation made by Michalsky et al.: “cancer cells within single tissue samples showed various extents of CB1 and CB2 staining, ranging from no immunoreactivity to strong immunoreactivity”. Such broad variation within single tissue samples suggests that the results obtained depended heavily on the part of the sample that was chosen for analysis, rendering comparisons difficult. Therefore, studies using more reliable and reproducible tissue analyses are needed. Given that only very small portions of surgically removed tumors are usually analyzed, it is not clear that traditional methods can provide conclusive data on receptor expression. New types of analyses that can provide information on the majority of a tumor’s bulk may thus be needed. In addition, the number of samples tested in both studies was relatively low, which may partly explain the observed variation.
Because CB2 expression has been linked to longer survival in both lung [15] and hepatocellular cancer [16], similar mechanisms may be active in PDAC. In particular, CB2 is a likely target for endo- and exocannabinoids with anticancer activity resulting from effects on motility and migration, reductions in invasiveness and angiogenesis, and the induction of apoptosis [17,46]. Additionally, Michalsky et al. [22] observed that PDAC cells exhibit elevated cannabinoid-hydrolyzing enzyme activity, which could be interpreted as evidence of an adaptive mechanism to evade the anticancer effects of endocannabinoids. There is also emerging evidence of a complex interplay between cannabinoid and opioid receptors. Both receptor types are G-protein-coupled and they can form receptor heteromers containing both cannabinoid and opioid receptor proteins (exemplified by the CB1-OPRL heteromer). This considerably modifies their individual properties, and stimulation of one part of the heteromer may change the responsiveness of the other. This could have major clinical implications; combined therapies using low doses of drugs targeting cannabinoid and opioid receptors (or novel bivalent drugs) have shown promising results in humans [47]. Our observations of shorter OS in patients with high OPRD gene expression and longer OS in those with high CB2 and OGFR gene expression could thus plausibly be interconnected.
We recognize that our study has limitations. First, the number of patients included in this retrospective study was relatively small. Second, although the morphine and piritramide groups had similar baseline characteristics, data on the quality of postoperative analgesia based on validated scores were unavailable. Since there is some evidence that suboptimal pain management may promote cancer recurrence [48], a substantial difference in pain intensity between the groups, if present, could have influenced our findings. In addition, the total opioid doses in both groups were relatively small (Table 2) given the extent of open pancreatic surgery, which could raise questions about the adequacy of the analgesia. It should be noted that various non-opioid co-analgesics are generally used when treating PDAC patients. In this regard, it is important that several clinical trials have confirmed that NSAIDs have an opioid-sparing effect in patients who received opioids in the postoperative period [49,50]. The use of NSAIDs could influence results by reducing the total dose of postoperative opioids and thus reducing the adverse effects of opioids. In our study, the total equivalent doses of opioids were similar in both groups, so significant differences in the quality of analgesia or significant differences in the postoperative administration of NSAIDs are unlikely. However, since information on the administration of co-analgesics was not available for most of the patients, we cannot exclude differences in corticosteroid administration, which may be associated with improved survival in PDAC [3]. Third, it was not possible to reliably retrieve data on blood product administration, a factor that is likely to influence the recurrence of cancers including PDAC [3]. Nevertheless, based on the reviewed records, it can reasonably be assumed that a majority of patients in both groups received blood products during their stay in hospital.
We used cancer-specific survival for 36 months in our survival analysis because PDAC is characterized by rapid progression, which makes it difficult to accurately determine the timing of recurrence—the achieved accuracy depends on the frequency/quality of follow-up examinations, and recurrence may not be detected for some time after it emerges. In a systematic review, Petrelli et al. observed that OS and DFS were only weakly correlated in PDAC and concluded that OS should be the preferred survival endpoint because most patients die directly from the cancer and related complications [51].
To conclude, the 65% improvement in CSS observed for the morphine analgesia group (from 15 to 22.4 months) when compared to piritramide is important and should influence postoperative PDAC management if confirmed in other studies. Moreover, our findings regarding the influence of CB2, OGFR, and OPRD gene expression on OS may improve prognostication in PDAC and enable better personalization of care in the future. However, given the limitations of our study, we suggest that these results should be seen primarily as hypothesis-generating. Prospective studies are needed to elucidate the relationship between different types of postoperative analgesia and cancer recurrence/survival in PDAC. Molecular biological studies should also be conducted to clarify the role of opioid and/or cannabinoid receptors and their agonists/antagonists in cancer promotion. Ideally, the effects of individual opioids should be prospectively studied and related to the influence of the expression and/or activity of opioid and/or cannabinoid receptors in cancer and immune system cells. Finally, the role of opioid and cannabinoid receptor heteromers warrants investigation.
5. Conclusions
Morphine analgesia improves CSS compared to piritramide analgesia after radical pancreatic cancer surgery. Cannabinoid receptor 2 and opioid growth factor receptor are highly expressed in pancreatic cancer tissue and their high expression improves OS, whereas high delta opioid receptor expression reduces OS. More studies are needed to elucidate the effects of opioid treatment and the expression of opioid and cannabinoid receptors on the treatment of pancreatic cancer and to determine their prognostic value.
Author Contributions
Conceptualization, L.V., P.P., J.S. (Josef Srovnal), E.B. and M.H.; methodology, M.V., P.P., J.S. (Josef Srovnal) and P.K.; validation, M.V., P.P., J.S. (Jozef Skarda), Z.K., P.M., J.S. (Josef Srovnal) and P.K.; formal analysis, L.V., E.B. and J.S. (Josef Srovnal); investigation, L.V., P.P., M.L. and P.S.; resources, P.P., L.V., M.L. and P.S.; data curation, L.V., P.P., M.L., P.S., P.K. and J.S. (Josef Srovnal); writing—original draft preparation, L.V. and E.B.; writing—review and editing, L.V., P.P., J.S. (Josef Srovnal), E.B., M.V., T.G., P.K., M.L., P.S., J.S. (Jozef Skarda), Z.K., P.M. and M.H.; visualization, L.V., J.S. (Josef Srovnal) and P.K.; supervision, T.G. and M.H.; funding acquisition, J.S. (Josef Srovnal), E.B., M.V., L.V., T.G. and M.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Boards of University Hospital Olomouc, Czech Republic (197/08), and University Hospital Brno, Czech Republic (10 June 2015-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available in FigShare at https://figshare.com/account/articles/21841071 (accessed on 6 August 2023) (DOI 10.6084/m9.figshare.21841071).
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. Josef Srovnal, Pavla Kourilova, Monika Vidlarova, and Marian Hajduch report that they obtained institutional funding from the Ministry of Health of the Czech Republic (NV18-03-00470), Ministry of Youth, School and Education of the Czech Republic (BBMRI—LM2018125, NCMG—LM2023067, EATRIS-CZ—LM2018133), Palacky University Olomouc (LF 2023_006), National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU. Josef Srovnal and Marian Hajduch are co-founders of spin-off company Intellmed, Ltd., and Cancer Research Czech Republic Foundation. The other authors have no conflicts of interest to declare.
Funding Statement
This study was supported by the Ministry of Health of the Czech Republic (NV18-03-00470), Ministry of Youth, School and Education of the Czech Republic (BBMRI—LM2018125, NCMG—LM2023067, EATRIS-CZ—LM2018133), Palacky University Olomouc (LF 2023_006), National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU and Cancer Research Czech Republic Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latenstein A.E.J., van Roessel S., van der Geest L.G.M., Bonsing B.A., Dejong C.H.C., Groot Koerkamp B., de Hingh I.H.J.T., Homs M.Y.V., Klaase J.M., Lemmens V., et al. Conditional Survival After Resection for Pancreatic Cancer: A Population-Based Study and Prediction Model. Ann. Surg. Oncol. 2020;27:2516–2524. doi: 10.1245/s10434-020-08235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Call T.R., Pace N.L., Thorup D.B., Maxfield D., Chortkoff B., Christensen J., Mulvihill S.J. Factors Associated with Improved Survival after Resection of Pancreatic Adenocarcinoma: A Multivariable Model. Anesthesiology. 2015;122:317–324. doi: 10.1097/ALN.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 4.Kobi M., Veillette G., Narurkar R., Sadowsky D., Paroder V., Shilagani C., Gilet A., Flusberg M. Imaging and Management of Pancreatic Cancer. Semin. Ultrasound CT MR. 2020;41:139–151. doi: 10.1053/j.sult.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Loveday B.P., Lipton L., Thomson B.N. Pancreatic Cancer: An Update on Diagnosis and Management. Aust. J. Gen. Gen. Pract. 2019;48:826–831. doi: 10.31128/AJGP-06-19-4957. [DOI] [PubMed] [Google Scholar]
- 6.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings K.C., Xu F., Cummings L.C., Cooper G.S. A Comparison of Epidural Analgesia and Traditional Pain Management Effects on Survival and Cancer Recurrence after Colectomy: A Population-Based Study. Anesthesiology. 2012;116:797–806. doi: 10.1097/ALN.0b013e31824674f6. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z.-Z., Li H.-J., Li M.-H., Huang S.-M., Li X., Liu Q.-H., Li J., Li X.-Y., Wang D.-X., Sessler D.I. Epidural Anesthesia-Analgesia and Recurrence-Free Survival after Lung Cancer Surgery: A Randomized Trial. Anesthesiology. 2021;135:419–432. doi: 10.1097/ALN.0000000000003873. [DOI] [PubMed] [Google Scholar]
- 9.Sessler D.I. Does Regional Analgesia Reduce the Risk of Cancer Recurrence? A Hypothesis. Eur. J. Cancer Prev. 2008;17:269–272. doi: 10.1097/CEJ.0b013e3282f0c005. [DOI] [PubMed] [Google Scholar]
- 10.Havlik R., Srovnal J., Klos D., Benedikova A., Lovecek M., Ghothim M., Cahova D., Neoral C., Hajduch M. Occult Tumour Cells in Peritoneal Lavage Are a Negative Prognostic Factor in Pancreatic Cancer. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc Czech Repub. 2013;157:233–238. doi: 10.5507/bp.2012.061. [DOI] [PubMed] [Google Scholar]
- 11.Park Y., Jun H.R., Choi H.W., Hwang D.W., Lee J.H., Song K.B., Lee W., Kwon J., Ha S.H., Jun E., et al. Circulating Tumour Cells as an Indicator of Early and Systemic Recurrence after Surgical Resection in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2021;11:1644. doi: 10.1038/s41598-020-80383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y., Wang P., Peng J., Wang X., Zhu Y., Shen N. Meta-Analysis Reveals the Prognostic Value of Circulating Tumour Cells Detected in the Peripheral Blood in Patients with Non-Metastatic Colorectal Cancer. Sci. Rep. 2017;7:905. doi: 10.1038/s41598-017-01066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stejskal P., Srovnal J., Berta E., Rehulkova A., Vecera L., Haiduk F., Michalek P., Hajduch M. Abstract 1956: Perioperative Opioid Analgesia Affects the Circulating Tumor Cells Levels in Colorectal Cancer Patients. Cancer Res. 2022;82:1956. doi: 10.1158/1538-7445.AM2022-1956. [DOI] [Google Scholar]
- 14.Srovnal J., Berta E., Rehulkova A., Vidlarova M., Prasil P., Vecera L., Stourac P., Kourilova P., Hajduch M. Abstract B41: Piritramide Analgesia Reduces CEA MRNA-Positive Circulating Tumor Cells’ Presence Compared to Morphine and Epidural Analgesia Following Radical Colon Cancer Surgery. Clin. Cancer Res. 2020;26:B41. doi: 10.1158/1557-3265.LiqBiop20-B41. [DOI] [Google Scholar]
- 15.Vidlarova M., Berta E., Prasil P., Prokopova A., Gurska S., Khoylou M., Rehulkova A., Kourilova P., Chudacek J., Szkorupa M., et al. Cannabinoid Receptor 2 Expression in Early-Stage Non-Small Cell Lung Cancers Identifies Patients with Good Prognosis and Longer Survival. Transl. Lung Cancer Res. 2022;11:2040–2050. doi: 10.21037/tlcr-22-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X., Liu Y., Huang S., Liu G., Xie C., Zhou J., Fan W., Li Q., Wang Q., Zhong D., et al. Overexpression of Cannabinoid Receptors CB1 and CB2 Correlates with Improved Prognosis of Patients with Hepatocellular Carcinoma. Cancer Genet. Cytogenet. 2006;171:31–38. doi: 10.1016/j.cancergencyto.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Carracedo A., Lorente M., Egia A., Blázquez C., García S., Giroux V., Malicet C., Villuendas R., Gironella M., González-Feria L., et al. The Stress-Regulated Protein P8 Mediates Cannabinoid-Induced Apoptosis of Tumor Cells. Cancer Cell. 2006;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Vecera L., Gabrhelik T., Prasil P., Stourac P. The Role of Cannabinoids in the Treatment of Cancer. Bratisl. Lek. Listy. 2020;121:79–95. doi: 10.4149/BLL_2020_012. [DOI] [PubMed] [Google Scholar]
- 19.Solinas M., Massi P., Cantelmo A.R., Cattaneo M.G., Cammarota R., Bartolini D., Cinquina V., Valenti M., Vicentini L.M., Noonan D.M., et al. Cannabidiol Inhibits Angiogenesis by Multiple Mechanisms. Br. J. Pharmacol. 2012;167:1218–1231. doi: 10.1111/j.1476-5381.2012.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisanti S., Picardi P., D’Alessandro A., Laezza C., Bifulco M. The Endocannabinoid Signaling System in Cancer. Trends Pharmacol. Sci. 2013;34:273–282. doi: 10.1016/j.tips.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L.X., Sharma S., Stolina M., Gardner B., Roth M.D., Tashkin D.P., Dubinett S.M. Delta-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 22.Michalski C.W., Oti F.E., Erkan M., Sauliunaite D., Bergmann F., Pacher P., Batkai S., Müller M.W., Giese N.A., Friess H., et al. Cannabinoids in Pancreatic Cancer: Correlation with Survival and Pain. Int. J. Cancer. 2008;122:742–750. doi: 10.1002/ijc.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Qu M., Gorur A., Sun Z., Cata J.P., Chen W., Miao C. Association of Mu-Opioid Receptor (MOR) Expression and Opioids Requirement with Survival in Patients with Stage I-III Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2021;11:686877. doi: 10.3389/fonc.2021.686877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagon I.S., Smith J.P., McLaughlin P.J. Human Pancreatic Cancer Cell Proliferation in Tissue Culture Is Tonically Inhibited by Opioid Growth Factor. Int. J. Oncol. 1999;14:577–584. doi: 10.3892/ijo.14.3.577. [DOI] [PubMed] [Google Scholar]
- 25.Haque M.R., Barlass U., Armstrong A., Shaikh M., Bishehsari F. Novel Role of the Mu-Opioid Receptor in Pancreatic Cancer: Potential Link between Opioid Use and Cancer Progression. Mol. Cell. Biochem. 2022;477:1339–1345. doi: 10.1007/s11010-022-04377-5. [DOI] [PubMed] [Google Scholar]
- 26.Shakhar G., Ben-Eliyahu S. Potential Prophylactic Measures against Postoperative Immunosuppression: Could They Reduce Recurrence Rates in Oncological Patients? Ann. Surg. Oncol. 2003;10:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Stamer U., Mpasios N., Stüber F., Laubenthal H., Maier C. Postoperative Schmerztherapie in Deutschland Ergebnisse einer Umfrage. Anaesthesist. 2002;51:248–257. doi: 10.1007/s00101-002-0288-7. [DOI] [PubMed] [Google Scholar]
- 28.Kinstner C., Likar R., Sandner-Kiesling A., Hutschala D., Pipam W., Gustorff B. Qualität der postoperativen Schmerztherapie in Österreich. Anaesthesist. 2011;60:827. doi: 10.1007/s00101-011-1911-2. [DOI] [PubMed] [Google Scholar]
- 29.Gabrhelík T., Pieran M. Léčba Pooperační Bolesti. Interní Medicína Pro Praxi. 2012;14:23–25. [Google Scholar]
- 30.Gramke H.-F., de Rijke J.M., van Kleef M., Raps F., Kessels A.G.H., Peters M.L., Sommer M., Marcus M.A.E. The Prevalence of Postoperative Pain in a Cross-Sectional Group of Patients after Day-Case Surgery in a University Hospital. Clin. J. Pain. 2007;23:543–548. doi: 10.1097/AJP.0b013e318074c970. [DOI] [PubMed] [Google Scholar]
- 31.Janssen P.A. Pirinitramide (R 3365), a Potent Analgesic with Unusual Chemical Structure. J. Pharm. Pharmacol. 1961;13:513–530. doi: 10.1111/j.2042-7158.1961.tb11864.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar N., Rowbotham D.J. Piritramide. Br. J. Anaesth. 1999;82:3–5. doi: 10.1093/bja/82.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Hinrichs M., Weyland A., Bantel C. Piritramid. Schmerz. 2017;31:345–352. doi: 10.1007/s00482-017-0197-y. [DOI] [PubMed] [Google Scholar]
- 34.Kay B. A Clinical Investigation of Piritramide in the Treatment of Postoperative Pain. Br. J. Anaesth. 1971;43:1167–1171. doi: 10.1093/bja/43.12.1167. [DOI] [PubMed] [Google Scholar]
- 35.Gottschalk A., Sharma S., Ford J., Durieux M.E., Tiouririne M. Review Article: The Role of the Perioperative Period in Recurrence after Cancer Surgery. Anesth. Analg. 2010;110:1636–1643. doi: 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 36.Harimaya Y., Koizumi K., Andoh T., Nojima H., Kuraishi Y., Saiki I. Potential Ability of Morphine to Inhibit the Adhesion, Invasion and Metastasis of Metastatic Colon 26-L5 Carcinoma Cells. Cancer Lett. 2002;187:121–127. doi: 10.1016/S0304-3835(02)00360-9. [DOI] [PubMed] [Google Scholar]
- 37.Zagon I.S., Rahn K.A., McLaughlin P.J. Opioids and Migration, Chemotaxis, Invasion, and Adhesion of Human Cancer Cells. Neuropeptides. 2007;41:441–452. doi: 10.1016/j.npep.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Gupta K., Kshirsagar S., Chang L., Schwartz R., Law P.-Y., Yee D., Hebbel R.P. Morphine Stimulates Angiogenesis by Activating Proangiogenic and Survival-Promoting Signaling and Promotes Breast Tumor Growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 39.Ustun F., Durmus-Altun G., Altaner S., Tuncbilek N., Uzal C., Berkarda S. Evaluation of Morphine Effect on Tumour Angiogenesis in Mouse Breast Tumour Model, EATC. Med. Oncol. 2011;28:1264–1272. doi: 10.1007/s12032-010-9573-5. [DOI] [PubMed] [Google Scholar]
- 40.Koodie L., Ramakrishnan S., Roy S. Morphine Suppresses Tumor Angiogenesis through a HIF-1alpha/P38MAPK Pathway. Am. J. Pathol. 2010;177:984–997. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zagon I.S., McLaughlin P.J. Opioid Growth Factor and the Treatment of Human Pancreatic Cancer: A Review. World J. Gastroenterol. 2014;20:2218–2223. doi: 10.3748/wjg.v20.i9.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zagon I.S., Verderame M.F., Hankins J., McLaughlin P.J. Overexpression of the Opioid Growth Factor Receptor Potentiates Growth Inhibition in Human Pancreatic Cancer Cells. Int. J. Oncol. 2007;30:775–783. doi: 10.3892/ijo.30.4.775. [DOI] [PubMed] [Google Scholar]
- 43.Zagon I.S., Smith J.P., Conter R., McLaughlin P.J. Identification and Characterization of Opioid Growth Factor Receptor in Human Pancreatic Adenocarcinoma. Int. J. Mol. Med. 2000;5:77–84. [PubMed] [Google Scholar]
- 44.Falk W., Magnuson A., Eintrei C., Henningsson R., Myrelid P., Matthiessen P., Gupta A. Comparison between Epidural and Intravenous Analgesia Effects on Disease-Free Survival after Colorectal Cancer Surgery: A Randomised Multicentre Controlled Trial. Br. J. Anaesth. 2021;127:65–74. doi: 10.1016/j.bja.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasil P., Berta E., Srovnal J., Gabrhelik T., Adamus M., Hajduch M. Morphinebut Not Piritramide-Based Postoperative Analgesia Negatively Influences Levels of Circulating Tumor Cells and Patients’ Survival Following Colorectal Cancer Surgery: 14AP6-1. Eur. J. Anaesthesiol. EJA. 2014;31:229. doi: 10.1097/00003643-201406001-00661. [DOI] [Google Scholar]
- 46.Ravi J., Sneh A., Shilo K., Nasser M.W., Ganju R.K. FAAH Inhibition Enhances Anandamide Mediated Anti-Tumorigenic Effects in Non-Small Cell Lung Cancer by Downregulating the EGF/EGFR Pathway. Oncotarget. 2014;5:2475–2486. doi: 10.18632/oncotarget.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sierra S., Gupta A., Gomes I., Fowkes M., Ram A., Bobeck E.N., Devi L.A. Targeting Cannabinoid 1 and Delta Opioid Receptor Heteromers Alleviates Chemotherapy-Induced Neuropathic Pain. ACS Pharmacol. Transl. Sci. 2019;2:219–229. doi: 10.1021/acsptsci.9b00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Page G.G. Immunologic Effects of Opioids in the Presence or Absence of Pain. J. Pain Symptom Manag. 2005;29:S25–S31. doi: 10.1016/j.jpainsymman.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Martinez L., Ekman E., Nakhla N. Perioperative Opioid-Sparing Strategies: Utility of Conventional NSAIDs in Adults. Clin. Ther. 2019;41:2612–2628. doi: 10.1016/j.clinthera.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Mulita F., Karpetas G., Liolis E., Vailas M., Tchabashvili L., Maroulis I. Comparison of Analgesic Efficacy of Acetaminophen Monotherapy versus Acetaminophen Combinations with Either Pethidine or Parecoxib in Patients Undergoing Laparoscopic Cholecystectomy: A Randomized Prospective Study. Med. Glas. 2021;18:27–32. doi: 10.17392/1245-21. [DOI] [PubMed] [Google Scholar]
- 51.Petrelli F., Tomasello G., Ghidini M., Lonati V., Passalacqua R., Barni S. Disease-Free Survival Is Not a Surrogate Endpoint for Overall Survival in Adjuvant Trials of Pancreatic Cancer: A Systematic Review of Randomized Trials. HPB. 2017;19:944–950. doi: 10.1016/j.hpb.2017.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are openly available in FigShare at https://figshare.com/account/articles/21841071 (accessed on 6 August 2023) (DOI 10.6084/m9.figshare.21841071).