Abstract
A randomized, controlled, clinical trial was conducted to examine the impact of a semistructured, 10-week, once weekly, 90-min/session bereavement support group intervention on immunological, neuroendocrine, and clinical health status in human immunodeficiency virus type 1-seropositive (HIV-1+) and HIV-1-seronegative (HIV-1−) homosexual men, compared to a standard of care control condition. A total of 119 homosexual men (74 HIV-1+ and 45 HIV-1−) were assessed at baseline, 10 weeks, and 6 months follow-up. At the 6-month follow-up assessment, the intervention groups exhibited significant beneficial effects compared to controls on changes in CD4 cell, total T-lymphocyte, and total lymphocyte counts, when baseline levels, antiretroviral medication use, CDC stage of disease, and other potentially confounding factors were accounted for. There was no statistically significant effect on the CD4/CD8 ratio or on the CD8 cell count. The effect on CD4 cell count was associated with group attendance and with changes in plasma cortisol level. Plasma cortisol levels decreased significantly among intervention subjects, compared to controls. A significantly reduced number of health care visits over the 6-month follow-up period among the intervention subjects supported the clinical relevance of the immunological changes observed for both HIV-1+ and HIV-1− individuals. These results indicate that behavioral interventions may have salutary immunological and clinical health effects following bereavement among HIV-1-infected individuals. The effect in HIV-1− individuals suggests that this bereavement support group intervention might have similar salutary effects in the general population. Potential effects of such interventions on clinical HIV disease progression are of interest and should be studied.
Bereavement is a severely stressful life event that occurs frequently among human immunodeficiency virus type-1 seropositive (HIV-1+) and HIV-1-seronegative (HIV-1−) individuals affected by the epidemic. Prior research in the general population has demonstrated that bereavement is associated with decrements in immunological function (3, 29), including natural killer cell cytotoxicity (NKCC) and lymphocyte proliferative response to mitogens (phytohemagglutinin [PHA], concanavalin A, and pokeweed). Earlier work with HIV-1-infected individuals suggested that there were no immunological associations specific to loss among close friends of HIV-1+ homosexual men (31) on a number of phenotypic measures (including CD4 and CD8 cell counts), a functional measure (lymphocyte proliferative response to PHA), and a marker of abnormal activation (neopterin level), though several associations with depressed mood were identified. In contrast, in a subsequent study of loss of an intimate partner (30), bereavement was associated with an increased serum neopterin level as well as a decreased lymphocyte proliferative response to PHA, and these effects were not mediated by depressed mood. Similar discrepancies have been found among studies examining relationships between psychosocial factors and immunological status among HIV-1+ individuals outside of bereavement (4, 24, 38). One possible explanation for the discrepancies involves the lack of control for concomitant, potentially impactful psychosocial factors, i.e., the number of background stressful life events, social support availability, and coping strategies (24). Our theory-driven, stressor-support-coping model incorporating these factors has been shown to be related to predicted alterations in immunological measures within (21, 22) and outside the setting of (23, 25) bereavement. Moreover, in bereavement, a previous study controlling for relationship type has demonstrated consistent findings in HIV-1+ individuals losing either a close friend or an intimate partner. In that study (22), controlling for other factors, decrements in NKCC occurred first, followed by decrements in lymphocyte proliferative response to PHA (the latter of which was related to increased plasma cortisol level, after controlling for plasma epinephrine and norepinephrine level).
Other explanations for the discrepancies observed in bereavement-related immunological changes include effects related specifically to grief level or to a complicated grief reaction (e.g., major depressive disorder) rather than to depressed mood level. This report relates the results of a randomized clinical trial of a support group intervention (20) aimed at grief resolution, improved stressor appraisal, increased social support utilization, and increased adaptive coping after the loss of a close friend or intimate partner due to HIV-1 infection. Based on prior work (22–25), we hypothesized that the intervention would increase both the CD4 cell count and clinical health status of HIV-1+ and HIV-1− homosexual men compared to those assigned to a standard of care control condition.
MATERIALS AND METHODS
Subjects.
The subjects totalled 119 HIV-1+ (n = 74) and HIV-1− (n = 45) homosexual men having lost a close friend or intimate partner to AIDS within the previous 6 months (rated as continuing to have negative impact at entry). Exclusion criteria were a CD4 cell count of less than 50 cells/mm3, active HIV-1-related opportunistic infection, or newly diagnosed cancer at entry; dependence on alcohol, other psychoactive substances or prescribed medication (but not lower levels of use—i.e., abuse or use without any disorder) at baseline or within the prior 6 months; any injecting substance use within the previous 2 years; current or prior history of major psychiatric disorder (e.g., HIV-1-associated dementia, schizophrenia, and major depressive disorder with melancholia); and current or recent use of prescribed medications known to substantially affect the immune system (e.g., systemic corticosteroids, beta blockers, and immunostimulants). Antiretroviral medication use did not warrant exclusion. Subjects were recruited by advertisements in local media, by pamphlets distributed in settings and at events with a large homosexual clientele, and by referrals from local hospitals and from members of our community liaison group developed for this trial. All subjects signed an informed consent form approved by the Medical Sciences Committee for the Protection of Human Subjects of the University of Miami School of Medicine. Prior to assessment, subjects were randomly assigned to either a 10-week bereavement support group intervention or a standard of care control condition. Subjects were typically in their late 30s or early 40s, were predominantly European American and living alone, and had some college education (Table 1). The average CD4 cell count at entry was 355 cells/mm3 among HIV-1+ subjects, within the moderate range of immunological progression according to the 1993 Centers for Disease Control and Prevention (CDC) immunological staging system (stage 1, ≥500 cells/mm3; stage 2, 200 to 499 cells/mm3; stage 3, <200 cells/mm3) (8), and significantly less than that of HIV-1− subjects (836 cells/mm3; P < 0.0001) (Table 2). Likewise, the CD4/CD8 ratio (P = 0.0001), CD8 cell count (P < 0.003), total T-lymphocyte count (P = 0.0002), and total lymphocyte count (P < 0.0001) were significantly lower at baseline among HIV-1+ subjects. However, there were no statistically significant differences on any of the study immunological measures by treatment assignment. Regarding the neuroendocrine measure, plasma cortisol level, there were no statistically significant baseline differences by HIV-1 serostatus or by treatment assignment. Clinically, most HIV-1+ subjects were in the early symptomatic stage (CDC stage B) (71.6%) at baseline, with a significant minority in the asymptomatic stage (CDC stage A) (27.0%) and a small minority with AIDS (CDC stage C) (1.4%). Regarding the health care service utilization outcome measure, there was an expected statistically significant baseline difference by HIV-1 serostatus (P = 0.03). HIV-1+ subjects had higher health care visit rates, consonant with the baseline immunological differences observed with regard to HIV-1 serostatus. In addition, there was a statistically significant difference on this measure by treatment assignment on the analyzed sample (P = 0.004), which was controlled through the use of a change measure of outcome, incorporating the baseline level, as well as by the use of a control for baseline physician visit utilization in the post hoc analyses of variance (ANOVAs) (see Statistical methods below).
TABLE 1.
Sociodemographic characteristics of the sample
| Characteristic | Grand mean | HIV-1− subjects | HIV-1+ subjectsa |
|---|---|---|---|
| n | 119 | 45 | 74 |
| Age (yr)b | 38.3 (9.5)c | 41.2 (11.1) | 36.5 (7.9) |
| Education (yr)b | 15.1 (2.2) | 15.5 (2.2) | 14.8 (2.1) |
| Income last month ($)d | 2,025.8 (2,246.8) | 2,561.1 (3,174.2) | 1,707.6 (1,374.3) |
| Health insurance (%)b,e | 61.9 | 75.0 | 54.1 |
| Annual income (%)f | |||
| $10,000 or less | 17.1 | 11.4 | 20.6 |
| $10,000–$19,999 | 23.9 | 27.3 | 21.9 |
| $20,000–$29,999 | 27.4 | 25.0 | 27.4 |
| $30,000–$39,999 | 16.2 | 22.7 | 13.7 |
| $40,000 or more | 15.4 | 13.6 | 16.4 |
| Living arrangement (%)g | |||
| Living alone | 60.7 | 60.0 | 61.1 |
| Living with intimate partner | 13.7 | 15.6 | 12.5 |
| Living with roommate | 14.5 | 17.8 | 12.5 |
| Living with parent | 5.1 | 4.4 | 5.6 |
| Other arrangement | 6.0 | 2.2 | 8.3 |
| Ethnicity (%) | |||
| African American | 6.7 | 4.4 | 8.1 |
| Hispanic American | 20.2 | 17.8 | 21.6 |
| European American | 71.4 | 77.8 | 67.6 |
| Other ethnicity | 1.7 | 0.0 | 2.7 |
| Employment (%) | |||
| Yes | 84.0 | 88.9 | 81.1 |
| No | 16.0 | 11.1 | 18.9 |
There were no seroconversions during the study. One subject confirmed to be HIV-1+ by antibody test at entry subsequently tested seronegative as well as negative by PCR. Loss of HIV-1 antibody together with a negative PCR result have been demonstrated previously (16) and may be more common than formerly thought (41). Hence, this subject was retained as seropositive; eliminating him from the sample did not affect the results reported herein.
Only age and health insurance were significantly different by HIV-1 serostatus (P ≤ 0.05). Education showed a nonsignificant trend toward a difference (P ≤ 0.07). These variables were not significantly different by treatment assignment and were not considered control variables.
Standard deviations appear in parentheses.
Means for income last month were based on nHIV-1− = 41 and nHIV-1+ = 69.
The percentages for health insurance data were based on nHIV-1− = 44 and on the full subsample (n = 74) of HIV-1+ subjects.
The percentages for annual income data were based on nHIV-1− = 44 and nHIV-1+ = 73.
The percentages for living arrangement data were based on nHIV-1− = 45 and nHIV-1+ = 72.
TABLE 2.
Means and 6-month changes in immunological, neuroendocrine, and clinical health outcome variables by HIV-1 serostatus and treatment assignmenta
| Outcome measurea | Treatment P valueb | HIV-1+ subject group
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention
|
Control
|
||||||||
| T1 | T2 | T3 | Δ(T3-T1) | T1 | T2 | T3 | Δ(T3-T1) | ||
| Immunological: | |||||||||
| CD4 cell count | 0.041 | 351.0 (42.9)c | 328.0 (47.1) | 329.4 (59.8) | −21.6 (39.3) | 432.6 (85.6) | 349.7 (34.7) | 372.0 (51.5) | −60.6 (55.5) |
| Total T lymphocyte count | 0.030 | 1,042.0 (60.9) | 997.0 (74.0) | 1,075.4 (99.2) | 33.4 (72.8) | 1,115.9 (132.7) | 958.0 (100.8) | 1,029.3 (64.1) | −86.6 (86.4) |
| Total lymphocyte count | 0.029 | 1,298.2 (69.8) | 1,268.3 (78.7) | 1,346.7 (103.8) | 48.5 (73.5) | 1,338.9 (142.6) | 1,204.3 (100.4) | 1,237.5 (76.4) | −101.4 (92.4) |
| CD8 cell count | 0.344 | 668.5 (45.7) | 657.7 (50.4) | 663.3 (64.7) | 30.1 (59.6) | 662.7 (120.1) | 609.1 (79.7) | 610.7 (55.0) | −52.0 (77.1) |
| CD4/CD8 ratiod | 0.786 | 0.47 (0.61) | 0.44 (0.49) | 0.41 (0.49) | −0.05 (0.21) | 0.63 (0.76) | 0.67 (0.54) | 0.60 (0.62) | −0.03 (0.36) |
| Neuroendocrine: | |||||||||
| Plasma cortisol level (μg/dl) | 0.0014 | 10.4 (0.7) | 11.0 (1.1) | 9.3 (0.9) | −1.2 (1.0) | 8.5 (1.9) | 11.6 (0.9) | 12.1 (1.8) | 3.6 (3.1) |
| Clinical: | |||||||||
| No. of health care visits prior 6 mo | 0.023e | 1.28 (0.33) | 2.52 (0.53) | 3.04 (0.54) | 1.76 (0.58) | 3.43 (0.92) | 2.79 (1.67) | 6.36 (2.70) | 2.93 (2.14) |
| HIV-1− subject group
| |||||||
|---|---|---|---|---|---|---|---|
| Intervention
|
Control
|
||||||
| T1 | T2 | T3 | Δ(T3-T1) | T1 | T2 | T3 | Δ(T3-T1) |
| 820.6 (35.4) | 866.0 (44.2) | 932.7 (79.3) | 112.1 (66.9) | 799.6 (66.7) | 761.5 (47.2) | 711.9 (36.2) | −87.7 (58.9) |
| 1,303.6 (60.0) | 1,239.0 (70.0) | 1,452.6 (106.7) | 149.0 (75.1) | 1,251.7 (79.2) | 1,219.2 (83.8) | 1,145.0 (58.3) | −106.7 (86.2) |
| 1,690.5 (74.1) | 1,632.8 (66.7) | 1,852.5 (136.3) | 162.0 (98.3) | 1,608.8 (101.9) | 1,568.1 (101.2) | 1,485.1 (81.0) | −123.7 (114.7) |
| 515.7 (44.1) | 460.6 (35.5) | 575.7 (57.2) | 60.0 (57.1) | 471.0 (38.9) | 432.1 (42.1) | 441.3 (39.1) | −29.7 (34.7) |
| 1.70 (0.83) | 1.85 (1.58) | 1.87 (0.94) | 0.17 (0.84) | 1.69 (0.82) | 1.85 (0.80) | 1.49 (0.96) | −0.001 (0.43) |
| 11.9 (1.2) | 9.6 (0.9) | 10.0 (1.2) | −1.9 (1.3) | 10.3 (1.2) | 11.8 (0.7) | 12.3 (1.6) | 2.0 (1.6) |
| 0.36 (0.15) | 1.27 (0.86) | 0.86 (0.23) | 0.50 (0.35) | 0.85 (0.30) | 1.0 (0.41) | 1.85 (0.60) | 1.0 (0.54) |
This table is based on the sample with measures at all time points (nimmune [nI] = 87, ncortisol [nC] = 65, nhealth visits [nH] = 74). For T1-T2, nI = 105, nC = 90, and nH = 90; for T1-T3, nI = 89, nC = 77, and nH = 81.
This is the P value for the T1-T3 contrast.
Values given in parentheses are standard errors unless otherwise indicated.
Medians are used as a measure of central tendency for CD4/CD8 ratio due to the presence of outliers. The number in parentheses is the interquartile range.
The planned RANOVA for health care was significant (P = 0.023), as its P value shows; the post hoc T1-T3 contrast P was not significant.
Bereavement support group technique.
Subjects randomly assigned to the support group intervention participated in an ongoing enrollment, 10-week (one 90-min session/week), semistructured group led by two cotherapists experienced with death and dying and HIV-1 spectrum disease. The two-tiered intervention—grief resolution on the first tier, overall life stressor management on the second—was conducted according to a standardized, published protocol with 10 specific session topics used in repeating cycles (20). Topics reflected the priority of the first tier, grief work, and were subsumed under three thematic foci: (i) making contact (what grieving is, relationships with health care providers and caregiving, and handling of relationship reminders), (ii) ventilation (interactions with family, past loss experiences, reactions to surviving, and implications for one’s spirituality and mortality), and (iii) moving on toward the future (managing distressing feelings, seeking social support and intimacy, and what was learned from the experience). In addition, both loss-related and general (nonloss) stressor management was emphasized by encouraging accurate appraisal of controllable aspects of stressors, maximizing social support utilization, and encouraging active coping strategies with controllable stressors (rather than passive, maladaptive strategies such as denial or avoidance).
Viral serology.
HIV-1 serostatus was determined for each subject by enzyme-linked immunosorbent assay (Cambridge Biotech, Worcester, Mass.) and confirmed by Western blotting (Cambridge Biotech) at entry. HIV-1− subjects received repeat testing at each assessment.
Outcome measures and methods. (i) Immunological measures.
The following phenotypic measures were evaluated: the CD3+ CD4+ (helper T-lymphocyte) cell count, the CD3+ CD8+ (suppressor or cytotoxic-T-lymphocyte) cell count, the CD4/CD8 ratio, the CD3+ (total mature T-lymphocyte) cell count, and the total lymphocyte count.
(ii) Immunological methods.
Lymphocyte phenotyping was conducted to quantify CD3+, CD4+, and CD8+ lymphocytes as well as the CD4/CD8 ratio. For this analysis, cells were stained with CD3-fluorescein isothiocyanate (FITC) and either CD4-RD1 or CD8-RD1. Gating was established on a histogram of forward scatter versus side scatter, and purity was defined using CD45-FITC by CD14-RD1. Cursor settings were defined with conjugated isotypic controls (mouse immunoglobulin G1 (IgG1)-RD1 with IgG2a-FITC). Whole blood (100 μl) was incubated with the above-mentioned combinations of antibodies for 10 min at 25°C prior to erythrocyte (RBC) lysis and fixation using the Q-prep procedure (18). Samples were analyzed with an Epics Elite flow cytometer. The cytometer and all reagents were from Coulter Corp. (Hialeah, Fla.). Total counts for each subset were determined by multiplying the percent positive value and the total lymphocyte count. Total lymphocyte counts were obtained by an automated complete blood count with a differential count by using a Cell Dyne 1500 (Sequoia-Turner, Mountain View, Calif.).
(iii) Neuroendocrine measure.
Plasma cortisol level was included as a possible factor associated with changes in the immunological measures.
(iv) Neuroendocrine method.
Plasma cortisol level (measured as concentration in micrograms per deciliter) was determined by a solid-phase radioimmunoassay technique in which antibody is bound to the tube (DSL, Webster, Tex.). The minimum cortisol detection limit was 0.2 μg/dl. The interassay coefficient of variation was <6.5%, and the intra-assay coefficient of variation was between 2 and 8% (33).
(v) Health care utilization measure and method.
The number of health care visits over the previous 6 months was obtained by self-report to the study physician performing physical examinations for CDC staging while blinded to each subject’s assignment and serostatus.
(vi) Control variables.
Statistical analyses incorporated control variables for effects of behavior on immune measures (32), and, specifically, those of import in HIV-1 infection (22, 24, 25) (see Statistical methods below). Checks on randomization were conducted for (i) sociodemographic characteristics (age, ethnicity, years of education, socioeconomic status, and current employment status); (ii) loss characteristics (type of relationship [close friend versus intimate partner], time since the loss, and how long the decreased was known) and loss burden (over the prior 6 months and total losses due to AIDS); (iii) psychological, clinical status: level of self-reported psychological distress (overall distress [39] and grief level [17]), clinically rated depression and anxiety (according to the Hamilton Rating Scale for Depression [26] and the Hamilton Anxiety Rating Scale [27]), bereavement reactions complicated by major depressive disorder (also related to item v below) (per the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders III—Revised [DSM-IIIR] adapted for the HIV-1 infected [SCID-NP-HIV] [47]), and counselling and social service use, (iv) related psychosocial factors; and (v) baseline differences on the immune, neuroendocrine, and health outcome variables. Regarding item iii, overall mood state distress was assessed by the 65-item Profile of Mood States (39). Widely used in biomedical research, the Profile of Mood States also yields a total mood disturbance score (Cronbach’s α = 0.96, on this sample) based upon the sum of five negative mood state subscale scores (“tension/anxiety” [α = 0.89], “depression/dejection” [α = 0.93], “anger/hostility” [α = 0.91], “fatigue/inertia” [α = 0.91], and “confusion/bewilderment” [α = 0.82]) minus one positive mood state subscale score (vigor/activity [α = 0.89]). Severity of grief was specifically controlled by the 13-item Texas Inventory of Grief (17). This instrument measures unresolved grief and acute mourning and has acceptable psychometric properties (Cronbach’s α = 0.91, on this sample). Clinically rated depression and anxiety were assessed by using the Structured Interview Guide to the Hamilton Anxiety and Depression rating scales (50), which is standardized for administration with the SCID (47). These clinical rating scales supplement self-report and combine two well known measures, the 17-item Hamilton Rating Scale for Depression (26) (Cronbach’s α = 0.80, on this sample) and the 14-item Hamilton Anxiety Rating Scale (27) (Cronbach’s α = 0.85, on this sample). We controlled for clinically rated depression and anxiety using both the full-scale scores as well as the scores obtained after deleting the items focusing upon somatic symptoms that may be due to HIV-1 infection itself rather than mood. (Note: psychological distress decreased in response to this intervention on each of the foregoing summary mood measures, as reported elsewhere [21].) Regarding item iv, overall major life stressor burden over the prior 6 months was obtained from the Life Experience Survey (44); this measure was represented as the number of major stressful life events acknowledged as experienced at any point over the prior 6-month interval on this standardized checklist, with minor adaptations for homosexual men with HIV-related concerns, and rated as having a negative impact (on a seven-point Likert scale from −3 to +3). Social support availability (total number of supportive persons) was obtained from the Social Support Questionnaire (six-item version) (43). Dispositional coping strategy was obtained from the Coping Orientations to Problems Experienced scale (7), which measures theoretically derived, conceptually distinct aspects of active, problem-focused; passive, adaptive, emotion-focused; and passive, potentially maladaptive, emotion-focused coping strategies. Factor analysis reduced the 13 coping scales to four coping variable predictors. Two were equally weighted composite scores: (i) active coping (α = 0.87, on this sample), the five problem-focused scales (active coping, planning, suppression of competing activities, restraint coping, and seeking instrumental support) as well as three emotion-focused scales (seeking emotional social support, positive reinterpretation and growth, and acceptance), and (ii) “disengagement/denial” (α = 0.80)—behavioral disengagement, mental disengagement, and denial. The other two coping variables were single subscale scores, (iii) the “focus on and venting of emotions” subscale score (α = 0.79) and the “turning to religion” subscale score (α = 0.92) (as described previously [22, 25]).
Regarding item v, the controls for confounding effects on immunological outcome measures were 1993 CDC stage of HIV-1 infection (8) (by a history and physical examination conducted by a study physician assessor), neuropsychological impairment (by the Mini-Mental Status Examination [MMSE] [19] and the Visual Scanning and Discrimination Speed Task [49]), past and current recreational substance use (alcohol, marijuana, cocaine, amphetamines, barbiturates, benzodiazepines, hallucinogens, and opioids) (by self-report, structured psychiatric interviewing for abuse and dependence diagnoses [SCID-NP-HIV] [47]), and urine toxicology screening [for the aforementioned psychoactive substances or classes of substances]), cigarette smoking, caffeine intake, sexual activity, sleep deprivation, exercise frequency, and prescribed medication use (i.e., antiretroviral medications [zidovudine, ddI, ddC, d4T, 3TC, saquinavir, ritonavir, indinavir, nelfinavir, nevirapine, and delavirdine] and psychotropic medications [benzodiazepines and antidepressants]) (by self-report); macronutrient nutritional status (serum albumin [37] and prealbumin [28] levels); and plasma levels of specific micronutrients (vitamins B6 [46] and B12 [35] and zinc [40]).
Methods for controls for confounding effects on immunological outcome measures. (i) CDC staging.
CDC staging for clinical disease progression was determined by a medical history and a complete physical examination at the baseline assessment. Medical history was confirmed by records obtained from primary care providers, whenever available. The 1993 CDC staging system (8) is as follows: stage A, asymptomatic (including acute HIV-1 infection, asymptomatic infection, and persistent generalized lymphadenopathy); stage B, early symptomatic (constitutional symptoms and/or non-AIDS-defining illnesses such as oral candidiasis, oral hairy leukoplakia, and peripheral neuropathy); and stage C, AIDS-defining illnesses (such as Pneumocystis carinii pneumonia, Mycobacterium avium-Mycobacterium intracellulare infection, and Kaposi’s sarcoma).
(ii) MMSE.
The MMSE (19), an 11-item test (score range, 0 to 30) widely employed clinically, was used to detect neuropsychological impairment; items assessed orientation, memory, attention, naming, comprehension of verbal and written commands, writing, and visuoconstructive ability. Test-retest reliability is high (at 0.89), and concurrent validity has been established. The Visual Scanning and Discrimination Speed Task (49), also used to test neuropsychological impairment, required scanning a series of five-figure items to identify the one figure (clown face, truck, etc.) that matched a prototype figure; total time (seconds) to completion was measured.
(iii) Alcohol use and use of other psychoactive substances.
Self-report of frequency (daily or more, less than daily and more than weekly, less than weekly and more than monthly, monthly or less, rare, and none) of previous (i.e., over the prior 6 months) and current (i.e., over the prior month) use of alcohol and other psychoactive substances, by category of substance (opioids, cocaine, amphetamines, sedatives/barbiturates, marijuana, hallucinogens, and others), was obtained by a form designed for this study. Alcohol use was also controlled for by self-reported ongoing quantity of use. Cigarette smoking was obtained as history of total pack-year exposure. Caffeine intake was obtained as the equivalent of cups of coffee per day in the week prior to assessment. Sexual activity was measured as the total number of lifetime sexual partners, the total number of partners in the prior month, and the number of new male partners over the prior 6 months. Sleep deprivation was quantified by a measure of sleep efficiency, obtained by self-report of the total number of hours in bed minus the number of hours in bed awake divided by the total number of hours in bed, on average, over the week prior to assessment. Exercise frequency was obtained as self-report of the total number of minutes spent exercising (aerobic and nonaerobic exercise) over the week prior to assessment.
(iv) Psychopathology.
Interviewing for psychopathology utilized the SCID-NP-HIV. The module for current affective disorder (specifically, major depressive episode) was used to determine bereavement complicated by major depressive disorder. The module for alcohol and psychoactive substance use disorders was used to determine the presence and history of abuse and dependence disorders, by specific substance used. Diagnostic criteria used were those of the Diagnostic and Statistical Manual of Mental Disorders III—Revised (1).
(v) Prescribed medication use.
Prescribed medication use was obtained by self-report to a physician assessor conducting the physical examination and confirmed by the subject’s primary care provider, whenever possible.
(vi) Macronutrient and micronutrient levels.
Serum albumin levels (half-life, ∼6 weeks) and serum prealbumin level (half-life, ∼2 weeks) were determined by standard-rate nephelometry. Vitamin B6 levels were determined by a bioassay of RBC aspartate aminotransferase activity. After a single thaw, 100 μl of RBCs were lysed in 1.6 ml of distilled water. One milliliter of the hemolysate was then pipetted into a labeled autoanalyzer cup and placed into a sample tray. For the unstimulated peak, the amino acid substrate solution was run for 5 min and at 20 min the NADH-malic dehydrogenase indicator reagent was added. At 28 min, the chart was checked for the first sample peak from the recorder (speed, 12 cm/h). The tray was then repositioned for the stimulated run. The pyridoxal 5′-phosphate reagent was started for 2 min, and the baseline was reset for the stimulated peak. The peak heights from the unstimulated and stimulated peaks were read. Dividing the stimulated value by the unstimulated value yielded the result, expressed as an activity coefficient. Vitamin B12 levels in serum were established by a radioisotope dilution assay, and plasma zinc levels were determined by flame atomic spectrophotometry.
Statistical methods.
All statistics were calculated by using SAS software (45). First, potential imbalances in randomization were examined by ANOVA, with HIV-1 serostatus and treatment assignment as blocking factors. Further, potentially confounding variables were ascertained by Spearman rank-order correlations between the baseline level of each control variable and changes in the immune and health care utilization outcome measures. All randomization checks showing effects in the aforementioned ANOVA and control-outcome variable correlations with P < 0.20 were then entered into a multiple-regression analysis with change on the respective immune or health care utilization outcome measure. Only variables retaining a P of β < 0.20 in these regression analyses (from either T1-T2 or T1-T3 associations) were included in the final, controlled test for intervention effect. This test was a repeated-measures analysis of covariance (RANCOVA) using the multivariate test statistics from the SAS General Linear Models procedure, with HIV-1 serostatus, treatment assignment, and their interaction as between-subjects factors and time point (baseline, T1; 10 weeks, T2; 6 months, T3) as the within-subjects factor. For comparison, identical models without control variables were also estimated using a repeated-measures ANOVA (RANOVA). To further control for baseline level of the dependent measure, post hoc tests on two measures of change (T1 to T2; T1 to T3) were also performed. For each of these, an ANOVA without control variables, an ANCOVA controlling for baseline level only, and an ANCOVA controlling for baseline level along with the other control variables determined by the aforementioned control variable method were estimated. This analysis controls for baseline level of the outcome variable by holding its effect constant. For all specifications, model results were confirmed by using rank-transformed data to ensure no undue influence of extreme data points (11, 12).
Secondary analyses were conducted to investigate potential factors accounting for any results found. Pearson product-moment correlations of any effects with group attendance were estimated as a planned intervention manipulation check. In order to determine a potential immunomodulating role of plasma cortisol level, Pearson product-moment correlations of change in plasma cortisol level with change in CD4 cell count were computed. Further, to analyze an intervention effect on this potential immunomodulator, a RANOVA on plasma cortisol level using all time points and two post hoc ANOVAs using change from T1 to each of the follow-up time points were also estimated. Though the additional control variable procedures specified above for immunological and health care utilization outcomes were not incorporated here, a control for baseline plasma cortisol level was included in the analytic models (ANCOVAs) to parallel the analyses on the other outcome measures described above.
RESULTS
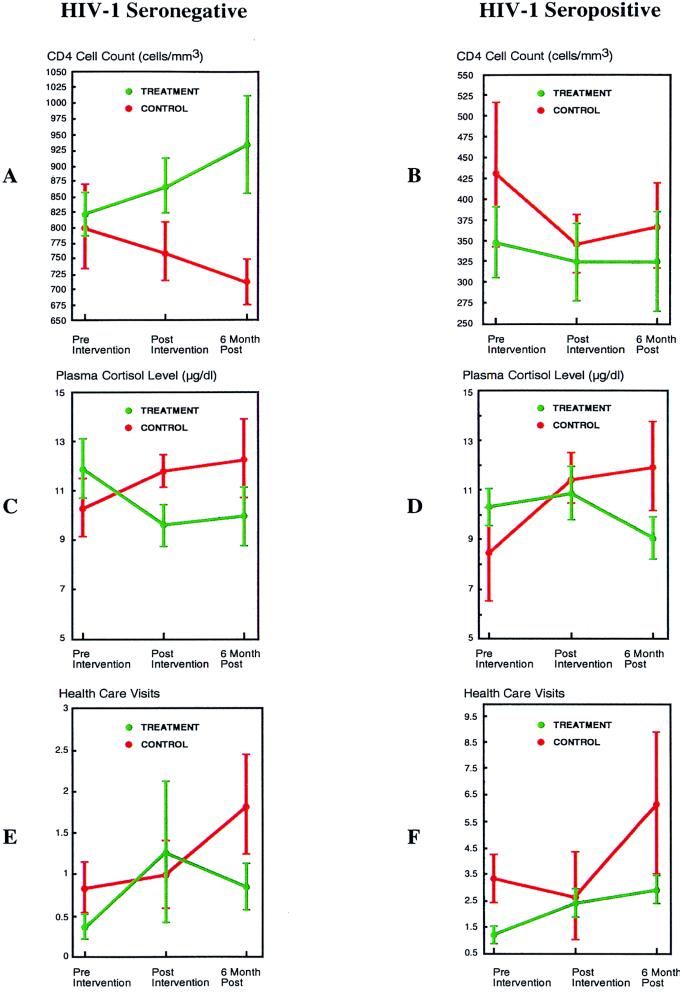
For the CD4 cell count, the RANCOVA demonstrated a statistically significant intervention effect [F(2,79) = 3.23; P = 0.045]. The covariates included in this analysis were total loss burden due to AIDS and the total scale scores for the clinical ratings of depression (26) and anxiety (27). In HIV-1− intervention subjects (n = 29) (Table 2), the CD4 cell count increased 112 cells/mm3 from T1 to T3, while that in HIV-1− control subjects (n = 16) decreased 88 cells/mm3, resulting in a difference of 200 cells/mm3 between treatment and control groups 6 months postintervention (Fig. 1A). In treated HIV-1+ individuals (n = 51), the CD4 cell count was stable, within laboratory error over 6 months (Table 2). However, that in HIV-1+ controls (n = 23) decreased 61 cells/mm3 (Fig. 1B). Both post hoc ANOVAs (using T1-T2 and T1-T3) demonstrated a statistically significant intervention effect on the CD4 cell count in the total sample [F(1,101) = 4.42; P = 0.04 (T1-T2); F(1,86) = 4.31; P = 0.04 (T1-T3)].
FIG. 1.
CD4 cell count, plasma cortisol level, and health care utilization change in HIV-1+ and HIV-1− individuals. (A and B) Regarding the CD4 cell count outcome, among HIV-1− intervention subjects (A), the mean CD4 cell count increased progressively, whereas for HIV-1− control subjects there was a progressive decrease. For HIV-1+ subjects (B), the mean CD4 cell count was higher at baseline (statistically controlled herein) among control subjects than among their intervention counterparts. However, the change in CD4 cell count reflected a decrease overall among control subjects, whereas for HIV-1+ intervention subjects the CD4 cell count remained stable. (C and D) Regarding plasma cortisol level, among HIV-1+ intervention subjects (D) mean plasma cortisol level stabilized initially postintervention and then decreased at 6 months, while the mean of HIV-1+ control subjects increased at T2 and again, though less substantially, at 6 months. In HIV-1− intervention subjects (C), unlike their HIV-1+ counterparts, mean plasma cortisol levels initially decreased postintervention, a change that was essentially maintained at 6 months. The HIV-1− control subjects showed a pattern similar to their HIV-1+ counterparts, with an increase in the mean at T2 and again, though less substantially, at T3. (E and F) Regarding the clinical outcome on health care visit utilization, among HIV-1+ control subjects (F) mean health care visit utilization increased, whereas for HIV-1+ intervention subjects there was a smaller, progressive increment. Among HIV-1− subjects (E), a similar pattern of change in the mean number of health care visits was observed. For all panels, standard errors are shown by vertical bars.
On the total T-lymphocyte count, a RANCOVA did not demonstrate a statistically significant effect [F(2,63) = 1.32; P = 0.27]; the covariates included were CDC stage, antiretroviral medication use, current frequency of smoking and sedative medication use, total loss burden due to AIDS over the prior 6 months, and the (total) Hamilton Depression and Anxiety Rating Scale scores. However, a RANOVA over all three time points revealed a trend toward a significant effect [F(2,82) = 2.43; P = 0.09], and a post hoc ANOVA from T1 to T3 demonstrated a statistically significant intervention effect [F(1,86) = 4.84; P = 0.03]. This effect remained after controlling for baseline count in an ANCOVA [F(1,85) = 4.89; P = 0.03]. However, an ANCOVA controlling for CD4 cell count was not statistically significant [F(1,84) = 1.49; P = 0.23]. In addition, these effects were not found in the ANOVA from T1 to T2, with [F(1,100) = 0.28; P = 0.59] or without [F(1,101) = 0.51; P = 0.47] baseline count controlled. Regarding the changes from T1 to T3 that were observed for HIV-1+ intervention subjects, total T-lymphocyte count increased 33 cells/mm3 over 6 months, while for HIV-1+ controls this count decreased 87 cells/mm3 (Table 2), a relative difference in mean change from baseline of 120 cells/mm3.
On the total lymphocyte count, a RANCOVA did not demonstrate a statistically significant intervention effect [F(2,53) = 1.22; P = 0.30]; the covariates included were prealbumin level, being urine toxicology positive, current sedative medication use, educational level, and highest prior (at least 6 months earlier) frequency of amyl nitrite use. The RANOVA over all three time points showed a trend toward a significant effect [F(2,83) = 2.48; P = 0.09], without control variables, and the post hoc ANOVA (T1-T3) demonstrated a statistically significant intervention effect [F(1,87) = 4.93; P = 0.03]; this analysis was also significant with the baseline level controlled [F(1,86) = 5.14; P = 0.03] (when ranks rather than raw data were analyzed). In addition, when CD4 cell count was controlled, the ANCOVA retained statistical significance [F(1,85) = 4.08; P = 0.05]. There was a modest increase from baseline in total lymphocyte count among HIV-1+ intervention subjects (of 49 cells/mm3 at T3) (Table 2), whereas that among HIV-1+ control subjects decreased 101 cells/mm3 at T3, a relative mean difference in change from baseline of 150 cells/mm3. Among HIV-1− intervention subjects, this measure increased 162 cells/mm3 at T3 but decreased 124 cells/mm3 among the controls, a relative mean difference in change from baseline of 286 cells/mm3.
No statistically significant intervention effects were found on the CD4/CD8 ratio or on the CD8 cell count, without control variables, with baseline level controlled alone, or with baseline level controlled in addition to other control variables determined by our aforementioned method.
The mode for group attendance was nine sessions for both the HIV-1+ and the HIV-1− intervention subjects. The Pearson product-moment correlation coefficient between frequency of groups attended and change in immunological outcome from T1 to T3 was r = 0.28 (P = 0.03), r = 0.32 (P = 0.02), and r = 0.29 (P = 0.02), for the CD4 cell count, the total T-lymphocyte count, and the total lymphocyte count, respectively.
The Pearson product-moment correlation coefficient between change in CD4 cell count and change in plasma cortisol level from T1 to T3 was r = −0.28 (P = 0.03), controlling for HIV-1 serostatus. The correlation among HIV-1+ subjects (r = −0.25) was larger in absolute value than that among HIV-1− subjects (r = −0.20). Change in plasma cortisol level by treatment was analyzed as described above for the immune measures. The three-time-point RANOVA demonstrated a significant difference by treatment in the time path of change in plasma cortisol level [F(2,60) = 3.92; P = 0.03], with the intervention groups showing a decrease and the control groups showing an increase in plasma cortisol level. For HIV-1+ intervention subjects, the mean plasma cortisol level at T1 decreased 1.2 μg/dl at T3, whereas the HIV-1+ control subjects showed a mean plasma cortisol level increase of 3.6 μg/dl from T1 to T3 (Fig. 1D), a relative mean difference in change from baseline of 4.8 μg/dl. For HIV-1− intervention subjects, the mean plasma cortisol level at T1 decreased 1.9 μg/dl at T3, whereas for HIV-1− control subjects, the mean plasma cortisol level increased 2.0 μg/dl at T3 (Fig. 1C), a relative mean difference in change from baseline of 3.9 μg/dl. In the ANOVA including T1 and T3, the intervention effect was statistically significant, whether [F(1,72) = 7.51; P = 0.008] or not [F(1,73) = 11.11; P = 0.001] the baseline level of plasma cortisol was controlled. The ANOVA including T1 and T2 was not statistically significant, though a trend in the same direction was observed, again whether [F(1,85) = 3.42; P = 0.07] or not [F(1,86) = 3.27; P = 0.075] the baseline level of plasma cortisol was controlled.
The effect of the intervention on change in health care utilization over the three time points was also examined. In the RANOVA [F(2,69) = 3.97; P = 0.02] and RANCOVA [F(2,43) = 3.55; P = 0.037] analyses on number of health care visits, an intervention effect was statistically significant; because of distributional issues involved with the use of the control variables, the RANCOVA was performed on ranks rather than on raw data. This suggests that the latter analysis corrected for a lack of normality in the data, which was required to substantiate the effect observed. The covariates included in the RANCOVA analysis were frequency of opioid use and marijuana use over the prior 6 months, current cocaine use frequency, length of time since the loss, sleep deprivation, prealbumin level (for macronutrient status), and levels of vitamin B6 and B12 (reflecting micronutrient status). For HIV-1+ control subjects, the number of health care visits over the prior 6 months (Table 2) increased a mean of 1.17 visits relative to those in the intervention condition (Fig. 1F). Interestingly, for healthy HIV-1− control subjects, a mean increase of 0.50 visits also was observed relative to those in the intervention condition (Fig. 1E).
In none of the primary analyses reported above on immunological, neuroendocrine, or health care utilization outcomes was a statistically significant interaction between HIV-1 serostatus and treatment assignment observed. Hence, the treatment findings on each of these outcome measures were not significantly different by HIV-1 serostatus.
DISCUSSION
The results demonstrate that a brief bereavement support group intervention was associated with salutary immunological effects immediately following intervention and at 6 months follow-up, for both HIV-1+ and HIV-1− homosexual men. To our knowledge, no other behavioral intervention studies have been reported that demonstrate such a longitudinal effect of this duration on immunological measures in the HIV-1 infected (2, 34). Further, the association of these changes with frequency of group attendance suggests a dose-response relationship, buttressing the intervention effect.
The increments observed in HIV-1− subjects postintervention and at 6 months contrasted to the simultaneous decrements in the HIV-1− control group suggest that this intervention not only protected against a decrement in CD4 cell count related to bereavement but also that it had a truly positive immunological effect. This conclusion is corroborated by a statistically significant post hoc mean contrast comparison on CD4 cell count change between the HIV-1− intervention and control groups. For HIV-1+ subjects, the difference in mean CD4 cell count over the follow-up time points suggests that only protection against a decrement was afforded by the intervention. The effect of HIV-1 load itself in plasma may have prevented a true increment in CD4 cell count from being observed among HIV-1+ intervention subjects herein, a broader issue in evaluating behavior-immune associations among the HIV-1 infected previously noted by Stein et al. (48). There was no evidence that the intervention effect observed was different based on HIV-1 serostatus. Although the post hoc mean contrast comparison on CD4 cell count change was not statistically significant between the HIV-1+ intervention and control groups, this could have been due to a lack of control for viral load (not routinely assessed clinically during the period of this study), a related smaller intervention effect size for HIV-1+ individuals, and the need for a commensurately larger HIV-1+ subsample size. The more-limited response to the intervention among HIV-1+ individuals may represent a direct, pathophysiological effect of HIV-1 infection rather than what might otherwise be potentially interpreted as a blunting of normal limbic-hypothalamic-pituitary-adrenal (LHPA) axis activity reflected in lesser degrees of intervention-associated change in plasma cortisol level. The phenomenon of blunting would predict a decreased correlation of changes in plasma cortisol level with changes in CD4 cell count in HIV-1+ subjects compared to their HIV-1− counterparts. However, the changes in plasma cortisol levels were comparable in magnitude for both the HIV-1+ and HIV-1− subjects (by treatment assignment); moreover, the correlation of change in plasma cortisol level to change in CD4 cell count was actually greater in the HIV-1+ subjects than among their HIV-1− counterparts. Together, these observations lend credence to the interpretation that the decreased immunological response to intervention observed among the HIV-1+ subjects herein is due to a direct pathophysiological effect of HIV-1 infection itself. Hence, future studies should include determinations of plasma HIV-1 RNA copy number.
The magnitude of the intervention effect merits specific comment. For HIV-1− individuals, the difference between the intervention and control groups in mean change at 6 months postintervention reached 200 cells/mm3. This is equivalent to more than four times the difference (48 cells/mm3) between the lower limit of the normal range for the CD4 cell count in our laboratory (547 cells/mm3) and the upper limit of 1993 CDC immunological stage 2 (499 cells/mm3), which is used to define the first step of clinically significant immunological decrement in CD4 cell count for an HIV-1+ individual (8). Though this change appears likely to be clinically significant, the clinical significance of CD4 cell count differences below the normal range may not apply to differences within the normal range. Nevertheless, these results do suggest a potential salutary clinical effect that this intervention might have outside of HIV-1-infected or -affected individuals, i.e., on the general population suffering a loss of a significant other from any cause.
Regarding HIV-1+ individuals, the CD4 cell count of bereaved HIV-1+ control subjects declined 60 cells/mm3, which is a change greater than the average decrement in CD4 cell count, 50 cells/mm3, sustained by HIV-1+ individuals over 1 year (twice the follow-up period used here). Hence, this effect may prove to be clinically significant, given the central role of the CD4 cell in coordinating immune responses. In conjunction with changes in plasma HIV-1 RNA copy number, decrements in CD4 cell count remain instrumental for clinical decision making in daily patient care (e.g., when to initiate antiretroviral drug therapy as well as primary prophylaxis of P. carinii pneumonia and of M. avium-M. intracellulare infections). Moreover, and as aforementioned, the CD4 cell count has been judged to be of sufficient clinical import to warrant incorporation into the 1993 CDC staging system itself (8). It should be noted that the CD4 cell count has been shown to persist as an independent predictor of clinical HIV disease progression, after accounting for plasma HIV-1 RNA copy number (6). Hence, a significant change in the CD4 cell count would be expected to affect the clinical progression of this infection. Further research with a larger HIV-1+ sample stratified by 1993 CDC disease stage, though difficult to accrue, would elucidate the impact of clinical HIV disease status on the estimate of the intervention effect.
Regarding the related lymphocyte populations that might also have been expected to be responsive to such an intervention, it is of interest that similar results were demonstrated for the intervention on total T-lymphocyte and total lymphocyte counts. Since the total T-lymphocyte count and the total lymphocyte count both include CD4+ T cells, post hoc analyses of these outcomes, controlling for the CD4 cell count, were conducted. For total T-lymphocyte count, this eliminated the intervention effects, suggesting no effect on other T lymphocytes, as expected given the lack of effect on the CD8 cell count. However, the total lymphocyte count did show an intervention effect after controlling for the CD4 cell count (P = 0.04), suggesting that another lymphocyte population (e.g., natural killer cells and/or B lymphocytes) may have been similarly affected. The total lymphocyte count has previously been shown to be useful in an immunological staging system of progression of HIV-1 infection in both homosexual men and injecting substance users by Zolla-Pazner et al. (51) and was predictive of progression independently of the CD4 cell count in these studies. The former measure was incorporated as the last step in a Guttman scalogram consisting of three hierarchical immunological changes, beginning with (i) CD4/CD8 ratio inversion, progressing to (ii) CD4 cell count decline to less than 500 cells/mm3, and culminating in (iii) total lymphocyte count decline to less than 1,500 cells/mm3. Hence, the findings on CD4 cell and total lymphocyte counts, taken together, indicate that the intervention effect may hold not only at the earlier stages of disease examined here (the asymptomatic and early symptomatic stages) but possibly in late-stage HIV-1 infection (AIDS) as well.
In order to examine the potential clinical effect of the immunological changes observed, we examined a clinical health variable: the number of health care visits reported by subjects to the blinded physician assessors conducting the study history and physical examinations. Over the 6 months following the intervention period, HIV-1+ subjects assigned to the intervention had fewer health care visits than their respective controls, suggesting a clinical benefit substantiating the significance of the immunological changes observed. The effect on the number of health care visits among the physically healthy, HIV-1− subjects is also noteworthy. This was relatively unexpected, given the greater press to seek health care services among HIV-1+ individuals. Nevertheless, the HIV-1− intervention subjects showed a mean of 0.5 fewer health care visits over the 6 months following the intervention period than their respective control subjects. This suggests that the bereavement support group intervention may be expected to have salubrious effects for the general population exposed to recent bereavement as well.
Though the consistency of both the immunological and clinical health care visit effects observed here is apparent, several aspects of the findings might be pursued in future studies. For example, the CD4 cell count, total T-lymphocyte count, and total lymphocyte count, while important, are only three immunological measures of relevance in HIV-1 infection. Several other measures should be examined for the breadth of such an immunological effect. These would include not only other phenotypic measures (e.g., the cytotoxic CD8+ T-lymphocyte or cytotoxic-T-lymphocyte [CTL] count, associated with long-term nonprogression), but also functional immune measures (lymphocyte proliferative response to mitogens, NKCC, and CTL-mediated cytotoxicity). In particular, functional tests using antigenic peptides and/or proteins of HIV-1 as stimuli would be of great interest. Markers of abnormal immunological activation (i.e., β2-microglobulin and neopterin levels) could also add to the power of the effects observed on phenotypic and functional immune measures. Of great interest, in addition, would be changes in the relative levels of Th1 cytokines (e.g., interleukin-2 [IL-2], gamma interferon, and IL-12), associated with maintaining cell-mediated immune responses exemplified by CTL activity linked with the deterrence of clinical HIV disease progression, versus Th2 cytokines (e.g., IL-4, IL-5, IL-6, IL-10, and IL-13), associated with stimulation of humoral immune responses and with greater likelihood of clinical HIV disease progression. Such cytokine effects might not be predominantly accounted for by the CD4 cell count alone. Ultimately, the effect that these changes in immunity might have on viral burden (i.e., plasma HIV-1 RNA copy number) is of great interest, since this measure has become a standard for assessing the clinical impact of antiretroviral drug therapies.
Given the effect observed on the level of plasma cortisol, the putative neuroendocrine mediator found to decrease in response to the intervention here, future studies of corticotropin-releasing hormone (CRH) (from cerebrospinal fluid) and of plasma adrenocorticotropic hormone (ACTH) are warranted. This would permit assessment of the response to intervention along the entire LHPA axis, allowing possible localization of the neuroendocrine mediation of the intervention effect, should it be replicated. Given that 90% of cortisol in plasma is bound to cortisol binding globulin (CBG) and that bound cortisol can neither reach many target tissues nor bind to its receptors, CBG level should be assessed to ascertain free cortisol level and further document the intervention effect observed in the present study. In addition, examining a cortisol effect as a ratio of cortisol to its endogenous antagonists (dehydroepiandrosterone [DHEA] and DHEA sulfate) might prove helpful. However, we do not expect that these measures would have affected the findings herein, as there would be no expected difference by treatment assignment on these specific measures in this randomized, controlled clinical trial. Moreover, both DHEA (or DHEA sulfate) and CBG levels would be expected to demonstrate a greater range of variation among women than men, owing to modulation by higher estrogen levels (13, 14), further supporting a lower likelihood of an effect of these measures on the cortisol results in this entirely male sample.
Notably, cortisol level has recently been proposed to be of central importance in the clinical progression of HIV-1 infection (10). This may be due to cortisol-induced suppression of the production of Th1 cytokines, enhancement of the production of Th2 cytokines, induction of apoptosis (programmed cell death), and, more directly, through increased replication of HIV-1 itself (10). Regarding specific cytokines, decreased IL-2 production, known to occur with increments in cortisol level, would be associated with decreased maturation of CTLs, the function of which is known to be preserved in long-term nonprogressors with HIV-1 infection (5). Moreover, a decreased count of CTLs has been observed in association with severe life stressors, such as bereavement, in the HIV-1 infected (15).
Perhaps yet more important is the potential interaction between neuroendocrine hormones of the LHPA axis and the genome of HIV-1 itself. For example, CRH is known to show hypersecretion in the setting of bereavement (42), and the proopiomelanocortin (POMC) CRH-responsive-element binding protein (PCRH-REB-1) binds to a specific region of the POMC promoter described as the POMC-CRH responsive element (PCRH-RE). A specific sequence of the PCRH-RE is 100% homologous with the long terminal repeat (LTR) of HIV-1, which is responsible for up-regulation of its replication (36). Hence, PCRH-REB-1 may bind to the LTR of HIV-1, and this interaction in the setting of bereavement could contribute an additional stimulus to disease progression beyond that which is expected—the endpoint of CRH hypersecretion, increased plasma cortisol levels—and the aforementioned associated immunological changes. Moreover, the vpr gene product of HIV-1, Vpr, a 15-kDa virion-associated HIV-1 protein, has been shown to interact with the activated glucocorticoid receptor complex and to influence its activity. Potentially, activation of glucocorticoid receptor type II in response to increased cortisol levels could be inhibited by this interaction, down-modulating the negative feedback loop of cortisol from periphery to brain, and contributing to chronic LHPA axis hyperactivity manifesting as part of the generalized glucocorticoid resistance reported in late-stage HIV-1 infection (9).
Finally, the potential clinical significance of the immunological and neuroendocrine findings was bolstered by the analysis of health care visit utilization. While there was a significant difference by treatment assignment in the HIV-1+ and HIV-1− intervention subjects versus their respective control subjects, in both cases the control subjects had higher levels of baseline health care visit use. This was likely related to the study design, since subjects assigned to the control condition were allowed to seek other treatment related to their bereavement-associated distress, consonant with the specification of the control condition as a community comparison standard of care condition rather than a no-treatment condition. Moreover, this increased baseline use of services in the control groups operates to create a bias against finding the bereavement support group intervention effect demonstrated herein, in which the control subjects were found to increase treatment utilization still further compared to the intervention subjects. It should be cautioned that the health care visit results may reflect changes in the likelihood of care utilization as a response to intervention rather than an actual decrement in HIV-1 specific clinical symptoms experienced. Hence, decreased progression to clinically defined AIDS (complementing the CD4 cell count changes related to 1993 CDC immunological staging reported herein) and increased survival time would further support a clinically significant intervention effect.
At this time, it may be concluded only that a bereavement support group of the type used in this study may have a significant, salutary immunological effect as well as a clinical health effect and that this possibility merits further investigation. Future studies would also have to account for the beneficial effects of the recent introduction of the protease inhibitors on the CD4 cell count and on clinical health status, though lack of adherence to these drugs in the general population may result in an increased prevalence of resistant virus over time and a return to more rapid clinical HIV disease progression rates. Regarding implications for clinical practice, a bereavement support group intervention should most certainly be viewed as investigational for immunological and clinical health outcomes. However, to the extent that such interventions do improve psychological status as well as functional status in activities of daily living, screening in HIV/AIDS clinical settings for the occurrence of loss of a significant other and offering bereavement intervention may well be indicated, regardless of any potential associated immunological or clinical health benefits.
ACKNOWLEDGMENTS
This work was supported by National Institute of Mental Health grants 1 R-01 MH48628, 1 R-01 MH48628S, and 1 R-01 MH53802 to K. Goodkin. P. Shapshak is supported by National Institute on Drug Abuse grants 1 R-01 DA04787 and 1 R-01 DA07909.
We acknowledge the group therapists, Barbara Leeds, Jack Burkhalter, and Scott Brinkmeier, for the intervention study. We also gratefully acknowledge the support of Michael Uselmann, Carlos Gonzales, Phillip Austin, and Rhonda Hudson Nelson for their support in recruitment of subjects from the greater Miami-Fort Lauderdale community who had suffered a recent loss to participate in this research study. We also thank the local community agencies regularly represented at our bimonthly community liaison group for their support in referring subjects to our trial: the People with AIDS Coalition (PWAC), Health Crisis Network (HCN), Body Positive, Center One, Cure AIDS Now, South Florida AIDS Network, the Community Research Initiative, la Liga Contra el SIDA, and the Equal Opportunity Family Health Centers, among others. Most importantly of all, we thank our subjects for enrolling in this research study at such a difficult time in their lives.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders III—revised. Washington, D.C: American Psychiatric Association; 1987. [Google Scholar]
- 2.Antoni M H, Baggett L, Ironson G, LaPerriere A, August S, Klimas N, Schneiderman N, Fletcher M A. Cognitive-behavioral stress management intervention buffers distress responses and immunological changes following notification of HIV-1 antibody seropositivity. J Consult Clin Psychol. 1991;59:906–915. doi: 10.1037//0022-006x.59.6.906. [DOI] [PubMed] [Google Scholar]
- 3.Bartrop R W, Luckhurst L, Lazarus L, Kiloh L G, Penny R. Depressed lymphocyte function after bereavement. Lancet. 1977;i:834–836. doi: 10.1016/s0140-6736(77)92780-5. [DOI] [PubMed] [Google Scholar]
- 4.Burack J H, Barrett D C, Stall R D, Chesney M A, Ekstrand M L, Coates T J. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270:2568–2573. [PubMed] [Google Scholar]
- 5.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A for the International AIDS Society—USA. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. JAMA. 1996;276:146–154. [PubMed] [Google Scholar]
- 7.Carver C S, Scheier M F, Weintraub J K. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 9.Chrousos, G. P., and T. Kino. 1997. The HIV-1 VPR gene product can cause glucocorticoid hypersensitivity or resistance: implications for the pathogenesis and clinical presentation of AIDS. Psychoneuroendocrinology 22(Suppl. 2):S160.
- 10.Clerici M, Bevilacqua M, Vago T, Villa M L, Shearer G M, Norbiato G. An immunoendocrinological hypothesis of HIV infection. Lancet. 1994;343:1552–1553. doi: 10.1016/s0140-6736(94)92944-0. [DOI] [PubMed] [Google Scholar]
- 11.Conover W J, Iman R L. Rank transformations as a bridge between parametric and nonparametric statistics. Am Statistician. 1981;35:124–133. [Google Scholar]
- 12.Conover W J, Iman R L. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- 13.Darj E, Axelsson O, Carlstrom K, Nilsson S, von Schoultz B. Liver metabolism during treatment with estradiol and natural progesterone. Gynecol Endocrinol. 1993;7:111–114. doi: 10.3109/09513599309152489. [DOI] [PubMed] [Google Scholar]
- 14.Ebeling P, Koivisto V A. Physiological importance of dehydroepiandrosterone. Lancet. 1994;343:1479–1481. doi: 10.1016/s0140-6736(94)92587-9. [DOI] [PubMed] [Google Scholar]
- 15.Evans D L, Leserman J, Perkins D O, Stern R A, Murphy C, Tamul K, Liao D, van der Horst C M, Hall C D, Folds J D, Golden R N, Petitto J M. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry. 1995;152:543–550. doi: 10.1176/ajp.152.4.543. [DOI] [PubMed] [Google Scholar]
- 16.Farzadegan H, Polis M A, Wolinsky S M, Rinaldo C R, Sninsky J J, Kwok S, Griffith R L, Kaslow R A, Phair J P, Polk B F, Saah A J. Loss of human immunodeficiency virus type 1 (HIV-1) antibodies with evidence of viral infection in asymptomatic homosexual men. Ann Int Med. 1988;108:785–790. doi: 10.7326/0003-4819-108-6-785. [DOI] [PubMed] [Google Scholar]
- 17.Faschingbauer T R, DeVaul R A, Zisook S. Development of the Texas Inventory of Grief. Am J Psychiatry. 1977;134:696–698. doi: 10.1176/ajp.134.6.696. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher M A, Baron G C, Ashman M R, Fischl M A, Klimas N G. Use of whole blood methods in assessment of immune parameters in immunodeficiency states. Diagn Clin Immunol. 1987;5:69–81. [PubMed] [Google Scholar]
- 19.Folstein M F, Folstein S E, McHugh P R. “Mini mental state”. A practical method for grading the mental status of patients for the clinician. J Psychiatry Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Goodkin K, Burkhalter J, Blaney N T, Leeds B, Feaster D J. Bereavement support group techniques for the HIV infected: integration of research with clinical practice. Omega J Death Dying. 1997;34:279–300. [Google Scholar]
- 21.Goodkin K, Blaney N T, Tuttle R S, Nelson R H, Baldewicz T, Kumar M, Fletcher M A, Leeds B, Feaster D J. Bereavement and HIV infection. Int Rev Psychiatry. 1996;8:201–216. [Google Scholar]
- 22.Goodkin K, Feaster D J, Tuttle R, Blaney N T, Kumar M, Baum M K, Shapshak P, Fletcher M A. Bereavement is associated with time-dependent decrements in cellular immune function in asymptomatic human immunodeficiency virus type 1-seropositive homosexual men. Clin Diagn Lab Immunol. 1996;3:109–118. doi: 10.1128/cdli.3.1.109-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodkin K, Fletcher M A, Cohen N. Clinical aspects of psychoneuroimmunology. Lancet. 1995;345:183–184. doi: 10.1016/s0140-6736(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 24.Goodkin K, Mulder C L, Blaney N T, Ironson G, Kumar M, Fletcher M A. Psychoneuroimmunology and HIV-1 infection revisited. Arch Gen Psychiatry. 1994;51:246–247. doi: 10.1001/archpsyc.1994.03950030082007. [DOI] [PubMed] [Google Scholar]
- 25.Goodkin K, Blaney N T, Feaster D, Fletcher M A, Baum M K, Mantero-Atienza E, Klimas N, Millon C, Szapocznik J, Eisdorfer C. Active coping style is associated with natural killer cell cytotoxicity in asymptomatic HIV-1 seropositive homosexual men. J Psychosom Res. 1992;36:635–650. doi: 10.1016/0022-3999(92)90053-5. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Ingenbleek Y, ven den Schriek H-G, de Nayer P, de Visscher M. Albumin, transferrin, and the thyroxine-binding prealbumin/retinol-binding protein (TBPA-RBP) complex in assessment of malnutrition. Clin Chim Acta. 1975;63:61–67. doi: 10.1016/0009-8981(75)90379-4. [DOI] [PubMed] [Google Scholar]
- 29.Irwin M, Daniels M, Smith T L, Bloom E, Weiner H. Impaired natural killer cell activity during bereavement. Brain Behav Immun. 1987;1:98–104. doi: 10.1016/0889-1591(87)90011-0. [DOI] [PubMed] [Google Scholar]
- 30.Kemeny M E, Weiner H, Duran R, Taylor S E, Visscher B, Fahey J L. Immune system changes after the death of a partner in HIV-positive gay men. Psychosom Med. 1995;57:547–554. doi: 10.1097/00006842-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kemeny M E, Weiner H, Taylor S E, Schneider S, Visscher B, Fahey J L. Repeated bereavement, depressed mood, and immune parameters in HIV seropositive and seronegative gay men. Health Psychol. 1994;13:14–24. doi: 10.1037//0278-6133.13.1.14. [DOI] [PubMed] [Google Scholar]
- 32.Kiecolt-Glaser J K, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Kumar M, Kumar A M, Morgan R, Szapocznik J, Eisdorfer C. Abnormal pituitary-adrenocortical response in early HIV-1 infection. J Acquired Immune Defic Syndr. 1993;6:61–65. [PubMed] [Google Scholar]
- 34.LaPerriere A R, Antoni M H, Schneiderman N, Ironson G, Klimas N, Caralis P, Fletcher M A. Exercise intervention attenuates emotional distress and natural killer cell decrements following notification of positive serologic status for HIV-1. Biofeedback Self-Regul. 1990;15:229–242. doi: 10.1007/BF01011107. [DOI] [PubMed] [Google Scholar]
- 35.Lau K S, Gottlieb C, Wasserman L R, Herbert V. Measurement of two serum vitamin B12 levels using radioisotope dilution and coated charcoal. Blood. 1965;36:202–208. [PubMed] [Google Scholar]
- 36.Licinio J, Gold P W, Wong M-L. A molecular mechanism for stress-induced alterations in susceptibility to disease. Lancet. 1995;346:104–106. doi: 10.1016/s0140-6736(95)92119-2. [DOI] [PubMed] [Google Scholar]
- 37.Lifshitz M S, de Cresce R P. The QM-300 protein analysis system. Clin Lab Med. 1988;8:633–642. [PubMed] [Google Scholar]
- 38.Lyketsos C G, Hoover D R, Guccione M, Senterfitt W, Dew M A, Wesch J, Van Raden M J, Treisman G J, Morgenstern H the Multicenter AIDS Cohort Study. Depressive symptoms as predictors of medical outcomes in HIV infection. JAMA. 1993;270:2563–2567. [PubMed] [Google Scholar]
- 39.McNair D M, Lorr M, Droppleman L F. Profile of mood states manual. San Diego, Calif: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 40.Milne D B, Ralston N V C, Wallionic D C. Zinc content of cellular components of blood: methods for cell separation and analysis evaluated. Clin Chem. 1985;31:65–69. [PubMed] [Google Scholar]
- 41.Root-Bernstein R S. Five myths about AIDS that have misdirected research and treatment. Genetica. 1995;95:111–132. doi: 10.1007/BF01435005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy A, Gallucci W, Avgerinos P, Linnoila M, Gold P. The CRH stimulation test in bereaved subjects with and without accompanying depression. Psychiatry Res. 1988;25:145–146. doi: 10.1016/0165-1781(88)90045-5. [DOI] [PubMed] [Google Scholar]
- 43.Sarason I G, Sarason B R, Shearin E N, Pierce G R. A brief measure of social support: practical and theoretical implications. J Soc Pers Relat. 1987;4:497–510. [Google Scholar]
- 44.Sarason I G, Johnson J H, Siegel J M. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 45.SAS Institute, Inc. SAS/STAT user’s guide, version 6. 4th ed. Vol. 2. Cary, N.C: SAS Institute, Inc.; 1989. [Google Scholar]
- 46.Skala J H, Gretz D, Waring P P. An automated continuous-flow procedure for simultaneous measurement of erythrocyte alanine and aspartate aminotransferase activities. Nutr Rev. 1987;7:731–741. [Google Scholar]
- 47.Spitzer R L, Williams J B W, Gibbon M, First M B. Structured clinical interview for DSM-III-R: non-patient version for HIV studies (SCID-NP-HIV). New York, N.Y: New York State Psychiatric Institute; 1988. [Google Scholar]
- 48.Stein M, Miller A H, Trestman R L. Depression, the immune system, and health and illness: findings in search of meaning. Arch Gen Psychiatry. 1991;48:171–177. doi: 10.1001/archpsyc.1991.01810260079012. [DOI] [PubMed] [Google Scholar]
- 49.Wilkie F L, Eisdorfer C, Morgan R, Loewenstein D A, Szapocznik J. Cognition in early human immunodeficiency virus infection. Arch Neurol. 1990;47:433–440. doi: 10.1001/archneur.1990.00530040085022. [DOI] [PubMed] [Google Scholar]
- 50.Williams J B W. Structured interview guide for the Hamilton-depression and anxiety scales (SIGH-AD). New York, N.Y: New York State Psychiatric Institute; 1988. [Google Scholar]
- 51.Zolla-Pazner S, Des Jarlais D C, Friedman S R, Spira T J, Marmor M, Holzman R, Mildvan D, Yancovitz S, Mathur-Wagh U, Garber J, El-Sadr W, Cohen H, Smith D, Kalyanaraman V S, Kaplan J E, Fishbein D B. Non-random development of immunologic abnormalities after infection with human immunodeficiency virus: implications for immunologic classification of the disease. Proc Natl Acad Sci USA. 1987;84:5404–5408. doi: 10.1073/pnas.84.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]