Abstract
Mitochondrial dysfunction plays a pivotal role in numerous complex diseases. Understanding the molecular mechanisms by which the “powerhouse of the cell” turns into the “factory of death” is an exciting yet challenging task that can unveil new therapeutic targets. The mitochondrial matrix protein CyPD is a peptidylprolyl cis-trans isomerase involved in the regulation of the permeability transition pore (mPTP). The mPTP is a multi-conductance channel in the inner mitochondrial membrane whose dysregulated opening can ultimately lead to cell death and whose involvement in pathology has been extensively documented over the past few decades. Moreover, several mPTP-independent CyPD interactions have been identified, indicating that CyPD could be involved in the fine regulation of several biochemical pathways. To further enrich the picture, CyPD undergoes several post-translational modifications that regulate both its activity and interaction with its clients. Here, we will dissect what is currently known about CyPD and critically review the most recent literature about its involvement in neurodegenerative disorders, focusing on Alzheimer’s Disease and Parkinson’s Disease, supporting the notion that CyPD could serve as a promising therapeutic target for the treatment of such conditions. Notably, significant efforts have been made to develop CyPD-specific inhibitors, which hold promise for the treatment of such complex disorders.
Keywords: Cyclophilin D (CyPD), mitochondrial permeability transition (mPTP), Alzheimer’s disease (AD), Parkinson’s disease (PD), mitochondria, neurodegeneration
1. Introduction
With the global increase in life expectancy and population, the prevalence of age-associated diseases has become a significant challenge in recent years. Among these diseases, neurodegenerative disorders stand out as a major social and health burden, and their rapid and alarming rise underscores the need for new therapeutic interventions. Aging is widely recognized as the most significant risk factor for the development of neurodegenerative disorders, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [1]. Neurodegenerative diseases can be categorized into two main types: early-onset familial cases, which are rare and account for 5% to 10% of all patients, and late-onset sporadic cases, which are more common and account for 90% to 95% of all patients. Familial cases typically manifest before the age of 65 and are caused by mutations in specific genes, such as α-synuclein, Pink1, and Parkin in PD, and amyloid precursor protein (APP) and presenilin (PS) in AD. On the other hand, sporadic cases are much more complex and result from a combination of genetic and environmental factors [2].
Neurons are highly specialized cells that require a substantial amount of energy to function properly. Therefore, it is not surprising that mitochondrial dysfunction has emerged as one of the hallmarks of dysfunctional neurons. However, the exact relationship between mitochondrial and neuronal dysfunction is not fully understood, and it remains unclear whether mitochondrial dysfunction is a concomitant cause or an indirect effect of neurodegeneration.
This review focuses on the mitochondrial protein Cyclophilin D (CyPD) and its involvement in neurodegeneration, with a particular focus on the two most spread neurodegenerative disorders, i.e., AD and PD.
2. The Superfamily of Peptidylprolyl cis-trans Isomerases
Peptidylprolyl cis-trans isomerases (PPIases), or rotamases, are conserved enzymes (EC 5.2.1.8) able to catalyze the cis-to-trans isomerization of proline residues, a rate-limiting step in protein folding [3]. The presence of an enzyme with PPIase activity was first described in 1984 by Fischer et al. [4] from porcine kidney extracts. In the same year, Handschumacher et al. [5] described the first natural receptor of the immunosuppressant Cyclosporine A (CsA) in the cytoplasm of bovine thymocytes and named it Cyclophilin. However, it was only in 1989 that the N-terminal sequencing of porcine PPIase revealed that the PPIase and Cyclophilin were the same protein [6]. Nowadays, the superfamily of PPIAses is divided into immunophilins, comprising Cyclophilins (CyPs) and FK506-binding proteins (FBKPs), and Parvulins. Parvulins, CyPs, and FKBPs constitute three distinct families of structurally unrelated PPIases whose members can be found in all cellular and extracellular compartments [7]. Moreover, it has been shown that CyPs, FKBPs, and Parvulins have different substrate specificities. In particular, CyPs and FBKPs efficiently recognize the AXPF motif, with CyPs more efficient on small hydrophobic residues (such as Ala) and FBKPs more specific on bigger residues (such as Phe or Leu) [7]. Parvulins are instead specific on the cis-trans isomerization of phosphorylated motifs such as pSer-Pro or pThr-Pro [7,8].
The enzymatic mechanism of the isomerization has not been completely established yet, and several models have been proposed [9]. It should be noted that the effective importance of the PPIase activity in vivo has been questioned, and some suggest that it could be less relevant than expected [10]. Indeed, in proteins, the cis isomers of the Xaa-Pro motifs represent only about 5–6% of the population [11], and several PPIAse-associated functions in the physiological context do not depend on their enzymatic activity [10]. In this scenario, PPIases would serve just as scaffolds or interaction hubs, and exert their function regardless of their enzymatic activity [10], which in several cases have been shown to be dispensable.
3. Generalities of CyPD
Cyclophilins were initially described as CsA-sensitive PPIases [12]. Today, there are at least 17 genes (and a plethora of pseudogenes) annotated as Cyclophilins in the human genome (Table 1) that differ from one another in terms of subcellular localization, function, and interaction clients. Indeed, while it was originally thought that CyPs were involved only in protein folding [3,4], it is now widely accepted that they exert many different roles in a wide variety of cellular processes, such as gene transcription [13], signal transduction, RNA maturation [14], host-pathogen interaction [15], and chemotaxis [16], not necessarily dependently on their PPIase activity.
CyPD, which is encoded by the ppif gene, is the only CyP present in the mitochondrial matrix. It should be noted that the nomenclature of CyPD and the relative ppif gene can generate some confusion. The ppif gene was first discovered by Bergsman et al. in 1991 [17], who named it hCyP3; the protein was then referred to as CyP3. In the subsequent years, the usage of the term CyPD referring to the mammalian mitochondrial cyclophilin became widespread, as reported by Woodfield et al. already in 1997 [18]. However, the HUGO gene nomenclature annotated improperly the gene encoding the cytosolic CyP-40 as ppid (peptidylprolyl cis-trans isomerases D) while the gene encoding for the mitochondrial CyPD was annotated as ppif (peptidylprolyl cis-trans isomerases F) [19,20]. This ambiguity can occasionally lead to confusion between CyP-40, which is also sometimes also referred to as CyPD, and the mitochondrial CyPD. Here, in line with most of the literature, CyPD will always refer to the gene product from ppif.
Table 1.
Human Cyclophilins. The human genome encodes for 17 different genes annotated as CyPs. Based on GeneCards [21] and Uniprot [22] data, the table shows for each of them the chromosomal localization, the name(s) of the respective proteins, their lengths, and subcellular localizations.
| Gene | Chromosome | Protein Name(s) | Length (AA) | Subcellular Localization |
|---|---|---|---|---|
| ppia | 7p13 | CyPA | 165 | Cytoplasm, Nucleus, ECM |
| ppib | 15q22.31 | CyPB | 216 | ER Lumen |
| ppic | 5q23.2 | CyPC | 212 | Cytoplasm |
| ppid | 4q32.1 | CyP-40, CyPD | 370 | Cytoplasm, Nucleus |
| ppie | 1p34.2 | CyP-33, CyPE | 301 | Nucleus |
| ppif | 10q22.3 | CyPD, CyP3, CyP-M, CyPF | 207 | Mitochondrial Matrix |
| ppig | 2q31.1 | SRCyP, SCAF10 | 754 | Nucleus, Nuclear Speckles |
| ppih | 1p34.2 | CyP-20, CyPH, USA-CyP | 177 | Cytoplasm, Nuclear Speckles |
| ppil1 | 6p21.2 | CYPL1, hCyPX, CGI-124 | 166 | Nucleus |
| ppil2 | 22q11.21 | CyP-60, CyC4, UBOX7 | 520 | Nucleus |
| ppil3 | 2q33.1 | CyPJ | 161 | Nucleus |
| ppil4 | 6q25.1 | PPIL4, HDCME13P | 492 | Nucleus |
| ppil6 | 6q21 | PPIL6, RSPH12 | 311 | Cytoplasm |
| ppwd1 | 5q12.3 | PPWD1 | 646 | Nucleus |
| ranbp2 | 2q13 | RANBP2, NUP358 | 3224 | Nucleus |
| cwc27 | 5q12.3 | CWC27, SDCCAG10 | 472 | Nucleus |
| nktr | 3p22.1 | CyPNK, P104 | 1462 | Cell Membrane |
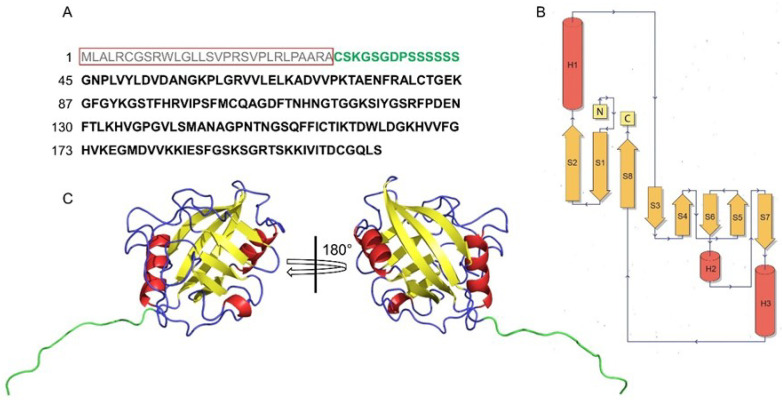
The numbering of CyPD residues (Table 2) can be quite confusing as well and has led to misunderstandings in the field. The human ppif gene encodes for a 207 residues protein whose 29-residues mitochondrial targeting sequence (MTS) is cleaved by the matrix peptidases upon entering the mitochondrion [23] (Figure 1A). Therefore, the mature CyPD consists of 178 residues. To further complicate the picture, crystal structures are based on a truncated form of CyPD lacking the first 14 residues (see below) and therefore consisting of 164 amino acids (Figure 1A). Thus, it has happened that the same residue has been numbered in literature in three different ways depending on the numbering system adopted. Here, we will number the residues according to the mature form of CyPD, reporting in brackets the numbering that takes the MTS into account when needed.
Table 2.
Numbering of CyPD. Due to the differences between the gene product of ppif, the mature CyPD, and the crystal structures of CyPD deposited in the PDB, the same residue can be numbered in at least three different ways. Here, we will mostly consider the numbering of the mature CyPD, i.e., without the mitochondrial targeting sequence (MTS). The corresponding numbering of residue i in the immature CyPD, i.e., with the MTS, will be i + 29, while in the crystal structure will be i-13. The crystal structure numbering of CyPD is the same as the numbering of CyPA.
| Mature CyPD | Immature CyPD (with MTS) |
Crystal/CyPA |
|---|---|---|
| i | i + 29 | i − 13 |
CyPD was first identified in 1992 by Connern and Halestrap [24], who described the presence of a PPIase distinct from CyPA in the mitochondrial matrix of rat livers.
It is now well-established that CyPD is a key protein in the mitochondrial proteome [25]. Indeed, it has been shown to interact with numerous diverse protein partners, including F-ATP synthase [26,27,28,29], the adenine nucleotide transporter (ANT) [30], the protein kinases GSK-3β [31] and Akt2 [32], p53 [33,34,35], the molecular chaperone TRAP-1 [36], complex III of the electron transport chain [37], and Bcl-2 [38] among others. Thus, it is not surprising that CyPD exerts many diverse biological functions in the mitochondrial homeostasis. For example, it has been shown to regulate the dynamic formation of mitochondrial supramolecular complexes such as the respirasome [37] and ATP Synthasome [39], although it should be noted that the existence of the latter is still a matter of controversy due to the large conformational changes that the ANT needs to adopt during its function [40,41]. Moreover, it was shown that it indirectly regulates the acetylation of mitochondrial proteins [42]. However, the best-characterized function of CyPD is the regulation of the mitochondrial permeability transition pore (mPTP). The mPTP is a calcium-dependent unselective channel in the inner mitochondrial membrane involved in calcium and ROS homeostasis, whose long-last opening leads to mitochondrial dysfunction and is associated with pathology [43]. For example, it has been associated with the detrimental effects of ischemia/reperfusion (I/R) of several organs, particularly the heart [44]. The initial observation that CsA inhibits pore opening [45] led to the subsequent observation that CyPD plays a crucial role as a regulator of the channel [46], even though it is not a part of its molecular structure [47]. Current evidence indicates that the mPTP is primarily formed by F-ATP synthase [28,29], although the involvement of ANT has not been excluded [43]. Indeed, it has been shown that CyPD interacts with the lateral stalk of F-ATP synthase [26], particularly with subunit OSCP (oligomycin sensitivity-conferring protein) [27], and that this interaction is associated with the opening of mPTP [27]. More details on the mPTP will be reported in a dedicated paragraph (Section 6).
4. Structural Features of CyPD
The first structure of a CyP was determined by two groups in 1991 [48,49], who independently resolved the crystal structure of CyPA. In fact, at that time, due to the great interest in the immunosuppressant properties of CsA, significant effort was put into the structural characterization of the CyPA:CsA system. Indeed, in less than two years four independent labs published about 10 structures of free and CsA-bound CyPA [50], resolving them by both NMR and crystallography. Subsequent work showed that all CyP structures shared the CLD domain [51], whose architecture is well conserved throughout evolution [51,52]. Mitochondrial CyPD structure was resolved only in 2005 [53], almost 15 years later than CyPA, while the crystal structure of CyPD:CsA complex was determined in 2007 [54]. Since then, more than 60 structures of the human CyPD have been deposited on the PDB database [55], and they are all derived from crystallography studies. It should be noted that all the CyPD structures deposited so far are based on the work of Schlatter et al. [53] and therefore they are all truncated forms of CyPD, which lack the first 14 residues (Figure 1A) and carry a lysine-to-isoleucine mutation at position 146 (133 in the crystal). So far, the only structure available of the complete mature wild-type CyPD (FL-CyPD) is the one predicted by AlphaFold [56,57] (Figure 1C), which is highly similar to the crystallographic one (RMSD 0.2–0.3 Å).
The CLD domain consists of a β-barrel formed by 8 antiparallel β-sheets that form a compact hydrophobic core [48] connected by several loops and 3 helical elements (Figure 1C): the two α-helices H1 and H3, that confine the barrel at opposite sides, and the small H2, a single helical turn located between the strands S6 and S7 also reported [54] as a 310 helix (Figure 1C).
Figure 1.
The CLD domain of human CyPD. (A) One-letter code amino acidic sequence of CyPD. The red box highlights the MTS, which is cleaved by matrix signal peptidases and therefore is not part of the mature protein. Residues in green are missing in all the crystallographic structures deposited so far in the PDB. (B) Secondary structure topology diagram of the CLD domain. Helices (H1–H3) are in red, sheets (S1–S8) are in yellow. (C) Two views of the cartoon-represented FL-CyPD predicted by AlphaFold (AF-P30405-F1), colored according to the secondary structure. Left: active site side. Right: back face side Residues in light blue correspond to the ones in (A).
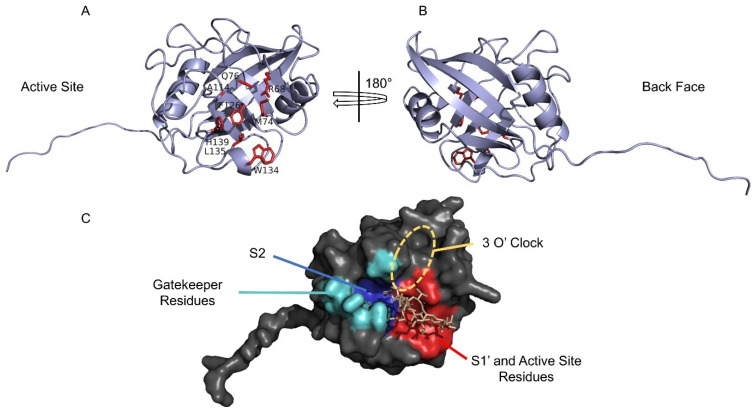
CyPD structure can be roughly divided into the active site side and the opposite face, the so-called “back face” (Figure 1C and Figure 2A,B). The back face of CyPD has been found to be a site of protein-protein interactions [52], further validating the idea of CyPs not only as PPIases but also, if not mostly, as interaction hubs.
Figure 2.
The active site and the back face of CyPD. (A–C) Different representations of the AlphaFold structure of CyPD. (A) The active site of CyPD includes a mixture of polar and non-polar highly conserved residues (sticks representation of sidechains, red). (B) The back face of CyPD is an underestimated site of protein-protein interactions. (C) The active site side includes several pockets. S1′ (red) and S2 (blue) are involved in the interaction with the substrate. The S1′ pocket, which accommodates the proline of the substrate, is surrounded by the active site residue (in red) and mediates the interaction with cyclosporine A. The S2 pocket, which is relatively non-specific, is surrounded by poorly conserved “gatekeeper residues” (cyan), which control the pocket and are therefore involved in substrate specificity. The “3 O’clock” pocket is a novel putative isoform-specific synthetic-inhibitor interaction site.
The active site of CyPs (Figure 2A) comprises highly conserved residues: these include the catalytic arginine Arg68 (R97) and a mixture of non-polar and polar residues, i.e., Met74 (M103), Gln76 (Q105), Ala114 (A143), Phe126 (F155), Trp134 (W163), Leu135 (L164), and His139 (H168). Altogether, they form an extensive binding surface measuring ~10 Å along the R68-H139 axis and ~15 Å along the W134-A114 axis [52]. It has also been observed that W134, the only tryptophan present in CyPD, is required for CsA binding and that this is abrogated by all other natural variants occurring at this position, with the exception of histidine [52]. Careful analysis of the substrate-binding surface reveals that it is formed by two different pockets (Figure 2C), the S1′ pocket, comprising the highly conserved proline-binding residues, and the S2 pocket, which mediates the interactions with substrate residues P2 and P3 (relative to the substrate proline P1) [52]. The CyPD S1′ pocket is formed by Phe126 (F155) at its base and Phe73 (F102), Met74 (M103), Leu135 (L164), and His139 (H168) at its sides [52]. On the other hand, the S2 pocket surface is formed by the main-chain atoms of the S5-S6 loop, including mostly the residues between Met113 (M142) and Gln124 (Q153), making it relatively uniform across CyPs. The pocket surface is critically guarded by a set of gatekeeper residues (Figure 2C), whose sidechains control the access to the active site and therefore determine substrate specificity [52]. This set of residues is less conserved and would explain the different substrate specificities among all CyPs. For CyPD, the gatekeeper residues include Thr86 (T115), Ser94 (S123), Arg95 (R124), Ala116 (A145), Thr120 (T149), Ser123 (S152), and Gln124 (Q153) [52].
Lastly, the design of a novel CyPs inhibitor led De Simone et al. to describe an additional pocket perpendicular to the active site, called the “3 O’clock” pocket [58]. The 3 O’clock pocket is formed by residues not involved in substrate binding and therefore poorly conserved between different CyPs, which would represent a good candidate for isoform-specific CyPs inhibitors [58].
5. Cyclosporine A
Cyclosporine A (CsA) is a cyclic undecapeptide produced by the ascomycete Tolypocladium inflatum. Ever since the discovery of its immunosuppressive activity, first reported in 1976 by Borel et al. [59], it has been used to prevent the rejection of transplanted organs. The immunosuppressive function of CsA specifically involves the suppression of the activation of T cells, which starts with its binding to the cytosolic Cyclophilin A (CyPA) in T cells [60]. The CyPA:CsA complex binds and inhibits in a PPIase-independent manner the phosphatase activity of calcineurin which is required for the activation and nuclear translocation of NF-AT, a transcription factor that activates the transcription of cytokine genes, including IL-2 and IL-4 [60].
CsA has a high hydrophobic character, due to the N-methylation along the protein backbone of 7 residues out of 11 [61]; only a single hydroxylic group is present [61]. It was observed that in apolar (chloroform) [62] and polar (methanol, DMSO) [63] solvents CsA adopts a twisted β-sheet conformation with three intramolecular hydrogen bonds, similar to what is observed in the crystal structure [62]. The solution structure of the CyPA-bound CsA [50,64] revealed remarkable differences with free CsA, as it adopts no regular secondary structure and contains no intramolecular hydrogen bonds [61]. On the other hand, it has been shown that CsA-bound CyPA [65] and CyPD [54] are very similar to their CsA-free counterparts, i.e., do not undergo major conformational changes upon binding. Moreover, the CyPA:CsA and CyPD:CsA complexes have the same geometry (RMSD of 0.5 Å), in which CsA binds the active site of CyP [54]. The mechanism of PPIase inhibition by CsA has been explained in terms of the twisted-amide model and catalysis through distortion [12].
Although Cyclophilins were first defined as intracellular receptors of CsA [12], it should be noted that it was recently demonstrated that not all the proteins annotated as CyPs in the human genome are able to bind CsA [52]. In particular, PPIL2, PPIL6, RANBP2, and SDCCAG-10, all lacking the catalytic tryptophan, do not display either Cyclosporin binding or PPIase activity [52].
Independent data revealed that the affinity of CsA towards CyPs is in the nanomolar range. Specifically, the was determined to be in the 10–200 nM range [66], while the was found between 6.8 nM and 588 nM [52]. For CyPD, the determined with isothermal calorimetry is 6.7 nM [67], while the determined by fluorescence analysis was found 12.5 nM [53].
6. The Permeability Transition Pore
As stated above, CypD is the master regulator of the mitochondrial permeability transition pore (mPTP), an unselective, high-conductance channel, whose opening is activated by calcium, oxidative stress, and membrane depolarization [68]. The channel exhibits a maximal conductance of up to 1.3 nS and a variety of subconductance states with variable durations [68]. The consequences of PTP opening depend on the open time of individual pores (channel kinetics) and the number of open pores at any given time (population dynamics) [68]. The high conductance pore, when fully open, allows the passage of large solutes, with MW up to 1.5 KDa [69]. Extensive and prolonged mPTP activation triggers the collapse of the mitochondrial protonmotive force (pmf), thus inhibiting the oxidative phosphorylation, and the flood of a mitochondrial matrix with cytosolic solutes, leading to mitochondrial swelling and eventually rupture of the outer mitochondrial membrane. This latter event may cause cell death by necrosis [70] or the release of proapoptotic proteins, thereby inducing apoptosis or similar processes [71].
CyPD modulates the mPTP by decreasing the Ca2+ load required for pore opening, an effect that is prevented in the presence of CsA [46]. Several laboratories successfully created CyPD-null (Ppif−/−) mice [47,70,72], which showed no overt phenotype and no noticeable changes in mitochondrial function. As expected, the mPTP in Ppif−/− mitochondria was desensitized to Ca2+ and was insensitive to CsA. Importantly, largely through the use of Ppif−/− mice, a wide variety of murine models of human degenerative diseases were created that allowed to demonstrate the involvement of mPTP misregulation in several pathologies [73,74,75,76,77,78,79,80].
Although the molecular identity of the mPTP is a long-standing mystery, recent efforts have made it possible to obtain a definitive model of the pore components. The original observation [81] that PTP opening was affected by the selective inhibitors of the adenine nucleotide translocator (ANT), atractylate (ATR, which favors PTP opening), and bongkrekate (BKA, which favors PTP closure) suggested the involvement of the ANT in mPTP formation. An early hypothesis viewed the mPTP as a multiprotein complex including the core constituents ANT and voltage-dependent anion channel (VDAC) associated with hexokinase 2 and CyPD acting as modulators [82]. However, this model did not stand the test of genetics, as reviewed by Bernardi et al. [83]. After extensive debate and experimental work [84,85,86,87], the most recent model proposes a dual nature of mPTP formation, one involving F-ATP synthase as the leading mPTP pathway and the other involving the ANT [43]. This is based on the evidence that in the absence of a fully assembled F-ATP synthase, a small CsA- and BKA-sensitive channel still forms [88].
F-ATP synthase (Figure 3) is a ~600 kDa multi-subunit enzyme that comprises two moieties, the membrane-embedded Fo sector, and the catalytic F1 sector, which are connected by two stalks, namely the peripheral stalk, which is structurally part of the Fo moiety, and the central stalk, which is related to the F1 sector. It should be noted that F-ATP synthase forms dimers within the inner mitochondrial membrane [89] via Fo-Fo interactions [90,91] that organize into long rows of oligomers that maintain the typical cristae morphology [92].
The globular evolutionary-conserved F1 sector is composed of 3 α- and 3 β-subunits that alternate in surrounding the central stalk, which in Mammals comprises subunits γ, δ, and ε [89]. According to Boyer’s catalysis model [93], the Fo sector allows the flow of H+ down their electrochemical gradient generated by the respiratory complexes across the membrane [94]. This induces the rotation of the c-ring that in turn generates a torque of the central stalk transmitted to the F1 sector where the ATP synthesis occurs [94]. Subunit γ rotation within α3β3 takes each of the three β subunits through the conformations βDP (bound to ADP), βTP (bound to ATP), βE (empty) thereby synthesizing three Mg2+-ATP molecules during each 360° rotation [95,96]. F-ATP synthase is a reversible rotary machinery able to hydrolyze ATP generating a pmf [89]. Both in the synthesis and in the hydrolysis directions, Mg2+ is essential for catalysis, and it can be replaced by other divalent cations, such as Mn2+ and Co2+ [97,98]. Intriguingly, Ca2+ ions only sustain ATP hydrolysis by F1 which is associated with proton translocation [99] and not coupled with the generation of pmf neither in Bacteria [98] nor in Mammals [97].
In humans [100], the Fo sector comprises 8 copies of subunit c, which is arranged in a circular structure (c-ring), and subunits a, A6L (ATP8), e, f, g, DAPIT(k) and 6.8PL(j). Subunits a and c form two semi-channels that allow the H+ flow by protonation/deprotonation reactions which involve conserved amino acid residues [94]. The cryo-EM structure of mammalian F-ATP synthase recently resolved by Sazanov’s group [101] revealed that subunit e, located at the basis of the peripheral stalk, is structurally linked to the lipid plug at the center of the c-ring. The mammalian peripheral stalk comprises subunits b, F6, d, and OSCP, and it contacts subunits a, A6L, f, e, and g of the membrane-embedded part of the Fo sector [102,103].
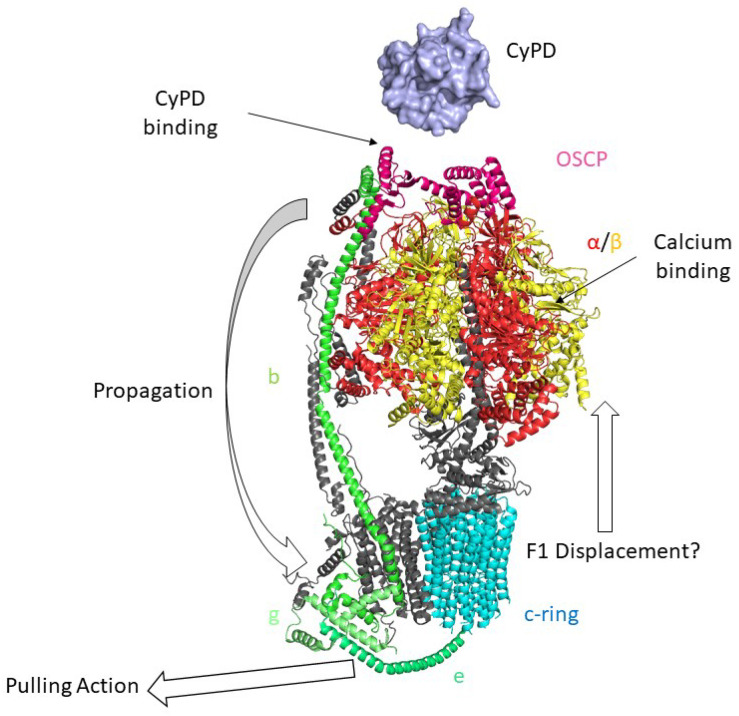
Figure 3.
Mammalian monomeric F-ATP Synthase and the “Death Finger” Hypothesis of mPTP formation. Cryo-EM structure of F-ATP Synthase is shown (PDB ID: 6TT7 [101]). According to the death finger hypothesis, calcium binding to the α (red)/β (yellow) subunits induces conformational changes that are transmitted from OSCP (pink), the binding site of CyPD (light blue, PDB ID: 2Z6W [54]) to the lateral stalk of F-ATP synthase (subunit b in light green) up to the g (lime) and e subunits (dark green). This would lead to the removal of lipids within the c-ring (cyan) and the displacement of the F1 sector from the Fo, leading to mPTP opening.
Structural and functional coupling of the Fo and F1 sectors is guaranteed by the OSCP subunit, since it prevents the co-rotation of the α3β3 subcomplex with the γ subunit during the proton flow, thereby ensuring enzyme efficiency [103]. Moreover, this coupling ensures enzyme sensibility to oligomycin, an antibiotic produced by Streptomycetes which binds the c-ring preventing its rotation [104], which is widely used in experimental characterization of mitochondria and F-ATP synthase.
The first clue that the F-ATP synthase could be involved in mPTP formation was the demonstration that CyPD interacts with the OSCP subunit of the F-ATP synthase peripheral stalk [26,27]. CyPD binding required relatively high concentrations (10 mM) of Pi, which probably acts by charge neutralization favoring the electrostatic interactions between the two proteins, and resulted in partial inhibition of ATP synthesis and hydrolysis; CsA dissociated CyPD from the F-ATP synthase removing this inhibitory effect [26]. A few years later clear evidence was obtained that a Ca2+-dependent channel can form from F-ATP synthase [27,105], as now supported by a variety of electrophysiological studies using highly purified enzyme preparations from bovine [28] and porcine [29] hearts. Based on extensive mutagenesis studies [106,107,108,109,110,111,112] and structural work [101] the “death finger model” (Figure 3) has been proposed as a plausible mechanism through which F-ATP synthase could generate a channel [43,113,114]. According to this model, binding of Ca2+ at the catalytic metal site located at the α/β subunits interface [106] causes a spatial rearrangement of the F1 sector that is transmitted from the OSCP subunit to the peripheral stalk at the point of entrance into the membrane, where subunit b forms a hairpin tightly associated with the C- and N-termini of subunits g and e, respectively. The C-terminus of subunit e, which contacts the c-ring, exerts a pulling effect on the lipids allowing formation of a channel within the c-ring by displacing the outer lipid plug. Binding of CyPD or of its chemical mimic benzodiazepine (Bz)-423 [27] favors the Ca2+-induced mechanical stress on OSCP, overcoming its ‘shock absorber’ function during rotation of subunit c.
Recently it has been observed that the synthetic c subunit of F-ATP synthase under high calcium concentrations adopts a cross-β conformation with channel properties influenced by CyPD [115,116]. This led to suggest an alternative but not mutually exclusive mechanism of mPTP formation in the context of neurodegenerative diseases that involves the participation of the c subunit together with mitochondrially targeted amyloid peptides Aβ and α-synuclein [117], whose involvement in pathology will be described in the following chapters of this review.
The role of the PPIase activity of CypD on the PTP modulation is still debated. It was originally suggested that CypD activates the PTP independent of its PPIase activity [118]. On the other hand, Ppif−/− fibroblasts were protected against oxidative stress, and this protection was lost in cells expressing a wild-type CypD but not in cells expressing the inactive R96G CypD, suggesting that the PPIase activity of CypD facilitates PTP activation [72]. However, the exact mechanism is unknown. Future studies will be needed to address the role of the PPIase activity in regulating the PTP. Interestingly, in a recent study the CyPD PPIase activity, measured in different tissues including the brain, correlates with an increased ATP synthasome assembly (an ATP synthase supercomplex containing ANT and the phosphate carrier) and the formation of high-order ATP synthase oligomers, decreasing the probability of PTP formation [39].
More details on mPTP can be found in recent specific reviews [43,119].
7. Post-Translational Modifications of CyPD
Post-translational modification (PTM) of proteins dramatically expands the cellular proteome and enables rapid responses to changes in the biochemical environment and fine-tuning of cellular metabolism [120]. Here, we will describe the principal PTMs that affect CyPD and its function within the mitochondrial proteome, i.e., acetylation, phosphorylation, and oxidation (Figure 4). These studies have mainly been performed in the heart [121,122,123,124,125,126] and liver tissues [127] and/or in cellular models [31,32,128], while very few data on CyPD PTMs have been obtained in the brain and especially in neurodegenerative disorders [129], a gap that would worthwhile to be filled.
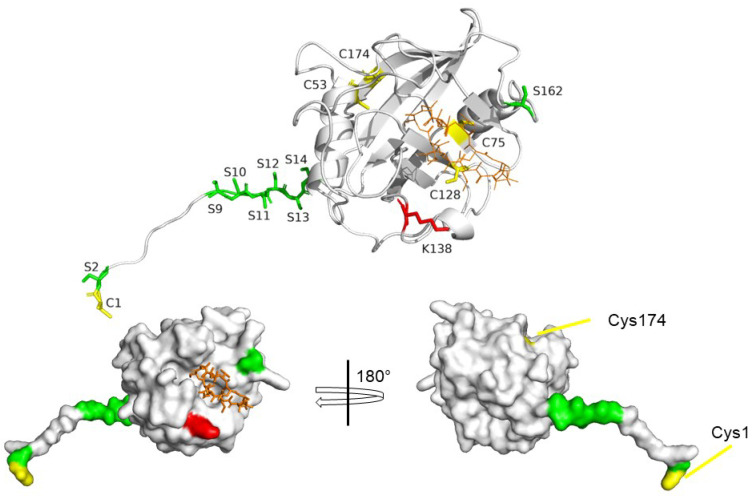
Figure 4.
Post-Translational Modification of CyPD. Cartoon (upper panel) and surface (lower panel) representation of CyPD (AlphaFold structure) active site side (upper panel, lower left panel) and back face side (lower right panel) and PTM target sites. Red: acetylation site. Yellow: cysteine residues. Green: serine residues involved in protein phosphorylation.
7.1. CyPD Acetylation
Protein acetylation refers to the transfer of the acetyl group of an acetyl coenzyme A (Ac-CoA) molecule to either the N-terminus of the polypeptide chain or the ε-amino group of lysine residues, which reduces the positive charge of the protein target [130]. The removal of the Ac-CoA from the protein is exquisitely enzymatic, being mediated by protein deacetylases. Mitochondrial deacetylases comprise three NAD+-dependent sirtuins, i.e., SIRT3, SIRT4 and SIRT5 [131]. While SIRT3, has the most robust deacetylase activity [132], SIRT4 and SIRT5 are thought to contribute to mitochondrial deacetylation to a lesser extent [42].
Lysine acetylation has been shown to be a widespread modification in the mitochondrial proteome: about 20% of mammalian liver [133] and heart [42] proteins have been found acetylated at a basal level, and more than 60% of the mitochondrial proteins contain acetylation sites [132]. Intriguingly, it has been demonstrated that CyPD removal alters the cardiac mitochondrial acetylome [42].
CyPD itself is a target of acetylation, in particular at the highly conserved Lys138 (K167 in human/K166 in murine) [121]. It has been demonstrated both in vitro and in vivo that acetylated CyPD is a substrate of SIRT3, and that CyPD acetylation is associated with an increase in mPTP opening [121,122,123,127,129]. The acetylated Lys138 is located in the catalytic site of CyPD, which is thought to be involved in the interaction with OSCP [27]. This interaction is sensitive to salts [27] at a concentration similar to the physiological one [134], suggesting that the acetylation of Lys138 enhances the interaction between CyPD and OSCP by lowering the sensitivity of the CyPD/OSCP complex to ionic strength. These findings are in line with the observation of a highly positively charged OSCP surface (pI = 9.97) in the F-ATP synthase structure [101].
7.2. CyPD Phosphorylation
Protein phosphorylation [135] involves the covalent introduction of a dianionic phosphate group to specific residues, a process that is mediated by protein kinases [136] and is reversed by protein phosphatases [137].
Different CyPD phosphoforms have been proposed [31,32,124,125,127]. While it is clear that the different phosphoforms have different effects on mPTP opening, much research is still needed to thoroughly characterize them all. In particular, it has been shown that CyPD is a substate of glycogen synthase kinase 3β (GSK-3β) [31], which phosphorylates CyPD and sensitizes mPTP opening [31,127,138]. It has been independently shown that Ser162 (S191) phosphorylation increased undoubtedly both the binding of CyPD to the OSCP subunit of F-ATP synthase and mPTP opening [124]. Intriguingly, the authors found that mutating the stretch of six serine residues at the N-terminus Ser9-14 (S38-43) into a stretch of six alanine residues increased the global phosphorylation levels of CyPD, probably at Ser162 (S191) and decrease mPTP propensity to open, suggesting that Ser9-14 may act as a negative regulator of the phosphorylation status of CyPD [124]. Similarly to the acetylated Lys138 (K166), Ser162 (S191) is located to the CsA-binding site side of CyPD, i.e., at the putative OSCP interacting surface. Both serine phosphorylation and lysine acetylation lower the positive charge of the respective target residues, suggesting that these two modifications co-operate to strengthen the interaction with OSCP by changing the electrostatic surface of the interaction site. Moreover, in silico studies suggested that both the phosphorylation of Ser162 (S191) and the acetylation of Lys138 (K166) affects the conformational ensemble of CyPD [139].
On the other hand, it has been reported that the germline deletion of the mitochondrial calcium uniporter (MCU) resulted in an increase in phosphorylation of CyPD at Ser13 (S42), and that this was associated both with an increase in CyPD binding to F-ATP synthase and mPTP propensity to open in heart cells [125]. These results seem in apparent discrepancy with those reported by Hurst et al. [124] and are difficult to rationalize on structural bases, as Ser13 is located far from the catalytic site. It is noticed, however, that: (i) the immunoprecipitation experiments were performed on the complete F-ATP synthase by Parks et al. [125], and on a single OSCP subunit by Hurst et al. [124], then one could hypothesize that phosphorylation of CyPD at Ser13 could also mediate its interaction towards another subunit of F-ATP Synthase (yet to be identified) other than OSCP, thus increasing the sensitization of mPTP opening; (ii) all the experiments performed by Parks et al. were carried out on murine cells, in which there are only three serine residues in tandem, while in human CyPD there are six (Ser9-14), thus suggesting that the regulation of phosphoCyPD-dependent mPTP opening in murine CyPD and human CyPD could be slightly yet at the same time strikingly different. In both cases, the N-term of CyPD appears to play a critical role.
It has also been reported that in patient-derived tumors phosphorylation of the N-terminal Ser2 by the kinase Akt2 has been associated with resistance to cell death [32]. Similarly to Ser9-14, the serine found in this work is structurally far from the active site of CyPD: it would be worth investigating how a point mutation at this site could lead to such a dramatic effect as the loss of enzymatic activity [32].
Altogether, it is clear that multiple phosphoforms of CyPD exist and that each has a different effect on the regulation of mPTP opening. While it was shown that CyPD is a target of at least two cytosolic kinases, i.e., GSK-3β [31] and Akt [32], that under specific conditions translocate within the mitochondrial matrix, there are still some fundamental data missing, such as: (i) what are the actual phosphoforms of CyPD, i.e., could multiple serine/threonine residues be phosphorylated at the same time? (ii) What is the stoichiometry of the different phosphoforms of CyPD? (iii) How can the N-terminal serine residues affect CyPD phosphorylation status and its regulation of the mPTP? (iv) What are the mechanisms that induce the translocation of cytosolic kinases within the mitochondrial matrix?
7.3. Oxidative Modifications of CyPD
A number of PTMs involve the chemical modification of proteins mainly at the level of cysteine residues [140] due to the reaction of their thiol group with reactive oxygen species (ROS), which include hydrogen peroxide, superoxide anion and the hydroxyl radical, and reactive nitrogen species (RNS), which include nitric oxide and its derivative peroxynitrite [141]. Mitochondria are the prime contributor of both ROS [142] and peroxynitrite [143].
The oxidative modification of CyPD has been investigated in the literature, with a particular focus on cysteine residues [126,128,144,145]. Of the five cysteine residues in the mature CyPD, Cys1 (C30) and Cys128 (C157) are solvent-exposed, while Cys53 (C82), Cys75 (C104), and Cys174 (C203) are more buried (Figure 4). According to the CyPD crystal structure [53,54], no disulfide bonds are observed, although the pairs Cys53-Cys174, located on the back face of the protein, and Cys75-Cys128 on the opposite side of the protein are close to each other. A closer analysis of the X-ray structure reveals that the first cysteine pair also displays a rather favorable side-chain geometry for S-S formation. It was Linard et al. who first reported that CyPD could be oxidized, that upon oxidation PPIAse activity is reduced by 20% and that Cys174 (C203) was the oxidation site [144]. The authors also suggested the formation of the Cys128-Cys174 disulfide bridge, but they could not detect it via mass spectrometry. Indeed, such a disulfide bridge would entail dramatic structural rearrangements of the protein which are quite unlikely to happen, especially considering that both Cys174 and Cys128 have other proximal cysteine residues and that such a conformational reorganization unlikely would retain the PPIase activity.
The role of the oxidative modifications of Cys174 (C203, C202 in murine models) on CyPD function, particularly on mPTP regulation, has been extensively investigated by Murphy’s group [126,128]. They found that treatment with a nitric oxide donor (GSNO) or mutation of Cys174 into a serine-desensitized H2O2-induced mPTP opening [128]. Moreover, the C174S mutants display a decreased binding on F-ATP synthase upon oxidation, compared to the wild-type counterpart [126]. Given that treatment with GSNO was effective only in wild-type cells, they concluded that Cys174 can be S-nitrosylated and that this PTM inhibits mPTP opening, as observed in ischemic preconditioning experiments [146]. The same group found that Cys174 (C203) is also a site of S-acylation and that the removal of the acyl group is necessary for mPTP opening [126], indicating the integration of multiple signals that converge on the modification of Cys174. Indeed, it has also been found that CyPD is part of the mitochondrial thioredoxin system [145], which indicates that CyPD oxidation is finely tuned within the mitochondrial matrix.
To conclude, also in the case of oxidative PTMs, there are several questions waiting to be answered. In particular, the structural modifications upon Cys174 (C203) oxidation and its relation to the regulation of mPTP should be addressed.
8. CyPD in Brain
Although CyPD is ubiquitously expressed in human tissues, its expression levels are tightly regulated and vary significantly among different districts [147,148,149]. Indeed, it has been found that while liver [147,148,149] and heart [149] mitochondria undergo CsA-sensitive swelling upon calcium treatment, adult brain mitochondria are highly resistant to this phenomenon [147,148,149], although both liver and brain mitochondria are able to swell, similarly, upon treatment with the channel-forming peptide alamethicin [147]. It was demonstrated that brain mitochondria resistance to swelling depends on the reduced CyPD levels, which were found almost three times lower than in heart and liver mitochondria [149]. More in detail, CyPD expression levels were found downregulated in mature (3-months old) rats when compared to newborn (1-day old) rats [149], which was also associated with different propensities to CsA-sensitive mPTP opening (lower in 3-months mice than in 1-day mice) [149]. Moreover, neuronal precursor PC12 cells treated with the differentiation factor NGF showed a dramatic reduction in CyPD expression level, confirming its differentiation-dependent down-regulation in mature neural cells [149]. Independently, another group found that neural CyPD levels increase during aging in both mice and humans [150,151,152] and that this correlates with calcium-handling capacity [150]. It should also be noted that Ppif−/− mice showed neurological symptoms, i.e., an increase in anxiety-type behavior [153]. This could be both a direct or indirect effect of the absence of CyPD since they also had an abnormal accumulation of white adipose tissue [153] which has been shown to be associated with an increase in anxiety-type behavior [154].
9. The Involvement of CyPD in Alzheimer’s Disease
AD represents the most common form of dementia [155]. The progressive neuronal and synaptic loss typical of AD has two prominent pathological features, i.e., the abnormal accumulation of amyloid β peptide (Aβ) and the phosphorylation of tau protein in the brain [155]. The neurotoxic Aβ peptide is the product of amyloid precursor protein (APP), a transmembrane protein, cleaved by β and γ secretase (presenilins) [156]. APP cleavage products form the pathological amyloid plaques [157]. According to the “amyloid cascade hypothesis” [158], Aβ accumulation is the primary cause of AD. While at first the emphasis was put on the toxicity of extracellular amyloid plaques, the attention was then switched to the pathological intracellular events associated with Aβ that ultimately resulted in plaque formation [157]. Indeed, it has been found that Aβ accumulates in several subcellular compartments, including mitochondria [159], which is associated with a dampening of cellular respiration [160] and an increase in oxidative stress [161]. Although the amyloid hypothesis has been challenged [162] particularly due to the failure of anti-Aβ therapies [163], Aβ-mediated cellular dysfunction is still a relevant feature of the AD pathology. Its presence in non-AD individuals [164] suggests its possible involvement in the very first subtle steps of AD pathogenesis. On the other hand, tau is a microtubule-binding protein usually found in axonal and dendritic microtubules [155,165]. Hyperphosphorylation of tau, a hallmark of AD and other neurological pathologies, induces its re-localization within the neuron and its aggregation into the toxic neurofibrillary tangles [155,165].
It has been found that CyPD and Aβ interact within AD brain mitochondria. Surface plasmon resonance analysis revealed a specific interaction between recombinant Aβ(1-42) and CyPD in the nanomolar range (164 nM), which was even higher in the case of Aβ(1-42) oligomers (4 nM) [150]. CO-IP and immunochemistry experiments confirmed the presence of this interaction in the AD brains of both transgenic animal models and human patients [150,166]. The structural features of these interactions have not been described, i.e., the CsA-sensitivity of the interaction has not been tested, so it is not possible to conclude whether it occurs on the active site side or the back face of CyPD. Indeed, from this point of view, only an in silico prediction of the interaction between CyPD and Aβ has been made, but the conclusions were only drawn from a subcellular localization and an energetic point of view [167].
Nevertheless, the pathophysiological consequences of the CyPD/Aβ interaction have been thoroughly investigated. The age-associated increase in CyPD expression levels observed in the brain have been found exacerbated in the context of AD, specifically in an Aβ-rich context, as it was observed in neurons in the temporal cortex and hippocampus neurons but not in the cerebellum [150,166]. Specifically, elevated Aβ levels are associated with an age-dependent dysregulation in calcium handling, an increase in mitochondrial swelling, mPTP opening, and cytochrome c release, all of which are reversed by CsA treatment or CyPD knockout [150,152,166]. Intriguingly, Ppif−/− mAPP mice, i.e., murine models of AD [168], exhibited a strong learning and spatial memory capacity than mAPP mice [150]. Indeed, the PKA/CREB signaling pathway, which is associated with synaptic plasticity [169], was found impaired in a CyPD/Aβ-dependent manner, and pharmacological or genetic inhibition of CyPD restored the axis activity in the context of AD [170]. Intriguingly, it has also been shown that CyPD levels decrease with the knock-out of tau and increase with its overexpression [171], strengthening the link between mPTP opening and the development of AD.
While brain CyPD levels steadily increase with age, the OSCP subunit of F-ATP synthase follows the exact opposite trend [152,172]. The reduction in OSCP levels, which is specific and does not concern other F-ATP synthase subunits, is also observed in the context of AD [172], which intriguingly has also been observed in AD patient-derived fibroblasts [173]. OSCP knock-down is associated with an increase of CsA-independent mPTP opening [172], consistent with the findings of other research groups [110], which is probably due to the opening of ANT-mediated mPTP [110]. Moreover, it has been found that Aβ interacts with OSCP [172] and that OSCP/CyPD interaction increases during aging [152], which is once again exacerbated in the context of AD [174].
The specific reduction in OSCP levels suggests a post-translational degradation of the protein since (i) OSCP knockdown also induces changes in the expression of other F-ATP synthase subunits [110] and (ii) the increase in CyPD expression was associated with an increase of OSCP ubiquitination [174], although other degradation pathways could be involved.
Despite the above studies reporting a detrimental effect of CyPD-mediated mPTP opening, it should be noted that it has also been associated with an important physiological role, i.e., the filopodiagenesis of neurons [175], which is impaired upon inhibition of CyPD. Similarly, it has been shown that an aberrant increase in SIRT3 expression, which promotes deacetylation of CyPD, hampers the mPTP flickering and is associated with motor impairment in a murine model of spastic paraplegia [129]. Moreover, CyPD knock-out impairs mPTP role in calcium handling, which was shown to lead to an increase of mitochondrial calcium and ultimately the activation of phospholipase A2, which induces the production of free fatty acids that impair oxidative phosphorylation and decreases the ATP levels [176]. Therefore, while CyPD inhibition has been shown to have ameliorative effects on the AD phenotype, it should be emphasized that it could also have detrimental effects on mitochondrial function, which should be preserved. Accordingly, two important pieces of information are missing. First, little attention has been put into characterizing the detailed structural features of the interaction between CyPD/Aβ/OSCP, which could be directly addressed with specific inhibitors without dramatically altering physiological mPTP opening and/or mitochondrial calcium handling. Second, CyPD PTMs in the context of AD have never been investigated. Specifically, we believe that both CyPD oxidation and CyPD acetylation could play a role in the development of AD, given that: (i) oxidative stress is a hallmark of AD [161,177] and, accordingly, treatment with antioxidants has been shown to be protective from Aβ-mediated insult similarly to genetic inhibition of CyPD [170]; (ii) aging and aging-associated diseases are characterized by a decline in NAD+ levels [178], which can lead to an inhibition of SIRT3 and an increase in CyPD acetylation [121], which induces mPTP opening [121]. Indeed, it has been shown that both brain mitochondrial acetylome and phosphoproteome are altered in AD [179,180], although little is known about the acetylation and phosphorylation status of CyPD in this compartment. Altogether, this would create a vicious cycle in which aberrant acetylated CyPD-dependent mPTP opening exacerbates oxidative stress, favoring oxidation of CyPD which, in turn, increases long-lasting mPTP opening. The investigation of these aspects could offer new insight into the participation of CyPD in the pathogenesis of AD.
10. The Involvement of CyPD in Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by a chronically progressive, age-related, fatally invalidating movement disorder. Although several neuronal types are affected, dopaminergic neurons in substantia nigra pars compacta (SN) are among the first neurons to degenerate, leading to dopamine depletion in the striatum and motor symptoms [2]. The vast majority of PD cases are considered idiopathic, for which the greatest risk factor is aging, as demonstrated by epidemiological quantitative evidence showing a closely linked connection between aging and PD [181]. Hallmarks of PD include the misfolding and aggregation of α-synuclein (α-Syn) into intracytoplasmic protein deposits known as “Levy bodies” (LB), oxidative stress leading to oxidized dopamine, mitochondrial dysfunction, and inflammation, all features that are facilitated by the age-related metabolic alterations [2,182,183].
Despite the great number of studies, the molecular mechanisms underlying dopaminergic neuronal death are still debated. As LB or an earlier oligomeric form of α-Syn are thought to be toxic [184,185] and able to spread in synaptically coupled brain networks [186,187], it has been proposed that LB triggers neuronal dysfunction and death. Consistently, a point mutation in the SNCA gene encoding α-Syn or duplication/triplication of SNCA increases the risk of developing PD [188,189]. However, post-mortem analysis of human brains showed that the correlation between LB and neuronal death is weak [183].
An alternative (and not mutually exclusive) hypothesis is that the loss of dopaminergic neurons is driven by mitochondrial dysfunction, leading to bioenergetic failure, oxidative stress, increased mtDNA damage, and mitophagy defects [182]. Mitochondria became suspects in PD etiology by the discovery that complex I activity is reduced in the SN [190] and skeletal muscle [191] of PD cases. Moreover, treatment of dopaminergic neurons with complex I inhibitors, notably 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP) and the pesticide rotenone, leads to increased ROS production [192], and activation of the NLRP3 inflammasome, which triggers the subsequent secretion of interleukin 1β [193], ultimately resulting in neuronal damage. Indeed, the use of these inhibitors is the basis of several animal and cellular models of PD. Consistently with these observations, a recent study demonstrated that selective disruption of complex I in mouse dopaminergic neurons by intersectional genetics was sufficient to cause progressive, human-type parkinsonism [194].
Another piece of evidence for a major contribution of mitochondrial dysfunction to motor deficits comes from studies of familial cases of PD. Recessive, early-onset forms of PD are linked to loss of function mutations in three gene products that are directly involved in mitochondrial biology, i.e., PINK1, encoding a mitochondria-targeted kinase, and Parkin/PARK2, encoding an E3 ubiquitin ligase, both involved in mitophagy and mitochondrial biogenesis and DJ-1, encoding a redox-regulated chaperone essential for oxidant defenses [2]. For example, in dopaminergic neurons derived from a patient with a homozygous mutation in DJ-1, mitochondrial oxidative stress triggers a pathogenic cascade that ultimately results in lysosomal dysfunction and α-Syn accumulation [195]. Mutations in genes associated with dominant forms of PD have also been linked to mitochondrial dysfunction. These include: (i) SNCA, encoding α-Syn [196,197], (ii) GBA, encoding the lysosomal glucosylceramidase β, which participates in glycolipid metabolism [198], and (iii) LRRK2, a kinase associated to the outer mitochondrial membrane that is involved in mitophagy [199].
Dopaminergic neurons in the SN are considered more vulnerable to mitochondrial dysfunction than other neuronal cells, due to their morphology (long and highly branched axon) and distinctive physiology, characterized by high and slow oscillations in intracellular calcium concentration triggered by the opening of the plasma membrane Cav1 calcium channels and the release of calcium from endoplasmic reticulum stores [183]. Different major factors underlying declining mitochondrial function have been proposed. According to one hypothesis [183], the typical calcium oscillations promote calcium entry into mitochondria at specialized junctions with the endoplasmic reticulum (MAMs) [200], stimulating the OXPHOS activity in the absence of strong ATP demand and leading to mitochondrial hyperpolarization, which causes an increase of ROS production and diminishes the autophagic capacity of neurons and their ability to deal with misfolded α-Syn fibrils [201]. However, in a recent clinical trial, a limited effect of the L-type channel inhibitor dihydropyridine isradipine was observed in newly diagnosed patients with PD [202], despite in dopaminergic neurons this drug reduced oxidative stress, mitochondrial and lysosomal dysfunctions, and α-Syn toxicity [203,204].
The involvement of the mPTP in the PD mechanisms has also been proposed, particularly its long-lasting opening, which causes mitochondrial depolarization, blocking of ATP production and mitochondrial swelling, and ultimately cell death [43]. Indeed, complex I toxins that increase ROS production also activate mPTP [205,206], while the inactivation of CyPD is beneficial in preserving mitochondrial bioenergetic in an acute insult model of MPTP-induced dopaminergic neurotoxicity [207]. Moreover, a solid piece of evidence in favor of the mPTP as a major contributor to PD pathogenesis comes from a study showing that genetic ablation of CyPD in PD-linked α-Syn mutant transgenic mice delayed the disease onset and extended lifespan, thus providing a demonstration that CyPD-induced mPTP regulates the PD development [208]. Interestingly, a recent study reveals that neurons bearing a PD-linked mutation in LRRK2 [209] or GBA [198] genes show NAD+ shortage, possibly as a consequence of the elevated activity of NADase SARM1, which has been observed to be reduced in neuronal cells from PD patients [210]. As observed above, NAD+ deficit could impair SIRT3 activity [211], thus increasing CyPD acetylation, which in turn is more prone to activate the mPTP [121], a hypothesis that has not yet been tested in animal or cellular models of PD. On the other hand, in vitro NMR experiments demonstrated that a truncated form of recombinant CyPD interacts directly with the proline-rich C-terminal domain of the soluble α-Syn, preventing its aggregation, as well as with preformed α-Syn fibrils favoring their disassembly, apparently mediated by the CyPD enzymatic activity [212]. While preventing protein disaggregation can be viewed as advantageous, disaggregation can produce small oligomeric species, whose toxicity is still debated, especially at the mitochondria level [213]. Further investigations are needed to evaluate whether this role of CyPD plays beneficial cellular effects.
It was reported that in neuronal cells treated with exogenous α-Syn a CsA-sensitive mPTP overactivation is triggered by the transition of α-Syn from its monomeric to oligomeric structure, probably as a consequence of a selective oxidation of the F-ATP synthase β subunit. Consistently, the threshold of mPTP opening by calcium ionophore was found lower in neuronal cells bearing SNCA triplication [197,214]. In familiar forms of PD, propagation of mPTP opening could also be favored by the inhibition of mitophagy, leading to the accumulation of fragmented mitochondria and failure in removing activated mPTP, which in turn increases ROS production [181]. Interestingly, in primary mouse cultured cerebral cortical neurons, it was demonstrated that activated mPTP contributes to neuronal Ca2+ overload by releasing Ca2+ into the cytoplasm during acute oxidative stress, while genetic ablation of CyPD reverses such release, leading to attenuation of the ATP decrease, and a significantly higher viability [215]. In accordance with these findings, a recent study showed that changes in membrane potential in peripheral blood mononuclear cells may function as an early indicator of neuronal death in PD, enabling earlier diagnosis [216].
As there is currently no disease-modifying treatment for PD, these studies suggest that mPTP may be a target for therapies. Efforts to identify mPTP inhibitors that are clinically useful are ongoing and very promising, as potent and metabolically stable inhibitors with an in vitro potency in the nanomolar range have recently been identified [217]. Nevertheless, as discussed by Rottenberg et al. [181], drugs, or other manipulations, should only inhibit damaging, long-lasting full openings of mPTP and not its short, partial openings that can be beneficial [129,175]. Interestingly, melatonin, which is known to have a protective effect against aging and neurodegeneration [218], has been shown to inhibit not transient [219], but only long-lasting mPTP openings [220]. Such a difference suggests that this latter inhibition could occur through a still unknown indirect mechanism. It is tempting to hypothesize that the observed melatonin interaction with SIRT3 [221] could also result in the modulation of CyPD acetylation and then mPTP opening [121], an effect still to be tested.
In conclusion, the emerging evidence demonstrates that mitochondrial dysfunction notably contributes to the development of PD and that CyPD modulation of mPTP opening plays a major role. CyPD itself may be a promising drug target, for which several inhibitors have been recently obtained [66], as discussed in the following section.
11. Pharmacological Inhibition of CyPD
Due to the established involvement of CyPD in various pathological conditions, much effort has been put into the development of small molecules able to inhibit its activity. It should be noted that the involvement of CyPD in pathology is almost always associated with its role in the regulation of the mPTP, for which there is still no detailed molecular structure [43]. While it is known that CsA desensitizes pore opening [45], it has not been fully established if only the CsA binding domain (i.e., the active site) of CyPD participates in mPTP regulation. As noted above, different studies revealed that several PTMs affecting mPTP regulation take place on the back face of CyPD [32,125,126,128], making it impossible to fully exclude its involvement in the binding of CyPD on mPTP. Therefore, while multiple, different binding sites of CyPD on mPTP could exist, all the research on CyPD inhibitors has focused solely on molecules binding to its active site.
The most significant challenge when it comes to the development of CyPD inhibitors is the molecular specificity. Indeed, the human genome encodes for at least 17 different CyPs (Table 1) with high homology to one another. According to Davis et al., the sequence identity between the PPIAse domains of different CyPs ranges from 61% to 86%, and the RMSD across all atoms is less than 2 Å, which lowers to 0.4–1.0 Å when the two most divergent structures (PPIL1 and PPWD1) are excluded [52]. Although several members of the CyP family have been poorly (if not at all) characterized and therefore their involvement in physiological processes has not been established, the development of isoform-specific inhibitors can greatly improve their safety profile by reducing the potential off-target effects. Particular relevance in this context is CyPA, which according to PaxDB is the most expressed CyP and one of the most abundant proteins in the cytoplasm [222].
Due to its immunosuppressant properties, non-immunosuppressive CsA-analogues have been synthesized. The simple addition of an isoleucine group at position 4 of CsA results in the non-immunosuppressive analogue N-methyl-4-isoleucine cyclosporin (NIM811) [223], which has been proposed for the treatment of hepatitis C virus infection [224]. The addition of the isoleucine group prevents the binding of the CyPA:CsA complex to calcineurin, abolishing the immunosuppressive properties of CsA. Another non-immunosuppressive CsA analogue is alisporivir (formerly Debio-025), which derives from the substitutions with an N-methylalanine and an N-ethylvaline at positions 3 and 4 respectively [225], which has also shown promising results in the treatment of patients co-infected by HCV and HIV [226]. CsA itself was found ineffective in patients with myocardial injury in phase III clinical trials [227], which raised the question of whether CyPD-dependent mPTP could be a relevant clinical target for such a condition [228].
CsA and its derivatives can be grouped into the general class of macrocyclic inhibitors, which also includes the macrolide sanglifehrin A (SfA), the cyclic decapeptide antanamide, and their analogues [229]. Macrocyclic inhibitors are however affected by several issues, including low selectivity over different CyPs and poor pharmacokinetic profiles [229]. Beyond the macrocyclic inhibitors, another numerous class of molecules comprehend the synthetic small-molecule inhibitors, which according to Haleckova et al. [229] can be further divided into:
-
(1)
N-4-Aminobenzyl-N′-(2-(2-arylpyrrolidin)-2-oxoethyl)urea-based compounds,
-
(2)
2-(Benzyloxy)arylurea-based compounds
-
(3)
Other small-molecule inhibitors
To date, the molecules in class I represent the most potent and best-characterized class of small-molecule CyPD inhibitors developed [229], although the issues of isoform-specificity and pharmacokinetic properties still stand. For instance, the inhibitor “C31” (i.e., 1-(4-aminobenzyl)-3-(2-(2-(2-(methylthio)phenyl)pyrrolidin-1-yl)-2-oxo-1-phenylethyl)urea), has been found to strongly inhibit CyPD PPIAse activity [230] and mPTP opening in isolated liver [230] and heart [231] mitochondria. Additionally, it has shown cytoprotective effects against I/R injury [230,231]. Notably, C31 retains its inhibitory effect on mPTP opening in Ppif−/− mitochondria, suggesting a dual inhibition mechanism involving both CyPD-dependent and CyPD-independent pathways [230,231]. However, it should be noted that there were no differences in mPTP inhibition between wild-type and Ppif−/− mitochondria [230,231], indicating that these two mechanisms, if they exist, do not have an additive or synergistic effect. Furthermore, while C31 demonstrated efficacy in protecting hepatocytes from I/R injury in vivo [230], no such protection was observed in the myocardium [231]. These findings underline the significance of testing the inhibitors on Ppif−/− models to assess CyPD-specific effects and emphasize the need for extensive in vivo administration studies.
To improve the selectivity of CyPD-inhibitors, at least two strategies have been proposed. First, De Simone et al. suggested the development of tri-vector molecules, which not only bind to the active site of CyPD, targeted by all the inhibitors developed so far, but make also extensive contacts on the poorly conserved 3’o-clock pocket [58]. The second strategy was proposed recently by Peterson et al. [66] and it was shown to actually lead to a remarkable enhancement in CyP-selectivity. The authors exploited the S2 pocket to develop, through iterated molecule engineering guided by X-ray co-crystal structure, the first potent isoform-specific macrocyclic inhibitors of CyPD and CyPE [66]. The pharmacokinetic properties of these molecules have not been tested, but their striking selectivity indicates the importance of the underestimated S2 pocket in CyP-targeted drug discovery. This finding will likely pave the way for the synthesis of highly specific compounds, serving as a starting point to enhance their pharmacokinetic properties and have optimized drugs.
Beyond the obvious difficult task to have potent molecules suited from the pharmacokinetic point of view, when it comes to CyPD there are some major challenges in the drug discovery process. The sole fact that CyPD resides within the mitochondrial matrix represents a significant obstacle since its inhibitors need to overcome more physiological and physical barriers than drugs directed towards cytosolic targets. This aspect assumes particular significance within the context of the central nervous system (CNS), where CyPD represents an appealing target given its involvement in pathologies such as AD [150] and PD [208]. To reach the CNS, molecules must overcome a great physical obstacle, i.e., the blood-brain barrier (BBB), which, based on current research findings, has exhibited limited permeability to CyPD inhibitors [232,233]. One possibility to overcome this problem would be the involvement of liposomal drug delivery systems, which is a promising therapeutical approach [234] that, to our knowledge, has not been tested for CyPD inhibitors.
Despite the pharmacological inhibition of CyPD, we speculate that at least two other different strategies could be adopted. First, the characterization of CyPD PTMs in the context of AD and/or PD could lead to targeting not directly CyPD itself but rather its modifying enzymes, such as GSK3β [31,127] or SIRT3 [121,122]. Second, since the genetic inhibition of CyPD has been shown to have ameliorative effects on the development of mPTP-mediated cellular dysfunction, it is tempting to also suggest the employment of antisense oligonucleotides (ASOs) to knock-down its expression. Indeed, while the pharmacological inhibition of protein targets has the challenging task of developing specific and BBB-permeable molecules when it comes to ASOs one should keep in mind that (i) they are highly selective and much easier to be designed, (ii) they are already being employed in the treatment of several neurological diseases, such as spinal muscular atrophy and Duchenne muscular dystrophy [235] and (iii) much work is being put in the design of drug delivery across the BBB, for which there are already promising results [236].
12. Conclusions
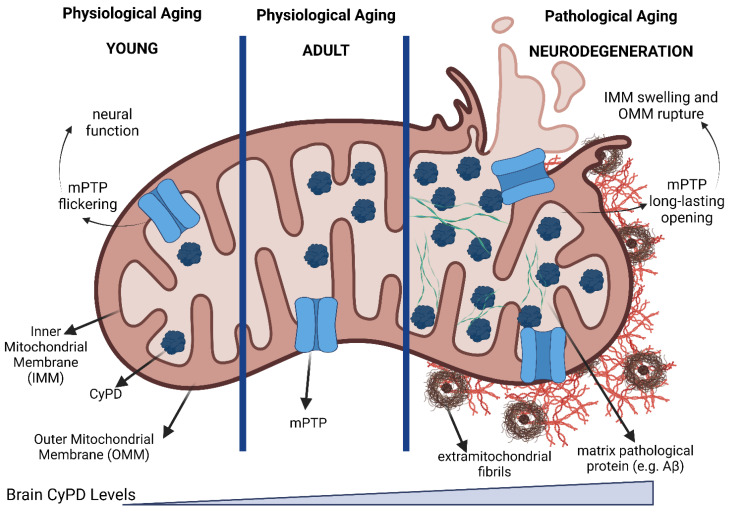
Neurodegenerative diseases (ND) represent a dramatic health and social burden that due to their molecular and biological complexity are still lacking an appropriate eradicative strategy. Aging remains a major risk factor for the development of various NDs, including AD and PD. The involvement of mitochondrial dysfunction in both these contexts has been extensively and convincingly documented, but many questions remain. CyPD has been shown to be almost absent in young brain mitochondria and to steadily increase with aging, which in turn is associated with a higher propensity for mPTP opening (Figure 5). In the context of AD-related mitochondrial dysfunction, CyPD levels are dramatically higher, and its inhibition has been shown protective at both the cellular and in vivo levels. It is tempting to speculate that aberrant CyPD-dependent mPTP opening may be a hallmark of ND-related mitochondrial dysfunction, which has been demonstrated for AD. However, it is important to note that mPTP also has physiological functions, as its flickering activity has been implicated in neuronal filopodiagenesis. Therefore, the complete inhibition of either mPTP or CyPD should not be the only focus of CyPD-targeted approaches, but rather fine-tuning its molecular function, such as reducing its abundance within mitochondria (through gene therapy) or modulating the enzymes responsible for its post-translational modifications. The complete ND-related picture of (i) CyPD levels, (ii) CyPD interactors, and (iii) CyPD PTMs could potentially provide novel CyPD-related molecular targets and offer a different perspective for the treatment of such pathologies.
Figure 5.
CyPD is a double-edged sword between health and pathology. During aging, CyPD levels in brain mitochondria steadily increase, which is associated with a different propensity of mPTP opening. In neurons, it has been shown that physiological mPTP activity has important effects, i.e., has been implicated in the neural filopodiagenesis. In the context of neurodegeneration, such as AD or PD, mitochondria are enriched of both CyPD and pathological proteins (such as Aβ, phosphorylated Tau, and α-Syn), which is associated with the aberrant mPTP opening that leads to both mitochondrial swellings, outer mitochondrial membrane rupture and, ultimately, cell death. Created with BioRender.com (accessed on 4 July 2023).
Author Contributions
Writing—original draft preparation, G.C., V.P.M., A.C., G.L.; writing—review and editing, G.C., G.L.; funding acquisition, A.C., G.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Original research in the authors’ laboratories was funded MUR, grant number LHFW42 and Telethon (GMR22T1067).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 2.Coleman C., Martin I. Unraveling Parkinson’s Disease Neurodegeneration: Does Aging Hold the Clues? J. Park. Dis. 2022;12:2321–2338. doi: 10.3233/JPD-223363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang K., Schmid F.X., Fischer G. Catalysis of Protein Folding by Prolyl Isomerase. Nature. 1987;329:268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- 4.Fischer G., Bang H., Mech C. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed. Biochim. Acta. 1984;43:1101–1111. [PubMed] [Google Scholar]
- 5.Handschumacher R.E., Harding M.W., Rice J., Drugge R.J., Speicher D.W. Cyclophilin: A Specific Cytosolic Binding Protein for Cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 6.Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F.X. Cyclophilin and Peptidyl-Prolyl Cis-Trans Isomerase Are Probably Identical Proteins. Nature. 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 7.Harikishore A., Sup Yoon H. Immunophilins: Structures, Mechanisms and Ligands. CMP. 2015;9:37–47. doi: 10.2174/1874467208666150519113427. [DOI] [PubMed] [Google Scholar]
- 8.Matena A., Rehic E., Hönig D., Kamba B., Bayer P. Structure and Function of the Human Parvulins Pin1 and Par14/17. Biol. Chem. 2018;399:101–125. doi: 10.1515/hsz-2017-0137. [DOI] [PubMed] [Google Scholar]
- 9.Fanghänel J., Fischer G. Insights into the Catalytic Mechanism of Peptidyl Prolyl Cis/Trans Isomerases. Front. Biosci. 2004;9:3453. doi: 10.2741/1494. [DOI] [PubMed] [Google Scholar]
- 10.Rein T. Peptidylprolylisomerases, Protein Folders, or Scaffolders? The Example of FKBP51 and FKBP52. BioEssays. 2020;42:1900250. doi: 10.1002/bies.201900250. [DOI] [PubMed] [Google Scholar]
- 11.Stewart D.E., Sarkar A., Wampler J.E. Occurrence and Role of Cis Peptide Bonds in Protein Structures. J. Mol. Biol. 1990;214:253–260. doi: 10.1016/0022-2836(90)90159-J. [DOI] [PubMed] [Google Scholar]
- 12.Galat A. Peptidylproline Cis-Trans-Isomerases: Immunophilins. Eur. J. Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanes S.D. Prolyl Isomerases in Gene Transcription. Biochim. Biophys. Acta BBA Gen. Subj. 2015;1850:2017–2034. doi: 10.1016/j.bbagen.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz D.S., Lee E.J., Mabon S.A., Misteli T. A Cyclophilin Functions in Pre-MRNA Splicing. EMBO J. 2002;21:470–480. doi: 10.1093/emboj/21.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamatis J.E., Pellizzari-Delano I.E., Gallardo-Flores C.E., Colpitts C.C. Emerging Roles of Cyclophilin A in Regulating Viral Cloaking. Front. Microbiol. 2022;13:828078. doi: 10.3389/fmicb.2022.828078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q., Leiva M.C., Fischkoff S.A., Handschumacher R.E., Lyttle C.R. Leukocyte Chemotactic Activity of Cyclophilin. J. Biol. Chem. 1992;267:11968–11971. doi: 10.1016/S0021-9258(19)49791-3. [DOI] [PubMed] [Google Scholar]
- 17.Bergsma D.J., Eder C., Gross M., Kersten H., Sylvester D., Appelbaum E., Cusimano D., Livi G.P., McLaughlin M.M., Kasyan K. The Cyclophilin Multigene Family of Peptidyl-Prolyl Isomerases. Characterization of Three Separate Human Isoforms. J. Biol. Chem. 1991;266:23204–23214. doi: 10.1016/S0021-9258(18)54484-7. [DOI] [PubMed] [Google Scholar]
- 18.Woodfield K.-Y., Price N.T., Halestrap A.P. CDNA Cloning of Rat Mitochondrial Cyclophilin. Biochim. Biophys. Acta BBA Gene Struct. Expr. 1997;1351:27–30. doi: 10.1016/S0167-4781(97)00017-1. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez-Aguilar M., Baines C.P. Structural Mechanisms of Cyclophilin D-Dependent Control of the Mitochondrial Permeability Transition Pore. Biochim. Biophys. Acta BBA Gen. Subj. 2015;1850:2041–2047. doi: 10.1016/j.bbagen.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elrod J.W., Molkentin J.D. Physiologic Functions of Cyclophilin D and the Mitochondrial Permeability Transition Pore. Circ. J. 2013;77:1111–1122. doi: 10.1253/circj.CJ-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., Stein T.I., Nudel R., Lieder I., Mazor Y., et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 22.The UniProt Consortium. Bateman A., Martin M.-J., Orchard S., Magrane M., Ahmad S., Alpi E., Bowler-Barnett E.H., Britto R., Bye-A-Jee H., et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023;51:D523–D531. doi: 10.1093/nar/gkac1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson N., Khan A., Virji S., Ward J.M., Crompton M. Import and Processing of Heart Mitochondrial Cyclophilin D. Eur. J. Biochem. 1999;263:353–359. doi: 10.1046/j.1432-1327.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 24.Connern C.P., Halestrap A.P. Purification and N-Terminal Sequencing of Peptidyl-Prolyl Cis-Trans-Isomerase from Rat Liver Mitochondrial Matrix Reveals the Existence of a Distinct Mitochondrial Cyclophilin. Biochem. J. 1992;284:381–385. doi: 10.1042/bj2840381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter G.A., Beutner G. Cyclophilin D, Somehow a Master Regulator of Mitochondrial Function. Biomolecules. 2018;8:176. doi: 10.3390/biom8040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giorgio V., Bisetto E., Soriano M.E., Dabbeni-Sala F., Basso E., Petronilli V., Forte M.A., Bernardi P., Lippe G. Cyclophilin D Modulates Mitochondrial F0F1-ATP Synthase by Interacting with the Lateral Stalk of the Complex. J. Biol. Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgio V., von Stockum S., Antoniel M., Fabbro A., Fogolari F., Forte M., Glick G.D., Petronilli V., Zoratti M., Szabó I., et al. Dimers of Mitochondrial ATP Synthase Form the Permeability Transition Pore. Proc. Natl. Acad. Sci. USA. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbani A., Giorgio V., Carrer A., Franchin C., Arrigoni G., Jiko C., Abe K., Maeda S., Shinzawa-Itoh K., Bogers J.F.M., et al. Purified F-ATP Synthase Forms a Ca2+-Dependent High-Conductance Channel Matching the Mitochondrial Permeability Transition Pore. Nat. Commun. 2019;10:4341. doi: 10.1038/s41467-019-12331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mnatsakanyan N., Llaguno M.C., Yang Y., Yan Y., Weber J., Sigworth F.J., Jonas E.A. A Mitochondrial Megachannel Resides in Monomeric F1FO ATP Synthase. Nat. Commun. 2019;10:5823. doi: 10.1038/s41467-019-13766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crompton M., Virji S., Ward J.M. Cyclophilin-D Binds Strongly to Complexes of the Voltage-Dependent Anion Channel and the Adenine Nucleotide Translocase to Form the Permeability Transition Pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 31.Rasola A., Sciacovelli M., Chiara F., Pantic B., Brusilow W.S., Bernardi P. Activation of Mitochondrial ERK Protects Cancer Cells from Death through Inhibition of the Permeability Transition. Proc. Natl. Acad. Sci. USA. 2010;107:726–731. doi: 10.1073/pnas.0912742107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh J.C., Siegelin M.D., Vaira V., Faversani A., Tavecchio M., Chae Y.C., Lisanti S., Rampini P., Giroda M., Caino M.C., et al. Adaptive Mitochondrial Reprogramming and Resistance to PI3K Therapy. JNCI J. Natl. Cancer Inst. 2015;107:dju502. doi: 10.1093/jnci/dju502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaseva A.V., Marchenko N.D., Ji K., Tsirka S.E., Holzmann S., Moll U.M. P53 Opens the Mitochondrial Permeability Transition Pore to Trigger Necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumutima J., Yao X.-Q., Hamelberg D. P53 Is Potentially Regulated by Cyclophilin D in the Triple-Proline Loop of the DNA Binding Domain. Biochemistry. 2021;60:597–606. doi: 10.1021/acs.biochem.0c00946. [DOI] [PubMed] [Google Scholar]
- 35.Zhao J., Liu X., Blayney A., Zhang Y., Gandy L., Mirsky P.O., Smith N., Zhang F., Linhardt R.J., Chen J., et al. Intrinsically Disordered N-Terminal Domain (NTD) of P53 Interacts with Mitochondrial PTP Regulator Cyclophilin D. J. Mol. Biol. 2022;434:167552. doi: 10.1016/j.jmb.2022.167552. [DOI] [PubMed] [Google Scholar]
- 36.Cannino G., Urbani A., Gaspari M., Varano M., Negro A., Filippi A., Ciscato F., Masgras I., Gerle C., Tibaldi E., et al. The Mitochondrial Chaperone TRAP1 Regulates F-ATP Synthase Channel Formation. Cell Death Differ. 2022;29:2335–2346. doi: 10.1038/s41418-022-01020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etzler J.C., Bollo M., Holstein D., Deng J.J., Perez V., Lin D., Richardson A., Bai Y., Lechleiter J.D. Cyclophilin D Over-Expression Increases Mitochondrial Complex III Activity and Accelerates Supercomplex Formation. Arch. Biochem. Biophys. 2017;613:61–68. doi: 10.1016/j.abb.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliseev R.A., Malecki J., Lester T., Zhang Y., Humphrey J., Gunter T.E. Cyclophilin D Interacts with Bcl2 and Exerts an Anti-Apoptotic Effect. J. Biol. Chem. 2009;284:9692–9699. doi: 10.1074/jbc.M808750200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beutner G., Alanzalon R.E., Porter G.A. Cyclophilin D Regulates the Dynamic Assembly of Mitochondrial ATP Synthase into Synthasomes. Sci. Rep. 2017;7:14488. doi: 10.1038/s41598-017-14795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruprecht J.J., Kunji E.R. Structural Changes in the Transport Cycle of the Mitochondrial ADP/ATP Carrier. Curr. Opin. Struct. Biol. 2019;57:135–144. doi: 10.1016/j.sbi.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruprecht J.J., Kunji E.R.S. Structural Mechanism of Transport of Mitochondrial Carriers. Annu. Rev. Biochem. 2021;90:535–558. doi: 10.1146/annurev-biochem-072820-020508. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.T.M., Wong R., Menazza S., Sun J., Chen Y., Wang G., Gucek M., Steenbergen C., Sack M.N., Murphy E. Cyclophilin D Modulates Mitochondrial Acetylome. Circ. Res. 2013;113:1308–1319. doi: 10.1161/CIRCRESAHA.113.301867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernardi P., Carraro M., Lippe G. The Mitochondrial Permeability Transition: Recent Progress and Open Questions. FEBS J. 2022;289:7051–7074. doi: 10.1111/febs.16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths E.J., Halestrap A.P. Protection by Cyclosporin A of Ischemia/Reperfusion-Induced Damage in Isolated Rat Hearts. J. Mol. Cell. Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 45.Broekemeier K.M., Dempsey M.E., Pfeiffer D.R. Cyclosporin A Is a Potent Inhibitor of the Inner Membrane Permeability Transition in Liver Mitochondria. J. Biol. Chem. 1989;264:7826–7830. doi: 10.1016/S0021-9258(18)83116-7. [DOI] [PubMed] [Google Scholar]
- 46.Halestrap A.P., Davidson A.M. Inhibition of Ca2+-Induced Large-Amplitude Swelling of Liver and Heart Mitochondria by Cyclosporin Is Probably Caused by the Inhibitor Binding to Mitochondrial-Matrix Peptidyl-Prolyl Cis-Trans Isomerase and Preventing It Interacting with the Adenine Nucleotide Translocase. Biochem. J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basso E., Fante L., Fowlkes J., Petronilli V., Forte M.A., Bernardi P. Properties of the Permeability Transition Pore in Mitochondria Devoid of Cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 48.Ke H.M., Zydowsky L.D., Liu J., Walsh C.T. Crystal Structure of Recombinant Human T-Cell Cyclophilin A at 2.5 A Resolution. Proc. Natl. Acad. Sci. USA. 1991;88:9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallen J., Spitzfaden C., Zurini M.G.M., Wider G., Widmer H., Wüthrich K., Walkinshaw M.D. Structure of Human Cyclophilin and Its Binding Site for Cyclosporin a Determined by X-Ray Crystallography and NMR Spectroscopy. Nature. 1991;353:276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- 50.Spitzfaden C., Braun W., Wider G., Widmer H., Wüthrich K. Determination of the NMR Solution Structure of the Cyclophilin A-Cyclosporin A Complex. J. Biomol. NMR. 1994;4:463–482. doi: 10.1007/BF00156614. [DOI] [PubMed] [Google Scholar]
- 51.Wang P., Heitman J. The Cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis T.L., Walker J.R., Campagna-Slater V., Finerty P.J., Paramanathan R., Bernstein G., MacKenzie F., Tempel W., Ouyang H., Lee W.H., et al. Structural and Biochemical Characterization of the Human Cyclophilin Family of Peptidyl-Prolyl Isomerases. PLoS Biol. 2010;8:e1000439. doi: 10.1371/journal.pbio.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlatter D., Thoma R., Küng E., Stihle M., Müller F., Borroni E., Cesura A., Hennig M. Crystal Engineering Yields Crystals of Cyclophilin D Diffracting to 1.7 Å Resolution. Acta Crystallogr. D Biol. Crystallogr. 2005;61:513–519. doi: 10.1107/S0907444905003070. [DOI] [PubMed] [Google Scholar]
- 54.Kajitani K., Fujihashi M., Kobayashi Y., Shimizu S., Tsujimoto Y., Miki K. Crystal Structure of Human Cyclophilin D in Complex with Its Inhibitor, Cyclosporin A at 0.96-Å Resolution. Proteins. 2007;70:1635–1639. doi: 10.1002/prot.21855. [DOI] [PubMed] [Google Scholar]
- 55.wwPDB Consortium Protein Data Bank: The Single Global Archive for 3D Macromolecular Structure Data. Nucleic Acids Res. 2019;47:D520–D528. doi: 10.1093/nar/gky949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Simone A., Georgiou C., Ioannidis H., Gupta A.A., Juárez-Jiménez J., Doughty-Shenton D., Blackburn E.A., Wear M.A., Richards J.P., Barlow P.N., et al. A Computationally Designed Binding Mode Flip Leads to a Novel Class of Potent Tri-Vector Cyclophilin Inhibitors. Chem. Sci. 2019;10:542–547. doi: 10.1039/C8SC03831G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borel J.F., Feurer C., Gubler H.U., Stähelin H. Biological Effects of Cyclosporin A: A New Antilymphocytic Agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda S., Koyasu S. Mechanisms of Action of Cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/S0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 61.Wüthrich K., Von Freyberg B., Weber C., Wider G., Traber R., Widmer H., Braun W. Receptor-Induced Conformation Change of the Immunosuppressant Cyclosporin A. Science. 1991;254:953–954. doi: 10.1126/science.1948082. [DOI] [PubMed] [Google Scholar]
- 62.Loosli H.-R., Kessler H., Oschkinat H., Weber H.-P., Petcher T.J., Widmer A. Peptide conformations. Part 31. The conformation of cyclosporin a in the crystal and in solution. Helv. Chim. Acta. 1985;68:682–704. doi: 10.1002/hlca.19850680319. [DOI] [Google Scholar]
- 63.Ko S.Y., Dalvit C. Conformation of Cyclosporin A in Polar Solvents. Int. J. Pept. Protein Res. 1992;40:380–382. doi: 10.1111/j.1399-3011.1992.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 64.Fesik S.W., Gampe R.T., Eaton H.L., Gemmecker G., Olejniczak E.T., Neri P., Holzman T.F., Egan D.A., Edalji R., Simmer R. NMR Studies of [U-13C]Cyclosporin A Bound to Cyclophilin: Bound Conformation and Portions of Cyclosporin Involved in Binding. Biochemistry. 1991;30:6574–6583. doi: 10.1021/bi00240a030. [DOI] [PubMed] [Google Scholar]
- 65.Thériault Y., Logan T.M., Meadows R., Yu L., Olejniczak E.T., Holzman T.F., Simmer R.L., Fesik S.W. Solution Structure of the Cyclosporin A/Cyclophilin Complex by NMR. Nature. 1993;361:88–91. doi: 10.1038/361088a0. [DOI] [PubMed] [Google Scholar]
- 66.Peterson A.A., Rangwala A.M., Thakur M.K., Ward P.S., Hung C., Outhwaite I.R., Chan A.I., Usanov D.L., Mootha V.K., Seeliger M.A., et al. Discovery and Molecular Basis of Subtype-Selective Cyclophilin Inhibitors. Nat. Chem. Biol. 2022;18:1184–1195. doi: 10.1038/s41589-022-01116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daum S., Schumann M., Mathea S., Aumüller T., Balsley M.A., Constant S.L., De Lacroix B.F., Kruska F., Braun M., Schiene-Fischer C. Isoform-Specific Inhibition of Cyclophilins. Biochemistry. 2009;48:6268–6277. doi: 10.1021/bi9007287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bernardi P., Rasola A., Forte M., Lippe G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Massari S., Frigeri L., Azzone G.F. A Quantitative Correlation between the Kinetics of Solutes and Water Translocation in Liver Mitochondria. J. Membr. Biol. 1972;9:71–82. doi: 10.1007/BF01868044. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa T., Shimizu S., Watanabe T., Yamaguchi O., Otsu K., Yamagata H., Inohara H., Kubo T., Tsujimoto Y. Cyclophilin D-Dependent Mitochondrial Permeability Transition Regulates Some Necrotic but Not Apoptotic Cell Death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 71.Rasola A., Bernardi P. Mitochondrial Permeability Transition in Ca2+-Dependent Apoptosis and Necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 73.Forte M., Gold B.G., Marracci G., Chaudhary P., Basso E., Johnsen D., Yu X., Fowlkes J., Rahder M., Stem K., et al. Cyclophilin D Inactivation Protects Axons in Experimental Autoimmune Encephalomyelitis, an Animal Model of Multiple Sclerosis. Proc. Natl. Acad. Sci. USA. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujimoto K., Chen Y., Polonsky K.S., Dorn G.W. Targeting Cyclophilin D and the Mitochondrial Permeability Transition Enhances Beta-Cell Survival and Prevents Diabetes in Pdx1 Deficiency. Proc. Natl. Acad. Sci. USA. 2010;107:10214–10219. doi: 10.1073/pnas.0914209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin L.J., Gertz B., Pan Y., Price A.C., Molkentin J.D., Chang Q. The Mitochondrial Permeability Transition Pore in Motor Neurons: Involvement in the Pathobiology of ALS Mice. Exp. Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millay D.P., Sargent M.A., Osinska H., Baines C.P., Barton E.R., Vuagniaux G., Sweeney H.L., Robbins J., Molkentin J.D. Genetic and Pharmacologic Inhibition of Mitochondrial-Dependent Necrosis Attenuates Muscular Dystrophy. Nat. Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parone P.A., Da Cruz S., Han J.S., McAlonis-Downes M., Vetto A.P., Lee S.K., Tseng E., Cleveland D.W. Enhancing Mitochondrial Calcium Buffering Capacity Reduces Aggregation of Misfolded SOD1 and Motor Neuron Cell Death without Extending Survival in Mouse Models of Inherited Amyotrophic Lateral Sclerosis. J. Neurosci. 2013;33:4657–4671. doi: 10.1523/JNEUROSCI.1119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schinzel A.C., Takeuchi O., Huang Z., Fisher J.K., Zhou Z., Rubens J., Hetz C., Danial N.N., Moskowitz M.A., Korsmeyer S.J. Cyclophilin D Is a Component of Mitochondrial Permeability Transition and Mediates Neuronal Cell Death after Focal Cerebral Ischemia. Proc. Natl. Acad. Sci. USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X., Carlsson Y., Basso E., Zhu C., Rousset C.I., Rasola A., Johansson B.R., Blomgren K., Mallard C., Bernardi P., et al. Developmental Shift of Cyclophilin D Contribution to Hypoxic-Ischemic Brain Injury. J. Neurosci. 2009;29:2588–2596. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palma E., Tiepolo T., Angelin A., Sabatelli P., Maraldi N.M., Basso E., Forte M.A., Bernardi P., Bonaldo P. Genetic Ablation of Cyclophilin D Rescues Mitochondrial Defects and Prevents Muscle Apoptosis in Collagen VI Myopathic Mice. Hum. Mol. Genet. 2009;18:2024–2031. doi: 10.1093/hmg/ddp126. [DOI] [PubMed] [Google Scholar]
- 81.Hunter D.R., Haworth R.A. The Ca2+-Induced Membrane Transition in Mitochondria: I. The Protective Mechanisms. Arch. Biochem. Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 82.Beutner G., Rück A., Riede B., Brdiczka D. Complexes between Porin, Hexokinase, Mitochondrial Creatine Kinase and Adenylate Translocator Display Properties of the Permeability Transition Pore. Implication for Regulation of Permeability Transition by the Kinases. Biochim. Biophys. Acta. 1998;1368:7–18. doi: 10.1016/S0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 83.Bernardi P., Di Lisa F., Fogolari F., Lippe G. From ATP to PTP and Back: A Dual Function for the Mitochondrial ATP Synthase. Circ. Res. 2015;116:1850–1862. doi: 10.1161/CIRCRESAHA.115.306557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He J., Ford H.C., Carroll J., Ding S., Fearnley I.M., Walker J.E. Persistence of the Mitochondrial Permeability Transition in the Absence of Subunit c of Human ATP Synthase. Proc. Natl. Acad. Sci. USA. 2017;114:3409–3414. doi: 10.1073/pnas.1702357114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He J., Carroll J., Ding S., Fearnley I.M., Walker J.E. Permeability Transition in Human Mitochondria Persists in the Absence of Peripheral Stalk Subunits of ATP Synthase. Proc. Natl. Acad. Sci. USA. 2017;114:9086–9091. doi: 10.1073/pnas.1711201114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carroll J., He J., Ding S., Fearnley I.M., Walker J.E. Persistence of the Permeability Transition Pore in Human Mitochondria Devoid of an Assembled ATP Synthase. Proc. Natl. Acad. Sci. USA. 2019;116:12816–12821. doi: 10.1073/pnas.1904005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernardi P. Mechanisms for Ca2+-Dependent Permeability Transition in Mitochondria. Proc. Natl. Acad. Sci. USA. 2020;117:2743–2744. doi: 10.1073/pnas.1921035117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neginskaya M.A., Solesio M.E., Berezhnaya E.V., Amodeo G.F., Mnatsakanyan N., Jonas E.A., Pavlov E.V. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore. Cell Rep. 2019;26:11–17.e2. doi: 10.1016/j.celrep.2018.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kühlbrandt W. Structure and Mechanisms of F-Type ATP Synthases. Annu. Rev. Biochem. 2019;88:515–549. doi: 10.1146/annurev-biochem-013118-110903. [DOI] [PubMed] [Google Scholar]
- 90.Spikes T.E., Montgomery M.G., Walker J.E. Structure of the Dimeric ATP Synthase from Bovine Mitochondria. Proc. Natl. Acad. Sci. USA. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu J., Zhang L., Zong S., Guo R., Liu T., Yi J., Wang P., Zhuo W., Yang M. Cryo-EM Structure of the Mammalian ATP Synthase Tetramer Bound with Inhibitory Protein IF1. Science. 2019;364:1068–1075. doi: 10.1126/science.aaw4852. [DOI] [PubMed] [Google Scholar]
- 92.Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D.M., Brèthes D., di Rago J.-P., Velours J. The ATP Synthase Is Involved in Generating Mitochondrial Cristae Morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boyer P.D. The ATP Synthase—A Splendid Molecular Machine. Annu. Rev. Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 94.Stock D., Leslie A.G., Walker J.E. Molecular Architecture of the Rotary Motor in ATP Synthase. Science. 1999;286:1700–1705. doi: 10.1126/science.286.5445.1700. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi R., Ueno H., Li C.-B., Noji H. Rotary Catalysis of Bovine Mitochondrial F1-ATPase Studied by Single-Molecule Experiments. Proc. Natl. Acad. Sci. USA. 2020;117:1447–1456. doi: 10.1073/pnas.1909407117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo H., Rubinstein J.L. Structure of ATP Synthase under Strain during Catalysis. Nat. Commun. 2022;13:2232. doi: 10.1038/s41467-022-29893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papageorgiou S., Melandri A.B., Solaini G. Relevance of Divalent Cations to ATP-Driven Proton Pumping in Beef Heart Mitochondrial F0F1-ATPase. J. Bioenerg. Biomembr. 1998;30:533–541. doi: 10.1023/A:1020528432609. [DOI] [PubMed] [Google Scholar]
- 98.Nathanson L., Gromet-Elhanan Z. Mutations in the Beta-Subunit Thr(159) and Glu(184) of the Rhodospirillum Rubrum F(0)F(1) ATP Synthase Reveal Differences in Ligands for the Coupled Mg2+- and Decoupled Ca2+-Dependent F(0)F(1) Activities. J. Biol. Chem. 2000;275:901–905. doi: 10.1074/jbc.275.2.901. [DOI] [PubMed] [Google Scholar]
- 99.Nesci S., Trombetti F., Ventrella V., Pirini M., Pagliarani A. Kinetic Properties of the Mitochondrial F1FO-ATPase Activity Elicited by Ca2+ in Replacement of Mg2+ Biochimie. 2017;140:73–81. doi: 10.1016/j.biochi.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Lai Y., Zhang Y., Zhou S., Xu J., Du Z., Feng Z., Yu L., Zhao Z., Wang W., Tang Y., et al. Structure of the Human ATP Synthase. Mol. Cell. 2023;83:2137–2147.e4. doi: 10.1016/j.molcel.2023.04.029. [DOI] [PubMed] [Google Scholar]
- 101.Pinke G., Zhou L., Sazanov L.A. Cryo-EM Structure of the Entire Mammalian F-Type ATP Synthase. Nat. Struct. Mol. Biol. 2020;27:1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 102.Colina-Tenorio L., Dautant A., Miranda-Astudillo H., Giraud M.-F., González-Halphen D. The Peripheral Stalk of Rotary ATPases. Front. Physiol. 2018;9:1243. doi: 10.3389/fphys.2018.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giorgio V., Fogolari F., Lippe G., Bernardi P. OSCP Subunit of Mitochondrial ATP Synthase: Role in Regulation of Enzyme Function and of Its Transition to a Pore. Br. J. Pharmacol. 2019;176:4247–4257. doi: 10.1111/bph.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Symersky J., Osowski D., Walters D.E., Mueller D.M. Oligomycin Frames a Common Drug-Binding Site in the ATP Synthase. Proc. Natl. Acad. Sci. USA. 2012;109:13961–13965. doi: 10.1073/pnas.1207912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alavian K.N., Beutner G., Lazrove E., Sacchetti S., Park H.-A., Licznerski P., Li H., Nabili P., Hockensmith K., Graham M., et al. An Uncoupling Channel within the C-Subunit Ring of the F1FO ATP Synthase Is the Mitochondrial Permeability Transition Pore. Proc. Natl. Acad. Sci. USA. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giorgio V., Burchell V., Schiavone M., Bassot C., Minervini G., Petronilli V., Argenton F., Forte M., Tosatto S., Lippe G., et al. Ca2+ Binding to F-ATP Synthase β Subunit Triggers the Mitochondrial Permeability Transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Antoniel M., Jones K., Antonucci S., Spolaore B., Fogolari F., Petronilli V., Giorgio V., Carraro M., Di Lisa F., Forte M., et al. The Unique Histidine in OSCP Subunit of F-ATP Synthase Mediates Inhibition of the Permeability Transition Pore by Acidic PH. EMBO Rep. 2018;19:257–268. doi: 10.15252/embr.201744705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo L., Carraro M., Sartori G., Minervini G., Eriksson O., Petronilli V., Bernardi P. Arginine 107 of Yeast ATP Synthase Subunit g Mediates Sensitivity of the Mitochondrial Permeability Transition to Phenylglyoxal. J. Biol. Chem. 2018;293:14632–14645. doi: 10.1074/jbc.RA118.004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo L., Carraro M., Carrer A., Minervini G., Urbani A., Masgras I., Tosatto S.C.E., Szabò I., Bernardi P., Lippe G. Arg-8 of Yeast Subunit e Contributes to the Stability of F-ATP Synthase Dimers and to the Generation of the Full-Conductance Mitochondrial Megachannel. J. Biol. Chem. 2019;294:10987–10997. doi: 10.1074/jbc.RA119.008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carrer A., Tommasin L., Šileikytė J., Ciscato F., Filadi R., Urbani A., Forte M., Rasola A., Szabò I., Carraro M., et al. Defining the Molecular Mechanisms of the Mitochondrial Permeability Transition through Genetic Manipulation of F-ATP Synthase. Nat. Commun. 2021;12:4835. doi: 10.1038/s41467-021-25161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morciano G., Pedriali G., Bonora M., Pavasini R., Mikus E., Calvi S., Bovolenta M., Lebiedzinska-Arciszewska M., Pinotti M., Albertini A., et al. A Naturally Occurring Mutation in ATP Synthase Subunit c Is Associated with Increased Damage Following Hypoxia/Reoxygenation in STEMI Patients. Cell Rep. 2021;35:108983. doi: 10.1016/j.celrep.2021.108983. [DOI] [PubMed] [Google Scholar]
- 112.Carraro M., Jones K., Sartori G., Schiavone M., Antonucci S., Kucharczyk R., di Rago J.-P., Franchin C., Arrigoni G., Forte M., et al. The Unique Cysteine of F-ATP Synthase OSCP Subunit Participates in Modulation of the Permeability Transition Pore. Cell Rep. 2020;32:108095. doi: 10.1016/j.celrep.2020.108095. [DOI] [PubMed] [Google Scholar]
- 113.Gerle C. Mitochondrial F-ATP Synthase as the Permeability Transition Pore. Pharmacol. Res. 2020;160:105081. doi: 10.1016/j.phrs.2020.105081. [DOI] [PubMed] [Google Scholar]
- 114.Mnatsakanyan N., Park H.-A., Wu J., He X., Llaguno M.C., Latta M., Miranda P., Murtishi B., Graham M., Weber J., et al. Mitochondrial ATP Synthase C-Subunit Leak Channel Triggers Cell Death upon Loss of Its F1 Subcomplex. Cell Death Differ. 2022;29:1874–1887. doi: 10.1038/s41418-022-00972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amodeo G.F., Krilyuk N., Pavlov E.V. Formation of High-Conductive C Subunit Channels upon Interaction with Cyclophilin D. Int. J. Mol. Sci. 2021;22:11022. doi: 10.3390/ijms222011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amodeo G.F., Lee B.Y., Krilyuk N., Filice C.T., Valyuk D., Otzen D.E., Noskov S., Leonenko Z., Pavlov E.V. C Subunit of the ATP Synthase Is an Amyloidogenic Calcium Dependent Channel-Forming Peptide with Possible Implications in Mitochondrial Permeability Transition. Sci. Rep. 2021;11:8744. doi: 10.1038/s41598-021-88157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amodeo G.F., Pavlov E.V. Amyloid β, α-Synuclein and the c Subunit of the ATP Synthase: Can These Peptides Reveal an Amyloidogenic Pathway of the Permeability Transition Pore? Biochim. Biophys. Acta. 2021;1863:183531. doi: 10.1016/j.bbamem.2020.183531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scorrano L., Nicolli A., Basso E., Petronilli V., Bernardi P. Two Modes of Activation of the Permeability Transition Pore: The Role of Mitochondrial Cyclophilin. Mol. Cell Biochem. 1997;174:181–184. doi: 10.1023/A:1006887921810. [DOI] [PubMed] [Google Scholar]
- 119.Frigo E., Tommasin L., Lippe G., Carraro M., Bernardi P. The Haves and Have-Nots: The Mitochondrial Permeability Transition Pore across Species. Cells. 2023;12:1409. doi: 10.3390/cells12101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khoury G.A., Baliban R.C., Floudas C.A. Proteome-Wide Post-Translational Modification Statistics: Frequency Analysis and Curation of the Swiss-Prot Database. Sci. Rep. 2011;1:90. doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hafner A.V., Dai J., Gomes A.P., Xiao C.-Y., Palmeira C.M., Rosenzweig A., Sinclair D.A. Regulation of the MPTP by SIRT3-Mediated Deacetylation of CypD at Lysine 166 Suppresses Age-Related Cardiac Hypertrophy. Aging. 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bochaton T., Crola-Da-Silva C., Pillot B., Villedieu C., Ferreras L., Alam M.R., Thibault H., Strina M., Gharib A., Ovize M., et al. Inhibition of Myocardial Reperfusion Injury by Ischemic Postconditioning Requires Sirtuin 3-Mediated Deacetylation of Cyclophilin D. J. Mol. Cell. Cardiol. 2015;84:61–69. doi: 10.1016/j.yjmcc.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 123.Castillo E.C., Morales J.A., Chapoy-Villanueva H., Silva-Platas C., Trevino-Saldana N., Guerrero-Beltrán C.E., Bernal-Ramìrez J., Torres-Quintanilla A., Garcìa N., Youker K., et al. Mitochondrial Hyperacetylation in the Failing Hearts of Obese Patients Mediated Partly by a Reduction in SIRT3: The Involvement of the Mitochondrial Permeability Transition Pore. Cell Physiol. Biochem. 2019;53:465–479. doi: 10.33594/000000151. [DOI] [PubMed] [Google Scholar]
- 124.Hurst S., Gonnot F., Dia M., Crola Da Silva C., Gomez L., Sheu S.-S. Phosphorylation of Cyclophilin D at Serine 191 Regulates Mitochondrial Permeability Transition Pore Opening and Cell Death after Ischemia-Reperfusion. Cell Death Dis. 2020;11:661. doi: 10.1038/s41419-020-02864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parks R.J., Menazza S., Holmström K.M., Amanakis G., Fergusson M., Ma H., Aponte A.M., Bernardi P., Finkel T., Murphy E. Cyclophilin D-Mediated Regulation of the Permeability Transition Pore Is Altered in Mice Lacking the Mitochondrial Calcium Uniporter. Cardiovasc. Res. 2019;115:385–394. doi: 10.1093/cvr/cvy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amanakis G., Sun J., Fergusson M.M., McGinty S., Liu C., Molkentin J.D., Murphy E. Cysteine 202 of Cyclophilin D Is a Site of Multiple Post-Translational Modifications and Plays a Role in Cardioprotection. Cardiovasc. Res. 2021;117:212–223. doi: 10.1093/cvr/cvaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Teodoro J.S., Varela A.T., Duarte F.V., Gomes A.P., Palmeira C.M., Rolo A.P. Indirubin and NAD+ Prevent Mitochondrial Ischaemia/Reperfusion Damage in Fatty Livers. Eur. J. Clin. Invest. 2018;48:e12932. doi: 10.1111/eci.12932. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen T.T., Stevens M.V., Kohr M., Steenbergen C., Sack M.N., Murphy E. Cysteine 203 of Cyclophilin D Is Critical for Cyclophilin D Activation of the Mitochondrial Permeability Transition Pore. J. Biol. Chem. 2011;286:40184–40192. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sambri I., Massa F., Gullo F., Meneghini S., Cassina L., Carraro M., Dina G., Quattrini A., Patanella L., Carissimo A., et al. Impaired Flickering of the Permeability Transition Pore Causes SPG7 Spastic Paraplegia. eBioMedicine. 2020;61:103050. doi: 10.1016/j.ebiom.2020.103050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu M., He F., Thompson E.W., Ostrikov K., Dai X. Lysine Acetylation, Cancer Hallmarks and Emerging Onco-Therapeutic Opportunities. Cancers. 2022;14:346. doi: 10.3390/cancers14020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ziętara P., Dziewięcka M., Augustyniak M. Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. Int. J. Mol. Sci. 2022;24:728. doi: 10.3390/ijms24010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baeza J., Smallegan M.J., Denu J.M. Mechanisms and Dynamics of Protein Acetylation in Mitochondria. Trends Biochem. Sci. 2016;41:231–244. doi: 10.1016/j.tibs.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim S.C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., et al. Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Mol. Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 134.Boreikaite V., Wicky B.I.M., Watt I.N., Clarke J., Walker J.E. Extrinsic Conditions Influence the Self-Association and Structure of IF1, the Regulatory Protein of Mitochondrial ATP Synthase. Proc. Natl. Acad. Sci. USA. 2019;116:10354–10359. doi: 10.1073/pnas.1903535116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bilbrough T., Piemontese E., Seitz O. Dissecting the Role of Protein Phosphorylation: A Chemical Biology Toolbox. Chem. Soc. Rev. 2022;51:5691–5730. doi: 10.1039/D1CS00991E. [DOI] [PubMed] [Google Scholar]
- 136.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The Protein Kinase Complement of the Human Genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 137.Barford D. Protein Phosphatases. Curr. Opin. Struct. Biol. 1995;5:728–734. doi: 10.1016/0959-440X(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 138.Juhaszova M., Zorov D.B., Kim S.H., Pepe S., Fu Q., Fishbein K.W., Ziman B.D., Wang S., Ytrehus K., Antos C.L., et al. Glycogen Synthase Kinase-3beta Mediates Convergence of Protection Signaling to Inhibit the Mitochondrial Permeability Transition Pore. J. Clin. Investig. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kumutima J., Yao X.-Q., Hamelberg D. Post-Translational Modifications of Cyclophilin D Fine-Tune Its Conformational Dynamics and Activity: Implications for Its Mitochondrial Function. J. Phys. Chem. B. 2022;126:10844–10853. doi: 10.1021/acs.jpcb.2c06208. [DOI] [PubMed] [Google Scholar]
- 140.Rinalducci S., Murgiano L., Zolla L. Redox Proteomics: Basic Principles and Future Perspectives for the Detection of Protein Oxidation in Plants. J. Exp. Bot. 2008;59:3781–3801. doi: 10.1093/jxb/ern252. [DOI] [PubMed] [Google Scholar]
- 141.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balaban R.S., Nemoto S., Finkel T. Mitochondria, Oxidants, and Aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 143.Radi R., Cassina A., Hodara R., Quijano C., Castro L. Peroxynitrite Reactions and Formation in Mitochondria. Free Radic. Biol. Med. 2002;33:1451–1464. doi: 10.1016/S0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 144.Linard D., Kandlbinder A., Degand H., Morsomme P., Dietz K.-J., Knoops B. Redox Characterization of Human Cyclophilin D: Identification of a New Mammalian Mitochondrial Redox Sensor? Arch. Biochem. Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 145.Folda A., Citta A., Scalcon V., Calì T., Zonta F., Scutari G., Bindoli A., Rigobello M.P. Mitochondrial Thioredoxin System as a Modulator of Cyclophilin D Redox State. Sci. Rep. 2016;6:23071. doi: 10.1038/srep23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kohr M.J., Sun J., Aponte A., Wang G., Gucek M., Murphy E., Steenbergen C. Simultaneous Measurement of Protein Oxidation and S-Nitrosylation during Preconditioning and Ischemia/Reperfusion Injury with Resin-Assisted Capture. Circ. Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Andreyev A., Fiskum G. Calcium Induced Release of Mitochondrial Cytochrome c by Different Mechanisms Selective for Brain versus Liver. Cell Death Differ. 1999;6:825–832. doi: 10.1038/sj.cdd.4400565. [DOI] [PubMed] [Google Scholar]
- 148.Berman S.B., Watkins S.C., Hastings T.G. Quantitative Biochemical and Ultrastructural Comparison of Mitochondrial Permeability Transition in Isolated Brain and Liver Mitochondria: Evidence for Reduced Sensitivity of Brain Mitochondria. Exp. Neurol. 2000;164:415–425. doi: 10.1006/exnr.2000.7438. [DOI] [PubMed] [Google Scholar]
- 149.Eliseev R.A., Filippov G., Velos J., VanWinkle B., Goldman A., Rosier R.N., Gunter T.E. Role of Cyclophilin D in the Resistance of Brain Mitochondria to the Permeability Transition. Neurobiol. Aging. 2007;28:1532–1542. doi: 10.1016/j.neurobiolaging.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 150.Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., et al. Cyclophilin D Deficiency Attenuates Mitochondrial and Neuronal Perturbation and Ameliorates Learning and Memory in Alzheimer’s Disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Du H., Guo L., Zhang W., Rydzewska M., Yan S. Cyclophilin D Deficiency Improves Mitochondrial Function and Learning/Memory in Aging Alzheimer Disease Mouse Model. Neurobiol. Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gauba E., Guo L., Du H. Cyclophilin D Promotes Brain Mitochondrial F1FO ATP Synthase Dysfunction in Aging Mice. J. Alzheimer’s Dis. 2016;55:1351–1362. doi: 10.3233/JAD-160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luvisetto S., Basso E., Petronilli V., Bernardi P., Forte M. Enhancement of Anxiety, Facilitation of Avoidance Behavior, and Occurrence of Adult-Onset Obesity in Mice Lacking Mitochondrial Cyclophilin D. Neuroscience. 2008;155:585–596. doi: 10.1016/j.neuroscience.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rebolledo-Solleiro D., Roldán-Roldán G., Díaz D., Velasco M., Larqué C., Rico-Rosillo G., Vega-Robledo G.B., Zambrano E., Hiriart M., Pérez de la Mora M. Increased Anxiety-like Behavior Is Associated with the Metabolic Syndrome in Non-Stressed Rats. PLoS ONE. 2017;12:e0176554. doi: 10.1371/journal.pone.0176554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tzioras M., McGeachan R.I., Durrant C.S., Spires-Jones T.L. Synaptic Degeneration in Alzheimer Disease. Nat. Rev. Neurol. 2023;19:19–38. doi: 10.1038/s41582-022-00749-z. [DOI] [PubMed] [Google Scholar]
- 156.O’Brien R.J., Wong P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Friedrich R.P., Tepper K., Rönicke R., Soom M., Westermann M., Reymann K., Kaether C., Fändrich M. Mechanism of Amyloid Plaque Formation Suggests an Intracellular Basis of Abeta Pathogenicity. Proc. Natl. Acad. Sci. USA. 2010;107:1942–1947. doi: 10.1073/pnas.0904532106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hardy J.A., Higgins G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 159.Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W., Xu H.W., Stern D., McKhann G., Du Yan S. Mitochondrial Aβ: A Potential Focal Point for Neuronal Metabolic Dysfunction in Alzheimer’s Disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 160.Casley C.S., Canevari L., Land J.M., Clark J.B., Sharpe M.A. β-Amyloid Inhibits Integrated Mitochondrial Respiration and Key Enzyme Activities. J. Neurochem. 2002;80:91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 161.Abramov A.Y., Canevari L., Duchen M.R. Beta-Amyloid Peptides Induce Mitochondrial Dysfunction and Oxidative Stress in Astrocytes and Death of Neurons through Activation of NADPH Oxidase. J. Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang S., Mims P.N., Roman R.J., Fan F. Is Beta-Amyloid Accumulation a Cause or Consequence of Alzheimer’s Disease? J. Alzheimers Park. Dement. 2016;1:007. [PMC free article] [PubMed] [Google Scholar]
- 163.Imbimbo B.P., Balducci C., Ippati S., Watling M. Initial Failures of Anti-Tau Antibodies in Alzheimer’s Disease Are Reminiscent of the Amyloid-β Story. Neural Regen. Res. 2022;18:117–118. doi: 10.4103/1673-5374.340409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Price J.L., McKeel D.W., Buckles V.D., Roe C.M., Xiong C., Grundman M., Hansen L.A., Petersen R.C., Parisi J.E., Dickson D.W., et al. Neuropathology of Nondemented Aging: Presumptive Evidence for Preclinical Alzheimer Disease. Neurobiol. Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kolarova M., García-Sierra F., Bartos A., Ricny J., Ripova D. Structure and Pathology of Tau Protein in Alzheimer Disease. Int. J. Alzheimer’s Dis. 2012;2012:e731526. doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Du H., Yan S.S. Mitochondrial Permeability Transition Pore in Alzheimer’s Disease: Cyclophilin D and Amyloid Beta. Biochim. Biophys. Acta. 2010;1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh P., Suman S., Chandna S., Das T.K. Possible Role of Amyloid-Beta, Adenine Nucleotide Translocase and Cyclophilin-D Interaction in Mitochondrial Dysfunction of Alzheimer’s Disease. Bioinformation. 2009;3:440–445. doi: 10.6026/97320630003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Drummond E., Wisniewski T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017;133:155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waltereit R., Weller M. Signaling from CAMP/PKA to MAPK and Synaptic Plasticity. Mol. Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 170.Du H., Guo L., Wu X., Sosunov A.A., McKhann G.M., Chen J.X., Yan S.S. Cyclophilin D Deficiency Rescues Aβ-Impaired PKA/CREB Signaling and Alleviates Synaptic Degeneration. Biochim. Biophys. Acta. 2014;1842:2517–2527. doi: 10.1016/j.bbadis.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jara C., Cerpa W., Tapia-Rojas C., Quintanilla R.A. Tau Deletion Prevents Cognitive Impairment and Mitochondrial Dysfunction Age Associated by a Mechanism Dependent on Cyclophilin-D. Front. Neurosci. 2021;14:586710. doi: 10.3389/fnins.2020.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beck S.J., Guo L., Phensy A., Tian J., Wang L., Tandon N., Gauba E., Lu L., Pascual J.M., Kroener S., et al. Deregulation of Mitochondrial F1FO-ATP Synthase via OSCP in Alzheimer’s Disease. Nat. Commun. 2016;7:11483. doi: 10.1038/ncomms11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pérez M.J., Ponce D.P., Aranguiz A., Behrens M.I., Quintanilla R.A. Mitochondrial Permeability Transition Pore Contributes to Mitochondrial Dysfunction in Fibroblasts of Patients with Sporadic Alzheimer’s Disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gauba E., Chen H., Guo L., Du H. Cyclophilin D Deficiency Attenuates Mitochondrial F1Fo ATP Synthase Dysfunction via OSCP in Alzheimer’s Disease. Neurobiol. Dis. 2019;121:138–147. doi: 10.1016/j.nbd.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sui S., Tian J., Gauba E., Wang Q., Guo L., Du H. Cyclophilin D Regulates Neuronal Activity-Induced Filopodiagenesis by Fine-Tuning Dendritic Mitochondrial Calcium Dynamics. J. Neurochem. 2018;146:403–415. doi: 10.1111/jnc.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hamilton J., Brustovetsky T., Brustovetsky N. The Effect of Mitochondrial Calcium Uniporter and Cyclophilin D Knockout on Resistance of Brain Mitochondria to Ca2+-Induced Damage. J. Biol. Chem. 2021;296:100669. doi: 10.1016/j.jbc.2021.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Misrani A., Tabassum S., Yang L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021;13:57. doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Covarrubias A.J., Perrone R., Grozio A., Verdin E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun L., Bhawal R., Xu H., Chen H., Anderson E.T., Haroutunian V., Cross A.C., Zhang S., Gibson G.E. The Human Brain Acetylome Reveals That Decreased Acetylation of Mitochondrial Proteins Associates with Alzheimer’s Disease. J. Neurochem. 2021;158:282–296. doi: 10.1111/jnc.15377. [DOI] [PubMed] [Google Scholar]
- 180.Ping L., Kundinger S.R., Duong D.M., Yin L., Gearing M., Lah J.J., Levey A.I., Seyfried N.T. Global Quantitative Analysis of the Human Brain Proteome and Phosphoproteome in Alzheimer’s Disease. Sci. Data. 2020;7:315. doi: 10.1038/s41597-020-00650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rottenberg H., Hoek J.B. The Mitochondrial Permeability Transition: Nexus of Aging, Disease and Longevity. Cells. 2021;10:79. doi: 10.3390/cells10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rani L., Sahu M.R., Mondal A.C. Age-Related Mitochondrial Dysfunction in Parkinson’s Disease: New Insights into the Disease Pathology. Neuroscience. 2022;499:152–169. doi: 10.1016/j.neuroscience.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 183.Surmeier D.J. Determinants of Dopaminergic Neuron Loss in Parkinson’s Disease. FEBS J. 2018;285:3657–3668. doi: 10.1111/febs.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roberts H.L., Brown D.R. Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules. 2015;5:282–305. doi: 10.3390/biom5020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iljina M., Garcia G.A., Horrocks M.H., Tosatto L., Choi M.L., Ganzinger K.A., Abramov A.Y., Gandhi S., Wood N.W., Cremades N., et al. Kinetic Model of the Aggregation of Alpha-Synuclein Provides Insights into Prion-like Spreading. Proc. Natl. Acad. Sci. USA. 2016;113:E1206–E1215. doi: 10.1073/pnas.1524128113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Luk K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M.-Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mao X., Ou M.T., Karuppagounder S.S., Kam T.-I., Yin X., Xiong Y., Ge P., Umanah G.E., Brahmachari S., Shin J.-H., et al. Pathological α-Synuclein Transmission Initiated by Binding Lymphocyte-Activation Gene 3. Science. 2016;353:aah3374. doi: 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chartier-Harlin M.-C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., et al. Alpha-Synuclein Locus Duplication as a Cause of Familial Parkinson’s Disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 189.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., et al. Alpha-Synuclein Locus Triplication Causes Parkinson’s Disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 190.Schapira A.H., Cooper J.M., Dexter D., Clark J.B., Jenner P., Marsden C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 191.Bindoff L.A., Birch-Machin M., Cartlidge N.E., Parker W.D., Turnbull D.M. Mitochondrial Function in Parkinson’s Disease. Lancet. 1989;2:49. doi: 10.1016/S0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 192.Ramsay R.R., Salach J.I., Dadgar J., Singer T.P. Inhibition of Mitochondrial NADH Dehydrogenase by Pyridine Derivatives and Its Possible Relation to Experimental and Idiopathic Parkinsonism. Biochem. Biophys. Res. Commun. 1986;135:269–275. doi: 10.1016/0006-291X(86)90972-1. [DOI] [PubMed] [Google Scholar]
- 193.Lee E., Hwang I., Park S., Hong S., Hwang B., Cho Y., Son J., Yu J.-W. MPTP-Driven NLRP3 Inflammasome Activation in Microglia Plays a Central Role in Dopaminergic Neurodegeneration. Cell Death Differ. 2019;26:213–228. doi: 10.1038/s41418-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.González-Rodríguez P., Zampese E., Stout K.A., Guzman J.N., Ilijic E., Yang B., Tkatch T., Stavarache M.A., Wokosin D.L., Gao L., et al. Disruption of Mitochondrial Complex I Induces Progressive Parkinsonism. Nature. 2021;599:650–656. doi: 10.1038/s41586-021-04059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nakamura K., Nemani V.M., Azarbal F., Skibinski G., Levy J.M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., et al. Direct Membrane Association Drives Mitochondrial Fission by the Parkinson Disease-Associated Protein Alpha-Synuclein. J. Biol. Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ludtmann M.H.R., Angelova P.R., Horrocks M.H., Choi M.L., Rodrigues M., Baev A.Y., Berezhnov A.V., Yao Z., Little D., Banushi B., et al. α-Synuclein Oligomers Interact with ATP Synthase and Open the Permeability Transition Pore in Parkinson’s Disease. Nat. Commun. 2018;9:2293. doi: 10.1038/s41467-018-04422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schöndorf D.C., Ivanyuk D., Baden P., Sanchez-Martinez A., De Cicco S., Yu C., Giunta I., Schwarz L.K., Di Napoli G., Panagiotakopoulou V., et al. The NAD+ Precursor Nicotinamide Riboside Rescues Mitochondrial Defects and Neuronal Loss in IPSC and Fly Models of Parkinson’s Disease. Cell Rep. 2018;23:2976–2988. doi: 10.1016/j.celrep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 199.Bonello F., Hassoun S.-M., Mouton-Liger F., Shin Y.S., Muscat A., Tesson C., Lesage S., Beart P.M., Brice A., Krupp J., et al. LRRK2 Impairs PINK1/Parkin-Dependent Mitophagy via Its Kinase Activity: Pathologic Insights into Parkinson’s Disease. Hum. Mol. Genet. 2019;28:1645–1660. doi: 10.1093/hmg/ddz004. [DOI] [PubMed] [Google Scholar]
- 200.Yang M., Li C., Yang S., Xiao Y., Xiong X., Chen W., Zhao H., Zhang Q., Han Y., Sun L. Mitochondria-Associated ER Membranes—The Origin Site of Autophagy. Front. Cell Dev. Biol. 2020;8:595. doi: 10.3389/fcell.2020.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wong E., Cuervo A.M. Autophagy Gone Awry in Neurodegenerative Diseases. Nat. Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Surmeier D.J., Nguyen J.T., Lancki N., Venuto C.S., Oakes D., Simuni T., Wyse R.K. Re-Analysis of the STEADY-PD II Trial-Evidence for Slowing the Progression of Parkinson’s Disease. Mov. Disord. 2022;37:334–342. doi: 10.1002/mds.28850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh A., Verma P., Balaji G., Samantaray S., Mohanakumar K.P. Nimodipine, an L-Type Calcium Channel Blocker Attenuates Mitochondrial Dysfunctions to Protect against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonism in Mice. Neurochem. Int. 2016;99:221–232. doi: 10.1016/j.neuint.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 204.Guzman J.N., Ilijic E., Yang B., Sanchez-Padilla J., Wokosin D., Galtieri D., Kondapalli J., Schumacker P.T., Surmeier D.J. Systemic Isradipine Treatment Diminishes Calcium-Dependent Mitochondrial Oxidant Stress. J. Clin. Invest. 2018;128:2266–2280. doi: 10.1172/JCI95898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Costantini P., Petronilli V., Colonna R., Bernardi P. On the Effects of Paraquat on Isolated Mitochondria. Evidence That Paraquat Causes Opening of the Cyclosporin A-Sensitive Permeability Transition Pore Synergistically with Nitric Oxide. Toxicology. 1995;99:77–88. doi: 10.1016/0300-483X(94)02997-9. [DOI] [PubMed] [Google Scholar]
- 206.Seaton T.A., Cooper J.M., Schapira A.H. Cyclosporin Inhibition of Apoptosis Induced by Mitochondrial Complex I Toxins. Brain Res. 1998;809:12–17. doi: 10.1016/S0006-8993(98)00790-2. [DOI] [PubMed] [Google Scholar]
- 207.Thomas B., Banerjee R., Starkova N.N., Zhang S.F., Calingasan N.Y., Yang L., Wille E., Lorenzo B.J., Ho D.J., Beal M.F., et al. Mitochondrial Permeability Transition Pore Component Cyclophilin D Distinguishes Nigrostriatal Dopaminergic Death Paradigms in the MPTP Mouse Model of Parkinson’s Disease. Antioxid. Redox Signal. 2012;16:855–868. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Martin L.J., Semenkow S., Hanaford A., Wong M. The Mitochondrial Permeability Transition Pore Regulates Parkinson’s Disease Development in Mutant α-Synuclein Transgenic Mice. Neurobiol. Aging. 2014;35:1132–1152. doi: 10.1016/j.neurobiolaging.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sison S.L., Ebert A.D. Decreased NAD+ in Dopaminergic Neurons. Aging. 2018;10:526–527. doi: 10.18632/aging.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Murata H., Khine C.C., Nishikawa A., Yamamoto K.-I., Kinoshita R., Sakaguchi M. C-Jun N-Terminal Kinase (JNK)-Mediated Phosphorylation of SARM1 Regulates NAD+ Cleavage Activity to Inhibit Mitochondrial Respiration. J. Biol. Chem. 2018;293:18933–18943. doi: 10.1074/jbc.RA118.004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shi H., Deng H.-X., Gius D., Schumacker P.T., Surmeier D.J., Ma Y.-C. Sirt3 Protects Dopaminergic Neurons from Mitochondrial Oxidative Stress. Hum. Mol. Genet. 2017;26:1915–1926. doi: 10.1093/hmg/ddx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torpey J., Madine J., Wood A., Lian L.-Y. Cyclophilin D Binds to the Acidic C-Terminus Region of α-Synuclein and Affects Its Aggregation Characteristics. Sci. Rep. 2020;10:10159. doi: 10.1038/s41598-020-66200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Grassi D., Howard S., Zhou M., Diaz-Perez N., Urban N.T., Guerrero-Given D., Kamasawa N., Volpicelli-Daley L.A., LoGrasso P., Lasmézas C.I. Identification of a Highly Neurotoxic α-Synuclein Species Inducing Mitochondrial Damage and Mitophagy in Parkinson’s Disease. Proc. Natl. Acad. Sci. USA. 2018;115:E2634–E2643. doi: 10.1073/pnas.1713849115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ludtmann M.H.R., Angelova P.R., Ninkina N.N., Gandhi S., Buchman V.L., Abramov A.Y. Monomeric Alpha-Synuclein Exerts a Physiological Role on Brain ATP Synthase. J. Neurosci. 2016;36:10510–10521. doi: 10.1523/JNEUROSCI.1659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barsukova A.G., Bourdette D., Forte M. Mitochondrial Calcium and Its Regulation in Neurodegeneration Induced by Oxidative Stress: Neuronal Calcium during Oxidative Stress. Eur. J. Neurosci. 2011;34:437–447. doi: 10.1111/j.1460-9568.2011.07760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Qadri R., Namdeo M., Behari M., Goyal V., Sharma S., Mukhopadhyay A.K. Alterations in Mitochondrial Membrane Potential in Peripheral Blood Mononuclear Cells in Parkinson’s Disease: Potential for a Novel Biomarker. Restor. Neurol. Neurosci. 2018;36:719–727. doi: 10.3233/RNN-180852. [DOI] [PubMed] [Google Scholar]
- 217.Stocco A., Smolina N., Sabatelli P., Šileikytė J., Artusi E., Mouly V., Cohen M., Forte M., Schiavone M., Bernardi P. Treatment with a Triazole Inhibitor of the Mitochondrial Permeability Transition Pore Fully Corrects the Pathology of Sapje Zebrafish Lacking Dystrophin. Pharmacol. Res. 2021;165:105421. doi: 10.1016/j.phrs.2021.105421. [DOI] [PubMed] [Google Scholar]
- 218.Polimeni G., Esposito E., Bevelacqua V., Guarneri C., Cuzzocrea S. Role of Melatonin Supplementation in Neurodegenerative Disorders. Front. Biosci. 2014;19:429–446. doi: 10.2741/4217. [DOI] [PubMed] [Google Scholar]
- 219.Jou M.-J. Melatonin Preserves the Transient Mitochondrial Permeability Transition for Protection during Mitochondrial Ca2+ Stress in Astrocyte. J. Pineal Res. 2011;50:427–435. doi: 10.1111/j.1600-079X.2011.00861.x. [DOI] [PubMed] [Google Scholar]
- 220.Andrabi S.A., Sayeed I., Siemen D., Wolf G., Horn T.F.W. Direct Inhibition of the Mitochondrial Permeability Transition Pore: A Possible Mechanism Responsible for Anti-Apoptotic Effects of Melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]
- 221.Reiter R.J., Tan D.X., Rosales-Corral S., Galano A., Jou M.-J., Acuna-Castroviejo D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018;19:2439. doi: 10.3390/ijms19082439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang M., Herrmann C.J., Simonovic M., Szklarczyk D., von Mering C. Version 4.0 of PaxDb: Protein Abundance Data, Integrated across Model Organisms, Tissues, and Cell-Lines. Proteomics. 2015;15:3163–3168. doi: 10.1002/pmic.201400441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rosenwirth B., Billich A., Datema R., Donatsch P., Hammerschmid F., Harrison R., Hiestand P., Jaksche H., Mayer P., Peichl P. Inhibition of Human Immunodeficiency Virus Type 1 Replication by SDZ NIM 811, a Nonimmunosuppressive Cyclosporine Analog. Antimicrob. Agents Chemother. 1994;38:1763–1772. doi: 10.1128/AAC.38.8.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma S., Boerner J.E., TiongYip C., Weidmann B., Ryder N.S., Cooreman M.P., Lin K. NIM811, a Cyclophilin Inhibitor, Exhibits Potent In Vitro Activity against Hepatitis C Virus Alone or in Combination with Alpha Interferon. Antimicrob. Agents Chemother. 2006;50:2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ptak R.G., Gallay P.A., Jochmans D., Halestrap A.P., Ruegg U.T., Pallansch L.A., Bobardt M.D., de Béthune M.-P., Neyts J., De Clercq E., et al. Inhibition of Human Immunodeficiency Virus Type 1 Replication in Human Cells by Debio-025, a Novel Cyclophilin Binding Agent. Antimicrob. Agents Chemother. 2008;52:1302–1317. doi: 10.1128/AAC.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Flisiak R., Horban A., Gallay P., Bobardt M., Selvarajah S., Wiercinska-Drapalo A., Siwak E., Cielniak I., Higersberger J., Kierkus J., et al. The Cyclophilin Inhibitor Debio-025 Shows Potent Anti-Hepatitis C Effect in Patients Coinfected with Hepatitis C and Human Immunodeficiency Virus. Hepatology. 2008;47:817–826. doi: 10.1002/hep.22131. [DOI] [PubMed] [Google Scholar]
- 227.Cung T.-T., Morel O., Cayla G., Rioufol G., Garcia-Dorado D., Angoulvant D., Bonnefoy-Cudraz E., Guérin P., Elbaz M., Delarche N., et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 228.Javadov S., Jang S., Parodi-Rullán R., Khuchua Z., Kuznetsov A.V. Mitochondrial Permeability Transition in Cardiac Ischemia-Reperfusion: Whether Cyclophilin D Is a Viable Target for Cardioprotection? Cell Mol. Life Sci. 2017;74:2795–2813. doi: 10.1007/s00018-017-2502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Haleckova A., Benek O., Zemanová L., Dolezal R., Musilek K. Small-Molecule Inhibitors of Cyclophilin D as Potential Therapeutics in Mitochondria-Related Diseases. Med. Res. Rev. 2022;42:1822–1855. doi: 10.1002/med.21892. [DOI] [PubMed] [Google Scholar]
- 230.Panel M., Ruiz I., Brillet R., Lafdil F., Teixeira-Clerc F., Nguyen C.T., Calderaro J., Gelin M., Allemand F., Guichou J.-F., et al. Small-Molecule Inhibitors of Cyclophilins Block Opening of the Mitochondrial Permeability Transition Pore and Protect Mice from Hepatic Ischemia/Reperfusion Injury. Gastroenterology. 2019;157:1368–1382. doi: 10.1053/j.gastro.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 231.Panel M., Ahmed-Belkacem A., Ruiz I., Guichou J.-F., Pawlotsky J.-M., Ghaleh B., Morin D. A Phenyl-Pyrrolidine Derivative Reveals a Dual Inhibition Mechanism of Myocardial Mitochondrial Permeability Transition Pore, Which Is Limited by Its Myocardial Distribution. J. Pharmacol. Exp. Ther. 2021;376:348–357. doi: 10.1124/jpet.120.000359. [DOI] [PubMed] [Google Scholar]
- 232.Waldmeier P.C., Zimmermann K., Qian T., Tintelnot-Blomley M., Lemasters J.J. Cyclophilin D as a Drug Target. Curr. Med. Chem. 2003;10:1485–1506. doi: 10.2174/0929867033457160. [DOI] [PubMed] [Google Scholar]
- 233.Valasani K.R., Sun Q., Fang D., Zhang Z., Yu Q., Guo Y., Li J., Roy A., ShiDu Yan S. Identification of a Small Molecule Cyclophilin D Inhibitor for Rescuing Aβ-Mediated Mitochondrial Dysfunction. ACS Med. Chem. Lett. 2016;7:294–299. doi: 10.1021/acsmedchemlett.5b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Agrawal M., Ajazuddin, Tripathi D.K., Saraf S., Saraf S., Antimisiaris S.G., Mourtas S., Hammarlund-Udenaes M., Alexander A. Recent Advancements in Liposomes Targeting Strategies to Cross Blood-Brain Barrier (BBB) for the Treatment of Alzheimer’s Disease. J. Control. Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 235.Rinaldi C., Wood M.J.A. Antisense Oligonucleotides: The next Frontier for Treatment of Neurological Disorders. Nat. Rev. Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 236.Min H.S., Kim H.J., Naito M., Ogura S., Toh K., Hayashi K., Kim B.S., Fukushima S., Anraku Y., Miyata K., et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew. Chem. Int. Ed. 2020;59:8173–8180. doi: 10.1002/anie.201914751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.