Abstract
Background: Breast cancer is the most common malignant tumor and cause of death in women. Factors that play role in tumor metastasis are lymph node involvement, lack of tumor differentiation and hormone receptor expression, high proliferation rate, and angiogenesis. In the present study, we tried to evaluate the microvessel density (MVD) using Immunohistochemistry for the CD34 marker to investigate the amount of angiogenesis in breast cancer and its relationship with other histopathological parameters and compare it with normal tissue.
Materials and Methods: 58 paraffin-embedded samples of breast cancer were enrolled. All blocks were
sectioned and stained for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2(HER 2/neu), ki67, and CD34 by immunohistochemistry (IHC) method.
Results: The mean age of patients in this study was 49.6 ± 10.6 years. Statistically, there was a significant relationship between the grade of the tumor (P = 0.01), absence of expression of estrogen receptor (P = 0.008), and progesterone receptor (P = 0.003) with MVD.
Conclusion: Due to the association between MVD, tumor grade, and absence of ER and PR expression, this valuable marker can play an important role in the prediction of prognosis in breast cancer patients and can lead to new-targeted therapy in the future.
Key Words: Breast cancer, Microvessel density, Angiogenesis
Introduction
Breast cancer is the most common cancer among women worldwide1,2,3 and metastasize through the lympho-vascular system 4-5.
Tumor size, patient's age, existence of vascular invasion and axillary lymph node involvement, and hormone receptor status are all clinicopathologic characteristics that influence the prognosis of breast cancer. These prognostic indicators aid in the assessment of illness outcome and the selection of targeted therapy1,3.
Angiogenesis is also one of the important factors in tumor metastasis 6-7-8. The most used technique to quantify angiogenesis is microvessel density (MVD). It is measured by counting small vessels in the tumor tissue by means of immunohistochemical staining. [1]
Many studies revealed that MVD correlates with advanced pathologic stage and poor prognosis of disease in different cancers1.
The aim of this study was to evaluate the MVD in breast cancer based on CD34 expression and its relationship with histopathological parameters.
MTERIALS AND METHODS
In this retrospective study, a total of 58 consecutive patients, who were diagnosed with invasive ductal carcinoma and underwent an excisional biopsy, lumpectomy, or modified radical mastectomy were enrolled. Paraffin-embedded blocks of samples collected by pathology department of Urmia University of Medical Sciences (UMSU), Urmia, Iran, between 2015 and 2017. Sample’s immunohistochemistry (IHC) staining results for hormone profile including estrogen receptor (ER) , progesterone receptor (PR) , human epidermal growth factor receptor 2 (HER2/neu) and Ki67 were also included. The prepared glass slides ( Hematoxylin and eosin (H&E) and IHC preparations ) obtained from the archive and were investigated by two pathologists. Tumor grading and staging were performed according to Nottingham modification of Bloom Richardson system and the American Joint Committee on Cancer(AICC) system, respectively. Histopathologic type of tumor, lymphovascular, perineural, and lymphatic invasions were re-evaluated. If the glass slides were broken or of poor quality, new sections were made and H&E staining was done. The study was approved by the Ethics Committee of UMSU.
Microvessel Density
Microvessel density (MVD) was evaluated according to Weidner method9. Mean MVD was 16.10 ± 6.37 and the median was 20. Therefore, tumors with MVD more than 20 were considered as high MVD, and tumors with MVD less than 20 were considered as low MVD.
Statistical analysis
The results were expressed as mean ±SD. SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) software package was used to investigate whether there is a significant relationship between all findings. The normality of data was evaluated with the Kolmogorov Smirnov test. The statistical differences between proportions were determined by χ2 analysis. Numerical data were evaluated using analysis of variance, followed by Tukey's post hoc test. P-values ≤ 0.05 were considered statistically significant.
Results
In this study, the age of patients ranged between 29 and 72 years. (Mean age was 49.6 ± 10.6) Left breast was involved in 58.6% (34 patients) and the right side was involved in 41.4% (24 patients). Mean size of tumor was 4.5 ± 2.6. In terms of tumor grade, 3(5.2%) were grade I, 30 (51.7%) were grade II, and 25(43.1%) were grade III.
Immunohistochemistry staining for ER, PR and HER2/neu showed that, of 58 cases, 32 (55.2%) were ER positive, 33 (56.9%) were PR positive, and 21 (36.2%) were Her2/neu positive. Patient ʼs demographic data and tumor characteristics are mentioned in Table 1 in details.
Table 1.
Demographic data of the enrolled cases
| Number (percent) | Microvessel density | P | |||
|---|---|---|---|---|---|
| Low (≤20) | High (>20) | ||||
| Histological grade: | Grade 1 | 3 (5.2%) | 3 (7.3%) | ||
| Grade 2 | 30 (51.7%) | 24 (58.5%) | 2 (18.1%) | 0.018** | |
| Grade 3 | 25 (43.1%) | 14 (34.2%) | 9 (81.9%) | ||
| Tumor site: | Right | 24 (41.4%) | 18 | 2 | |
| Left | 33 (56.9%) | 22 | 9 | ||
| Bilateral | 1 (1.7%) | 1 | - | ||
| Tumor size: | <2 cm | 7 (12.1%) | 4 | 1 | |
| 2_5 cm | 39 (67.2%) | 31 | 7 | ||
| >5 cm | 12 (20.7%) | 6 | 3 | ||
| Lymph-vascular invasion: |
Present | 44 (75.9%) | 29 (70.7%) | 10 (90.9%) | 0.17 |
| Not identified | 14 (24.1%) | 12 (29.3%) | 1 (9.1%) | ||
| Perineural invasion: | Present | 22 (37.9%) | 18 (43.9%) | 3 (27.2%) | 0.39 |
| Not identified | 36 (62.1%) | 22 (56.1%) | 7 (63.6%) | ||
| Nipple involvement | Present | 10 (17.2 %) | 5 (12.1%) | 4 (36.3%) | 0.06 |
| Not identified | 48 (82.8%) | 35 (85.3%) | 7 (63.7%) | ||
| Skin involvement | Present | 12 (20.7%) | 7 (17%) | 4 (36.3%) | 0.17 |
| Not identified | 46 (79.3%) | 33 (80.4%) | 7 (63.7%) | ||
| Axillary lymph node involvement: |
Present | 45 (77.6%) | 34 (83%) | 7 (63.7%) | 0.164 |
| Not identified | 13 (22.4%) | 7 (17%) | 4 (36.3%) | ||
| Estrogen receptor: | Positive | 32 (55.2 %) | 26 (63.4%) | 2 (18.1%) | 0.008** |
| Negative | 26 (44.8%) | 15 (36.6%) | 9 (81.9%) | ||
| Progestrone receptor: |
Positive | 33 (56.9%) | 28 (68.2%) | 2 (18.1%) | 0.003** |
| Negative | 25 (43.1%) | 13 (31.8%) | |||
| Her 2: | Positive | 21 (36.2%) | 12 (29.2%) | 6 (54.5%) | 0.118 |
| Negative | 37 (63.8%) | 29 (70.8%) | 5 (45.5%) | ||
| Tumor stage: | 1 | 1 (1.7%) | 1 (2.5%) | 9 (81.9%) | |
| 2 | 31 (53.4%) | 21 (51.2%) | 6 (54.5 %) | 0.886 | |
| 3 | 26 (44.9%) | 19 (46.3%) | 5 (45.5%) | ||
*Her2: Human epidermal growth factor receptor2
P value < 0.05 is significant.
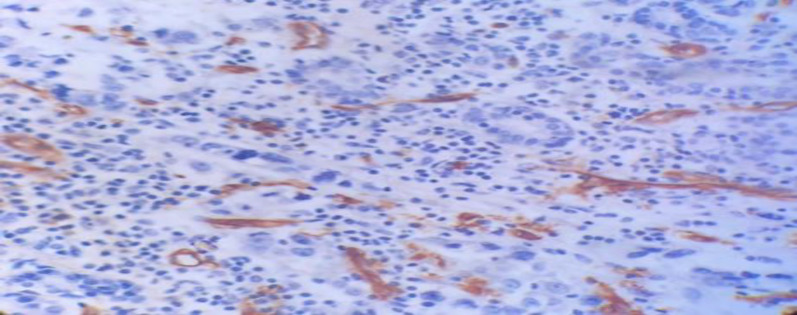
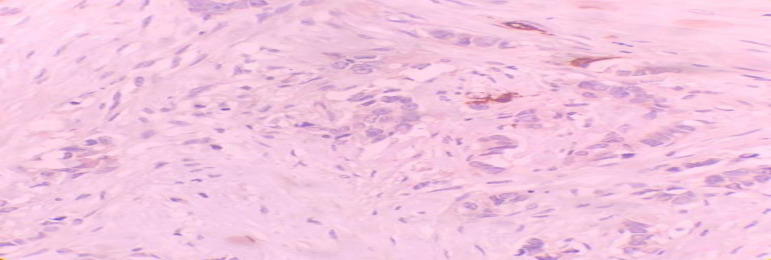
After CD34 staining, microvessel density (MVD) was evaluated according to Weidner method. Mean MVD was 16.10 ± 6.37 and the median was 20. So, tumors with MVD more than 20 were considered as high MVD “…shown in Figure 1…” and tumors with MVD less than 20 were considered as low MVD “…shown in Figure 2…” 41 patients (70.7%) had low MVD, 11(19%) have high MVD and in six patients (10.3%) tumor could not be assessed. Statistical analysis showed significant relationship between MVD and histological grade of tumor (P=0.018) which the higher the tumor grade the higher the MVD.
Figure1.
Microvascular proliferation in breast cancer stained by CD34 marker, showing high vascular proliferation (arrow), (IHC, 10X)
Figure 2.
Microvascular proliferation in breast cancer stained by CD34 marker, showing very low vascular proliferation (arrow), (IHC, 10X)
Moreover, there was an inverse association between MVD and both ER (P=0.008) and PR expression (P=0.003). However, no association was found between MVD and tumor stage, perineural and lymphovascular invasions, nipple, skin and axillary lymph node involvement and Her2 expression (P=0.886, P=0.39, P=0.17, P=0.06, P=0.17, P=0.164, and P=118, respectively). The relationship between MVD and clinicopathological parameters is shown in Table 1.
Discussion
Breast cancer is the most common cancer among women worldwide5. The incidence of breast cancer in Iran is lower compared to the neighboring countries11, and above 30 % of patients are under the age of 30. In contrast, in western countries, only 6% are under 4011,12. In this study, the mean age of the patients was 49.6 ± 10.6 years.
Today, using biomarkers is becoming the method of choice in diagnosis of neoplastic diseases. Although the most important clinicopathologic factors in biologic behavior and treatment of breast cancer are patients age , tumor size and histologic type , axillary lymph node involvement, vascular invasion and estrogen , progesterone and human epidermal growth factor receptor 2 (also known as ER,PR and HER2, respectively) expression1, the identification of more specific markers would help us making better treatment decisions.
One of the most important prognostic factors in breast cancer is metastasis. Lymph node involvement, lack of differentiation and hormone receptor expression, high proliferation rate, and angiogenesis are important predictors of metastasis6,7. Angiogenesis is one of the features of malignancy playing a vital role in tumor growth, local invasion, and distant metastasis13. Tumors may regress and undergo necrosis in the absence of vascular development and angiogenesis. One of the most useful techniques to quantify angiogenesis is microvessel density (MVD). Previous studies have shown that tumor microvascular density (MVD) correlates with the aggressiveness of multiple tumors1,14. In this study, the mean MVD was measured in 52 cases and was 16.10 ± 6.37. Our study revealed a statistically significant relationship between MVD and tumor grade, as higher MVD was seen in higher tumor grades. Moreover, we found a higher MVD in tumors without ER and PR expression. Similar to our results, Bujor et al. showed a significant relationship between high microvessel density, tumor grade and lack of estrogen, and progesterone receptor expression13. We found no significant statistical relationship between lympho-vascular invasion, perineural invasion, nipple, skin or axillary lymph node involvement, HER2 expression, and tumor stage with microvessel density. Similar to our results, Chen et al. showed that there was no relationship between tumor size, lympho-vascular invasion, lymph node involvement, and HER2 expression with high CD34 expression. They showed high CD34 expression in 27.3 % of cases 10.
Consistent studies by Sener et al. have also shown that MVD has no significant association with a patient’s age, tumor grade, vascular invasion, and HER2 expression1.
They have also found no relationship between MVD and ER and PR expression, which is inconsistent with our experiment1. The results of Biesaga et al. are also similar to ours. They have shown a significant association between MVD and lack of ER and PR expression13.
Some other consistent studies have also reported no relationship between MVD and axillary lymph node metastasis 15,16. However, the study of Bosari et al. has shown that MVD in patients with lymph node involvement is significantly higher than that in patients without lymph node involvement 17.
There are different results in the articles regarding the relationship between MVD and prognostic factors, which may be due to a variety of reasons. One is the calculation methods of MVD (identification of hot-spot area versus counting a single area)1, and the other is using different antibodies such as CD31, CD34, CD105, and Factor VIII to detect microvessels. These markers have different specificities in staining vascular structure1.
Statements of ethics
The study was conducted according to the World Medical Association Declaration of Helsinki, and the study was confirmed by the Ethics Committee of Urmia University of Medical Sciences. The written informed consent was obtained from all participants enrolled in this study.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
Funding sources
The study was funded by research grants from Urmia University of Medical Sciences.
References
- 1.Şener E, Şipal S, Gündoğdu C. Comparison of microvessel density with prognostic factors in invasive ductal carcinomas of the breast. Turk Patoloji Derg. 2016;32(3):164–70. doi: 10.5146/tjpath.2016.01366. [DOI] [PubMed] [Google Scholar]
- 2.Esfarjani S, Ahvazi N, Doberjovi M, et al. Comparative study of some breast cancer risk factors in patients with breast cancer and leukemia in Ahvaz Shafa Hospital. Sci Medl J. 2010;9(3):263–70. [Google Scholar]
- 3.Taghavi A, Fazeli Z, Vahedi M, et al. Increased trend of breast cancer mortality in Iran. Asian Pac J Cancer Prev. 2012;13(1):367–70. doi: 10.7314/apjcp.2012.13.1.367. [DOI] [PubMed] [Google Scholar]
- 4.Nasir A, Holzer TR, Chen M, et al. Differential expression of VEGFR2 protein in HER2 positive primary human breast cancer: potential relevance to anti-angiogenic therapies. Cancer cell int. 2017;17(1):56. doi: 10.1186/s12935-017-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Li J, Wang B, et al. Tumor angiogenesis and bone metastasis-Correlation in invasive breast carcinoma. J Immunol Methods. 2018;452:46–52. doi: 10.1016/j.jim.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Chandran VI, Eppenberger-Castori S, Venkatesh T, et al. HER2 and uPAR cooperativity contribute to metastatic phenotype of HER2-positive breast cancer. Oncoscience. 2015;2(3):207–24. doi: 10.18632/oncoscience.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampelj M, Arko D, Cas-Sikosek N, et al. Urokinase plasminogen activator (uPA) and plasminogen activator inhibitor type-1 (PAI-1) in breast cancer-correlation with traditional prognostic factors. Radiol oncol. 2015;49(4):357–64. doi: 10.2478/raon-2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraby MR, Opdahl S, Akslen LA, et al. Quantifying tumour vascularity in non-luminal breast cancers. J clin pathol. 2017;70(9):766–74. doi: 10.1136/jclinpath-2016-204208. [DOI] [PubMed] [Google Scholar]
- 9.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Xu S, Xu W, et al. Expression of cluster of differentiation 34 and vascular endothelial growth factor in breast cancer, and their prognostic significance. Oncol lett. 2015;10(2):723–9. doi: 10.3892/ol.2015.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jazayeri SB, Saadat S, Ramezani R, et al. Incidence of Primary Breast Cancer in Iran: Ten-year National Cancer Registry Data Report. Cancer Epidemiol. 2015;39(4):519–27. doi: 10.1016/j.canep.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Goldblum JR, Mckenney JK, Lamps LW, Myers JL. Rosai and Ackerman's surgical pathology. 11th edn. Philadelphia, PA, USA: Elsevier Health Sciences; 2018. [Google Scholar]
- 13.Bujor IS, Cioca A, CEAUȘU RA, et al. Evaluation of Vascular Proliferation in Molecular Subtypes of Breast Cancer. In Vivo. 2018;32(1):79–83. doi: 10.21873/invivo.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iakovlev VV, Gabril M, Dubinski W, et al. Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Lab Invest. 2012;92(1):46–56. doi: 10.1038/labinvest.2011.153. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Kimura T, Ishii N, et al. The methodology of quantitation of microvessel density and prognostic value of neovascularization associated with long‐term survival in Japanese patients with breast cancer. Breast cancer Res Treat. 1999;53(1):19–31. doi: 10.1023/a:1006193024382. [DOI] [PubMed] [Google Scholar]
- 16.Jyotsna NB, Poonam R, Vinay K, et al. Angiogenesis in breast cancer and its correlation with estrogen, progesterone receptors and other prognostic factors. J Clin Diagn Res . 2015;9(1):EC05–7. doi: 10.7860/JCDR/2015/10591.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosari S, Lee AK, DeLellis RA, et al. Microvessel quantitation and prognosis in invasive breast carcinoma. Human pathol. 1992;23(7):755–61. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]