Abstract
Objective
To evaluate the persistence and effectiveness of TNF inhibitors (TNFi) vs non-TNFi among newly diagnosed JIA patients after initiation of biologic DMARD (bDMARD).
Methods
Using longitudinal patient-level data extracted from electronic medical records in a large Midwestern paediatric hospital from 2009 to 2018, we identified JIA patients initiating TNFi and non-TNFi treatment. Treatment effectiveness was assessed based on disease activity. Inverse probability of treatment weighting of propensity score was used to estimate the treatment effectiveness and Kaplan–Meier analyses were conducted to assess persistence.
Results
Of 667 JIA patients, most (92.0%) were prescribed one of the class of TNFi as their initial biologic treatment. Etanercept was the most frequently prescribed (67.1%) treatment, followed by adalimumab (27.5%). Only around 5% of patients were prescribed off-label bDMARDs as their first-course treatment; however, >20% were prescribed off-label biologics as their second-course therapy. Some 7.2% of patients received four or more bDMARDs. The median persistence of the first-course bDMARD is 320 days, with TNFi being significantly longer than the non-TNFi (395 vs 320 days, P = 0.010). The clinical Juvenile Disease Activity Score (cJADAS) reduction of TNFi users (6.6, 95% CI 5.7, 7.5) was significant greater compared with non-TNFi users (3.0, 95% CI 1.5, 4.6, P < 0.0001) at 6-month follow-up visit.
Conclusion
Persistence was significantly longer among patients initiating TNFi as their first biologic therapy than those receiving non-TNFi. Patients receiving TNF therapy had significant greater reduction of cJADAS at the 6-month follow-up visit compared with patients in the non-TNF cohort.
Keywords: juvenile idiopathic arthritis, biological therapy, prescribing patterns, treatment outcome, anti-TNFi
Rheumatology key message
Switching between biologic DMARDs occurs frequently in daily clinical practice.
Persistence was significantly longer among patients receiving TNF inhibitors (TNFi) as their first biologic therapy.
Patients with TNFi therapy had significant greater reduction of cJADAS at the 6-month follow-up visit compared with non-TNFi cohort.
Introduction
JIA is a heterogeneous collection of inflammatory arthritis diseases with onset before a child turns 16 years old in which the arthritis persists for a minimum of 6 weeks during which no other cause is identified [1, 2]. JIA demonstrates a significant negative impact on quality of life. The primary goals of treatment are to improve the patient’s health through control of the inflammation, which improves the symptoms, prevents joint damage, mitigates disease progression, maximizes health-related quality of life, and minimizes or avoids disability [3–6].
Several biologic agents have been recently approved for JIA treatment. There are now multiple therapies available for JIA, increasing the complexity of treatment decisions [7, 8]. TNF inhibitors (TNFi) have proven to be particularly effective in polyarthritis treatment and are increasingly available [7, 9, 10]. Etanercept, a TNFi, was approved by the US Food and Drug Administration (FDA) for JIA earlier than other biologic DMARDs (bDMARDs), and many patients were prescribed etanercept as their initial bDMARD. The second TNFi approved, adalimumab, serves as another popular choice for JIA therapy. In the USA, etanercept and adalimumab are the only two TNFi agents approved for children with JIA [11]. Golimumab and infliximab have been approved for treating JIA in Europe by the European Medicines Agency, but are not approved by the FDA for children in the USA.
In 2019, the ACR published a set of treatment recommendations to assist in selecting safe and effective therapies for non‐systemic JIA. These guidelines offered expanded options for utilizing biologic agents, specifically options for agents other than TNFi [2, 12]. Currently, there are two non-TNFi approved for the indication of non-systemic JIA: a T-cell activation inhibitor (CTLA-4Ig; abatacept) and an anti-IL-6 receptor antagonist (tocilizumab). However, there are several medications used off-label for JIA in the USA.
Little is known about how TNFi compare to non-TNFi in terms of clinical effectiveness and safety for JIA treatment. Although several studies have compared biologic agents with different mechanisms, they targeted narrow treatment strategies and focused only on adult RA [13–22]. A comparative effectiveness study with over 9000 adult RA patients found that treatment responses appeared better for patients starting on non-TNFi compared with TNFi as first-course treatment [17]. Few studies have addressed polyarticular JIA, and those studies used only a few randomized controlled trials with placebo to make comparisons [23–25]. Moreover, no studies have looked at non-TNFi in comparison with TNFi in children with JIA. Without a better understanding of the comparative effectiveness and safety of these numerous options, the physician’s task of choosing between them is more difficult.
This study examined children in six of the seven JIA subcategories, including oligoarthritis, polyarthritis RF-positive, polyarthritis RF-negative, enthesitis-related arthritis (ERA), PsA and undifferentiated JIA, with the only exclusion being systemic JIA. The objective of the study was to compare the treatment effectiveness of TNFi vs non-TNFi on JIA disease activity outcomes. We analysed the persistence of different treatments and described the patterns of switching to alternative bDMARDs starting from the first bDMARD therapy.
Methods
Study design
This cohort study used longitudinal patient-level data from a large Midwestern paediatric rheumatology centre in the USA, from 1 January 2009 to 31 December 2018. The study data were extracted from electronic medical records (EMRs), including information on demographics, medical history, laboratory results, drug prescriptions and disease outcomes. This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Patients
During this 10-year time frame, the EMRs contained a total of 2082 patients diagnosed with JIA. Detailed patient eligibility screening is shown in supplementary Fig. S1, available at Rheumatology online. The study included all patients <19 years of age with a diagnosis of non-systemic JIA who received at least one dose of bDMARD and had at least one follow-up visit. Patients with missing diagnosis date were excluded, as we could not determine whether the first bDMARD was captured in the EMRs. Patients who met the inclusion and exclusion criteria were stratified by the ILAR six disease subcategories of non-systemic JIA: oligoarthritis, polyarthritis RF-positive, polyarthritis RF-negative, ERA, PsA and undifferentiated JIA.
Persistence
Persistence was defined as the duration from treatment initiation to discontinuation. Treatment initiation (i.e. the index date) was defined as the date on which a patient first was prescribed their (first- or second-ever) biologic agent. Discontinuation date was defined as the date when the prescription was discontinued or switched to another biologic agent for any reason. Temporary stops shorter than 90 consecutive days, commonly for surgery or adverse events, followed by recommencement of the same biologic agents, were not considered discontinuations [26, 27].
Treatment
After first-course therapy (i.e. first-ever bDMARD), a next course of treatment was identified if the prescription was discontinued (i.e. stopped for a minimum of 90 days) and then, within 12 months of the discontinuation date, the patient received a new bDMARD prescription (either the same or different as the previous course). Medications in the study population included the following: bDMARDs (TNFi: etanercept, adalimumab, infliximab, certolizumab pegol and golimumab; non-TNFi: abatacept, anakinra, canakinumab and tocilizumab), and conventional synthetic DMARDS (MTX, HCQ, SSZ and LEF). Glucocorticoids include intra-articular glucocorticoid injections (IAGCIs) and systemic glucocorticoids (triamcinolone dexamethasone, betamethasone methylprednisolone, prednisone and prednisolone). All medications were identified by their brand and generic name within the EMR databases (supplementary Table S1, available at Rheumatology online).
Assessment of disease activity
Treatment response was evaluated using the clinical Juvenile Disease Activity Score (cJADAS) with active joint counts truncated at 10 (cJADAS-10) [28]. The cJADAS-10 ranges from 0 to 30, with higher values indicating greater disease activity. The cutoff value of 5 was used to separate inactive/low disease activity from moderate/high disease activity [28]. Moderate and high disease activity were grouped together because of similar treatment approaches [29]. Based on their cJADAS, we recorded whether patients achieved inactive/low disease activity at 6 months (the visit closest to 180 days, within a window of 90–274 days) and at 12 months (the visit closest to 365 days, within a window of 275–455 days) after treatment initiation. Treatment response was also evaluated based on the number of joints with limited range of motion and patient-reported pain level. Pain levels range from 0–10, with 0 representing ‘no pain’ and 10 representing ‘unbearable pain’.
Covariates
The study database contains comprehensive patient-level information on demographics, disease characteristics, clinical assessments and laboratory results. The demographic parameters extracted were age, gender, race, ethnicity and health insurance status. Disease characteristics included JIA category, disease duration, date of diagnosis, date of symptom onset and calendar year of patient receiving their first biologic therapy. The utilized clinical assessments were cJADAS, the physician’s global assessment of disease activity, the parent’s/patient’s assessment of overall well-being, ESR (mm/h), RF (positive/negative), ANA (positive/negative), active joint count, the number of joints with limited range of motion, report of morning stiffness, patient-reported pain level and report of uveitis (yes/no). Each of these covariates were considered in the analysis. A patient’s previous treatments were also considered covariates, including any prior conventional synthetic DMARDs (yes/no), prior NSAID treatment (yes/no), or prior glucocorticoid and/or IAGCI treatment (yes/no). All clinical findings were determined by faculty level board-certified paediatric rheumatologists.
Statistical analysis
Patient demographics and baseline disease characteristics were summarized and assessed using descriptive statistics. Patient characteristics were compared between cohorts using standardized mean differences, before and after weighting. When comparing survival rates between TNFi and non-TNFi as well as between individual medications, only first- and second-course therapy were considered. Kaplan–Meier analysis was used to estimate the survival function of time to treatment discontinuation. If a patient was still receiving biologic treatment on the last day of the study period, this last day was censored for the survival function. A log-rank test was used to compare persistence rates between biologic agents. The analysis of primary clinical outcomes was performed in an intention-to-treat manner. Any changes in the primary outcomes assessed 6 and 12 months after the initiation of biologic were attributed to that biologic regardless of what medications the patients were taking at the time of outcome assessment. In order to adjust for potential confounding, inverse probability of treatment weighting analysis was performed. We used a propensity score approach to account for differences in observed factors that might confound the effect of treatment on outcome [30, 31]. Each patient was assigned a weight based on their propensity score, defined as the probability of the patient receiving non-TNFi as their initial treatment. The data were trimmed according to the distribution of propensity score to minimize confounding [32]. Patients with a propensity score <0.05 were removed from analysis. Missing baseline and follow-up visit data were imputed by applying multiple imputation using fully conditional specification [33]. Multiple comparison was also performed. All covariates were considered in statistical analysis. Statistical significance was set at α = 0.05. Analyses were performed in Statistical Analysis System software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics of patients and follow-up
The study cohort comprised 667 eligible children with non-systemic JIA (supplementary Fig. S1, available at Rheumatology online). The eligible children were classified into categories as follows: 257 (38.5%) as polyarthritis RF-negative, 173 (25.9%) as oligoarticular, 78 (11.7%) as PsA, 68 (10.2%) as ERA, 44 (6.6%) as polyarthritis RF- positive and 47 (7.1%) as undifferentiated arthritis. The mean age at diagnosis was 9.1 years (s.d. = 5.2). Of the total patients, 614 (92.0%) started with TNFi as their first biologic treatment and the remaining 53 (8.0%) started with non-TNFi.
Patients starting with non-TNFi had higher disease activity and larger prevalence of positive ANA. Patients treated with non-TNFi had significant higher prevalence of prior systemic glucocorticoids and/or IAGCI use compared with children treated with TNFi (P < 0.01). Table 1 shows the baseline demographic and clinical characteristics of patients by initial biologic treatment.
Table 1.
Baseline demographic and clinical characteristics at the time of initiation of biologic agents
| TNFi (n = 614) | Non-TNFi (n = 53) | SMD Before IPTW | SMD After IPTW | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean (s.d.) | 9.1 (5.2) | 9.5 (4.9) | 0.09 | 0.11 |
| Female, n (%) | 419 (68.2) | 43 (81.1) | 0.30 | 0.26 |
| Race, n (%) | ||||
| White | 554 (90.2) | 46 (86.8) | 0.07 | 0.06 |
| Black | 32 (5.2) | 5 (9.4) | 0.16 | 0.03 |
| Other | 24 (3.9) | 1 (1.9) | ref | ref |
| Health insurance, n (%) | ||||
| Publica | 163 (26.5) | 15 (28.3) | 0.04 | 0.09 |
| Private | 395 (64.3) | 33 (62.3) | 0.10 | 0.07 |
| Multiple-type | 49 (8.0) | 4 (7.5) | 0.06 | 0.01 |
| Other | 3 (0.5) | 1 (1.9) | ref | ref |
| Clinical characteristics | ||||
| RF+, % | 46 (7.5) | 4 (7.5) | 0.10 | 0.04 |
| Time to bDMARDs since diagnosis, months, mean (s.d.) | 27.9 (45.8) | 19.5 (30.5) | 0.22 | 0.08 |
| cJADAS, mean (s.d.) | 10.7 (6.3) | 12.1 (6.4) | 0.19 | 0.16 |
| ILAR categories, n (%) | ||||
| oJIA | 153 (24.9) | 20 (37.7) | 0.02 | 0.02 |
| pJIA RF+ | 41 (6.7) | 3 (5.7) | 0.04 | 0.05 |
| pJIA RF– | 237 (38.6) | 20 (37.7) | 0.28 | 0.10 |
| ERA | 74 (12.1) | 4 (7.6) | 0.15 | 0.11 |
| PsA | 67 (10.9) | 1 (1.9) | 0.38 | – |
| Undifferentiated arthritis | 42 (6.8) | 5 (9.4) | ref | ref |
| Physician’s global assessment, mean (s.d.) | 3.1 (2.2) | 3.1 (1.8) | 0.03 | 0.02 |
| Overall well-being, 0–10, mean (s.d.). | 3.2 (2.6) | 3.7 (2.7) | 0.13 | 0.21 |
| Pain NRS, 0–10, median (Q1–Q3) | 4 (1– 6) | 4 (3–6) | 0.13 | 0.15 |
| Active joints, 0–71, median (Q1–Q3) | 3 (1–9) | 5 (2–13) | 0.08 | 0.17 |
| Number of joints with LOM, median (Q1–Q3) | 3 (1–6) | 4 (1–9) | 0.15 | 0.16 |
| ESR, median (Q1–Q3) | 10 (7–19) | 8 (7–16) | 0.23 | 0.02 |
| ANA positive, % | 121 (19.7) | 16 (30.2) | 0.01 | 0.15 |
| Ever had uveitis, n (%) | 29 (4.7) | 1 (1.9) | 0.18 | 0.06 |
| Morning stiffness, n (%) | ||||
| None | 139 (22.6) | 20 (37.7) | 0.34 | 0.16 |
| ≤15 min | 118 (19.2) | 9 (17.0) | 0.16 | 0.15 |
| >15 min | 245 (39.9) | 18 (34.0) | ref | ref |
| Past treatments | ||||
| Prior csDMARD, % | 415 (67.6) | 41 (77.4) | 0.22 | 0.11 |
| Prior GC and/or IAGCI, % | 289 (47.1) | 35 (66.0) | 0.39 | 0.14 |
| Prior NSAID, % | 502 (81.8) | 43 (81.1) | 0.02 | 0.10 |
For each variable, an SMD <10% was considered to be an inconsequential imbalance between the TNFi and non-TNFi cohorts. In the IPTW-weighted sample, weighted standardized mean differences were calculated by incorporating each patient’s stabilized weight.
SMD >10% in magnitude. cJADAS: clinical Juvenile Arthritis Disease Activity Score; csDMARD: conventional synthetic DMARD; ERA: enthesitis-related arthritis; GC: glucocorticoid; IAGCI: intra-articular glucocorticoid injection; IPTW: inverse probability of treatment weighting; LOM: limited range of motion; NRS: numeric rating scale; oJIA: oligoarticular JIA; Overall well-being: parent’s/patient’s assessment of overall well-being; pJIA: polyarthritis; PsA: juvenile PsA; SMD: standardized mean difference; TNFi: TNF inhibitor.
Treatment patterns
From the study cohort, 77.9% of children received at least two biologic agents, 14.8% received three biologic agents and 7.2% received four or more biologic agents. Among patients on TNFi, 412 (67.1%) were prescribed etanercept, 169 (27.5%) adalimumab, 18 (2.9%) infliximab, 12 (2.0%) golimumab and 3 (0.5%) certolizumab pegol. Among non-TNFi users, 62.3% were prescribed abatacept and 33.96% tocilizumab.
In second-course therapy, 276 TNFi users (45.0%) were prescribed the same medication as their first-course therapy, 267 (43.5%) transferred to a different TNFi and 71 (11.5%) began to use a non-TNFi. For patients who used non-TNFi as their initial medication, 17 (32.1%) were prescribed the same medication as first-course therapy, 12 (22.7%) used a different non-TNFi as their second-course therapy and 24 (45.2%) transferred to a TNF inhibitor. The Sankey diagram in Fig. 1 depicts the change and relative proportions of different biologic agents selected on first- and second-course biologic treatment.
Fig. 1.
Changes between first- and second-course biologic treatment for treating JIA
Sankey diagram depicts the relative proportions of different biologic agents selected on first- and second-course biologic treatment.
Off-label bDMARDs not approved for use in JIA (such as golimumab, infliximab, anakinra and ustekinumab) were used as first-course therapy for 35 children (5.3%). However, in second-course therapy, 78 children (20.9%) were prescribed an off-label biologic agent.
Persistence
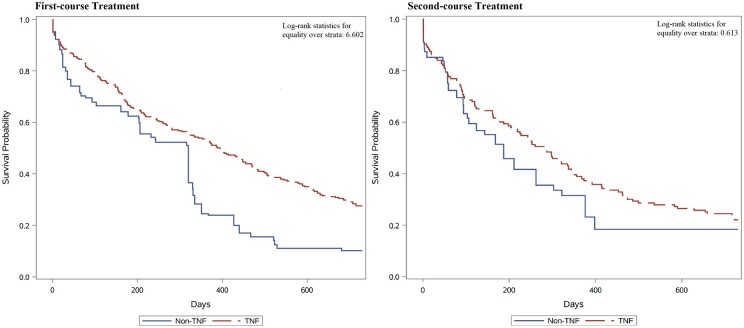
Fig. 2 presents the Kaplan–Meier curves of treatment persistence of first- and second-course bDMARDs. The median persistence time of first-course biologic therapy over 24 months was 320 days (mean 330). Children prescribed with TNFi were persistent for significantly longer than children prescribed with non-TNFi (log-rank P = 0.010) in first-course treatment, and persistence time was similar among TNFi and non-TNFi users in second-course treatment.
Fig. 2.
Kaplan–Meier survival curves for persistence of first- and second-course bDMARDs
Children prescribed with TNFi were persistent for significantly longer than children prescribed with non-TNFi (log-rank P-value = 0.010) in first-course treatment. bDMARD: biologic DMARD; TNFi: TNF inhibitor.
The median persistence times for first-course TNFi and non-TNFi were 395 days (mean 391) and 320 days (mean 269), respectively. Roughly one-third of TNFi users (33.9%) discontinued therapy within 6 months, which was lower than non-TNFi users (37.6%). One-year persistence rates were 53.2% for patients on TNFi, which was much higher than the persistence rate (24.5%) for patients on non-TNFi. In first-course therapy, persistence was significantly longer among children receiving etanercept than among those receiving adalimumab (P = 0.022), infliximab (P = 0.007), tocilizumab (P = 0.013) or abatacept (P = 0.005; see Fig. 3A).
Fig. 3.
Kaplan–Meier survival curves for biologic treatment persistence by medication
(A) Comparison of the drug survival of biologic agents as first-course therapy (adalimumab vs etanercept: Šidák-adjusted P-value 0.022; etanercept vs infleximab: Šidák-adjusted P-value 0.007; etanercept vs tocilizumab: Šidák-adjusted P-value 0.013; etanercept vs abtacept: Šidák-adjusted P-value 0.005; etanercept vs golimumab: Šidák-adjusted P-value 0.004). (B) Comparison of the drug survival of biologic agents as second-course therapy. In second-course therapy, persistence was not significant difference among children receiving each medication (adalimumab vs etanercept: raw P-value 0.739, Šidák-adjusted P-value 1.000; abatacept vs tocilizumab: raw P-value 0. 779, Šidák-adjusted P-value 1.000).
For second-course treatment, median persistence time overall was 211 days (mean 291), with no significant difference between TNFi (280 days) and non-TNFi (187 days, P = 0.434). One-year persistence rates ranged between 39.0% for patients on TNFi and 31.5% for patients on non-TNFi. Discontinuations were similar among TNFi such as adalimumab, etanercept, golimumab and infliximab. In second-course therapy, persistence was not significant difference among children receiving TNFi. For every TNFi, drug survival was lower in second-course therapy than in first-course therapy (Fig. 3B).
Treatment outcomes
Patients’ disease outcomes improved after they received biologic therapy. These outcomes included disease activity (cJADAS), number of joints with limited range of motion, active joint count and patient-reported pain level. Patients who were prescribed non-TNFi had higher disease activity at baseline on several measures. Patients with TNFi therapy had significant greater reduction (Δ) of cJADAS at the 6-month visit compared with patients in the non-TNF cohort (P < 0.0001). The mean of cJADAS decreased from 10.7 at baseline, to 5.6 (95% CI 4.8, 6.4) at 6-month follow-up and to 6.0 (95% CI 4.9, 7.1) at 12-month follow-up for TNFi, and decreased from 12.1 at baseline to 8.1 (95% CI 5.5, 10.7) at 6-month follow-up and to 7.2 (95% CI 5.5, 9.0) at 12-month follow-up for non-TNFi (Table 2). This corresponds to a reduction (Δ) from the baseline cJADAS by a mean of 6.6 (95% CI 5.7, 7.5) at 6-month follow-up visit if patients were treated with TNFi vs 3.0 (95% CI 1.5, 4.6, P < 0.0001) if they were treated with non-TNFi.
Table 2.
Estimated mean potential outcomes treated on TNFi and non-TNFi at 6 and 12 monthsa
| Outcome | TNFi | Non-TNFi | ATE | P-value |
|---|---|---|---|---|
| At 6 months (N = 667) | ||||
| cJADAS (95% CI) | 5.6 (4.8, 6.4) | 8.1 (5.5, 10.7) | 2.5 (−0.1, 5.1) | 0.056 |
| Achievement of inactive/low disease activity % (95% CI) | 58.0 (51.1, 64.8) | 43.9 (20.1, 67.8) | −14.1 (−38.7, 10.7) | 0.258 |
| Pain score | 2.6 (2.2, 2.9) | 3.3 (2.2, 4.4) | 0.7 (−0.5, 1.9) | 0.256 |
| ΔcJADAS (95% CI) | 6.6 (5.7, 7.5) | 3.0 (1.5, 4.6) | −3.6 (−5.2, −1.9) | <0.0001 |
| Number of joints with LOM (95% CI) | 3.0 (2.4, 3.7) | 3.5 (1.8, 5.1) | 0.4 (−1.2, 2.1) | 0.609 |
| Active joint count (95% CI) | 2.6 (1.9, 3.3) | 4.5 (2.5, 6.6) | 1.9 (−0.03, 3.9) | 0.054 |
| At 12 months (N = 667) | ||||
| cJADAS (95% CI) | 6.0 (4.9, 7.1) | 7.2 (5.5, 9.0) | 1.2 (−0.7, 3.0) | 0.207 |
| Achievement of Inactive/low disease activity % (95% CI) | 56.1 (49.0, 63.2) | 43.6 (22.5, 64.6) | −12.5 (−35.1, 10.1) | 0.275 |
| Pain score (95% CI) | 2.8 (2.3, 3.3) | 3.6 (2.6, 4.7) | 0.8 (−0.1, 1.7) | 0.078 |
| ΔcJADAS (95% CI) | 6.2 (4.9, 7.5) | 3.9 (1.4, 6.4) | −2.3 (−5.0, 0.4) | 0.090 |
| Number of joints with LOM (95% CI) | 3.1 (2.2, 4.0) | 2.3 (0.3, 4.3) | −0.8 (−2.3, 0.8) | 0.326 |
| Active joint count (95% CI) | 2.6 (1.6, 3.6) | 3.1 (1.4, 4.9) | 0.5 (−1.1, 2.1) | 0.522 |
Variables in IPTW for comparing between TNFi and non-TNFi users included age, gender, ethnicity, disease duration, JIA subtypes, RF, ESR, ANA, cJADAS at baseline, physician global assessment at baseline, morning stiffness at baseline, number of joint with limited range of motion at baseline, active joint count at baseline, patient overall well-being at baseline, uveitis history and patient-reported pain level. ATE: average treatment effect; cJADAS: clinical Juvenile Arthritis Disease Activity Score; IPTW: inverse probability of treatment weighting; LOM: limited range of motion; TNFi: TNF inhibitor. The reduction (Δ) of cJADAS at the 6-month or 12-month visit compared with baseline visit.
Similarly, the mean of patient-reported pain level and number of joints with limited range of motion was slightly higher in the non-TNFi cohort than in the TNFi cohort at baseline. In both TNFi and non-TNFi cohorts, these disease responses at 12-months were similar between treatments. There was no significant difference between TNFi and non-TNFi in the odds of achieving an inactive/low disease activity. Among TNFi users, 58.0% achieved inactive/low disease activity at the 6-month follow-up and 56.1% achieved inactive/low disease activity at the 12-month follow-up. For non-TNFi users, 43.9% achieved inactive/low disease activity at the 6-month follow-up, and 43.6% achieved inactive/low disease activity at the 12-month follow-up. Patient-reported pain level decreased from 3.6 at baseline to 2.6 (95% CI 2.2, 2.9) at 6-month follow-up and remained the same level (2.8, 95% CI 2.3, 3.3) at 12-month follow-up for TNFi, and decreased from 4.2 at baseline to 3.3 (95% CI 2.2, 4.4) at 6-month follow-up to 3.6 (95% CI 2.6, 4.7) at 12-month follow-up for non-TNFi.
Discussion
Over the last decade, significant therapeutic advances in JIA management have reflected a better understanding of JIA pathogenesis. With several new non-TNFi approved by the FDA for JIA indication and present in the market since 2013, there are now multiple therapies available for JIA, increasing the complexity of treatment decisions [34]. In this study we found that 90% of patients were prescribed on TNFi as the first-course biologic agents. These results are consistent with a national biologic cohort study of children and young people with JIA in the UK [35]. We also found that switching to a new biologic agent in JIA treatment occurs frequently in clinical practice. Nearly a quarter of children with JIA had been treated with three or more biologic agents, and ∼7% of children received four or more biologic agents. Etanercept was the most frequently prescribed first-course bDMARD therapy, followed by adalimumab. Similar studies have been performed for more frequently used TNFi such as adalimumab and etanercept [36]. However, studies on the comparative effectiveness and treatment persistence for newly approved non-TNFi (i.e. abatacept, tocilizumab) are still rare, likely because the non-TNFi have become available on the market only in recent years. Only few studies have examined persistence for discontinuation among children with JIA who were treated with TNFi using medical claims data [37, 38], and these studies vary greatly in design, patient demographics and disease characteristics. Lee et al. characterized the use of TNFi in children and young adults with JIA/RA [37]. Ringold et al. measured adherence and persistence to TNFi and MTX using data from a large Pharmacy Benefit Management firms [38].
To the best of our knowledge, this longitudinal observational cohort study is the first to compare the clinical effectiveness of non-TNFi vs TNFi among children with non-systemic categories of JIA in real-world clinical practice in the USA. We examined effectiveness based on several different outcome measures, including cJADAS, patient-reported pain level, achievement of inactive/low disease activity, active joint count and number of joints with limited range of motion. Patients with TNF therapy had significant greater reduction of cJADAS at the 6-month visit compared with patients in the non-TNF cohort. The disease activity and patient-reported pain level were similar between patients receiving TNFi and those receiving non-TNFi at the 6-month follow-up visits. The study failed to find the significant difference in disease activity, patient-reported pain level and the number of joints with limited range of motion between TNFi and non-TNFi at the 12-month follow-up. One possible reason for the difference between outcomes at 6 months and 12 months is that, at 12 months after initial biologic treatment, patients are more likely to switch between bDMARDs. Therefore, the effects of initial treatment at 12 months are more affected by the alternative bDMARDs for those who switched. Compared with patients in the TNFi treatment group, patients in non-TNFi treatment group showed worse disease activity at the baseline, but similar cJADAS at the 6- and 12-month follow-ups. Our findings are consistent with prior studies. In a national biologic cohort studies in the UK, the effectiveness of TNFi vs non-TNFi as second-course therapy was compared among children and young people with JIA, and no evidence was found that switching to different classes of biologic therapy was more beneficial [35]. Horneff et al. conducted a nationwide observational study using the German biologics in pediatric rheumatology (BIKER) registry found no difference in the efficacy of tocilizumab, etanercept or adalimumab as first- or second-course biological therapy in polyarticular JIA [23].
Interestingly, this study found only roughly 5% of JIA patients were prescribed off-label biologic agents as their first-course treatment; however, >20% of patients were prescribed off-label biologics as their second-course therapy. Tocilizumab and abatacept are considered viable alternatives as initial biologic therapy and are recommended for patients who have failed primary TNFi for RA or PsA in adult disease management [39–41]. Although infliximab and golimumab are not licensed for JIA, they are considered potential treatment options, particularly in RF-positive JIA patients where TNF therapy is ineffective or not tolerated [42]. Because JIA is a rare disease, and only around 20% of JIA patients are prescribed biologic agents as initial therapy, the number of patients who are exposed to non-TNFi is even lower compared with TNFi [36]. It would be informative to evaluate the effectiveness among children with JIA receiving non-TNFi vs TNFi, especially in a large population, as their first- and second-course biologic treatment.
Medication persistence and adherence play an important role in achieving optimal disease management of rheumatic conditions [43]. We found etanercept was associated with superior drug survival compared with other JIA-approved TNFi included in the analysis. Adalimumab’s drug survival was intermediate compared with etanercept and golimumab. In 2008, most patients were given etanercept as the first bDMARD, and physicians were more tolerant of active arthritis and less likely to switch patients to newer and less familiar bDMARD. The available treatments have changed during the study period. By 2018, etanercept and adalimumab were the most prescribed first-line bDMARDs. At the same time, physician tolerance of active arthritis lowered, and the number of alternative options was greater, both of which lead to increased frequency of medication discontinuation or switching. Moreover, during the study period, adalimumab formulation contained preservatives that made it a painful injection. The citrate-free formula, which stings less or not at all, made adalimumab similar to other biologics, but this did not come onto the market until 2018, near the end of the study. This likely affected the persistence of adalimumab therapy as it was less painful, and may have affected adherence, thus influencing the outcomes. While there was no difference in persistence rates between TNFi and non-TNFi for first-course therapy, the persistence rate for abatacept was greater than persistence rates for TNFi in second-course therapy. Our findings are largely in line with previous research, although there are also several inconsistencies. Horneff et al., found that etanercept is also frequently used as first-course biologic in Germany. However, compliance was highest with tocilizumab and lowest with adalimumab. Etanercept had drug survival intermediate to tocilizumab and adalimumab [23]. Treatment persistence is considered a surrogate for long-term clinical effectiveness. Poor persistence and nonadherence are serious issues that can reduce the effectiveness of biologic therapies [44].
A major strength of this study is that it is the first study that compared real-world effectiveness and drug survival of non-TNFi vs TNFi using a large sample of JIA patients with the full granularity of data from hospital EMRs, which provided the disease presentation during the real-world clinical practice. It can take time to accumulate enough patients for analysis, especially for a rare disease such as JIA. EMRs consist of integrating large amounts of medical information collected throughout the patient’s life, providing accurate, up-to-date and complete information on patients’ diagnoses, disease characteristics and treatment at the point of care [45–47]. Unlike survey data utilized by some previous studies or randomized controlled trials with selected patient populations running for limited periods of time, EMRs are optimal to fully understand patient experience and to obtain the most complete overview of initial biologic treatment.
This study has a few limitations. First, the existence of unmeasured confounders, such as patients’ comorbidity, insurance reimbursement policies and physician behaviour, could have affected the treatment assignment to patients as well as disease outcomes. The logistic regression model for propensity score estimation could not predict the treatment very well as most of patients in the non-TNFi group had propensity scores <0.25 and the maximum value of propensity score is around 0.5. Second, there is a limited number of non-TNFi users, especially as a first-line biologic treatment. Third, the treatments were determined by medication prescriptions written in EMRs, which does not necessarily reflect medication adherence. The proportion of patients who receive a prescription but do not take the medication is not known. Similarly, the accuracy of medication discontinuation dates and the reason for discontinuation are unknown. Fourth, as our study sample was limited to patients from a single medical centre, it may be limited to the preferences of the centre and types of biologic therapies available in that geographic location or approved by insurance formulary. In addition, situations will change drastically depending on the structures of healthcare systems. In single-payer healthcare systems, access to off-label treatment may less than in multi-payer healthcare systems. National or multicentre studies are suggested for future studies. However, these results nonetheless provide a first glance into the initial biologic prescribing patterns, disease outcomes and drug survival of non-systemic JIA in real-world clinical practice.
Conclusion
In summary, our results indicate that switching between bDMARDs (i.e. instances of failure with one drug and movement to an alternative) occurs frequently in daily clinical practice. Persistence was significantly longer among patients initiating TNFi as their first biologic therapy than those receiving non-TNFi. Patients with TNF therapy had significantly greater reduction of cJADAS at the 6-month visit compared with patients in the non-TNFi cohort. The study failed to find significant differences between the effectiveness of TNFi vs non-TNFi after 12 months of treatment. Further studies should focus on real-world effectiveness and safety to more precisely evaluate the various benefits and detriments of newly approved biologic therapy, especially in a large population, so that first-line therapy can be more appropriately prescribed and switching between medications can be reduced.
Supplementary Material
Acknowledgement
The authors thank Dr Francis Grover for his editorial assistance.
Funding: This study was partially funded by the Patient-Centered Outcomes Research Institute (ME-1408-19894).
Disclosure statement: The authors declare that there is no conflict of interest concerning this research. J.J.G. has received research grant or unrestricted grant funding from the following: the Ohio Department of Jobs and Family Services (Medicaid Agency), Ortho-McNeil Janssen Scientific Affairs LLC, Eli-Lilly Company, Novartis Company and Roche-Genentech Company. The opinions and conclusions expressed in this manuscript are solely those of the authors.
Data availability statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Xiaomeng Yue, Division of Pharmacy Practice and Administrative Sciences, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center.
Bin Huang, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center; Department of Pediatrics, University of Cincinnati College of Medicine.
Ana L Hincapie, Division of Pharmacy Practice and Administrative Sciences, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center.
Patricia R Wigle, Division of Pharmacy Practice and Administrative Sciences, James L. Winkle College of Pharmacy, University of Cincinnati Academic Health Center.
Yuxiang Li, Department of Environmental and Public Health Sciences, University of Cincinnati College of Medicine.
Tingting Qiu, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center.
Daniel J Lovell, Department of Pediatrics, University of Cincinnati College of Medicine; Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Esi M Morgan, Department of Pediatrics, University of Cincinnati College of Medicine; Department of Environmental and Public Health Sciences, University of Cincinnati College of Medicine; Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
References
- 1. Petty RE, Southwood TR, Manners P et al. ; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 2. Ringold S, Angeles‐Han ST, Beukelman T et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non‐systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Care Res (Hoboken) 2019;71:717–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé R, Breedveld FC et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braun J, Van Den Berg R, Baraliakos X et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gossec L, Smolen J, Gaujoux-Viala C et al. ; European League Against Rheumatism. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 2012;71:4–12. [DOI] [PubMed] [Google Scholar]
- 6. Oen K, Guzman J, Dufault B et al. ; the Research in Arthritis in Canadian Children emphasizing Outcomes (ReACCh-Out) investigators. Health‐related quality of life in an inception cohort of children with juvenile idiopathic arthritis: a longitudinal analysis. Arthritis Care Res (Hoboken) 2018;70:134–44. : [DOI] [PubMed] [Google Scholar]
- 7. Verstegen RH, McMillan R, Feldman BM, Ito S, Laxer RM. Towards therapeutic drug monitoring of TNF inhibitors for children with juvenile idiopathic arthritis: a scoping review. Rheumatology (Oxford) 2020;59:386–97. [DOI] [PubMed] [Google Scholar]
- 8. Ringold S, Weiss PF, Beukelman T et al. ; American College of Rheumatology. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum 2013;65:2499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lovell DJ, Reiff A, Ilowite NT et al. ; Pediatric Rheumatology Collaborative Study Group. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum 2008;58:1496–504. [DOI] [PubMed] [Google Scholar]
- 10. Prince FH, Twilt M, ten Cate R et al. Long-term follow-up on effectiveness and safety of etanercept in juvenile idiopathic arthritis: the Dutch national register. Ann Rheum Dis 2009;68:635–41. [DOI] [PubMed] [Google Scholar]
- 11. Lovell DJ, Ruperto N, Goodman S et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810–20. [DOI] [PubMed] [Google Scholar]
- 12. Beukelman T, Patkar NM, Saag KG et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken) 2011;63:465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malottki K, Barton P, Tsourapas A et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess 2011;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrold LR, Reed GW, Kremer JM et al. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis 2015;74:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrold LR, Reed GW, Solomon DH et al. Comparative effectiveness of abatacept versus tocilizumab in rheumatoid arthritis patients with prior TNFi exposure in the US Corrona registry. Arthritis Res Ther 2016;18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrold LR, Litman HJ, Connolly SE et al. Comparative effectiveness of abatacept versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis who are anti-CCP positive in the United States Corrona registry. Rheumatol Ther 2019;6:217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisell T, Dehlin M, Di Giuseppe D et al. ; ARTIS Study Group. Comparative effectiveness of abatacept, rituximab, tocilizumab and TNFi biologics in RA: results from the nationwide Swedish register. Rheumatology (Oxford) 2019;58:1367–77. [DOI] [PubMed] [Google Scholar]
- 18. Kim HL, Lee MY, Park SY et al. Comparative effectiveness of cycling of tumor necrosis factor-α (TNF-α) inhibitors versus switching to non-TNF biologics in rheumatoid arthritis patients with inadequate response to TNF-α inhibitor using a Bayesian approach. Arch Pharmacal Res 2014;37:662–70. [DOI] [PubMed] [Google Scholar]
- 19. Gottenberg JE, More J, Perrodeau E et al. Comparative effectiveness of rituximab, abatacept, and tocilizumab in adults with rheumatoid arthritis and inadequate response to TNF inhibitors: prospective cohort study. BMJ 2019;364:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauper K, Nordström DC, Pavelka K et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann Rheum Dis 2018;77:1276–82. [DOI] [PubMed] [Google Scholar]
- 21. Svedbom A, Stahle M. Real-world comparative effectiveness of adalimumab, etanercept and methotrexate: a Swedish register analysis. J Eur Acad Dermatol Venereol 2020;34:525–32. [DOI] [PubMed] [Google Scholar]
- 22. Reed GW, Gerber RA, Shan Y et al. Real-world comparative effectiveness of tofacitinib and tumor necrosis factor inhibitors as monotherapy and combination therapy for treatment of rheumatoid arthritis. Rheumatol Ther 2019;6:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horneff G, Klein A, Klotsche J et al. Comparison of treatment response, remission rate and drug adherence in polyarticular juvenile idiopathic arthritis patients treated with etanercept, adalimumab or tocilizumab. Arthritis Res Ther 2016;18:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilowite N, Porras O, Reiff A et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol 2009;28:129–37. [DOI] [PubMed] [Google Scholar]
- 25. Ruperto N, Lovell DJ, Quartier P et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet 2008;372:383–91. [DOI] [PubMed] [Google Scholar]
- 26. McErlane F, Foster HE, Davies R et al. Biologic treatment response among adults with juvenile idiopathic arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 2013;52:1905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puig L, Carrascosa J-M, Daudén E, Sulleiro S, Guisado C. Drug survival of conventional systemic and biologic therapies for moderate-to-severe psoriasis in clinical practice in Spain: prospective results from the SAHARA study. J Dermatolog Treat 2020;31:344–8. [DOI] [PubMed] [Google Scholar]
- 28. Consolaro A, Giancane G, Schiappapietra B et al. Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol 2016;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magni-Manzoni S, Ruperto N, Pistorio A et al. Development and validation of a preliminary definition of minimal disease activity in patients with juvenile idiopathic arthritis. Arthritis Rheum 2008;59:1120–7. [DOI] [PubMed] [Google Scholar]
- 30. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sugihara M. Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharm Stat 2010;9:21–34. [DOI] [PubMed] [Google Scholar]
- 32. Da Costa B, Gahl B, Jüni P. Tools & techniques-statistics: propensity score techniques. EuroIntervention 2014;10:761–7. [DOI] [PubMed] [Google Scholar]
- 33. Yuan YC. Multiple imputation for missing data: Concepts and new development (version 9.0). Rockville, MD: SAS Institute Inc., 2010;49:12. [Google Scholar]
- 34. Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kearsley-Fleet L, Heaf E, Davies R et al. Frequency of biologic switching and the outcomes of switching in children and young people with juvenile idiopathic arthritis: a national cohort study. Lancet Rheumatol 2020;2:e217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davies R, Carrasco R, Foster HE et al. Treatment prescribing patterns in patients with juvenile idiopathic arthritis (JIA): analysis from the UK Childhood Arthritis Prospective Study (CAPS). Semin Arthritis Rheum 2016;46:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee WJ, Briars L, Lee TA et al. Use of tumor necrosis factor‐alpha inhibitors in children and young adults with juvenile idiopathic arthritis or rheumatoid arthritis. Pharmacotherapy 2016;36:1201–9. [DOI] [PubMed] [Google Scholar]
- 38. Ringold S, Grant S, Girdish C, Wallace CA, Sullivan SD. Methotrexate and injectable tumor necrosis factor-α inhibitor adherence and persistence in children with rheumatic diseases. J Rheumatol 2013;40:80–6. [DOI] [PubMed] [Google Scholar]
- 39. Kearsley-Fleet L, Beresford MW, Davies R et al. Short-term outcomes in patients with systemic juvenile idiopathic arthritis treated with either tocilizumab or anakinra. Rheumatology (Oxford) 2019;58:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nikfar S, Saiyarsarai P, Tigabu BM, Abdollahi M. Efficacy and safety of interleukin-1 antagonists in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int 2018;38:1363–83. [DOI] [PubMed] [Google Scholar]
- 41. Iannone F, Santo L, Bucci R et al. Drug survival and effectiveness of ustekinumab in patients with psoriatic arthritis. Real-life data from the biologic Apulian registry (BIOPURE). Clin Rheumatol 2018;37:667–75. [DOI] [PubMed] [Google Scholar]
- 42.NHS England. Clinical commissioning policy statement: biologic therapies for the treatment of juvenile idiopathic arthritis (JIA). Contract No.: NHS England E03X04 E 2015, 3, https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/e03pd-bio-therapies-jia-oct15.pdf [cited on 16 January 2021].
- 43. Harnett J, Wiederkehr D, Gerber R et al. Primary nonadherence, associated clinical outcomes, and health care resource use among patients with rheumatoid arthritis prescribed treatment with injectable biologic disease-modifying antirheumatic drugs. J Manag Care Spec Pharm 2016;22:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grijalva CG, Chung CP, Arbogast PG et al. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care 2007;45(10 Suppl 2) S66–76. [DOI] [PubMed] [Google Scholar]
- 45.FDA Budget Matters: A Cross-Cutting Data Enterprise for Real World Evidence. https://www.fda.gov/news-events/fda-voices-perspectives-fda-leadership-and-experts/fda-budget-matters-cross-cutting-data-enterprise-real-world-evidence [updated 2018 June; cited 16 Jan 2021].
- 46. Poon EG, Jha AK, Christino M et al. Assessing the level of healthcare information technology adoption in the United States: a snapshot. BMC Med Inform Decis Mak 2006;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang B, Qiu T, Chen C et al. Comparative effectiveness research using electronic health records data: ensure data quality. SAGE Research Methods Cases 2020. doi: 10.4135/9781529726480.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.