Abstract
Background
Excess mortality in people with vs without type 2 diabetes (T2DM) has fallen, but it is unclear whether men/women at all ages have benefited and which causes of death have driven these trends.
Methods
All-cause and cause-specific mortality rates and excess mortality [by mortality rate ratios (MRRs) relative to the non-diabetic general population] were examined in 1 268 018 Australians with T2DM registered on the National Diabetes Services Scheme (2002–2014).
Results
Age-standardized mortality decreased in men (−2.2%/year; Ptrend < 0.001) and women with T2DM (−1.3%/year; Ptrend < 0.001) throughout 2002–14, which translated to declines in the MRRs (from 1.51 to 1.45 in men; 1.59 to 1.46 in women; Ptrend < 0.05 for both). Declining mortality rates in T2DM were observed in men aged 40+ years and women aged 60+ years (Ptrends <0.001), but not at younger ages. However, the only age group in which excess mortality declined relative to those without diabetes was 80+ years (Ptrends < 0.05); driven by reductions in excess cancer-related deaths in men and cardiovascular disease (CVD) in women. Among age groups <80 years, CVD and cancer MRRs remained similar or increased over time, despite falls in both CVD and cancer mortality rates. MRRs for non-CVD/non-cancer-related deaths increased in 60–79 year-olds, but were otherwise unchanged.
Conclusions
Declining excess mortality attributable to T2DM from 2002–14 was driven entirely by reductions in those aged 80+ years. Declines in total mortality among those with T2DM were apparent in more age groups, but often to a lesser extent than in the general population, thereby serving to increase the excess risk associated with T2DM.
Keywords: Trends, cause of death, diabetes complications, follow-up studies
Key Messages
Age-standardized all-cause mortality in the type 2 diabetes population has fallen more than in the general population, signifying a reduction in overall excess mortality risk associated with diabetes.
However, age-specific data indicate that this trend in the total diabetes population is predominantly influenced by trends in those aged 80+ years (the group in which nearly half of all deaths occur); indeed, in those aged <80 years, falling mortality in those with type 2 diabetes has lagged background general population trends, such that excess mortality risk associated with diabetes has increased.
The increase in excess all-cause mortality in the type 2 diabetes population aged <80 years compared with the non-diabetic general population aged <80 years reflects that risks of both cardiovascular disease (CVD) and cancer-related deaths have been steady or rising in those with type 2 diabetes (dependent on age and sex); moreover, excess non-CVD/non-cancer-related deaths have increased in people with type 2 diabetes aged 60–79 years.
Concurrently, the distribution of cause-specific mortality in type 2 diabetes has evolved. The proportion of deaths attributable to CVD has declined, offset by an increase in non-CVD/non-cancer-related mortality (the latter marked by rising excess risks associated with non-traditional complications of diabetes, i.e. dementia and pulmonary disease).
Introduction
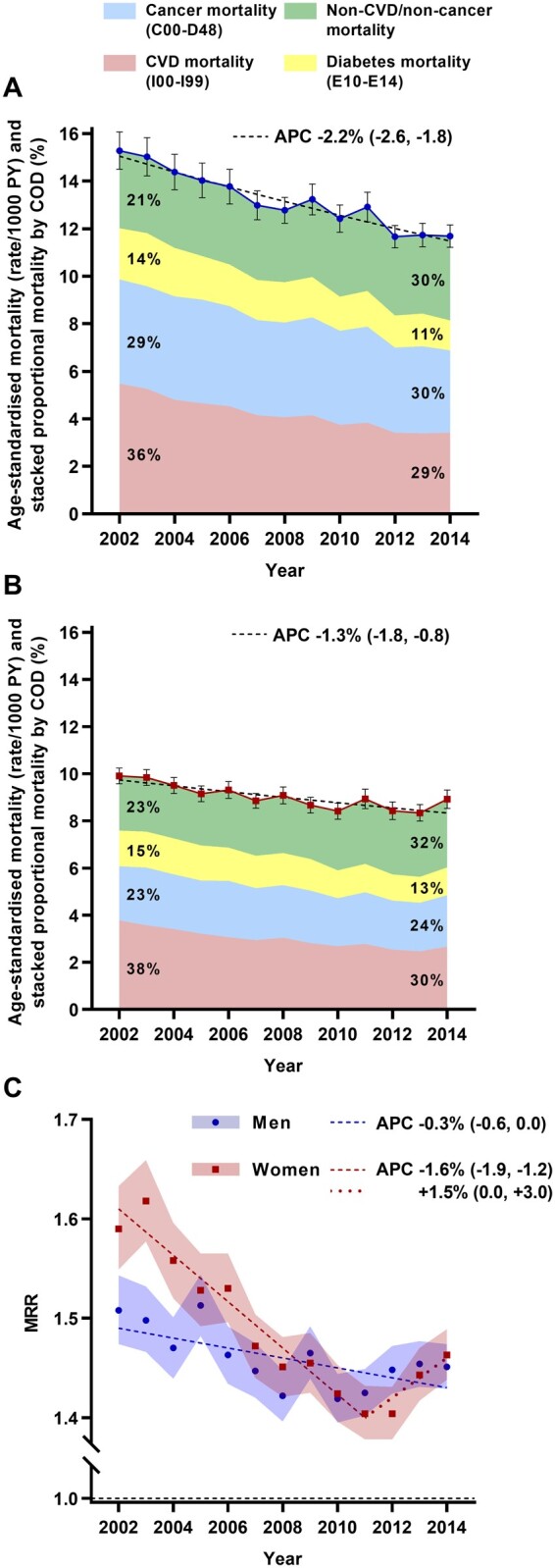
Mortality rates in people with diabetes have fallen in most locations over the past three decades.1–6 This has largely reflected declines in deaths due to cardiovascular disease (CVD), cancer and diabetes per se, which together represent the three leading causes of death in people with diabetes.1,2,7 Together with the emergence of other novel and potentially fatal complications of diabetes,8 these trends have, over time, altered the distribution of causes of death within the diabetes population. In the USA for example, CVD is now responsible for ∼one-third of deaths among adults with diabetes, down from almost 50% in the late 1980s.1 A similar reduction in the proportion of deaths due to CVD was reported in Australians with type 2 diabetes between 1997 and 2010.9
Given all-cause and CVD mortality have also trended lower in the general population, falling mortality in diabetes does not necessarily equate to lower excess mortality risk. Certainly the magnitude of the fall in those with diabetes has been greater than in those without diabetes in most populations studied;1,3,4,9 however, the consistency of this risk reduction across age and sex strata, and by various causes of death (COD), remains unknown. This is particularly relevant in the context of the progressive diversification of diabetes complications beyond traditional macro- and micro-vascular disease, particularly in type 2 diabetes.8 A major barrier to examining trends in excess mortality by age, sex and COD has been the large numbers of deaths required to detect meaningful trends at such levels of granularity. To address this, we have examined trends in excess mortality in ∼1.25 million Australians with type 2 diabetes registered on the National Diabetes Services Scheme (NDSS) between 2002 and 2014.
Methods
Setting
The origins of the NDSS have been described previously.2,9,10 Briefly, this registry arose from a national scheme introduced in 1987 to provide people with subsidised consumables used in the management of diabetes. With registration completed by any medical practitioner or diabetes nurse educator, the NDSS has a broad capture rate (∼80–90% of all Australians with known diabetes).11,12 Registrants on the NDSS during the period 2002–14 were linked to the National Death Index and the Pharmaceutical Benefits Scheme (for medication prescription data) by the Australian Institute of Health and Welfare (AIHW), who use established probabilistic matching methodologies.13 The year 2002 was chosen as the start date on the basis of administrative changes resulting in improved data quality compared with earlier years of the NDSS. The endpoint of 2014 was the final full calendar year with complete COD data.
Participants
Diabetes type is nominated for all individuals on the NDSS by the registering practitioner. However, given known uncertainty in the assignment of this classification, insulin use and age at diagnosis/registration were also used to assign diabetes type for the purposes of this analysis. These criteria were adapted from previous NDSS registry studies10 and are described in detail in the Supplementary Material, available as Supplementary data at IJE online. The current study includes all individuals who met the criteria for type 2 diabetes and who were registered on the NDSS at any time during the period 2002–14.
Mortality outcomes
The National Death Index provided date of death and COD [International Classification of Diseases, Tenth Revision format (ICD-10)]. General population mortality data came from General Records of Incidence of Mortality (GRIM), published by the AIHW.14,15 In line with the GRIM data, we used the underlying ICD-10 COD code to classify cause of death. CODs were initially grouped into four broad categories: all CVD (I00–I99), all cancer (C00–D48), diabetes (E10–E14) and all other non-CVD/non-cancer CODs. We then analysed more specific CODs that accounted for at least 5% of the deaths within these categories. Additional COD code grouping methodology is described in the Supplementary Material, available as Supplementary data at IJE online.
Statistical analysis
All NDSS registrants were followed from 1 January 2002 (or date of registration, if later) until death or 31 December 2014, whichever occurred earlier. Mortality rate ratios (MRRs) were calculated as observed numbers of deaths relative to expected numbers of deaths, and its 95% confidence intervals (CIs) reflected the Poisson distribution exact method (using Stata version 14.2; StataCorp, College Station, TX). Expected numbers of deaths were calculated via the product of the total person-years of follow-up and the relevant background population rate (these rates having been re-calculated from the published GRIM rates after removing the NDSS from the general population; i.e. so that the comparator more closely reflected the non-diabetic general population). For the purpose of reporting MRR results, we defined broader age bands than were used in its calculation (i.e. 10–39, 40–59, 60–69, 70–79 and 80+ years) and for the 10–39 years group examined trends over three time periods (2002–05; 2006–10; 2011–14), rather than annually (i.e. because of small numbers of deaths occurring within narrower bands).
Annual age-specific and age-standardized mortality rates were also calculated and reported alongside MRRs. Age-standardized mortality was derived by indexing the age-specific mortality rates to the age strata of the 2001 Australian population (excluding those aged <10 years), using the direct method.
Trends over time in mortality rates and MRRs were analysed using Joinpoint Trend Analysis Software version 4.7.16,17 Monte Carlo permutation tests were applied to identify points (up to two) where linear trends in the log-transformed rate or MRR changed—either in direction or magnitude at P < 0.05. Annual percentage changes (APC; Δ%/year) for each identified time period were generated with 95% CIs that assumed a t distribution.
Results
Study cohort and clinical profile
Derivation of the final analytic sample with type 2 diabetes (n = 1 268 018) is described in the Supplementary Material, with clinical characteristics displayed in Supplementary Table S1, available as Supplementary data at IJE online. Overall, mean ages at diagnosis and registration were 58 ± 14 years and 59 ± 14 years, respectively, and men comprised 54.2% of the population. Mean age at death was 78 ± 11 years. The numbers of registrants with type 2 diabetes increased from 471 906 in 2002 to 1 033 369 in 2014, but the demographic profile remained relatively constant over time.
All-cause mortality
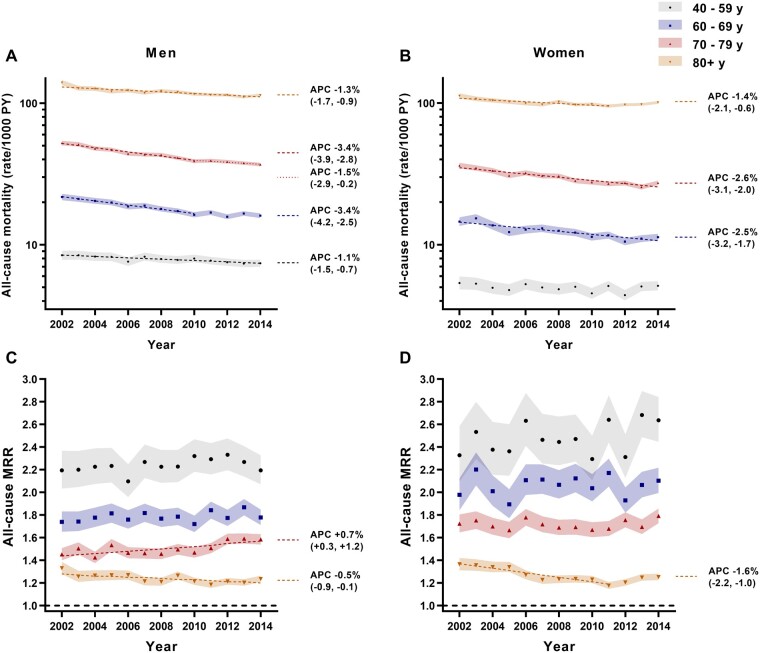
During 9 200 680 person-years follow-up of the NDSS type 2 diabetes population (median follow-up time 7.0 years), 262 903 deaths were recorded. Age-standardized all-cause mortality rates among those with type 2 diabetes decreased from 2002–14 in both sexes (P < 0.001 for both; Figure 1A and B). Corresponding trends in the MRR (comparing mortality in those with diabetes to those without) were similar (Figure 1C); however, women demonstrated a steeper decline from their higher initial MRR in 2002 to a nadir in 2011 [although there was an upturn thereafter, the average APC throughout 2002–14 still indicated an overall decline: −0.8%/year (95% CI −1.2, −0.4)].
Figure 1.
All-cause age-standardized mortality rate per 1000 person-years (PY) in men (A) and women (B) with type 2 diabetes, and corresponding MRRs (C) by calendar year. Stacked shadings in (A) and (B) represent proportions of deaths attributable to the four broad COD categories (data labels displayed for 2002 and 2014 only). 95% CIs are depicted by error bars in (A) and (B) and shading in (C). Regression lines (dashed/dotted) are displayed for time periods over which the APC in age-standardized mortality or MRR was different from zero at P < 0.05.
The reduction in age-standardized all-cause mortality for type 2 diabetes reflected falling mortality in all age groups except younger men (<40 years) and women (<60 years) (Figure 2A and B). However, the only age group in which falling mortality translated to a fall in excess mortality relative to the non-diabetic general population (MRR) was 80+ years (Figure 2C and D). As with the mortality rate, the all-cause MRR decreased throughout 2002–14 in men in this age group, but only up to 2011 in women. In all other age groups, all-cause MRRs remained similar or increased (70–79 year-old men). In a sensitivity analysis comparing those aged 80+ years with all other age groups combined (i.e. given fewer deaths at younger ages produce less precise estimates), MRRs for the total group aged <80 years increased between 2002 and 2014 [+0.5 %/year (95% CI +0.4, +0.7) in men; +0.3 %/year (95% CI +0.2, +0.5) in women]. This was confirmed by an interaction between age (80+ vs <80 years) and calendar year (P < 0.001 for both sexes).
Figure 2.
Age-specific all-cause mortality rates per 1000 person-years (PY) in men (A) and women (B) with type 2 diabetes, and corresponding MRRs (C [men] and D [women]) by calendar year. 95% CIs are depicted by shading. Regression lines (dashed/dotted) are displayed for time periods over which the APC in mortality rate or MRR was different from zero at P < 0.05. Thus, termination of a regression line prior to 2014 indicates a joinpoint at the year of termination, with the APC thereafter not different from zero (P > 0.05). Where time trends at P<0.05 were present either side of a joinpoint, multiple APCs are reported. Note: corresponding data for the 10–39 year age group are reported in Supplementary Table S2, available as Supplementary data at IJE online.
Cause-specific mortality
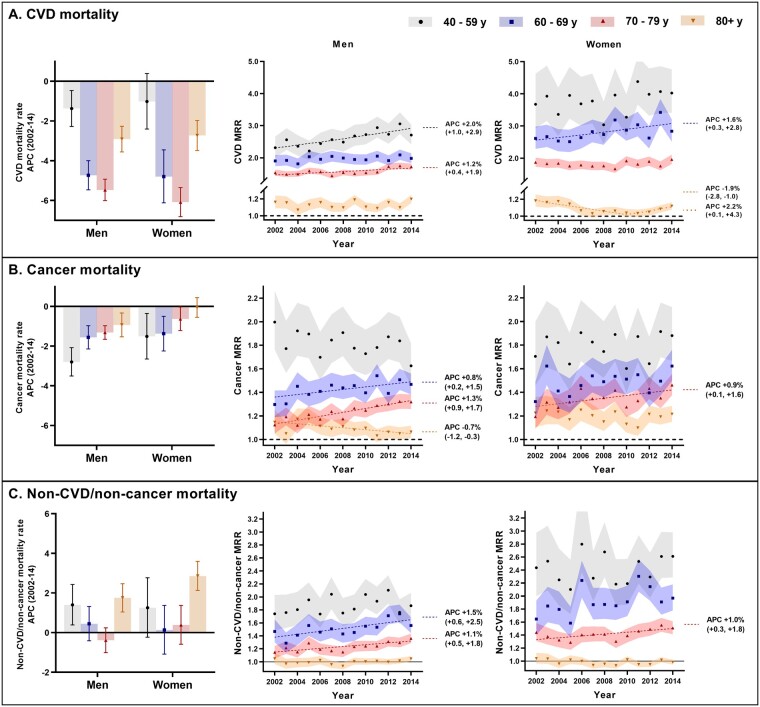
The fall in age-standardized all-cause mortality in type 2 diabetes coincided with declines in age-standardized CVD (Supplementary Figure S1, available as Supplementary data at IJE online) and cancer mortality rates (Supplementary Figure S2, available as Supplementary data at IJE online; P < 0.001 for men, P = 0.093 for women). Non-CVD/non-cancer mortality remained similar in men (P = 0.95), but increased over time in women (P = 0.001; Supplementary Figure S3, available as Supplementary data at IJE online). Trends in cause-specific mortality rates and MRRs by age are reported in Figure 3. CVD mirrored all-cause mortality insofar as the reduction in age-standardized CVD mortality in type 2 diabetes over time was observed in all age groups except younger men (<40 years) and women (<60 years). Despite this, trends for the MRR were mostly flat or rising, indicating that these falls in CVD mortality were similar to, or lagging, the non-diabetic general population. Although women with type 2 diabetes aged 80+ years demonstrated a decline in MRR between 2002 and 2010, this was offset by an increase between 2010 and 2014, resulting in no overall change.
Figure 3.
Age- and sex-specific CVD (A), cancer (B) and non-CVD/non-cancer (C) mortality rate trends (left panels) and corresponding MRRs (centre and right panels). Error bars in the left panels represent 95% CIs for the APC in mortality rate (for simplicity, this is the APC for the whole period, 2002–14—i.e. with no joinpoints). In the centre and right panels, 95% CIs for the MRR are depicted by shading. Regression lines (dashed/dotted) are displayed for time periods over which the APC in MRR was different from zero at P < 0.05. Thus, termination of a regression line prior to 2014 indicates a joinpoint at that year, with any changes in the MRR thereafter not different from zero (P > 0.05). Note: corresponding data for the 10–39 year age group are reported in Supplementary Table S2, available as Supplementary data at IJE online.
Cancer mortality in type 2 diabetes declined in all subgroups, except women aged 80+ years. However, corresponding MRRs remained at similar levels, or increased over time, indicating that these falls were again in line with, or lagging, the non-diabetic general population (except for older men, in whom the MRR decreased over time).
In contrast to CVD and cancer, non-CVD/non-cancer mortality rates in type 2 diabetes did not fall in any age group, and indeed, increased over time in men and women aged <40 years and 80+ years. However, it was only in the other age groups (i.e. 60–69 and 70–79 years) that the MRR changed, with increases noted in both men and women.
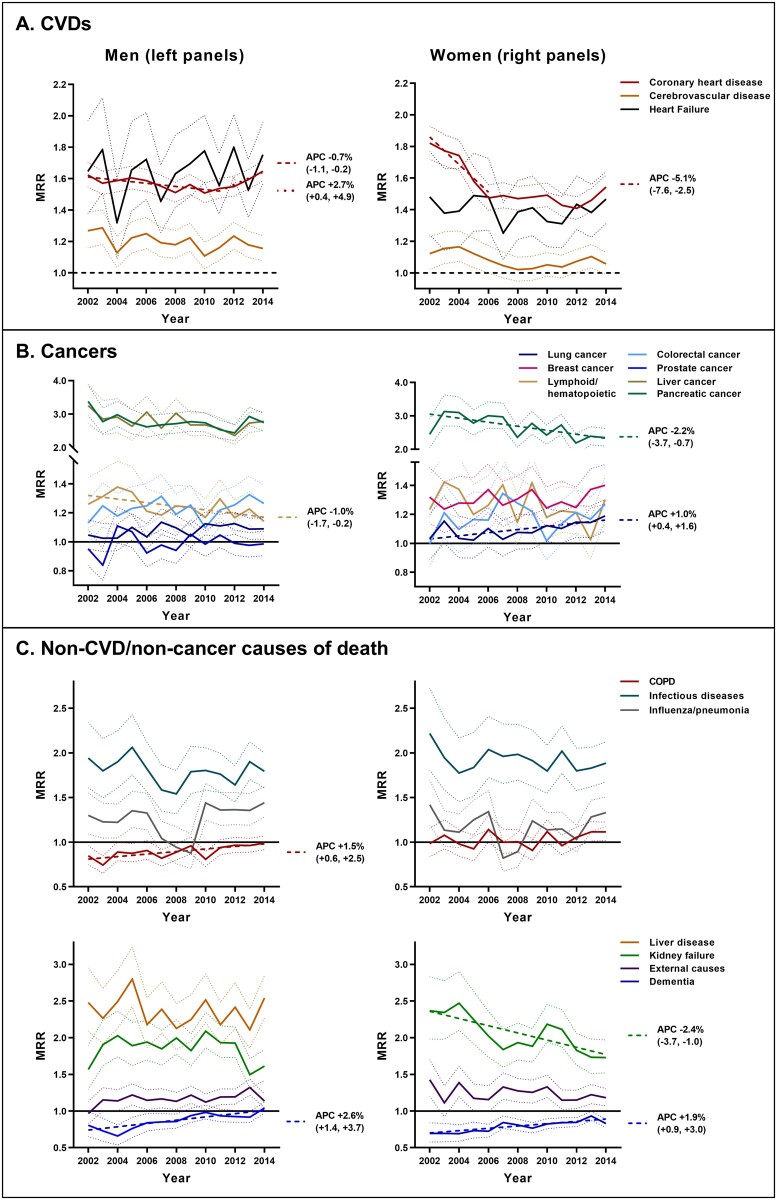
CVD-related CODs (Figure 4A)
Figure 4.
MRR by calendar year for the specific causes of death that contributed >5% of all CVD deaths (A), cancer-related deaths (B) and non-CVD/non-cancer mortality (C). 95% CIs are depicted by dotted lines. Regression lines (dashed) are displayed for time periods during which the APC in MRR was different from zero at P < 0.05.
The MRR for the leading CVD-related COD—coronary heart disease—showed early declines in both men (up to 2011) and women (up to 2006). Although there was no change thereafter in women, a reversal of the trend in men from 2011–14 corresponded to no overall change between 2002 and 2014 [average APC +0.2 %/year (95% CI -0.4, +0.7)]. Cerebrovascular disease and heart failure MRRs remained at similar levels throughout the study in both men and women.
Cancer-related CODs (Figure 4B)
Of the three most common cancer deaths (lung and colorectal cancers, and prostate/breast cancer in men/women respectively), excess mortality in type 2 diabetes was apparent only for colorectal cancer in men and breast cancer in women (with no changes in the MRR over time). However, excess lung cancer mortality increased over time, with the MRR >1.0 by 2014 (P = 0.005 in women, P = 0.064 in men).
Of the other cancer types, declines in MRR were noted for pancreatic cancer in women (P = 0.008) and liver cancer in men (P = 0.080). Excess deaths from lymphoid/ haematopoietic cancers also decreased in men.
Non-CVD/non-cancer CODs (Figure 4C)
Influenza/pneumonia, other infectious diseases, kidney failure, liver disease (men only) and external CODs all returned MRR values >1.0. Of these, reductions over time were observed only for kidney failure, and only in women. The MRR for chronic obstructive pulmonary disease (COPD) was initially <1.0 in men, but increased over time to reach parity by 2014 (i.e. to a level similar to women, in whom the MRR remained stable at ∼1.0). Dementia MRRs—though <1.0 in both men and women at study inception—increased over time, reaching ∼1.0 in men and ∼0.9 in women.
Discussion
Our data provide important caveats to the prevailing view of improving mortality in people with diabetes. Although there was a decline in excess all-cause mortality overall, age-specific analyses revealed that this was entirely driven by trends in older people (80+ years). Indeed, increasing all-cause MRRs in those aged <80 years indicated that the additional mortality risk attributable to diabetes in these individuals may actually be worsening, even in the face of a falling mortality rate. The lack of progress in those aged <80 years was borne out in corresponding trends by COD category—i.e. CVD and cancer MRRs increased or remained unchanged in most age groups <80 years (again notwithstanding falling rates of mortality from these causes), whereas non-CVD/non-cancer MRRs increased in those aged 60–79 years (driven by non-traditional morbidities in diabetes—COPD and dementia). Addressing the stagnation in excess CVD and cancer mortality at younger ages will probably require further progress with respect to the biggest contributor to CVD deaths—coronary heart disease (its MRR having stopped falling in 2007 in women, and even reversed its decline in men post-2011)—and the most common cancers for which people with type 2 diabetes are at excess risk (i.e. lung, breast and colorectal).
Variation in excess mortality trends by age
Although mortality rates and MRRs declined in the total population in this study, these trends belie that the excess mortality risk attributable to type 2 diabetes actually worsened over time in people aged <80 years. That the oldest individuals influenced total population trends to such an extent can be explained by the mean age at death (78 years), translating to almost 50% of deaths falling within this age group. It is also noteworthy that respective cause-specific MRRs appearing to drive the downward trends in those aged 80+ years—cancer in older men and CVD in older women—had reached ∼1.0 by the end of the study. Indeed, in the case of CVD mortality in older women, this had even begun to reverse, with the MRR trending higher post-2010. Unless improvements can be achieved in other CODs and other age groups, the decline in all-cause MRR observed at the total population level may stall (as already indicated by the upturn identified in women post-2011).
Differences in MRR trends by age are consistent with time trends in the incidence of major diabetes complications in the USA (1990–2010), which have also exhibited larger declines in older (75+ years), compared with younger individuals.18 However, other reports of age-specific trends in excess mortality are scarce. Studies from the UK/Canada (1996–2009)3 and Hong Kong (2001–16)6 both reported excess mortality reductions in all age groups except the youngest (20–44 years). Such improvements across a broader age range can probably be attributed to these populations having started at markedly higher levels of excess risk [baseline all-cause MRRs in the UK population being 2.14 (both sexes combined), Canada = 1.90 and Hong Kong = 2.82 (men) and 3.28 (women), respectively; compared with 1.51 (men) and 1.59 (women) in the current study]. Of course, we found that not only did the MRR fail to improve in those aged <80 years, but that it actually worsened over time for several CODs/age groups. Nevertheless, it is important to recognise that in most of these cases, underlying mortality rates were still falling; i.e. upward-trending MRRs reflected smaller improvements in those with type 2 diabetes compared with the general population, as opposed to rising mortality per se (an exception being non-CVD/non-cancer mortality). Given the strong association of complications and mortality with disease duration,19 we investigated whether the more favourable trends in older people could be attributed to a relatively smaller increase in diabetes duration over time; however, exploratory analyses (not shown) suggested potentially the opposite (duration having increased ∼5 years in the 80+ group between 2002 and 2014, vs 2-4 years in other age groups).
Clinical/public health implications
Rising excess mortality in people aged <80 years points to lagging quality of care in younger and middle-aged adults with type 2 diabetes. Younger people with type 2 diabetes tend to have a more adverse risk factor profile, with higher rates of overweight/obesity and dyslipidaemia.20 Improvements in disease management through the 2000s may have had proportionately less impact in this younger phenotype. The lower likelihood of younger people meeting glycaemic control targets21—with even middle-aged (45–64 years) people with diabetes becoming less likely to achieve target glycated haemoglobin (HbA1c) levels22—may also contribute to a progressive rise in relative risk in these age groups. Whereas Australian National Diabetes Audits have exposed a broad lack of improvement over the past decade in HbA1c target achievement,23 these surveys are restricted to patients of specialist care centres and do not report age-specific trends. Investment in systems capable of more representative national surveillance of cardiometabolic risk factors in people with diabetes would more readily identify the clinical inertia and gaps in care that our data suggest are present. In addition to implications for disease management in younger people, findings with respect to falling excess mortality in older people with type 2 diabetes—though encouraging—appear to be a portent to people presenting with a broader set of potential complications.
CVD-specific mortality
Falling all-cause mortality in type 2 diabetes has been frequently ascribed to improved CVD outcomes; however, MRRs were unchanged or even increased across most age groups. In accounting for more than half of all CVD deaths, coronary heart disease heavily influenced these results. Although its MRR decreased in the early 2000s—perhaps as a result of more aggressive risk factor management in the aftermath of diabetes being labelled a coronary risk equivalent24—its steadiness thereafter in women and subsequent increase in men would have curtailed any gains in excess CVD mortality overall. Heart failure was the underlying COD in <10% of CVD deaths, but with this proportion drifting upward and no apparent improvement in the MRR, it could be expected to make a progressively more prominent contribution to excess CVD mortality in this population.
Cancer-specific mortality
Cancer mortality declined in people with type 2 diabetes in broad alignment with background population trends (except for the 60–69 and 70–79 years age groups, who demonstrated a smaller reduction and therefore an increase in excess mortality). Concerningly, lung cancer mortality (the most common fatal cancer in this study—which we previously found to be inversely associated with diabetes),13 worsened over time such that, by 2014, rates had exceeded the non-diabetic general population (MRR >1.0). The two cancers that we previously found to demonstrate highest excess risk in diabetes (pancreatic and liver)13 continue to show the highest MRRs, despite some improvement throughout the study period.
Non-CVD/non-cancer mortality
As in previous studies,10,25–28 excess mortality risk in people with type 2 diabetes was noted for multiple non-CVD/non-cancer-related causes of death. Of these, only kidney failure mortality showed a decline, and moreover, only in women. However, this result should be interpreted with caution given our recent report from the same linked dataset that the incidence of end-stage kidney disease has increased in younger adults over the same time period.10 Given COPD accounts for a high proportion of non-CVD/non-cancer deaths, its upward MRR trend in men is concerning even if the MRR value itself does not yet indicate excess mortality risk in type 2 diabetes. The same can be said for the increase in dementia in both men and women, which surpassed COPD in terms of proportional mortality by the end of the study. Even though MRR values pointed to a historical protective effect of diabetes, the heightened risk for dementia in type 2 diabetes29—in combination with falling CVD and cancer mortality (i.e. competing risks)—may soon see dementia emerge as a major contributor to excess mortality in this population.
Strengths and limitations
With near whole-of-population coverage, the NDSS is one of the largest diabetes registries worldwide. Aside from the sample size advantage, this offers assurance of the generalizability of reported mortality and MRR trends. On the other hand, the NDSS primarily serves as an administrative database and is limited in its collection of relevant covariates for analyses of mediation/confounding (e.g. HbA1c, lifestyle). The use of general population mortality rates precluded any flexibility with COD definitions (i.e. reliance on the underlying COD). We have previously shown that this can result in under-reporting of CVD deaths in type 2 diabetes.9 In addition, although we removed the NDSS cohort from the general population to derive background mortality rates used in MRR calculations, ∼10–20% of people with diagnosed diabetes would have remained (based on estimated capture rates of the NDSS). However, although this would impact absolute MRR levels, confounding of time trends is unlikely. Likewise, it is unlikely that changes in screening practices impacted our findings, given incidence of diagnosed type 2 diabetes in the NDSS remained at similar levels throughout 2002–14 (data not shown), and excess mortality reductions were restricted to the oldest age group, in whom screening rates are highest.30
Conclusion
Whereas mortality has declined in people with type 2 diabetes overall, excess mortality—relative to the non-diabetic general population—has fallen only in those aged 80+ years. Unchanged or increasing MRRs in all other age groups indicate that improvements in the management of diabetes and its complications may not have translated to the prevention of premature deaths in type 2 diabetes. Moreover, as the major drivers of reductions in excess mortality in those aged 80+ years approach rates in the general population (i.e. CVD and cancer), continuing improvements at the total population level may stall—as has already been observed in women from 2011. Aside from efforts to broaden improved CVD and cancer mortality outcomes to include younger people, a key challenge in furthering progress will be to address ever-diversifying complications and associated mortality risks in diabetes.
Ethics approval
The study was approved by the human research ethics committees of Alfred Health (Project No. 15/15) and AIHW (EO 2015/1/148).
Supplementary data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
The AIHW linked the NDSS to the NDI and Pharmaceutical Benefits Scheme (PBS; an Australian government programme designed to subsidise the cost of prescription medications). The NDSS is an Australian Government initiative administered by Diabetes Australia since 1987. The NDI—a database of all deaths registered in Australia since 1980—is managed by the AIHW [who are also the publishers of the General Records of Incidence of Mortality (GRIM) data used for MRR calculations]. J.W.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability
The data underlying this article cannot be shared publicly due to privacy reasons.
Funding
This work was supported by the National Health and Medical Research Council of Australia (grant number 1002663) and the Victorian Government’s Operational Infrastructure Support Program.
Conflict of interest
None declared.
Contributor Information
Julian W Sacre, Clinical Diabetes and Epidemiology, Baker Heart and Diabetes Institute, Melbourne, Australia.
Jessica L Harding, Department of Surgery, Division of Transplantation, Emory University School of Medicine, Atlanta, GA, USA.
Jonathan E Shaw, Clinical Diabetes and Epidemiology, Baker Heart and Diabetes Institute, Melbourne, Australia.
Dianna J Magliano, Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, Australia; School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
References
- 1. Gregg EW, Cheng YJ, Srinivasan M et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 2018;391:2430–40. [DOI] [PubMed] [Google Scholar]
- 2. Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000-2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 2016;39:1018–26. [DOI] [PubMed] [Google Scholar]
- 3. Lind M, Garcia-Rodriguez LA, Booth GL et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 2013;56:2601–8. [DOI] [PubMed] [Google Scholar]
- 4. Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K. Steering Group of the National Diabetes R. The Danish National Diabetes Register: trends in incidence, prevalence and mortality. Diabetologia 2008;51:2187–96. [DOI] [PubMed] [Google Scholar]
- 5. Read SH, Kerssens JJ, McAllister DA; On behalf of the Scottish Diabetes Research Network Epidemiology Group et al. Trends in type 2 diabetes incidence and mortality in Scotland between 2004 and 2013. Diabetologia 2016;59:2106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu H, Lau ESH, Ma RCW et al. Secular trends in all-cause and cause-specific mortality rates in people with diabetes in Hong Kong, 2001-2016: a retrospective cohort study. Diabetologia 2020;63:757–66. [DOI] [PubMed] [Google Scholar]
- 7. Harding JL, Andes LJ, Gregg EW et al. Trends in cancer mortality among people with vs without diabetes in the USA, 1988-2015. Diabetologia 2020;63:75–84. [DOI] [PubMed] [Google Scholar]
- 8. Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol 2016;4:537–47. [DOI] [PubMed] [Google Scholar]
- 9. Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997-2010. Diabetes Care 2014;37:2579–86. [DOI] [PubMed] [Google Scholar]
- 10. Koye DN, Magliano DJ, Reid CM et al. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia, 2002-2013. Am J Kidney Dis 2019;73:300–8. [DOI] [PubMed] [Google Scholar]
- 11.Australian Institute of Health and Welfare. Diabetes Prevalence in Australia: An Assessment of National Data Sources. Canberra, Australia: AIHW, 2009. [Google Scholar]
- 12. Davis WA, Peters KE, Makepeace A et al. Prevalence of diabetes in Australia: insights from the Fremantle diabetes study phase II. Intern Med J 2018;48:803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015;38:264–70. [DOI] [PubMed] [Google Scholar]
- 14.Australian Institute of Health and Welfare. General Record of Incidence of Mortality (GRIM) Data. 2019. https://www.aihw.gov.au/reports/life-expectancy-death/grim-books/contents/grim-books (7 February 2019, date last accessed)
- 15.Australian Institute of Health and Welfare. Cancer Data in Australia. 2019. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/acim-books (2 September 2019, date last accessed).
- 16.Joinpoint Regression Program. Statistical Methodology and Applications Branch, Surveillance Research Program. https://surveillance.cancer.gov/joinpoint/. 4.7.0.0 version (February 2019) ed: National Cancer Institute; 2019.
- 17. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statist. Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 18. Gregg EW, Li Y, Wang J et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–23. [DOI] [PubMed] [Google Scholar]
- 19. Huo L, Magliano DJ, Ranciere F et al. Impact of age at diagnosis and duration of type 2 diabetes on mortality in Australia 1997-2011. Diabetologia 2018;61:1055–63. [DOI] [PubMed] [Google Scholar]
- 20. Saydah SH, Siegel KR, Imperatore G, Mercado C, Gregg EW. The cardiometabolic risk profile of young adults with diabetes in the U.S. Diabetes Care 2019;42:1895–902. [DOI] [PubMed] [Google Scholar]
- 21. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–24. [DOI] [PubMed] [Google Scholar]
- 22. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther 2017;8:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Association of Diabetes Centres. Australian National Diabetes Audit - Australian Quality Self-Management Audit (ANDA-AQSMA) 2016 Final Report. Melbourne, Australia; 2016.
- 24. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 25. Magliano DJ, Harding JL, Cohen K, Huxley RR, Davis WA, Shaw JE. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 2015;38:1274–80. [DOI] [PubMed] [Google Scholar]
- 26. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 2012;35:1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rao Kondapally Seshasai S, Kaptoge S, Thompson A; Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 2007;30:734–43. [DOI] [PubMed] [Google Scholar]
- 29. Chatterjee S, Peters SA, Woodward M et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Evron JM, Herman WH, McEwen LN. Changes in screening practices for prediabetes and diabetes since the recommendation for hemoglobin A1c testing. Diabetes Care 2019;42:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy reasons.