Abstract
Serum samples from 14 patients with Legionella pneumonia were examined for the presence of cytokines. In spite of high levels of serum C-reactive protein in all patients during the acute phase in only four cases (one involving interleukin-1β [IL-1β], three involving IL-6, and none involving tumor necrosis factor alpha) was the concentration of cytokines more than 100 pg/ml. Th2 cytokines IL-4 and IL-10 were detected in only one patient each. In contrast, significant increases of serum gamma interferon (IFN-γ) and IL-12 levels were observed during the acute phase in 6 and 11 cases, respectively. Interestingly, although serum IFN-γ levels diminished thereafter, in seven cases IL-12 levels remained high or increased further during the convalescent phase. In an additional 22 cases clinically suspected to be but not diagnosed as Legionella pneumonia, increases of serum IL-12 levels were observed in 16 cases, whereas the remaining 6 cases showed no detectable IL-12. Our results demonstrate the relative predominance of Th1 cytokine production in Legionella pneumonia. Although the role and significance of prolonged increases in IL-12 levels in Legionella disease are unknown, our results should prompt further investigation of the host immune response in terms of Th1 and Th2 balance in legionellosis.
Legionnaires’ disease is an important cause of epidemic and sporadic pneumonia in humans (1). The most common causative agent, Legionella pneumophila, is a facultative intracellular bacterium that attacks mononuclear phagocytes. Diagnosis is still difficult because this organism does not grow on routine bacteriological media, so application of specific techniques, such as PCR, for identification of bacteria is required for accurate diagnosis (7). Marston et al. (13) documented more than 3,000 cases of Legionnaires’ disease that were reported to the Centers for Disease Control and Prevention, Atlanta, Ga., from 1980 through 1989. They pointed out that Legionnaires’ disease was underreported, most likely because of underdiagnosis. Epidemiological data predict that an estimated 17,000 to 23,000 cases of community-acquired Legionella pneumonia occur annually in the United States. Unfortunately, the mortality rate is still high, particularly in immunocompromised hosts (8).
Development of cell-mediated immunity in response to L. pneumophila plays a key role in the inhibition of bacterial growth and resolution of legionellosis. Although effector mechanisms of cell-mediated immunity directed against this organism in the lung are not completely understood, in vitro studies indicate that gamma interferon (IFN-γ)-activated macrophages inhibit the intracellular growth of the bacterium (17, 23). Recently, two subsets of CD4+ T cells, Th1 and Th2, have been defined on the basis of their cytokine profiles (12, 20). Th1 cells produce interleukin-2 (IL-2) and IFN-γ and are involved in cell-mediated immunity, while Th2 cells produce IL-4, IL-5, IL-6, and IL-10 and are associated with humoral immunity. Furthermore, it has been demonstrated that some of these immunoregulatory cytokines possess cross-regulatory properties. For example, IL-12 not only enhances Th1 clones and their cytokine production but also suppresses Th2 clones and their respective cytokines (24). Despite accumulating evidence indicating a crucial role for Th1/Th2 cytokine balance in animal models of infectious diseases (4, 19), only limited data exist for human bacterial diseases in terms of Th1 and Th2 cytokine profiles. In this study, we examined the immune status of patients with Legionella disease by examining Th1 and Th2 cytokines in serial serum samples obtained from patients with Legionella pneumonia.
Clinical specimens such as sputum, bronchoalveolar lavage fluid, serum, and urine from suspected cases of Legionella disease were sent to our department. We defined a case of Legionella disease as radiographically confirmed pneumonia accompanied by at least one of the following: (i) isolation of Legionella from respiratory secretions; (ii) a fourfold increase (≥1:128) in antibody titer (microagglutination kit; Denka-seiken, Tokyo, Japan); (iii) detection of urinary antigen (Legionella urinary antigen enzyme immunoassay; Binax, Portland, Maine), or (iv) detection of Legionella DNA by PCR (2). In confirmed cases of Legionella pneumonia, information such as basic personal data and data concerning underlying diseases, including previous and/or concurrent infections, medications, symptoms, outcomes, and results of specific laboratory tests, was collected. For determination of serum cytokine levels, 75 samples from 36 cases were stored in aliquots at −80°C until assayed for cytokines. Levels of IL-1β, IL-4, IL-6, IL-10, tumor necrosis factor alpha (TNF-α), IFN-γ, and IL-12 (p40 and p70) in serum were quantified by enzyme-linked immunosorbent assay with a detection limit in the picogram-per-milliliter range (IL-1β and IFN-γ were from Otuka Pharmaceutical; IL-4, IL-6, and IL-10 were from Genzyme; TNF-α was from PerSeptive Diagnostics; IL-12 was from Biokine T Cell Diagnostics, Woburn, Mass.). In preliminary studies, it was confirmed that these cytokines were not detectable in sera of healthy volunteers (n = 5).
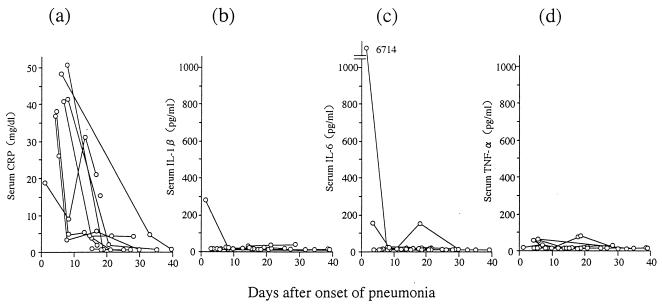
Fourteen patients were confirmed as having Legionella pneumonia. They were diagnosed by serum antibody (five cases), culture (one case), urinary antigen detection (six cases), and/or PCR (six cases). Among these cases, 11 were diagnosed by a single method while three were diagnosed by multiple methods. Etiologic organisms were L. pneumophila (12 cases) and Legionella bozemanii (one case), and the pathogen in remaining case was presumed to be L. pneumophila or Legionella dumoffii pneumonia, as significant increases of antibody titer against both organisms were observed. One patient with L. pneumophila pneumonia died, whereas the others survived. Figure 1 shows levels of C-reactive protein (CRP), IL-1β, IL-6, and TNF-α in the sera of 14 patients with Legionella pneumonia. All patients had high serum CRP levels in the acute phase. In contrast, in only four cases (one involving IL-1β, three involving IL-6, and none involving TNF-α) were cytokine concentrations >100 pg/ml.
FIG. 1.
Concentrations during the course of infection of CRP (a), IL-1β (b), IL-6 (c), and TNF-α (d) in sera of 14 patients with Legionella pneumonia.
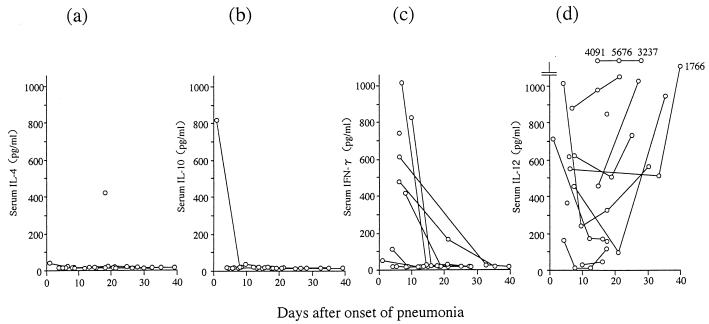
Figure 2 shows the concentrations in serum of IL-4, IL-10, IFN-γ, and IL-12. IL-4 and IL-10 were detected in only one case each. The patient showing the highest level of IL-10 in serum also had detectable levels of IL-1β and IL-6, as shown in Fig. 1. This patient died 48 days after the onset of pneumonia because of complications involving interstitial pneumonitis of unknown etiology. The concentrations of IFN-γ and IL-12 in the sera of these patients were clearly different from those of other cytokines; significant increases in the levels of IFN-γ (range, 414 to 1,028 pg/ml) and IL-12 (range, 161 to 1,006 pg/ml) in serum were observed in the acute phases of 6 and 11 cases, respectively. Although serum IFN-γ levels diminished thereafter, seven cases sustained high levels or showed even further increases of IL-12 levels in the 20 days after the onset of pneumonia. With one exception, these patients showed no signs of exacerbation of the pneumonia or other complications during the observation period.
FIG. 2.
Concentrations during the course of infection of Th2 (IL-4 [a] and IL-10 [b]) and Th1 (IFN-γ [c] and IL-12 [d]) cytokines in sera of 14 patients with Legionella pneumonia.
We also examined serum levels of IL-12 in an additional 22 cases of pneumonia clinically suspected to be but not diagnosed as Legionella pneumonia. Those cases fell into two distinct groups; 16 cases showed significant increases of levels of IL-12 in serum, as in the confirmed cases of Legionella pneumonia (range, 230 to 1,049 pg/ml), whereas IL-12 was not detected in the remaining six cases (data not shown). Considering the fact that diagnosis of Legionella disease is still difficult and more than 10,000 cases may be overlooked annually in the United States, it is likely that Legionella pneumonia is involved in these cases.
The major finding of our study is the relative predominance of cellular immune responses in patients with Legionella pneumonia, as evidenced by the significant increase of levels of Th1 cytokines (IFN-γ and IL-12) in serum. In addition, our data demonstrate for the first time the potential of IL-12 as a critical mediator of host immunity against legionellosis.
The balance of Th1/Th2 cytokine responses is believed to play an important role in orchestrating the immune response against invading microbes (12, 20). Of special importance are the Th1 cytokines (IFN-γ and IL-12) and the Th2 cytokines (IL-4 and IL-10). Several experimental models of infectious diseases, such as those caused by Leishmania spp. (4, 19), Toxoplasma gondii (6), Mycobacterium spp. (3), Listeria monocytogenes (5), and Candida albicans (21), shed light on the critical role of the Th1/Th2 balance in innate and adaptive immune responses to infections. However, in the clinical setting only a few diseases, such as pleuritis caused by Mycobacterium tuberculosis (11), leprosy (22), and leishmaniasis (14), have been analyzed in terms of their immune status and Th1/Th2 balance. Newton et al. (18) showed that suppression of Th1 activity by marijuana significantly sensitized mice to a lethal challenge of L. pneumophila. Kitsukawa et al. (9) detected mRNA encoding IFN-γ but not IL-4 in the supernatant of human peripheral blood mononuclear leukocytes cultured with L. pneumophila. Our results confirm these early findings and further demonstrate the crucial role of Th1-polarized immune responses in patients with Legionella pneumonia.
IL-12, a recently described cytokine, appears to possess the characteristics necessary to link the innate and cognate cellular immune systems (24). The ability of IL-12 to induce production of IFN-γ and other phagocytic cell-activating cytokines is particularly important during acute bacterial infections. In addition, IL-12 induces the differentiation of Th1 cells from uncommitted T cells, thus initiating cell-mediated immunity, which generally protects against intracellular parasites in the chronic stages of infections. Interestingly, in the present study, we observed sustained high levels of IL-12 in the sera of patients with Legionella pneumonia even in the convalescent phase, although signs of continuous colonization of the lungs or exacerbation of pneumonia were not observed. In this regard, Naot et al. (16) and Morley et al. (15) reported possible reactivation of Legionella pneumonia in immunocompromised patients. In addition, Kohler et al. (10) reported that 10 of 23 patients with Legionella pneumonia excreted antigen in their urine for 42 days or longer despite full recovery from Legionnaires’ disease and an absence of clinical disease during this phase. We also observed continued urinary antigen excretion for more than two weeks postrecovery in four of our patients (data not shown). These data strongly suggest the continuous presence in these patients of bacteria or bacterial components or products, which may be associated with IL-12 production.
In the present study, we could not compare serum cytokines in pneumonia cases of different etiologies: we have only presented cytokine profiles of Legionella disease as a preliminary report. Whether these cytokine characteristics are specific to Legionella disease, in addition to whether blood monocytes from patients with Legionella pneumonia would preferentially synthesize Th1-type cytokines in response to Legionella antigens, remains to be determined in future studies. It would also be of great interest to determine if IL-12 could be used as a diagnostic indicator for certain infectious diseases which preferentially stimulate cell-mediated immunity. These issues would have important implications for our understanding of the pathological and immunological status of patients with legionellosis.
Acknowledgments
We thank Shogo Kuwahara and Paul H. Edelstein for their critical readings of the manuscript and their helpful suggestions. We also thank F. G. Issa for expert editorial assistance.
REFERENCES
- 1.Edelstein P H, Meyer R D. Legionella pneumonias. In: Pennington J E, editor. Respiratory infections: diagnosis and management. 3rd ed. New York, N.Y: Raven Press; 1994. pp. 381–402. [Google Scholar]
- 2.Engleberg N C, Carter C, Weber D R, Cianciotto N P, Eisenstein B I. DNA sequence of mip, a Legionella pneumophila gene associated with macrophage infectivity. Infect Immun. 1989;57:1263–1270. doi: 10.1128/iai.57.4.1263-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 4.Guler M L, Gorham J D, Hsieh C S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 6.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaulhac B, Nowicki M, Bornstein N, Meunier O, Prevost G, Piemont Y, Fleurette J, Monteil H. Detection of Legionella spp. in bronchoalveolar lavage fluids by DNA amplification. J Clin Microbiol. 1992;30:920–924. doi: 10.1128/jcm.30.4.920-924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby B D, Snyder K M, Meyer R D, Finegold S M. Legionnaire’s disease: report of sixty-five nosocomially acquired cases and review of the literature. Medicine (Baltimore) 1980;59:188–205. [PubMed] [Google Scholar]
- 9.Kitsukawa K, Nakamoto A, Koito H, Matsuda Y, Saito A, Yamamoto H. Interferon-γ production by human T lymphocytes upon Legionella pneumophila stimulation in vitro. Clin Exp Immunol. 1995;99:76–81. doi: 10.1111/j.1365-2249.1995.tb03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler R B, Winn W C, Jr, Wheat L J. Onset and duration of urinary antigen excretion in Legionnaires disease. J Clin Microbiol. 1984;20:605–607. doi: 10.1128/jcm.20.4.605-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Zhang M, Hofman F M, Gong J, Barnes P F. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucey D R, Clerici M, Shearer G M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marston B J, Lipman H B, Breiman R F. Surveillance for Legionnaire’s disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- 14.Melby P C, Andrade-Narvaez F, Darnell B J, Valencia-Pacheco G. In situ expression of interleukin-10 and interleukin-12 in active human cutaneous leishmaniasis. FEMS Immunol Med Microbiol. 1996;15:101–107. doi: 10.1111/j.1574-695X.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 15.Morley J N, Smith L C, Baltch A L, Smith R P. Recurrent infection due to Legionella pneumophila in a patient with AIDS. Clin Infect Dis. 1994;19:1130–1132. doi: 10.1093/clinids/19.6.1130. [DOI] [PubMed] [Google Scholar]
- 16.Naot Y, Brown A, Elder E M, Shonnard J, Luft B J, Remington J S. IgM and IgG antibody response in two immunosuppressed patients with Legionnaire’s disease. Evidence of reactivation of latent infection. Am J Med. 1982;73:791–794. doi: 10.1016/0002-9343(82)90759-8. [DOI] [PubMed] [Google Scholar]
- 17.Nash T W, Libby D M, Horwitz M A. IFN-γ activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 18.Newton C A, Klein T W, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 20.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 21.Romani L, Menacci L, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S E, Bistoni F. IL-12 is both required and prognostic in vivo for T helper 1 differentiation in murine candidiasis. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 22.Sieling P A, Modlin R L. Cytokine patterns at the site of mycobacterial infection. Immunobiology. 1994;191:378–387. doi: 10.1016/S0171-2985(11)80443-2. [DOI] [PubMed] [Google Scholar]
- 23.Skerrett S J, Martin T R. Recombinant murine interferon-γ reversibly activates rat alveolar macrophages to kill Legionella pneumophila. J Infect Dis. 1992;166:1354–1361. doi: 10.1093/infdis/166.6.1354. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]