Abstract
A rapid immunochromatographic test was compared to the hemagglutination inhibition assay for separate determinations of dengue virus-specific immunoglobulin M (IgM) and IgG levels in paired serum specimens from 92 patients (34 with primary dengue virus infection, 35 with secondary dengue virus infection, and 23 without dengue virus infection). The rapid test showed 99% sensitivity in the diagnosis of dengue virus infection. The majority (30 of 34 [88%]) of patients with primary infection showed positive IgM but negative IgG, while 34 of 35 (97%) patients with secondary infection showed positive IgG with or without IgM. Specificity in nonflavivirus infections was 96% (1 of 23 positive). The rapid test should be a useful aid in rapid diagnosis of dengue virus infection.
Dengue virus, a flavivirus, is found in areas of the tropics and subtropics. Four serotypes of the virus (dengue-1 to -4) are observed; these are closely related but antigenically distinct (8). The virus, which causes disease in humans, is transmitted by mosquito, principally Aedes aegypti. In terms of morbidity, mortality, and economic costs, dengue is the most important mosquito-borne disease in the world, with an estimated 50 to 100 million cases per year (6, 7). Furthermore, the incidence and spread of the disease are increasing (4, 14).
Primary infection with dengue virus results in a self-limiting disease characterized by mild to high fever lasting 3 to 7 days, severe headache with pain behind the eyes, muscle and joint pain, and a rash (14, 15). Secondary infection with a different dengue virus serotype is the more common form of the disease in many parts of Southeast Asia and South America (3). This form of the disease is more serious and can result in dengue hemorrhagic fever and dengue shock syndrome. The major clinical symptoms can include high fever, hemorrhagic events, and circulatory failure, and the fatality rate can be as high as 30%. Early diagnosis of dengue shock syndrome is particularly important, as patients may die within 12 to 24 h if appropriate treatment is not administered (12, 15).
Primary dengue virus infection is characterized by elevations in specific immunoglobulin M (IgM) levels 3 to 5 days after the onset of symptoms; this generally persists for 30 to 60 days (9, 10). IgG levels also become elevated after 10 to 14 days, and these remain detectable for life, though at a hemagglutination inhibition assay (HAI) titer of ≤1:640 (5). During secondary infection, IgM levels generally rise more slowly and reach lower levels than in primary infection, while IgG levels rise rapidly from 1 to 2 days after the onset of symptoms (5, 9). The HAI titer rises to ≥1:2,560, and these levels persist for 30 to 40 days before returning to levels of ≤1:640 (5).
Traditionally, HAI has been used to classify dengue infections as primary or secondary. The current definition depends upon an assay of paired serum specimens separated by at least 7 days (fourfold rise in titer), though any acute-phase specimen with an HAI titer of ≥1:2,560 is defined as coming from a patient with secondary infection (17). Despite its general acceptance as a standard laboratory test for dengue diagnosis, variations in methods used in different laboratories can make it difficult to compare HAI results. Furthermore, HAI requires extraction of sera as well as the preparation of a series of dilutions for each sample. In addition, sera may need to be collected after hospital discharge to confirm the diagnosis. Consequently, a simple, commercially available assay for dengue diagnosis would offer distinct advantages over HAI. In this study, the Dengue Rapid Test (PanBio Pty., Ltd., Brisbane, Australia) was compared to HAI with paired sera collected from patients presenting at Singapore General Hospital.
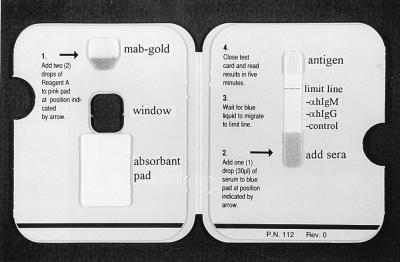
In the Dengue Rapid Test, both IgM and IgG levels are determined with a capture assay format (Fig. 1). A drop of serum is added to the serum pad in the test device, and it migrates along the nitrocellulose membrane, where IgG and IgM are captured by lines of either anti-human IgG or anti-human IgM striped onto the membrane. At the same time, a gold-labelled anti-dengue virus monoclonal antibody (MAb) is rehydrated by the addition of two drops of buffer to the conjugate pad. After the serum reaches the limit line (<2 min), the card is closed. This allows the rehydrated gold-labelled anti-dengue virus MAb to complex with dengue virus types 1 to 4 stabilized in the pad at the top of the nitrocellulose membrane. In addition, closure of the card draws the flow of gold-complexed antigen in the reverse direction, down the nitrocellulose membrane and into the large absorbent pad, which allows binding of gold-complexed antigen to the bound IgM or IgG. After 5 min, the assay result is read visually through the window on the front panel of the card. The captured gold-labelled antigen-antibody complexes appear as maroon lines. Tests are read blind to eliminate reader bias, with the intensity of the lines observed in the rapid test scored as nonreactive, weakly positive, or strongly positive, depending on the intensity of the reaction. In addition to the anti-IgG and anti-IgM lines, a control line is included to ensure that the test result is valid. Positive control (HAI titer, 1:2,560) and negative control (HAI titer, 1:10) sera are also included with each run. Since the IgG cutoff in the test has been set to detect the high IgG levels characteristic of secondary dengue infection, primary dengue infection is defined by a visible IgM line without a visible IgG line while secondary dengue infection is defined as a visible IgG line with or without a positive IgM line. A negative result is defined by the absence of both an IgM line and an IgG line (control line only visible).
FIG. 1.
Inside view of the PanBio Dengue Rapid Test device, showing general instructions for use. The locations of the antigen pad, gold-labelled MAb conjugate pad, and absorbent pad are shown, as well as the anti-human (αh) IgG, anti-human IgM, control, and limit lines.
Sera were tested for HAI antibodies as described previously (2) except that the assay was modified to a microtiter plate format. Serum inhibitors were removed by pretreatment with kaolin absorption, and naturally occurring nonspecific agglutinins were absorbed out with packed gander cells. Serum dilutions were carried out in borate-buffered saline, pH 9.0, containing 1% Proteose Peptone (Difco, Detroit, Mich.). Antigens were produced by sucrose acetone extraction of the brains of suckling mice infected with dengue virus type 1 or 2, purchased from the Virus Research Institute, Ministry of Public Health, Bangkok, Thailand. Gander blood was obtained from the Singapore Primary Productions Department and used at a final concentration of 0.4%. Results were read after an incubation period of 2 h. A positive control (HAI titer, 1:2,560) and a negative control (HAI titer, <1:10) were included in each assay. Dengue virus infection was defined by World Health Organization criteria (17). That is, a fourfold rise in titer in sera collected 7 days apart defined dengue virus infection (primary or secondary), and an HAI titer of ≥1:2,560 in any serum specimen defined secondary dengue infection.
All patients who participated in this study were suspected to have dengue infection, based on their clinical symptoms. Patients were admitted to Singapore General Hospital from June to October 1996. Dengue virus serotypes 1 and 2 were circulating in Singapore at this time. The acute-phase sera were collected at the time of hospital admission, while the convalescent-phase sera were collected 7 to 21 days later. By HAI and the criteria given above, patients were classified as having primary dengue virus infection (n = 34), secondary dengue virus infection (n = 35), or no dengue virus infection (n = 23). Viral isolation or type-specific antibody assays were not performed.
The combined use of IgM and IgG has been shown to increase sensitivity in the detection of dengue virus infection, since IgM is a good marker of primary infection while elevation of IgG levels is an excellent marker of secondary infection (9, 16). In this study, all but one patient with dengue virus infection (99%) were detected by the Dengue Rapid Test when paired sera were used (Table 1). The false-negative result occurred in a patient with primary infection who showed low HAI titers in paired sera (1:10 and 1:40). Eight other patients with primary infection also showed the same HAI titers in paired sera, and these were detected in the rapid test. The Dengue Rapid Test also had excellent specificity (96%) for patients showing similar clinical presentations without dengue infection (Table 1). The one false-positive case showed an elevation in the level of rapid test IgM but not IgG in the convalescent-phase sera, and this patient showed an HAI titer of 1:10 in both acute- and convalescent-phase sera.
TABLE 1.
Sensitivity and specificity of the Dengue Rapid Test with paired sera
| HAI diagnosis (n)a | Dengue Rapid Test result (n)
|
Specificity or sensitivity (%) | ||
|---|---|---|---|---|
| Negative (IgG− IgM−) | Primary (IgG− IgM+) | Secondary (IgG+) | ||
| Negative (23) | 22 | 1 | 0 | 96b |
| Primary (34) | 1 | 30 | 3 | 97c |
| Secondary (35) | 0 | 1 | 34 | 100c |
Based on World Health Organization criteria.
Specificity.
Sensitivity.
Traditionally, HAI has been used to distinguish between primary and secondary dengue virus infections, with a titer of ≥1:2,560 considered indicative of secondary dengue virus infection (17). The IgG readings in the Dengue Rapid Test showed excellent correlation with HAI, with the majority of IgG-positive tests corresponding to an HAI titer of ≥1:2,560 (χ2 = 73.4; P < 0.0001 [chi-square test]) (Table 2). Consequently, the rapid test had a high predictive value in classifying dengue virus infections as primary or secondary (Table 1). The majority (30 of 34 [88%]) of patients with primary dengue virus infection showed elevations of IgM levels but not IgG levels, while three patients with primary dengue virus infection showed elevations of both IgM and IgG levels and were consequently classified as having secondary dengue virus infection by the rapid test. The HAI titers in the convalescent-phase sera of these three patients were 1:80, 1:640, and 1:1,280. All but one patient (34 of 35 [97%]) with secondary dengue virus infection showed elevations of IgG levels with or without IgM (Table 1). Of these 34 cases of secondary dengue, 26 (76%) showed positive IgM readings while the remainder showed undetectable IgM in the rapid test.
TABLE 2.
Comparison of Dengue Rapid Test IgG score and HAI titera
| Dengue Rapid Test IgG scoreb | HAI titer, ≥1:2,560
|
|
|---|---|---|
| No. positive/no. tested | % Positive | |
| NR | 1/55 | 2 |
| WP | 6/8 | 75 |
| SP | 27/29 | 93 |
χ2 = 73.4; P < 0.0001 (chi-square test for independence).
NR, nonreactive; WP, weakly positive; SP, strongly positive (arbitrary scale).
Previous studies have suggested that diagnosis based on IgM alone may take up to 7 days after the onset of infection (5, 9, 10, 11, 16). This is reflected when the rapid test results in acute-phase sera are analyzed, with only 57% of dengue cases detected by this test in the early acute phase of illness. In acute-phase sera, 16 of 34 cases (47%) of primary infection and 23 of 35 cases (66%) of secondary infection were detected by the rapid test (not shown). All cases of primary infection diagnosed with the rapid test on acute-phase sera showed a positive-IgM–negative-IgG profile, while the majority (19 of 23) of secondary dengue cases diagnosed through use of the acute-phase sera showed elevations of IgG levels in the rapid test, with 11 of these patients also showing elevations of IgM levels. In contrast, HAI detected only 15 of 35 cases (43%) of secondary infection and no cases of primary infection with the acute-phase sera (HAI titer, ≥1:2,560). The sensitivity of the rapid test compares favorably to HAI in that a second serum specimen would need to be assayed in less than half of the cases presented. However, as with all serological tests, it is important to stress the use of the rapid test as a diagnostic aid, the results of which should be taken in conjunction with clinical symptoms and other available laboratory results. That is, physicians making patient management decisions should not rely solely on this or any other serology test for clinical guidance unless the result is positive. We would suggest that a patient with a negative test result and persisting symptoms be retested 3 to 4 days later to confirm the diagnosis of dengue virus infection.
Commercially available dot blot enzyme-linked immunosorbent assays for dengue diagnosis have been described, and sensitivities and specificities similar to the Dengue Rapid Test have been reported (1, 13, 18). A commercial dipstick dot blot enzyme-linked immunosorbent assay for IgM and IgG (Integrated Diagnostics, Baltimore, Md.) has been reported to have sensitivity of 95 to 98% and specificity of 100% (18). The Dengue Blot test (Genelabs Diagnostics, Singapore, Singapore) has been reported to have sensitivity ranging from 92 to 98% and specificity of 81 to 88% (1, 13), though low sensitivity for primary dengue virus infections and high cross-reactivity in patients with malaria have been reported (10, 13). The clinical diagnoses of the nonflaviviral diseases tested in this study were not known, though in another study only 1 of 10 sera from patients with malaria showed cross-reactivity in the PanBio Dengue Rapid Test (9a). Despite the similar performances of these tests, the Dengue Rapid Test has the advantage of being easier to perform in that it does not require dilution or pretreatment of sera to remove competing IgG or rheumatoid factor, washing steps and multiple incubations are not performed, preparation and dilution of reagents used in the test are unnecessary, and a laboratory incubator (50°C for the dipstick test) is not required. Furthermore, the rapid test takes only 5 min to run, compared with at least 3 h for the dot blot assays. Consequently, the Dengue Rapid Test should be a useful aid in the diagnosis of dengue fever. It is rapid, easy to perform, and overcomes some of the limitations associated with HAI. It has potential for use at the point of care or in laboratories where the volume of testing is low or sporadic or where equipment is not available. However, as with all serology-based assays, it is essential to interpret the results in conjunction with other laboratory tests and clinical symptoms.
REFERENCES
- 1.Cardosa M J, Baharudin F, Hamid S, Hooi T P, Nimmanitya S. A nitrocellulose membrane based IgM capture enzyme immunoassay for etiological diagnosis of dengue virus infections. Clin Diagn Virol. 1995;3:343–350. doi: 10.1016/0928-0197(94)00049-z. [DOI] [PubMed] [Google Scholar]
- 2.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 3.Gubler D J. Dengue haemorrhagic fever: a global update. Virus Inf Exchange Newsl. 1991;8:2–3. [Google Scholar]
- 4.Gubler D J. World distribution of dengue. Dengue Bull. 1996;20:1–4. [Google Scholar]
- 5.Gubler D J. Serological diagnosis of dengue/dengue haemorrhagic fever. Dengue Bull. 1996;20:20–23. [Google Scholar]
- 6.Halstead S B. Selective primary health care: strategies for control of disease in the developing world. XI. Dengue. Rev Infect Dis. 1984;6:251–264. doi: 10.1093/clinids/6.2.251. [DOI] [PubMed] [Google Scholar]
- 7.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 8.Henchal E A, Putnak J R. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Innis B L, Nisalak A, Nammanitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H. An enzyme-linked immunosorbent assay to characterise dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 9a.Lam, S. K. Personal communication.
- 10.Lam S K. Rapid dengue diagnosis and interpretation. Malays J Pathol. 1993;15:9–12. [PubMed] [Google Scholar]
- 11.Lam S K. Application of rapid laboratory diagnosis in dengue control. Asia Pac J Mol Biol Biotechnol. 1995;3:351–355. [Google Scholar]
- 12.Lam S K. Dengue haemorrhagic fever. Rev Med Microbiol. 1995;6:39–48. [Google Scholar]
- 13.Lam S K, Fong M Y, Chungue E, Doraisingham S, Igarashi A, Khin M A, Kyaw Z T, Nisalak A, Roche C, Vaughn D W, Vorndam V. Multicentre evaluation of dengue IgM dot enzyme immunoassay. Clin Diagn Virol. 1996;7:93–98. doi: 10.1016/s0928-0197(96)00257-7. [DOI] [PubMed] [Google Scholar]
- 14.Monath T P, Heinz F X. Flaviviruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. New York, N.Y: Lippincott-Raven; 1996. pp. 1016–1021. [Google Scholar]
- 15.Nimmannitya S. Clinical management of dengue fever/dengue haemorrhagic fever/dengue shock syndrome. Dengue Bull. 1996;20:13–19. [Google Scholar]
- 16.Ruechusatawat K, Morita K, Tanaka M, Vongcheree S, Rojanasuphot S, Warachit P, Kanai K, Thongtradol P, Nimnakorn P, Kanungkid S, Igarashi A. Daily observation of antibody levels among dengue patients detected by enzyme-linked immunosorbent assay (ELISA) Jpn J Trop Med Hyg. 1994;22:9–12. [Google Scholar]
- 17.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment and control. Geneva, Switzerland: World Health Organization; 1986. [Google Scholar]
- 18.Wu S-J L, Hanson B, Paxton H, Nisalak A, Vaughn D W, Rossi C, Henchal E A, Porter K R, Watts D M, Hayes C G. Evaluation of a dipstick enzyme-linked immunosorbent assay for detection of antibodies to dengue virus. Clin Diagn Lab Immunol. 1997;4:452–457. doi: 10.1128/cdli.4.4.452-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]