Abstract
BACKGROUND:
To determine if low-frequency repetitive transcranial magnetic stimulation targeting the primary motor cortex contralateral (M1CL) to the affected corticospinal tract in patients with hemiparetic stroke augments intensive training–related clinical improvement; an extension of the NICHE trial (Navigated Inhibitory rTMS to Contralesional Hemisphere Trial) using an alternative sham coil.
METHODS:
The present E-FIT trial (Electric Field Navigated 1Hz rTMS for Post-stroke Motor Recovery Trial) included 5 of 12 NICHE trial outpatient US rehabilitation centers. The stimulation protocol remained identical (1 Hz repetitive transcranial magnetic stimulation, M1CL, preceding 60-minute therapy, 18 sessions/6 wks; parallel arm randomized clinical trial). The sham coil appearance mimicked the active coil but without the weak electric field in the NICHE trial sham coil. Outcomes measured 1 week, and 1, 3, and 6 months after the end of treatment included the following: upper extremity Fugl-Meyer (primary, 6 months after end of treatment), Action Research Arm Test, National Institutes of Health Stroke Scale, quality of life (EQ-5D), and safety.
RESULTS:
Of 60 participants randomized, 58 completed treatment and were included for analysis. Bayesian analysis of combined data from the E-FIT and the NICHE trials indicated that active treatment was not superior to sham at the primary end point (posterior mean odds ratio of 1.94 [96% credible interval of 0.61–4.80]). For the E-FIT intent-to-treat population, upper extremity Fugl-Meyer improvement ≥5 pts occurred in 60% (18/30) active group and 50% (14/28) sham group. Participants enrolled 3 to 6 months following stroke had a 67% (31%–91% CI) response rate in the active group at the 6-month end point versus 50% in the sham group (21.5%–78.5% CI). There were significant improvements from baseline to 6 months for both active and sham groups in upper extremity Fugl-Meyer, Action Research Arm Test, and EQ-5D (P<0.05). Improvement in National Institutes of Health Stroke Scale was observed only in the active group (P=0.004). Ten serious unrelated adverse events occurred (4 active group, 6 sham group, P=0.72).
CONCLUSIONS:
Intensive motor rehabilitation 3 to 12 months after stroke improved clinical impairment, function, and quality of life; however, 1 Hz-repetitive transcranial magnetic stimulation was not an effective treatment adjuvant in the present sample population with mixed lesion location and extent.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03010462.
Keywords: odds ratio, outpatients, quality of life, stroke, therapeutics, upper extremity
Repetitive transcranial magnetic stimulation (rTMS) has been widely adopted as a treatment strategy in psychiatric applications1,2 but has yet to gain traction for neurorecovery such as following stroke. Low-frequency rTMS can lead to a lasting reduction of cortical excitability in the motor cortex3 and was an appealing strategy post–unilateral stroke for targeting the primary motor cortex contralateral (M1CL) to the affected corticospinal tract, based on an early model of interhemispheric competition4,5 and meta-analyses published between 2012 and 2019.6,7 This model was considered simplistic given the heterogeneity of stroke and incomplete understanding of structural and functional underpinnings of hemiparesis; however, non-navigated 1-Hz rTMS targeting M1CL in patients with stroke demonstrated modest short-term effects on motor function8 and warranted further investigation. Our prior NICHE trial (Navigated Inhibitory rTMS to Contralesional Hemisphere Trial),9 based on promising early data,10 aimed to augment the restoration of clinical motor status associated with hand-arm motor training, by combining with rTMS. The rTMS (1 Hz, M1CL) was applied using neuronavigation for more robust modulation than non-navigated,11 preceding goal-oriented motor training for each of the 18 sessions. A clinical benefit was observed in both the rTMS and sham study groups, and the findings were interpreted as a strong benefit of the intensive training, without advantage conferred by the rTMS. However, given that the sham coil delivered low-intensity stimulation (<10% real), and that low-intensity rTMS stimulation can result in structural and functional changes,12 it remained unclear if both arms of the study were influenced by rTMS. The E-FIT trial was an extension of the NICHE trial, using an alternative sham coil, without the induced low-intensity cortical electric field, but with a similar appearance and auditory and tactile patient experience. The trial design and interventions were otherwise unchanged except that patients with hemorrhagic stroke were not included in the present trial. The primary goal of the present trial was to determine if 1 Hz rTMS delivered before each motor training session would augment the clinical benefit of training.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Trial Design
The E-FIT trial was a multisite, prospective, sham-controlled phase III supplementary trial to the NICHE trial. All sites (5 outpatient US rehabilitation centers), and local principal investigators participated in the completed NICHE trial (NCT02089464). The local institutional review board approved the trial at all sites. The trial was sponsored by Nexstim Ltd who contracted with Medfiles Ltd (Kuopio, Finland) and its subcontractor Quretec Ltd (Tartu, Estonia), with a full-service Contract Research Organization. The companies were responsible for E-FIT data management and biostatistical services. The trial was monitored by an independent data and safety monitoring board continuing in the same role as the NICHE trial and comprising 2 physician members, expert in the field of the trial, as well as an independent biostatistician. Interim data analyses were not performed beyond the data and safety monitoring board safety data review (per trial protocol).
Participants
Adult participants (18+ years) with residual hemiparesis (Chedoke–McMaster arm and hand stage of 3–6) from a unilateral ischemic stroke 3 to 12 months prior were eligible to volunteer for the study (for full inclusion and exclusion criteria, see the Supplemental Material). All potential subjects provided written informed consent before any study-related procedures.
Intervention and Instrumentation
The intervention for the present E-FIT trial was identical to the NICHE trial,9 with the only difference being the sham coil used. A structural magnetic resonance imaging was acquired on enrolled participants for individual-specific navigated transcranial magnetic stimulation (TMS) treatment targeting. Treatment comprised 18 sessions (3×/wk, 6 weeks) of Nexstim navigated brain therapy (NBT, 1 Hz rTMS 900 pulses, 110% of resting motor threshold, for the extensor digitorum communis muscle before each standardized motor therapy session. An additional week was permitted to replace missed sessions. The specific sequence in a given session was (1) 20-minute prefunctional upper limb therapy (individualized from the Cedoke-McMaster arm score), (2) 10-minute rest, (3) NBT delivered at rest targeting M1CL (≈15 minutes), (4) 5- to 10-minute rest, (5) 60-minute structured session of goal-directed, task-oriented rehabilitation therapy (individualized from the Chedoke-McMaster hand score). Baseline outcome measures were repeated at 1 week, 1, 3, and 6 months after the end of treatment. Details on the rTMS instrumentation, mapping, and treatment protocols, and the motor therapy can be found in the Supplemental Material). Standardized protocol training was provided across the sites for NBT, arm motor training, and assessments.
Outcome Measures
Primary and secondary measures were collected by nontreating clinician raters, masked to treatment assignment, and trained by a single rater (raters were reassessed every 6 months to maintain standardization throughout the study duration). The primary outcome measure was the upper extremity Fugl-Meyer score (UEFM), testing the proportion of participants in each group achieving a clinical improvement 6 months from end of treatment relative to baseline. Secondary outcome measures were: UEFM score in points, Action-Research Arm Test, National Institutes of Health Stroke Scale, and quality of life assessed using the EQ-5D questionnaire. Serious adverse events (SAEs) and treatment-emergent adverse events were recorded.
Sample Size, Randomization, and Blinding
A minimum of 60 patients were planned to be randomized to the study to have >80% power to detect a difference in the proportions of clinical improvement of >30% when combined with NICHE trial data. Randomization to rTMS or sham rTMS (computer-based random number generator) of 1:1 ratio was conducted per site, in randomly occurring blocks of 3 to 6 patients. Patients 3 to 6 months poststroke were randomized separately from patients 6 to 12 months poststroke.
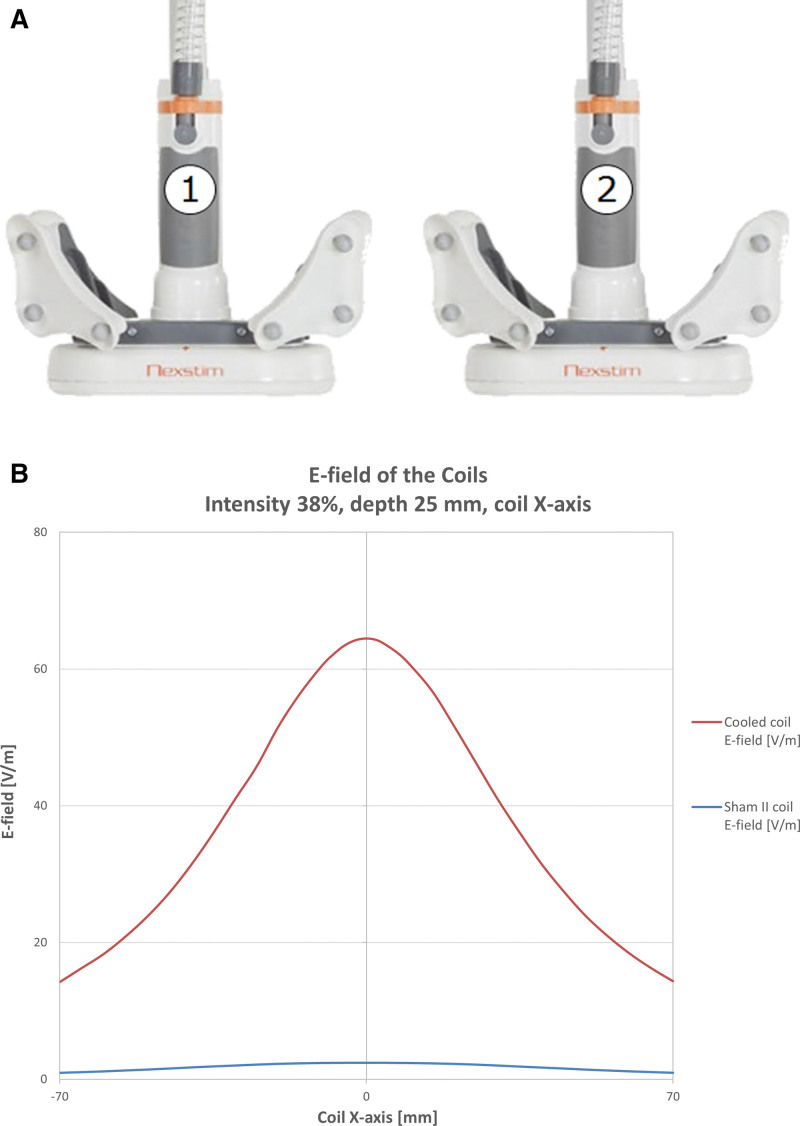
Patients were naive to TMS. Only the TMS administrator was aware of, and responsible for, local site randomization. The effectiveness of the blinding was assessed before the start of treatment, at end of treatment, and at the end of the study, by asking the patient which treatment condition they thought they had received. The effectiveness of blinding on outcome assessment therapists was collected at the first follow-up visit, and again at the primary outcome assessment visit at 6 months after end of treatment. The sham condition was delivered using the NBT system to navigate and localize a sham TMS coil to the same position on the patient’s head as the active TMS coil would have been located. The sham coil (Figure 1) was outwardly identical to the active TMS coil and caused similar auditory and sensory scalp responses as the active coil and was validated before the trial (see the Supplemental Material sham coil validation). The sham treatment protocol including the number of pulses, frequency, and duration was consistent with the active treatment.
Figure 1.
Sham vs real transcranial magnetic stimulation (TMS) coil and corresponding electric fields. The coils are identical in appearance (A) and are distinguished by reduced electric field than in the sham coil, approximates zero (B) in the brain.
Statistical Analysis
The primary efficacy analysis was generated from the intent-to-treat population, with a separate calculation conducted on the per protocol population. The analysis populations were identically defined for the present E-FIT trial and patients with ischemic stroke on the active arm from the NICHE trial. For all analyses where data from NICHE were borrowed and combined with E-FIT data, data from the corresponding population were used (intent-to-treat, per protocol). Missing values for the primary and secondary outcome measures were imputed using the last observation carried forward principle.
The statistical analysis was performed in 2 phases. (1) The primary efficacy analysis was a Bayesian analysis of the E-FIT data combined with data from the active trial arm of the previously completed NICHE trial The primary hypothesis was that the proportion of patients with clinically important improvement in UEFM (≥5 points) from baseline to 6 months posttreatment would be greater in patients receiving 1 Hz navigated brain stimulation-guided rTMS compared with those receiving sham rTMS. The null hypothesis of no difference would be rejected if the lower bound of the 96% credible interval for the odds ratios comparing active to sham was >1. (2) A non-Bayesian analysis of the E-FIT trial data alone was performed to demonstrate robustness of the result. Logistic regression, adjusting for the stratification variable of time since stroke, was used to estimate the odds ratio for the treatment effect. Secondary outcomes were analyzed using Bayesian linear mixed models in the combined data and rank-based tests in the E-FIT only analysis. Bayesian models were fit using WinBUGS version 1.4. All other analyses were completed using SAS version 9.3 or higher (SAS Institute, Cary, NC).
The number of subjects with SAEs and treatment emergent adverse events were summarized by group and included all subjects who received the study treatment.
Further details of the analysis method, as well as intend to treat and per protocol study group definitions are provided in the Supplemental Material.
RESULTS
The results presented here are those for the E-FIT trial population, building directly on the NICHE trial results published by Harvey et al.9 For analyses of safety and efficacy, the Bayesian statistical analysis combined the E-FIT data with data borrowed from the active treatment arm of the NICHE trial.
A total of 60 participants were enrolled and randomized to the E-FIT trial between March 2017 and January 2018. The last close-out visit took place August 2018, and the trial database was locked the same month. Subject enrollment and retention during the trial, analysis populations, and exclusions/losses are presented in the flow diagram of Figure 2.
Figure 2.
E-FIT Trial (Electric Field Navigated 1 Hz Repetitive Transcranial Magnetic Stimulation for Poststroke Motor Recovery the E-FIT Trial) flow diagram. MMSE indicates Mini‐Mental State Examination.
Of the 58 subjects completing the baseline visit, 30 subjects were randomized to receive active rTMS while 28 were randomized to the sham group. The mean UEFM score at baseline was 39.7 in all subjects, 37.4 in the subjects randomized to receive active rTMS, and 42.2 in patients in the sham group. There were no significant differences in the trial outcome measures between the active and the sham group at baseline (Table 1). In the per protocol population (25 active, 25 sham subjects), there were also no significant differences in the trial outcome measures between the active and the sham group at baseline.
Table 1.
Baseline Characteristics
Efficacy Analysis
E-FIT+NICHE
For the Bayesian analysis combining data from the E-FIT trial with the NICHE trial, the posterior mean odds ratio was 1.94 with a 96% credible interval of 0.61 to 4.80, indicating insufficient evidence to conclude the active treatment was superior to sham. The estimate in the per protocol population was similar (mean odds ratio, 1.95 [96% credible interval, 0.58–4.93]).
E-FIT
In the analysis of E-FIT data only, the estimated odds ratio was 1.49 with a 95% CI of 0.53 to 4.22. In the E-FIT trial intent-to-treat population, improvement of 5 or more points from baseline was observed in 60% (18/30) active arm patients compared with 50% (14/28) in the sham arm (Table 2). Participants enrolled between 3 and 6 months of stroke had 67% (31%–91% CI) response rate in the active group at the 6-month end point, versus 50% in the sham group (21.5%–78.5% CI). See Table 2 for time since stroke subanalyses results.
Table 2.
Likelihood to Achieve an Increase of at Least 5 Points on UEFM Between Baseline and 6-Month Follow-Up Visit
Results of the Bayesian linear mixed effects models indicated no significant treatment effects (active versus sham) on change in any of the outcomes from baseline to 6 months (see Table 3 for UEFM and action-research arm test data). When analyzing the change in secondary outcome measures (Table 3), significant improvements were observed for both groups in UEFM (active, 5.17±8.24; mean±SD; P=0.0013; sham, 5.00±7.29; P=0.0002), action-research arm test (active, 4.93±6.99; P=0.0004; sham, 5.68±7.96; P=0.0003), and EQ-5D (active, 10.70±23.32; P=0.037; sham, 8.96±18.80; P=0.0065). National Institutes of Health Stroke Scale improved significantly for the active group only (active, −0.73±1.31; P=0.004; sham, −0.50±1.40; P=0.070). Impairment and functional improvements are illustrated in Figure 3. There were no significant differences between the active versus sham groups in the amount of improvement on any outcome.
Table 3.
rTMS Treatment Effect (Odds Ratio) for UEFM and ARAT per Assessment Time Point Relative to Baseline
Figure 3.
Clinical improvement with time. Change in impairment (upper extremity Fugl-Meyer, A), and function (Action Research Arm Test [ARAT], B) measures with time in the intend to treat population indicate significant clinical improvement in both active and sham groups from baseline to 6 mo post, but no difference between groups.
Covariate Analyses
Preplanned covariate analyses modeled the primary end point (a binary response of UEFM score improvement), the absolute changes (in points) in the tests of motor function (UEFM, and action-research arm test) using hours of occupational therapy (OT) outside the trial and overall hours of therapy (occupational, physical, and home exercise) as covariates during treatment phase and over the entire 6 months of follow-up. There were no statistically significant effects of the covariates on the primary or secondary outcome measures in either treatment group.
Safety Analysis
In the study, 1013 NBT treatment sessions were provided (520 in the active and 493 in the sham arm). All SAEs that occurred in the E-FIT trial population were categorized as unrelated to the NBT device use. In the E-FIT trial safety population encompassing 58 patients, 10 SAEs took place in 9 subjects. Four SAEs in 4 subjects took place in the active treatment arm while 6 SAE in 5 subjects occurred in the sham treatment arm. There was no significant difference in the frequency of SAE between study arms (P=0.72).
Blinding
Blinding of both study subjects and outcome assessors was successful. In both trial arms at end of trial, 56% to 64% of subjects believed they had received active treatment (P=0.51; Table S3). Similarly, at the first follow-up visit and at the end of trial, the outcome assessor therapists were not able to identify which treatment arm the patients had belonged to (Table S3).
DISCUSSION
The present E-FIT trial data, when combined with the active arm of the NICHE trial, or when examined independently, showed that a regimen of motor hand-arm training combined with either active low-frequency rTMS to M1CL, or with sham rTMS, improves clinical arm motor status for at least 6 months after treatment. There was not a difference between study groups and thus these findings confirm that the rTMS adjuvant to motor training confers no advantage over sham rTMS, regardless of the type of sham coil used.
Rationale for the E-FIT Trial and Principal Findings
Conventional non-navigated 1 Hz rTMS targeting M1CL in patients with stroke can lead to modest and short-term benefits on motor function of the paretic hand.8,13 Harvey et al10 progressed this further by demonstrating a single-center clinical trial in patients with stroke, that 84% of subjects receiving a 6-week treatment course of active 1 Hz navigated brain stimulation-rTMS targeting M1CL followed by standardized task-oriented OT, attained clinically important functional improvement 6 months after the end of therapy. The NICHE trial9 was a phase III multicenter double-blinded, randomized, sham-controlled trial, with a protocol base on the completed single-center trial with minor modifications. The aim was to establish definitive evidence of the safety and efficacy of Nexstim navigated brain stimulation-guided 1 Hz rTMS as an adjuvant to standardized OT in patients with chronic stroke. The 199-subject NICHE trial tested the same device intervention with an identical trial protocol. The patient population was also identical to that of the present trial protocol, with the exception that the NICHE trial enrolled patients with ischemic or hemorrhagic stroke while the present trial was limited to patients with ischemic stroke only. NICHE showed that 66% of all subjects gained clinically important improvement of motor function on the UEFM (>5 points),14 and there were no statistically significant differences in outcomes between the active NBT-rTMS (72% of subjects with ischemic stroke improved at least 5 points on UEFM) and sham-rTMS (65% improved) trial arms. The mean improvement on UEFM was 8.1 points. In an analysis of the potential reasons for the lack of separation of trial arms in NICHE, it was reasoned that the sham coil used in NICHE may well have been physiologically active leading to a situation where both active and control groups received cortical stimulation, albeit through a different mechanisms of action. Given the excellent clinical outcomes in NICHE and after correspondence with FDA, the E-FIT trial was conducted as a supplemental trial to NICHE with the aim of making possible a Bayesian statistical analysis of a combined dataset of E-FIT supplemented by data borrowed from the patients with ischemic stroke in the active treatment arm of the NICHE trial. In addition, analysis of E-FIT dataset alone would enable the demonstration of the robustness of the Bayesian analysis. In comparison to NICHE, E-FIT was conducted with the alternative sham coil design that ensured true sham rTMS delivery to the control group, allowing comparison of active trial arm results to a true sham group. Otherwise, the study protocol and treatment provided to subjects in the E-FIT trial and those in the active group of the NICHE trial were identical.
The overall clinical response rate (patients gaining at least 5 points on UEFM) was high (60% and 50% in active and sham treatment arms, respectively). Similarly, the average improvement exceeded 5 points in both treatment arms, and there was a statistically significant improvement in all secondary outcome measures in both trial arms except in the National Institutes of Health Stroke Scale for the sham group. However, there were no statistically significant differences in any outcome measure between trial arms. This was the case for both the Bayesian statistical analysis combining the E-FIT data with the NICHE active trial arm data as well as analysis of E-FIT data only.
The therapy provided in the E-FIT trial clearly benefited the participating subjects. Based on the results, providing active 1 Hz NBT-rTMS to M1CL as adjunct to the standardized OT provided to all patients in the trial, does not improve clinical outcomes beyond those obtained with the OT alone. The standardized OT protocol provided in the trial was highly effective resulting in good clinical outcomes.15
Does Early Intervention Show More Promise?
Recent clinical trial evidence16 shows that task-specific motor intervention within the first 2 to 3 months poststroke, was more effective in recovery of arm motor function, than in the chronic phase. This is consistent with findings in animal studies supporting a critical window for plasticity.17,18 Recent controlled trials with 1 Hz rTMS applied before the therapy sessions have demonstrated a clinical benefit in the early recovery period for neurosurgery-related upper extremity paresis,19 and in participants with poststroke aphasia.20
Our study was designed to enroll participants after the steep recovery trajectory thought to plateau around 3 months.21 When examining outcome in the early time window (3–6 months poststroke) of our enrollment range, the difference in clinical response rate on the primary end point was 67% active versus 50% sham, a nonsignificant difference and thus an effect of earlier intervention is inconclusive. As such, the early group may have missed or was on the cusp of the critical early plasticity period.
Considerations for Future Studies
The rationale for inhibitory rTMS targeting M1CL may be dependent on the anatomic features of the lesion, and how it may disrupt transcallosal22 or more distributed networks23,24 and should be categorized in future trials. The interhemispheric competition model as an explanation for the pathophysiology of unilateral stroke has been questioned based on cumulative human data,25,26 and a refined bimodal balance-recovery model27 should be considered in future trial designs. While targeting M1CL with 1 Hz rTMS may be indicated in some individuals with specific lesion profiles,28 group-level data in patients with stroke often fails to demonstrate evidence of hyperexcitability in M1CL.29 Targeting M1 of the affected corticospinal tract using excitatory rTMS may be more appropriate in patients with sufficient residual corticospinal tract integrity. Our sample population of participants with mixed lesion location and extent included ≈30% with lesions of the brainstem. The location of the lesion and how it impacts a more distributed functional network, including M1CL excitability, will likely influence whether an inhibitory protocol targeting M1CL is rational, and is considered a limitation of the present study. Further investigation of rTMS on upper limb clinical recovery in the early poststroke period is encouraged, considering treatment targeting from baseline neuroantomical (lesion-network) or neurophysiological (TMS) profile and reasoning based on contemporary models.
The number of treatment sessions might be considered in the future. Whereas the E-FIT and NICHE trials comprised 18 sessions, other recent (successful) trials described above using 1 Hz rTMS combined with therapy, observed a benefit with as few as 7 to 10 sessions. Future studies may also consider tracking the time course of clinical benefit across the treatment regimen, to examine if rTMS+motor therapy needs fewer treatment sessions than sham rTMS+motor therapy.
The generalization of the improvements from targeted arm training to a larger global impact on quality of life in the present study is remarkable. These data are supported by recent independent findings of poststroke intensive arm training using telerehabilitation platforms30 and underscores the importance of motor rehabilitation in overall poststroke recovery. Future trials should assess quality of life, even if the intervention is confined to a single limb.
The outcome of the present study was defined by the predetermined statistics examining proportion of clinical responders, as well as group-level mean change in outcome measure score. These methods are typical in clinical trials and important for the context of interpretation. However, features of individual responders may be masked due to this analytical design. Additionally, group effects may not be generalizable to individuals.31 Understanding the baseline features of individuals that might predict outcomes can be achieved through modern machine-learning approaches24 and ultimately inform inclusion criteria for prospective clinical trials.
Conclusions
Intensive motor rehabilitation combined with low-frequency rTMS to M1CL or with sham rTMS, 3 to 12 months after stroke, can improve clinical impairment, function, and quality of life. The proportion of participants with clinical improvement 6 months after the intervention was indistinguishable between active or sham rTMS groups when the data were combined with the NICHE trial data, or evaluated alone, thus confirming the findings of the NIHCE trial that rTMS as applied in our combinatorial approach was ineffective to further enhance a significant training effect.
ARTICLE INFORMATION
Acknowledgments
Design of the therapy protocol Mary Ellen Stoykov, OTRL, PhD. Trial staff training for the occupational therapy provided in the trial was provided by Marilyn Bubula Harvey, OTRL. Trial staff training and certification for outcome assessments was provided by Michael Ellis, PT, DPT. Article preparation assistance from Sapna Kumar, MSE.
Sources of Funding
This study was supported by a grant from the Nexstim Corporation, Helsinki, Finland.
Disclosures
Dr Laine is employed and reports stock holdings in Nexstim Corporation. Dr Fregni reports compensation from Neurive for consultant services and research funding from National Institutes of Health. Dr Rogers reports grants from Nexstim. Dr Harvey reports compensation from Neuro-Innovators LLC for consultant services.
Supplemental Material
Expanded participant enrollment criteria
Expanded materials and methods
Figures S1–S2
Tables S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- M1CL
- motor cortex contralateral
- NBT
- navigated brain therapy
- NICHE
- Navigated Inhibitory rTMS to Contralesional Hemisphere Trial
- rTMS
- repetitive transcranial magnetic stimulation
- SAE
- serious adverse event
- UEFM
- upper extremity Fugl-Meyer
For Sources of Funding and Disclosures, see page 2263.
This article is part of the Null Hypothesis Collection, a collaborative effort between CBMRT, AHA Journals, and Wolters Kluwer, and has been made freely available through funds provided by the CBMRT. For more information, visit https://www.ahajournals.org/null-hypothesis.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.123.043164.
Contributor Information
Dylan J. Edwards, Email: dylan.edwards@jefferson.edu.
Charles Y. Liu, Email: cliu@usc.edu.
Kari Dunning, Email: DUNNINKK@UCMAIL.UC.EDU.
Felipe Fregni, Email: Fregni.Felipe@mgh.harvard.edu.
Jarmo Laine, Email: Jarmo.Laine@nexstim.com.
Benjamin E. Leiby, Email: benjamin.leiby@jefferson.edu.
Lynn M. Rogers, Email: lynnrogers2008@u.northwestern.edu.
REFERENCES
- 1.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018) [published correction appears in Clin Neurophysiol 2020;131(5):1168-1169]. Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 2.Chail A, Saini RK, Bhat PS, Srivastava K, Chauhan V. Transcranial magnetic stimulation: a review of its evolution and current applications. Ind Psychiatry J. 2018;27:172–180. doi: 10.4103/ipj.ipj_88_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5 [DOI] [PubMed] [Google Scholar]
- 4.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848 [DOI] [PubMed] [Google Scholar]
- 5.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661 [DOI] [PubMed] [Google Scholar]
- 6.Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, Paulus W, Pennisi M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Ther Adv Neurol Disord. 2019;12:1756286419878317. doi: 10.1177/1756286419878317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756 [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med. 2008;40:298–303. doi: 10.2340/16501977-0181 [DOI] [PubMed] [Google Scholar]
- 9.Harvey RL, Edwards D, Dunning K, Fregni F, Stein J, Laine J, Rogers LM, Vox F, Durand-Sanchez A, Bockbrader M, et al. ; NICHE Trial Investigators *. Randomized sham-controlled trial of navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke. 2018;49:2138–2146. doi: 10.1161/STROKEAHA.117.020607 [DOI] [PubMed] [Google Scholar]
- 10.Harvey RL, Roth HR, Tappan RS, Kermen R, Laine J, Stinear J, Rogers LM. Abstract 152: The contrastim stroke study: improving hand and arm function after stroke with combined non-invasive brain stimulation and task-oriented therapy - a pilot study. Stroke. 2014;45:A152–A152. [Google Scholar]
- 11.Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54–64. doi: 10.1007/s10548-010-0165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretti J, Rodger J. A little goes a long way: Neurobiological effects of low intensity rTMS and implications for mechanisms of rTMS. Curr Res Neurobiol. 2022;3:100033. doi: 10.1016/j.crneur.2022.100033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakuda W, Abo M, Sasanuma J, Shimizu M, Okamoto T, Kimura C, Kakita K, Hara H. Combination protocol of low-frequency rTMS and intensive occupational therapy for post-stroke upper limb hemiparesis: a 6-year experience of more than 1700 Japanese patients. Transl Stroke Res. 2016;7:172–179. doi: 10.1007/s12975-016-0456-8 [DOI] [PubMed] [Google Scholar]
- 14.Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009 [DOI] [PubMed] [Google Scholar]
- 15.Harvey RL, Winstein CJ; Everest Trial Group. Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23:32–44. doi: 10.1177/1545968308317532 [DOI] [PubMed] [Google Scholar]
- 16.Dromerick AW, Geed S, Barth J, Brady K, Giannetti ML, Mitchell A, Edwardson MA, Tan MT, Zhou Y, Newport EL, et al. Critical period after stroke study (CPASS): a phase II clinical trial testing an optimal time for motor recovery after stroke in humans. Proc Natl Acad Sci U S A. 2021;118:e2026676118. doi: 10.1073/pnas.2026676118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hensch TK, Bilimoria PM. Re-opening windows: manipulating critical periods for brain development. Cerebrum. 2012;2012:11. [PMC free article] [PubMed] [Google Scholar]
- 19.Ille S, Kelm A, Schroeder A, Albers LE, Negwer C, Butenschoen VM, Sollmann N, Picht T, Vajkoczy P, Meyer B, et al. Navigated repetitive transcranial magnetic stimulation improves the outcome of postsurgical paresis in glioma patients - A randomized, double-blinded trial. Brain Stimul. 2021;14:780–787. doi: 10.1016/j.brs.2021.04.026 [DOI] [PubMed] [Google Scholar]
- 20.Zumbansen A, Kneifel H, Lazzouni L, Ophey A, Black SE, Chen JL, Edwards D, Funck T, Hartmann AE, Heiss W-D, et al. ; NORTHSTAR-study group. Differential effects of speech and language therapy and rTMS in chronic versus subacute post-stroke aphasia: results of the NORTHSTAR-CA trial. Neurorehabil Neural Repair. 2022;36:306–316. doi: 10.1177/15459683211065448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Havenon A, Tirschwell DL, Heitsch L, Cramer SC, Braun R, Cole J, Reddy V, Majersik JJ, Lindgren A, Worrall BB. Variability of the modified rankin scale score between day 90 and 1 year after ischemic stroke. Neurol Clin Pract. 2021;11:e239–e244. doi: 10.1212/CPJ.0000000000000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thickbroom GW, Cortes M, Rykman A, Volpe BT, Fregni F, Krebs HI, Pascual-Leone A, Edwards DJ. Stroke subtype and motor impairment influence contralesional excitability. Neurology. 2015;85:517–520. doi: 10.1212/WNL.0000000000001828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy JM, Mark JI, Cramer SC. Functional connectivity drives stroke recovery: shifting the paradigm from correlation to causation. Brain. 2022;145:1211–1228. doi: 10.1093/brain/awab469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tozlu C, Edwards D, Boes A, Labar D, Tsagaris KZ, Silverstein J, Pepper Lane H, Sabuncu MR, Liu C, Kuceyeski A. Machine learning methods predict individual upper-limb motor impairment following therapy in chronic stroke. Neurorehabil Neural Repair. 2020;34:428–439. doi: 10.1177/1545968320909796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonnell MN, Stinear CM. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul. 2017;10:721–734. doi: 10.1016/j.brs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Branscheidt M, Schambra H, Steiner L, Widmer M, Diedrichsen J, Goldsmith J, Lindquist M, Kitago T, Luft AR, et al. ; SMARTS Study Group. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann Neurol. 2019;85:502–513. doi: 10.1002/ana.25452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162 [DOI] [PubMed] [Google Scholar]
- 28.Lin C, Schaper FL, Cooke D, Liu C, Corbetta M, Edwards D, Fox MD. rTMS to the contralesional M1 modulates the trajectory of post-stroke motor recovery. Brain Stimul. 2021;14:1601. doi: 10.1016/j.brs.2021.10.043 [Google Scholar]
- 29.Vucic S, Stanley Chen KH, Kiernan MC, Hallett M, Benninger DH, Di Lazzaro V, Rossini PM, Benussi A, Berardelli A, Currà A, et al. Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders Updated report of an IFCN committee. Clin Neurophysiol. 2023;150:131–175. doi: 10.1016/j.clinph.2023.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer SC, Le V, Saver JL, Dodakian L, See J, Augsburger R, McKenzie A, Zhou RJ, Chiu NL, Heckhausen J, et al. Intense arm rehabilitation therapy improves the modified rankin scale score: association between gains in impairment and function. Neurology. 2021;96:e1812–e1822. doi: 10.1212/WNL.0000000000011667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci U S A. 2018;115:E6106–E6115. doi: 10.1073/pnas.1711978115 [DOI] [PMC free article] [PubMed] [Google Scholar]