Abstract
INTRODUCTION:
Colorectal cancer (CRC) is one of the major life-threatening complications in patients with Crohn's disease (CD). Previous studies of CD-associated CRC (CD-CRC) have involved only small numbers of patients, and no large series have been reported from Asia. The aim of this study was to clarify the prognosis and clinicopathological features of CD-CRC compared with sporadic CRC.
METHODS:
A large nationwide database was used to identify patients with CD-CRC (n = 233) and sporadic CRC (n = 129,783) over a 40-year period, from 1980 to 2020. Five-year overall survival (OS), recurrence-free survival (RFS), and clinicopathological characteristics were investigated. The prognosis of CD-CRC was further evaluated in groups divided by colon cancer and anorectal cancer (RC). Multivariable Cox regression analysis was used to adjust for confounding by unbalanced covariables.
RESULTS:
Compared with sporadic cases, patients with CD-CRC were younger; more often had RC, multiple lesions, and mucinous adenocarcinoma; and had lower R0 resection rates. Five-year OS was worse for CD-CRC than for sporadic CRC (53.99% vs 71.17%, P < 0.001). Multivariable Cox regression analysis revealed that CD was associated with significantly poorer survival (hazard ratio 2.36, 95% confidence interval: 1.54–3.62, P < 0.0001). Evaluation by tumor location showed significantly worse 5-year OS and RFS of CD-RC compared with sporadic RC. Recurrence was identified in 39.57% of CD-RC cases and was mostly local.
DISCUSSION:
Poor prognosis of CD-CRC is attributable primarily to RC and high local recurrence. Local control is indispensable to improving prognosis.
KEYWORDS: Crohn's disease, colorectal cancer, recurrence
INTRODUCTION
Colorectal cancer (CRC) is one of the major life-threatening complications in patients with Crohn's disease (CD). The incidence of CRC among patients with CD is higher than in the general population (1–5), and in 1 report, the prevalence of CD-associated CRC (CD-CRC) was 0.23% during a median follow-up of 12.55 years (6). Long duration of CD, CD diagnosis before 30 years of age, family history of CRC, primary sclerosing cholangitis (PSC), and extensive colorectal lesions have been reported to be risk factors for CD-CRC (5,7–12).
The characteristics of CD-CRC vary with geography and race. In Western countries, right-sided colon cancer occurs at a higher frequency in patients with CD than in general population (6,13–16). By contrast, in Asia, CD-CRC has been increasing since the year 2000, and most lesions are anorectal (2,6,17–19). A genomewide association study of patients with CD identified different associated variants between patients of Asian and European ancestry (20). These variants and environmental factors likely underlie some of the differential features of CD-CRC between populations.
Most studies of CD-CRC have evaluated only small numbers of patients, and no large series from Asia have been reported. The aim of this study was to clarify the prognosis and clinicopathological features of CD-CRC compared with sporadic CRC using a large nationwide database from the Japanese Society for Cancer of the Colon and Rectum (JSCCR) (21).
METHODS
Database
Patients with CD diagnosed with CRC between 1980 and 2020 were included in this study. Patient data were retrospectively collected from 43 institutions that participated in the JSCCR project. We also used a large nationwide CRC database from the Multi-Institutional Registry of Large-Bowel Cancer in Japan. The data were extracted along with clinicopathological factors, including sex, age, familial history of CRC, year of diagnosis, serum carcinoembryonic antigen (CEA), surgical treatment, adjuvant chemotherapy, tumor location, number of lesions, tumor depth, morphological and histological type, lymph node or distant metastasis, and cancer-specific outcomes. Regarding year of diagnosis, in 2008, treatment guidelines for CD anal lesions were established by the Japan Ministry of Health, Labor, and Welfare (22). Therefore, the period was divided into 1980–2008 and 2009–2020. Regarding the number of lesions, 2 or more lesions were counted as “multiple” regardless of whether they were simultaneous or metachronous tumors. Staging used in the study was the TNM classification, and morphological and histological type was based on the JSCCR definitions (23).
Study design
The clinicopathological features, 5-year overall survival (OS), and 5-year recurrence-free survival (RFS) for patients with CD-CRC were compared with outcomes in sporadic CRC. We also further analyzed the data based on lesion location in the colon (CC) or anus/rectum (RC). To adjust for confounding by unbalanced covariables, multivariable Cox regression analysis was performed.
Statistical analysis
Statistical analyses were performed using JMP 16.2.0 (SAS Institute Inc, Cary, NC). To compare patients and clinical characteristics, we used the Welch t test, Pearson χ2 test, or Fisher exact test. Data are expressed as mean and SD, median and interquartile range (IQR), or number of patients and proportion (%). We used the Kaplan–Meier method to estimate 5-year OS and RFS, the log-rank test to assess survival curves, and the Z test to assess estimated survival. The patients who died from other causes or were still alive at the date of the last follow-up were treated as censored in this study.
To adjust for confounding by unbalanced covariables, we used a multivariable Cox regression model to estimate hazard ratios (HRs) for death according to age, sex, family history of CRC, elevation of CEA, surgical treatment, adjuvant chemotherapy, number of lesions, morphological type, histological type, tumor depth, lymph node metastasis, distant metastasis, and residual tumor. Significance was assessed using 95% confidence intervals (CIs). In addition, P < 0.05 was considered to indicate significance.
Ethics
This multicenter retrospective study was approved by the University of Tokyo Ethics Review Board (2019220NI-(2)).
RESULTS
Characteristics and survival analysis of CD-CRC
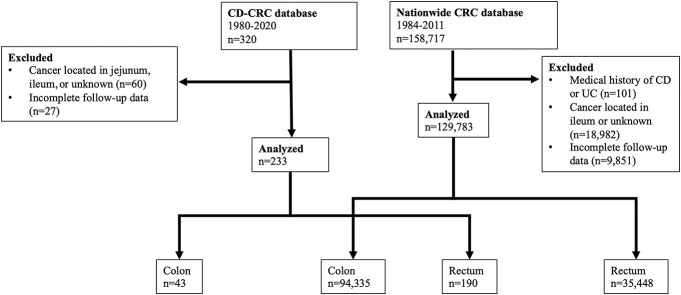
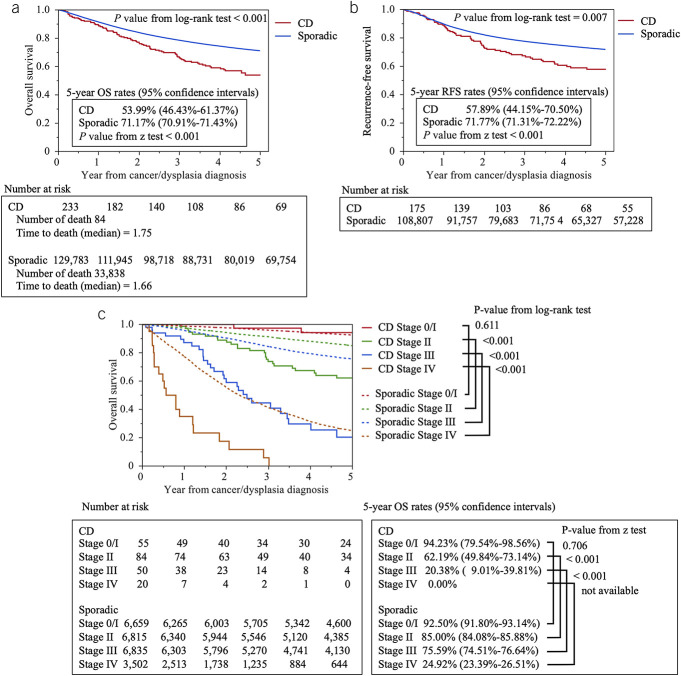
The patient flow chart is shown in Figure 1. A total of 233 patients with CD-CRC and 129,783 patients with sporadic CRC were evaluated. Table 1 summarizes and shows a comparison of the characteristics of these 2 patient groups. The median age at CD diagnosis was 24 years. The CD-CRC group had 240 months of CD duration. The CD-CRC group also had a younger mean age (P < 0.001) and a higher male prevalence than the sporadic CRC group (P = 0.023). The CD-CRC group had a smaller proportion of patients diagnosed in 1980–2008 and higher proportion in 2009–2020 compared with the sporadic CRC group (P < 0.001). The proportion with a family history of CRC was significantly lower in the CD-CRC group.
Figure 1.
Flowchart of patient selection. CD, Crohn's disease; UC, ulcerative colitis.
Table 1.
Patient characteristics, CD-CRC, and sporadic CRC
CEA elevation was comparable between the 2 groups, and the most common treatment for both groups was surgery. The CD-CRC group had a higher prevalence of adjuvant chemotherapy (43.56%) than with the sporadic CRC patients (23.29%; P < 0.001).
Among patients with CD-CRC, 9.01% had right-sided CC, 9.44% had left-sided CC, and 81.55% had RC. Among the sporadic CRC patients, 28.81% had right-sided CC, 43.87% left-sided CC, and 27.31% RC (P < 0.001). A total of 10.49% of CD-CRC patients had multiple lesions, which was a significantly higher proportion than in the sporadic CRC group (0.91%; P < 0.001).
For CD-CRC patients, the unclassified type of lesion morphology was most prevalent, whereas the most common morphology in the sporadic CRC patients was ulcerated with clear margin type. Among the CD-CRC group, 46.02% had mucinous adenocarcinoma, in contrast to more than 90% of sporadic CRC patients having well or moderately differentiated adenocarcinoma. Lymph node metastasis and distant metastasis were more common among sporadic CRC cases, as was R0 resection (90.99% vs 77.43% in CD-CRC; P < 0.001).
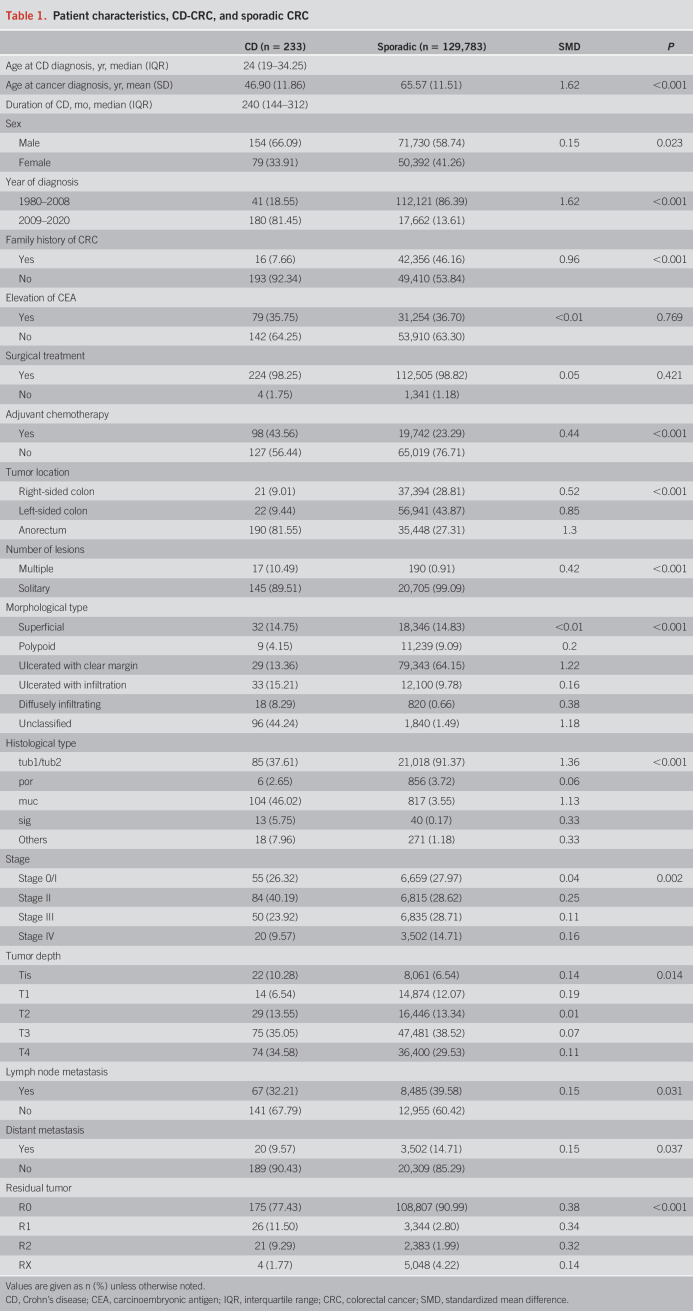
For both groups, patients available for follow-up were included in the survival analyses. The overall median follow-up time was 2.72 years (IQR: 1.08–5.00) for CD-CRC and 5.00 years (IQR: 2.11–5.00) for sporadic CRC. Eighty-four deaths (36.05%) occurred among CD-CRC patients and 33,838 deaths (26.07%) among sporadic CRC patients. The median time to death was 1.75 years (IQR: 0.89–2.93) for CD-CRC and 1.66 years (IQR: 0.79–2.90) for sporadic CRC. The 5-year OS of CD-CRC was significantly worse than that of sporadic CRC (53.99% vs 71.17%; P < 0.001; Figure 2a). The 5-year RFS of CD-CRC after R0 resection was significantly worse than that of sporadic CRC (57.89% vs 71.77%; P < 0.001; Figure 2b). For disease stages II, III, and IV, 5-year OS was lower in the CD-CRC group than in the sporadic CRC group (stage 0/I: 94.23% vs 92.50%, P = 0.611; stage II: 62.19% vs 85.00%, P < 0.001; stage III: 20.38% vs 75.59%, P < 0.001; stage IV: 0.00% vs 24.92%, P < 0.001, log-rank test; Figure 2c). In all cases of stage IV disease in the CD-CRC group, death occurred in less than 5 years, during the early phase.
Figure 2.
Survival analysis of CD-CRC. (a) Five-year overall survival (OS) of CD-CRC and sporadic CRC patients. (b) Five-year recurrence-free survival (RFS) of CD-CRC and sporadic CRC patients. (c) Five-year OS of CD-CRC and sporadic CRC patients by stage. CD, Crohn's disease; CRC, colorectal cancer.
CD-RC was associated with poor prognosis
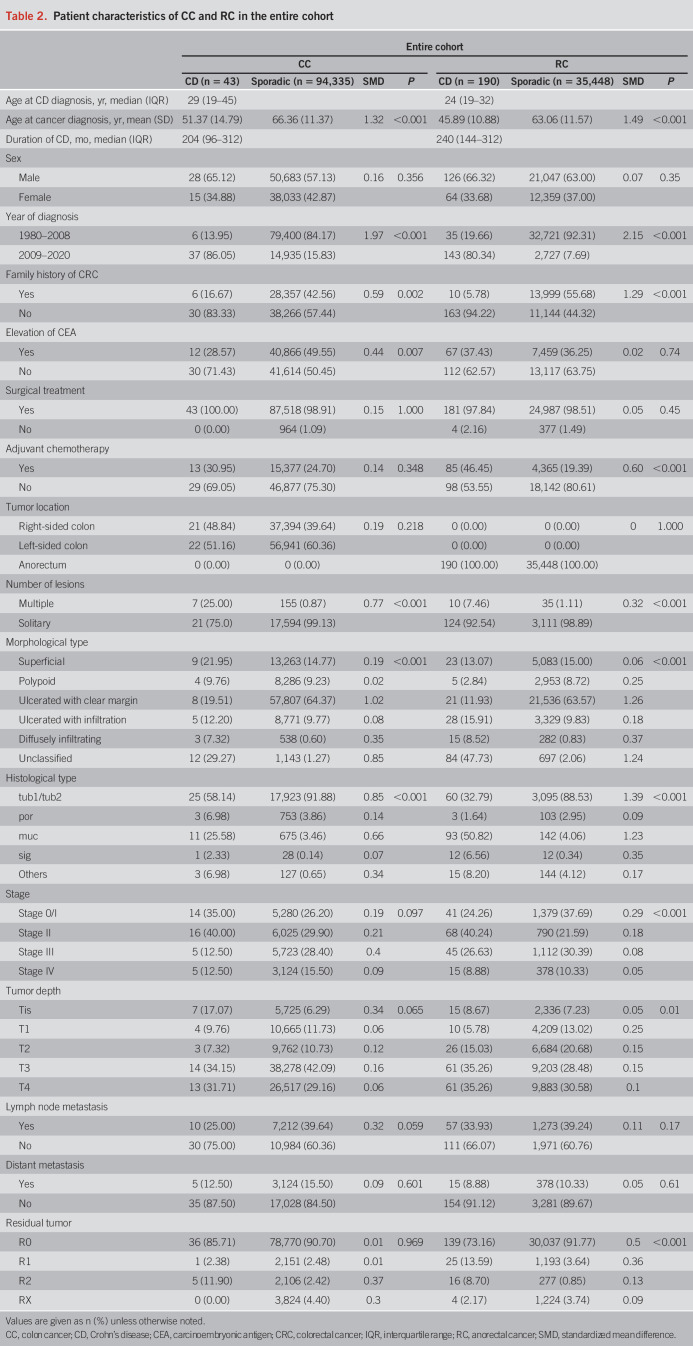
Next, we compared patients with CD-CRC and sporadic CRC based on tumor location (CC vs RC; Figure 1). Table 2 summarizes the characteristics of 43 patients with CD-CC and 190 patients with CD-RC compared with 94,335 patients with sporadic CC and 35,448 patients with sporadic RC. There was a higher prevalence of multiple lesions in CD-CC (25%) and a higher incidence of mucinous adenocarcinoma in CD-RC (50.82%). The overall median follow-up was 2.26 years (IQR: 0.71–5.00) for CD-CC, 5.00 years (IQR: 2.08–5.00) for sporadic CC, 2.82 years (IQR: 1.30–5.00) for CD-RC, and 5.00 years (IQR: 2.17–5.00) for sporadic RC. The median time to death was 1.00 year (IQR: 0.28–2.26) for CD-CC, 1.59 years (IQR: 0.74–2.85) for sporadic CC, 1.83 years (IQR: 0.94–2.97) for CD-RC, and 1.80 years (IQR: 0.89–3.02) for sporadic RC. The 5-year OS of CD-RC was significantly worse than that of sporadic RC (51.61% vs 68.58%; P < 0.001), whereas the 5-year OS of CD-CC vs sporadic CC was comparable (Figure 3a, Supplementary Figure S1a, see Supplementary Digital Content 1, http://links.lww.com/AJG/C928). The 5-year RFS of CD-RC was also significantly worse than that of sporadic RC (53.00% vs 69.06%; P < 0.001), whereas the CD-CC and sporadic CC groups did not differ in 5-year RFS (Figure 3b, Supplementary Figure S1b, see Supplementary Digital Content 1, http://links.lww.com/AJG/C928).
Table 2.
Patient characteristics of CC and RC in the entire cohort
Figure 3.
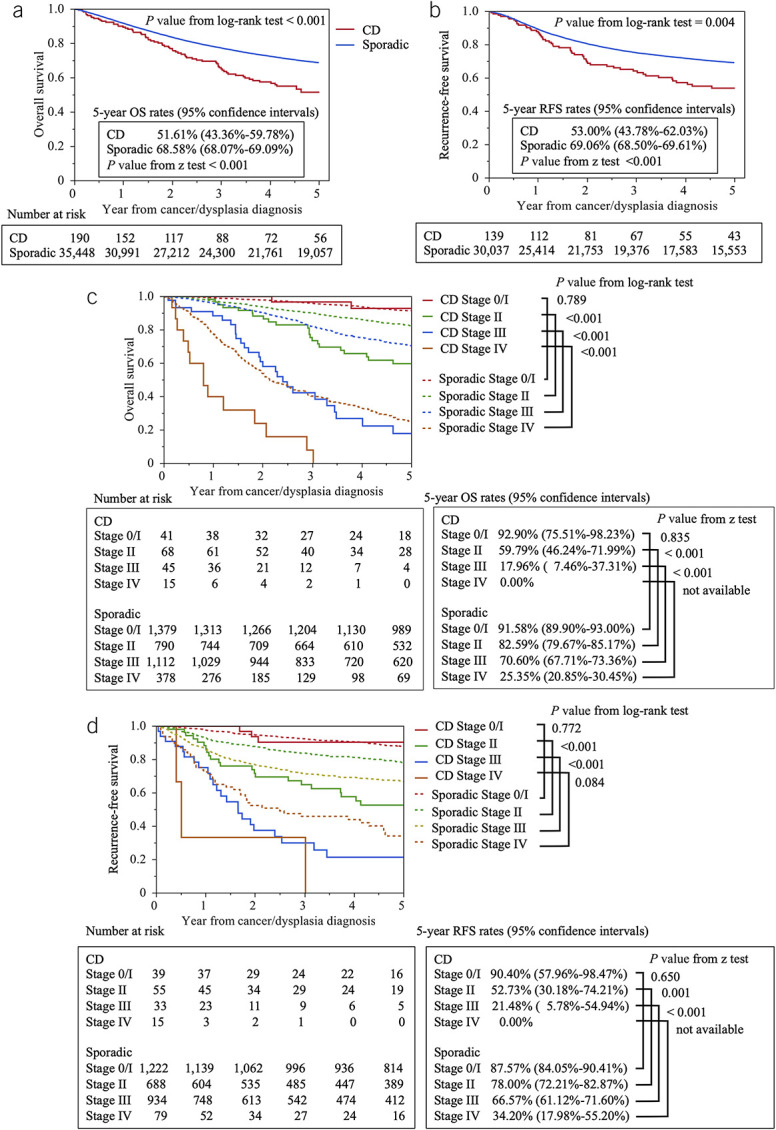
Survival analysis of CD-RC. (a) Five-year overall survival (OS) of CD-RC and sporadic RC patients. (b) Five-year recurrence-free survival (RFS) of CD-RC and sporadic RC patients. (c) Five-year OS of CD-RC and sporadic RC patients by stage. (d) Five-year RFS of CD-RC and sporadic RC patients by stage. CD, Crohn's disease; RC, anorectal cancer.
According to the survival analysis by stage, 5-year OS of CD-RC was significantly lower than that of sporadic RC except for stage 0/I (stage 0/I: 92.90% vs 91.58%, P = 0.789; stage II: 59.79% vs 82.59%, P < 0.001; stage III: 17.96% vs 70.60%, P < 0.001; stage IV: 0.00% vs 25.35%, P < 0.001, log-rank test; Figure 3c), whereas only stage IV CD-CC had a worse prognosis compared with sporadic CC (see Supplementary Figure S1c, Supplementary Digital Content 1, http://links.lww.com/AJG/C928). All CD-CC patients with stage IV disease died before 5 years. The 5-year RFS of CD-RC also was significantly lower than that of sporadic RC except for stage 0/I (stage 0/I: 90.40% vs 87.57%, P = 0.772; stage II: 52.73% vs 78.00%, P < 0.001; stage III: 21.48% vs 66.57%, P < 0.001; stage IV: 0.00% vs 34.20%, P = 0.084, log-rank test; Figure 3d), whereas CD-CC was not associated with a worse prognosis compared with sporadic CC (see Supplementary Figure S1d, Supplementary Digital Content 1, http://links.lww.com/AJG/C928).
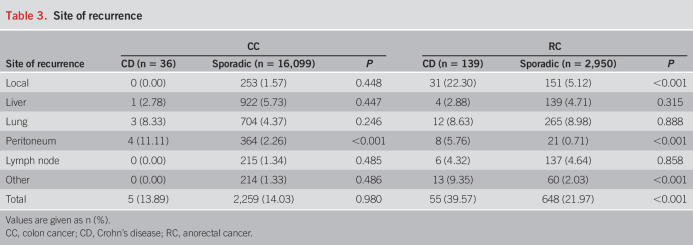
A higher recurrence rate was found with CD-RC (39.57%) than sporadic RC (21.97%, P < 0.001), but the recurrence rates did not differ between CD-CC and sporadic CC. The most frequent sites of recurrence in CD-RC were local (22.30%; Table 3).
Table 3.
Site of recurrence
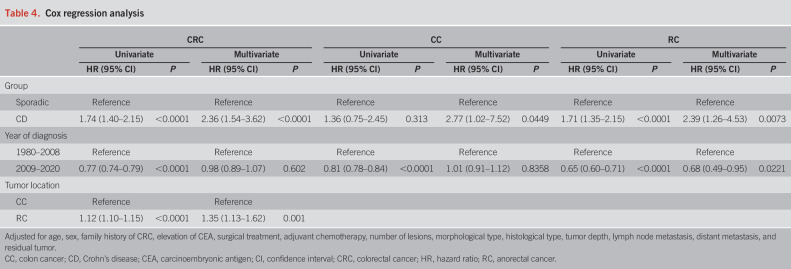
Multivariable Cox regression analysis
We performed multivariable Cox regression analysis using the total cohort to assess whether CD was independently associated with survival (Table 4). After adjusting for age, sex, family history of CRC, elevation of CEA, surgical treatment, adjuvant chemotherapy, number of lesions, morphological type, histological type, tumor depth, lymph node metastasis, distant metastasis, and residual tumor, CD was associated with significantly poorer survival (univariate HR 1.74, 95% CI: 1.40–2.15, P < 0.0001; multivariate HR 2.36, 95% CI: 1.54–3.62, P < 0.0001). The evaluation using the RC cohort also associated CD with significantly poorer survival (univariate HR 1.71, 95% CI: 1.35–2.15, P < 0.0001; multivariate HR 2.39, 95% CI: 1.26–4.53, P = 0.0073, Table 4). In the CC cohort, CD was also associated with poorer survival (multivariate HR 2.77, 95% CI: 1.02–7.52, P = 0.0449).
Table 4.
Cox regression analysis
As this study covered a 40-year period, the year of diagnosis was important for the survival analysis. We performed an analysis using the total cohort divided by periods, 1980–2008 and 2009–2020 (Table 4), coinciding with when the treatment guidelines for CD anal lesions were established in Japan (22). After adjusting for confounding by unbalanced covariables, the year of diagnosis during 2009–2020 was not associated with survival in the CRC cohort compared with 1980–2008 (multivariate HR 0.98, 95% CI: 0.89–1.07, P = 0.602). However, the evaluation using the RC cohort revealed that diagnosis during 2009–2020 was significantly associated with better survival (univariate HR 0.65, 95% CI: 0.60–0.71, P < 0.0001; multivariate HR 0.68, 95% CI: 0.49–0.95, P = 0.0221), but the evaluation using the CC cohort showed that diagnosis during 2009–2020 was not associated with survival compared with 1980–2008 (multivariate HR 1.01, 95% CI: 0.91–1.12, P = 0.8358).
We also assessed whether tumor location was independently associated with survival. RC was associated with significantly poorer survival compared with CC (univariate HR 1.12, 95% CI: 1.10–1.15, P < 0.0001; multivariate HR 1.35, 95% CI: 1.13–1.62, P = 0.001, Table 4).
DISCUSSION
We found that the 5-year OS of CD-CRC was significantly worse than that of sporadic CRC. Multivariable Cox regression analysis revealed that CD was associated with significantly poorer overall survival. A recent meta-analysis also showed a significantly worse prognosis with CD-CRC, but most studies have not included populations in Asia (6,24,25). In Western countries, CD-CRC tends to be more left-sided, similar to the findings in Asia, but the incidence of right-sided tumor is higher than the incidence of sporadic CRC (6,8,26–29). In this study, 81.5% of CD-CRC cases were located in the anorectum, similar to other reports from Asia (21,22). A higher incidence of CD-RC has been observed in Asia compared with Western countries. However, we must raise awareness of the risk of CD-RC worldwide because several cases have been reported in Western countries (26,30–32).
The 5-year OS of CD-RC was significantly worse than that of sporadic RC. Although some reports from Western populations have identified the major histological type of CD-RC as squamous cell carcinoma (SCC) (6), we found a significantly higher frequency of mucinous adenocarcinoma and signet ring cell carcinoma with CD-RC. These histological types are well known for aggressive behavior and poor prognosis (33). Furthermore, CD-RC included 5 cases of SCC (2.6% of SCC cases) in this study, whereas CD-CC and sporadic CRC did not include any cases of SCC. The treatment for anal SCC differs from the standard treatment for rectal cancer (34). We considered the possibility that different treatments may affect prognosis. To avoid hampering the interpretation of the results, we also analyzed the population excluding SCC (see Supplementary Figure S2, Supplementary Digital Content 2, http://links.lww.com/AJG/C929). Even after excluding SCC, the survival of CD-RC was significantly worse than that of sporadic RC.
Our study revealed an obviously worse prognosis with CD-RC, especially with stage II or III disease. However, clear evidence is lacking for surgery, chemotherapy, or radiotherapy. CD-RC is linked to characteristics, such as younger age at cancer diagnosis, a tendency to have multiple lesions, advanced tumor depth, and aggressive histomorphological features. One of the reasons for advanced invasion could be delayed diagnosis because of neglected surveillance in patients with CD. With the geographical differences, it is necessary to establish tailor-made diagnostic and therapeutic strategies. In Western countries, regular colonoscopy is recommended for CD-CRC surveillance because of increasing detection of CRC, reducing the risk of advanced cancer (25,35–37), but is often difficult because of anorectal stenosis or anal fistulas. Computed tomography and magnetic resonance imaging are useful for determining the extent of lesions but not necessarily for early detection. Fluorodeoxyglucose-positron emission tomography does not easily allow for inflammation and cancer to be distinguished and is linked to low detection of mucinous carcinoma (38). Tumor markers can easily be measured, but most cases diagnosed by elevated CEA are in the advanced stage. Perianal examinations under anesthesia have been performed in addition to endoscopic surveillance at a specialized institute for inflammatory bowel diseases in Japan, with a 5.8% cancer detection rate among patients with CD (39). In a large national Japanese cohort, approximately 70% of CD-RC cases were diagnosed by symptoms (21). With CD-RC, it is difficult to obtain information related to the diagnosis of malignancy because perianal CD itself involves a similar suite of symptoms, such as inflammation, stenosis, bleeding, anal pain, and mucus discharge. Especially in patients with anal stenosis and a history of multiple surgeries for anal fistula, fibrosis and chronic inflammation could make diagnosis much more challenging. Therefore, it is important to perform regular and repeated examinations, not only colonoscopy, but also proctological examinations with biopsies under anesthesia.
Even when R0 resection was possible, local recurrence was not well controlled in this study. Although the 5-year RFS did not differ between CD-CC and sporadic CC, it was extremely poor for CD-RC, and local recurrence accounted for more than half of the total recurrence sites. To improve the prognosis of CD-RC, local control is crucial. Preoperative chemoradiotherapy (CRT) would be expected to be an effective strategy. It has been performed for sporadic RC to improve OS with R0 resection, reduce local recurrence, and preserve the anal sphincter muscle with tumor shrinkage. Several reports have found that CRT could be safely performed for CD-RC, but no reports have addressed improved survival or reduced recurrence (40–42). Furthermore, CD itself can be exacerbated during CRT. Taken together, better prognosis of CD-RC could be expected with extended radical resection, to avoid leaving tumor on the resected plane, combined with preoperative CRT.
This study had several limitations. First, all data were obtained from Japanese patients, so the global generalizability of the results is unclear. Second, the study was retrospective. However, large numbers of both CD-CRC and sporadic CRC cases were included, representing multiple institutions and, thus, Japanese clinical practice over the past 4 decades. Previous reports have shown that diagnosis in a later period is associated with better survival outcomes (25,35,36). In our study, diagnosis during 2009–2020 was significantly associated with better survival of RC, but not CC. This discrepancy may be due to the development of diagnosis and treatment algorithms.
In conclusion, we found that more than 80% of CD-CRC cases presented as an anorectal lesion. The 5-year OS of CD-RC was significantly worse than that of sporadic RC, with high local recurrence. Optimal cancer surveillance for early detection and extended radical resection with preoperative CRT for local control are key to improving the prognosis of CD-CRC.
CONFLICTS OF INTEREST
Guarantor of the article: Takayuki Ogino, MD, PhD.
Specific author contributions: T.O.: contributed to the design of the work, the analysis, and interpretation of data for the work and revised the manuscript critically for important intellectual content. T.M., M.F., Y.S., and H.E.: contributed to the conception of the work and revised the work critically for important intellectual content. R.N., H.I., M.U., K.F., K.O., H.N., K.W., K.O., K.Y., H.O., S.T., Y.M., Y.O., Y.S., H.U., T.K., M.I., H.K., K.H., Y.K., K.T., F.K., T.H., K.M., T.N., Y.S., T.Y., J.A., K.M., J.O., E.S., Y.A., K.K., K.U., T.Y., S.S., S.I., Y.A., and K.A.: contributed to the acquisition of the data for the work and revised the work critically for important intellectual content. All the authors approved the final version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support: This work was supported by the Japanese Society of Cancer of the Colon and Rectum. The funding source had no role in study design, data collection, data analysis and interpretation, preparation of the manuscript, or decision to publish.
Potential competing interests: None to report.
ACKNOWLEDGMENT
The authors sincerely appreciate all participants in this project for their great efforts in data registration and San Francisco Edit for English language editing.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C928 and http://links.lww.com/AJG/C929.
Deceased on November 9, 2021.
Contributor Information
Tsunekazu Mizushima, Email: tmizushima@oph.gr.jp.
Makoto Fujii, Email: m.fujii.sahs.med@osaka-u.ac.jp.
Yuki Sekido, Email: ysekido@gesurg.med.osaka-u.ac.jp.
Hidetoshi Eguchi, Email: heguchi@gesurg.med.osaka-u.ac.jp.
Riichiro Nezu, Email: rnezu@osaka-centralhp.jp.
Hiroki Ikeuchi, Email: ikeuci2s@hyo-med.ac.jp.
Uchino Motoi, Email: uchino2s@hyo-med.ac.jp.
Kitaro Futami, Email: fkitaro1917.1229@gmail.com.
Kinya Okamoto, Email: k.oka@watch.ocn.ne.jp.
Hisashi Nagahara, Email: nagahara@yoshida-hosp.or.jp.
Kazuhiro Watanabe, Email: k-wata@med.tohoku.ac.jp.
Koji Okabayashi, Email: okabayashikoji@gmail.com.
Kazutaka Yamada, Email: k-yamada@magma.jp.
Hiroki Ohge, Email: ohge@hiroshima-u.ac.jp.
Shinji Tanaka, Email: colon@hiroshima-u.ac.jp.
Yusuke Mizuuchi, Email: mizuy@med.kyushu-u.ac.jp.
Yoshiki Ohkita, Email: nyokkin@clin.medic.mie-u.ac.jp.
Yu Sato, Email: yu.sato@med.toho-u.ac.jp.
Hideki Ueno, Email: ueno_surg1@ndmc.ac.jp.
Toru Kono, Email: takayukiogino0113@yahoo.co.jp.
Michio Itabashi, Email: itabashi.michio@twmu.ac.jp.
Hideaki Kimura, Email: hkim@yokohama-cu.ac.jp.
Koya Hida, Email: hidakoya@kuhp.kyoto-u.ac.jp.
Yusuke Kinugasa, Email: kinugasa.srg1@tmd.ac.jp.
Kenichi Takahashi, Email: ketakajb3@gmail.com.
Fumikazu Koyama, Email: fkoyama@naramed-u.ac.jp.
Tsunekazu Hanai, Email: thanahana@eagle.ocn.ne.jp.
Toshihiro Noake, Email: noaket2003@ybb.ne.jp.
Yoshifumi Shimada, Email: shimaday@med.niigata-u.ac.jp.
Takayuki Yamamoto, Email: nao-taka@sannet.ne.jp.
Junya Arakaki, Email: golferpower777@yahoo.co.jp.
Keiji Mastuda, Email: keiji@med.teikyo-u.ac.jp.
Junji Okuda, Email: junji.okuda@ompu.ac.jp.
Eiji Sunami, Email: eijisunami@gmail.com.
Yoshito Akagi, Email: yoshisg@med.kurume-u.ac.jp.
Kenji Kastumata, Email: k-katsu@tokyo-med.ac.jp.
Kay Uehara, Email: kuehara@med.nagoya-u.ac.jp.
Takeshi Yamada, Email: y-tak@nms.ac.jp.
Shin Sasaki, Email: shin.sasa.0727@gmail.com.
Soichiro Ishihara, Email: soichiro.ishihara@gmail.com.
Yoichi Ajioka, Email: ajioka@med.niigata-u.ac.jp.
Kenichi Sugihara, Email: sugi.srg2@gmail.com.
REFERENCES
- 1.Freeman HJ. Colorectal cancer risk in Crohn's disease. World J Gastroenterol 2008;14(12):1810–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yano Y, Matsui T, Hirai F, et al. Cancer risk in Japanese Crohn's disease patients: Investigation of the standardized incidence ratio. J Gastroenterol Hepatol 2013;28(8):1300–5. [DOI] [PubMed] [Google Scholar]
- 3.Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn's disease: A meta-analysis of population-based cohort studies. Am J Gastroenterol 2005;100(12):2724–9. [DOI] [PubMed] [Google Scholar]
- 4.Jess T, Horváth-Puhó E, Fallingborg J, et al. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: A Danish population-based cohort study. Am J Gastroenterol 2013;108(12):1869–76. [DOI] [PubMed] [Google Scholar]
- 5.Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in Crohn's disease: A Scandinavian population-based cohort study. Lancet Gastroenterol Hepatol 2020;5(5):475–84. [DOI] [PubMed] [Google Scholar]
- 6.Uchino M, Ikeuchi H, Hata K, et al. Intestinal cancer in patients with Crohn's disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36(2):329–36. [DOI] [PubMed] [Google Scholar]
- 7.Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol 2008;14(17):2662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekbom A, Adami HO, Helmick C, et al. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet 1990;336(8711):357–9. [DOI] [PubMed] [Google Scholar]
- 9.Askling J, Dickman PW, Ekbom A, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 2001;120(6):1356–62. [DOI] [PubMed] [Google Scholar]
- 10.Gillen CD, Walmsley RS, Prior P, et al. Ulcerative colitis and Crohn's disease: A comparison of the colorectal cancer risk in extensive colitis. Gut 1994;35(11):1590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji SG, Juran BD, Mucha S, et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet 2017;49(2):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitali F, Wein A, Rath T, et al. The outcome of patients with inflammatory bowel disease-associated colorectal cancer is not worse than that of patients with sporadic colorectal cancer-a matched-pair analysis of survival. Int J Colorectal Dis 2022;37(2):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C, Schardey J, Zhang T, et al. Survival outcomes and clinicopathological features in inflammatory bowel disease-associated colorectal cancer: A systematic review and meta-analysis. Ann Surg 2022;276(5):e319–e330. [DOI] [PubMed] [Google Scholar]
- 14.Stahl TJ, Schoetz DJ, Jr, Roberts PL, et al. Crohn's disease and carcinoma: Increasing justification for surveillance? Dis Colon Rectum 1992;35(9):850–6. [DOI] [PubMed] [Google Scholar]
- 15.Connell WR, Sheffield JP, Kamm MA, et al. Lower gastrointestinal malignancy in Crohn's disease. Gut 1994;35(3):347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiran RP, Khoury W, Church JM, et al. Colorectal cancer complicating inflammatory bowel disease: Similarities and differences between Crohn's and ulcerative colitis based on three decades of experience. Ann Surg 2010;252:330–5. [DOI] [PubMed] [Google Scholar]
- 17.Mizushima T, Ohno Y, Nakajima K, et al. Malignancy in Crohn's disease: Incidence and clinical characteristics in Japan. Digestion 2010;81(4):265–70. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, Ikeuchi H, Bando T, et al. Clinicopathological characteristics of cancer associated with Crohn's disease. Surg Today 2017;47(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Lee HS, Park SH, et al. Pathologic features of colorectal carcinomas associated with Crohn's disease in Korean population. Pathol Res Pract 2017;213(3):250–5. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch D, Wangsa D, Zhu YJ, et al. Dynamics of genome alterations in Crohn's disease-associated colorectal carcinogenesis. Clin Cancer Res 2018;24(20):4997–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noguchi T, Ishihara S, Uchino M, et al. Clinical features and oncological outcomes of intestinal cancers associated with ulcerative colitis and Crohn's disease. J Gastroenterol 2022;58(1):14–24. [DOI] [PubMed] [Google Scholar]
- 22.Proposed guidelines for the management of patients with Crohn's disease. Annual reports of Research Group of Intractable Inflammatory Bowel Disease subsidized by the Ministry of Health, Labor and Welfare of Japan. 2008. (in Japanese). [Google Scholar]
- 23.Japanese Society for Cancer of the Colon and Rectum: Japanese Classification of Colorectal Carcinoma. 3rd English Edition. Kanehara: Tokyo, Japan, 2019. [Google Scholar]
- 24.Vetter LE, Merkel S, Bénard A, et al. Colorectal cancer in Crohn's colitis is associated with advanced tumor invasion and a poorer survival compared with ulcerative colitis: A retrospective dual-center study. Int J Colorectal Dis 2021;36(1):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bye WA, Ma C, Nguyen TM, et al. Strategies for detecting colorectal cancer in patients with inflammatory bowel disease: A Cochrane systematic review and meta-analysis. Am J Gastroenterol 2018;113(12):1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmieri C, Müller G, Kroesen AJ, et al. Perianal fistula-associated carcinoma in Crohn's disease: A multicentre retrospective case control study. J Crohns Colitis 2021;15(10):1686–93. [DOI] [PubMed] [Google Scholar]
- 27.Birch RJ, Burr N, Subramanian V, et al. Inflammatory bowel disease-associated colorectal cancer epidemiology and outcomes: An English population-based study. Am J Gastroenterol 2022;117(11):1858–70. [DOI] [PubMed] [Google Scholar]
- 28.Matsuno H, Mizushima T, Nezu R, et al. Detection of anorectal cancer among patients with Crohn's disease undergoing surveillance with various biopsy methods. Digestion 2016;94(1):24–9. [DOI] [PubMed] [Google Scholar]
- 29.Higashi D, Katsuno H, Kimura H, et al. Current state of and problems related to cancer of the intestinal tract associated with Crohn's disease in Japan. Anticancer Res 2016;36(7):3761–6. [PubMed] [Google Scholar]
- 30.Shwaartz C, Munger JA, Deliz JR, et al. Fistula-associated anorectal cancer in the setting of Crohn's disease. Dis Colon Rectum 2016;59(12):1168–73. [DOI] [PubMed] [Google Scholar]
- 31.Beaugerie L, Carrat F, Nahon S, et al. High risk of anal and rectal cancer in patients with anal and/or perianal Crohn's disease. Clin Gastroenterol Hepatol 2018;16(6):892–9.e2. [DOI] [PubMed] [Google Scholar]
- 32.Galata C, Hirsch D, Reindl W, et al. Clinical and histopathologic features of colorectal adenocarcinoma in Crohn's disease. J Clin Gastroenterol 2018;52(7):635–40. [DOI] [PubMed] [Google Scholar]
- 33.Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013;258(5):775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Anal Carcinoma. Version 2. NCCN: Plymouth Meeting, PA, 2017. [Google Scholar]
- 35.Herrinton LJ, Liu L, Levin TR, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology 2012;143(2):382–9. [DOI] [PubMed] [Google Scholar]
- 36.Te Groen M, Derks M, den Broeder N, et al. Quality of surveillance impacts the colitis-associated advanced neoplasia risk: A multicenter case-control study. Clin Gastroenterol Hepatol 2022:S1542-3565(22)01177-6. doi: 10.1016/j.cgh.2022.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Wang YR, Cangemi JR, Loftus EV, Jr, et al. Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am J Gastroenterol 2013;108(3):444–9. [DOI] [PubMed] [Google Scholar]
- 38.Delbeke D, Martin WH. PET and PET-CT for evaluation of colorectal carcinoma. Semin Nucl Med 2004;34:209–23. [DOI] [PubMed] [Google Scholar]
- 39.Hirano Y, Futami K, Higashi D, et al. Anorectal cancer surveillance in Crohn's disease. J Anus Rectum Colon 2018;2(4):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch SL, van Rooijen SJ, Bokkerink GM, et al. Acute toxicity and surgical complications after preoperative (chemo)radiation therapy for rectal cancer in patients with inflammatory bowel disease. Radiother Oncol 2017;123(1):147–53. [DOI] [PubMed] [Google Scholar]
- 41.Iesalnieks I, Gaertner WB, Gla H, et al. Fistula-associated anal adenocarcinoma in Crohn's disease. Inflamm Bowel Dis 2010;16(10):1643–8. [DOI] [PubMed] [Google Scholar]
- 42.Alsughayer A, Grass F, McKenna NP, et al. Does IBD portend worse outcomes in patients with rectal cancer? A case-matched analysis. Dis Colon Rectum 2020;63(9):1265–75. [DOI] [PubMed] [Google Scholar]