Abstract
With the recent Health Canada approval of olaparib for high-risk, HER2-negative early breast cancer, physicians are now facing the practical challenges of integrating olaparib into current management of triple-negative breast cancer (TNBC) and HR-positive, HER2-negative (HR+/HER2−) early breast cancer. This review provides perspectives on some of the challenges related to identification of olaparib candidates, with a focus on the latest guidance for germline BRCA testing and considerations regarding high-risk disease definitions. Updated treatment pathways are explored for both disease states, including other adjuvant treatment options such as pembrolizumab, capecitabine, and abemaciclib. Gaps in the current literature regarding the sequential or combined use of these adjuvant therapies are noted and future, potentially informative, studies are briefly examined.
Keywords: adjuvant therapy, BRCA, capecitabine, early-stage breast cancer, genetic testing, olaparib, poly(ADP-ribose) polymerase inhibitors, pembrolizumab
1. Introduction
Pathogenic or likely pathogenic variants in BRCA1 and BRCA2, commonly referred to as mutations in BRCA, are associated with an increased risk of breast, ovarian, prostate, and pancreatic cancer. Germline BRCA (gBRCA) mutations can explain up to 10% of cases of breast cancer and occur most frequently in patients with a breast cancer diagnosis at or before the age of 40, a triple-negative breast cancer (TNBC) diagnosis at or before age 60, and a male breast cancer diagnosis [1,2]. Patients with gBRCA mutations often experience more aggressive disease, with increased risk of other or secondary malignancies [2,3]. Additionally, some studies have found that BRCA mutations are associated with lower breast cancer-specific survival rates [4].
Due to its effect on lifetime risk of cancers, gBRCA status is a significant factor in the personalization of breast cancer treatment. A mastectomy and contralateral prophylactic mastectomy should be considered per standard of care, rather than a conservative surgery for risk reduction purposes [1,5,6]. Subsequently, this surgical decision can have downstream implications for radiation treatment [1].
Poly(ADP) ribose polymerase inhibitors (PARPi) selectively induce cell death in BRCA-mutated tumours through synthetic lethality, due to defects in the homologous recombination repair pathway in these cells [7]. Two PARPi therapies, olaparib and talazoparib, have been approved by Health Canada for use in patients with gBRCA-mutated metastatic breast cancer [8,9]. More recently, positive results were reported from the OlympiA trial, which evaluated if olaparib would provide a benefit as an adjuvant therapy in patients with early breast cancer at high risk of recurrence [1,8,10,11,12,13].
In August 2022, Health Canada approved the use of adjuvant olaparib for HER2-negative gBRCA-mutated early breast cancer, adding to the evolving armamentarium of (neo)adjuvant treatment strategies [8,14,15]. With its approval comes a new set of considerations surrounding its use in clinical practice, such as patient identification, integration with existing treatment options, and toxicity management. This opinion piece aims to discuss some of these issues, including selection of patients for hereditary cancer genetic testing, exploring strategies for the identification of high-risk disease, and navigation of new treatment pathways for TNBC and hormone receptor-positive/HER2-negative (HR+/HER2-) early breast cancer, with a specific focus on Canadian clinical practice.
2. Efficacy and Safety of Olaparib in Early Breast Cancer
2.1. The OlympiA Trial: Key Patient Characteristics and Eligibility Criteria
OlympiA was a Phase 3, double-blinded, randomized trial evaluating the safety and efficacy of 12 months of adjuvant olaparib therapy versus placebo in high-risk, gBRCA-mutated, HER2-negative early breast cancer following definitive local treatment and adjuvant or neoadjuvant chemotherapy. Patients had completed at least six cycles of neoadjuvant or adjuvant chemotherapy containing anthracyclines, taxanes, or both agents; platinum chemotherapy was allowed. All local therapy, including radiation therapy, had to be completed at least 2 weeks and not more than 12 weeks before trial entry. In HR-positive patients, adjuvant endocrine therapy and adjuvant bisphosphonates were allowed concurrently with olaparib and administered according to institutional guidelines [13].
OlympiA examined four patient populations considered to have HER2-negative disease at high risk of recurrence (Table 1) [13,16]. Patients with TNBC who had received neoadjuvant therapy were required to have residual invasive breast cancer in the breast or resected lymph nodes (i.e., no pathological complete response [pCR]) to qualify, while patients with TNBC who had received adjuvant therapy were required to have axillary node-positive disease or an invasive primary tumour ≥2 cm. For patients with HR-positive disease who had received neoadjuvant therapy, risk assessment involved evaluation of their clinical-pathologic stage and nuclear grade via the CPS + EG scoring system (refer to Jeruss et al., 2008, for a detailed description of the calculation) [13,17]. These patients were considered at high risk if they had no pCR and a CPS + EG score of 3 or greater. Conversely, patients with HR-positive disease who had received adjuvant therapy were at high risk if they had at least 4 pathologically-confirmed positive lymph nodes [13].
Table 1.
High-risk Patient Populations in the OlympiA Trial [13].
| HER2-Negative Disease | Prior Therapy | High-Risk Criteria |
|---|---|---|
| TNBC | Neoadjuvant | Non-pCR |
| Adjuvant | ≥pT2 or ≥pN1 | |
| HR-positive | Neoadjuvant | Non-pCR and CPS + EG score ≥3 * |
| Adjuvant | ≥4 LN+ |
* Refer to Jeruss et al., 2008 for CPS + EG score calculation [17]. CPS + EG, clinical stage, pathologic stage, ER status, and tumour grade; HR, hormone receptor; LN, lymph node; pCR, pathological complete response; TNBC, triple-negative breast cancer.
2.2. Efficacy Outcomes in the OlympiA Trial
Overall, the OlympiA trial enrolled 1836 patients and included patients with Stage IB to IIIC disease [13]. At a pre-specified, event-driven interim analysis, treatment with olaparib was associated with a statistically significant improvement in invasive disease-free survival (IDFS), the primary endpoint, with a 3-year IDFS rate of 85.9% in the olaparib group and 77.1% in the placebo group (difference, 8.8%; stratified hazard ratio, 0.58; 99.5% confidence interval (CI), 0.41–0.82; p < 0.0001). Similarly, treatment with olaparib led to a significant improvement in 3-year distant disease-free survival (DDFS) (stratified hazard ratio, 0.57; 99.5% CI, 0.39–0.83; p < 0.0001). There was no significant difference in overall survival (OS) between the olaparib and placebo arms at this first interim analysis [13]; however, at the second interim analysis, a significant OS improvement was observed with a hazard ratio of 0.68 (98.5% CI, 0.47–0.97; p = 0.009, with a significance threshold of 0.015), translating to an absolute benefit of 3.4% at 4 years [16].
A subgroup analysis of both IDFS and OS revealed a benefit with olaparib for all stratification groups and subgroups, including both patients with TNBC (stratified hazard ratio for 4-year OS, 0.640; 95% CI, 0.459–0.884) and patients with HR-positive disease (stratified hazard ratio for 4-year OS, 0.897; 95% CI, 0.449–1.784). There was no evidence suggesting statistical heterogeneity in the treatment effect across various stratification factors, which included HR status, prior chemotherapy, prior platinum therapy, and BRCA1/2 status [13,16].
2.3. Adverse Event Profile
In the OlympiA trial, the most common adverse events (AEs) reported in the olaparib treatment group were gastrointestinal toxicities, fatigue, and hematologic toxicities; these were also the most common reasons for discontinuation. Notably, the only Grade 3 AE with an incidence higher than 5% was anemia, and 5.8% of patients treated with olaparib required blood transfusion versus 0.9% in the placebo group [13]. Experience from ovarian cancer suggests that upfront patient education and proactive monitoring and management of these toxicities are key for maintaining patients on olaparib [18,19,20,21,22].
Adverse events of special interest included pneumonitis, myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) [13]. None of these AEs of special interest occurred at a greater incidence in the olaparib group than in the placebo group over a median of 2.5 years [13]. Longer-term follow-up is required to assess the risk of MDS/AML as well as new primary cancers.
MDS is of particular interest as it is a class warning for PARPi therapies arising from trials in the ovarian cancer setting [20]. While the causes of PARPi-associated leukemogenesis remain unclear, one underlying risk factor may be previous exposure to platinum and alkylating agents, as MDS/AML has been more commonly observed in heavily pretreated patients [20,23]. The SOLO-2 trial evaluated olaparib maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer. In this setting where olaparib was taken until disease progression or discontinued at investigator discretion, the MDS/AML rate was 8% after 5 years of follow-up and a mean total duration of olaparib therapy of 29.1 months (vs. an MDS/AML rate of 4% with placebo) [24]. In the SOLO-1 trial, olaparib maintenance followed first-line platinum-based chemotherapy and was administered for a finite period of 2 years (or continued if evidence of disease per investigator discretion). After 7 years of follow-up, the MDS/AML rate was 1.5% (vs. 0.8% with placebo) [25].
In the breast cancer setting, the rate of MDS/AML in OlympiA was 0.1% where the planned duration of olaparib therapy was 12 months [13], and there were no cases of MDS/AML in the OlympiAD trial, in which olaparib was given for a median total treatment duration of 8.2 months in the metastatic breast cancer setting [8,26].
Recognizing the potential for rare but serious hematologic toxicity, patients on olaparib should be monitored routinely [8,20]. Proactive monitoring measures include baseline assessment, monthly monitoring of complete blood counts (CBC) throughout treatment, and periodic monitoring beyond 12 months [8]. Within the ovarian cancer setting, clinician opinion is that CBC should continue to be assessed every 3–4 months following completion of olaparib therapy.
3. Identification of Olaparib Candidates
3.1. Identifying Patients at High-Risk for Recurrence
Based on the results of the OlympiA trial, the use of adjuvant olaparib in high-risk, HER2-negative early breast cancer is recommended in several guidelines and is approved by regulatory bodies in numerous countries, including Canada [6,8,27,28,29]. The Health Canada indication statement for olaparib does not define “high-risk”; however, several clinical practice guidelines recommend the selection of olaparib candidates based on the trial criteria (Table 2). Additionally, Canadian Agency for Drugs and Technologies in Health (CADTH) recommends reimbursement for olaparib under conditions that match the trial criteria (see Section 4.1 and Section 4.2) [30].
Table 2.
Guideline Recommendations for Olaparib Eligibility in Early Breast Cancer.
| Guideline | Recommendation for Olaparib Eligibility | Recommendation for gBRCA Testing to Inform Treatment Decisions |
|---|---|---|
| The American Society of Clinical Oncology (ASCO) | One year of adjuvant olaparib for patients with early-stage, gBRCA-mutated, HER2-negative cancer with a high risk of recurrence after completion of (neo)adjuvant chemotherapy and local treatment, including radiation. “High risk” is defined as the four patient subpopulations that were eligible for the OlympiA trial (see Section 2, Table 1) [27]. | n/a |
| NCCN Clinical Practice Guidelines in Oncology (The NCCN Guidelines®) | One year of adjuvant olaparib should be considered for patients with gBRCA-mutated HER2-negative disease who fall into the four high-risk populations enrolled in OlympiA [6]. | In addition to other personal and family history criteria, testing should be done whenever it will aid adjuvant treatment decisions with olaparib in high-risk, HER2-negative breast cancer [6,31]. |
| 2021 St. Gallen International Consensus Guidelines | Adjuvant olaparib for patients with Stage II or III HER2-negative disease meeting OlympiA trial criteria (support from >93% panelists), or patients with Stage II or III HER2-negative cancers regardless of estrogen receptor status or prior treatment with platinum-based chemotherapy (support from 64% of panelists) [28]. | gBRCA testing is recommended for patients meeting the OlympiA trial criteria in order to identify candidates for olaparib therapy [28]. |
gBRCA, germline BRCA; HER2, human epidermal growth factor 2.
Notably, the OlympiA trial protocol permitted inclusion of patients with Stage IB to IIIC disease, provided the criteria for high-risk disease were met (see Table 1) [13]; however, the St. Gallen International Consensus Guideline only recommends olaparib for patients with Stage II or III disease [28]. For the purposes of adopting olaparib into Canadian clinical practice, the authors of this manuscript define high-risk early breast cancer per the OlympiA trial criteria.
3.2. Hereditary Cancer Genetic Testing to Identify Olaparib Candidates
With the OlympiA trial demonstrating the efficacy benefits of olaparib in gBRCA-mutated early breast cancer, a number of guideline bodies have updated their recommendations for hereditary cancer genetic testing to facilitate testing to inform treatment decisions (see Table 2). Access to hereditary cancer genetic testing in Canada remains variable and continues to evolve with the discovery of new risk genes, targeted therapies, and improvements in genetic technologies. Some provinces and institutions have updated their criteria to provide publicly funded testing in scenarios where germline variant status will qualify a patient for an approved targeted therapy and all other criteria for that therapy are met [32].
Potential olaparib candidates may also be eligible for publicly funded hereditary cancer genetic testing through customary means based on personal and family history. Ontario’s most current testing criteria include individuals with a personal history of breast cancer at ≤45 years of age and triple-negative breast cancer at ≤60 years of age [32]. Unfortunately, standardized national criteria for hereditary cancer testing have not been established and Canadians face inequitable access to this pathway to personalized medicine. For now, practitioners should seek out their current regional guidance for accessing funded genetic services and become familiar with alternative genetic testing mechanisms, such as research initiatives and commercial providers for patients willing to pay for genetic testing.
3.2.1. Timing Considerations for Hereditary Cancer Genetic Testing
As gBRCA status is relevant for both surgical and adjuvant systemic treatment decisions, it is ideal for hereditary cancer genetic testing to be completed early in the treatment pathway [1,14]. Mutation status will influence the surgical options presented: a total mastectomy (uni- or bilateral) may be considered due to increased risk of a second ipsi- and/or contralateral cancer [1,14]. In scenarios where BRCA results are received after surgery and radiotherapy, a risk-reducing mastectomy may be performed later, but may negatively affect cosmetic results and increase the risk of surgical complications [1]. Accordingly, multiple guidelines suggest that germline testing be initiated as early as possible or during the workup for invasive breast cancer [6,14].
In the specific context of making PARPi treatment decisions, gBRCA status requires confirmation within the timeframe required to initiate olaparib treatment per OlympiA protocol: not more than 12 weeks after completion of local therapy, including radiation therapy [13]. In addition to testing during pre-surgical workup, a second timepoint for considering gBRCA testing exists post-surgery. If, following surgery (with or without radiotherapy) and chemotherapy, the patient is found to be at high risk for disease recurrence, then they may be a potential candidate for olaparib [6,13,27,28] (see Section 3.1 for definitions of “high risk” disease). Optimally, results of genetic testing should be obtained before any planned radiotherapy treatment since it might provide an opportunity to offer increased options for alternative risk reducing surgery in patients with clinically relevant genetic alterations. Initiating gBRCA testing following surgery requires an expedited turnaround of test results, which may be challenging in some jurisdictions.
3.2.2. Mainstreaming Genetic Testing
Within the context of Canadian practice, current turnaround times for hereditary cancer genetic testing may pose a significant challenge for surgical planning and initiating PARPi therapy. This is especially the case when traditional testing pathways, which require upfront referral to the cancer genetics clinic for pre-test counselling, are followed; access to testing may be delayed by months. One solution to these delays is to implement mainstreamed genetic testing, a pathway in which testing is initiated by a non-genetics clinician in patient populations that meet eligibility criteria based on their own personal cancer history [33]. Beyond personal cancer history, some mainstreaming protocols may also consider family history and tumour biology. Because the specialist who first sees the patient is often a surgeon or medical oncologist in this disease setting, they are the focus of mainstreaming efforts [14,32,34].
Within the mainstreaming pathway, the surgeon or medical oncologist will provide pretest counselling, obtain patient consent, and directly order the genetic test [33]. In Canada, results are generally also disclosed by the ordering physician; for most patients, the cancer genetics clinic will only be involved in the consultation process when a pathogenic or likely pathogenic variant in BRCA1/2 or other targeted genes is detected [35,36]. Depending on the region, the Genetic Services may also connect with patients when a Variant of Unknown Significance (VUS) is detected. In cases where no known variant is detected, a referral to Genetic Services is generally not required but is recommended if the patient has many questions or concerns due to personal or family history of cancers. By eliminating upfront pre-test counselling by Genetic Services, the turnaround time for testing is minimized. The cancer genetics clinic is also able to focus resources on patients who test positive, thereby easing resource constraints [34].
To date, mainstreamed genetic testing has been implemented in various practice sites across Canada [34,35,36]. Several strategies exist to help implement or improve efficiency in the mainstreaming pathway: screening tools can be used to help surgeons and oncologists identify patients who are eligible for testing; pre-test counselling checklists and patient handouts can support non-genetics clinicians in educating patients and obtaining consent in a 5–10 min timeframe [37]. Standardized results letters and phone calls can also be used to disclose results to patients, and some locales have mechanisms in place to reflexively refer patients to the cancer genetics clinic whenever a positive result is obtained [33,35,36,38]. Mainstreaming requires cross-departmental coordination, and any site seeking to establish a mainstreaming pathway should do so in collaboration with their local cancer genetics service [32,35].
4. Treatment Pathways for HER2-Negative Early Breast Cancer
4.1. Adjuvant Treatment Options for Early High-Risk TNBC
Three key adjuvant therapy trials inform treatment choice in the early, high-risk TNBC patient population: CREATE-X, which assessed capecitabine versus standard therapy in patients with residual disease after NACT; KEYNOTE-522, which assessed the addition of neoadjuvant/adjuvant pembrolizumab versus placebo; and OlympiA for olaparib (see Table 3) [13,39,40]. The KEYNOTE-522 and OlympiA trials supported Health Canada approvals for the use of adjuvant pembrolizumab and olaparib in high-risk early breast cancer, respectively [8,41]; however, a Health Canada-approved indication was not pursued for capecitabine [42]. Despite this, most provinces in Canada fund capecitabine for early breast cancer based on the CREATE-X trial (see Table 3).
Table 3.
Key Adjuvant Therapy Trials in Patients with Early High-Risk TNBC, Health Canada, and CADTH Guidance.
| Capecitabine|CREATE-X (n = 910) [39] | Pembrolizumab|KEYNOTE-522 (n = 1174) [40] | Olaparib|OlympiA (n = 1836) [13] | |
|---|---|---|---|
| Population |
|
|
|
| Definition of “High Risk” per Trial Criteria |
|
|
TNBC †‡
|
| Intervention |
|
Experimental arm:
|
|
| Primary Endpoint |
ITT Population: HER2- Median follow-up: 3.6 years [39]
|
ITT Population: TNBC Median follow-up: 15.5 months [40]
|
ITT Population: HER2- Median follow-up: 2.5 years [13]
|
| Exploratory Subgroup Analyses of DFS/IDFS |
Subgroup: ER- and PgR- Median follow-up: 3.6 years [39]
|
n/a |
Subgroup: TNBC Median follow-up: 2.5 years [13]
|
| Secondary Endpoint: OS |
ITT Population: HER2- Median follow-up: 3.6 years [39]
|
ITT Population: TNBC Median follow-up: 39.1 months [43]
|
ITT Population: HER2- Median follow-up: 3.5 years [16]
|
| Exploratory Subgroup Analyses of OS |
Subgroup: ER- and PgR- Median follow-up: 3.6 years [39]
|
n/a |
Subgroup: TNBC Median follow-up: 3.5 years [16,44]
|
| Health Canada Indication and CADTH Recommendation |
Health Canada: [41]
|
Health Canada: [8]
|
* According to the AJCC, 7th edition. † Risk assessment was performed at the time of surgery. ‡ The OlympiA trial included patients with both TNBC and HR+/HER2- breast cancer. Only high-risk TNBC criteria are shown here. For high-risk HR+/HER2- disease criteria, refer to Table 4. § Not significant. ‖ Significance boundary of 0.015. Summary of key trials for adjuvant capecitabine, pembrolizumab, and olaparib in early-stage, high-risk TNBC. A, doxorubicin or epirubicin; AdjCT, adjuvant chemotherapy; AdjTx, adjuvant treatment; BID, bis in die (twice daily); C, cyclophosphamide; CADTH, Canadian Agency for Drugs and Technologies in Health; Cb, carboplatin; DFS, disease-free survival; eBC, early breast cancer; EFS, event-free survival; ER, estrogen receptor; HR, hazard ratio; HR+/HER2-, hormone receptor-positive/human epidermal growth factor receptor 2-negative; IA1/2, interim analysis 1 or 2; IDFS, invasive disease-free survival; ITT, intention-to-treat; LN, lymph node; N, node; NACT, neoadjuvant chemotherapy; NS, not significant; OS, overall survival; pCR, pathological complete response; PgR, progesterone receptor; PD-L1, programmed death ligand 1; PO, per os (orally); Q3W, every 3 weeks; T, paclitaxel; TNBC, triple-negative breast cancer.
When assessing the eligibility of a patient for any of these three adjuvant options, it is imperative to understand the differences between the study populations enrolled in their respective pivotal trials, the high-risk disease definition used by each trial, and the timing of these treatments relative to chemotherapy and surgery (Table 3, Figure 1). Prescribers should also note the duration of these new therapies and the potential toxicities endured by patients during extended adjuvant therapies. Notably, all patients enrolled in OlympiA carried a germline BRCA mutation [13]. Conversely, KEYNOTE-522 and CREATE-X did not mandate genetic testing and outcomes related to this specific subgroup of patients were not reported in either trial. While it is helpful to note these differences between the pivotal trials, it is also critical to avoid cross-trial comparisons, as pre-specified endpoints and statistical plans are different between them all.
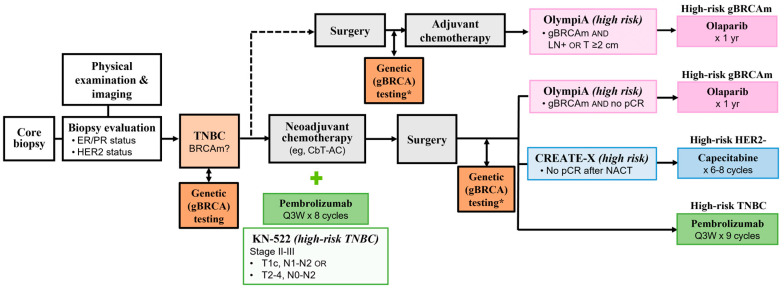
Figure 1.
Treatment pathways for early, high-risk TNBC, with adjuvant therapy options and the “high risk” definitions used in their respective pivotal trials highlighted in pink (olaparib), blue (capecitabine), or green (pembrolizumab). For TNBC, the neoadjuvant chemotherapy pathway is more frequently pursued within the current standards of care; surgery followed by adjuvant chemotherapy is a less common pathway (dotted line). BRCAm, BRCA-mutated; CbT-AC, carboplatin and paclitaxel/doxorubicin or epirubicin and cyclophosphamide; ER, estrogen receptor; gBRCAm, germline BRCA-mutated; LN, lymph node; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PR, progesterone receptor; Q3W, every 3 weeks. * If not done earlier.
4.2. Adjuvant Treatment Options for High-Risk HR+/HER2- Early Breast Cancer
Adjuvant therapy options for high-risk, early-stage HR+/HER2- disease include capecitabine, the CDK4/6 inhibitor (CDK4/6i) abemaciclib, and olaparib; safety and efficacy for these therapies were assessed in the CREATE-X, monarchE, and OlympiA Phase 3 trials, respectively (Table 4, Figure 2) [13,39,55]. Olaparib and abemaciclib both have Health Canada approval for this indication, while capecitabine, although funded in most provinces for high-risk HER2-negative early breast cancer, does not (see Table 4). As stated previously, while it is critical to note key differences between the pivotal trials when selecting a treatment option, it is also imperative to avoid cross-trial comparisons, as pre-specified endpoints and statistical plans differ between the trials.
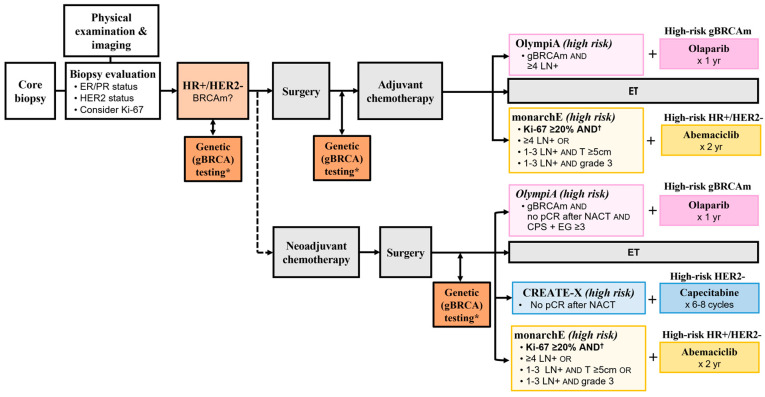
Figure 2.
Treatment pathways for high-risk HR+/HER2- early breast cancer. “High-risk disease” criteria for the OlympiA trial (pink), CREATE-X trial (blue), and CADTH recommendation for abemaciclib (yellow) are overlayed. In HR+/HER2- disease, surgery followed by adjuvant chemotherapy is commonly pursued within the current standards of care; the neoadjuvant chemotherapy pathway is less common (dotted line). ER, estrogen receptor; ET, endocrine therapy; BRCAm, BRCA mutation; CADTH, Canadian Agency for Drugs and Technologies in Health; gBRCA, germline BRCA; gBRCAm, germline BRCA-mutated; HR, hormone receptor; LN, lymph node; NACT, neoadjuvant chemotherapy; pCR, pathological complete response; PR, progesterone receptor. * If not done earlier. † For abemaciclib: The CADTH reimbursement recommendation requires Ki-67 ≥20% and specific clinicopathological features per monarchE Cohort 1. Cohort 2 did not receive a funding recommendation [56].
The CREATE-X trial included both HR-positive and TNBC patients and demonstrated a disease-free survival benefit in its intention-to-treat population (Table 4); a subgroup analysis showed a greater benefit in TNBC patients (HR, 0.58; 95% CI, 0.39–0.87) and less promising results in HR-positive patients (DFS HR, 0.81; 95% CI, 0.55–1.17; n = 601) [39]. Because of its uncertain benefit in HR-positive disease, as well as the existence of other adjuvant treatment options, physicians tend not to consider capecitabine in HR-positive early breast cancer. Within the OlympiA trial, which also included both HR-positive and TNBC patients, only 18% of the intention-to-treat population had HR-positive disease. Accordingly, the CADTH reimbursement recommendation for olaparib noted that due to the small sample size of patients with HR-positive disease, there is uncertainty with the results of its HR-positive subgroup analysis (OS HR, 0.897; 95% CI, 0.449–1.784; n = 325 patients) [16,30].
In the HR+/HER2- early breast cancer setting, there are varying definitions for “high-risk disease”. Notably, for abemaciclib, the Health Canada indication and CADTH reimbursement recommendation specify a requirement for Ki-67 ≥20% regardless of lymph node involvement (see Table 4) [56,57]. The CADTH reimbursement recommendation further limits funded use of abemaciclib in early breast cancer to patients with features aligned with Cohort 1 and Ki-67 ≥20% [56]; an overview of this monarchE pre-specified analysis was published by Royce and colleagues [29]. Interestingly, although the U.S. Food and Drug Administration (FDA) previously also required a Ki-67 score ≥20% for the approved use of abemaciclib, this requirement was removed in March 2023 [58,59]. The decision followed a 4-year interim analysis update demonstrating that the IDFS benefit of abemaciclib is not dependent on Ki-67 index [60]. Given these data, as well as the decision by the FDA, there is potential for an update to the Health Canada indication in the future.
The Health Canada indication for olaparib does not include the detailed definitions of high-risk disease presented in the OlympiA trial [8,13]. However, the CADTH reimbursement recommendation states that olaparib should be reimbursed in patients who meet OlympiA trial criteria (Table 4); various clinical practice guidelines similarly recommend the use of olaparib in patients meeting OlympiA eligibility criteria [6,27,28,30].
Table 4.
Key Adjuvant Therapy Trials in Patients with High-Risk HR+/HER2- Early Breast Cancer, Health Canada, and CADTH Guidance
| Capecitabine|CREATE-X (n = 910) [39] |
Abemaciclib|monarchE (n = 5367) [55] |
Olaparib|OlympiA (n = 1836) [13] |
|
|---|---|---|---|
| Population |
|
|
|
| Definition of “High Risk” per Trial Criteria |
|
Cohort 1
|
HR+/HER2- *†
|
| Intervention |
|
|
|
| Primary Endpoint |
ITT Population: HER2- Median follow-up: 3.6 years [39]
|
ITT Population: HR+/HER2- Median follow-up: 15.5 months [55]
|
ITT Population: HER2- Median follow-up: 2.5 years [13]
|
| Exploratory Subgroup Analysis of DFS/IDFS |
Subgroup: ER+ or PgR+ Median follow-up: 3.6 years [39]
|
CADTH Population: ‡ Cohort 1, Ki-67 ≥20% [29,56] Median follow-up: 27 months
|
Subgroup: HR+/HER2- Median follow-up: 2.5 years [13]
|
| Secondary Endpoint: OS |
ITT Population: HER2-Median follow-up: 3.6 years [39]
|
ITT Population: HR+/HER2- Median follow-up: 27 months [55]
|
ITT Population: HER2-Median follow-up: 3.5 years [16]
|
| Exploratory Subgroup Analysis of OS |
Subgroup: ER+ or PgR+Median follow-up: 3.6 years [39]
|
CADTH Population: ‡ Cohort 1, Ki-67 ≥20% [29,56] Median follow-up: 27 months
|
Subgroup: HR+/HER2-Median follow-up: 3.5 years [16,44]
|
| Health Canada Indication and CADTH Recommendation |
Health Canada: [57]
|
Health Canada: [8]
|
* Risk assessment was performed at the time of surgery. † The OlympiA trial included patients with both TNBC and HR+/HER2- breast cancer. Only high-risk HR+/HER2- disease criteria are shown here. For high-risk TNBC criteria, refer to Table 3. ‡ A gated hierarchical testing strategy included IDFS in patients with a Ki-67 score ≥20% from cohort 1 alone [29]. § Significance boundary of 0.015. Summary of key trials for adjuvant capecitabine, abemaciclib, and olaparib in high-risk, HR+/HER2- early breast cancer. AdjCT, adjuvant chemotherapy; BID, bis in die (twice daily); CADTH, Canadian Agency for Drugs and Technologies in Health; DFS, disease-free survival; eBC, early breast cancer; HR, hazard ratio; HR+/HER2-, hormone receptor-positive/human epidermal growth factor receptor 2-negative; IA1/2, interim analysis 1 or 2; ITT, intention-to-treat; LN, lymph node; NACT, neoadjuvant chemotherapy; NS, not significant; Q3W, every 3 weeks; RT, radiation therapy; SOC ET, standard of care endocrine therapy.
Figure 2 depicts the possible treatment pathways for HR+/HER2- disease, including these three adjuvant treatment options and the varying definitions for high-risk disease that are relevant to Canadian practice. Note that risk criteria in the OlympiA trial varied depending on the timing of chemotherapy: for patients receiving neoadjuvant therapy, a CPS + EG score was used to assess their risk; for patients without neoadjuvant therapy, the requirement was disease in 4 pathologically confirmed lymph nodes. Additionally, of the three trials, only OlympiA required the presence of a gBRCA mutation [8,13].
4.3. Considerations for Sequencing Olaparib with Other Therapies in the Adjuvant Setting
4.3.1. Olaparib, Radiation, and Endocrine Therapy
With the introduction of new adjuvant treatment options, clinicians are seeking guidance on the sequencing or combining of therapies. Adjuvant olaparib can be given concurrently with endocrine therapy, consistent with the protocol in the OlympiA trial [6,13]. In cases where radiation is indicated, it is common for radiation therapy to follow chemotherapy. Olaparib must be given at least 2 weeks after completion of radiation therapy, as PARP inhibition has a known radiosensitizing effect [6,13,62]. Additionally, per the OlympiA trial protocol, olaparib therapy should be initiated within 12 weeks of completion of the last treatment, which may include surgery, radiation, or chemotherapy [13]. With regard to timing, the CADTH reimbursement recommendation notes that some situations may warrant treatment initiation beyond this 12-week timeframe for certain patients with high-risk breast cancer, such as legacy patients [30].
4.3.2. Olaparib and Other Adjuvant Treatment Options
Currently, the NCCN Guidelines® suggest that the sequential or combined use of pembrolizumab, olaparib, and/or capecitabine may be considered in select patients with a high risk of recurrence and who meet criteria for treatment with one of more of these agents, although the guidelines also state that there are presently no data on sequencing or combining adjuvant pembrolizumab with olaparib in patients [6]. The absence of combination data represents a key knowledge gap in the treatment of HER2-negative early breast cancer, and various ongoing trials are evaluating the efficacy and safety of concurrent therapies with PARPi treatments including olaparib (Table 5).
Olaparib and Immunotherapy
With recent approvals of both immunotherapy and PARPi treatment in TNBC [8,41,63], there is notable interest in the feasibility of combining these two drug classes. While limited, there is published experience with olaparib in combination with pembrolizumab in patients with breast cancer (see Table 5). Outcomes from these studies suggest that efficacy is unaltered, and that patient toxicity is acceptable with a manageable safety profile. Notably, the ongoing phase II/III KEYLYNK-009 study is evaluating the clinical benefit of pembrolizumab plus olaparib maintenance therapy after first-line chemotherapy with pembrolizumab in locally recurrent inoperable or metastatic TNBC [64]. Results from this study, as well as other trials focusing on sequential/combination therapies, will inform the integration of olaparib with immune-oncology therapies in routine practice.
Olaparib and Abemaciclib
Our search of the literature and ClinicalTrials.gov registry revealed one ongoing National Cancer Institute trial investigating olaparib in combination with abemaciclib in recurrent ovarian cancer (see Table 5). This dose escalation study is examining concurrent use of these two agents. In this early phase of clinical adoption of olaparib therapy, clinicians have expressed substantial concern regarding the potential cumulative toxicity of this combination; it is anticipated that oncologists will choose either abemaciclib or olaparib, giving consideration to their respective toxicity profiles and duration of therapy. Although the survival benefit observed in the OlympiA trial was in a study population in which only 18% of patients had HR-positive breast cancer, the monarchE trial has not yet reached maturity for its OS analysis. This currently translates in many physicians having a clinical preference for prescribing olaparib for gBRCA-mutated, HR-positive patients [16,55,59].
Table 5.
Select Clinical Trials of Olaparib/PARPi Combination Therapy in Breast Cancer and Other Solid Tumours*.
| Trial | Population | Intervention | Outcomes |
|---|---|---|---|
| Olaparib and Pembrolizumab in Breast Cancer | |||
|
KEYLYNK-0072 [65] (NCT04123366) Phase II, single-arm, open-label study |
Previously treated advanced solid tumours with mutations in homologous recombination repair genes and/or homologous recombination deficiency (including breast cancer) (N = 168) |
|
|
|
TOPACIO/KEYNOTE-162 † [66] (NCT02657889) Phase II, single arm, open-label study |
Advanced/metastatic TNBC (irrespective of BRCA status or PD-L1 expression) (N = 55) |
|
|
|
KEYLYNK-009 [64] (NCT04191135) Phase II/III, randomized, open-label study |
Locally recurrent inoperable or metastatic TNBC (estimated N = 932) |
|
|
|
NCT05203445 [67] Phase II single-arm, open-label study |
Newly diagnosed TNBC or HR+/HER2- BC (N = 23) |
|
|
| Olaparib and Pembrolizumab in Other Solid Tumours | |||
|
KEYLYNK-010 [68] (NCT03834519) Phase III, randomized, open-label study |
mCRPC (molecularly unselected) (N = 793) |
Arms:
|
|
|
KEYNOTE-365 [70] (NCT02861573) Phase Ib/II, non-randomized, multicohort, open-label study (Cohort A) |
mCRPC (molecularly unselected) (Cohort A: N = 102) |
Cohort A:
|
|
|
ENGOT-OV43/KEYLYNK-001 [71] (NCT03740165) Phase III, randomized, double-blind study |
1L ovarian cancer (BRCA non-mutated) (N = 1367) |
Arms:
|
|
|
KEYLYNK-012 [72] (NCT04380636) Phase III, randomized, placebo- and active-controlled, double-blind study |
Unresectable stage III NSCLC (N = 870) |
Arms:
|
|
|
KEYLYNK-013 [73] (NCT04624204) Phase III, randomized, double-blind study |
Limited-stage SCLC (N = 672) |
Arms:
|
|
| Olaparib and Abemaciclib in Solid Tumours | |||
|
NCI-2020-10084 [74] (NCT04633239) Phase I/Ib, open-label, dose escalation study |
Recurrent ovarian cancer (N = 42) |
|
|
* Based on a non-systematic search of medical literature and ClinicalTrials.gov based on the following keywords: “olaparib” or “PARP inhibitor” + “pembrolizumab”, “abemaciclib”, or “capecitabine”. † Note: Niraparib clinical trial included. AE, adverse events; BID, bis in die (twice daily); cap, capsules; CbT, carboplatin-paclitaxel; CRT, chemoradiotherapy; HRD, homologous recombination deficiency; HRRm, homologous recombination repair mutation; IMAE, immune-mediated adverse events; IRAE, immune-related adverse events; IV, intravenous; mCRPC; metastatic castration-resistant prostate cancer; NHA, next-generation hormonal agent; NSCLC, non-small cell lung cancer; PD, progressive disease; PD-L1, programmed death ligand 1; PO, per os (orally); Q3W, every 3 weeks; SCLC, small cell lunger cancer; tabs, tablets; TRAE, treatment-related adverse events; TNBC, triple-negative breast cancer.
Olaparib and Capecitabine
There is a paucity of information regarding the sequential use or combination of olaparib with capecitabine [6]. Like abemaciclib, there is concern surrounding potential cumulative toxicity from combined use of capecitabine and olaparib, and it is probable that many oncologists will choose one over the other in practice. Some physicians are also considering sequential use of capecitabine followed by olaparib in patients with high-risk, gBRCA-mutated TNBC, although there are no data to support this strategy.
5. Conclusions
The recent approval of olaparib in Canada for HER2-negative early breast cancer offers a novel option for personalized treatment of gBRCA-mutated, high-risk early breast cancers. This new indication for olaparib presents a need for early determination of gBRCA status to facilitate systemic therapy planning, as well as surgical decision-making and familial risk identification. Mainstreaming led by oncologists or surgeons offers a potential path to streamlined, patient-centered genetic testing to ensure that results are received in time for treatment decisions. In addition to gBRCA status, the identification of high-risk disease is also critical to personalizing care for patients with HER2-negative breast cancer, as multiple adjuvant therapy options are available for both high-risk TNBC and high-risk HR+/HER2- disease. Capecitabine, olaparib, and pembrolizumab are notable options for high-risk TNBC, whereas abemaciclib, capecitabine, and olaparib are options for high-risk HR+/HER2- disease. Selection between these adjuvant treatments should be guided by the patient’s germline BRCA status and the respective criteria for high-risk disease. For patients who are eligible for multiple treatment options, however, there are very limited data to guide the selection, sequencing, or combination of these therapies.
Furthermore, recent data presented at the 2023 ASCO Annual Meeting demonstrated that another CDK4/6i regimen, ribociclib with endocrine therapy, shows an IDFS benefit in the adjuvant setting for Stage IB-III early breast cancer [75]. This emerging option may be incorporated into future guidelines and/or algorithms but has not received approval from either the FDA or Health Canada at the time of this publication. PARPi combinations are being explored in a variety of solid tumours, which may provide insights into the safety of these treatment regimens. However, few of these studies focus specifically on early breast cancer, highlighting a need for more trials in this disease setting. Any future trials or real-world evidence examining the combination or sequencing of these therapies, or the comparative efficacy or safety of these treatment options, will provide useful information for evolving the clinical management of early-stage, HER2-negative breast cancer.
Acknowledgments
The authors would like to thank and acknowledge the writing assistance provided by Karen Chiang, MSc and Marcia Bos, BScPhm of FUSE Health.
Author Contributions
Conceptualization, J.-W.H., J.-F.B., L.P. and T.M.; resources, J.-W.H., L.P. and T.M.; writing—original draft preparation, J.-W.H., writing—review and editing, J.-W.H., J.-F.B., L.P. and T.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
J.W.H. declares the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J-W.H. received funding from AstraZeneca for this opinion piece; J-W.H. has served as an advisor/consultant for AstraZeneca, Eli Lilly, Roche, Knight Therapeutics, Novartis, Pfizer, Seagen, and Merck; J-W.H. has received grants/honoraria from AstraZeneca and Pfizer; J-W.H. has served as a speaker for AstraZeneca and Novartis; J-W.H has also participated in clinical trials sponsored by AstraZeneca, Novartis, and Pfizer. J-F.B. declares the following potential conflicts of interest: J-F.B. has served as an advisor/consultant for Roche, Eli Lilly, Pfizer, Merck, Exact Sciences, Novartis, and AstraZeneca; J-F.B. has received grants/honoraria from Roche, Novartis, Pfizer, Abbvie, Merck, Eli Lilly, Bristol-Myers Squibb, Exact Sciences, and AstraZeneca through institutional funding at clinical trial sites; J-F.B. has served as a speaker for Roche, Novartis, Pfizer, Merck, Exact Sciences, and AstraZeneca; J-F.B. has participated in a steering committee for a trial sponsored by AstraZeneca (DESTINY-Breast11). T.M. declares the following potential conflicts of interest: T.M. has served as an advisor/consultant for Purdue, GlaxoSmithKline, and Pfizer; T.M. has received grants/honoraria from Purdue, Amgen, AstraZeneca, Leo Pharma, ApoBiologix, and Novartis. L.P. declares the following potential conflict of interest: L.P. has served as an advisor/consultant for AstraZeneca. The funders had no role in the writing of the manuscript, or the decision to publish an opinion piece.
Funding Statement
Funding was provided by AstraZeneca Canada to support medical writing and administrative coordination of this manuscript. The funder did not contribute to the content or writing of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pujol P., Barberis M., Beer P., Friedman E., Piulats J.M., Capoluongo E.D., Foncillas J.G., Ray-Coquard I., Penault-Llorca F., Foulkes W.D., et al. Clinical Practice Guidelines for BRCA1 and BRCA2 Genetic Testing. Eur. J. Cancer. 2021;146:30–47. doi: 10.1016/j.ejca.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 2.Cortesi L., Rugo H.S., Jackisch C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021;16:255–282. doi: 10.1007/s11523-021-00796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becourt S., Cohen-Haguenauer O., Ledoux F., Nguyen O., Cuvier C., Giacchetti S., Cahen-Doidy L., Bourstyn E., Espie M., Teixeira L. Comparison of Clinicopathological (CP) Features and Outcome of Breast Cancers (BC) in BRCA-Mutation Carriers Patients, with a Family History without BRCA-Mutation and with Sporadic Disease. J. Clin. Oncol. 2018;36:e13522. doi: 10.1200/JCO.2018.36.15_suppl.e13522. [DOI] [Google Scholar]
- 4.Baretta Z., Mocellin S., Goldin E., Olopade O.I., Huo D. Effect of BRCA Germline Mutations on Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Medicine. 2016;95:e4975. doi: 10.1097/MD.0000000000004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung N.M., Boughey J.C., Pierce L.J., Robson M.E., Bedrosian I., Dietz J.R., Dragun A., Gelpi J.B., Hofstatter E.W., Isaacs C.J., et al. Management of Hereditary Breast Cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J. Clin. Oncol. 2020;38:2080–2106. doi: 10.1200/JCO.20.00299. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network® NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer. Version 4.2023. 2023. [(accessed on 13 April 2023)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.Lord C.J., Ashworth A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LYNPARZA® (Olaparib) Product Monograph 2022. [(accessed on 2 April 2023)]. Available online: https://pdf.hres.ca/dpd_pm/00066912.PDF.
- 9.TALZENNA® (Talazoparib) Product Monograph 2022. [(accessed on 2 April 2023)]. Available online: https://pdf.hres.ca/dpd_pm/00064613.PDF.
- 10.O’Connor M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Drew Y. The Development of PARP Inhibitors in Ovarian Cancer: From Bench to Bedside. Br. J. Cancer. 2015;113((Suppl. S1)):S3–S9. doi: 10.1038/bjc.2015.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateo J., Lord C.J., Serra V., Tutt A., Balmaña J., Castroviejo-Bermejo M., Cruz C., Oaknin A., Kaye S.B., de Bono J.S. A Decade of Clinical Development of PARP Inhibitors in Perspective. Ann. Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi S., Brackstone M., Hong N.J.L., Grenier D., Donovan E., Lu F.-I., Skarpathiotakis M., Lee J., Boileau J.-F., Perera F., et al. A Canadian National Guideline on the Neoadjuvant Treatment of Invasive Breast Cancer, Including Patient Assessment, Systemic Therapy, and Local Management Principles. Breast Cancer Res. Treat. 2022;193:1–20. doi: 10.1007/s10549-022-06522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agostinetto E., Gligorov J., Piccart M. Systemic Therapy for Early-Stage Breast Cancer: Learning from the Past to Build the Future. Nat. Rev. Clin. Oncol. 2022;19:763–774. doi: 10.1038/s41571-022-00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tutt A.N.J., Garber J., Gelber R.D., Phillips K.-A., Eisen A., Johannsson O.T., Rastogi P., Cui K.Y., Im S.-A., Yerushalmi R., et al. VP1-2022: Pre-Specified Event Driven Analysis of Overall Survival (OS) in the OlympiA Phase III Trial of Adjuvant Olaparib (OL) in Germline BRCA1/2 Mutation (GBRCAm) Associated Breast Cancer. Ann. Oncol. 2022;33:566–568. doi: 10.1016/j.annonc.2022.03.008. [DOI] [Google Scholar]
- 17.Jeruss J.S., Mittendorf E.A., Tucker S.L., Gonzalez-Angulo A.M., Buchholz T.A., Sahin A.A., Cormier J.N., Buzdar A.U., Hortobagyi G.N., Hunt K.K. Combined Use of Clinical and Pathologic Staging Variables to Define Outcomes for Breast Cancer Patients Treated with Neoadjuvant Therapy. J. Clin. Oncol. 2008;26:246–252. doi: 10.1200/JCO.2007.11.5352. [DOI] [PubMed] [Google Scholar]
- 18.Tew W.P., Lacchetti C., Ellis A., Maxian K., Banerjee S., Bookman M., Jones M.B., Lee J.-M., Lheureux S., Liu J.F., et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020;38:3468–3493. doi: 10.1200/JCO.20.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore K.N., Monk B.J. Patient Counseling and Management of Symptoms During Olaparib Therapy for Recurrent Ovarian Cancer. Oncologist. 2016;21:954–963. doi: 10.1634/theoncologist.2015-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madariaga A., Bowering V., Ahrari S., Oza A.M., Lheureux S. Manage Wisely: Poly (ADP-Ribose) Polymerase Inhibitor (PARPi) Treatment and Adverse Events. Int. J. Gynecol. Cancer. 2020;30:903–915. doi: 10.1136/ijgc-2020-001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Care Ontario Olaparib—Patient Monograph. [(accessed on 12 August 2022)]. Available online: https://www.cancercareontario.ca/en/drugformulary/drugs/infosheet/54086.
- 22.Cancer Care Ontario Olaparib—Provider Monograph. [(accessed on 12 August 2022)]. Available online: https://www.cancercareontario.ca/en/drugformulary/drugs/monograph/54086.
- 23.Navitski A., Al-Rawi D.H., Liu Y., Rubinstein M.M., Friedman C.F., Rampal R.K., Mandelker D.L., Cadoo K., O’Cearbhaill R.E. Baseline Risk of Hematologic Malignancy at Initiation of Frontline PARP Inhibitor Maintenance for BRCA1/2-Associated Ovarian Cancer. Gynecol. Oncol. Rep. 2021;38:100873. doi: 10.1016/j.gore.2021.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poveda A., Floquet A., Ledermann J.A., Asher R., Penson R.T., Oza A.M., Korach J., Huzarski T., Pignata S., Friedlander M., et al. Olaparib Tablets as Maintenance Therapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Final Analysis of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2021;22:620–631. doi: 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed] [Google Scholar]
- 25.DiSilvestro P., Banerjee S., Colombo N., Scambia G., Kim B.-G., Oaknin A., Friedlander M., Lisyanskaya A., Floquet A., Leary A., et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2022;41:609–617. doi: 10.1200/JCO.22.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 27.Tung N.M., Zakalik D., Somerfield M.R. Adjuvant PARP Inhibitors in Patients with High-Risk Early-Stage HER2-Negative Breast Cancer and Germline BRCA Mutations: ASCO Hereditary Breast Cancer Guideline Rapid Recommendation Update. J. Clin. Oncol. 2021;39:2959–2961. doi: 10.1200/JCO.21.01532. [DOI] [PubMed] [Google Scholar]
- 28.Burstein H.J., Curigliano G., Thürlimann B., Weber W.P., Poortmans P., Regan M.M., Senn H.J., Winer E.P., Gnant M., Aebi S., et al. Customizing Local and Systemic Therapies for Women with Early Breast Cancer: The St. Gallen International Consensus Guidelines for Treatment of Early Breast Cancer 2021. Ann. Oncol. 2021;32:1216–1235. doi: 10.1016/j.annonc.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Royce M., Osgood C., Mulkey F., Bloomquist E., Pierce W.F., Roy A., Kalavar S., Ghosh S., Philip R., Rizvi F., et al. FDA Approval Summary: Abemaciclib with Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. 2022;40:1155–1162. doi: 10.1200/JCO.21.02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canadian Agency for Drugs and Technologies in Health (CADTH) CADTH Reimbursement Recommendation for Olaparib (Lynparza) Can. J. Health Technol. 2023;3:1–23. [Google Scholar]
- 31.National Comprehensive Cancer Network® NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. 2023. [(accessed on 12 June 2023)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf.
- 32.Cancer Care Ontario Hereditary Cancer Testing Eligibility Criteria: Version 3. 2022. [(accessed on 4 February 2023)]. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/70161.
- 33.George A., Riddell D., Seal S., Talukdar S., Mahamdallie S., Ruark E., Cloke V., Slade I., Kemp Z., Gore M., et al. Implementing Rapid, Robust, Cost-Effective, Patient-Centred, Routine Genetic Testing in Ovarian Cancer Patients. Sci. Rep. 2016;6:29506. doi: 10.1038/srep29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alberta Health Services Mainstreaming Cancer Genetics 2019/2020 Year End Report 2020. [(accessed on 8 December 2022)]. Available online: https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-cancer-mainstreaming-cancer-genetics-2020.pdf.
- 35.Cancer Care Ontario Enhancing Clinical Cancer Genetic Service Delivery in Ontario 2020. [(accessed on 13 March 2023)]. Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/67891.
- 36.BC Cancer Hereditary Cancer Program Hereditary Cancer Program Mainstreaming: Increasing Access to Hereditary Cancer Genetic Testing NA. [(accessed on 9 September 2022)]. Available online: http://www.bccancer.bc.ca/coping-and-support-site/Documents/Hereditary%20Cancer%20Program/HCP_MainstreamingOrientationSlides.pdf.
- 37.Familial Cancer Clinic—Princess Margaret Cancer Centre Learn about Genetic Testing 2018. [(accessed on 23 May 2023)]. Available online: https://www.uhn.ca/PatientsFamilies/Health_Information/Health_Topics/Documents/Learn_about_Genetic_Testing.pdf.
- 38.Ashton-Prolla P., Giacomazzi J., Schmidt A.V., Roth F.L., Palmero E.I., Kalakun L., Aguiar E.S., Moreira S.M., Batassini E., Belo-Reyes V., et al. Development and Validation of a Simple Questionnaire for the Identification of Hereditary Breast Cancer in Primary Care. BMC Cancer. 2009;9:283. doi: 10.1186/1471-2407-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuda N., Lee S.-J., Ohtani S., Im Y.-H., Lee E.-S., Yokota I., Kuroi K., Im S.-A., Park B.-W., Kim S.-B., et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 40.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 41.KEYTRUDA® (Pembrolizumab) Product Monograph 2023. [(accessed on 2 April 2023)]. Available online: pdf.hres.ca/dpd_pm/00070003.PDF.
- 42.XELODA® (Capecitabine) Product Monograph 2021. [(accessed on 2 April 2023)]. Available online: https://pdf.hres.ca/dpd_pm/00060777.PDF.
- 43.Schmid P., Cortes J., Dent R., Pusztai L., McArthur H., Kümmel S., Bergh J., Denkert C., Park Y.H., Hui R., et al. Event-Free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022;386:556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 44.Geyer C.E., Garber J.E., Gelber R.D., Yothers G., Taboada M., Ross L., Rastogi P., Cui K., Arahmani A., Aktan G., et al. Overall Survival in the OlympiA Phase III Trial of Adjuvant Olaparib in Patients with Germline Pathogenic Variants in BRCA1/2 and High-Risk, Early Breast Cancer. Ann. Oncol. 2022;33:1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alberta Health Services Outpatient Cancer Drug Benefit Program 2023. [(accessed on 24 February 2023)]. Available online: https://www.albertahealthservices.ca/assets/programs/ps-1025651-drug-benefit-list.pdf.
- 46.BC Cancer BC Cancer Benefit Drug List 2023. [(accessed on 24 February 2023)]. Available online: http://www.bccancer.bc.ca/systemic-therapy-site/Documents/Policy%20and%20Forms/Benefit%20Drug%20List.pdf.
- 47.Government of New Brunswick New Brunswick Drug Plans Formulary 2023. [(accessed on 24 February 2023)]. Available online: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/NBDrugPlan/NewBrunswickDrugPlansFormulary.pdf.
- 48.Government of Nova Scotia Nova Scotia Formulary 2023. [(accessed on 24 February 2023)]. Available online: https://novascotia.ca/dhw/pharmacare/documents/formulary.pdf.
- 49.Health PEI Health PEI Formulary Drugs: Oncology 2023. [(accessed on 24 February 2023)]. Available online: https://www.princeedwardisland.ca/sites/default/files/publications/oncologyformulary.pdf.
- 50.King’s Printer for Ontario DIN/PIN/NPN Detail—Xeloda (Capecitabine) [(accessed on 24 February 2023)]. Available online: https://www.formulary.health.gov.on.ca/formulary/detail.xhtml?drugId=02238454.
- 51.Manitoba Health Manitoba Drug Benefits Formulary 2023. [(accessed on 24 February 2023)]. Available online: https://residents.gov.mb.ca/file?id=6256968&key=LABEL_FILE_POLICY&index=0.
- 52.Régie de l’assurance maladie du Québec (RAMQ) List of Medications. [(accessed on 24 February 2023)]. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/liste_med_2023-02-01_en.pdf.
- 53.Saskatchewan Cancer Agency Saskatchewan Cancer Agency Drug Formulary 2023. [(accessed on 24 February 2023)]. Available online: http://www.saskcancer.ca/images/pdfs/health_professionals/drug_formulary/drug_formulary/SCA_Drug_Formulary_-_2023-02-01.pdf.
- 54.Canadian Agency for Drugs and Technologies in Health (CADTH) CADTH Reimbursement for Recommendation for Pembrolizumab (Keytruda) Can. J. Health Technol. 2022;2:1–21. [Google Scholar]
- 55.Johnston S.R.D., Harbeck N., Hegg R., Toi M., Martin M., Shao Z.M., Zhang Q.Y., Martinez Rodriguez J.L., Campone M., Hamilton E., et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (MonarchE) J. Clin. Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canadian Agency for Drugs and Technologies in Health (CADTH) CADTH Reimbursement Recommendation Abemaciclib (Verzenio) Can. J. Health Technol. 2022;2:1–16. [Google Scholar]
- 57.VERZENIO® (Abemaciclib) Product Monograph 2022. [(accessed on 4 February 2023)]. Available online: https://pdf.hres.ca/dpd_pm/00064295.PDF.
- 58.VERZENIO® (Abemaciclib) Prescribing Information 2023. [(accessed on 4 April 2023)]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/208716s010s011lbl.pdf.
- 59.Food and Drug Administration (FDA) FDA Expands Early Breast Cancer Indication for Abemaciclib with Endocrine Therapy. [(accessed on 4 April 2023)]. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-early-breast-cancer-indication-abemaciclib-endocrine-therapy.
- 60.Helwick C. MonarchE Update Benefit of Abemaciclib Increases over Time. [(accessed on 4 April 2023)]. Available online: https://ascopost.com/news/december-2022/monarche-update-benefit-of-abemaciclib-increases-over-time/
- 61.Johnston S.R.D., Toi M., O’Shaughnessy J., Rastogi P., Campone M., Neven P., Huang C.-S., Huober J., Jaliffe G.G., Cicin I., et al. Abemaciclib plus Endocrine Therapy for Hormone Receptor-Positive, HER2-Negative, Node-Positive, High-Risk Early Breast Cancer (MonarchE): Results from a Preplanned Interim Analysis of a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2023;24:77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barcellini A., Loap P., Murata K., Villa R., Kirova Y., Okonogi N., Orlandi E. PARP Inhibitors in Combination with Radiotherapy: to Do or Not to Do? Cancers. 2021;13:5380. doi: 10.3390/cancers13215380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howard F.M., Villamar D., He G., Pearson A.T., Nanda R. The Emerging Role of Immune Checkpoint Inhibitors for the Treatment of Breast Cancer. Expert. Opin. Investig. Drugs. 2022;31:531–548. doi: 10.1080/13543784.2022.1986002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saji S., Cussac A.L., Andre F., Robson M.E., Harbeck N., Schmid P., Cescon D.W., Ahn J.S., Nanda R., Fan L., et al. 68TiP KEYLYNK-009: A Phase II/III, Open-Label, Randomized Study of Pembrolizumab (Pembro) + Olaparib (Ola) vs. Pembro + Chemotherapy after Induction with First-Line (1L) Pembro + Chemo in Patients (Pts) with Locally Recurrent Inoperable or Metastatic TNBC. Ann. Oncol. 2020;31:S1268. doi: 10.1016/j.annonc.2020.10.088. [DOI] [Google Scholar]
- 65.Maio M., Shapira-Frommer R., Yap T.A., Ciuleanu T., Gomez H., Hill A., Lugowska I., Ozyilkan O., Vera K., Im S.-A., et al. Abstract CT178: Olaparib plus Pembrolizumab in Patients with Previously Treated Advanced Solid Tumors with Homologous Recombination Repair Mutation (HRRm) and/or Homologous Recombination Deficiency (HRD): Initial Results of the Phase 2 KEYLYNK-007 Study. Cancer Res. 2021;81:CT178. doi: 10.1158/1538-7445.AM2021-CT178. [DOI] [Google Scholar]
- 66.Vinayak S., Tolaney S.M., Schwartzberg L., Mita M., McCann G., Tan A.R., Wahner-Hendrickson A.E., Forero A., Anders C., Wulf G.M., et al. Open-Label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019;5:1132–1140. doi: 10.1001/jamaoncol.2019.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Memorial Sloan Kettering Cancer Center Phase II of Neoadjuvant Olaparib in Combination with Pembrolizumab in Patients with Triple Negative Breast Cancer (TNBC) or Hormone Receptor-Positive HER2-Negative Breast Cancer and Germline Mutations in DNA Damage Repair Genes. 2022. [(accessed on 28 February 2023)]. Available online: https://clinicaltrials.gov.
- 68.Yu E.Y., Park S.H., Goh J.C.H., Shin S.J., Mehra N., McDermott R., Sala Gonzalez M.A., Fong P.C., Greil R., Retz M., et al. 1362MO Pembrolizumab + Olaparib vs. Abiraterone (Abi) or Enzalutamide (Enza) for Patients (Pts) with Previously Treated Metastatic Castration-Resistant Prostate Cancer (MCRPC): Randomized Open-Label Phase III KEYLYNK-010 Study. Ann. Oncol. 2022;33:S1163–S1164. doi: 10.1016/j.annonc.2022.07.1494. [DOI] [Google Scholar]
- 69.Klaassen Z. ESMO 2022: Pembrolizumab + Olaparib Versus Abiraterone or Enzalutamide for Patients with Previously Treated MCRPC: Randomized Open-Label Phase 3 KEYLYNK-010 Study. [(accessed on 1 March 2023)]. Available online: https://www.urotoday.com/conference-highlights/esmo-2022/esmo-2022-prostate-cancer/139443-esmo-2022-pembrolizumab-olaparib-versus-abiraterone-or-enzalutamide-for-patients-with-previously-treated-mcrpc-randomized-open-label-phase-3-keylynk-010-study.html.
- 70.Yu E.Y., Piulats J.M., Gravis G., Fong P.C.C., Todenhöfer T., Laguerre B., Arranz J.A., Oudard S., Massard C., Heinzelbecker J., et al. Pembrolizumab plus Olaparib in Patients with Metastatic Castration-Resistant Prostate Cancer: Long-Term Results from the Phase 1b/2 KEYNOTE-365 Cohort A Study. Eur. Urol. 2023;83:15–26. doi: 10.1016/j.eururo.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Coleman R.L., Fujiwara K., Sehouli J., Salutari V., Zola P., Madry R., Korach J., Pautier P., Cibula D., Lheureux S., et al. ENGOT-Ov43/Keylynk-001: A Phase III, Placebo- and Active-Controlled Trial of Pembrolizumab plus Chemotherapy with Olaparib Maintenance for First-Line Treatment of Advanced BRCA-Nonmutated Epithelial Ovarian Cancer. Gynecol. Oncol. 2020;159:89–90. doi: 10.1016/j.ygyno.2020.05.070. [DOI] [Google Scholar]
- 72.Jabbour S.K., Cho B.C., Bria E., Kato T., Bhosle J., Gainor J.F., Reguart N., Wang L., Morgensztern D., Shentu Y., et al. Rationale and Design of the Phase III KEYLYNK-012 Study of Pembrolizumab and Concurrent Chemoradiotherapy Followed by Pembrolizumab with or without Olaparib for Stage III Non-Small-Cell Lung Cancer. Clin. Lung Cancer. 2022;23:e342–e346. doi: 10.1016/j.cllc.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rimner A., Lai W.-C.V., Califano R., Jabbour S.K., Rudin C.M., Faivre-Finn C., Cho B.C., Kato T., Yu J., Chafin W., et al. Rationale and Design of the Phase 3 KEYLYNK-013 Study of Pembrolizumab with Concurrent Chemoradiotherapy Followed by Pembrolizumab with or without Olaparib for Limited-Stage Small-Cell Lung Cancer. Clin. Lung Cancer. 2022;23:e325–e329. doi: 10.1016/j.cllc.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Library of Medicine NCT04633239 Testing the Addition of Abemaciclib to Olaparib for Women with Recurrent Ovarian Cancer. [(accessed on 2 March 2023)]. Available online: https://clinicaltrials.gov/ct2/show/NCT04633239.
- 75.Slamon D.J., Stroyakovskiy D., Yardley D.A., Huang C.-S., Fasching P.A., Crown J., Bardia A., Chia S., Im S.-A., Martin M., et al. Ribociclib and Endocrine Therapy as Adjuvant Treatment in Patients with HR+/HER2− Early Breast Cancer: Primary Results from the Phase III NATALEE Trial. J. Clin. Oncol. 2023;41:LBA500. doi: 10.1200/JCO.2023.41.17_suppl.LBA500. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.