Abstract
The effect of selected cytokines on the antifungal activity of human microglia was studied with encapsulated and acapsular strains of Cryptococcus neoformans. None of the cytokines tested increased the fungistatic activity of microglia, suggesting that killing of cryptococci within the central nervous system is dependent on other host defense mechanisms.
Cryptococcus neoformans is the leading mycologic cause of central nervous system (CNS) disease, especially in patients with suppressed cell-mediated immunity. Macrophages are considered the first line of defense against this pathogen. Within the CNS, microglial cells are regarded as resident macrophages. Recent in vitro studies have demonstrated that human microglia can readily phagocytize opsonized cryptococci (11, 14). However, it is unclear whether microglial cells can also kill ingested cryptococci. Lee et al. (12) demonstrated that nonactivated human microglial cells can temporarily inhibit the growth of ingested cryptococci but that eventually the cryptococci outgrow the microglia and cause cell lysis. Blasi et al. (1) demonstrated that gamma interferon (IFN-γ) enhances anticryptococcal activity of murine microglial cells. Moreover, Hill and Aguirre (9) demonstrated that mice can clear cerebral foci of C. neoformans provided that they have functional CD4+ T cells. Therefore, we hypothesized that human microglial cells need to be activated with cytokines, especially the T-helper 1 cytokine IFN-γ, to be able to kill ingested cryptococci.
To test this hypothesis, human fetal microglial cells were obtained from brain tissues of 16- to 22-week-old aborted fetuses, by previously described techniques (17). Greater than 99% of cells stained positively with anti-CD68, a marker for human macrophages, and <1% stained with antibodies to the astrocyte marker glial fibrillary acid protein (Dako, Carpinteria, Calif.). Cell viability, assessed by trypan dye exclusion, was >98%. Microglia were added to 48-well plates (5 × 104 cells/well) in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, Mo.), containing 10% bovine serum albumin. Microglial cells were treated for 24 h with IFN-γ (200 U/ml, based on previous studies [6] and the literature [1]); granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/ml; R&D Systems, Minneapolis, Minn.), which is known to activate macrophages and enhances antifungal activity in this concentration range (18); a combination of both cytokines; or culture medium alone. The thinly (<0.5-μm) encapsulated C. neoformans strain NIH 37 (serotype A) and the acapsular C. neoformans strain NIH 3413 (National Institutes of Health, Bethesda, Md.) were kept on Sabouraud dextrose agar slants (Merck, Darmstadt, Germany) at 4°C. Cryptococci were plated at 30°C and harvested after 2 to 4 days, washed in Hanks’ balanced salt solution, and opsonized with 10% normal human pooled serum, which has been shown to facilitate microglial cell ingestion of cryptococci via complement receptors (14). After the opsonized yeasts were washed in phosphate-buffered saline (Sigma), cryptococci were added to the microglia at an effector-to-target ratio of 50:1, based on our studies with monocytes (2) and studies by Lee et al. (11, 12) and Miller and Mitchell (15). The cytokines were added again, and after 24 h of incubation at 37°C in a humidified 5% CO2 incubator, supernatants were harvested. To lyse microglial cells harboring cryptococci, 0.8 ml of sterile water was added twice to tissue culture wells and to the culture supernatants. The bottoms of the wells were then carefully scraped to make sure that the cryptococci did not stick to the plate. Samples were plated in triplicate in serial dilutions on Sabouraud’s agar, and CFU were counted after 48 h of incubation at 30°C. As a control, the original inoculum of cryptococci (t = 0) and yeast cells cultured for 24 h at 37°C in Dulbecco’s modified Eagle’s medium without microglia (t = 24 h) underwent the same handling procedure. Incubation of cryptococci without microglia with cytokines did not affect cryptococcal growth (data not shown). All experiments were performed in triplicate and were repeated with microglial cells from the indicated numbers of different brain specimens. Data are expressed as percent growth relative to the control culture of cryptococci, according to the formula (CFUexp − CFUt = 0)/(CFUt = 24 − CFUt = 0) × 100%, i.e., cryptococcal growth unrestricted by microglial cells at 24 h equals 100%. Killing by microglial cells is defined as cryptococcal growth at 24 h (CFUexp) that is less than the original inoculum (CFUt = 0). Growth inhibition is defined as a decrease in cryptococcal growth relative to the control at 24 h (CFUt = 24) but exceeding the original inoculum, i.e., growth ranging between 0 and 100%. Differences between experimental values and the 24-h control value (in CFU) were analyzed by the paired, two-tailed Student t test.
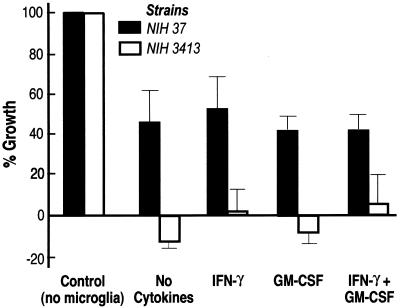
As shown in Fig. 1, microglia significantly (P ≤ 0.05) inhibited the growth of both encapsulated and acapsular cryptococci. However, there was no significant difference between growth inhibition by microglial cells that had been pretreated with cytokines and that by untreated microglia. When acapsular and encapsulated cryptococci were compared, there was greater (P < 0.05) inhibition of growth of the acapsular strain by microglia (Fig. 1). Although microglial cells totally suppressed the growth of the acapsular strain, there was no killing of acapsular cryptococci, i.e., the difference between experimental values and the original inoculum was not statistically significant. Extension of the duration of cytokine treatment of microglia from 24 to 72 h, variation of the GM-CSF concentration, or the addition of lipopolysaccharide (1 μg/ml) to IFN-γ (1, 5, 7) did not increase the growth inhibition of encapsulated cryptococci (data not shown). Staining of cryptococci with Uvitex (14) revealed that all cryptococci were ingested by microglia after 24 h.
FIG. 1.
Growth inhibition of encapsulated (NIH 37) and acapsular (NIH 3413) C. neoformans by human microglia. Growth inhibition is expressed as percent growth relative to cryptococcal growth at 24 h in cultures that did not contain microglia (control). Microglia were either not treated with cytokines or stimulated with IFN-γ (200 U/ml), GM-CSF (10 ng/ml), or a combination of both cytokines for 24 h prior to constitution of phagocytosis mixtures. Data represent means ± standard errors of the means of five (NIH 37) and three (NIH 3413) separate experiments with cells from different brain tissue specimens.
The inability of human microglia to kill ingested cryptococci, even after stimulation with macrophage-activating cytokines, contrasts with the findings for murine microglial cells (1). Similar observations have been reported for intracellular killing of Toxoplasma gondii by human versus murine microglial cells (3, 5). Although IFN-γ-activated murine microglial cells readily kill T. gondii (4), activation of human microglial cells with IFN-γ has no effect on intracellular survival of this parasite (5). The explanation of this animal species difference in killing of intracellular pathogens appears to be related to relatively inefficient generation of the microbicidal free radical nitric oxide by cytokine-activated human microglia compared to that by murine microglia (5, 17).
Individuals with intact cell-mediated immunity rarely contract meningitis due to C. neoformans var. neoformans. Studies analyzing the role of T-cell subsets in pulmonary murine cryptococcal infections suggest that CD4+ T cells are needed to prevent cryptococci from systemically spreading, whereas CD8+ T cells (and to a lesser extent CD4+ T cells) are essential in eliminating local infection, at least partly through cytotoxic lysis of infected macrophages (10, 16). Histologic examination of both mice (8) and humans (13) with cryptococcosis has revealed that, when T-cell function is intact, granulomata and multinucleated giant cells are observed in affected organs (including the brain), with reduced cryptococcal growth compared to that in animals and humans with impaired T-cell function. Taken together, our data suggest that human microglia are unable independently to eliminate cryptococci from the brain and support the notion that T cells play a neuroprotective role against this opportunist. Also, the finding that IFN-γ did not stimulate fungicidal activity of human microglia suggests that the contribution of T cells to defense of the CNS against C. neoformans is not simply related to their ability to produce this cytokine.
Acknowledgments
We thank Al Pheley for his advice on statistical analysis.
This study was supported by National Institutes of Health grant DA-04381.
REFERENCES
- 1.Blasi E, Barluzzi R, Mazolla R, Tancini B, Saleppico S, Puliti M, Pitzurra L, Bistoni F. Role of nitric oxide and melanogenesis in the accomplishment of anticryptococcal activity by the BV-2 microglial cell line. J Neuroimmunol. 1995;58:111–116. doi: 10.1016/0165-5728(95)00016-u. [DOI] [PubMed] [Google Scholar]
- 2.Chaka E S, Scharringa J, Verheul A F M, Verhoef J, van Strijp J A G, Hoepelman J M. Quantitative analysis of phagocytosis and killing of Cryptococcus neoformans by human peripheral blood mononuclear cells using flow cytometry. Clin Diagn Lab Immunol. 1995;2:753–759. doi: 10.1128/cdli.2.6.753-759.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao C C, Hu S, Gekker G, Novick W J, Jr, Remingotn J S, Peterson P K. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. J Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 4.Chao C C, Anderson W R, Hu S, Martella A, Gekker G, Peterson P K. Activated microglia inhibit Toxoplasma gondii via a nitric oxide mechanism. Clin Immunol Immunopathol. 1993;67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 5.Chao C C, Gekker G, Hu S, Peterson P K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 6.Chao C C, Hu S, Peterson P K. Modulation of human microglial cell superoxide production by cytokines. J Leukocyte Biol. 1995;58:65–70. doi: 10.1002/jlb.58.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Flesch I E A, Schwamberger G, Kaufmann S H E. Fungicidal activity of IFN-γ activated macrophages: extracellular killing of Cryptococcus neoformans. J Immunol. 1989;142:3219–3224. [PubMed] [Google Scholar]
- 8.Hill J O. CD4+ T cells cause multinucleated giant cells to form around C. neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992;175:1685–1695. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill J O, Aguirre K M. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–2340. [PubMed] [Google Scholar]
- 10.Hill J O, Harmsen A G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. J Exp Med. 1991;173:755–758. doi: 10.1084/jem.173.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S C, Kress Y, Dickson D W, Casadevall A. Human microglia mediate anti-Cryptococcus neoformans activity in the presence of specific antibody. J Neuroimmunol. 1995;62:43–52. doi: 10.1016/0165-5728(95)00097-l. [DOI] [PubMed] [Google Scholar]
- 12.Lee S C, Kress Y, Zhao M-L, Dickson D W, Casadevall A. Cryptococcus neoformans survive and replicate in human microglia. Lab Invest. 1995;73:871–879. [PubMed] [Google Scholar]
- 13.Lee S C, Dickson D W, Casadevall A. Pathology of cryptococcal meningo-encephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 14.Lipovsky, M. M., G. Gekker, H. Shuxian, A. I. M. Hoepelman, and P. K. Peterson. Morphine enhances complement receptor-mediated phagocytosis of Cryptococcus neoformans by human microglia. Clin. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 15.Miller M F, Mitchell T G. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mody C H, Chen G H, Jackson C, Curtis J L, Toews G B. Depletion of murine CD8+ T cells in vivo decreases pulmonary clearance of a moderately virulent strain of C. neoformans. J Lab Clin Med. 1993;121:765–773. [PubMed] [Google Scholar]
- 17.Peterson P K, Hu S, Anderson R W, Chao C C. Nitric oxide production and neurotoxicity mediated by activated microglia from human versus mouse brain. J Infect Dis. 1995;170:457–460. doi: 10.1093/infdis/170.2.457. [DOI] [PubMed] [Google Scholar]
- 18.Smith P D, Lamerson C L, Banks S M, Saini S S, Wahl L M, Calderone R A, Wahl S M. Granulocyte-macrophage colony stimulating factor augments human monocyte fungicidal activity for Candida albicans. J Infect Dis. 1990;161:999–1005. doi: 10.1093/infdis/161.5.999. [DOI] [PubMed] [Google Scholar]