Abstract
An immunohistochemistry method using formalin-fixed tissues, a direct immunofluorescence method using cryostat sections, an enzyme-linked immunosorbent assay (ELISA), and a PCR method were compared for diagnosis in a litter of weaned pigs that had been experimentally inoculated with wild-type porcine epidemic diarrhea virus (PEDV) and killed between 6 and 60 h after onset of diarrhea. The immunohistochemistry method proved to be as reliable as direct immunofluorescence for diagnosis of PEDV in tissues collected postmortem. The good reliability of ELISA for investigating clinical samples was confirmed, whereas the PCR method used was ineffective.
Porcine epidemic diarrhea virus (PEDV) is a widespread coronavirus causing enteric disease in swine (9). Based on their good reliability and sensitivity, direct immunofluorescence (IF) using cryostat sections of gut tissue and enzyme-linked immunosorbent assay (ELISA) of fecal material are the most commonly used detection methods at present (3, 4, 7, 9). However, additional methods have been recently developed with potential for use in PEDV diagnostics, in particular, an immunohistochemistry method (IHC) using formalin-fixed, paraffin-embedded tissue (2) and a polymerase chain reaction (PCR) using feces (8, 10). No comparative data are available regarding the reliability of these four methods. The aim of this study was to assess the suitability of IHC for use with formalin-fixed paraffin-embedded gut tissues for diagnosis of PEDV infection in comparison with IF, ELISA, and PCR. The tests were performed on specimens obtained from an experiment designed to simulate as closely as possible the natural disease. Specimens were sampled taking into consideration that IHC and IF are postmortem methods whereas ELISA and PCR are used in clinical practice mostly for investigation of fecal specimens from living pigs.
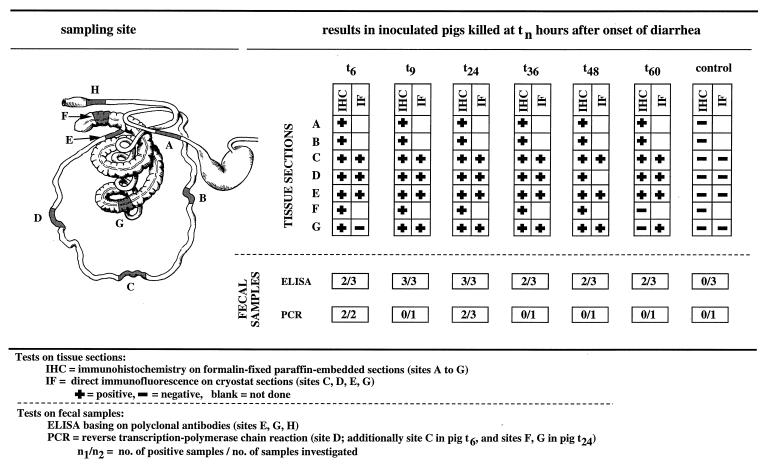
A litter of seven healthy, 5-week-old, weaned pigs born from a sow serologically negative for PEDV was purchased from a conventional farm known to be free of relevant diseases. The pigs were housed together on a flat-deck unit. One uninoculated control pig was euthanized at the start of the experiment. The remaining six animals were inoculated per os with 1 ml of wild-type PEDV (isolate CV777) stock diluted in 4 ml of phosphate-buffered saline. The virus stock consisted of a bacterium-free filtrate of small-intestine homogenate with contents derived from a 1-day-old specific-pathogen-free piglet inoculated as previously described (6). The animals were observed at regular intervals for signs of diarrhea. After an incubation period of 39 h, all virus-inoculated pigs had watery feces. All animals were euthanized by electrocution followed by exsanguination, except for pig t9, which died as a result of concomitant fibrinous polyserositis, polyarthritis, and meningitis due to Streptococcus suis. The animals were designated pigs t6, t9, t24, t36, t48, and t60 according to the time of death in hours after onset of diarrhea. The intestines were removed as soon as possible after the death of the pigs; sampling sites were designated A to G as shown in Fig. 1. Specimens from each animal were collected and processed as indicated below.
FIG. 1.
Performance of different tests for detection of PEDV.
IHC.
Tissues from sites A to G were fixed in formalin for 48 h and embedded in paraffin wax by standard methods. IHC detection of PEDV antigen was performed by using monoclonal antibody mcAb204, which was previously shown to be specific for M protein of PEDV (11). The reaction product was visualized by using a streptavidin-horseradish peroxidase immunogold-silver method as previously described (2). A duplicate of each section was incubated with phosphate-buffered saline instead of the primary antibody as a negative control. The amount of viral antigen in each site in the small intestine was determined, evaluating the approximate percentage of positive villi and villous enterocytes as described elsewhere (1). All tissues were also examined histopathologically by using sections stained with hematoxylin and eosin.
IF.
Tissue specimens from sites C, D, E, and G were embedded in Methocel and frozen in a CO2-alcohol bath. Cryostat sections were cut, and a direct IF test using a porcine PEDV hyperimmune serum as previously described (7) was employed.
ELISA.
Intestinal contents were sampled from sites E, G, and H, frozen in liquid nitrogen, stored at −70°C, and investigated by using an antigen detection ELISA based on polyclonal antibodies against PEDV as previously described (3).
RT-PCR.
Tissue samples from sites C, D, F, and G were frozen in liquid nitrogen and stored at −70°C until investigation with a reverse transcription (RT)-PCR protocol described previously (10). Briefly, RNA was extracted in 4 M guanidinium thiocyanate and then in phenol and chloroform and reverse transcription was accomplished with a specific antisense primer (AACAAAGCCTGCCAATAAG) from the genetic region coding for open reading frame 3. The cDNA obtained was then amplified by PCR using the same primer and a sense primer (GTTAGCTCTTTTTCTAGACC) representing a part of the leader sequence of PEDV. The amplification products within the expected size of 394 bp were analyzed on 1.5% agarose gels.
Experimental infection of conventionally reared weaned pigs with wild-type PEDV resulted clinically in watery diarrhea and histopathologically in villous atrophy and extensive vacuolation and flattening of villous enterocytes, thus mimicking the naturally occurring disease and results of experiments previously described (5, 9). The results of all tests for PEDV performed on intestinal specimens collected at necropsy are summarized in Fig. 1. They indicate that large amounts of virus were present in the gut throughout the time period examined (from 6 to 60 h after onset of diarrhea), which therefore appears to be ideally suited for sampling. ELISA allowed diagnosis in each infected pig, but the presence of occasional negative results stressed the need to test several specimens or specimens from several diseased animals in clinical practice. PCR often gave negative results in cases where other tests indicated the presence of virus; therefore, we discontinued the use of this test on all samples available and PCR results were not included in the evaluation of the data. A technical problem was apparently rendering the PCR ineffective under the present protocol, but this method may be improved by using a virus purification and concentration step before extraction of RNA and RT-PCR (10).
In contrast to the tests using fecal material, the antigen detection methods using gut tissues (IHC and IF) showed a very high sensitivity, allowing a diagnosis for almost every sample investigated. In correspondence with previous reports, large amounts of antigen were detected in the small intestine throughout the experiment, in particular, in the mid-jejunum (site C), where more than 75% of the villi and of the villous enterocytes were found positive for virus antigen by IHC. The remaining parts of the small intestine (sites A, B, D, and E) yielded comparably large amounts of virus antigen between 9 and 36 h after onset of diarrhea. In contrast to this, negative results in the duodenum have been reported (12, 13). The number of positive superficial epithelial cells was very low in the large intestine (sites F, G, and H), which is in agreement with previous reports (7, 12, 13). A direct IF test using cryostat sections, which must be performed on samples taken from euthanized animals to prevent autolytic change of the mucosa, has been reported as the most sensitive and reliable method for detection of PEDV (9). However, formaldehyde fixation simplifies handling of tissue specimens for practitioners and allows improvement of the quality of the sections.
In conclusion, among postmortem detection methods, IHC using formalin-fixed tissue and IF using cryostat sections are equivalent in reliability, but IHC is more practical. Moreover, histopathology allows a discriminating evaluation of the causal role of the virus detected on the basis of the severity and type of lesions observed. Our results confirm the good reliability of ELISA for investigating fecal samples for PEDV.
Acknowledgments
This study was supported by the Swiss Federal Veterinary Services (grants 012.93.7 and 012.91.7).
The skillful technical assistance of the laboratory technical staff of the Institute of Veterinary Pathology, Zurich University, is greatly appreciated.
REFERENCES
- 1.Bernasconi C. Dissertation thesis. Zurich, Switzerland: University of Zurich; 1996. [Google Scholar]
- 2.Bernasconi C, Guscetti F, Utiger A, Van Reeth K, Ackermann M, Pospischil A. Experimental infection of gnotobiotic piglets with a cell culture adapted porcine epidemic diarrhoea virus: clinical, histopathological, and immunohistochemical findings. In: Schwyzer M, Ackermann M, Bertoni G, Kocherhans R, McCullogh K, Engels M, Wittek R, Zanoni R, editors. Immunobiology of viral infections. Proceedings of the Third Congress of the European Society for Veterinary Virology. 1995. pp. 542–546. [Google Scholar]
- 3.Callebaut P, Debouck P, Pensaert M. Enzyme-linked immunosorbent assay for the detection of the coronavirus-like agent and its antibodies in pigs with porcine epidemic diarrhea. Vet Microbiol. 1982;7:295–306. doi: 10.1016/0378-1135(82)90009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvajal A, Lanza I, Diego R, Rubio P, Carmenes P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J Vet Diagn Invest. 1995;7:60–64. doi: 10.1177/104063879500700109. [DOI] [PubMed] [Google Scholar]
- 5.Coussement W, Ducatelle R, Debouck P, Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. I. Histological and histochemical study. Vet Pathol. 1982;19:46–56. doi: 10.1177/030098588201900108. [DOI] [PubMed] [Google Scholar]
- 6.Debouck P, Pensaert M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am J Vet Res. 1980;41:219–223. [PubMed] [Google Scholar]
- 7.Debouck P, Pensaert M, Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV 777. Vet Microbiol. 1981;6:157–165. [Google Scholar]
- 8.Kweon C-H, Lee J-G, Han M-G, Kang Y-B. Rapid diagnosis of porcine epidemic diarrhea virus infection by polymerase chain reaction. J Vet Med Sci. 1997;59:231–232. doi: 10.1292/jvms.59.231. [DOI] [PubMed] [Google Scholar]
- 9.Pensaert M B. Porcine epidemic diarrhea. In: Leman A D, Straw B E, Mengeling W L, D’Allaire S, Taylor D J, editors. Diseases of swine. 7th ed. Ames: Iowa State University Press; 1992. pp. 293–298. [Google Scholar]
- 10.Tobler K, Kocherhans R, Ackermann M. Detection of porcine epidemic diarrhoea coronavirus by RT-PCR. In: Becker Y, Darai G, editors. PCR: protocols for diagnosis of human and animal virus diseases. Berlin, Germany: Springer-Verlag; 1995. pp. 483–490. [Google Scholar]
- 11.Utiger A, Rosskopf M, Guscetti F, Ackermann M. Preliminary characterization of a monoclonal antibody specific for a viral 27kD glycoprotein family synthesized in porcine epidemic diarrhoea virus infected cells. Adv Exp Med Biol. 1994;342:197–202. doi: 10.1007/978-1-4615-2996-5_31. [DOI] [PubMed] [Google Scholar]
- 12.van Nieuwstadt A P, Zetstra T. Use of two enzyme-linked immunosorbent assays to monitor antibody responses in swine with experimentally induced infection with porcine epidemic diarrhea virus. Am J Vet Res. 1991;52:1044–1050. [PubMed] [Google Scholar]
- 13.Witte K H, Prager D, Ernst H, Nienhoff H. Die Epizootische Virusdiarrhoe (EVD) Tieraerztl Umsch. 1981;36:235–250. [Google Scholar]