Abstract
Urogenital isolates (n = 93) of Chlamydia trachomatis were differentiated into serovars and variants by serotyping with monoclonal antibodies and genotyping by restriction fragment length polymorphism (RFLP) analysis of the PCR-amplified omp1 gene, respectively. The types of 87 of the 93 isolates (94%) were identical, as determined by both methods. Among these 87 isolates, 3 isolates were identified as the recently described new serovariant Ga/IOL-238 by omp1 nucleotide sequence analysis of the variable domains. Of the remaining six isolates, three isolates serotyped as both L2 and Ba but were identified as Ba/A-7 by genotyping by RFLP analysis of omp1. The omp1 nucleotide sequences of variable domains VD1, VD2, and VD4 of these urogenital Ba strains were identical to the sequences of the variable domains of Ba/J160, an ocular Ba type. The three remaining isolates were serotyped as J, but the patterns obtained by RFLP analysis of omp1, which were identical for the three isolates, differed from that of prototype serovar J/UW36. omp1 nucleotide sequence analysis revealed that these strains are genovariants of serovar J/UW36. Nucleotide sequence differences between serovar J/UW36 and this J genovariant, designated Jv, were found in both variable and constant domains. In conclusion, this study shows that the PCR-based genotyping of clinical C. trachomatis isolates by RFLP analysis of omp1 has a higher discriminatory power and is more convenient than serotyping. Variants of C. trachomatis serovars Ba, G, and J were identified and characterized.
Chlamydia trachomatis is the most common bacterial sexually transmitted disease (STD) and is currently classified into 15 serovars: A, B, Ba (AP-2), C, D, E, F, G, H, I, J, K, L1, L2, and L3. This classification is based on immunoepitope analysis of the major outer membrane protein (MOMP) with polyclonal and monoclonal antibodies (MAbs) (11, 16). The MOMP is the immunodominant antigen of C. trachomatis and contains four variable domains (VDs) that are flanked and interspaced by five constant domains (CDs). Three of the variable domains (VD1, VD2, and VD4) are surface exposed and contain antigenic epitopes (21). Differences in reactivities with MAbs and polyclonal antibodies have led to the identification of a large number of C. trachomatis serovariants: Ba (UW113, J104, J160, TW439, U/CT77), Da (TW448, MT199), D−(NL32 6, TB39, MT157, RB205), D* (MTS2, ICD033), Ga (IOL238), Ia (NL1540, MT165), I− (MT518, MT741, MT1196), and L2a (UW396) (3, 4, 12, 16, 23). Characterization of the nucleotide sequences of the omp1 genes of these serovariants (except Ga) demonstrates that almost all nucleotide sequence differences result in amino acid substitutions (2, 3). A single amino acid substitution may lead to different reactivities of the MAbs (1, 2). In addition to these reported serovariants, a much larger group of genovariants (up to 30% of clinical isolates [24]) has been described on the basis of analysis of the omp1 gene either by restriction fragment length polymorphism (RFLP) analysis (19, 20) or nucleotide sequence analysis (5, 7, 13, 24).
In order to study the epidemiology of C. trachomatis infections, laboratory techniques for differentiating C. trachomatis serovars and variants have recently been developed (6, 18–20). These techniques include standard MOMP serotyping, RFLP analysis of the PCR-amplified omp1 gene, and nucleotide sequencing of the omp1 gene (10). The need for multiple passages in cell culture and a large panel of MAbs are major drawbacks of MOMP serotyping. Nucleotide sequencing of the omp1 gene, which provides definite typing results, is still very laborious and not suitable for typing the isolates from a large number of clinical samples. Alternatively, typing by RFLP analysis of the omp1 gene is a simple, rapid, and powerful tool in epidemiology studies (6, 14, 15, 19, 20). This method enables the successful differentiation of not only all known serovars and serovariants (15) but also genovariants, such as Ba/A-7 and Dv (19, 20). An additional advantage of this method is its applicability to typing the C. trachomatis isolates in clinical specimens after direct amplification of the omp1 gene by PCR, without prior cell culture and DNA extraction (14, 15). In this study we evaluated whether genotyping by RFLP analysis of omp1 reveals more variants than conventional serotyping for the identification of C. trachomatis serovars and variants in a group of clinical isolates obtained from the urogenital tracts of patients attending an STD clinic in Amsterdam, The Netherlands. Furthermore, variants obtained by either serotyping or genotyping were further analyzed by DNA sequencing of the omp1 gene to identify point mutations resulting in amino acid substitutions or the loss or gain of restriction enzyme recognition sites.
MATERIALS AND METHODS
Clinical isolates.
Ninety-three C. trachomatis strains were isolated from urogenital tract samples obtained from male and female patients attending an STD clinic in Amsterdam between 1985 and 1990. Serial passages in HeLa 229 cell culture were performed until at least 75% of the cells were infected, as determined with a direct fluorescent-antibody assay (MicroTrak; Syva). The isolates were stored at −80°C until use.
Serotyping.
The clinical isolates (enriched by passage in cell culture) were serotyped by using MAbs in a dot enzyme immunoassay as described in detail elsewhere (16). Briefly, sheets of grided nitrocellulose (Schleicher and Schuell, Dassel, Germany) were cut into pieces of 8 by 12 cm2, fixed on an inert support (such as used X-ray film), and spotted with antigens. Hybridoma culture supernatants diluted 1:4 in phosphate-buffered saline (PBS; pH 7.2) containing 1% bovine serum albumin (Organon Teknika, Boxtel, The Netherlands) were incubated for 2 h at room temperature on a shaker. After vigorous washing with PBS with 0.05% Tween 20 for 30 min on a shaker, the sheets were incubated with rabbit anti-mouse peroxidase-labeled conjugate (Dako, Glostrup, Denmark). Subsequently, the sheets were vigorously washed with PBS with 0.05% Tween 20 for 30 min on a shaker, followed by washing with PBS. Finally, the sheets were incubated with substrate 4-chloro-1-naphthol (Sigma) for 30 min, washed with tap water, and air dried. Immunoglobulin G MAbs to the chlamydial lipopolysaccharide (16) were included as a control to quantify the amount of spotted antigen. The final color reaction was positive when a gray or black spot was clearly visible.
Genotyping by RFLP analysis.
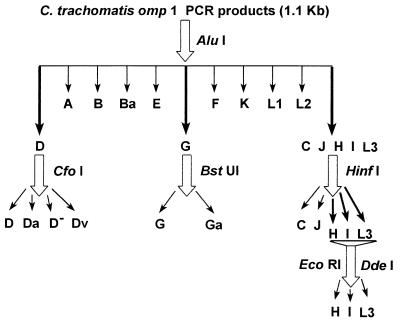
The chlamydial omp1 gene was amplified by PCR as described previously (14, 15). The primers used for generating an approximately 1.1-kb fragment of the omp1 gene were SERO1A and SERO2A (6) (Table 1). In brief, 250 μl of resuspended cell culture, corresponding to one-eighth of a monolayer of a shell vial (diameter, 1 cm), was pelleted, and subsequently, a proteinase K treatment was performed. One microliter of this proteinase K lysate was resuspended in 9 μl of distilled water, and the mixture was boiled for 10 min and chilled on ice. The PCR mixture (final volume, 50 μl) contained 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3), 200 μM (each) deoxynucleotide triphosphate (dATP, dTTP, dGTP, and dCTP), 50 pmol of each primer, and 1 U of Taq polymerase (Amplitaq; Roche Molecular Systems, Branchburg, N.Y.). The reaction mixture was overlaid with a few drops of liquid paraffin to prevent evaporation. The PCR amplification was carried out in a thermocycler (Biomed, Theres, Germany) starting with 6 min of denaturation at 95°C and continuing for 49 cycles of amplification. Each cycle consisted of denaturation at 95°C for 1 min, annealing at 45°C for 2 min, and chain elongation at 72°C for 3 min. All enzymes except BstUI were purchased from Boehringer Mannheim, Almere, The Netherlands; BstUI was purchased from New England Biolabs, Leusden, The Netherlands. For genotyping by RFLP analysis (Fig. 1), the omp1 PCR products were digested with AluI and were electrophoresed through a 7% polyacrylamide gel (acrylamide/bisacrylamide, 29/1) to differentiate serovars B, Ba, D, E, F, G, K, and the C complex (C, J, H, I, and L3). Serovars belonging to the C complex were further typed by RFLP analysis after digestion with HinfI and the combination of EcoRI and DdeI. Serovar G was further differentiated into G and Ga by RFLP analysis after digestion with BstUI (16). Serovar D isolates were subdifferentiated into D, Da, D−, or Dv after digestion with CfoI. C. trachomatis types were identified according to the RFLP patterns of the prototype strains as described elsewhere (15) (Fig. 1). In some cases the specificities of the fragments obtained by RFLP analysis were confirmed by Southern blot hybridization with a probe of the omp1 PCR product randomly labeled with [α-32P]dCTP-l as described previously (15).
TABLE 1.
Primers used for omp1-based PCR or DNA sequencing of the omp1 gene
| Primer | Strand | Nucleotide sequence | Positiona |
|---|---|---|---|
| OMP1 | Sense | 5′-TGACGCTATCAGCATGCG-3′ | 165–182 |
| CM3A | Antisense | 5′-GAATACATCAAAACGATCCC-3′ | 389–408 |
| OMP11 | Sense | 5′-GGAAACTCCGCTTCCTTCAAC-3′ | 448–468 |
| OMP6AS | Antisense | 5′-TGAGCGTATTGGAAAGAAGC-3′ | 640–659 |
| OMP6S | Sense | 5′-TCTTTCCAATACGCTCAATC-3′ | 643–662 |
| SERO2A | Antisense | 5′-TTTCTAGA(T/C)TTCAT(T/C)TTGTT-3′ | 1057–1076 |
| SERO1A | Sense | 5′-ATGAAAAAACTCTTGAAATCGG-3′ | 1–22 |
| OMP10 | Antisense | 5′-TCTTGCATGTGTTTGCCATAAGCGG-3′ | 350–326 |
| OMP12 | Antisense | 5′-CAATAGAGGCATCCTTAGTCCC-3′ | 508–487 |
| VD41 | Sense | 5′-TACATTGGAGTTAAATGGTCT-3′ | 868–888 |
According to the omp1 nucleotide sequence of C. trachomatis serovar strain J/UW36 (25).
FIG. 1.
Schematic presentation of the PCR-based strategy for the differentiation of C. trachomatis serovars and variants by genotyping by RFLP analysis of omp1.
Automated DNA sequencing.
The omp1 nucleotide sequences of the VDs of variants Ga, Ba, and J as determined by serotyping or genotyping by RFLP analysis of omp1 were analyzed by automated DNA sequencing. The omp1 nucleotide sequence of CDs from J variants were also analyzed. To determine the sequence of each VD region the following primer sets were used: OMP1-CM3A (VD1), OMP11-OMP6AS (VD2), OMP6S-SERO2A (VD3), and VD41-SERO2A (VD4) (see Table 1 for the nucleotide sequences). In addition, the omp1 nucleotide sequence of each CD region from the J variants was also analyzed by using the following primer pairs: SERO1A-OMP10 (CD1), OMP1-OMP6AS (CD2), OMP11-OMP12 (CD3), OMP6S-SERO2A (CD4), and VD41-SERO2A (CD5) (see Table 1 for the nucleotide sequences). The template DNA was prepared as follows. The omp1 PCR products (±1.1 kb) were separated through 1% agarose (NuSieve; FMC Biozym, Rockland, Maine) by agarose gel electrophoresis and were subsequently purified by using a QIAquick-spin PCR purification kit (Qiagen, Düsseldorf, Germany). The DNA was eluted in 10 mM Tris-HCl (pH 8.3). The purified template DNA (0.5 to 1 μg) was labeled with the PRISM ready reaction terminator kit (Perkin-Elmer/Applied Biosystems, Foster City, Calif.) in a final volume of 20 μl containing 3.2 pmol of primer (Table 1). The reaction was carried out in a thermocycler (Biomed). The samples were denatured at 95°C for 1 min and subjected to 25 cycles of denaturation at 95°C for 30 s, annealing at 53°C for 30 s, and elongation at 60°C for 4 min. The labeled DNA was extracted twice with phenol-H2O-chloroform (68/18/14; vol/vol/vol), precipitated with ethanol, and resuspended in 5 μl of denaturant mixture (50 mM EDTA, 20% formamide). The samples were boiled for 2 min and chilled on ice, and 4 μl was immediately loaded onto a 6% polyacrylamide sequence gel (acrylamide/bisacrylamide, 19/1; 8.3 M urea). The sequencing was carried out on an automated DNA sequencer (373A; Perkin Elmer/Applied Biosystems) for 11 h. The data were collected and analyzed with 373A computer software. DNA sequencing was performed in both orientations for all serovars to confirm the nucleotide sequence. Furthermore, nucleotide sequencing of the serovar E (UW5) prototype, whose DNA sequence is known, was included to confirm the accuracy of the sequencing reactions.
RESULTS
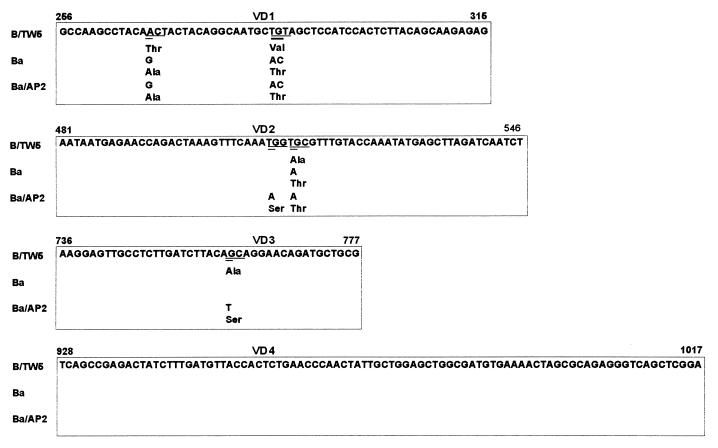
The results of genotyping by RFLP analysis of omp1 (see Fig. 1 for a schematic presentation) corresponded to the serotyping results for 87 of the 93 clinical isolates tested in this study. Eleven were typed as serovar D, 23 were typed as serovar E, 18 were typed as serovar F, 2 were typed as serovar G, 3 were typed as serovar Ga (16), 14 were typed as serovar H, 4 were typed as serovar I, 8 were typed as serovar J, and 4 were typed as serovar K. The 11 isolates typed as serovar D were further differentiated by RFLP analysis of omp1 after digestion with CfoI; 6 of those isolates showed a pattern identical to that of serovar D, 4 showed a pattern identical to that of serovariant D−, and 1 showed a pattern identical to that of serovariant Da. Six isolates had different results by serotyping and genotyping by RFLP analysis of omp1. Serotyping with MAbs showed that three of the isolates with discordant results serotyped both as L2 and Ba, making distinction between L2 and Ba impossible. These strains were identified as serovar Ba by genotyping by RFLP analysis of omp1, with the RFLP pattern after digestion with AluI being identical to that of strain Ba/A-7 (20). When the omp1 nucleotide sequences of the VDs of three Ba serovars strains were compared to the omp1 nucleotide sequence of prototype B/TW5, three substitutions in VD1 and one substitution in VD2 were found in these Ba strains (Fig. 2). One of the nucleotide substitutions in VD1 (nucleotide 268; A→G) resulted in an additional AluI restriction site. All nucleotide substitutions resulted in amino acid substitutions (Fig. 2). Comparison of the omp1 nucleotide sequences of our Ba isolates with the VDs of other B and Ba strains showed that our Ba strains were not identical to the ocular prototype Ba/AP-2 (Fig. 2) but had sequences identical to those of strain Ba/J160 (not included in Fig. 2), a strain isolated from a patient with trachoma (3).
FIG. 2.
Nucleotide and amino acid sequence comparison of the omp1 VDs of the prototype B/TW5, the genital Ba strain found in this study, and prototype Ba/AP-2. The nucleotide substitutions are double underlined. The codons having nucleotide substitutions are underlined. The VDs are boxed.
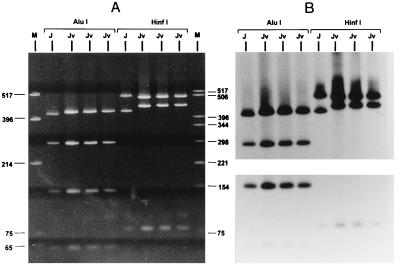
The other three isolates (isolates 424, 443, and 453) with discordant serotyping and genotyping results serotyped as serovar J, but their pattern by RFLP analysis of omp1 was different from that of prototype J/UW36, as shown in Fig. 3A. In Fig. 3B the omp1 specificity of the restriction fragments was confirmed by Southern blot hybridization with a probe of the omp1 PCR product randomly labeled with [α-32P]dCTP. This J genovariant (designated Jv) had almost the same RFLP pattern after digestion with AluI as prototype J/UW36 (Fig. 3A, lane 2; Fig. 3B [Southern blot hybridization], lane 1), except that the largest fragment obtained by RFLP analysis of omp1 was slightly longer, as shown in lanes 3, 4, and 5 of Fig. 3A and lanes 2, 3, and 4 of Fig. 3B. The RFLP pattern of this J genovariant after digestion with HinfI (Fig. 3A, lanes 7, 8, and 9) was clearly different from that of prototype J/UW36 (Fig. 3A, lane 6). Nucleotide sequencing of approximately 1.1 kb of the omp1 gene of the J prototype and two isolates of this J variant (isolates 424 and 443) confirmed the findings obtained by typing by RFLP analysis. The observed RFLP pattern of Jv obtained after digestion with AluI, with a slightly longer upper fragment by RFLP analysis of omp1 (Fig. 3, lanes 3, 4, and 5), is due to a mutation in VD2 resulting in the loss of the AluI restriction site at nucleotide 499, as shown by the nucleotide sequences of the omp1 genes of serovars J and Jv (Fig. 4). The next AluI site is at nucleotide 506, resulting in a 7-bp longer fragment by RFLP analysis, as shown in Fig. 3 (lanes 3, 4, and 5). The clearly different HinfI RFLP pattern of Jv compared to that of prototype J/UW36 (Fig. 3, lanes 6 to 9) could be explained as follows. In the case of the smaller lower fragment due to one point mutation in VD2, resulting in the loss of a HinfI restriction site at nucleotide 519, a longer fragment by RFLP analysis compared to that of the J prototype (441 bp for 543-78 versus 465 bp for 519-78) was generated. For Jv the largest fragment by RFLP analysis of omp1 after digestion with HinfI is slightly smaller compared to that for the J prototype. However, this length difference observed by RFLP analysis cannot be explained by the nucleotide sequence that was obtained since no additional HinfI site was found responsible for the generation of a slightly smaller fragment for Jv (possible options are the 3′ region of VD2 or the 3′ region of CD5; see Discussion).
FIG. 3.
(A) RFLP patterns of omp1 after restriction with AluI and HinfI for serovar J/UW36 (lanes 2 and 6, respectively) and genovariant Jv (isolates 424, 443, and 453) lanes 3, 4, and 5 and lanes 7, 8, and 9, respectively). Lane 1, molecular weight marker (pUC19 digested with HinfI); lane 10, pBR322 digested with HinfI. (B) Southern blot hybridization results for panel A by using a probe of the omp1 PCR product randomly labeled with [α-32P]dCTP as described previously (15). The film in the upper panel of panel B was exposed for 2 h and the film in the lower panel was exposed for 4 h to obtain visible and equal intensities.
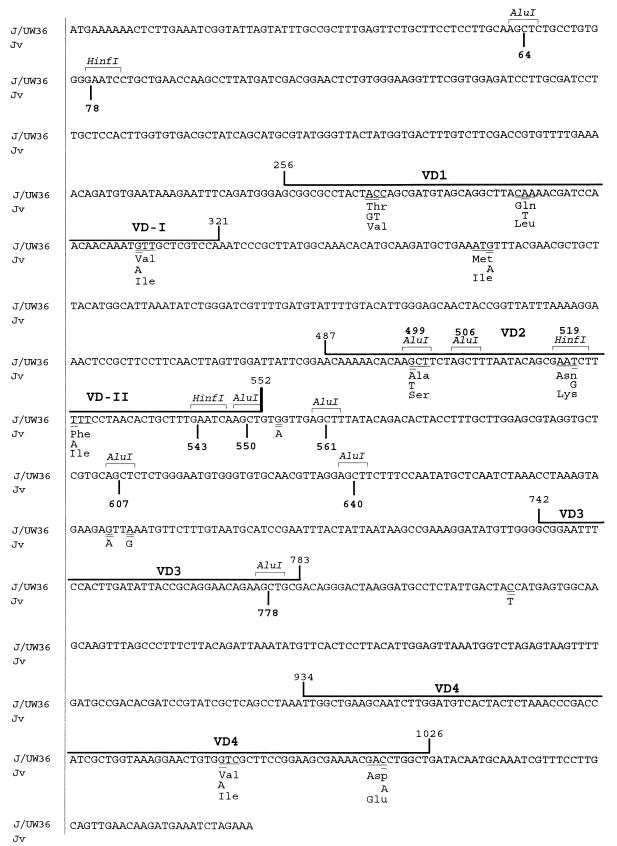
FIG. 4.
Comparison of nucleotide and amino acid sequences of omp1 genes from prototype J/UW36 and J1 and J2 genovariants found in this study. The nucleotide substitutions are double underlined. The codons having nucleotide substitutions are underlined. The VDs are boxed.
The 14 nucleotide substitutions in the two Jv isolates analyzed were identical, and their sequences were identical to the sequence of prototype J/UW36. These nucleotide substitutions were distributed throughout the omp1 gene: four mutations in VD1, three in VD2, two in VD4, one in CD2, three in CD3, and one in CD4 (Fig. 4). All nucleotide substitutions in the VDs were missense mutations, resulting in amino acid substitutions, whereas nucleotide substitutions in all except one of the CDs were silent mutations (Fig. 4). The sequences of all CDs, VD1, and VD4 had the highest degrees of homology with the CDs and VD1 and VD4 of prototype J, as follows: CD1, 100%; CD2, 99%; CD3, 98%; CD4, 99%; CD5, 100%; VD1, 94%; and VD4, 98%. The nucleotide sequence of VD2 of Jv had the highest degree of homology to prototype C: Three nucleotide substitutions (G→T, T→G, and T→A) and deduced amino acid substitutions (Ala→Ser, Asn→Lys, and Phe→Ile) showed that the sequence of prototype C serovar, except for a single nucleotide at nucleotide 541, is identical to that of prototype J. The nucleotide sequence of VD3 of Jv was identical to the VD3 sequences of prototypes C, H, I, and J.
Serovariant Ga was identified by serotyping as described previously (16) and was differentiated from serovar G by genotyping by RFLP analysis of omp1 by an additional BstUI restriction site (16) (Fig. 1). The omp1 nucleotide sequences of the VDs of the Ga variants revealed two nucleotides substitutions compared to the nucleotide sequence of serovar G/UW57. The nucleotide substitutions were found in VD2 (nucleotide 547; T→A) and VD4 (nucleotide 1003; T→G) and resulted in amino acid substitutions of Leu→Ile in VD2 and Ser→Ala in VD4. On the other hand, the sequences of VD1 and VD3 were identical to those of VD1 and VD3 of serovar G/UW57. The nucleotide substitution in VD4 (nucleotide 1003; TCG→GCG) resulted in an additional BstUI restriction site.
DISCUSSION
The comparison of the serotyping results versus the genotyping results by RFLP analysis of omp1 gave 94% concordance for 93 C. trachomatis isolates from the urogenital tract. These data validate the fact that genotyping by RFLP analysis of omp1 is a reliable tool for typing C. trachomatis isolates in epidemiological studies. Furthermore, these data are in agreement with those from other comparative studies (8, 19). Gaydos et al. (8) reported that genotyping by RFLP analysis of omp1 and serotyping yielded identical results for 42 of 43 (98%) clinical samples infected with a single serovar but not for 7 samples with suspected double infections. Rodriguez et al. (19) reported that genotyping by RFLP analysis of omp1 and serotyping yielded identical results for 147 of 150 (98%) clinical C. trachomatis strains, while the remaining 3 isolates were serotyped as serovar F or G but were identified as serovar G by genotyping by RFLP analysis of omp1. In both studies nucleotide sequence analysis of omp1 was not performed to further analyze the isolates with discordant typing results. In this study clinical isolates with atypical serotyping results or aberrant genotyping results by RFLP analysis of omp1 were additionally characterized by nucleotide sequence analysis of the omp1 gene.
Three Ba isolates which were identified by genotyping by RFLP analysis of omp1 and by nucleotide sequencing analysis of omp1 were not clearly serotyped as Ba. Although serovar L2 and the AP-2 strain of Ba could be discriminated by serotyping (16), the genital Ba strains found in this study could not be discriminated from L2 by serotyping. These Ba/L2 serovars were identified as serovar Ba by genotyping by RFLP analysis of omp1 since their RFLP patterns were identical to that of strain Ba/A-7, as reported by Sayada et al. (20). The nucleotide sequences of the VD1, VD2, and VD4 of the omp1 genes of these Ba strains were identical to the sequences of these VDs of Ba/J160, an ocular Ba type (3). Although nucleotide sequence information for Ba/A-7 was not available, it may well be possible that Ba/J160, Ba/A-7, and the Ba strains found in this study are identical. Three percent of the urogenital C. trachomatis infections found in this study were caused by this Ba strain. Seven different Ba strains have been described to date (3, 4, 20); of these strains strain Ba/UW113 was isolated from the urogenital tract, while the other strains were isolated from eyes. The sequences of VD2 and VD4 of our genital tract Ba isolates were clearly different from those of VD2 and VD4 of Ba/UW113, the only urogenital Ba strain characterized by sequencing (3), by a single point mutation, resulting in an amino acid substitution in both VDs. In contrast, 100% sequence similarity was found in the VD1, VD2, and VD4 regions between our Ba strains and the ocular Ba/J160 strain, possibly indicating that this strain can infect both ocular and genital sites. In this study, as well as in a previous study (22) involving the genotyping of 350 urogenital isolates by RFLP analysis of omp1, this A-7-like Ba strain was the only serovar B-related type observed. Moreover, Ba strains have also been observed in urogenital specimens in Canada (6), but it is unknown whether they resemble Ba/J160 or A-7. These data indicate that Ba serovars are responsible for both ocular and genital infections.
In this study for the first time the complete sequence of the omp1 gene of serovar J/UW36 has been determined. By using these sequence data a J genovariant, designated Jv, was identified in 3 of the 11 J serovars by RFLP analysis and nucleotide sequence analysis of omp1. Poole et al. (17) described a J′ strain with three nucleotide substitutions in VD4, two of which were identical to those found in Jv. However, they restricted the sequence analysis only to VD4. Moreover, the deduced omp1 amino acid sequences of Jv showed multiple amino acid substitutions in the VDs (Fig. 4); these, however, did not influence the reactivity of the MAbs used to identify serovar J. The observed differences in the RFLP patterns of Jv and J/UW36 prototype serovar after digestion with AluI and HinfI could be explained by the nucleotide sequences of the omp1 genes of J and Jv, except that the largest HinfI fragment of Jv was slightly smaller compared to the size of the largest fragment of J. Cloning of these largest HinfI cleavage fragments of J and Jv into a plasmid vector (after HinfI restriction of the omp1 PCR product and excision of this fragment from the gel), followed by sequencing, confirmed the expected sequence of the 5′ HinfI site at nucleotide 543 and the expected sequence of the 3′ end at nucleotide 1076. Also, the six point mutations in Jv were confirmed, and no internal deletion was found in this Jv fragment. Interestingly, when the cloned upper HinfI fragments of J and Jv were cut out of the vector, they still showed, even under denaturating conditions, differences in migration, while nucleotide sequencing proved that both fragments are of the same length. Furthermore, the two largest HinfI fragments of 533 bp from J and Jv migrate faster than the 517-bp fragment standard and are closer to the 506-bp fragment standard. To our knowledge, this particular phenomenon has not been documented previously. This migration anomaly may be due to the charge differences resulting from the six nucleotide substitutions present in the Jv HinfI fragment. Although further investigation is in progress, it is clearly proven by RFLP analysis and nucleotide sequence analysis that Jv is a variant of serovar J.
In this study three isolates were identified as Ga variants (strain IOL-238) by serotyping. The Ga variant was defined by a positive staining reaction with MAb 8.3H8, which did not react with the prototype G/UW57 (16). The VD4 nucleotide sequences of the omp1 genes of these Ga variants were found to be identical to that of the genovariant G strain IOL-238 reported by Poole et al. (17), who only sequenced VD4. In this study, additional sequence analysis of VD1, VD2, and VD3 of omp1 showed that a missense mutation was also found in VD2, and this resulted in an amino acid substitution. Therefore, the recognition site of MAb 8.3H8 is probably located in VD2 or VD4.
It has been speculated that omp1 genovariants occur as a result of point mutations and recombination events selected by immune pressure (8, 9, 12). The sequences of Ba, Ga, and Jv variants had several point mutations compared with the sequences of prototypes B, G, and J, respectively. Although the nucleotide sequence of VD2 of serovar Jv showed the highest degree of homology with that of VD2 of serovar C, our data do not support the hypothesis of a recombination (9) between C and J, since sequences of all CDs of Jv and prototype J were highly homologous (Fig. 4). The mutations found in variants Ba, Ga, and Jv in this study, as well as in other variants of serovars Ba, D, I, and L2 found by others (2, 3, 12), are most frequently observed in the surface-exposed VD1, VD2, and VD4 (21). These mutations, which always appear to be missense mutations, might therefore have an important role in protecting C. trachomatis and helping it to escape the host immunity to omp1. In contrast, non-surface-exposed VD3 appears to be more conserved. Interestingly, all except one of the nucleotide substitutions found in the CDs of genovariant Jv were silent mutations, as was also observed for Ba when analyzing the nucleotide sequences of the CDs of different ocular Ba strains reported by Dean et al. (3). The amino acid compositions of CDs must be stable since they are involved in transmembrane interactions. In addition, the point mutations found in these variants are not likely to have originated in vitro, either by cell culture or by PCR amplification, since identical variants of Ba, Jv, and Ga, as reported in this study, were isolated from nonrelated patients. To exclude the existence of an in vitro mutation due to culture, a comparison should have been made by analyzing Jv directly in the original clinical sample (before culture). Unfortunately, in this study the original clinical samples were no longer available.
The occurrence of C. trachomatis variants has been described by using different typing methods (19, 24). Rodriguez et al. (19) reported that by using RFLP analysis of omp1 with AluI and HpaII-EcoRI-HinfI restriction, 3% of clinical isolates belonged to variants of serovars B, C, and I. Yang et al. (24) have found by DNA sequencing that up to 30% of the clinical C. trachomatis isolates had omp1 nucleotide sequence variations, which were more frequently found in isolates belonging to serovars D, E, G, H, K, and Ba than those belonging to serovars F and J. Our results indicated that 3% genovariants (3 of 93; Jv) were found by genotyping by RFLP analysis of omp1 and that 3% of the isolates (3 of 93; genital Ba) showed atypical results by serotyping. The number of variants may be underestimated since only strains with atypical serotyping patterns or atypical RFLP patterns were subjected to omp1 nucleotide sequence analysis in this study. Nevertheless, to study the epidemiology of C. trachomatis infections, genotyping by RFLP analysis of omp1 is simpler and more rapid than labor-intensive serotyping and DNA sequencing methods. omp1 DNA sequencing is necessary to characterize possible new C. trachomatis variants identified by either serotyping or genotyping by RFLP analysis of omp1.
In conclusion, for typing of clinical C. trachomatis isolates, PCR-based genotyping by RFLP analysis of omp1 showed a higher discriminatory power and is more convenient than serotyping. Therefore, this genotyping approach is strongly recommended for future epidemiological studies of C. trachomatis. In addition, a substantial number of C. trachomatis variants (Ba, Ga, and Jv) were found in clinical isolates from a population of patients with STDs. Identification and characterization of omp1 variants present in the human urogenital tract are of great value for molecular epidemiology studies with C. trachomatis and provide information necessary for the development of a vaccine directed against the MOMP-1 epitope.
REFERENCES
- 1.Batteiger B E. The major outer membrane protein of a single Chlamydia trachomatis can possess more than one serovar-specific epitope. Infect Immun. 1996;64:542–547. doi: 10.1128/iai.64.2.542-547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean D, Patton M, Stephens R S. Direct sequence evaluation of the major outer membrane protein gene variant regions of Chlamydia trachomatis subtypes D′, I′, and L2′. Infect Immun. 1991;59:1579–1582. doi: 10.1128/iai.59.4.1579-1582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean D, Schachter J, Dawson C R, Stephens S. Comparison of the major outer membrane protein variant sequence regions of B/Ba isolates: a molecular epidemiologic approach to Chlamydia trachomatis infections. J Infect Dis. 1992;166:383–392. doi: 10.1093/infdis/166.2.383. [DOI] [PubMed] [Google Scholar]
- 4.Dean D. Molecular characterization of a new Chlamydia trachomatis serological variant from a trachoma endemic region of Africa. In: Orfila J, Byrne G I, Chernesky M A, et al., editors. Chlamydial infections. Proceedings of the Eighth International Symposium on Human Chlamydial Infections, Chantilly, France. Bologna, Italy: Società Editrice Esculapio; 1994. pp. 259–262. [Google Scholar]
- 5.Dean D, Miller K. Molecular and mutation trends analyses of omp1 alleles for serovar E of Chlamydia trachomatis. Implications for the immunopathogenesis of disease. J Clin Invest. 1997;99:475–483. doi: 10.1172/JCI119182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost E H, Deslandes S, Veilleux S, Bourgaux-Ramoisy D. Typing Chlamydia trachomatis by detection of restriction fragment length polymorphism in the gene encoding the major outer membrane protein. J Infect Dis. 1991;163:1103–1107. doi: 10.1093/infdis/163.5.1103. [DOI] [PubMed] [Google Scholar]
- 7.Frost E H, Deslandes S, Gendron D, Bourgaux-Ramoisy D, Bourgaux P. Variation outside variable segments of the major outer membrane protein distinguishes trachoma from urogenital isolates of the same serovar of Chlamydia trachomatis. Genitourin Med. 1995;71:18–23. doi: 10.1136/sti.71.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaydos C H, Bobo L, Welsh L, Hook III E W, Viscidi R, Quinn T C. Gene typing of Chlamydia trachomatis by polymerase chain reaction and restriction endonuclease digestion. Sex Transm Dis. 1992;19:303–308. [PubMed] [Google Scholar]
- 9.Hayes L J, Yearsley P, Treharne J D, Ballard R A, Fehler G H, Ward M E. Evidence for naturally occurring recombination in the gene encoding the major outer membrane protein of lymphogranuloma venereum isolates of Chlamydia trachomatis. Infect Immun. 1994;62:5659–5663. doi: 10.1128/iai.62.12.5659-5663.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaltenboeck B, Kousoulas K G, Storz J. Structure of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo C C, Wang S-P, Holmes K K, Grayston J T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983;41:865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lampe M F, Suchland R J, Stamm W E. Nucleotide sequence of the variable domains within the major outer membrane protein gene from serovariants of Chlamydia trachomatis. Infect Immun. 1993;61:213–219. doi: 10.1128/iai.61.1.213-219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampe M F, Wong K G, Stamm W E. Sequence conservation in the major outer membrane protein gene among Chlamydia trachomatis strains isolated from the upper and lower urogenital tract. J Infect Dis. 1995;172:589–592. doi: 10.1093/infdis/172.2.589. [DOI] [PubMed] [Google Scholar]
- 14.Lan J, Ossewaarde J M, Walboomers J M M, Meijer C J L M, van den Brule A J C. Improved PCR sensitivity for direct genotyping of Chlamydia trachomatis serovars by using a nested PCR. J Clin Microbiol. 1994;32:528–530. doi: 10.1128/jcm.32.2.528-530.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan J, Walboomers J M M, Roosendaal R, van Doornum G J, MacLaren D M, Meijer C J L M, van den Brule A J C. Direct detection and genotyping of Chlamydia trachomatis in cervical scrapes by using polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31:1060–1065. doi: 10.1128/jcm.31.5.1060-1065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ossewaarde J M, Rieffe M, de Vries A, Derksen-Nawrocki R P, Hooft H J, van Doornum G J J, van Loon A M. Comparison of two panels of monoclonal antibodies for serovar determination of Chlamydia trachomatis. J Clin Microbiol. 1994;32:2968–2974. doi: 10.1128/jcm.32.12.2968-2974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole E, Lamont I. Chlamydia trachomatis serovar differentiation by direct sequence analysis of the variable segment 4 region of the major outer membrane protein gene. Infect Immun. 1992;60:1089–1094. doi: 10.1128/iai.60.3.1089-1094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez P, Vekris A, de Barbeyrac B, Dutilh B, Bonnet J, Bebear C. Typing of Chlamydia trachomatis by restriction endonuclease analysis of the amplified major outer membrane protein gene. J Clin Microbiol. 1991;29:1132–1136. doi: 10.1128/jcm.29.6.1132-1136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez P, de Barbeyrac B, Persson K, Dutilh B, Bebear C. Evaluation of molecular typing for epidemiology study of Chlamydia trachomatis genital infections. J Clin Microbiol. 1993;31:2238–2240. doi: 10.1128/jcm.31.8.2238-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayada C, Denamur E, Orfila J, Catalan F, Elion J. Rapid genotyping of the Chlamydia trachomatis major outer membrane protein by the polymerase chain reaction. FEMS Microbiol Lett. 1991;83:73–78. doi: 10.1016/0378-1097(91)90447-i. [DOI] [PubMed] [Google Scholar]
- 21.Stephens R S, Wager E A, Schoolnik G K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988;167:817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van de Laar M J W, Lan J, van Duynhoven Y T H P, Fennema J S A, Ossewaarde J M, van den Brule A J C, van Doornum G J J, Coutinho R A, van den Hoek J A R. Differences in clinical manifestations of genital chlamydial infections related to serovars. Genitourin Med. 1996;72:261–265. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S-P, Grayton J T. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J Infect Dis. 1991;163:403–405. doi: 10.1093/infdis/163.2.403. [DOI] [PubMed] [Google Scholar]
- 24.Yang C-L, Maclean I, Brunham R. DNA sequence polymorphism of the Chlamydia trachomatis omp1 gene. J Infect Dis. 1993;168:1125–1130. doi: 10.1093/infdis/168.5.1225. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y, Zhang Y-X, Watkins N G, Caldwell H D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989;57:1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]